Abstract
Production of interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) was confirmed by enzyme-linked immunosorbent assay in a medium where human epidermal keratinocytes were cocultured with Trichophyton mentagrophytes for 1 to 12 h. IL-8 and TNF-α mRNAs were also detected in the keratinocytes cocultured with T. mentagrophytes.
The keratinized tissues of skin and hair of humans and animals are commonly infected with dermatophytes. The physical and chemical structure of the skin represents a form of defense barrier against fungal pathogens. A cutaneous immune system is probably responsible for initiating immune responses that work to prevent and to eliminate the infecting organisms (16). In addition, such responses may also induce some inflammatory agents that result in much of the symptomatology of these infections (1-5, 9, 11, 17).
Six isolates of a most common dermatophyte, Trichophyton mentagrophytes (four isolates from human patients and two isolates from animal patients), were used in this study. All isolates were identified by conventional methods (morphological characteristics of macroconidia and spiral hyphae, urease reaction, and hair perforating test) (10) and by molecular analysis of chitin synthase 1 gene sequences (8). These strains were been maintained by culturing at 25°C on 1/10 Sabouraud's glucose agar containing peptone (0.1%), glucose (0.2%), KH2PO4 (0.1%), MgSO47H2O (0.1%), and agar (2%) and were subcultured more than twice every 2 weeks before use.
Human epidermal keratinocytes of foreskin (Cosmo Bio, Tokyo, Japan) were cultured in the serum-free medium K110 keratinocyte growth medium (Cosmo Bio) at 37°C and 5% CO2 for 5 days.
Coculture of epidermal keratinocytes and microconidia of T. mentagrophytes was carried out based on a previously described method (7). In the preliminary studies it was determined that the number of the microconidia per human epidermal keratinocyte needed for optimal stimulation varied widely among dermatophytes tested (data not shown). On the basis of these studies, a 1:1 ratio of microconidia to keratinocytes was chosen for subsequent experiments. Moreover, no good results were obtained in preliminary experiments when heat-killed microconidia were used. Therefore, microconidia were collected by being scraped from the white powdery colonies of T. mentagrophytes grown on 1/10 Sabouraud's glucose agar and were suspended with 0.04% Tween 80 distilled water (pH 7.4) in a glass homogenizer. These microconidia were washed twice in phosphate-buffered saline and were resuspended in K110 medium. The human epidermal keratinocytes (104 cells/well) were incubated on a microplate with 96 wells at 37°C and 5% CO2 for 16 h, and then the microconidia of T. mentagrophytes (104 cells/well) were distributed to the wells. After 1 to 12 h of coculture these supernatants were obtained and kept at −20°C until use for cytokine assay with enzyme-linked immunosorbent assay (ELISA).
Every assay was carried out with a Cytoscreen Immunoassay kit (BioSource International, Camerillo, Calif.), and this assay system was sensitive to more than 1 pg/ml. All experiments were done in triplicate; results were expressed as means ± standard deviations. Comparisons among tests were done by the Student's t test, with statistical significance considered to be P < 0.01.
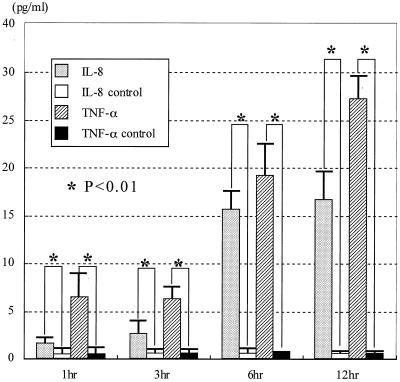
After 1 to 12 h of coculture of epidermal keratinocytes and microconidia of T. mentagrophytes, the interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) levels in the supernatants were determined by ELISA. The culture supernatants of keratinocytes alone were used as the control. IL-8 and TNF-α were detected in the supernatant and increased following coculture, although the levels of other cytokines (IL-1β, IL-6, and monocyte chemoattractant protein 1 [MCP-1]) were very low or undetectable in these assays (Fig. 1). The IL-8 level in the supernatant increased at 3 h after coculture of keratinocytes with T. mentagrophytes. On the other hand, TNF-α levels in the supernatant increased suddenly after coculturing. However, IL-1β, IL-6, and MCP-1 levels were not detected in each culture supernatant of epidermal keratinocytes and T. mentagrophytes examined at every culturing time.
FIG. 1.
After 1, 3, 6, and 12 h of coculture with the microconidia and keratinocytes, cytokine levels in the supernatants were measured by the ELISA method. IL-8 and TNF-α levels in the supernatant were increased following coculture. The statistical significance was determined by the nonpaired t test.
The human epidermal keratinocytes (3.5 × 105 cells) were incubated in the cell culture dish at 37°C and 5% CO2 for 16 h, and then the microconidia of T. mentagrophytes (3.5 × 105 cells) were distributed to the dishes. After 1 to 12 h of coculture these keratinocytes were obtained and kept at −80°C until use for the isolation of RNA. Total RNAs were extracted from the keratinocyte samples by an RNeasy total RNA kit (Qiagen, Valencia, Calif.). Reverse transcription (RT) of the poly(A)+ RNA was carried out with an Ominiscript Reverse Transcriptase kit (Qiagen). The RNase-Free DNase set (Qiagen) was used for dissolving DNase while purifying RNA. RT-PCR primers were prepared on the basis of the sequences conserved in human cytokines. The primer sequences used for amplification of human ILs were the following: 5′-GCA GTC TAC ACA GCT TCG GG-3′ (primer IL-1β 1S; nucleotides [nt] 88 to 107 in mRNA for human interferon-gamma-inducing factor IL-1β; DDBJ/EMBL/GenBank accession no. D49950) and 5′-CCG CCT CAG CCT CCC AAA G-3′ (primer IL-1β 1R; nt 820 to 810) (14), 5′-GCC TTC GGT CCA GTT GCC TT-3′ (primer IL-6 1S; nt 22 to 41 in mRNA for human IL-6; DDBJ/EMBL/GenBank accession no. X04602) and 5′-GCA GAA TGA GAT GAG TTG TC-3′ (primer IL-6 1R; nt 586 to 567) (6), and 5′-ATG ACT TCC AAG CTG GCC GT-3′ (primer IL-8 1S; nt 102 to 122 in mRNA for human TNF; DDBJ/EMBL/GenBank accession no. M10988) and 5′-TCC TTG GCA AAA CTG CAC CT-3′ (primer IL-8 1R; nt 164 to 183) (12). Other primer pair sequences used for amplification of human MCP-1 and TNF-α were 5′-ATG AAA GTC TCT GCC GCC CT CA-3′ (primer MCP-1 S1; nt 598 to 617 in mRNA for the human MCP gene; DDBJ/EMBL/GenBank accession no. M10988) and 5′-GAG ATC TGT GCT GAC CCC AA-3′ (primer MCP-1 R1; nt 197 to 216) (13) and 5′-AGG CGC TCC CCA AGA AGA CA-3′ (primer TNF-α S2; nt 129 to 148 in mRNA for human TNF; DDBJ/EMBL/GenBank accession no. M10988) and 5′-TCC TTG GCA AAA CTG CAC CT-3′ (primer TNF-α R1; nt 164 to 183) (15). The cDNA samples from epidermal keratinocytes were amplified by PCR in a reaction mixture (30 μl) containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, 200 mM each deoxynucleoside triphosphate, 1.0 U of Taq polymerase (Takara, Kyoto, Japan), and 0.5 μg of a pair of primers. The PCR amplification was carried out for 30 cycles consisting of template denaturation (1 min at 94°C), primer annealing (1 min at 55°C), and polymerization (2 min at 72°C). The PCR products were electrophoresed through 3% (IL-1β, IL-6, and IL-8) or 2% (MCP-1 and TNF-α) agarose gels and then were stained with ethidium bromide and observed under UV irradiation.
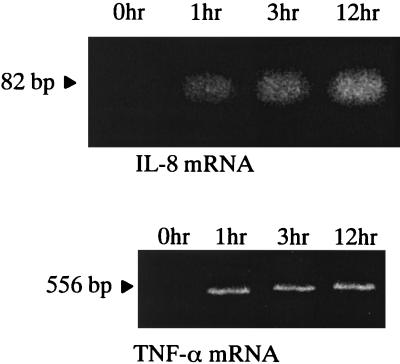
After coculturing epidermal keratinocytes and T. mentagrophytes at 0, 1, 3, and 12 h, IL-8 and TNF-α mRNAs in human epidermal keratinocytes were detected by RT-PCR (Fig. 2). IL-8 and TNF-α mRNAs were detected in epidermal keratinocytes after 1 to 12 h of coculture, although these mRNAs in epidermal keratinocyte were not detectable at the beginning of coculture. These results suggested that T. mentagrophytes stimulated the production of IL-8 and TNF-α mRNAs in human epidermal keratinocytes.
FIG. 2.
The mRNA of IL-8 and TNF-α in human epidermal keratinocytes cocultured with T. mentagrophytes. After 0, 1, 3, and 12 h of coculture with human epidermal keratinocytes and T. mentagrophytes, mRNAs of IL-8 and TNF-α in the keratinocytes were determined by RT-PCR. IL-8 and TNF-α of mRNAs were detected after 1 to 12 h of coculture but not at 0 h of coculture.
The data in this study revealed that T. mentagrophytes directly induced IL-8 and TNF-α production from human epidermal keratinocytes without activated macrophages. This finding was also confirmed by detecting mRNAs of IL-8 and TNF-α in the keratinocytes cocultured with T. mentagrophytes. In addition, T. mentagrophytes could not induce IL-1β, IL-6, or MCP-1 in this study. These results suggested that IL-8 and TNF-α might be main cytokines released from human epidermal keratinocytes when T. mentagrophytes infects human and animals. Therefore, T. mentagrophytes seems to have direct and unique effects on cytokine production from keratinocytes. Further analysis is required to understand the process of IL-8 and TNF-α production from keratinocytes and the relationships to the host defense mechanisms against T. mentagrophytes infections.
Nucleotide sequence accession numbers.
The sequences determined in this study were deposited in the DDBJ, EMBL, and GenBank databases and were given accession numbers as follows: primer IL-1β 1S, no. D49950; primer IL-6 1S, no. X04602; primer IL-8 1S, no. M10988; primer MCP-1 S1, no. M10988; primer TNF-α S2, no. M10988.
REFERENCES
- 1.Ansel, J., P. Perry, J. Brown, D. Damm, T. Phan, C. Hart, T. Luger, and S. Hefeneider. 1990. Cytokine modulation of keratinocyte cytokines. J. Investig. Dermatol. 94(Suppl.):101S-107S. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, H. S. I., S. Reitamo, P. Erkko, M. Ceska, B. Moser, and M. Baggiolini. 1992. Interleukin-8 immunoreactivity in the skin healthy subjects and patients with palmoplanter pustulosis and psoriasis. J. Investig. Dermatol. 98:96-100. [DOI] [PubMed] [Google Scholar]
- 3.Barker, J. N. W., V. Sarma, R. S. Mitra, V. M. Dixit, and B. J. Nickoloff. 1990. Marked synergism between tumor necrosis factor-α and interferon-α in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J. Clin. Investig. 85:605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74-80. [DOI] [PubMed] [Google Scholar]
- 5.Boxman, I. L. A., C. Ruwhof, O. C. Boerman, C. W. G. M. Löwik, and M. Ponec. 1996. Role of fibroblasts in the regulation of proinflammatory interleukin IL-1, IL-6 and IL-8 levels induced by keratinocyte-derived IL-1. Arch. Dermatol. Res. 288:391-398. [DOI] [PubMed] [Google Scholar]
- 6.Hirano, T., K. Yasukawa, H. Harada, T. Taga, Y. Watanabe, T. Matsuda, S. I. Kashiwamura, K. Nakajima, K. Koyama, A. Iwamatsu, S. Tsunasawa, F. Sakiyama, H. Matsui, Y. Takahara, T. Taniguchi, and T. Kishimoto. 1986. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324:73-76. [DOI] [PubMed] [Google Scholar]
- 7.Jiang, Y., T. R. Russell, D. T. Graves, H. Cheng, S. Hong, and A. M. Levitz. 1996. Monocyte chemoattractant protein 1 and interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect. Immun. 64:4450-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kano, R., Y. Nakamura, T. Watari, S. Watanabe, H. Takahashi, H. Tsujimoto, and A. Hasegawa. 1998. Molecular analysis of chitin synthase 1 (CHS 1) gene sequences of Trichophyton mentagrophytes complex and T. rubrum. Curr. Microbiol. 37:236-239. [DOI] [PubMed] [Google Scholar]
- 9.Kondo, T., and T. Ohshima. 1996. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int. J. Legal Med. 108:231-236. [DOI] [PubMed] [Google Scholar]
- 10.Kwon-Chung, K. J., and J. E. Bennett. 1992. Dermatophytoses, p. 143-147. In K. J. Kwon-Chung and J. E. Bennett (ed.), Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 11.Mateo, R. B., J. S. Reichner, and J. E. Albina. 1994. Interleukin-6 activity in wounds. Am. J. Physiol. 266:R1840-R1844. [DOI] [PubMed] [Google Scholar]
- 12.Matsuhima, K., K. Morishita, T. Yoshimura, S. Lavu, Y. Kobayashi, W. Lew, E. Appella, H. F. Kung, E. J. Leonard, and J. J. Oppenheim. 1988. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J. Exp. Med. 167:1883-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyy, Y.-J., Y.-S. Li, and P. E. Kolattukudy. 1990. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem. Biophys. Res. Commun. 169:346-351. [DOI] [PubMed] [Google Scholar]
- 14.Ushio, S., M. Namba, T. Okura, K. Hattori, Y. Nukada, K. Akita, F. Tanabe, K. Konishi, M. micallef, M. Fujii, K. Torigoe, T. Tanimoto, S. Fukuda, M. Ikeda, H. Okamura, and M. Kurimoto. 1996. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 156:4274-4279. [PubMed] [Google Scholar]
- 15.Wang, A. M., A. A. Creasey, M. B. Ladner, L. S. Lin, J. Strickler, J. N. Van Arsdell, R. Yamamoto, and D. F. Mark. 1985. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science 228:149-154. [DOI] [PubMed] [Google Scholar]
- 16.Wang, B., N. Ruiz, A. Pentland, and M. Caparon. 1997. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect. Immun. 65:2119-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe, S., R. Kano, H. Sato, Y. Nakamura, and A. Hasegawa. 2001. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J. Investig. Dermatol. 116:769-773. [DOI] [PubMed] [Google Scholar]