Abstract
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease that can lead to cirrhosis in up to 30% of patients. Cirrhotic patients are at risk of high morbidity and mortality due to cirrhosis decompensation and hepatocellular carcinoma (HCC). This retrospective study assessed the rates of decompensated cirrhosis and HCC in patients with AIH-related cirrhosis. A total of 774 AIH patients were included, with 40% developing cirrhosis. Over a median follow-up of 8.2 years (IQR 2.9–12.3), the annual incidence of decompensated cirrhosis was 4.25%, with a mean time of 8.2 years from cirrhosis diagnosis to decompensation. Nineteen cirrhotic patients (6.2%) developed HCC, with a yearly incidence rate of 0.63%. Most HCC cases occurred within the first years of cirrhosis diagnosis. The rate of decompensated cirrhosis in AIH patients was lower than in other cirrhotic liver diseases, suggesting AIH may follow a different clinical course. The annual incidence of HCC was also significantly lower than the threshold for HCC surveillance. This indicates the need to reassess current surveillance guidelines, particularly in the late years following a cirrhosis diagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-96342-7.
Keywords: Autoimmune hepatitis, Cirrhosis, Cirrhosis decompensation hepatocellular carcinoma
Subject terms: Liver, Liver diseases
Introduction
Autoimmune hepatitis (AIH) is a rare and heterogeneous chronic liver disease characterized by circulating autoantibodies, hypergammaglobulinemia, and specific liver histological abnormalities1–3. Clinical manifestations of AIH range from asymptomatic disease to acute hepatitis and occasionally fulminant hepatic failure4,5. The first-line treatment for AIH comprises two core components: glucocorticoids and immunosuppression, leading to 80–90% remission6. Diagnosis of autoimmune hepatitis justifies life-long therapy in most patients to prevent the development of cirrhosis and end-stage liver disease7. Approximately one-third of patients with AIH have evidence of cirrhosis at the time of initial diagnosis8,9. In a systemic review published by Tansel et al., which included 25 studies with 6528 patients exploring the rate of progress of Patients with AIH to cirrhosis, the results were very heterogeneous, with HCC incidence ranging from 0.1 to 8.1% annually10.
Decompensated cirrhosis is defined by the development of one of the following complications: jaundice, ascites, hepatic encephalopathy, or variceal hemorrhage. Patients with acute decompensation and organ failure(s) are at high risk of short-term death12. Overall, the rate of decompensation for cirrhosis patients with compensated disease was 11.8% annually, adjusted for age and gender11. While numerous studies have concluded that the rate of cirrhosis decompensation could be lower in AIH patients than in those with other etiologies, no previous research has examined the rate of decompensation of cirrhosis in AIH patients.
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer, the sixth most common cancer, and the third leading cause of cancer-related death worldwide. In 2020, roughly 906,000 new HCC cases and 830,000 deaths were reported globally13. Cirrhosis represents the leading risk factor for the development of HCC, as it is present in more than 80–90% of cases14. However, estimates of HCC risk among patients with AIH in cohort studies have varied widely15–18. Two prominent North European cohort studies reported an elevated general cancer risk in patients with AIH compared to age- and sex-matched controls while showing a minimal increase in HCC development19,20. In a recent multicenter retrospective observational study, it was shown that the risk of HCC in AIH is low even after the development of cirrhosis and is associated with risk factors, including obesity, cirrhosis, and AIH/PSC variant syndrome13. Considering the presumed low incidence of HCC among AIH patients, this raises the question of whether to conduct surveillance in these patients, given that the accepted threshold for cost-effective surveillance for HCC is 1.5% annually10,13.
Therefore, this study aimed at describing the incidence of cirrhosis, decompensation, and HCC in AIH patients and providing new insights into the progression of autoimmune hepatitis within a large retrospective AIH cohort study with long-term follow-up. The thoroughness of our research, conducted within a large and diverse patient population, provides confidence in the reliability of our findings.
Methods
Setting
Clalit Health Services (CHS) is Israel’s largest Health Maintenance Organization (HMO), catering to the healthcare needs of approximately 4.8 million members, constituting 52% of the Israeli population. It operates a network of 14 hospitals and around 1500 clinics spread nationwide, alongside laboratories, imaging facilities, and pharmacies integrating outpatient and inpatient data, significantly enhancing the precision and reliability of identifying diagnoses and procedures.
Ethics approval and informed consent
This retrospective study was approved by Clalit Health Services and Emek Medical Center (approval number 0091 − 23). The need for informed consent was waived by the Institutional Review Board due to the retrospective nature of the study, in accordance with relevant ethical guidelines. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Patients
This retrospective, population-based study encompassed approximately 2.7 million adults and relied on data extracted from the comprehensive CHS computerized database. This electronic medical record (EMR) repository aggregates information from diverse sources, including records from primary care physicians, community specialty clinics, hospital admissions, laboratory tests, and pharmacy transactions. A registry of chronic disease diagnoses was compiled meticulously by employing diagnosis-specific algorithms, utilizing the International Classification of Diseases Ninth Revision (ICD-9) code framework (ICD-9 codes for cirrhosis and AIH are 571.5 and 571.8, respectively). Additionally, laboratory test results and disease-specific medication usage were extracted. The diagnosis of cirrhosis was further validated with clinical documentation, imaging (ultrasound, CT, MRI), and, in some cases, histology. The earliest recorded date of diagnosis for AIH, cirrhosis and other hepatic complications (encephalopathy, esophageal varices, ascites and others), and hepatocellular carcinoma from any source, was established as the defining date of diagnosis. This longitudinal dataset spans two decades and offers an extensive electronic health service data repository diagnosis.
Data collection
The dataset included adult patients aged 18 years or older at the time of autoimmune hepatitis (AIH) diagnosis between 2000 and late 2020. Diagnoses of liver cirrhosis and other hepatic complications, including hepatocellular carcinoma (HCC), were established using ICD 9 codes. Patients with pre-existing cirrhosis, ascites, encephalopathy, varices, or HCC before AIH diagnosis, as well as those with concurrent liver diseases, were excluded. Additionally, participants under 18 were excluded to ensure the study population was representative of adults. These comprehensive exclusion criteria were designed to enhance precision and provide a more accurate assessment of complication development after an AIH diagnosis in adults, tracing them over an extended follow-up period.
Available data included demographic information such as sex, age, and BMI at AIH diagnosis, liver histology, serological markers (antinuclear antibodies, smooth muscle antibodies, anti-liver kidney microsome type 1 antibodies), and serum IgG levels. Further data collected involved AIH treatment specifics (type of immunosuppression, dosage, initiation date, and duration), laboratory results at diagnosis, follow-up assessments, and survival status.
The study’s baseline was set at the time of cirrhosis diagnosis, with the primary outcomes being the development of cirrhotic complications (encephalopathy, ascites, variceal bleeding, or HCC). Patient follow-up adhered to each center’s standard practices. Importantly, due to the study’s retrospective nature, not all data points were consistently available for every patient.
Statistical analysis
Categorical variables were presented using counts and percentages, and continuous variables were presented with mean and standard deviation.
The Kaplan–Meier curve, with a 95% CI, was estimated for mortality. It presents the time-to-death for each follow-up year since the onset of AIH diagnosis.
Complication rates due to cirrhosis among the study population were compared between males and females using the Chi-square test (or the Fisher’s exact test). P < 0.05 was considered statistically significant.
In addition, cumulative incidence function (CIF) curves with 95%CI were presented for complications due to cirrhosis for the entire study cohort.
Statistical analyses were made using SAS Enterprise Guide 8.3 software (SAS Institute Inc., Cary, NC, USA).
Results
At the time of the data extraction, data were collected from 774 patients with an initial diagnosis of AIH between 2000 and 2020. The median age at AIH diagnosis was 51.5 years (interquartile range 37–65.5), with 449 (58.0%) patients above 50 years of age and 635 (81%) females. Of the study population, 273 (30.6%) were overweight (BMI 25.0–29.9 kg/m2, and 170 (22%) were obese (BMI ≥ 30.0 kg/m2). Definitive diagnosis of coexisting autoimmune diseases was witnessed in 42.6% of the study cohort, with thyroid diseases being the most common (10.9%), followed by Crohn’s disease (10.5%). Most patients used azathioprine as their primary therapy (n = 718, 92.8%). Additionally, 19.1% used mycophenolate mofetil (MMF) as a second-line therapy, while less than 5% used 6-MP. At the time of AIH diagnosis, 181 (23.4%) patients had cirrhosis. During follow-up, an additional 126 patients developed cirrhosis, resulting in a total of 307 patients who were diagnosed with cirrhosis among AIH patients (39.7%) (Table 1).
Table 1.
Characteristics of the autoimmune hepatitis cohorts.
| Total number of AIH patients | 774 |
| Mean age at diagnosis (years ± SD) | 51.5 ± 18.1 |
| Age ≥ 50 years (n, %) | 449 (58%) |
| Female (%) | 82% |
| Male (%) | 18% |
| Overweight (n, %) | 237 (30.6%) |
| Obesity (n, %) | 170 (22%) |
| Treated with azathioprine (%) | 92.8% |
| Treated with MMF (%) | 19.1% |
| Treated with purinethol/ 6MP (%) | 4.7% |
| Mean follow-up (years ± SD) | 7.2 ± 4.5 |
| Cirrhosis at diagnosis (% patients) | 23.4% |
| Death during follow-up (death, % death) | 126 (16.2%) |
| Autoimmune diseases (n, %) | 330 (42.6%) |
| IBD | 81 (10.5%) |
| CD | 20 (2.6%) |
| RA | 58 (7.5%) |
| SLE | 56 (7.2%) |
| DM | 23 (3%) |
| THY | 84 (10.9%) |
| MS | 8 (1%) |
AIH, autoimmune hepatitis; MMF, mycophenolate mofetil; 6-MP, 6- mercaptopurine; BMI, body mass index; SD- standard deviation; CD, coeliac disease; DM, diabetes mellitus; IBD, inflammatory bowel disease; MS, multiple sclerosis; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; THY, thyroid disease
Data are missing for BMI (n = 111 patients).
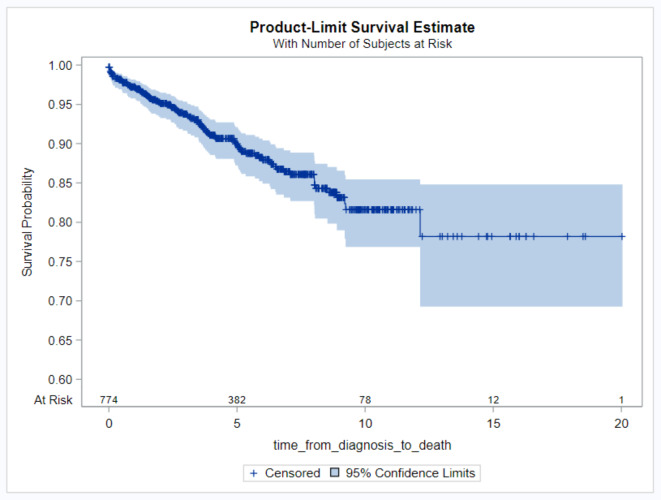
During a median follow-up of nearly 8.2 years (interquartile range 2.9–12.3), 102 patients showed signs of decompensated cirrhosis, including esophageal variceal bleeding, ascites, or encephalopathy at an incidence rate of 42.5 cases/ 1000 patient-years, while 85 patients had died (Fig. 1).
Fig. 1.
Overall survival of patients with AIH. Overall survival after AIH diagnosis was plotted using the Kaplan–Meier method.
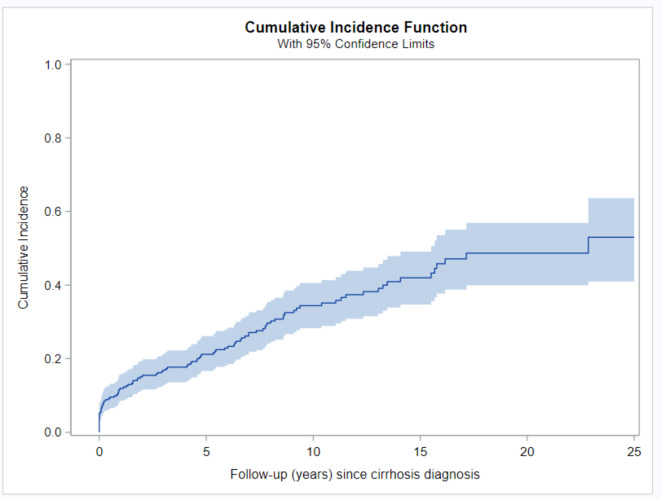
The mean time from cirrhosis diagnosis to the occurrence of decompensation was 8.2 years (interquartile range 2.9–12.3). The cumulative risk of decompensation increased over time from 12% (95% CI 8.6–15.9%) at the one-year timepoint to 21.2% (95% CI 16.7–26.1%) at the five-year timepoint, to 34.4% (95% CI 28.3–40.6%) at the 10- year timepoint from cirrhosis diagnosis, to more than 50% after the 25- year follow up from cirrhosis diagnosis. (Fig. 2; Table 2). No statistically significant difference between genders was observed in the risk of developing any complications of decompensation (p > 0.05). (supp. Table 1, Supplement). A more detailed analysis of the development of each specific complication of decompensated cirrhosis is provided in the Supplement (supp. Tables 2–4, supplementary Fig. 1–3).
Fig. 2.
Development of DC after diagnosis of cirrhosis in patients with AIH. The cumulative incidence of decompensated cirrhosis after the diagnosis of cirrhosis was estimated using the cumulative incidence function. AIH, autoimmune hepatitis; DC, decompensated cirrhosis.
Table 2.
Cumulative incidence of development of DC after diagnosis of cirrhosis in patients with AIH at multiple time points.
| FU (years) | Cumulative incidence | 95% Confidence interval | |
|---|---|---|---|
| 0 | 4.6% | 2.6% | 7.3% |
| 1 | 12.0% | 8.6% | 15.9% |
| 5 | 21.2% | 16.7% | 26.1% |
| 10 | 34.4% | 28.3% | 40.6% |
| 15 | 42.0% | 34.7% | 49.1% |
| 20 | 48.7% | 39.9% | 56.9% |
| 25 | 53.0% | 40.9% | 63.6% |
AIH, autoimmune hepatitis; DC, decompensated cirrhosis; CI, confidence interval.
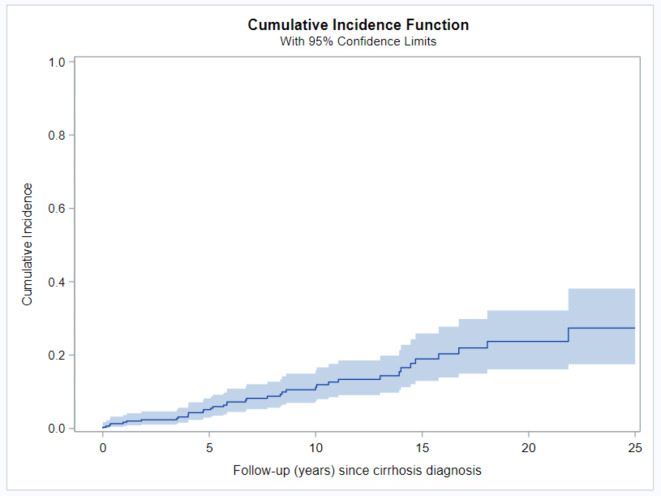
Table 4.
Cumulative incidence of development of liver transplantation after diagnosis of cirrhosis in patients with AIH at multiple time points.
| FU (years) | Cumulative incidence | 95% Confidence interval | |
|---|---|---|---|
| 0 | 0.3% | 0.0% | 1.7% |
| 1 | 1.7% | 0.6% | 3.7% |
| 5 | 5.1% | 2.9% | 8.2% |
| 10 | 11.2% | 7.5% | 15.8% |
| 15 | 19.0% | 12.9% | 25.9% |
| 20 | 23.7% | 16.1% | 32.2% |
| 25 | 27.4% | 17.5% | 38.2% |
AIH, autoimmune hepatitis; CI, confidence interval.
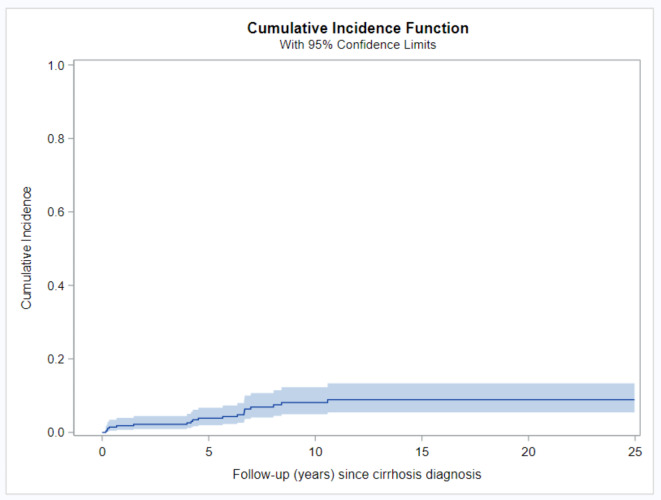
Nineteen of the cirrhotic patients (6.2%) developed HCC after cirrhosis diagnosis at an incidence rate of 6.3 cases/ 1000 patient-years; the median time from cirrhosis to the occurrence of HCC was 9.8 years (interquartile range 4.8–14.6). The cumulative risk of HCC was 1.8% (95% CI 0.7–3.9%) at the one-year timepoint and increased over the years to 3.8% (95% CI 1.9–6.6%) at the Five-year timepoint and 7.9% (95% CI 4.8–12%) at the 10- year time point from cirrhosis diagnosis (Fig. 3; Table 3). No statistically significant difference between genders was observed in the risk of developing HCC in AIH patients (p > 0.05) (Supp. Table 1, Supplement). No cases of HCC were identified in non-cirrhotic AIH patients within our cohort.
Fig. 3.
Development of HCC after diagnosis of cirrhosis in patients with AIH. The cumulative incidence of HCC after the diagnosis of cirrhosis was estimated using the cumulative incidence function. AIH, autoimmune hepatitis; HCC, hepatocellular carcinoma.
Table 3.
Cumulative incidence of development of HCC after diagnosis of cirrhosis in patients with AIH in multiple time points.
| FU (years) | Cumulative incidence | 95% Confidence interval | |
|---|---|---|---|
| 0 | 0.0% | 0.0% | 1.3% |
| 1 | 1.8% | 0.7% | 3.9% |
| 5 | 3.8% | 1.9% | 6.6% |
| 10 | 7.9% | 4.8% | 12.0% |
| 15 | 8.6% | 5.3% | 13.0% |
| 20 | 8.6% | 5.3% | 13.0% |
| 8.6% | 5.3% | 13.0% | |
AIH, autoimmune hepatitis; DC, decompensated cirrhosis; CI, confidence interval.
Liver transplantation was conducted in 38 patients (12.4%) after a cirrhosis diagnosis. The median time from cirrhosis to the occurrence of liver transplantation was 9.8 (interquartile range 5–13.9). The cumulative risk of transplantation increased over time from 1.7% (95% CI 0.6–3.7%) at the one-year timepoint to 5.1% (95% CI 2.9–8.2%) at the five-year timepoint to 11.2% (95% CI 7.5–15.8%) at the 10- year timepoint from cirrhosis diagnosis after 25 years from cirrhosis diagnosis; more than a quarter of the cirrhotic patients underwent liver transplantation (Fig. 4; Table 4). No statistically significant difference between genders was observed in the risk of conducting liver transplantation (p > 0.05) (Supp. Table 1, Supplement). Among patients who underwent liver transplantation, there were no cases of incidental HCC found on explant pathology.
Fig. 4.
Development of liver transplantation after diagnosis of cirrhosis in patients with AIH. The cumulative incidence of liver transplantation after the diagnosis of cirrhosis was estimated using the cumulative incidence function. AIH, autoimmune hepatitis.
Discussion
In this study of 307 cirrhotic patients out of nearly 800 diagnosed with autoimmune hepatitis, followed for a median duration of 8 years, we observed a different pattern in the progression of liver disease compared to what is generally accepted in the broader cirrhotic population. The annual incidence rate of decompensation among these AIH patients was 4.2%, which is a notably lower rate than that typically reported for cirrhosis in other entities. Additionally, the annual incidence rate of HCC following cirrhosis diagnosis was 0.6% annually, even over an extended follow-up period, significantly below the accepted threshold typically used to justify routine HCC surveillance. Importantly, no statistically significant difference between genders was observed in the risk of developing any complications related to decompensation and cancer development.
Cirrhosis is the leading cause of liver-related death globally and represents the end stage of progressive liver fibrosis characterized by the distortion of hepatic architecture21. In its initial stages, cirrhosis is typically compensated, with most patients remaining asymptomatic; it is often discovered incidentally during medical evaluation of unrelated circumstances22. In the context of autoimmune hepatitis, up to 30% of AIH patients are found to have confirmed cirrhosis at the time of diagnosis8,9. Similarly, cirrhosis was observed in 23% of our study cohort at the time of diagnosis. This finding may suggest a prolonged subclinical disease course lasting months or even years before diagnosis23. Over the years, clinicians in our study have increasingly been able to recognize AIH before the onset of cirrhosis (Supplementary Fig. 4, Supplement). This improvement in early detection reflects the growing knowledge, awareness, and availability of diagnostic tools that could significantly aid in early intervention, potentially preventing the development of complications from the disease in the future and thereby offering a much more favorable prognosis.
Acute decompensation (AD) is defined as the acute development of one or more significant complications of liver disease (ascites, hepatic encephalopathy, gastrointestinal hemorrhage, Jaundice)24–28. The rate of decompensation in patients with compensated cirrhosis was found to be 11% overall. The rate of decompensation was higher in the first year11. In our study, we found that the rate of decompensation was 4.2%, which is much lower than the known rate accepted; this finding may indicate that AIH-related cirrhosis follows a different clinical course, possibly due to earlier intervention or more effective management strategies tailored to AIH patients. Notably, ascites was the most common complication of decompensation in our cohort. Over 10% of cirrhotic patients developed ascites within the first year, and nearly one-third experienced this complication over a 10-year follow-up period. In contrast, esophageal bleeding showed a peak incidence within the first five years following cirrhosis diagnosis, indicating a need for more intensive monitoring during this period. This observation may reflect cases of late diagnosis of cirrhosis at a more advanced stage, which could progress to complications in subsequent years.
Cirrhosis represents the main risk factor for the development of HCC as it is present in more than 80–90% of cases14. The average HCC incidence rate among patients with cirrhosis is 2–4 per 100 person-years. In our study, however, the annual incidence rate of HCC was found to be 0.6%, significantly lower than the internationally accepted threshold for HCC surveillance18,29. Notably, 1.8% of the cirrhotic patients in our study developed HCC within the first year, constituting 20% of the total HCC cases in our study. This suggests that some of the cases of HCC may arise from a prolonged period of advanced undiagnosed cirrhosis, underscoring the importance of intensive follow-up during the first year after cirrhosis diagnosis for early detection and management of HCC. Furthermore, most of our HCC patients (91% of patients) developed HCC within the first 10 years following cirrhosis, with relatively few cases emerging afterward. This may indicate a clear variation in the absolute risk of HCC over time, suggesting the need to reassess surveillance guidelines for AIH patients with cirrhosis, considering the risk over the years following cirrhosis diagnosis.
Inflammatory bowel disease (IBD) and autoimmune hepatitis (AIH) exhibit a bidirectional epidemiological association, with evidence suggesting a shared pathophysiological link between the two conditions30. Proposed mechanisms include shared genetic susceptibility, particularly HLA haplotypes, gut-liver axis dysregulation through bacterial translocation, and overlapping immune pathways involving T-cell activation and autoantibody production31. In our study, 10.5% of AIH patients had concurrent IBD, aligning with previous literature, which estimates that approximately 7–10% of AIH patients develop IBD, particularly ulcerative colitis (UC) with pan colonic involvement. Notably, AIH-IBD overlap has been explored in a small study of 17 patients with both IBD and AIH. These patients exhibited an earlier onset of liver disease and poorer treatment outcomes, including higher rates of immunosuppression failure, increased risk of liver transplantation, and greater mortality32. This highlights the need for further prospective studies to understand the clinical implications better and improve management strategies for this patient population.
Our research findings provide comprehensive insights into the progression of autoimmune hepatitis (AIH) through a significant follow-up, showcasing both strengths and limitations. The study benefits from a large dataset comprising a substantial number of patients with AIH, enabling robust statistical analysis that enhances the reliability of the results. Additionally, the study’s longitudinal design, with a median follow-up of nearly eight years, allows for a detailed view of the natural course of the disease.
However, several limitations must be acknowledged. The study’s retrospective nature introduces the potential for biases and confounding factors, which could influence the interpretation of the results. Moreover, our study primarily involves a single-country population, limiting the generalizability of findings to patients of different ethnic backgrounds. Furthermore, while the treatment of AIH can lead to the regression of significant fibrosis and cirrhosis, our study did not include successive assessments of fibrosis in treated patients, leaving gaps in understanding how fibrosis progresses or regresses with treatment. Additionally, we assumed that once cirrhosis was diagnosed, most patients were regularly followed with ultrasound and AFP tests every six months. Still, our dataset did not explicitly evaluate this follow-up schedule. To address these limitations, further prospective multicenter studies conducted globally are warranted. Such studies would help validate and extend our findings, ensuring their applicability across diverse patient populations and healthcare settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to express our sincere gratitude to Naama Schwartz for her exceptional contributions to our data analysis and statistical evaluation. Her expertise and dedication were instrumental in the successful completion of this research. We deeply appreciate her meticulous attention to detail and her invaluable support throughout this project.
Abbreviations
- AIH
Autoimmune hepatitis
- HCC
Hepatocellular carcinoma
- DC
Decompensated cirrhosis
- MMF
Mycophenolate mofetil
- 6-MP
6-Mercaptopurine
- BMI
Body mass index
- SD
Standard deviation
- CD
Coeliac disease
- DM
Diabetes mellitus
- IBD
Inflammatory bowel disease
- MS
Multiple sclerosis
- SLE
Systemic lupus erythematosus
- RA
Rheumatoid arthritis
- THY
Thyroid disease
Author contributions
M.T.: conception and design, analysis and interpretation of the data, formal analysis, critical revision of the article for important intellectual content; final approval of the article. E.Z.: study design; critical revision of the article for important intellectual content; final approval of the article. N.A.F.: study design; critical revision of the article for important intellectual content; final approval of the article. R.H.: conception and design; analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article.
Data availability
The datasets generated and/or analyzed during the current study are notPublicly available because of clalit health service policy but are available from thethe corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was waived by the Institutional Review Board (IRB) of Clalit Health Services and Emek Medical Center (approval number 0091-23).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lohse, A. W. et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the international AIH pathology group: Results of a workshop on AIH histology hosted by the European reference network on hepatological diseases and the European society of pathology. Liver Int.42, 1058–1069 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Galaski, J. et al. Update of the simplified criteria for autoimmune hepatitis: Evaluation of the methodology for immunoserological testing. J. Hepatol.74, 312–320 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Mack, C. L. et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology72, 671–722 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Zachou, K. et al. Review article: Autoimmune hepatitis - current management and challenges. Aliment. Pharmacol. Ther.38(8), 887–913. 10.1111/apt.12470 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Vierling, J. M. Autoimmune hepatitis and overlap syndromes: Diagnosis and management. Clin. Gastroenterol. Hepatol.13(12), 2088–2108. 10.1016/j.cgh.2015.08.012 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Ramamoorthy, S. & Cidlowski, J. A. Corticosteroids: Mechanisms of action in health and disease. Rheum. Dis. Clin. North. Am.42, 15–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pape, S., Schramm, C. & Gevers, T. J. G. Clinical management of autoimmune hepatitis. United European. Gastroenterol. J.7(9), 1156–1163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czaja, A. J. & Freese, D. K. Diagnosis and treatment of autoimmune hepatitis. Hepatology36(2), 479–497. 10.1053/jhep.2002.34944 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Manns, M. P. et al. Diagnosis and management of autoimmune hepatitis. Hepatology51(6), 2193–2213. 10.1002/hep.23584 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Tansel, A. et al. Incidence and determinants of hepatocellular carcinoma in autoimmune hepatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol.15(8), 1207–1217e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming, K. M., Aithal, G. P., Card, T. R. & West, J. The rate of decompensation and clinical progression of disease in people with cirrhosis: A cohort study. Aliment. Pharmacol. Ther.32(11–12), 1343-50. 10.1111/j.1365-2036.2010.04473.x (2010). [DOI] [PubMed]
- 12.Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology144(7), 1426-37, 1437.e1-9. 10.1053/j.gastro.2013.02.042 (2013). [DOI] [PubMed]
- 13.Colapietro, F., Maisonneuve, P. & Lytvyak, E. Incidence and predictors of hepatocellular carcinoma in patients with autoimmune hepatitis. J. Hepatol.80, 53–61 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet391, 1301–1314 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Montano-loza, A. J., Carpenter, H. A. & Czaja, A. J. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am. J. Gastroenterol.103(8), 1944–1951. 10.1111/j.1572-0241.2008.01922.x (2008). [DOI] [PubMed] [Google Scholar]
- 16.Grønbæk, L., Vilstrup, H. & Jepsen, P. Autoimmune hepatitis in Denmark: Incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol.60(3), 612–617. 10.1016/j.jhep.2013.10.020 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Yeoman, A. D. et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for Follow-up and screening. Hepatology48(3), 863–870. 10.1002/hep.22432 [DOI] [PubMed]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines: Autoimmune hepatitis. J. Hepatol.63(4), 971–1004 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Sharma, R. et al. Cancer risk in patients with autoimmune hepatitis: A nationwide population-based cohort study with histopathology. Am. J. Epidemiol.191, 298–331 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, M. D., Jepsen, P., Vilstrup, H. & Gronbaek, L. Increased cancer risk in autoimmune hepatitis: A Danish nationwide cohort study. Am. J. Gastroenterol.117, 129–137 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Anthony, P. P. et al. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the world health organization. J. Clin. Pathol.31 (5), 395–414. 10.1136/jcp.31.5.395 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth, G. A., Abate, D. & Abate, K. H. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet392, 1736–1788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pape, S., Schramm, C. & Gevers, T. J. Clinical management of autoimmune hepatitis. United Eur. Gastroenterol. J.7(9), 1156–1163. 10.1177/2050640619872408 (2019). [DOI] [PMC free article] [PubMed]
- 24.Moore, K. P. et al. The management of ascites in cirrhosis: Report on the consensus conference of the international ascites club. Hepatology38, 258–266 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Blei, A. T. & Córdoba, J. Practice parameters committee of the American college of gastroenterology: Hepatic encephalopathy. Am. J. Gastroenterol.96, 1968–1976 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Tsao, G. & Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N. Engl. J. Med.362, 823–832 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Arvaniti, V., D’Amico, G. & Fede, G. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology139, 1246–1256 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Gustot, T. et al. Severe sepsis in cirrhosis. Hepatology50, 2022–2033 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology68, 723–750 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Chi, G., Pei, J. & Li, X. Inflammatory bowel disease and risk of autoimmune hepatitis: A univariable and multivariable Mendelian randomization study. PLoS One19(6), e0305220. 10.1371/journal.pone.0305220 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dey, A. et al. The evolution of very early onset inflammatory bowel disease, autoimmune hepatitis, and primary sclerosing cholangitis in a young girl. Case Rep. Gastroenterol.15(3), 939–947 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey, J. M. D. et al. Autoimmune hepatitis with inflammatory bowel disease is distinct and May be more refractory to traditional treatment: 504. Am. J. Gastroenterol.109, S149 (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are notPublicly available because of clalit health service policy but are available from thethe corresponding author on reasonable request.