Abstract
The time course of cell-mediated and humoral immune responses was elucidated in eight women with human papillomavirus type 16 (HPV-16) infection by performing serial HPV-16 E6 and E7 cytotoxic T-lymphocyte (CTL) assays and HPV-16 virus-like particle (VLP) antibody analyses. Four subjects had a single incident of HPV-16 DNA detection, and four subjects had two periods of HPV-16 DNA detection. In two of the women in the latter group, the second episode of HPV-16 detection occurred in the presence of high titers of HPV-16 VLP antibody, bringing into question the protective role of humoral immunity in preventing repeated infection. However, all four subjects rapidly became HPV-16 DNA negative following the second detection of HPV-16 DNA, suggesting the presence of immunological memory. In addition, one subject rapidly became negative for HPV-16 DNA despite having no evidence of CTL or VLP antibody response prior to the second HPV-16 DNA detection, suggesting the presence of immunological responses at an undetectable level. Overall, seven of eight subjects (88%) had detectable HPV-16 E6 and/or E7 CTL responses and seven of eight women (88%) had detectable HPV-16 VLP antibody responses.
The association between human papillomavirus (HPV) and the development of cervical cancer is well known, with HPV type 16 (HPV-16) being the most common type found in these cancers (27). Seventy to 90% of women who acquire HPV infection clear it within 1 to 2 years (21), yet the contributions of cell-mediated and humoral immunities in eliminating HPV infection and preventing reinfection or reemergence of the same infection are not well understood. A number of studies have investigated the role of helper T lymphocytes in providing protection against the development of HPV-associated lesions by measuring T-cell proliferative responses (7, 8, 11, 15, 16, 18, 19, 22, 32) or interleukin-2 release (2, 9, 36), but the results are inconsistent. However, our studies, which compared the T-cell proliferative responses (22) or cytotoxic T-lymphocyte (CTL) responses (23) of women with HPV infections with those of women with squamous intraepithelial lesions (SIL), suggested a protective role for the cell-mediated immune response against the development of SIL.
Several studies have demonstrated the presence of antibody responses to HPV virus-like particles (VLPs) in women who are infected with HPV-16. A cross-sectional study of HPV-16 serum antibody showed a seroprevalence of 46% in college women with HPV-16 infections (37). A longitudinal study of college women demonstrated a seroconversion rate of 67.1% (with a median seroconversion time of 8.3 months) among women with HPV-16 infections (4). In the same study, HPV-16-positive women who seroconverted were 5.7 times more likely to have SIL than were women who did not seroconvert, suggesting that the presence of HPV-16 VLP antibody served as a marker of disease progression rather than protection. In a follow-up study (3), the seroconversion rate for HPV-16-infected subjects was shown to be 59.5% within 18 months from the time of HPV DNA detection. Seroconversion for HPV-16 occurred most frequently between 6 and 12 months after DNA detection.
To elucidate the roles of CTL and antibody responses in controlling HPV-16 infection, eight women in whose cervixes HPV-16 DNA had been detected were monitored with serial HPV-16 E6 and E7 CTL assays and HPV-16 VLP immunoglobulin G (IgG) antibody detection assays in this study. Both CTL and VLP antibody responses were detected in the majority of these women. However, the role of VLP antibody in preventing a second HPV-16 infection was questioned, since two women acquired a second HPV-16 infection while having detectable levels of HPV-16 VLP antibody.
MATERIALS AND METHODS
Subjects.
The eight subjects in the present study were participants in a longitudinal study of HPV initiated in 1991 (21). The subjects had been monitored by cervical HPV DNA testing by PCR (35), cytology, and colposcopy every 4 months. The eight women were selected for immunological studies, which were initiated in 1996, because they had evidence of recent HPV-16 infection. They were asked to provide a blood specimen for the establishment of Epstein-Barr virus-transformed B lymphoblastoid cell lines (EBV-LCL) to serve as targets for the HPV-16 E6 and E7 CTL assay. In addition, a plasma sample from the same blood draw was cryopreserved for HPV-16 VLP antibody analysis. Informed consent was obtained for the immunological studies, according to the guidelines of the Committee on Human Research at the University of California, San Francisco. Four women were European-American, three were Hispanic, and one was Asian. They had been in the study for a mean of 58 months (range, 33 to 94 months) and had a mean age of 23.6 years (range, 20 to 26 years) at the time blood was drawn for EBV transformation. In addition to the routine analyses mentioned above, serial HPV-16 E6 and E7 CTL assays were performed, and plasma samples were frozen for HPV-16 VLP IgG antibody analyses at subsequent visits every 4 months.
PCR analysis.
Cervical specimens collected with Dacron swabs were placed in sample transport media (Digene Diagnostics, Silver Spring, Md.) and digested using a commercial digestion solution (Digene Diagnostics) and proteinase K. DNA was precipitated with ammonium acetate ethanol. PCR analysis for multiple HPV types (including low-risk types 6, 11, 42, and 44 and high-risk types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, and 58) was performed, and β-hemoglobulin was amplified as a positive control (20, 21). A previously published report comparing this method and another method using a reverse blot strip (Roche Molecular Systems, Inc., Alameda, Calif.) found the agreement between the two tests to be 87.8%, yielding a κ statistic of 0.63 (P < 0.001) (20).
HPV-CTL assay.
Sixty milliliters of heparinized whole blood was collected, and the HPV-CTL assay was performed as described previously (23, 25). Briefly, peripheral blood mononuclear cells were isolated using a Ficoll-Hypaque density gradient centrifugation and then stimulated for 7 days at 37°C in an atmosphere of 5% CO2 with 1 μg of E6- or E7-glutathione S-transferase per ml of cells. Both CD4- and CD8-positive T lymphocytes have been shown to be responsible for the HPV-16 E6- and E7-specific responses (24). At the same time, a mixed lymphocyte culture was set up as a positive cytotoxicity control by using an irradiated (40-Gy) allogeneic EBV-LCL (106 cells/well) as a stimulator, and it demonstrated an adequate response (>10% lysis) in all assays.
For the preparation of target cells, an autologous EBV-LCL was infected 1 day prior to the CTL assay with a recombinant vaccinia virus expressing HPV-16 E6 or E7 or with a nonrecombinant vaccinia virus derived from strain WR at a multiplicity of infection of 1. The target cells were labeled with 200 μCi of sodium chromate (Na251CrO4; specific activity, 5 mCi/ml) (Amersham Corp., Arlington Heights, Ill.) for 90 min at 37°C on the day of the assay. The labeled cells were washed and plated in triplicate in 96-well round-bottom plates at 5 × 103 cells/well. Effector lymphocytes were added at four different effector-to-target ratios ranging from 100:1 to 12.5:1. Supernatants were harvested, and the 51Cr disintegrations were counted after 5 h of incubation (Cobra E5005; Packard Instruments, Meriden, Conn.). The standard deviations (SD) of the triplicate results for all effector-to-target ratios from all assays were averaged. The CTL response was considered positive when the specific lysis for the experimental results was at least 3 SD (18%) higher than the percent specific lysis for the combined negative controls for at least two of the four effector-to-target ratios.
For assays performed after November 1998, additional steps to select for CD3-positive T lymphocytes were added to reduce the background activity due to natural killer cells (24). The CD3-positive T lymphocytes were selected by magnetically depleting CD11b-, CD16-, CD19-, and CD56-positive cells (Pan T-Cell isolation kit; Miltenyi Biotec, Auburn, Calif.). The quality of the selection was monitored by fluorescence-activated cell sorter analysis (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, Calif.) using a combination of CD3 and CD16 antibodies (Caltag, Burlingame, Calif.). The natural killer cells were reduced to less than 1% by all lots of magnetic beads. The 3-SD cutoff for defining a positive response was reduced from 18% for the assays of nondepleted lymphocytes to 6.5% for the assays of natural-killer-cell-depleted lymphocytes.
Purification of HPV-16 VLPs.
VLPs were generated in Trichoplusia ni cells (High Five; Invitrogen, Carlsbad, Calif.) infected with an HPV-16 L1/L2 recombinant baculovirus, clone 114/K (gift from John T. Schiller, National Cancer Institute, Bethesda, Md.), as described previously (17). VLPs were purified using a protocol that combined ultracentrifugation and low-pressure liquid chromatography methods (6, 14, 17). Briefly, infected cells were harvested, sonicated on ice, and pelleted through 40% sucrose in phosphate-buffered saline, pH 7.2 (PBS). The pellet was resuspended in 27% (wt/wt) CsCl-PBS and centrifuged overnight in an SW-28 rotor to remove the contaminating buoyant layer. The clarified lysate was then centrifuged for 48 h at 45,000 rpm in a Vti50 vertical rotor. The CsCl gradient was collected in 2-ml fractions, and each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The fractions containing L1 protein were pooled and loaded onto a POROS 50HS strong cation-exchange resin (PerSeptive Biosystems, Framingham, Mass.) column, preequilibrated with 50 mM MOPS (morpholine propanesulfonic acid), pH 7, containing 0.33 M NaCl. After the solution containing VLP was loaded, the column was washed with 10 column volumes and proteins were eluted with a linear salt gradient (0.33 to 1.5 M NaCl) in the same buffer. Fractions containing L1 protein were collected, the salt concentration of the solution was adjusted to 0.33 M NaCl, and the material was applied to a heparin-Sepharose (Amersham Parmacia Biotech, Uppsala, Sweden) column preequilibrated with 50 mM MOPS (pH 7)-0.33 M NaCl. Proteins were eluted with a linear salt gradient (0.33 to 1.5 M NaCl) in the same buffer, and fractions containing L1 protein were pooled and stored at 4°C. Analysis of a Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel with NIH Image software showed that HPV-16 L1 protein comprised 90% of the total protein.
HPV-16 VLP ELISA.
Plasma samples from the same whole-blood specimens collected from each woman were tested for the presence of HPV-16 VLP antibody. Additional serum samples were available for four subjects from an earlier visit. For the enzyme-linked immunosorbent assay, (ELISA), flat-bottom 96-well polystyrene plates (PolySorp, Nunc; Naperville, Ill.) were coated with a 100-μl/well concentration of an HPV-16 VLP solution (0.4 μg of VLP protein per ml of PBS). Following overnight incubation at 4°C, 300 μl of blocking solution (10% Superblock [Pierce, Rockford, Ill.]) in PBS containing 0.05% Tween 20 [Sigma, St. Louis, Mo.]) was added to each well. After incubation at room temperature for 3 h, the blocking solution was replaced with PBS. The plates were covered with a plastic sealer and stored at −20°C. Before use, the plates were washed three times with wash solution (PBS-0.05% Tween 20) in an automatic plate washer (Skanwasher 300; Skatron, Lier, Norway), and then 100 μl of sample dilution buffer (10% Superblock and 0.05% Tween 20 in PBS) was added to each well. One microliter of serum was added to each well by use of a MultiPROBE II robotic liquid handling system (Packard Instruments). Plates were incubated at 37°C for 1 h on a microplate shaker and then washed twice, rotated 180°, and washed two more times. Gamma chain-specific goat anti-human IgG, conjugated with horseradish peroxidase (Zymed, San Francisco, Calif.), was diluted 1:4,000 in conjugate buffer (10% Superblock, 2.5% polyethylene glycol [molecular weight, 20,000; Sigma], and 0.5% Igepal CA-630 [Sigma] in PBS), and 100 μl was added to each well. Plates were incubated at 37°C for 30 min on a microplate shaker and then washed as described above. Freshly prepared 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution (Kierkegaard & Perry, Gaithersburg, Md.), prewarmed to 50°C, was added to each well in 100-μl volumes. The plates were incubated at room temperature in the dark for 20 min. The first plate in a series was monitored until the weakly positive control reached a predetermined optical density (OD) value. The positive controls were human serum samples previously shown to be reactive in the assay. Three controls—a weakly positive control, a moderately to strongly positive control, and a negative control—were included on each plate. The enzyme reaction was stopped by the addition of 100 μl of 1% dodecyl sulfate per well to all the plates. The plates were read at 405 nm, with a reference wavelength of 490 nm, in an automated microtiter plate reader (Molecular Devices, Menlo Park, Calif.). The cutoff for positive results was determined from the reactivities of plasma samples from 108 self-reported virgins from Costa Rica. The mean and SD of OD values for the control subjects were calculated, and values greater than the mean plus 3 SD were excluded. The analysis was repeated on the remaining samples until no further OD values could be excluded by this criterion. The cutoff, established after four rounds of analysis with the exclusion of six samples, was an OD value of greater than 0.136 unit.
HPV-16 variant analysis.
Because four women had two periods of HPV-16 DNA detection, a possibility that the second period of detection represented an acquisition of a new HPV variant was examined. By analysis of samples from various parts of the world with a liquid hybridization assay, variants designated European prototype, Asian, Asian-American, African-1, and African-2 have been described previously (13). For the present study, a single-tube nested E6 PCR was performed on HPV-16-positive samples (39). Briefly, amplification reactions were performed with 100-μl volumes containing 10 mM Tris (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) deoxynucleotide triphosphate, 2 nM (each) external primer (CGTAACCGAAATCGGTTGAAC and GGACCATCTATTTCATCCTCCT), 200 nM (each) internal primer (GCTCATAACAGTAGAG and ACCGGTTAGTATAAAAG), and 2.5 U of AmpliTaq (Perkin-Elmer, Foster City, Calif.). To each reaction mixture, 3 μl of crude DNA preparation was added. PCR was performed with a Uno II Thermocycler (Biometra, Tampa, Fla.) under conditions of 95°C for 3 min for the hot start; 30 cycles of 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min; 35 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 1 min; and a terminal extension of 72°C for 10 min.
The nested E6 protein fragment was semipurified and sequenced by double-stranded PCR sequencing using the internal nested primers. Briefly, 30 to 70 ng of PCR product was incubated with 10 U of exonuclease 1 and 2 U of shrimp alkaline phosphatase (Amersham Life Sciences, Cleveland, Ohio) for 15 min at 37°C and inactivated by incubation at 80°C for 15 min. These samples were sequenced directly by the Biomolecular Resource Center at University of California, San Francisco, with an automated sequencer (model 3700; Applied Biosystems, Foster City, Calif.).
RESULTS
Four women had a single episode of HPV-16 DNA detection (Table 1), and four women had episodes of HPV-16 DNA detection at two time points separated by at least two negative tests for HPV-16 DNA (Table 2). Overall, seven of eight subjects (88%) had positive HPV-16 E6 and/or E7 CTL responses and seven of eight subjects (88%) had detectable HPV-16 VLP antibody responses during the study. Five of eight subjects (63%) had positive HPV-16 E6 CTL responses, five of eight subjects (63%) had positive HPV-16 E7 CTL responses, and three of eight subjects (38%) had positive responses for both. None of the subjects developed high-grade SIL.
TABLE 1.
HPV-16 DNA, HPV-16 E6 and E7 CTL, and HPV-16 VLP IgG antibody results for four subjects with a single period of HPV-16 DNA detection
| Subject | Duration of HPV-16 DNA detection (mo) | Presence or absence of CTL or Aba
|
|||
|---|---|---|---|---|---|
| During HPV-16 DNA detection period
|
After clearance of HPV-16 infectionb
|
||||
| CTL (E6/E7) | VLP Ab | CTL (E6/E7) | VLP Ab | ||
| 1 | 65 | − | + | + | + |
| 2 | 37 | + | + | + | + |
| 3 | 10 | NAc | − | + | − |
| 4 | 17 | NA | NA | + | + |
Ab, antibody.
Data cover the 20 months following the period of HPV-16 detection.
NA, analysis not initiated during the corresponding period of detection.
TABLE 2.
HPV-16 DNA, HPV-16 E6 and E7 CTL, and HPV-16 VLP IgG antibody results for four subjects with two periods of HPV-16 DNA detection
| Subject | Duration of HPV-16 DNA detection (mo) | Presence or absence of CTL or Aba
|
|||||
|---|---|---|---|---|---|---|---|
| After clearance of 1st HPV-16 infection
|
During 2nd HPV-16 DNA detection period
|
After clearance of 2nd HPV-16 infection
|
|||||
| CTL (E6/E7) | VLP Ab | CTL (E6/E7) | VLP Ab | CTL (E6/E7) | VLP Ab | ||
| 5b | 11 | + | + | − | + | − | + |
| <4 | |||||||
| 6 | Unknown | − | − | + | + | + | + |
| <4 | |||||||
| 7b | 28 | − | + | + | + | + | + |
| <4 | |||||||
| 8 | <4 | NAc | NA | NA | NA | − | + |
| <4 | |||||||
Ab, antibody.
Subject was HPV-16 VLP antibody positive during the first period of HPV-16 DNA detection.
NA, analysis not initiated during the corresponding period of detection.
For the women who had a single episode of HPV-16 DNA detection, the duration of detection by PCR lasted for 10 to 65 months. During the period of HPV-16 DNA detection, an HPV-16 E6 and/or E7 CTL response was seen in one of two subjects tested. HPV-16 VLP antibodies were detected in two of three subjects tested. During a period of up to 20 months after the clearance of HPV-16 DNA, all subjects demonstrated an HPV-16 E6 and/or E7 CTL response, and three of four subjects had positive HPV-16 VLP antibody responses.
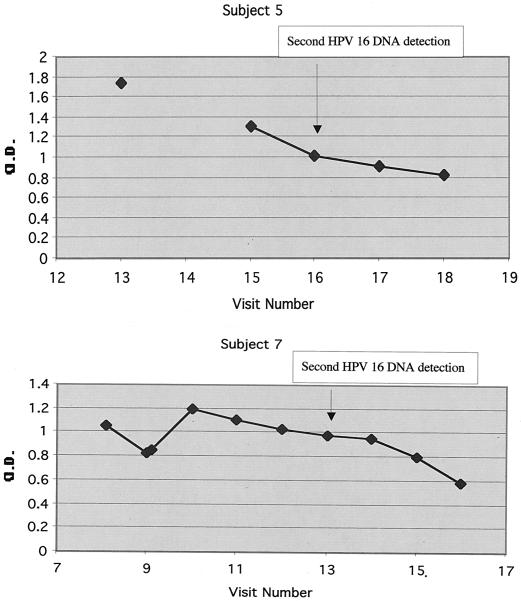
For three of the four women with HPV-16 DNA detected at two time points, the first HPV-16 DNA detection occurred after the women entered the study, while one of the women (subject 6) entered the study with prevalent HPV-16 infection. The first period of detection of HPV-16 DNA ranged from less than 4 months (i.e., cleared by the following visit) to 28 months. Overall, HPV-16 VLP antibody was detected in all four subjects with two episodes of HPV-16 DNA detection, while HPV-16 E6 and/or E7 CTL responses were detected in all but one subject (subject 8). Two subjects for whom antibody data were available at the time of the first HPV-16 DNA detection maintained positive HPV-16 VLP antibody responses throughout the study period; i.e., these women had circulating HPV 16 VLP antibodies at the times of the second episodes of HPV-16 DNA positivity (Fig. 1).
FIG. 1.
Titers of HPV-16 VLP IgG antibody for subjects 5 and 7. The visits at which HPV-16 DNA was detected for the second time are indicated by arrows. The first period of HPV-16 DNA detection spanned visits 11 through 13 for subject 5 and visits 4 through 10 for subject 7. The 3-SD cutoff defining positivity was an OD of 0.136.
Concerning the second period of HPV-16 DNA detection, all four subjects became negative for HPV-16 DNA within the 4 months following the second detection, suggesting the presence of immunological memory. For subject 5, data were unavailable for HPV-16 E6 and/or E7 CTL responses at the time of the first HPV-16 DNA detection but CTL responses were detected after the clearance of HPV-16 DNA following the first episode of detection. The findings for subject 6 were interesting, since no CTL or antibody responses were detectable after the clearance of her first HPV-16 infection; however, both responses emerged rapidly during the second period of HPV-16 DNA detection, suggesting the presence of immunological memory despite the failure to detect a circulating immune response to the earlier infection.
To address the question of whether the two periods of HPV-16 DNA detection in the same subject were the results of a new infection or the reemergence of a latent infection, HPV-16 variant analysis was performed. Subject 5 had the African-1 variant of HPV-16 at the time of the first HPV-16 DNA detection and the European prototype at the time of the second HPV-16 DNA detection, suggesting that the second incidence represented a new infection. For subject 8, the first HPV-16 DNA detection was of a mixed infection of the European prototype and the African-2 variant while only the European prototype was detected in the second period of HPV-16 DNA detection, suggesting the reemergence of the European prototype and the clearance of the African-2 variant. For subjects 6 and 7, either the amounts of the cervical samples were insufficient or the sequencing analysis was inadequate for performance of HPV-16 variant analysis for either period of HPV-16 DNA detection.
DISCUSSION
Although data for only eight subjects are presented here, several interesting observations can be made for this cohort with detailed analyses of HPV infection and the corresponding immunological responses. The presence of immunological memory was suggested by the rapid clearance of HPV-16 DNA in all four subjects with a second episode of HPV-16 DNA detection. Other evidence for the presence of immunological memory includes the rapid emergence of HPV-16 E6 and/or E7 CTL and HPV-16 VLP antibody in subject 6 at the time of the second HPV-16 DNA detection despite undetectable CTL and VLP responses after the clearance of HPV-16 DNA from the first period of detection.
Almost all subjects in this study had detectable CTL responses to HPV-16 E6 and/or E7 protein, and all eight subjects cleared HPV-16 DNA, implying an association of the CTL responses with the clearance of the HPV-16 infection. The CTL results in the present study, which showed that five of eight subjects had a positive response to the HPV-16 E6 or E7 protein, were similar to those of an earlier study by Nakagawa et al. (23). As found in this study, the detection of CTL responses to HPV-16 E6 and/or E7 did not necessarily correspond with the timing of HPV-16 DNA disappearance. This may be due to the immunological pressure necessary to control latent infection during HPV-16 DNA-negative periods, in that CTL are at work even though the infection cannot be detected. In addition, CTL responses to proteins other than E6 and E7, which were not tested, may play an important role in the control of HPV-16 infection.
As was observed in a previous study (23), HPV CTL responses were transient and rarely detectable in two consecutive visits. On the other hand, antibody responses were more likely to be sustained over a longer period of time. The lack of sustained demonstration of HPV CTL responses compared to that of VLP antibody responses is not surprising, since CTL are thought to persist for days to weeks while the B-cell response is known to persist for months to years. In addition, the sensitivity of the CTL assay may be low, requiring repeated testing for optimal assessment (25). In this study, HPV-16 VLP antibodies persisted for as long as 42 months after the last documented HPV-16 infection (subject 1). On the other hand, tests for the HPV-16 VLP antibody became negative in subjects 4, 6, and 8 during this study period. This phenomenon of the HPV-16 VLP antibody becoming undetectable over time in a subset of subjects has been previously reported (3).
Although data for the HPV-16 variant analysis were available for only two subjects, the second incidence of detection in subject 5 appeared to represent a new infection, while that of subject 8 seemed to be due to the reemergence of an earlier infection. However, it is important to note that without a means to distinguish an HPV DNA-negative clearance from an HPV DNA-negative latent infection, it is not possible to determine with certainty whether any incidence of HPV DNA detection represents a new infection or the reemergence of a latent infection.
Many studies have focused on the association between HPV-specific antibodies and SIL or cervical cancer (1, 10, 26, 29-31, 33, 34, 38, 40). Few studies have focused on the role of HPV-specific antibodies in preventing HPV infection. The ability of a pathogen-specific antibody to prevent infection is the basis of many vaccines, such as those for influenza virus. Data in this study showed that reinfection by HPV-16 or reemergence of HPV-16 can occur in the presence of circulating HPV-16 VLP antibody. Although it is possible that an antibody was not effective because reinfection was caused by a different variant of HPV-16, this finding was surprising since data from Pastrana et al. demonstrating cross-specificity of the HPV-16 VLP antibody with different HPV-16 variants seemed to predict cross-variant protection (28). Although the antibody did not prevent an episode of second HPV-16 DNA positivity, the IgG antibody response may have had a role in controlling HPV infection, since all four subjects became negative for HPV-16 DNA by the following visit.
Currently, a number of human clinical trials are under way to evaluate the safety and efficacy of HPV-16 VLP as a prophylactic vaccine (5). The results of the phase I trial published by the National Cancer Institute have shown that the HPV-16 VLP are highly immunogenic; i.e., nearly all women who received the vaccine achieved serum antibody levels that were nearly 40-fold higher than those found in subjects with natural infections (12). However, whether a high systemic antibody level translates to the prevention of HPV-16 infection remains to be evaluated. Our data, though derived from only two subjects, suggest that low levels of circulating HPV-16 VLP antibody may not be sufficient to prevent HPV-16 reinfection or HPV-16 reemergence.
Acknowledgments
We acknowledge Li Wang for sharing her technical expertise.
This work was supported by the National Institutes of Health (grants NCI CA51323, NCI K07 CA75974, M01 RR01271, and AI42058).
REFERENCES
- 1.Baay, M. F., J. M. Duk, M. P. Burger, J. Walboomers, J. ter Schegget, K. H. Groenier, H. W. de Bruijn, E. Stolz, and P. Herbrink. 1995. Antibodies to human papillomavirus type 16 E7 related to clinicopathological data in patients with cervical carcinoma. J. Clin. Pathol. 48:410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bontkes, H. J., T. D. de Gruijl, A. Bijl, R. H. Verheijen, C. J. Meijer, R. J. Scheper, P. L. Stern, J. E. Burns, N. J. Maitland, and J. M. Walboomers. 1999. Human papillomavirus type 16 E2-specific T-helper lymphocyte responses in patients with cervical intraepithelial neoplasia. J. Gen. Virol. 80:2453-2459. [DOI] [PubMed] [Google Scholar]
- 3.Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. [DOI] [PubMed] [Google Scholar]
- 4.Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. [DOI] [PubMed] [Google Scholar]
- 5.Connett, H. 2001. HPV vaccine moves into late stage trials. Nat. Med. 7:388. [DOI] [PubMed] [Google Scholar]
- 6.Cook, J. C., J. G. Joyce, H. A. George, L. D. Schultz, W. M. Hurni, K. U. Jansen, R. W. Hepler, C. Ip, R. S. Lowe, P. M. Keller, and E. D. Lehman. 1999. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr. Purif. 17:477-484. [DOI] [PubMed] [Google Scholar]
- 7.Cubie, H. A., M. Norval, L. Crawford, L. Banks, and T. Crook. 1989. Lymphoproliferative response to fusion proteins of human papillomaviruses in patients with cervical intraepithelial neoplasia. Epidemiol. Infect. 103:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Gruijl, T. D., H. J. Bontkes, M. J. Stukart, J. M. Walboomers, A. J. Remmink, R. H. Verheijen, T. J. Helmerhorst, C. J. Meijer, and R. J. Scheper. 1996. T cell proliferative responses against human papillomavirus type 16 E7 oncoprotein are most prominent in cervical intraepithelial neoplasia patients with a persistent viral infection. J. Gen. Virol. 77:2183-2191. [DOI] [PubMed] [Google Scholar]
- 9.de Gruijl, T. D., H. J. Bontkes, J. M. Walboomers, M. J. Stukart, F. S. Doekhie, A. J. Remmink, T. J. Helmerhorst, R. H. Verheijen, M. F. Duggan-Keen, P. L. Stern, C. J. Meijer, and R. J. Scheper. 1998. Differential T helper cell responses to human papillomavirus type 16 E7 related to viral clearance or persistence in patients with cervical neoplasia: a longitudinal study. Cancer Res. 58:1700-1706. [PubMed] [Google Scholar]
- 10.Dillner, L., A. Zellbi, E. Avall-Lundqvist, P. Heino, C. Eklund, C. A. Pettersson, O. Forslund, B. G. Hansson, M. Grandien, P. Bistoletti, et al. 1995. Association of serum antibodies against defined epitopes of human papillomavirus L1, E2, and E7 antigens and of HPV DNA with incident cervical cancer. Cancer Detect. Prev. 19:381-393. [PubMed] [Google Scholar]
- 11.Gill, D. K., J. M. Bible, C. Biswas, B. Kell, J. M. Best, N. A. Punchard, and J. Cason. 1998. Proliferative T-cell responses to human papillomavirus type 16 E5 are decreased amongst women with high-grade neoplasia. J. Gen. Virol. 79:1971-1976. [DOI] [PubMed] [Google Scholar]
- 12.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 13.Ho, L., S.-Y. Chan, R. D. Burk, B. C. Das, K. Fujinaga, J. P. Icenogle, T. Kahn, N. Kiviat, W. Lancaster, P. Mavromara-Nazos, et al. 1993. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J. Virol. 67:6413-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 15.Kadish, A. S., G. Y. Ho, R. D. Burk, Y. Wang, S. L. Romney, R. Ledwidge, and R. H. Angeletti. 1997. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J. Natl. Cancer Inst. 89:1285-1293. [DOI] [PubMed] [Google Scholar]
- 16.Kadish, A. S., S. L. Romney, R. Ledwidge, R. Tindle, G. J. Fernando, S. Y. Zee, M. A. Van Ranst, and R. D. Burk. 1994. Cell-mediated immune responses to E7 peptides of human papillomavirus (HPV) type 16 are dependent on the HPV type infecting the cervix whereas serological reactivity is not type-specific. J. Gen. Virol. 75:2277-2284. [DOI] [PubMed] [Google Scholar]
- 17.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luxton, J. C., R. C. Rose, T. Coletart, P. Wilson, and P. S. Shepherd. 1997. Serological and T-helper cell responses to human papillomavirus type 16 L1 in women with cervical dysplasia or cervical carcinoma and in healthy controls. J. Gen. Virol. 78:917-923. [DOI] [PubMed] [Google Scholar]
- 19.Luxton, J. C., A. J. Rowe, J. C. Cridland, T. Coletart, P. Wilson, and P. S. Shepherd. 1996. Proliferative T cell responses to the human papillomavirus type 16 E7 protein in women with cervical dysplasia and cervical carcinoma and in healthy individuals. J. Gen. Virol. 77:1585-1593. [DOI] [PubMed] [Google Scholar]
- 20.Moscicki, A. B., N. Hills, S. Shiboski, K. Powell, N. Jay, E. Hanson, S. Miller, L. Clayton, S. Farhat, J. Broering, T. Darragh, and J. Palefsky. 2001. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 285:2995-3002. [DOI] [PubMed] [Google Scholar]
- 21.Moscicki, A. B., S. Shiboski, J. Broering, K. Powell, L. Clayton, N. Jay, T. M. Darragh, R. Brescia, S. Kanowitz, S. B. Miller, J. Stone, E. Hanson, and J. Palefsky. 1998. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J. Pediatr. 132:277-284. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa, M., D. P. Stites, S. Farhat, A. Judd, A.-B. Moscicki, A. J. Canchola, J. F. Hilton, and J. M. Palefsky. 1996. T-Cell proliferative response to human papillomavirus type 16 peptides: relationship to cervical intraepithelial neoplasia. Clin. Diagn. Lab. Immunol. 3:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa, M., D. P. Stites, S. Farhat, J. R. Sisler, B. Moss, F, Kong, A. B. Moscicki, and J. M. Palefsky. 1997. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J. Infect. Dis. 175:927-931. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa, M., D. P. Stites, J. M. Palefsky, Z. Kneass, and A. B. Moscicki. 1999. CD4-positive and CD8-positive cytotoxic T lymphocytes contribute to human papillomavirus type 16 E6 and E7 responses. Clin. Diagn. Lab. Immunol. 6:494-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa, M., D. P. Stites, S. Patel, S. Farhat, M. Scott, N. K. Hills, J. M. Palefsky, and A. B. Moscicki. 2000. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J. Infect. Dis. 182:595-598. [DOI] [PubMed] [Google Scholar]
- 26.Onda, T., T. Kanda, S. Zanma, T. Yasugi, S. Watanabe, T. Kawana, K. Ueda, H. Yoshikawa, Y. Taketani, and K. Yoshiike. 1993. Association of the antibodies against human papillomavirus 16 E4 and E7 proteins with cervical cancer positive for human papillomavirus DNA. Int. J. Cancer 54:624-628. [DOI] [PubMed] [Google Scholar]
- 27.Palefsky, J. M., and E. A. Holly. 1995. Molecular virology and epidemiology of human papillomavirus and cervical cancer. Cancer Epidemiol. Biomark. Prev. 4:415-428. [PubMed] [Google Scholar]
- 28.Pastrana, D. V., W. C. Vass, D. R. Lowy, and J. T. Schiller. 2001. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279:361-369. [DOI] [PubMed] [Google Scholar]
- 29.Sasagawa, T., M. Inoue, M. Lehtinen, W. Zhang, S. E. Gschmeissner, M. A. Nasser Hajibagheri, J. Finch, and L. Crawford. 1996. Serological responses to human papillomavirus type 6 and 16 virus-like particles in patients with cervical neoplastic lesions. Clin. Diagn. Lab. Immunol. 3:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasagawa, T., M. Inoue, O. Tanizawa, M. Yutsudo, and A. Hakura. 1992. Identification of antibodies against human papillomavirus type 16 E6 and E7 proteins in sera of patients with cervical neoplasias. Jpn. J. Cancer Res. 83:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah, K. V., R. P. Viscidi, A. J. Alberg, K. J. Helzlsouer, and G. W. Comstock. 1997. Antibodies to human papillomavirus 16 and subsequent in situ or invasive cancer of the cervix. Cancer Epidemiol. Biomark. Prev. 6:233-237. [PubMed] [Google Scholar]
- 32.Shepherd, P. S., A. J. Rowe, J. C. Cridland, T. Coletart, P. Wilson, and J. C. Luxton. 1996. Proliferative T cell responses to human papillomavirus type 16 L1 peptides in patients with cervical dysplasia. J. Gen. Virol. 77:593-602. [DOI] [PubMed] [Google Scholar]
- 33.Silins, I., Z. Wang, E. Avall-Lundqvist, B. Frankendal, U. Vikmanis, M. Sapp, J. T. Schiller, and J. Dillner. 1999. Serological evidence for protection by human papillomavirus (HPV) type 6 infection against HPV type 16 cervical carcinogenesis. J. Gen. Virol. 80:2931-2936. [DOI] [PubMed] [Google Scholar]
- 34.Sun, Y., J. Eluf-Neto, F. X. Bosch, N. Munoz, J. M. Walboomers, C. J. Meijer, K. V. Shah, B. Clayman, and R. P. Viscidi. 1999. Serum antibodies to human papillomavirus 16 proteins in women from Brazil with invasive cervical carcinoma. Cancer Epidemiol. Biomark. Prev. 8:935-940. [PubMed] [Google Scholar]
- 35.Ting, Y., and M. M. Manos. 1990. Detection and typing of genital human papillomaviruses, p. 356-367. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.
- 36.Tsukui, T., A. Hildesheim, M. H. Schiffman, J. Lucci III, D. Contois, P. Lawler, B. B. Rush, A. T. Lorincz, A. Corrigan, R. D. Burk, W. Qu, M. A. Marshall, D. Mann, M. Carrington, M. Clerici, G. M. Shearer, D. P. Carbone, D. R. Scott, R. A. Houghten, and J. A. Berzofsky. 1996. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 56:3967-3974. [PubMed] [Google Scholar]
- 37.Viscidi, R. P., K. L. Kotloff, B. Clayman, K. Russ, S. Shapiro, and K. V. Shah. 1997. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin. Diagn. Lab. Immunol. 4:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viscidi, R. P., Y. Sun, B. Tsuzaki, F. X. Bosch, N. Munoz, and K. V. Shah. 1993. Serologic response in human papillomavirus-associated invasive cervical cancer. Int. J. Cancer 55:780-784. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler, C. M., T. Yamada, A. Hildesheim, and S. A. Jenison. 1997. Human papillomavirus type 16 sequence variants: identification by E6 and L1 lineage-specific hybridization. J. Clin. Microbiol. 35:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wideroff, L., M. Schiffman, P. Haderer, A. Armstrong, C. E. Greer, M. M. Manos, R. D. Burk, D. R. Scott, M. E. Sherman, J. T. Schiller, R. N. Hoover, R. E. Tarone, and R. Kirnbauer. 1999. Seroreactivity to human papillomavirus types 16, 18, 31, and 45 virus-like particles in a case-control study of cervical squamous intraepithelial lesions. J. Infect. Dis. 180:1424-1428. [DOI] [PubMed] [Google Scholar]