Abstract
Poorly differentiated gastric cancer (PDGC) is characterized by high invasiveness, rapid progression, and poor prognosis for patients. Differentiation therapy has long been a promising approach by manipulating the differentiation state of tumor cells to inhibit tumor growth, offering fewer side effects. Decitabine (DAC), is known as an inhibitor of DNA methylation, thus reactivating the transcription of previously methylated silenced genes associated with differentiation to induce a more differentiated state. This study used the differentiation-inducing agents DAC to treat two PDGC cell lines, MKN45 and NUGC4, and explored the impact of DAC on cell proliferation and influence of their sensitivity to Natural Killer cells (NK cells) mediated cytotoxicity. The results demonstrated a significant reduction in cell proliferation, migration, and invasion without affecting cell viability after DAC treatment. Additionally, transcriptomic analysis revealed that DAC-treated PDGC cells upregulated multiple immune-related genes, including the gene encoding for tumor necrosis factor alpha (TNF-α). Co-culture study of NK cells and PDGC cells showed that DAC treatment enhanced the sensitivity of these cancer cells to NK cell-mediated cytotoxicity, and TNF-α played a crucial role in promoting NK cell cytotoxicity. Following the subcutaneous implantation of tumors in nude mice, DAC administration significantly inhibited the growth of PDGC tumors and induced the upregulation of differentiation related genes. In summary, DAC effectively reduces the malignant characteristics of the PDGC cells by promoting their transition towards a higher state of differentiation and enhancing their sensitivity to NK cell-mediated killing, providing new insights for the mechanisms of the antitumor effects of DAC.
Keywords: Decitabine, PDGC, Differentiation therapy, Immunity, NK cells
Subject terms: Cancer, Cell biology
Introduction
Gastric cancer (GC) is a malignancy of the digestive tract with high incidence and mortality rates globally1, ranking as the fifth most common and third deadliest cancer globally. Risk factors include Helicobacter pylori infection, increasing age, high-salt diets, and low intake of fruits and vegetables2. The Lauren classification is the predominant method for classifying gastric cancer, categorizing it into intestinal and diffuse types3. These two categories exhibit substantial differences in their clinical characteristics, genetics, and morphological features4. Intestinal-type gastric cancer features tumor cells that are cohesive and capable of forming gland-like structures; on the other hand, diffuse-type gastric cancer is marked by non-cohesive tumor cells that exhibit weak interactions and usually infiltrate the matrix as individual cells or in small clusters, forming non-cohesive cell groups3. Poorly differentiated gastric cancer (PDGC) and gastric signet ring cell cancer (GSRCC) are both classified as diffuse gastric cancer5.
PDGC is a highly heterogeneous malignant tumor, characterized by significant abnormalities in cell morphology and structure6. The tumor lacks normal cell differentiation features, exhibiting high proliferative and invasive properties and allowing it to invade nearby tissues and organs. Its rapid growth and division facilitate swift tumor expansion and spread, potentially metastasizing to distant sites in the body7. Surgical resection is typically the first-line treatment for early PDGC8; this is followed by chemotherapy and radiotherapy, using high-energy radiation to kill or shrink the tumor, commonly employed as adjuvant treatments after surgery9. Furthermore, targeted therapies employ drugs aimed at specific cancer cell growth signaling pathways or molecular targets. While these targeted drugs can be effective in some PDGC patients, their benefit is generally diminished for patients with advanced disease, particularly those receiving trastuzumab, compared to those with well-differentiated cancers10. Due to its high malignancy and aggressiveness, PDGC commonly presents with advanced-stage diagnosis and its limited responsiveness to conventional treatments leads to a generally unfavorable prognosis11.
Traditional chemotherapy and radiotherapy primarily function by directly killing tumor cells to inhibit tumor growth; however, these methods inevitably cause damage to normal cells12,13. The concept of differentiation therapy stems from the fact that hormones or cytokines can promote the differentiation of ex vivo tumor cells, thereby altering their phenotype. Differentiating agents activate the intrinsic differentiation potential of cancer cells, leading to the restoration of their normal cellular differentiation state, thus eliminating malignant characteristics and effecting the transformation of cancer cells back to normal cells. Compared to conventional cancer treatments, differentiation therapy drugs generally have lower toxic side effects14. Since the late 1970s, researchers have progressively explored differentiation therapy, discovering that various signaling molecules and drugs including retinoic acid (RA), cyclic adenosine monophosphate (cAMP), and sodium butyrate can induce the terminal differentiation of tumor cells in diseases such as acute myeloid leukemia (AML), embryonal carcinoma, and neuroblastoma in vitro15,16. The treatment of acute promyelocytic leukemia is a landmark success of differentiation therapy, where the disease can be cured through the combined use of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). This cure not only represents a significant breakthrough in the field of differentiation therapy but also marks a milestone in the development of this new therapeutic approach17.
Besides the breakthrough of differentiation therapy in non-solid tumors, there have been numerous studies exploring the potential of differentiation therapy for solid tumors. A Study by Yan et al. found that treatment with ATRA enhanced the expression of IKB kinase α (IKKα), thereby guiding the differentiation of poorly differentiated nasopharyngeal carcinoma (NPC) cells towards normal cells18, demonstrating the potential of differentiation therapy in solid tumor treatment. Based on these findings, further exploration of differentiation therapy for solid tumors, such as PDGC, holds the immense potential.
As a cell cycle S-phase specific cytidine analog, decitabine (5-aza-2’-deoxycytidine, DAC) is phosphorylated upon entry into the cell to form DAC triphosphate (5-aza-dCTP), which, like cytosine, is incorporated into the DNA strand by DNA polymerase during DNA replication. It binds to DNA methyltransferases (DNMTs), inhibiting their catalytic activity, thereby preventing DNA methylation, affecting gene expression and cell function, and exerting its anticancer effects19,20. Low doses of DAC can induce changes in the gene expression profile, promoting cellular differentiation21,22. DAC acts by inhibiting DNMT activity, resulting in DNA demethylation and the activation of transcription for genes silenced by methylation, especially those involved in cell differentiation and proliferation. This process encourages tumor cells to progress to a mature differentiated state, diminishing their malignancy. Additionally, DAC can induce apoptosis in tumor cells and inhibit their growth and dissemination23. In May 2006, DAC was approved by the FDA for the treatment of myelodysplastic syndromes (MDS) 19,24.
Natural killer (NK) cells, as key immune cells within the lymphocyte family, are responsible for generating, forming, and maintaining multicellular immune responses within the body25. The function of NK cells is tightly regulated by activating and inhibitory receptors to protect the body from pathogens and cancer threats. They can quickly activate in the absence of prior specific antigen exposure to identify and eliminate pathogen-infected or abnormal cells, such as virus-infected cells, tumor cells, and cells lacking MHC-I molecules26,27. In addition to their direct cytotoxic functions, NK cells can secrete cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor (TNF), which modulate the activity of other immune cells like macrophages and dendritic cells, thereby enhancing the immune response28,29.
It is well known that DNA-damaging anticancer drugs, such as gemcitabine, have been shown to enhance the expression of innate immune receptors on the surface of tumor cells30. Studies have found that Tumor cells treated with gemcitabine showed increased expression levels of CD95 (a death receptor), and transcription levels of two ligands for the activating natural killer cell receptor NKG2D (Natural Killer Group 2, Member D), namely MICB (MHC class I polypeptide-related sequence B) and ULBP2 (UL16 binding protein 2), were upregulated as well. This suggests that such drugs act on tumor cells by enhancing the expression of immune receptors, thereby increasing their susceptibility to immune cell attacks30.
This study utilized the S-phase specific drug DAC to treat two PDGC cell lines, MKN45 and NUGC4. By assessing the impact of DAC on the cell morphology, migratory and invasive capabilities, and gene expression differentiation of these cells, we further explored its ability to suppress the pathogenicity of PDGC cells in vitro and in vivo through enhanced NK cell cytotoxicity. This study uncovers novel antitumor roles of DAC via the regulation of immune-related genes in the treatment of PDGC, offering new insights and approaches for the differentiation therapy of solid-type poorly differentiated gastric tumors.
Materials and methods
Mice
Four-week-old male BALB/c Nude mice (Gempharmatech Co., Ltd.) were housed in a 12h light/12h dark cycle under specific pathogen-free conditions. They are provided ad libitum access to food and water. We carried out all mouse experiments in compliance with the regulations of Institutional Animal Ethics Committees at The Hangzhou Institute of Medicine, Chinese Academy of Sciences. The studies were approved by the Institutional Animal Ethics Committees at the Hangzhou Institute of Medicine, Chinese Academy of Sciences. The 3Rs rule was respected. All methods are compliant with the ARRIVE guidelines (https://arriveguidelines.org).
Cell cultures
Gastric cancer cell lines MKN45 and NUGC4 (iCell Bioscience Inc., Shanghai, China) are maintained in RPMI 1640 medium (Gibco, C11875500BT) enhanced with 10% FBS (Gibco, 10270-106) and 1% P/S (biosharp, BL505A), with passaging performed using trypsin (biosharp, BL512A). Human malignant non-Hodgkin lymphoma-derived natural killer cells NK-92MI (iCell Bioscience Inc., Shanghai, China) were cultured in a medium supplemented with 0.2 mM myo-Inositol (MCE, HY-B1411), 0.1 mM β-mercaptoethanol (Gibco, 21985023), 0.02 mM folic acid (MCE, HY-16637), 12.5% horse serum (Pricella, 164215), 12.5% FBS (Noverse, NFBS-2500A), and 1% P/S (Biosharp, BL505A). Cells were placed in 10 cm cell culture dishes (LABSELECT, 12311) and cultured in an incubator set to 37 °C with 5% CO2. All cell lines were cultured for no more than 15 passages and were free from mycoplasma contamination.
Cell proliferation and viability
Cell suspension was mixed with trypan blue staining solution (Invitrogen, T10282) and allowed to stand at room temperature for 3 min. Then, 10 μL of sample was loaded into the chamber of a hemocytometer, and cell counting was performed under a microscope.
β-gal assay
Following 5 days of DAC treatment (Biosmile, HY-A0004), cells were fixed and stained using a cellular senescence β-galactosidase staining kit (Beyotime, C0602). Subsequently, crystals were washed off with 70% ethanol and replaced with PBS (Servicebio, G4250-500ML) for imaging under a microscope.
Transwell assay
Matrigel (BD, 356234) was mixed with 1640 medium at a ratio of 1:8 and added to the upper chamber of the Transwell insert (Corning, 3422), incubated at 37 °C for 30 min to form gelation. Subsequently, 10g/L BSA-containing 1640 medium (Biofroxx, 4240GR100) was added to the upper chamber, and incubated at 37 °C for 30 min before adding the cell suspension to the upper chamber, while 600 μL of 10% FBS-containing 1640 medium was added to the lower chamber. After incubating for 24h, the insert was fixed in 4% paraformaldehyde, then stained with 0.2% crystal violet, and imaged under a 20 microscope. Cell counting was performed using ImageJ, and the number of cells migrating to the bottom of the membrane was compared by generating column charts using GraphPad.
Scratch assay
After 5 days of DAC treatment, cells were digested using trypsin and counted, then 5 106 cells per well were seeded in a 12-well plate, cultured for 24h, and scratched vertically using a 200 μL pipette tip, followed by washing cells three times with PBS and adding 1640 medium. The medium was changed every 24h, and images were captured under 4 microscope.
Flow cytometry
Mouse spleens were collected and mechanically dissociated. The splenocytes were filtered through 70 μm strainer to obtain a suspension of individual splenocyte cells. Conjugated antibodies for CD45-APC/Cyanine7 (Cat. # 103116), CD3- PerCP/Cyanine5.5 (Cat. # 100218), CD19- FITC (Cat. #115506), CD49a-PE (Cat. #142604), CD49b-APC (Cat. #108910), CD27-PE/Cyanine7 (Cat. #124216), and CD11b-BV421 (Cat. #101236) were purchased from Biolegend. Cells were stained in flow staining buffer (PBS plus 1% FBS), incubated with antibodies at room temperature for 30 min in the dark, then washed with flow staining buffer, and finally subjected to sample detection on a BD flow cytometer, with analysis performed using FlowJo software.
Cell cycle analysis
Cells treated with DAC for 5 days were collected according to the manufacturer’s instructions and analyzed using a cell cycle detection kit (Beyotime, C1052). First, cells were washed twice with PBS, then fixed with 70% ethanol. And then they were washed twice with PBS, followed by staining with iodide propidium containing RNase I at room temperature in the dark for 30 min, and finally subjected to flow cytometric analysis using a Beckman flow cytometer, with analysis performed using FlowJo software.
Cell co-culture
Cells treated with DAC for 5 days were collected to obtain cell suspension, followed by staining with 5 μM CFSE for 15 min at room temperature, then adding serum-containing 1640 medium to terminate staining. NK-92MI cells were mixed with PDGC cells at a ratio of E:T = 3:1 in a 12-well plate with 1640 medium plus 10% FBS for 4h co-culture. After co-culture, the cells were collected, resuspended in 300 μL PBS, and 2 μL PI was added for staining for 30 min, followed by flow cytometric analysis.
Enzyme-linked immunosorbent assay
The cell culture medium was centrifuged at 3000 rpm for 20 min at 4 °C, the supernatant was collected. The Human TNF-alpha Standard TMB ELISA Development Kit (PEPRO TECH, 900-T25) combined with an enzyme plate (Corning, 9018) were used to measure the TNF-alpha level in cell culture supernatant. The corresponding absorbance was read using a microplate reader, and a standard curve was generated to calculate the TNF-α content in the sample.
Tumor implantation
Subcutaneous injection of 100 μL Matrigel containing 2 106 MKN45 cells was performed to establish the tumor model. Starting from the 5th day after injection, intraperitoneal injection of 0.4 mg/kg DAC was administered every 2 days, with 100μL per mouse each time, for a duration of 3 weeks. Tumor size and mouse weight were measured and recorded before each injection, and tumor volume was calculated using the formula V 0.52 length width2.
Real-time quantitative PCR
Total RNA was extracted from cells treated with DAC for 5 days and dissected tumor tissues, following the manufacturer’s instructions for TRIzol reagent (Yeasen, 10606ES60). Real-time quantitative PCR (qRT-PCR) was performed using reverse transcription reagents (TIANGEN, KR118-02) and SYBR Green (Yeasen, 11201ES08), and the relative quantification of gene expression were determined based on the Cq value, normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) standards.
Transcriptome sequencing
RNA sequencing services were provided by Azenta Life Sciences Co., Ltd., and heat maps were generated using Hiplot software developed by Shanghai Tengyun Biotech Co., Ltd.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 software. Results were presented as mean ± standard error of the mean (SEM). Statistical differences between two groups were analyzed using the Student’s t-test, with significance levels indicated as: * for P < 0.05, ** for P < 0.01, and *** for P < 0.001.
Results
The effect of DAC on cell morphology and proliferation
In most malignant tumors, DNA methylation pattern undergoes significant alteration, thereby affecting gene expression20. We selected two human gastric cancer cell lines with a low degree of differentiation, MKN45 and NUGC4, for differentiation induction studies using a DNA methyltransferase inhibitor, DAC. DAC has been utilized as a treatment for acute Myelodysplastic Syndromes (MDS) and standard therapeutic dosage of DAC is 15–20 mg/m2, which results in plasma concentration around 1 μM approximately 2 h after administration of DAC31,32. In addition, DAC at concentrations ranging from 0.1 to 5 μM eradicated proliferative potential of multiple solid tumor cells in vitro31. Taking into account both standard therapeutic dosage of DAC for MDS patients and experimental outcomes in vitro, we employed 0.5 μM and 1 μM concentrations of DAC in our experiment in vitro.
After treating cells with DAC for 5 days, significant morphological differences were observed between the drug-treated cells and the control group. The impact of DAC on the morphology of the two cell lines was evident. As the drug concentration increased, the treated cells tended to shift from round to spindle-shaped, and the cell size increased as well (Fig. 1a). Cellular senescence can cause similar morphological changes, and aging cells typically increase in volume and express β-galactosidase, which is highly active at pH 6.033,34. To exclude the possibility of cellular aging on morphological changes, cells were stained with β-galactosidase, using X-Gal as the substrate, which results in a deep blue reaction product in aging cells. The staining intensity of DAC-treated MKN45 and NUGC4 cells was compared to control cells, and no significant differences were found, confirming that the observed morphological changes were not attributable to aging (Fig. 1b). Instead, these morphological changes were likely a result of cellular differentiation.
Fig. 1.
Effects of DAC on cell morphology and proliferation of PDGC cells. (a) Representative images of MKN45 and NUGC4 Cells after DAC treatment at a concentration of 0.5 μM or 1 μM DAC 5 days. Red arrows indicate the cells with increased cell volume after DAC induction. (b) The β-galactosidase staining kit was used to stain PDGC cells treated with different concentrations of DAC, using X-Gal as the substrate. Senescent cells generate a deep blue reaction product, with the intensity of the blue color indicating the concentration of β-galactosidase in the cells. (c) The proliferation of PDGC cells after DAC treatment. Decitabine inhibited the proliferation capacity of the MKN45 and NUGC4 cell lines by 30%-50%. (d) Cell viability test with trypan blue. (e) After fixing the cells induced by decitabine for 5 days, propidium iodide was used to stain the cell nucleic acids, and flow cytometry was used to analyze cell cycles for PDGC cells. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 20 μm.
To test effects of decitabine on proliferation of PDGC cells, CCK-8 assay was utilized following a 5-day treatment of DAC. We found that 0.5 μM and 1 μM of DAC could reduce cell proliferation by 30%-50% (Fig. 1c) without significantly affecting cell survival rate determined by trypan blue staining (< 10%) (Fig. 1d). Annexin V staining analysis did not reveal any apparent increase in apoptosis following treatment with either concentration of DAC (data not shown). DAC acts as an S-phase-specific agent that interferes with DNA synthesis during the S phase. To investigate whether the suppressed cell proliferation is attributed to the blockade of S phase, we performed the cell cycle analysis by flow cytometry, showing DAC treatment increased the proportion of cells in the S phase by ~ 10% (Fig. 1e).
The effect of DAC on cell invasion and migration in vitro
The impact of tumor metastasis on poor cancer prognosis is profound, and it represents a crucial factor in the context of PDGC. We further explored the effects of DAC on metastasis associated cell behaviors. The invasion assay in vitro showed the treatment of DAC at a concentration of 0.5 μM significantly reduced the invasiveness of MKN45 and NUGC4 cells by 25–30% (Fig. 2a). As the drug concentration increased to 1 μM, there was a 50% reduction in invasion capability. We also assessed the migration capability of the NUGC4 cell line by creating scratches on a monolayer of cells and measuring the wound area. The results indicate that the migration capability of DAC-treated cells was significantly reduced compared to control cells (Fig. 2b).
Fig. 2.
Effects of DAC on cell invasion and migration. (a) PDGC cells were treated by DAC for 5 days, then were cultured for 24 h in transwell plate, followed by microscopic observation. The number of cells that migrated to the membrane’s lower layer was counted and analyzed using ImageJ. (b) Wound healing assay of DAC-induced NUGC4 cells was performed using low serum medium. Observations were recorded on day 0 and day 4. Wound area was quantified using ImageJ software. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 20 μm (a); 50 μm (b).
These results indicate that treatment with different concentrations of DAC can affect cellular morphology and simultaneously inhibit cell proliferation, invasion, and migration. In addition, these effects are negatively correlated with drug concentrations.
Effects of DAC on expression of immune-related genes in PDGC cells
Low dose of DAC induces changes in gene expression and promotes cell differentiation21,22. Following initial analyses of DAC’s impact on cell morphology and behavior, further investigation was conducted into its effects on cell differentiation and the expression of immune-related genes.
Inadequate cell differentiation often stems from the downregulated or aberrant expression of genes associated with differentiation process, obstructing the normal progression of cell differentiation. As a result, this impacts morphology and function of the cells. CDX2 and CK2 are considered sensitive markers of differentiation for adenocarcinoma of the gastrointestinal tract and other tissues35,36, while CK7 expression is characteristic of signet ring cells, indicative of low differentiation37. To confirm the expressional changes of these gene markers related to differentiation, qRT-PCR was performed. As shown in Fig. 3a, it reveals that DAC treatment upregulated CDX2 by ~ twofold and CK2 expressions by ~ 5 folds in both in MKN45 and NUGC4 cells. DAC treatment downregulated CK7 expression in MKN45 cells. These results suggest that DAC promotes differentiation in MKN45 and NUGC4 cells.
Fig. 3.
DAC treatment affect expression of immune-related genes in PDGC cells. (a) RNA was extracted from cells treated with decitabine for 5 days, followed by reverse transcription. Real-time quantitative fluorescence PCR was then used to measure the relative mRNA expression of differentiation-related genes in both cell lines. CDX2 and CK2 are considered positive markers of differentiation in some cancer type, while CK7 positivity is a feature of poor differentiation. (b) Transcriptome sequencing was performed on DAC-induced PDGC cells, and a heatmap of gene expression differences related to immune inflammation was generated. Red indicates high expression, while blue indicates low expression. (c) KEGG pathway enrichment plot for differentially expressed genes. (d) Evaluation of expressional differences of immune related genes by qRT-PCR. (e) Measurement of TNF-α level from cell culture of DAC-treated PDGC cells by ELISA assay. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
To further compare the expression profiles before and after DAC treatment, we analyzed transcriptome profiling by RNA-seq. Interestingly, we found many genes with significant expressional change are related to immune and chemotactic factors. Key pathways analyzed included the TNF signaling pathway, chemokine signaling pathway, and Th1 and Th2 cell differentiation. Transcriptomic data revealed that most genes within these three pathways were upregulated (Fig. 3b). Genes associated with immunity, differentiation, proliferation, and apoptosis were selected from these pathways to create a heatmap (Fig. 3b). We applied a threshold of Q value ≤ 0.05 and identified the 30 most significantly enriched pathways to create a KEGG pathway enrichment map for differentially expressed genes (Fig. 3c). Next, we conducted qPCR and confirmed the expressional change of these immune-related genes. TNF, known as inflammation related cytokine, was upregulated in both MKN45 and NUGC4 cell line (Fig. 3d). Inflammation related genes IL1B were upregulated as well. In addition, we observed an increase of expression level of HLA-DMB that encodes beta chain of MHCII. We also confirmed some genes encoding for chemokine and chemokine receptors. Among them, the majority of examined genes exhibited upregulated expression or a trend of upregulation. CX3CL1 and CCR10 were upregulated in both MKN45 and NUGC4 cell line.
Based on RNA-seq data showing upregulated TNF mRNA expression in PDGC following DAC treatment, we hypothesized that these cancer cells might actively secrete the proinflammatory cytokine TNF-α during DAC-induced differentiation. TNF-α is a pleiotropic cytokine classically associated with immune cells, where it enhances innate immunity, modulates immune cell activation, and triggers apoptosis in target cells38. To test this hypothesis, we quantified TNF-α protein levels in the culture supernatant of DAC-treated PDGC (MKN45 and NUGC4 cell lines) using enzyme-linked immunosorbent assay (ELISA). Notably, TNF-α secretion increased in a DAC concentration- and treatment duration-dependent manner in both cell lines (Fig. 3e). These data confirm that TNF-α is secreted by PDGCs during DAC-induced differentiation, suggesting a potential autocrine or paracrine role of TNF-α in impacting tumor progression.
These results indicate that DAC treatment modulates the expression of genes related to differentiation and inflammation in the PDGC cells, providing a basis for further exploration of its effects on the interaction between PDGC cells and immune cells.
The effect of DAC on NK cell-mediated cytotoxicity against PDGC in vitro
Natural Killer (NK) cells, as innate immune cells, play an important role in tumor immune suppression39. Since our studies have shown that DAC treated PDGC exhibit increased expression of inflammation related genes and other immune related genes, we proceeded to explore the effects of DAC induction on NK cell-mediated cytotoxicity against PDGC. We performed NK cell cytotoxicity against PDGC by co-culture of NK-92MI cells with DAC-treated PDGC at an E:T ratio of 1:3. Flow cytometry was used to assess the viability of the gastric cancer cells and to evaluate the cytotoxic capability of the NK-92MI cells. Based on prior evidence demonstrating that PDGC secrete TNF-α during DAC-induced differentiation, we utilized conditioned medium harvested from DAC-treated PDGC to mimic paracrine signaling in co-culture systems with NK-92MI cells and DAC-treated PDGCs. This experimental design aimed to recapitulate the crosstalk between cancer cells and NK cells that was mediated by soluble factors, including TNF-α in tumor microenvironment. As shown in Fig. 4a, compared with control group without DAC treatment, there was minimal cytotoxic effects from the group with 0.5 μM treatment. As the concentration of DAC increased from 0.5 μM to 1 μM, the rate of killing of PDGC cells by NK cells increased by ~ 25% (Fig. 4a). It is worth noting that the killing efficiency of NK-92MI cells significantly improved in the presence of the cell culture supernatant collected from DAC-induced differentiated PDGC cells (Fig. 4a & b). These data suggest some soluble immunomodulatory factors including TNF-α from the cell culture supernatant enhanced the killing efficiency of NK-92MI cells.
Fig. 4.
DAC treatment enhanced the sensitivity of NK cell cytotoxicity against PDGC cells via TNF-α. (a) The evaluation of NK-92MI cell cytotoxicity against PDGC cells. NK-92MI cells were mixed with PDGC cells at a ratio of E:T = 3:1 in a 12-well plate with 1640 medium plus 10% FBS for 4h co-culture. Cell lysis was analyzed by flow cytometry. Cell culture supernatants ( +): The cytotoxicity assay was performed in the presence of cell culture supernatant from DAC-treated MKN45 or NUGC4 cells. Cell culture supernatants (–): The cytotoxicity assay was performed in the absence of cell culture supernatant from DAC-treated MKN45 or NUGC4 cells. (b) The effect of the fully human IgG1 monoclonal anti-TNF-α antibody, Adalimumab, on the cytotoxicity of NK-92MI cells was evaluated. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
To validate the role of TNF-α in the mechanism by which NK-92MI cells kill differentiated gastric cancer cells, co-culture experiments were conducted in the presence of human monoclonal anti-TNF-α antibody—adalimumab40. And we found that adalimumab reduced the cytotoxic efficiency of NK-92MI cells against PDGC cells (Fig. 4b). These results indicate that DAC-induced TNF-α secretion from PDGC cells enhances the cytotoxic sensitivity of NK-92MI cells.
The impact of DAC on tumor growth in vivo
To explore whether DAC promote PDGC cell differentiation and enhance cytotoxic sensitivity of NK cells in vivo, we utilized BALB/c Nude mice with functional NK cells for this evaluation. After conducting a comparative analysis of the tumorigenicity of MKN45 and NUGC4 cell lines in nude mice, we selected the human PDGC cell line MKN45 due to its more reliable and consistent tumorigenic properties.
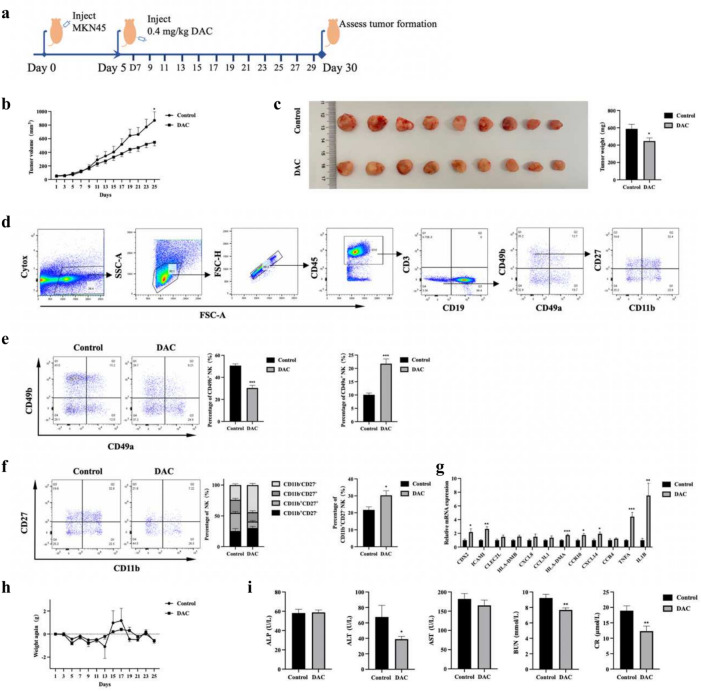
We inoculated logarithmically growing MKN45 cells subcutaneously into the axillae of 4-week-old male BALB/c Nude mice. After confirming successful tumor inoculation, DAC treatment was initiated on the 5th day post-inoculation. The mice received 0.4 mg/kg of DAC via intraperitoneal injection every two days. The control group was injected with solvent of DAC, dimethyl sulfoxide (DMSO). The treatment lasted for three weeks, with mouse weight and tumor size monitored (Fig. 5a). At the end of the treatment, it was observed that DAC treatment delayed tumor growth with reduced tumor mass and volume (Fig. 5b,c). H&E staining showed tumor from DAC treatment group exhibited a loose texture and tumor cell exhibited a larger size (Supplementary Fig. 1a).
Fig. 5.
DAC administration inhibits tumor growth in nude mice. (a) Experimental design of tumor model with DAC administration. The tumor model was established by subcutaneous injection of 2 × 106 MKN45 cells into the axilla of 4-week-old male BALB/c Nude mice. After confirming successful tumor inoculation, DAC treatment began on the 5th day post-inoculation. The experimental group received intraperitoneal injection of 0.4 mg/kg DAC every two days, while the control group received injection of the solvent DMSO. (b) During the 3-week treatment process, mice tumor volumes were monitored, and the tumor volume was calculated using the formula V = 0.52 × length × width2. (c) Illustration of tumor dissection and tumor weight in mice. (d) NK cell subpopulation gating. Splenic cells were isolated from tumor-bearing nude mice, stained with antibodies with fluorescence, and then detected using flow cytometry. (e) The proportion of NK cell subpopulations. (f) Effect of DAC treatment on the maturation of CD49b+NK cells. The proportion of mature NK cells (CD11b+CD27-) within the cNK population exhibited an upregulated trend. (g) Relative mRNA expression from tumors. (h) Assessment of body weight after DAC treatment. (i) Toxicity test to mice after DAC treatment. ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BUN blood urea nitrogen, CR creatinine. Error bars represent mean ± SEM; n = 9 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
Spleen cells from tumor-bearing nude mice were extracted and the proportion and maturation of NK were analyzed by flow cytometry. The gating strategy was shown as in Fig. 5d. The expression of CD27 and CD11b have been used to define the maturation of NK cells41. The classification of developing NK cells is as follows: precursor (Stage I, CD11b- CD27-), immature (Stage II, CD11b- CD27+), proinflammatory (stage III, CD11b+CD27+), or cytotoxic (stage IV, CD11b+ CD27-). The stage IV exhibits highest cytotoxicity capability. Although DAC treatment decreased the proportion of CD49b+ NK cells, which were considered as circulating NK cells, it promoted the maturation with stage IV increase by ~ 40% (Fig. 5e,f). In contrast, DAC treatment reduced the maturation of CD49a+ NK cells by ~ 50% (Supplementary Fig. 1b).
The immune gene expression profile in tumor by qPCR revealed a notable regulation of CDX2 gene, with an increase of ~ 100%, suggesting a high level of cellular differentiation. Additionally, the genes associated with inflammation, such as TNF-α, exhibited a significant increase in expression. In general, the pattern of gene expression change is consistent with it in vitro (Fig. 5g). Next, we evaluated whether DAC treatment caused any toxicity to mice. There was no significant change for body weight gain between DAC-treated group and control group (Fig. 5h). Furthermore, liver and kidney toxicity test revealed no notable toxicity to these two organs (Fig. 5i).
Discussion
In our study, we investigated the impacts of DAC on the differentiation of PDGC cells and associated expression profiles of immune related genes, which sensitize the NK cell cytotoxicity toward PDGC cells. DAC is considered to be converted into decitabine triphosphate to form its active metabolite, which is incorporated into the DNA during the S phase of cell cycle to suppress the activity of DNMTs42. Our data showed that DAC treatments for 5 days at concentrations of 0.5μM or 1μM altered the morphology and slowed the proliferation of PDGC cells without affecting the viability at both concentrations. In addition, DAC treatment significantly inhibited the PDGC cell invasion and migration. Gene expression profile by RNA-seq analysis showed immune-related genes including TNF genes were upregulated. Consistently, ELISA assay showed increased level of TNF in the cell culture supernatant after DAC treatment. We found cell culture supernatant sensitized the NK cell cytotoxicity against PDGC cells, whereas the anti-TNF antibody blocked the increased sensitivity of NK cell cytotoxicity. Furthermore, DAC administration effectively inhibited tumor growth caused by subcutaneously implanted PDGC cells in nude mice. Notably, TNF gene expression was upregulated within tumor. Our in vitro and in vivo findings indicate that DAC treatment induce alternations of immune-related gene expression and remodels immune microenvironment of PDGC tumor, which is a novel underlying mechanism of DAC-induced anticancer effects.
Numerous studies have demonstrated that the impact of DAC on cancer cell is dependent on its concentration. High doses of DAC cause the apoptosis while low doses of it leads to differentiation43,44. A study by Ramakrishnan et al. showed DAC at concentrations of 0.1 and 1 µM are non-cytotoxic to bladder cancer lines and inhibit their proliferation mainly by differentiation effect43. Likewise, our finding showed that when DAC was administrated at concentrations of 0.1 and 1 µM, it prompted PDGC cell differentiation while impeding their proliferation. The cell viability remained comparable and there was no significant difference in terms of apoptosis following DAC treatment. We believe that these concentrations are ideal for studies of differentiation related study for PDGC cells. It was reported that cell size was linked to cell stemness and there was a positive correlation between increased cell size and differentiation phenotypes18,45. Our data showed that DAC treatment increased the size of PDGC cell, coinciding with the observation of large size induced by DAC treatment in bladder cancer cells43. X-Gal staining was used to test whether the increase in cell size was attributed to cellular senescence and the staining intensity exhibited no discernible difference, further supporting the hypothesis that the alternation in cell morphology was primarily due to DAC-induced differentiation. Furthermore, DAC treatment significantly decreased PDGC cells’ capability of invasion and migration, indicating a reduced stemness following DAC treatment. This result was consistent with the reports that stemness was connected to enhanced invasive features46.
The process of differentiation is closed linked to the alteration of cell signaling pathways, which play an important role in establishing the characteristic traits associated with a more specialized and mature state of a cell47. CDX2, a lineage-specific transcription factor, plays a crucial role in the proliferation and differentiation of gastrointestinal epithelial cells48. Consequently, it serves as a good differentiation marker gene for gastrointestinal epithelial cells. Our data in vitro exhibited an increase in the expression level of CDX2 gene, consistent with the report that CDX2 could serve as a prognostic marker, indicative of improved survival in gastric cancer49. In addition, DAC treatment led to an increase of CDX2 gene expression in both MKN45 and NUGC4 cells. Cytokeratins are an important component of intermediate filaments of epithelial cells. The pattern of cytokeratin expression is relatively specific in tissues50. In our study, DAC treatment upregulated the expression of CK2, indicating a more differentiated state of PDGC cells49. The gene expression profile of PDGC cells following DAC treatment demonstrated that expression of multiple immune-related gene was altered, including inflammation-related genes such as TNF and IL1B. Interesting, the genes encoding NK cell activation ligands were upregulated including ICAM1 in PDGC cells following DAC treatment. Notably, the tumor samples from DAC-treated mice exhibited a gene expression profile that was similar to the profile observed in DAC-treated PDGC cells. For example, there was an upregulation for certain signature genes such as CDK2, TNF and CCR10 (Figs. 3c & 5g).
Previous studies reported the stemness of cancer cells possessed the potential to shape their microenvironment and impact its interactions with tumor infiltrated lymphocytes51. Miranda et al. reported that stemness was negatively correlated with immune cell infiltration across solid cancers51. Our data here were consistent with these previous reports, suggesting a more differentiated status of cancer cells could confer a favorable environment to support activation of tumor infiltrated cells that limited the growth of cancer. Indeed, we observed an enhancement of NK cell cytotoxicity toward DAC-treated PDGC cells in comparison to untreated PDGC cells. We speculated that there were two possibilities to interpret the enhancement of cytotoxicity of NK cells induced by the differentiated PDGC cell. The one is the upregulation of activating ligands of NK cells, leading to a more potent immune response. The other is elevated secretion of inflammatory cytokines including TNF, contributing the NK cell cytotoxicity. In our current study, we observed an additional enhancement of NK cell cytotoxicity when the cytotoxicity assay was conducted in the presence of cell culture supernatant obtained from PDGC cells treated with DAC (Fig. 4a). This finding provides support for the notion that certain cytokines secreted from the PDGC cells can sensitize the NK cell cytotoxicity. We postulated that TNF might increase the NK cell cytotoxicity. Our ELISA assay validated the increased level of TNF secreted from differentiated PDGC cells, which is consistent with the qPCR data indicating the elevated mRNA level (Fig. 4c). More importantly, the sensitization of NK cell cytotoxicity was abrogated when the cytotoxicity assay was conducted in the presence of anti-TNF antibody. Collectively, this evidence in our study support TNF secreted from the process of differentiation of PDGC cells play a crucial role of regulation of NK cell cytotoxicity. This finding is consistent with previous reports showing that NK cells express TNF receptor and TNF signaling pathway enhance NK Cell production of IFN-γ and their cytotoxic capabilities52,53. Furthermore, there was an increase of TNF level by ~ fourfold from tumor subcutaneous implanted in mice after DAC treatment, indicating the important role in tumor inhibition by enhancement of NK cell cytotoxicity (Fig. 5g).
DAC, as a hypomethylating agent, has been used for treatment of adult patients with MDS24. It has demonstrated activity in other hematologic disorders including acute myelogenous leukemia (AML)54, chronic myelogenous leukemia (CML)55. Our study here demonstrated DAC treatment moderately inhibited the tumor growth in nude mice that were subcutaneously implanted with MKN45 cells. There are a couple of explanations for this modest effect. One is that the nude mice were used as the experimental model because the MKN45 cells are of human origin. The nude mice are immunodeficient, although NK cells are present in the mice. The mice don’t possess a robust enough immune system to effectively fight against the cancer. In addition, we observed an increased expression of HLA-DMA and HLA-DMB in vitro and in vivo (Figs. 3c & 5g), which may facilitate the presentation of tumor antigens in immunocompetent mice and lead to additional tumor inhibition. The other is the possibility of impacts of DAC on NK cells. From our data, we observed a reduction in the percentage of CD49b+ NK cells in spite of an increase of mature CD11b+ CD27- NK cells. Consistently, Li et al. reported that decitabine affects NK cell phenotype and function56,57 and its impact on NK cells is concentration dependent. To maximize the effects of decitabine on tumor growth in vivo, Decitabine administration with different dose are underway.
Our findings demonstrated that DAC treatment upregulated the expression of numerous immune-related genes, including chemokines. The expression of these chemokines may alter the tumor microenvironment by lymphocyte recruitment. For instance, CXCL10 is known to enhance NK cell migration through its interaction with CXCR3 on NK cells58. Additionally, CXCL10 promotes the recruitment CD8+ T and Th1-type CD4+ effector T cell to infected tissue and tumor microenvironment59. Furthermore, Saudemont et al. reported that CXCL10 stimulates NK cells to express B7-H1, which induces T cell proliferation and the production of interferon γ60. Based on this, we infer that DAC treatment may also impact adaptive immunity, facilitating cooperation between CD8+ T cells and tumor-infiltrating NK cells to enhance antitumor immunity. Further investigation is required to explore the effects of DAC treatment on adaptive immunity and its interplay with NK cells.
The limitation of this study is the unresolved mechanism by which decitabine induce the upregulation of TNF expression in PDGC cells. We have speculated two possible explanations: (1) The increased expression may be related to cellular differentiation processes; (2) Decitabine may increase TNF expression levels through demethylation of the TNF promoter region. Our data showed both human normal gastric epithelial cells GES-1 and NGEC exhibited relatively low basal levels of TNF expression compared to MKN45 cell line (data not shown). Therefore, it is plausible that decitabine, a DNA-demethylating agent, demethylated the promoter region of the TNF gene, resulting in its upregulation. This proposed mechanism warrants further investigation.
In summary, our study showed DAC promoted the differentiation of MKN45 and NUGC4 cells. The differentiation was associated with enlarged morphology, reduced capability of invasion and altered expression profile of immune genes. The upregulation of TNF expression sensitized the NK cell cytotoxicity toward PDGC in vitro and in vivo. These data show the potential of differentiation drug, reducing cancer cell malignancy, and enhancing their clearance by immune cells via modulation of interaction between cancer cell and immune cells such as NK cells. It slows the growth rate of PDGC in mice and boosts the function of immune cells within the mice. Overall, although DAC is principally employed in treating hematological disorders, further research is needed to thoroughly understand its effects and therapeutic potential against solid tumors.
Supplementary Information
Acknowledgements
The authors acknowledge the support from the Scientific Experiment Center, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences.
Author contributions
Man Lv: conceptualization, methodology, formal analysis, software, experimental work, writing-original draft, data curation. Wang Yue: methodology, formal analysis. Ziyin Yuan: experimental work, data curation. Lina Zhai: formal analysis, software, visualization. Haroon Iqbal: formal analysis, software. Uzair Ur-Rehman: methodology, formal analysis. Xin Ning: validation, data curation. Huiying Wei: methodology, visualization. Jun Xin: formal analysis, visualization, data curation. Zihui Jin: formal analysis, validation. Zhou Yi: formal analysis, validation. Baichuan Wang: visualization, formal analysis. Wangkai Chen: formal analysis, data curation. Xiao Run: conceptualization, supervision, project administration, resources, review & editing.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. YXD23H0303.
Data availability
The original data of RNA sequencing described in the paper has been deposited in the China National Center for Bioinformation database with accession number HRA008979. All other data are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95741-0.
References
- 1.Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68, 394–424. 10.3322/caac.21492 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet396, 635–648. 10.1016/s0140-6736(20)31288-5 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand.64, 31–49. 10.1111/apm.1965.64.1.31 (1965). [DOI] [PubMed] [Google Scholar]
- 4.Machlowska, J., Baj, J., Sitarz, M., Maciejewski, R. & Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci.10.3390/ijms21114012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J. N., Wang, Q. W., Zhang, Q. W., Tang, Z. R. & Li, X. B. Poorly differentiated is more significant than signet ring cell component for lymph node metastasis in mixed-type early gastric cancer: A retrospective study from a large-volume hospital. Surg. Endosc.35, 1558–1565. 10.1007/s00464-020-07532-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, S. F. et al. Lymph node micrometastasis of poorly differentiated node-negative gastric cancer risks a worse-than-expected survival outcome under standard management algorithm. Eur. J. Surg. Oncol.48, 783–788. 10.1016/j.ejso.2021.11.117 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Hatta, W., Gotoda, T., Koike, T. & Masamune, A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig. Endosc.32, 180–190. 10.1111/den.13531 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Allen, C. J. et al. Chemotherapy versus chemotherapy plus chemoradiation as preoperative therapy for resectable gastric adenocarcinoma: A propensity score-matched analysis of a large, single-institution experience. Ann. Surg. Oncol.28, 758–765. 10.1245/s10434-020-08864-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, J. et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J. Clin. Oncol.30, 268–273. 10.1200/jco.2011.39.1953 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Sun, L. B. et al. Comparison between better and poorly differentiated locally advanced gastric cancer in preoperative chemotherapy: A retrospective, comparative study at a single tertiary care institute. World J. Surg. Oncol.12, 280. 10.1186/1477-7819-12-280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, I. S. et al. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: Mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg. Oncol.26, 8–12. 10.1016/j.suronc.2016.12.001 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Della Pepa, C. et al. Ovarian cancer standard of care: Are there real alternatives?. Chin. J. Cancer34, 17–27. 10.5732/cjc.014.10274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhermain, F. Radiotherapy of high-grade gliomas: Current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin. J. Cancer33, 16–24. 10.5732/cjc.013.10217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan, M. & Liu, Q. Differentiation therapy: A promising strategy for cancer treatment. Chin. J. Cancer35, 3. 10.1186/s40880-015-0059-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotem, J. & Sachs, L. In vivo control of differentiation of myeloid leukemic cells by recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3. Blood71, 375–382 (1988). [PubMed] [Google Scholar]
- 16.Sachs, L. The molecular control of hematopoiesis and leukemia: From basic biology to the clinic. Hematol. Am. Soc. Hematol. Educ. Progr.10.1182/asheducation-2000.1.1 (2000). [DOI] [PubMed] [Google Scholar]
- 17.de Thé, H. Differentiation therapy revisited. Nat. Rev. Cancer18, 117–127. 10.1038/nrc.2017.103 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Yan, M. et al. IKKα restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nat. Commun.5, 3661. 10.1038/ncomms4661 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Patel, A. A., Cahill, K., Saygin, C. & Odenike, O. Cedazuridine/decitabine: From preclinical to clinical development in myeloid malignancies. Blood Adv.5, 2264–2271. 10.1182/bloodadvances.2020002929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabbour, E., Issa, J. P., Garcia-Manero, G. & Kantarjian, H. Evolution of decitabine development: Accomplishments, ongoing investigations, and future strategies. Cancer112, 2341–2351. 10.1002/cncr.23463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, P. A. & Taylor, S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell20, 85–93. 10.1016/0092-8674(80)90237-8 (1980). [DOI] [PubMed] [Google Scholar]
- 22.Momparler, R. L. Pharmacology of 5-Aza-2’-deoxycytidine (decitabine). Semin. Hematol.42, S9-16. 10.1053/j.seminhematol.2005.05.002 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Momparler, R. L. Molecular, cellular and animal pharmacology of 5-aza-2’-deoxycytidine. Pharmacol. Ther.30, 287–299. 10.1016/0163-7258(85)90053-1 (1985). [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian, H. et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer106, 1794–1803. 10.1002/cncr.21792 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Vivier, E. et al. Natural killer cell therapies. Nature626, 727–736. 10.1038/s41586-023-06945-1 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science331, 44–49. 10.1126/science.1198687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol.18, 85–100. 10.1038/s41571-020-0426-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang, Z., Wang, Z., Li, F., Feng, C. & Mu, X. Current progress of CAR-NK therapy in cancer treatment. Cancers (Basel)10.3390/cancers14174318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster, I. S., Coudert, J. D., Andoniou, C. E. & Degli-Esposti, M. A. “Natural Regulators”: NK cells as modulators of T cell immunity. Front. Immunol.7, 235. 10.3389/fimmu.2016.00235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gravett, A. M., Dalgleish, A. G. & Copier, J. In vitro culture with gemcitabine augments death receptor and NKG2D ligand expression on tumour cells. Sci. Rep.9, 1544. 10.1038/s41598-018-38190-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karahoca, M. & Momparler, R. L. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2’-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin. Epigenet.5, 3. 10.1186/1868-7083-5-3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blum, W. et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J. Clin. Oncol.25, 3884–3891. 10.1200/jco.2006.09.4169 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Angello, J. C., Pendergrass, W. R., Norwood, T. H. & Prothero, J. Cell enlargement: One possible mechanism underlying cellular senescence. J. Cell. Physiol.140, 288–294. 10.1002/jcp.1041400214 (1989). [DOI] [PubMed] [Google Scholar]
- 34.Chen, Q. M. et al. Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J. Cell. Sci.113(Pt 22), 4087–4097. 10.1242/jcs.113.22.4087 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Beck, F., Chawengsaksophak, K., Waring, P., Playford, R. J. & Furness, J. B. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc. Natl. Acad. Sci. U. S. A.96, 7318–7323. 10.1073/pnas.96.13.7318 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collin, C., Moll, R., Kubicka, S., Ouhayoun, J. P. & Franke, W. W. Characterization of human cytokeratin 2, an epidermal cytoskeletal protein synthesized late during differentiation. Exp. Cell Res.202, 132–141. 10.1016/0014-4827(92)90412-2 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Z. S. et al. Clinicopathological characteristics of signet-ring cell carcinoma derived from gastric fovelar epithelium. J. Dig. Dis.23, 396–403. 10.1111/1751-2980.13120 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley, J. R. TNF-mediated inflammatory disease. J. Pathol.214, 149–160. 10.1002/path.2287 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Shimasaki, N., Coustan-Smith, E., Kamiya, T. & Campana, D. Expanded and armed natural killer cells for cancer treatment. Cytotherapy18, 1422–1434. 10.1016/j.jcyt.2016.06.013 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther.117, 244–279. 10.1016/j.pharmthera.2007.10.001 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Chiossone, L. et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood113, 5488–5496. 10.1182/blood-2008-10-187179 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Santini, V., Kantarjian, H. M. & Issa, J. P. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann. Intern. Med.134, 573–586. 10.7326/0003-4819-134-7-200104030-00011 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Ramakrishnan, S. et al. Decitabine, a DNA-demethylating agent, promotes differentiation via NOTCH1 signaling and alters immune-related pathways in muscle-invasive bladder cancer. Cell Death Dis.8, 3217. 10.1038/s41419-017-0024-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng, W. et al. Decitabine-induced changes in human myelodysplastic syndrome cell line SKM-1 are mediated by FOXO3A activation. J. Immunol. Res.2017, 4302320. 10.1155/2017/4302320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Q., Rycaj, K., Chen, X. & Tang, D. G. Cancer stem cells and cell size: A causal link?. Semin. Cancer Biol.35, 191–199. 10.1016/j.semcancer.2015.07.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues, F. S. et al. IKKβ kinase promotes stemness, migration, and invasion in KRAS-driven lung adenocarcinoma cells. Int. J. Mol. Sci.10.3390/ijms21165806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joung, J. et al. A transcription factor atlas of directed differentiation. Cell186, 209-229.e226. 10.1016/j.cell.2022.11.026 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satake, S. et al. Cdx2 transcription factor regulates claudin-3 and claudin-4 expression during intestinal differentiation of gastric carcinoma. Pathol. Int.58, 156–163. 10.1111/j.1440-1827.2007.02204.x (2008). [DOI] [PubMed] [Google Scholar]
- 49.Masood, M. A., Loya, A. & Yusuf, M. A. CDX2 as a prognostic marker in gastric cancer. Acta Gastroenterol. Belg.79, 197–200 (2016). [PubMed] [Google Scholar]
- 50.Dmello, C. et al. Multifaceted role of keratins in epithelial cell differentiation and transformation. J. Biosci.10.1007/s12038-019-9864-8 (2019). [PubMed] [Google Scholar]
- 51.Miranda, A. et al. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. U. S. A.116, 9020–9029. 10.1073/pnas.1818210116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almishri, W. et al. TNFα augments cytokine-induced NK cell IFNγ production through TNFR2. J. Innate Immun.8, 617–629. 10.1159/000448077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mason, A. T. et al. Regulation of NK cells through the 80-kDa TNFR (CD120b). J. Leukoc. Biol.58, 249–255. 10.1002/jlb.58.2.249 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Feng, X. et al. Decitabine: An effective and safe treatment for myelodysplastic syndrome and acute myeloid leukemia. J. Cancer Res. Ther.15, 1471–1476. 10.4103/0973-1482.204849 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Itzykson, R. et al. Decitabine versus hydroxyurea for advanced proliferative chronic myelomonocytic leukemia: Results of a randomized phase III trial within the EMSCO network. J. Clin. Oncol.41, 1888–1897. 10.1200/jco.22.00437 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Li, X. et al. Concentration-dependent decitabine effects on primary NK cells viability, phenotype, and function in the absence of obvious NK cells proliferation-original article. Front. Pharmacol.12, 755662. 10.3389/fphar.2021.755662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cany, J. et al. Decitabine enhances targeting of AML cells by CD34(+) progenitor-derived NK cells in NOD/SCID/IL2Rg(null) mice. Blood131, 202–214. 10.1182/blood-2017-06-790204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ran, G. H. et al. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Signal. Transduct. Target. Ther.7, 205. 10.1038/s41392-022-01058-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peperzak, V. et al. CD8+ T cells produce the chemokine CXCL10 in response to CD27/CD70 costimulation to promote generation of the CD8+ effector T cell pool. J. Immunol.191, 3025–3036. 10.4049/jimmunol.1202222 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Saudemont, A., Jouy, N., Hetuin, D. & Quesnel, B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7–H1 that stimulates T cells. Blood105, 2428–2435. 10.1182/blood-2004-09-3458 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data of RNA sequencing described in the paper has been deposited in the China National Center for Bioinformation database with accession number HRA008979. All other data are available from the corresponding author on reasonable request.