Abstract
The humoral immune response against Leishmania braziliensis histone H1 by patients with cutaneous leishmaniasis is described. For this purpose, the protein was purified as a recombinant protein in a prokaryotic expression system and was assayed by enzyme-linked immunosorbent assay (ELISA) with a collection of sera from patients with cutaneous leishmaniasis and Chagas' disease. The assays showed that L. braziliensis histone H1 was recognized by 66% of the serum samples from patients with leishmaniasis and by 40% of the serum samples from patients with Chagas' disease, indicating that it acts as an immunogen during cutaneous leishmaniasis. In order to locate the linear antigenic determinants of this protein, a collection of synthetic peptides covering the L. braziliensis histone H1sequence was tested by ELISA. The experiments showed that the main antigenic determinant is located in the central region of this protein. Our results show that the recombinant L. braziliensis histone H1 is recognized by a significant percentage of serum samples from patients with cutaneous leishmaniasis, but use of this protein as a tool for the diagnosis of cutaneous leishmaniasis is hampered by the cross-reaction with sera from patients with Chagas' disease.
Histones are evolutionarily conserved proteins which associate with DNA to form the chromatin structural unit in eukaryotes, the nucleosome. The name histone H1 is applied to a family of small basic proteins which take part in the stabilization of the nucleosomes and facilitate the assembly of chromatin into higher-order structures. Histone H1 proteins have been described in different trypanosomatids like Crithidia fasciculata (7), Trypanosoma cruzi (1), Trypanosoma brucei (4), Leishmania major (9), and Leishmania braziliensis (13). All of these H1 proteins are smaller than their counterparts from higher eukaryotes due to their lack of a central globular domain. This fact has been related to the imperfect condensation of chromatin in trypanosomatid chromosomes during cell division (8).
The first report of the elicitation of a humoral immune response against parasite histones during infection was made in 1995, in which a response against Leishmania infantum H2A during canine visceral leishmaniasis (CVL) was described (16). Similar responses against histone H3, histone H2B, and a fragment of histone H4 from L. infantum were described thereafter (17, 18). The investigators mapped the linear epitopes of histones using synthetic peptides. Their findings led to the conclusion that the humoral response against the L. infantum histones during CVL was triggered by the less conserved regions of the molecule, which correspond to the amino- and carboxy-terminal ends of the protein (15).
The term leishmaniasis is applied to a spectrum of diseases caused by different species of the genus Leishmania. This parasite is endemic in 88 countries worldwide, and 350 million people are considered to be at risk of leishmaniasis. Of the 2 million new cases discovered every year, about 1.5 million are cutaneous leishmaniasis (CL) or mucocutaneous leishmaniasis (MCL; MCL is also known as New World leishmaniasis). L. braziliensis is one of the major causative agents of CL and MCL in wide areas of Central and South America.
In this paper, we report on the first analysis of the human humoral immune response against an L. braziliensis histone H1 from individuals with CL. The analysis included investigation of the potential use of this protein for the diagnosis of leishmaniasis, as well as the mapping of the linear antigenic determinants of L. braziliensis histone H1.
MATERIALS AND METHODS
Expression and purification of recombinant L. braziliensis histone H1.
The coding region of L. braziliensis histone H1 was amplified from clone 3.3 (13) by PCR with the following specific primers that include BamHI and HindIII sites, respectively (underlined): primer HIV-BH1 (5′-GGATCCTTCAAAGATGTTCGCTAA-3′) and HIV-HD3 (5′-AAGCTTGATGTGCCCGTAGGAGA-3′). The amplification was carried out for 30 cycles with 40 ng of each primer and 20 ng of clone 3.3 as the template. The PCR product was digested with the corresponding restriction enzymes, purified from agarose gels (Qiaex II; Qiagen), and cloned in frame into plasmid pQE31 (Qiagen) to give the construct pQELbH1.
Estimation of the optimal conditions for the induction of recombinant L. braziliensis histone H1 in the Escherichia coli Topp3 prokaryotic expression system (Stratagene) was achieved after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12) analysis of cultures induced with different isopropyl-β-d-thiogalactopyranoside concentrations and induction times. In order to solubilize recombinant L. braziliensis histone H1, a spectrum of different buffers was tested. The cell pellet was sonicated in sonication buffer (0.3 M NaCl, 50 mM NaHPO4 [pH 8], 1 mM phenylmethylsulfonyl fluoride) and centrifuged at 13,000 × g for 15 min at 4°C. The cell debris was again treated with the same buffer but with the addition of 0.1% sodium dodecyl sulfate, Triton X-100, or Tween 20. Another aliquot of the culture was lysed under denaturing conditions with 8 M urea-10 mM Tris-HCl-100 mM sodium phosphate at a pH close to the protein's isoelectric point (pH 12). The soluble recombinant protein was purified by Ni2+-nitrilotriacetic acid-agarose affinity chromatography (Qiagen). The resin was washed twice with the same solubilization buffer at pH 7.5 and 6.5, and finally, the attached recombinant protein was eluted in the same buffer at pH 4.5.
Synthesis of peptides.
A library of overlapping peptides was synthesized at the Instituto de Inmunología San Juan de Dios (Bogotá, Colombia) by the simultaneous multiple-solid-phase synthetic method with a polyamine resin and by use of 9-fluorenylmethoxy carbonyl chemistry (10). The peptides had a purity of 96%, as detected by mass spectroscopy, amino acid analysis, and high-performance liquid chromatography.
Sera.
Sixty-eight serum samples from individuals with different pathologies were tested, as follows: 24 serum samples from patients with CL diagnosed by culture and microscopic visualization of parasites (the samples were collected by the Laboratorio de Microbiología, Facultad de Biología, San Antonio Abad University of Cuzco, Cuzco, Peru); 8 serum samples from Peruvian individuals living in the same area as the previous group of patients but with no history of contact with Leishmania; 10 serum samples from patients from Brazil with chronic Chagas' disease (ChD) diagnosed by enzyme-linked immunosorbent assay (ELISA) and the complement fixation test; 4 serum samples from Spanish individuals who had no antecedents of contact with the L. braziliensis parasite and who had never traveled to areas where Leishmania is endemic; and 22 serum samples from Spanish patients with different autoimmune diseases. All 68 serum samples were assayed by an ELISA with a Leishmania total protein extract as the antigen. All serum samples from Peruvian (eight serum samples) and Spanish (four serum samples) individuals without a previous history of contact with Leishmania were negative, and consequently, for statistical purposes they were used as controls in the ELISAs.
ELISA measurements.
The recombinant L. braziliensis histone H1 protein was diluted in carbonate-bicarbonate buffer (pH 9.6) to a concentration of 5 μg/ml, and each of the different peptides was diluted to a concentration of 20 μg/ml. The ELISA plates (Immulon 4HBX; Dynex) were sensitized with 100 μl of antigen per well, incubated overnight at 4°C, and washed three times with 0.05% phosphate-buffered saline (PBS)-Tween 20 (PBS-T) buffer. All the sera were diluted 1:100 in PBS-T with 5% nonfat dried milk, 100 μl of the mixture was added to each well, and the plates were incubated at 37°C for 1 h, after which the plates were washed again. A mixture of anti-human immunoglobulin A (IgA), IgG, and IgM (heavy and light chains) conjugated with peroxidase (Jackson ImmunoResearch) was used as the second antibody; the mixture was diluted 1:2,000 in PBS-T, and 100 μl was added to each well. After incubation at 37°C for 1 h and the corresponding washings, the mixture was developed with ortho-phenylenediamine (Sigma) as the substrate. The absorbance was read at 450 nm. These ELISA trials were performed in triplicate, and the mean values for the three assays are presented. Only the results of the ELISAs for which there was a difference of less than 5% among the different assays were considered.
Detection of antinuclear antibodies in serum samples.
Antinuclear antibodies in the patients' sera were detected with an INNO-LIA ANA kit (Innogenetics). This system allows the detection of antibodies directed against different nuclear and cytoplasmic antigens like Sm (SmB and SmD), RNP (RNP-70k, RNP-A and RNP-C), P ribosomal proteins, and histones.
RESULTS
Expression and purification of recombinant L. braziliensis histone H1.
Overexpression of recombinant L. braziliensis histone H1 in the pQE31-E. coli Topp3 expression system was achieved after induction with 2 mM isopropyl-β-d-thiogalactopyranoside in Luria-Bertani medium at 37°C for 2 h. Solubilization of the recombinant protein was obtained only under highly denaturing conditions with 8 M urea, 10 mM Tris-HCl, and 100 mM sodium phosphate (pH 12). None of the nondenaturing conditions tested was able to bring recombinant L. braziliensis histone H1 into solution (data not shown).
Antigenicity of recombinant L. braziliensis histone H1.
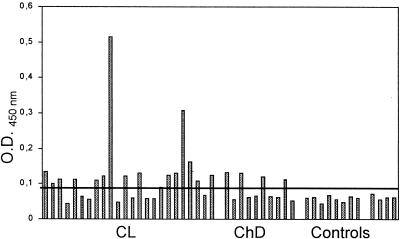
In order to analyze the immune recognition of recombinant L. braziliensis histone H1 by sera from patients with CL, it was used as an antigen in ELISAs. The cutoff value was established as the mean plus 3 standard deviations (SDs) for the control sera from Peruvian and Spanish individuals. Sixteen of 24 serum samples from patients with CL (66.6%) had reactivity values above the cutoff point (Table 1; Fig. 1), indicating that anti-H1 antibodies are present in Leishmania-infected people. Similarly, 4 of the 10 serum samples from patients with ChD (40%) reacted with the recombinant L. braziliensis histone H1. None of the control sera from either Peruvian or Spanish individuals showed any reactivity with the antigen.
TABLE 1.
Percentage of serum samples from patients with CL and ChD positive for reactivity to recombinant L. braziliensis histone H1 and the synthetic peptides, homologous to regions of L. braziliensis histone H1.
| Serum sample source | % Serum samples positive
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Recombinant L. braziliensis histone H1 | 23061 | 23063 | 23065 | 23067 | 23069 | 23071 | 23073 | |
| Patients with CL | 66.6 | 12.5 | 0 | 11.7 | 46.8 | 18.75 | 12.9 | 0 |
| Patients with ChD | 40 | 0 | 0 | 0 | 40 | 0 | 10 | 0 |
FIG. 1.
Antigenicity of recombinant L. braziliensis histone H1. The reactivities of 24 serum samples from patients with CL, 10 serum samples from patients with ChD, and 12 control serum samples (8 from Peruvian patients and 4 from Spanish patients) were assayed by ELISA. The cutoff line was established as the mean reactivity value plus 3 SDs for the control sera.
Mapping of the linear antigenic determinants of L. braziliensis histone H1.
In order to define the location of the main antigenic determinants recognized by sera from patients with leishmaniasis, overlapping synthetic peptides derived from the L. braziliensis histone H1 amino acid sequence were designed. The peptides were assayed individually by ELISA with sera from patients with leishmaniasis and ChD. The peptides (and their amino acid sequences) were as follows: 23061 (MFANSSAAAVTAASNSPQRS), 23063 (SNSPQRSPRPSPKKAAVKKA), 23065 (KKAAAKKAAAKKAAPKKAAP), 23067 (KAAPKRAAPKRAAPKKAAPK), 23069 (APKKAAAKRAAKKSAPKKAV), 23071 (APKKAVKKAVKAAKKAVKKA), and 23073 (AVKKAAKKATKRTAKKAAKK).
The reactivities of the sera from patients with CL to the L. braziliensis histone H1 peptides were highly variable. The recognition of peptides 23063 and 23073 was null, as none of the sera from patients with leishmaniasis gave optical density (OD) values above the cutoff point (Table 1). A second group of peptides (peptides 23061, 23065, 23069, and 23071) was recognized by less than 20% of the serum samples assayed, and finally, peptide 23067 was recognized by 46.8% of the serum samples from patients with leishmaniasis assayed (Table 1). Moreover, sera from patients with ChD reacted with L. braziliensis histone H1 peptides as well: peptide 23067 reacted with 40% of the serum specimens from patients with ChD, and peptide 23071 reacted with 10% of the serum specimens from patients with ChD assayed. None of the peptides showed any reactivity with the control sera.
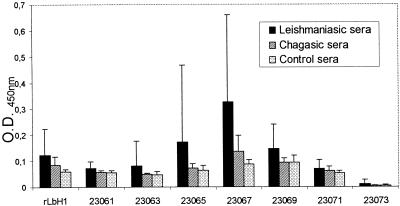
By use of a different analysis of all of these data, it was noted that the sera from patients with leishmaniasis had the highest OD values for a particular group of peptides among the peptides assayed: peptides 23065, 23067, and 23069 (Fig. 2). These results indicate that the region of L. braziliensis histone H1 covered by these peptides acts as a prominent antigenic determinant during CL. This response was not found for the sera from patients with ChD; sera from patients with ChD had slightly elevated OD values only for peptide 23067.
FIG. 2.
Reactivities of serum samples from patients with CL and ChD and control serum samples against recombinant L. braziliensis histone H1 (rLbH1) and synthetic peptides homologous to regions of L. braziliensis histone H1. Columns represent mean reactivities, and bars give the SDs for patient and control serum samples.
Specificities of anti-H1 antibodies.
All the serum specimens from patients with CL that gave a positive reaction for recombinant L. braziliensis histone H1 and two that gave a negative result were tested with the INNO-LIA ANA kit. None of the serum samples assayed was positive, so the presence of anticytoplasmic or antinuclear antibodies in those serum samples can be rejected. In particular, none of the serum samples reacted with the human histones tested with the INNO-LIA ANA kit, so we can be assured that the anti-histone H1 antibodies present in the sera from patients with CL were specifically elicited by L. braziliensis histone H1.
In order to determine the specificity of the anti-L. braziliensis histone H1 reaction, 22 serum samples from individuals with different autoimmune diseases were tested for their reactivities against recombinant L. braziliensis histone H1 by ELISA, as described above. The reactivities of these sera were null, so it can be stated that the reactivities of the sera from patients with leishmaniasis against recombinant L. braziliensis histone H1 are specific to these patients and are not related to any autoimmune disease.
DISCUSSION
Previous work has shown that all the core histones from L. infantum induce a significant humoral response during CVL (16, 17, 18). Among them, histones H3 and H2A are recognized the most (81 and 78%, respectively) by sera from dogs with visceral leishmaniasis. On the other hand, histone H2B and the amino-terminal fragment of histone H4 from L. infantum are recognized by a lower proportion of serum samples (63 and 47%, respectively). Nevertheless, to our knowledge no data are available regarding the humoral response of humans to Leishmania histones during infection with this parasite. Moreover, the antigenicity of the parasite histone H1 had not been studied in humans or other mammals.
This work demonstrated the presence of anti-histone H1 antibodies in sera from patients with CL as well as in sera from patients with chronic ChD. The fact that the titers of antibodies against Leishmania antigens are low during CL has impaired the development of kits for the immunological diagnosis of CL (2, 5). In this context, the high level of recognition (66.6%) of the L. braziliensis recombinant L. braziliensis histone H1 by sera from patients with CL must be noted.
The occurrence of cross-reactions between sera from patients with leishmaniasis and sera from patients with ChD to numerous Leishmania antigens is well established. This fact has invariably hampered the use of Leishmania antigens for the diagnosis of this parasitic disease. Due to the interesting recognition of recombinant L. braziliensis histone H1 shown by sera from patients with CL, we wanted to know if that antigen cross-reacted with sera from patients with ChD. In this way, we found that 40% of the serum samples from patients with ChD tested gave OD values above the cutoff value. In an attempt to avoid this lack of specificity, we designed a collection of synthetic peptides covering the L. braziliensis histone H1 sequence in order to localize its antigenic determinants. The reactivities of these synthetic peptides were very low or null (Table 1) with the exception of the ones whose sequences mapped along the central region of the protein (Fig. 2). Among these peptides, peptide 23067 was recognized by the largest number of serum samples from patients with CL (46.8%), but it was also detected by 40% of the serum samples from patients with ChD. On the other hand, none of the serum samples from patients with ChD reacted with peptide 23069, but its rate of recognition by sera from patients with CL was as low as 18.75%. From these results we can conclude that there is a prominent antigenic determinant in the central region of the protein whose sequence matches those of peptides 23065, 23067, and 23069, but unfortunately, this epitope has limited utility for the diagnosis of CL caused by L. braziliensis.
The existence of antibodies against the nucleosome in human sera acts as an indication of several autoimmune diseases, such as systemic lupus erythematosus (3, 14). The presence in human sera of antibodies against different host tissues during ChD is well established (11) and has been related to the similarities of some parasitic antigens and host molecules in terms of their protein conformations and their protein sequences (6). Due to the high percentage of serum samples from patients with CL that reacted to L. braziliensis histone H1, we decided to demonstrate whether the antibodies in those serum samples were specific for the parasite's histone H1 or reacted to human histones as well. For this purpose we used the INNO-LIA ANA kit (Innogenetics). None of the serum samples that reacted to recombinant L. braziliensis histone H1 recognized any of the nuclear or cytoplasmic antigens tested with the kit, so it can be concluded that the anti-histone H1 humoral response is specific to the parasite's histone H1 and is independent of host antigens. On the other hand, none of the 22 serum samples from patients with autoimmune diseases that reacted to nuclear proteins or human histones recognized recombinant L. braziliensis histone H1.
All these results point to the conclusion that the humoral immune response against L. braziliensis histone H1 is elicited specifically by the parasite protein and that L. braziliensis histone H1 is a prominent antigen during leishmaniasis caused by members of the L. braziliensis complex.
The search for antigens with high specificities and sensitivities is essential in order to develop tools for the serologic diagnosis of leishmaniasis. The cross-reaction shown with sera from patients with ChD hampers the use of recombinant L. braziliensis histone H1 for this purpose, but the possibility of using some of the peptides evaluated in the present study, in addition to other Leishmania-derived molecules, in a multicomponent antigen remains open.
Acknowledgments
We thank Rosa Luz Pacheco of the Departamento de Microbiología, Universidad San Antonio Abad, Cuzco, Peru, for kindly providing the sera from patients with leishmaniasis used in this work. In addition, the sera from patients with autoimmune diseases were provided by Aurora Jurado of the Laboratorio de Inmunología del Hospital Universitario de Canarias.
This work was supported by grants from the Spanish Fondo de Investigaciones Sanitarias (grant FIS 99/1038) and Comunidad Autónoma Canaria (grant COF 2000/12).
REFERENCES
- 1.Aslund, L., L. Carlsson, J. Henriksson, M. Rydoiker, G. C. Toro, N. Galanti, and U. Petterson. 1994. A gene family encoding heterogeneous histone H1 proteins in Trypanosoma cruzi. Mol. Biochem. Parasitol. 65:317-330. [DOI] [PubMed] [Google Scholar]
- 2.Brito, M. E., M. G. Mendonça, Y. M., Gomes, M. L. Jardim, and F. G. C. Abath. 2000. Identification of potentially diagnostic Leishmania braziliensis antigens in human cutaneous leishmaniasis by immunoblot analysis. Clin. Diagn. Lab. Immunol. 7:318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burlingame, R. W., M. L. Boey, G. Starkebaum, and R. L. Rubin. 1994. The central role of chromatin in autoimmune reponses to histones and DNA in systemic lupus erythematosus. J. Clin. Investig. 94:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burri, M., W. Schlimme, B. Betschart, U. Kampfer, J. Schaller, and H. Hecker. 1993. Biochemical and functional characterization of histone H1-like proteins in procyclic Trypanosoma brucei brucei. Parasitol. Res. 79:649-659. [DOI] [PubMed] [Google Scholar]
- 5.Cerdeñosa, N., C. Riera, P. Cortés, F. March, C. Muñoz, M. Portús, and G. Prats. 1995. Detection and characterization by immunoblot analysis of potentially diagnostic Leishmania infantum polypeptides in human visceral leishmaniasis. Parasite Immunol. 17:509-516. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Neto, E., V. Coelho, L. Guilherme, A. Fiorelli, L. Stolf, and J. Kalil. 1996. Autoimmunity in Chagas' disease—identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T-cell clones in heart lesions of a chronic Chagas' disease cardiomyopathy patient. J. Clin. Investig. 98:1709-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duschak, V. G., and J. J. Cazzulo. 1990. The histones of the insect trypanosomatid Chritidia fasciculata. Biochim. Biophys. Acta 1040:159-166. [DOI] [PubMed] [Google Scholar]
- 8.Espinoza, I., G. C. Toro, V. Hellman, and N. Galanti. 1996. Histone H1 and core histones in Leishmania and Crithidia: comparison with Trypanosoma. Exp. Cell Res. 224:1-7. [DOI] [PubMed] [Google Scholar]
- 9.Fasel, N. J., D. C. Robyr, J. Mauel, and T. A. Glaser. 1994. Identification of a histone H1-like gene expressed in Leishmania major. Mol. Biochem. Parasitol. 62:321-324. [DOI] [PubMed] [Google Scholar]
- 10.Houghten, R. A. 1985. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 82:5131-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kierszenbaum, F. 1999. Chagas' disease and the autoimmunity hypothesis. Clin. Microbiol. Rev. 12:210-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 27:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Martínez, E., M. C. Thomas, V. Alonso, E. Carmelo, A. C. González, A. Del Castillo, and B. Valladares. 2002. Cloning and molecular characterization of the DNA encoding histone H1 from Leishmania braziliensis. J. Parasitol. 88:199-203. [DOI] [PubMed] [Google Scholar]
- 14.Muller, S., D. Bonnier, M. Thiry, and M. H. V. Van Regenmortel. 1989. Reactivity to autoantibodies in systemic lupus erythematosus with synthetic core histone peptides. Int. Arch. Allergy Appl. Immunol. 89:288-296. [DOI] [PubMed] [Google Scholar]
- 15.Requena, J. M., C. Alonso, and M. Soto. 2000. Evolutionarily conserved proteins as prominent antigens during Leishmania infections. Parasitol. Today 16:246-249. [DOI] [PubMed] [Google Scholar]
- 16.Soto, M., J. M. Requena, L. Quijada, M. García, F. Guzmán, M. E. Patarroyo, and C. Alonso. 1995. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol. Lett. 48:209-214. [DOI] [PubMed] [Google Scholar]
- 17.Soto, M., J. M. Requena, L. Quijada, L. C. Gómez, F. Guzmán, M. E. Patarroyo, and C. Alonso. 1996. Characterization of the antigenic determinants of the Leishmania infantum histone H3 recognized by antibodies elicited during canine visceral leishmaniasis. Clin. Exp. Immunol. 106:454-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soto, M., J. M. Requena, L. Quijada, M. J. Perez, C. G. Nieto, F. Guzman, M. E. Patarroyo, and C. Alonso. 1999. Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin. Exp. Immunol. 115:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]