Abstract
Parelaphostrongylus tenuis is a neurotropic nematode common in white-tailed deer (Odocoileus virginianus) of eastern North America. This parasite is the causative agent of a debilitating neurologic disease in atypical hosts, including domestic livestock. In order to identify proteins of potential significance in the host-parasite relationship, a cDNA library was produced from adult P. tenuis mRNA. Screening the library with antisera from infected red deer (Cervus elaphus elaphus) and immunized AO strain rats, we identified clones with sequence similarities to aspartyl protease inhibitors from several parasitic nematodes. Antibody that was generated against this recombinant protein of P. tenuis (Pt-API-1) detected the native protein in E/S products, in muscle and gonad, and on the surface of the cuticle of adult male and female P. tenuis. The native protein was detected in internal structures of first-stage (L1) and third-stage (L3) larvae. Reverse transcription-PCR confirmed expression of Pt-api-1 in L1, L3, and adult male and female worms. Expression of Pt-API-1 throughout the life cycle of P. tenuis suggests an essential function. Antibodies specific for recombinant Pt-API-1 were detected by enzyme-linked immunosorbent assay in sera from 12 red deer experimentally infected with P. tenuis. Antibodies were detected within 28 to 56 days postinfection. Responses were sustained or biphasic in animals with patent infections, consistent with expression of Pt-API-1 by L1. Our results are compatible with findings in other parasitic nematodes showing that aspartyl protease inhibitors are highly immunogenic.
Parelaphostrongylus tenuis, commonly known as meningeal worm, is a parasitic nematode common in white-tailed deer (Odocoileus virginianus) in eastern North America (4). Adult nematodes reside in the subdural space of the central nervous system (CNS) and in associated blood vessels and sinuses. Female worms deposit eggs into venous blood vessels (3), and eggs hatch in capillaries of the lungs. First-stage larvae (L1) enter air spaces of the lungs, move up the mucociliary escalator, and leave the host in the mucous coat of voided fecal pellets. L1 actively penetrate the foot of gastropod intermediate hosts, where they develop and molt twice (3). The definitive host becomes infected through accidental ingestion of infective third-stage larvae (L3). L3 leave the alimentary canal and reach the CNS in approximately 10 days (2). Larvae develop within the dorsal horns of the gray matter of the spinal cord, enter the subdural space 40 days later, and migrate towards the brain (2). The prepatent period is 3 to 4 months (4). Adult P. tenuis organisms have been shown to survive for 3.7 years in white-tailed deer and likely survive much longer (15).
Although infections are asymptomatic in white-tailed deer, P. tenuis can cause a debilitating neurologic disease in all other cervids of North America and a variety of domestic ungulates, including llamas, sheep, and goats (3). These hosts become infected by ingestion of infected gastropods or L3 while grazing. The parasite persists in the parenchyma of the CNS, causing neurologic abnormalities including general incoordination or some form of paralysis. Disease is caused by physical trauma to the parenchyma of the CNS by developing and migrating worms. Anthelmintic drugs fail to eliminate infections (22), and infections are often fatal in animals showing signs of disease.
Little is known of the nature of parasitism by P. tenuis. We prepared a cDNA library from adult P. tenuis in order to identify excretory and secretory (E/S) products used by the nematode to parasitize its host. Such molecules may be applied in diagnosis, vaccination, or therapeutic intervention. We have identified a putative aspartyl protease inhibitor that is expressed by larval and adult stages and released in E/S products by adult worms. The protein induced an antibody response in red deer (Cervus elaphus elaphus) experimentally infected with P. tenuis, suggesting that it may be useful for diagnosis of infection in such atypical hosts.
MATERIALS AND METHODS
Parasites and E/S.
Live, adult P. tenuis organisms were dissected from the crania of white-tailed deer, and E/S products were collected from adult worms (14). L1 were extracted from feces of an experimentally infected white-tailed deer (16) by using a modification of the Baermann technique (31). L3 were cultured in lab-reared terrestrial gastropods (Deroceras sp.) as described by Anderson (1).
Sera.
Three groups of four white-tailed (O. virginianus) and red deer (C. elaphus elaphus) were infected experimentally with 10, 25, or 100 L3 of P. tenuis (13). Animals received an equivalent secondary inoculation of P. tenuis L3 at various intervals to assess the potential for establishment of L3 from the secondary inoculation (13). Sera from 11 infected red deer were collected 112 to 140 days postinfection and pooled for cDNA library screening. Serum from an infected white-tailed deer was used for affinity purification of antibody.
Three AO strain rats were immunized with 50 μg of E/S protein from adult P. tenuis mixed with Freund's complete adjuvant (Sigma, St. Louis, Mo.). After 40 days, animals were boosted with 50 μg of E/S protein mixed with Freund's incomplete adjuvant (Sigma). Blood was collected 41 days later and sera were stored at −20°C.
Three AO strain rats were immunized with 50 μg of the purified His-tagged recombinant Pt-API-1 (rPt-API-1) or with an irrelevant His-tagged recombinant, dihydrofolate reductase (DHFR), mixed with Freund's incomplete adjuvant (Sigma). Blood was collected after 1 month and sera were stored at −20°C.
All rats were housed in the James A. Baker Institute vivarium according to the guidelines of the American Association for Accreditation of Laboratory Animal Care.
Construction and screening of the expression library.
RNA was extracted (Trizol reagent; Gibco BRL, Grand Island, N.Y.) from 221 adult P. tenuis worms. Poly(A)+ RNA was purified (Poly AT Tract mRNA Isolation System IV; Promega, Madison, Wis.), precipitated, and converted into double-stranded cDNA (ZAP cDNA Synthesis kit; Stratagene, La Jolla, Calif.). The yield of mRNA from adult P. tenuis organisms was 11.7 μg, representing 0.7% of total RNA. The cDNA was size fractionated on a Sepharose CL-2B column (Amersham Pharmacia Biotech, Piscataway, N.J.). Aliquots of each fraction were electrophoresed on a 5% nondenaturing acrylamide gel (30). Fractions with cDNA of ≥500 bp were pooled. One hundred nanograms of cDNA was cloned into the bacteriophage Uni-ZAP XR vector (Stratagene), and an aliquot was packaged (Gigapack III Gold Packaging Extract; Stratagene). The primary library contained 1.5 × 106 PFU. Average insert size was 1,200 bp, and the percent nonrecombinants was 3%. The library was either amplified prior to screening or the primary library was screened. The amplified library contained 1.5 × 1010 PFU.
Approximately 120,000 plaques from the amplified library were screened with pooled sera collected from red deer 112 to 140 days following experimental infection with P. tenuis. Deer sera were diluted (1:100) and absorbed with Escherichia coli phage lysate (Stratagene) bound to nitrocellulose (Schleicher & Schuell, Keene, N.H.). In a separate experiment, 45,000 plaques from the primary library were screened with serum (1:1,000) from a rat immunized with E/S products from adult P. tenuis organisms. Plaque lifts were obtained following standard procedures (30) (Pico-Blue Immunoscreening Kit; Stratagene). Deer antibody was detected using alkaline phosphatase-conjugated affinity-purified rabbit anti-deer immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) at 0.2 μg/ml, followed by colorimetric development (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium; Bio-Rad Laboratories, Mississauga, Ontario, Canada). Rat antibody was detected using horseradish peroxidase (HRP)-conjugated goat anti-rat IgG (ICN Pharmaceuticals, Inc., Aurora, Ohio) at 1.6 μg/ml, followed by chemiluminescent development and autoradiography (ECL reagent; Amersham Pharmacia Biotech). Positive plaques were subjected to two or three additional rounds of plating until purified.
Sequencing and analysis.
Plasmid clones in the pBluescript SK vector were obtained by in vivo excision (Stratagene). Sequencing was performed with an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) on the coding strand using T3 universal primer (Gibco BRL) and a custom primer (5′-CTG CTC TCC CGA CGA TAC AAC-3′; Gibco BRL). The opposite strand was sequenced using T7 universal primer (Gibco BRL) and a custom primer (5′-TTG AGT TGT ATC GTC GGG AGA G-3′; Gibco BRL). The sequence was edited and the open reading frame (ORF) was deduced using ORF Finder at the National Center for Biotechnology Information (NCBI; Bethesda, Md). Sequences were compared with nucleotide and protein sequences deposited in nonredundant databases using the basic local alignment search tool (BLAST, version 2) (NCBI). Comparison to expressed sequence tag (EST) sequences was performed using tBLASTn (NCBI) and NemaBLAST (Washington University BLAST, version 2). Sequences with a minimum BLAST score of 77 and with a probability of ≤2 × e−13 at the protein level were compared to the P. tenuis sequence using MacVector (version 6.5.3; Accelrys, Princeton, N.J.). The presence of a signal peptide was determined using SignalP version 1.1 (Center for Biological Sequence Analysis).
Protein expression and purification.
The recombinant plasmid pBluescript SK/Pt-api-1 was digested with BamHI and KpnI (Gibco BRL) to release a 931-bp fragment. The insert was subcloned into a similarly digested expression vector (pQE31) which was used to transform chemically competent M15[pREP 4] cells (QIAexpress; Qiagen, Valencia, Calif.). Recombinant fusion proteins were recovered from bacterial cultures under nondenaturing conditions and affinity purified using nickel chelation chromatography (Ni-nitrilotriacetic acid agarose; Qiagen). Purified proteins were dialyzed against phosphate-buffered saline (PBS; pH 7.2) and concentrated (Centricon-10; Amicon, Beverly, Mass.). The protein concentration was estimated using a Bio-Rad (Hercules, Calif.) protein assay, and samples were stored at −20°C.
Affinity purification of antibodies.
Purified rPt-API-1 was resolved with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide gels) under reducing conditions (23), transferred to polyvinylidene difluoride (PVDF) membranes following the instructions of the manufacturer (Millipore Corporation, Bedford, Mass.), and detected with anti-His antibody (Qiagen). The remaining membranes bearing rPt-API-1 were used to affinity purify antibody (7) from the serum of a white-tailed deer infected with P. tenuis. In order to identify the native protein, the affinity-purified antibodies were tested with Western blotting of E/S products of adult P. tenuis.
PAGE and Western blotting.
E/S products (200 μg) from adult female P. tenuis organisms were resolved in Tris-Tricine by SDS-PAGE (34) under nonreducing conditions and transferred to PVDF membranes (14). Strips were cut from Western blots and tested with rat anti-rPt-API-1 or anti-DHFR (1:2,500) antibodies and developed using HRP-conjugated goat anti-rat IgG, as described for the library screening.
Immunohistochemistry.
Adult male and female P. tenuis worms were embedded in tissue freezing medium (TFM; Electron Microscopy Services, Ft. Washington, Pa.). A slug (Deroceras sp.), infected 2 months previously with L1 of P. tenuis, was similarly embedded in TFM. L1 extracted from deer feces were embedded in TFM within glycerin capsules (9). Sections (6 to 8 μm) were cut (Cryocut 1800 cryotome; Reichert-Jung, Cambridge Instruments, Heidelberg, West Germany), and slides were stored at −80°C until use. Immunohistochemistry was performed as described by Crump et al. (10), with the following modifications. A 5% nonfat dry milk solution was used as the diluent for primary and secondary antibodies. Sections were blocked with 10% normal goat serum in 5% nonfat dry milk. Endogenous peroxidase activity was blocked by treatment with 0.3% H2O2, 1% NaN3 in Tris-buffered saline, pH 7.6. Rat anti-rPt-API-1 and anti-DHFR antibodies were diluted 1:50 and detected with HRP-conjugated goat anti-rat IgG (16.2 μg/ml) using 3-amino-9-ethyl-carbazole (Sigma) as substrate. Sections were counterstained with Harris modified hematoxylin (Fisher Scientific, Pittsburgh, Pa.), and coverslips were mounted with Glycergel (Dako Corporation, Carpinteria, Calif.). Sections were examined with a Nikon Labophot microscope (Nippon Kogaku K.K., Tokyo, Japan), and digital images were captured (Nikon, Nippon Kogaku K.K.).
RT-PCR.
Adult worms were homogenized in 1 ml of RNA Bee (Tel-Test, Inc., Friendswood, Tex.), and RNA was isolated following instructions from the manufacturer. Larval stages (L1 and L3) were disrupted by incubation in an RNA extraction buffer (20) at 65°C for 15 min, followed by addition of RNA Bee (15:1). RNA samples were treated with amplification-grade DNase I (Gibco BRL) and further purified using the cleanup protocol of an RNeasy mini kit (Qiagen). First-strand cDNA synthesis from 200 ng of total RNA was primed using oligo(dT) primers for reverse transcription (RT) (Superscript Preamplification System; Gibco BRL). Negative control reactions for cDNA synthesis were performed on total RNA from all life stages in the absence of reverse transcriptase. The PCR mix was prepared following instructions from the manufacturer (Taq DNA polymerase; Gibco BRL). Transcripts were amplified from cDNA using primers for the gene of interest (400-bp product from Pt-api-1; forward, 5′-AAC GCC TTA CTG TTG GAA CG-3′, and reverse, 5′-ATT TCG CTT GGT GTC AGG TC-3′), and primers for a ubiquitous gene (200-bp product from heat shock protein-70 (HSP-70); forward, 5′-ACC GTC GCC TTC ACT AAA GA-3′, and reverse, 5′-TTC AAC CCA AGC ATC TCC AT-3′) (Integrated DNA Technologies, Coralville, Iowa). The negative controls for RT were included in PCRs to demonstrate the absence of cDNA and contaminating genomic DNA. Phage with inserts for Pt-api-1 or HSP-70 were included as positive controls for PCRs. Samples were denatured at 94°C for 5 min followed by 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min. Following a final extension of 10 min at 72°C, amplified DNA products were analyzed by electrophoresis in a 2% agarose gel (Seakem LE; FMC BioProducts, Rockland, Maine) with a 100-bp DNA ladder (Gibco BRL). DNA fragments were stained with ethidium bromide (0.5 μg/ml) and visualized using UV light (GDS 7500 Darkroom; UVP, Upland, Calif.). Images were captured using the public domain NIH Image program (written by Wayne Rasband at the National Institutes of Health; available at http://rsbweb.nih.gov/pub/nih-image/ or from the National Technical Information Service, 5285 Port Royal Rd., Springfield, VA 22161 [PB93-504868]).
Enzyme-linked immunosorbent assays (ELISAs).
All procedures were performed at room temperature with a well volume of 100 μl. Wells of polystyrene microtiter plates (Falcon; Fisher Scientific, Nepean, Ontario, Canada) were coated with 4 μg of rPt-API-1/ml in PBS (pH 7.2) for 3 h. Wells were washed three times with 0.05% Tween 20 in PBS and incubated for 3 h with 0.2% Aurora blocking reagent (ICN) in PBS. Red deer sera were diluted 1:100 in blocking solution and incubated in wells for 2 h. Wells were washed three times, and HRP-conjugated affinity-purified rabbit anti-deer IgG (Canadian Life Technologies, Burlington, Ontario, Canada) was added at 1 μg/ml. After 1 h, wells were washed three times and 2′,2-azinobis, 3-ethyl-benzthiazoline-6-sulfonic acid (Bio-Rad Laboratories, Canada) containing 0.3% H2O2 was added. After 30 min, optical densities were read at 405 nm using a microplate reader (Titertek Plus Model MS2; ICN).
Nucleotide sequence accession number.
The nucleotide sequence data reported for P. tenuis in this paper are available in the GenBank, EMBL, and DDBJ databases under the accession number AF277291.
RESULTS
Library screening and analysis of inserts.
Red deer sera identified 10 positive recombinant clones among the 120,000 tested from the amplified library. Of 45,000 plaques screened in the primary library using rat anti-E/S, 20 of 21 positives were identical to Pt-api-1 (0.04% of the primary cDNA library). Six clones were plaque purified, excised as recombinant pBluescript SK plasmids, and digested with XhoI and PstI to release and size the cDNA insert. The six clones were virtually identical in size (900 bp) and sequence. One clone (GenBank accession number AF277291) was sequenced on both DNA strands. Using the deduced amino acid sequence as a query, a BLASTp search revealed similarity with an aspartyl protease inhibitor from Onchocerca volvulus (GenBank P21250 [35]). Additional similarities included amino acid sequences from adults of Ostertagia ostertagi (GenBank CAD10783), Caenorhabditis elegans (GenBank AAC46663), Dirofilaria immitis (GenBank AAA70419 [18]), and Acanthocheilonema viteae (GenBank S23229 [37]). The deduced amino acid sequence of P. tenuis was used as a query in a tBLASTn search. This search revealed several orthologous ESTs each from Ascaris suum (two orthologues; n = 4 and 4), Brugia malayi (n = 10), and Parastrongyloides trichosuri (n = 16). Full-length ORFs were deduced from the consensus nucleotide sequences of the ESTs for each species. Sequence alignments of orthologous full-length ORFs are shown in Fig. 1. An additional orthologue from A. suum was identified by a BLASTp search. The amino acid sequence of this aspartyl protease inhibitor (GenBank P19400 [26]) showed 19% identity to P. tenuis. This ORF was different from both of the A. suum ORFs deduced from ESTs.
FIG. 1.
ClustalW alignment (MacVector 6.5.3) of the deduced amino acid sequence of rPt-API-1 with orthologous sequences from other nematodes identified by BLASTp and tBLASTn searches. Amino acid positions are numbered along the margins. Conserved residues are indicated by the shaded boxed areas. The percent identities of orthologues to Pt-API-1 are indicated in the box at the lower right. Asterisks indicate four invariant conserved cysteine residues. The overline at the N terminus indicates the predicted signal peptide of P. tenuis.
Due to its similarity to this family of nematode aspartyl protease inhibitors, the gene from P. tenuis was assigned the designation Pt-api-1. Pt-API-1 and all orthologous protein sequences have a predicted signal peptide and four conserved invariant cysteine residues. The predicted masses of the preprotein and the mature protein are 27.3 and 23.4 kDa, respectively.
Identification of the native protein in E/S products.
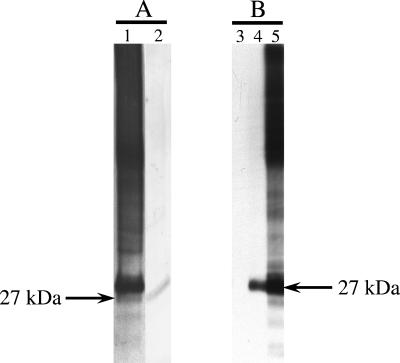
E/S products from adult P. tenuis worms were resolved under nonreducing conditions in Tris-Tricine by SDS-PAGE and blotted to PVDF. Immunoblotting was performed using affinity-purified deer antibodies to rPt-API-1 and rat antibodies to rPt-API-1. Affinity-purified deer antibodies to rPt-API-1 bound a protein of 27 kDa (Fig. 2A). Rat anti-rPt-API-1 antibodies bound a protein of 27 kDa, whereas rat anti-DHFR did not react with E/S proteins (Fig. 2B). Pooled sera from infected red deer (Fig. 2A) and rat anti-E/S (Fig. 2B) also bound a protein of 27 kDa. When resolved under reducing conditions in Tris-glycine by SDS-PAGE (data not shown), the mass of the protein was estimated to be 33 kDa.
FIG. 2.
Native Pt-API-1 is present in the E/S products of adult P. tenuis worms. Serum antibodies from deer infected with P. tenuis (A) or from rats immunized with rPt-API-1 (B) specifically recognize a 27-kDa molecule in the E/S products of adult P. tenuis. E/S products were blotted and incubated with pooled sera from 11 red deer at 112 to 140 days postexperimental infection (lane 1), antibody (from white-tailed deer) affinity-purified against rPt-API-1 (lane 2), pooled sera collected from rats immunized with rDHFR (lane 3), pooled sera collected from rats immunized with rPt-API-1 (lane 4), and pooled sera collected from rats immunized with E/S products from adult P. tenuis (lane 5).
Immunohistochemistry.
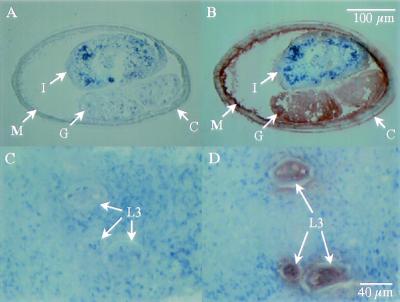
Rat anti-rPt-API-1 detected the native protein in gonad, muscle, and on the surface of the cuticle of adult female (Fig. 3B) and male (data not shown) P. tenuis organisms. The native protein was not detected in the intestine of adult worms. Native Pt-API-1 was detected in internal structures of L3 (Fig. 3D) and L1 (data not shown).
FIG. 3.
Immunohistochemical detection of Pt-API-1 in adult and larval stages of P. tenuis. (A and C) Sections were treated with pooled sera collected from rats immunized with rDHFR. (B and D) Sections were treated with pooled sera collected from rats immunized with rPt-API-1. (A and B) Adult female; (C and D) infective L3 in situ within foot of terrestrial gastropod. M, muscle; G, gonad; C, cuticle; I, intestine.
RT-PCR.
PCR was performed on first-strand cDNA from L1, L3, and adult male and female P. tenuis organisms. PCR using gene-specific primers for Pt-api-1 and HSP-70 confirmed transcription of both genes by L1, L3, and adult male and female P. tenuis worms (data not shown). Negative controls for RT of total RNA from all life stages failed to yield amplicons and demonstrated an absence of contaminating cDNA and genomic DNA (data not shown).
ELISA.
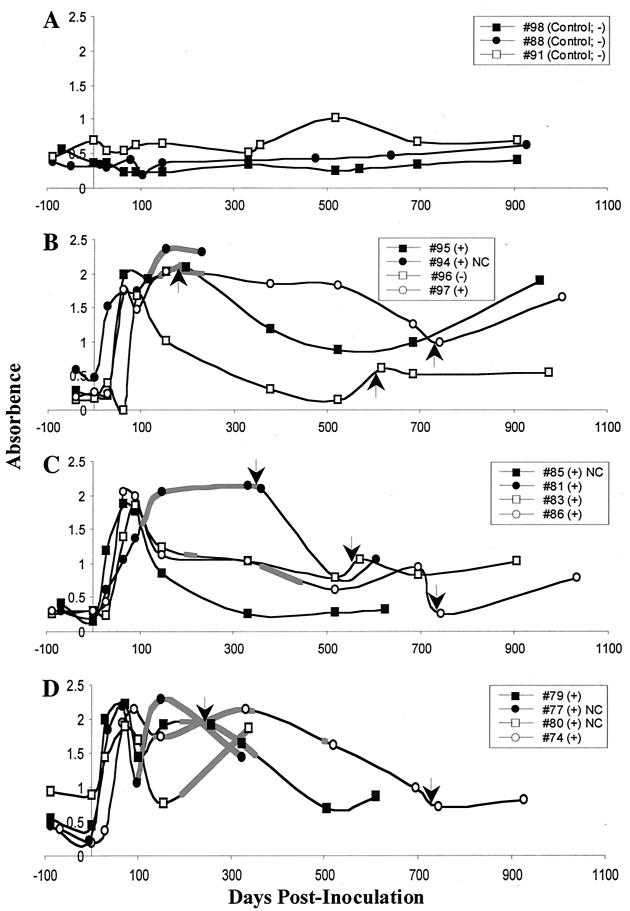
Serum antibodies specific for rPt-API-1 were detected in each of 12 red deer inoculated with P. tenuis. The three groups of inoculated red deer showed similar patterns of seroconversion (Fig. 4B to D). Sera from uninoculated animals (Fig. 4A) yielded absorbance readings ranging from 0.15 to 1.0 (average, 0.42). Antibodies were detected in all infected animals by 28 to 56 days postinfection (Fig. 4B to D). Of the 12 inoculated animals, 11 had adult P. tenuis organisms in the CNS at necropsy (13). rPt-API-1-specific antibodies were elevated for nearly 2 years in some animals (Fig. 4B and D). There was no correlation between level of nematode exposure and level of rPt-API-1-specific antibody. rPt-API-1-specific antibody did correlate with the patent periods of infections. Among the deer inoculated with 10 or 100 L3, all but one developed a patent infection and these deer produced biphasic antibody responses to rPt-API-1. Biphasic responses were not mounted in any of the animals inoculated with 25 L3, even those with patent infections. This result is unexplained. The decline in antibody was prolonged in these animals compared to deer inoculated but uninfected (Fig. 4B, no. 96) or to those with occult infection (Fig. 4C, no. 85). Two deer in the group exposed to 100 L3 were euthanized when they developed neurologic signs (13). Both animals had elevated serum antibodies to rPt-API-1 (Fig. 4D). Specific antibody responses to secondary inoculation of L3 were variable and weak. In addition, none of the animals developed patent infections following secondary inoculation (13). Taken together, the results support the conclusion that L3 in the secondary inoculation failed to establish.
FIG. 4.
rPt-API-1 is specifically recognized by serum antibody from red deer (C. elaphus elaphus) infected with P. tenuis in an ELISA. Deer were uninoculated controls (A) or were inoculated orally with 10 (B), 25 (C), or 100 (D) infective larvae (L3) of P. tenuis. Arrows indicate time of equivalent secondary inoculation of L3. Thicker lines indicate periods during which infections were patent by fecal examination. Legend lists individual animals and presence (+) or absence (−) of adult P. tenuis at necropsy. NC, animals did not receive a secondary inoculation of P. tenuis L3.
DISCUSSION
Among the nematode aspartyl protease inhibitor family, amino acid sequence identity with rPt-API-1 ranged from 74% for the orthologue from O. ostertagi to 19% for protease inhibitor-3 (PI-3) of A. suum. Conserved structural features were found in rPt-API-1: the presence of a signal peptide, conservation of four invariant cysteine residues, and the lack of N- or O-glycosylation motifs (the predicted protein sequences from C. elegans and A. suum 2 bear N-glycosylation sites). Enzyme inhibitory activities have been demonstrated for orthologues Ov33 of O. volvulus (35) and PI-3 of A. suum (26). Structural studies of the complex between PI-3 from A. suum and porcine pepsin revealed that the N-terminal β-strand of PI-3 pairs with a strand in pepsin to create a β-sheet that bridges the two proteins (27). The N-terminal residues in PI-3 block the first three substrate binding pockets in pepsin (27). This novel mode of inhibition demonstrates the value of identifying variant orthologues.
The 74% identity of Pt-API-1 to the orthologue from O. ostertagi is not surprising. O. ostertagi has the closest phylogenetic relationship to P. tenuis of all full-length orthologues identified. Both are bursate nematodes in the order Strongylida. Also in the order Strongylida are Haemonchus contortus and Ancylostoma caninum. Partial ORFs from these orthologues showed similar high identities (71%) to Pt-API-1.
Native Pt-API-1 was detected by immunohistochemistry in adult and larval stages of P. tenuis. Similar observations have been made in O. volvulus (24, 35) and D. immitis (18). Such unrestricted expression suggests a metabolic role for aspartyl protease inhibitors in these parasitic nematodes; however, secretion of Pt-API-1 in E/S products of adult P. tenuis suggests that this life stage deploys the enzyme for interaction with the host. The tissue source of the secreted inhibitor is not known, although the presence of large quantities of inhibitor in the gonads of females (Fig. 3) suggests release during egg laying. We detected the inhibitor on the surface of adult worms, suggesting that it also may be shed from the surface into E/S products. Ov33 is secreted by adult male O. volvulus (33), although the tissue source is not known. Secretion by blood- or tissue-dwelling adult stages is compatible with roles for Pt-API-1 and Ov33 in migration, niche establishment, or manipulation of the host immune system. An aspartyl protease inhibitor from Ascaris lumbricoides specifically interferes with antigen processing in B lymphocytes by blocking the activity of cathepsin E (8).
Bm33 and Ov33 were identified using infected patient sera in a manner similar to our identification of Pt-API-1. Ov33 is immunodominant and induces IgE and IgG4 in infected humans (19), suggesting that it influences lymphocyte function beyond conventional antigenic stimulation by promoting type 2 cytokine production. This observation holds considerable interest, as protective immunity against nematodes is facilitated by type 2 cytokines (reviewed in reference 17). Antibody to Pt-API-1 is produced by red deer infected with P. tenuis, indicating that Pt-API-1 is also highly immunogenic. The isotype and cytokine responses induced by Pt-API-1 have not yet been investigated in any species.
Diagnosis of P. tenuis infections in atypical hosts is challenging because most never develop patent infections (5, 6, 29). In such hosts, including llamas, sheep, and goats, definitive diagnosis of infection is restricted to postmortem recovery and identification of nematodes from the CNS. Red deer show mixed responses to P. tenuis infection. Some animals develop patent infections of short duration while others maintain occult infections (13). We found a potential application for rPt-API-1 in the diagnosis of P. tenuis infection in red deer. rPt-API-1-specific antibodies were detected in sera of 12 red deer inoculated with P. tenuis 28 to 56 days previously, well in advance of the minimum prepatent period. This response is likely induced by Pt-API-1 released from L3, L4, or subadult parasites. While Pt-API-1-specific antibodies declined to baseline levels by 280 days postinoculation in an infected deer with occult infection, elevated levels of Pt-API-1-specific antibodies were sustained for up to 2 years in animals that developed patent infections. This result is compatible with release of Pt-API-1 from the female uterus during egg laying or with the expression of Pt-API-1 by L1 that hatch in the lung. Sustained production of antibodies specific for Pt-API-1 may, thus, identify atypical cervid hosts with patent infections. This is important because these animals are capable of disseminating the parasite. Antibody to Pt-API-1 was not apparent following secondary inoculation with L3. In addition, none of the red deer developed patent infections following secondary inoculation (13). The absence of patent infections indicates that L3 from secondary inoculations did not establish, and this is compatible with the development of acquired immunity to P. tenuis (13).
Aspartyl protease inhibitors have been employed in the serologic diagnosis of filarial nematode infections. Serum antibody specific for Ov33-3 was detected in patients infected with O. volvulus (25, 35). Antibodies against Bm33 have been found in B. malayi-infected patients (12). Serum antibodies specific for DiT33 were found in D. immitis-infected dogs (21, 33) and cats (18, 33). In a manner analogous to our observation of elevated antibodies to Pt-API-1 in red deer with patent P. tenuis infections, serum antibody to Ov33 correlated with the presence of O. volvulus microfilariae in the skin of experimentally infected chimpanzees (24).
The strong, specific antibody response in red deer between 28 and 56 days postinoculation encourages interest in Pt-API-1 for diagnosis of P. tenuis infections in atypical hosts. Many cervid (28, 32, 36) and noncervid (5, 6, 11, 29) atypical hosts succumb to neurologic disease by 120 days postinfection with P. tenuis. Our results show that Pt-API-1-specific serum antibodies are present in red deer prior to this time and also during active neurologic disease. A reliable serologic test would aid the effective management of disease induced by P. tenuis. Future efforts will assess the diagnostic potential of Pt-API-1 in other atypical hosts.
Acknowledgments
M.S.D. and N.M. contributed equally to the work described in this report.
We thank Denise Clark, David Coombs, Dion Durnford, Margarida Krause, and Gary Saunders for advice and assistance with library production and screening, restriction mapping, sequence editing, and interpretation of results. We also thank Lucy Gagliardo for assistance with immunohistochemistry and Fernanda Romaris, Dan Zarlenga, and Helen Bird for advice on RT-PCR.
This work was supported by grants (to M.D.B.B.) from the Natural Sciences and Research Council (NSERC) of Canada; Elk Research Council; Alberta Elk Association; Ontario Ministry of Agriculture Food and Rural Affairs; Ontario Deer and Elk Farmers Association; and the Saskatchewan Elk Breeders, Game Farmers, Fallow Deer Producers, and White-tail and Mule Deer Producers Associations. In addition, the work was supported by grants (to J.A.A.) from the U.S. Department of Agriculture Animal Health and Disease Research Program (NYCV-473391) and through a postdoctoral fellowship (to M.S.D.) from NSERC of Canada.
REFERENCES
- 1.Anderson, R. C. 1963. The incidence, development, and experimental transmission of Pneumostrongylus tenuis Dougherty (Metastrongyloidea: Protostrongylidae) of the meninges of the white-tailed deer (Odocoileus virginianus borealis) in Ontario. Can. J. Zool. 41:775-792. [Google Scholar]
- 2.Anderson, R. C. 1965. The development of Pneumostrongylus tenuis in the central nervous system of white-tailed deer. Pathol. Vet. 2:360-379. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. C. 1992. Nematode parasites of vertebrates: their development and transmission, p. 151-208. CAB International, Oxon, United Kingdom.
- 4.Anderson, R. C., and A. K. Prestwood. 1981. Lungworms, p. 266-317. In W. R. Davidson, F. A. Hayes, V. F. Nettles, and F. E. Kellogg (ed.), Diseases and parasites of the white-tailed deer. Tall Timbers Research Station, Tallahassee, Fla.
- 5.Anderson, R. C., and U. R. Strelive. 1966. Experimental cerebrospinal nematodiasis (Pneumostrongylus tenuis) in sheep. Can. J. Zool. 44:889-894. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, R. C., and U. R. Strelive. 1969. The effect of Pneumostrongylus tenuis (Nematoda: Metastrongyloidea) on kids. Can. J. Comp. Med. 33:280-286. [PMC free article] [PubMed] [Google Scholar]
- 7.Beall, J. A., and G. F. Mitchell. 1986. Identification of a particular antigen from a parasite cDNA library using antibodies affinity purified from selected portions of Western blots. J. Immunol. Methods 86:217-223. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, K., T. Levine, J. S. Ellis, R. J. Peanasky, I. M. Samloff, J. Kay, and B. M. Chain. 1992. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur. J. Immunol. 22:1519-1524. [DOI] [PubMed] [Google Scholar]
- 9.Carlisle, M. S., D. D. McGregor, and J. A. Appleton. 1990. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology 70:126-132. [PMC free article] [PubMed] [Google Scholar]
- 10.Crump, A., W. L. Donaldson, J. Miller, J. H. Kydd, W. R. Allen, and D. F. Antczak. 1987. Expression of major histocompatibility complex (MHC) antigens on horse trophoblast. J. Reprod. Fertil. Suppl. 35:379-388. [PubMed] [Google Scholar]
- 11.Dew, T. L., D. D. Bowman, and R. B. Grieve. 1992. Parasite-specific immunoglobulin in the serum and cerebrospinal fluid of white-tailed deer (Odocoileus virginianus) and goats (Capra hircus) with experimentally induced parelaphostrongylosis. J. Zoo Wildl. Med. 23:281-287. [Google Scholar]
- 12.Dissanayake, S., M. Xu, C. Nkenfou, and W. F. Piessens. 1993. Molecular cloning and serological characterization of a Brugia malayi pepsin inhibitor homolog. Mol. Biochem. Parasitol. 62:143-146. [DOI] [PubMed] [Google Scholar]
- 13.Duffy, M. S. 2000. Life history studies of Parelaphostrongylus tenuis and Elaphostrongylus cervi with investigations into the potential for reliable antemortem immunodiagnosis. Ph.D. thesis. University of New Brunswick, Fredericton, New Brunswick, Canada.
- 14.Duffy, M. S., and M. D. B. Burt. 2002.. Identification of antigens with potential for immunodiagnosis of Parelaphostrongylus tenuis and Elaphostrongylus cervi infections in red deer (Cervus elaphus elaphus). J. Parasitol. 88:587-593. [DOI] [PubMed] [Google Scholar]
- 15.Duffy, M. S., T. A. Greaves, N. J. Keppie, and M. D. B. Burt. 2002. Meningeal worm is a long-lived parasitic nematode in white-tailed deer. J. Wildl. Dis. 38:448-452. [DOI] [PubMed] [Google Scholar]
- 16.Duffy, M. S., N. J. Keppie, and M. D. B. Burt. 1999. The potential for false-positive diagnosis of protostrongyliasis by extraction of larvae from feces. J. Wildl. Dis. 35:783-785. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 18.Frank, G. R., R. R. Mondesire, K. S. Brandt, and N. Wisnewski. 1998. Antibody to the Dirofilaria immitis aspartyl protease inhibitor homologue is a diagnostic marker for feline heartworm infections. J. Parasitol. 84:1231-1236. [PubMed] [Google Scholar]
- 19.Garraud, O., C. Nkenfou, J. E. Bradley, F. B. Perler, and T. B. Nutman. 1995. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J. Immunol. 155:1316-1325. [PubMed] [Google Scholar]
- 20.Gomez-Escobar, N., E. Lewis, and R. M. Maizels. 1998. A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp. Parasitol. 88:200-209. [DOI] [PubMed] [Google Scholar]
- 21.Hong, X. Q., J. Santiago Mejia, S. Kumar, F. B. Perler, and C. K. S. Carlow. 1996. Cloning and expression of DiT33 from Dirofilaria immitis: a specific and early marker of heartworm infection. Parasitology 112:331-338. [DOI] [PubMed] [Google Scholar]
- 22.Kocan, A. A. 1985. The use of ivermectin in the treatment and prevention of infection with Parelaphostrongylus tenuis (Dougherty) (Nematoda: Metastrongyloidea) in white-tailed deer (Odocoileus virginianus Zimmermann). J. Wildl. Dis. 21:454-455. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lucius, R., A. Kern, F. Seeber, T. Pogonka, J. Willenbucher, H. R. Taylor, M. Pinder, H. W. Ghalib, H. Schulz-Key, and P. Soboslay. 1992. Specific and sensitive IgG4 immunodiagnosis of onchocerciasis with a recombinant 33 kD Onchocerca volvulus protein (Ov33). Trop. Med. Parasitol. 43:139-145. [PubMed] [Google Scholar]
- 25.Lucius, R., H. Schulz-Key, D. W. Buttner, A. Kern, B. Kaltmann, J. Prod'hon, F. Seeber, R. D. Walter, K. C. Saxena, and H.-J. Diesfeld. 1988. Characterization of an immunodominant Onchocerca volvulus antigen with patient sera and a monoclonal antibody. J. Exp. Med. 167:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martzen, M. R., B. A. McMullen, N. E. Smith, K. Fujikawa, and R. J. Peanasky. 1990. Primary structure of the major pepsin inhibitor from the intestinal parasitic nematode Ascaris suum. Biochemistry 29:7366-7372. [DOI] [PubMed] [Google Scholar]
- 27.Ng, K. K. S., J. F. W. Petersen, M. M. Cherney, C. Garen, J. J. Zalatoris, C. Rao-Naik, B. M. Dunn, M. R. Martzen, R. J. Peanasky, and M. N. G. James. 2000. Structural basis for the inhibition of porcine pepsin by Ascaris pepsin inhibitor-3. Nat. Struct. Biol. 7:653-657. [DOI] [PubMed] [Google Scholar]
- 28.Pybus, M. J., W. M. Samuel, D. A. Welch, J. Smits, and J. C. Haigh. 1992. Mortality of fallow deer (Dama dama) experimentally infected with meningeal worm, Parelaphostrongylus tenuis. J. Wildl. Dis. 28:95-101. [DOI] [PubMed] [Google Scholar]
- 29.Rickard, L. G., B. B. Smith, E. J. Gentz, A. A. Frank, E. G. Pearson, L. L. Walker, and M. J. Pybus. 1994. Experimentally induced meningeal worm (Parelaphostrongylus tenuis) infection in the llama (Lama glama): clinical evaluation and implications for parasite translocation. J. Zoo Wildl. Med. 25:390-402. [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Samuel, W. M., and J. B. Gray. 1982. Evaluation of the Baermann technic for recovery of lungworm (Nematoda, Protostrongylidae) larvae from wild ruminants. Proc. Bienn. Symp. North. Wild Sheep Goat Counc. 3:232-243. [Google Scholar]
- 32.Samuel, W. M., M. J. Pybus, D. A. Welch, and C. J. Wilke. 1992. Elk as a potential host for meningeal worm: implications for translocation. J. Wildl. Manage. 56:629-639. [Google Scholar]
- 33.Santiago Mejia, J., C. Nkenfou, M. W. Southworth, F. B. Perler, and C. K. S. Carlow. 1994. Expression of an Onchocerca volvulus Ov33 homolog in Dirofilaria immitis: potential in immunodiagnosis of heartworm infection. Parasite Immunol. 16:297-303. [DOI] [PubMed] [Google Scholar]
- 34.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 35.Tume, C. B., J. L. Ngu, J. L. McKerrow, J. Seigel, E. Sun, P. J. Barr, I. Bathurst, G. Morgan, C. Nkenfou, T. Asonganyi, and G. Lando. 1997. Characterization of a recombinant Onchocerca volvulus antigen (Ov33) produced in yeast. Am. J. Trop. Med. Hyg. 57:626-633. [PubMed] [Google Scholar]
- 36.Tyler, G. V., C. P. Hibler, and A. K. Prestwood. 1980. Experimental infection of mule deer with Parelaphostrongylus tenuis. J. Wildl. Dis. 16:533-540. [DOI] [PubMed] [Google Scholar]
- 37.Willenbucher, J., W. Hofle, and R. Lucius. 1993. The filarial antigens Av33/Ov33-3 show striking similarities to the major pepsin inhibitor from Ascaris suum. Mol. Biochem. Parasitol. 57:349-351. [DOI] [PubMed] [Google Scholar]