Abstract
Background
Radiodermatitis (RD) is the primary acute adverse effect experienced by patients receiving radiotherapy (RT) for head and neck cancer (HNC). This study aimed to investigate the correlation between triglyceride (TG) levels and the severity of RD, as well as the underlying mechanisms involved.
Methods
Data were collected from 248 patients with locally advanced HNC treated with intensity-modulated radiation therapy (IMRT). Clinical characteristics and blood profiles prior to RT were collected. After RT, RD severity was assessed. A binary logistic regression analysis was used to determine risk factors. Mouse models of RD were established by administering radiating at a dose of 9 Gy over two consecutive days. TG levels in the mice and cells were quantified using an automatic biochemical analyzer and a TG assay kit, respectively. Cell viability was detected by the Cell Counting Kit-8 (CCK-8) assay, while apoptotic cell percentages were measured via flow cytometry. Western blotting assay was used to analyze the protein levels in the cells of interest.
Results
The TG level was the sole independent risk factor for grade 3 or higher (grade 3+) RD. Radiation was found to increase the TG content in both mouse blood and skin cells. Skin cells with high TG contents presented more severe radiation-induced damage when the radiation dose administered was 9 Gy over two consecutive days. The administration of 200 µmol/L palmitic acid (PA) or 2 Gy radiation independently did not affect HaCaT cell proliferation or apoptosis rates. Their combination was shown to induce skin cell injury. Mechanistically, autophagy was excessively activated. Furthermore, the protein concentrations of phospho-PI3K, phospho-Akt, and phospho-mTOR were notably decreased.
Conclusions
TGs are crucially involved in the development of RD. Increased TG levels after radiation treatment suppress the PI3K/Akt/mTOR pathway, induce autophagy, and exacerbate RD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02553-2.
Keywords: Head and neck cancer, Radiodermatitis, Triglyceride, Autophagy, PI3K/Akt/mTOR pathway
Background
Radiotherapy (RT) is the main approach for locally advanced head and neck cancer (HNC). Nevertheless, radiation unavoidably causes tissue damage within the targeted area [1, 2]. The skin is extremely sensitive to radiation-induced damage because of its role as the initial organ exposed during treatment and its rapid cell turnover and proliferation rates [3–5]. The neck skin is particularly sensitive to radiation because environmental stressors can compromise its barrier integrity [6]. Hence, the risk of radiodermatitis (RD) appears to be highest in patients with HNC, reaching as high as 100% [7]. RD can be categorized into two types: acute and chronic. Acute RD typically manifests during treatment, approximately 2–3 weeks after the start of radiotherapy, and can lead to temporary treatment interruptions, potentially reducing the effectiveness of RT. Chronic RD can manifest from three months to several years after RT, presenting symptoms such as hyperpigmentation, vascular alterations, alopecia, skin atrophy, and dermal fibrosis. This form of RD can significantly impair patients’ quality of life [8–10]. The prevention and treatment of RD, therefore, are particularly crucial for patients with HNC.
Triglycerides (TGs) are compounds composed of glycerol esterified with three fatty acids. They constitute the majority of natural fats and oils. According to Huang et al., blood triglyceride levels increase following radiotherapy in nasopharyngeal carcinoma patients [11]. High levels of triglycerides in the blood cause excessive accumulation of triglycerides in various parts of the body [12]. High TG levels have been linked to lipotoxicity in multiple organs, and the skin is not exempt [13, 14]. In addition, some studies have reported that fibrates and statins, which are commonly used to decrease TG levels, can mitigate RD in cells and patients [15, 16]. However, the mechanism by which high TG levels affect RD is not clear.
Autophagy is an adaptive cellular process that degrades and recycles abnormal cytoplasmic components and organelles to supply nutrients to cells. Excessive autophagy activation initiates a cell death program. On the one hand, radiation can induce cellular autophagy [17]. On the other hand, exposure to high TG levels can also induce autophagy [18–20]. Mammalian target of rapamycin (mTOR), a serine/threonine kinase, inhibits the process of autophagy [21]. Research indicates that autophagy initiation is facilitated by the inhibition of the phosphatidylinositol 3-kinase (PI3K)/ Protein Kinase B (Akt)/mTOR signaling pathway [22]. In addition, autophagy is also an important mediator of RD [23–25]. It can be speculated that a mechanism of RD involves radiation-induced increases in TG levels, which induce autophagy and inhibit the PI3K/Akt/mTOR signaling pathway. Therefore, we investigated the role and mechanism of TGs in RD, aiming to enhance the management, prevention and treatment of RD.
Patients and methods
Patients
From May 2020 to October 2023, 248 patients with locally advanced HNC undergoing intensity-modulated radiation therapy (IMRT) were enrolled at Shandong Cancer Hospital. Each participant provided their consent to join the research project. The eligibility requirements included: (1) individuals aged 18 or above; (2) newly diagnosed with locally advanced HNC in the nasopharynx, larynx, oropharynx, or hypopharynx according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system; and (3) daily IMRT treatment with 2.0 Gy fractions. The overall dosage varied between 60 and 70 Gy. The exclusion criteria encompassed: (1) the presence of multiple primary cancers; (2) a history of prior radiotherapy; and (3) the presence of skin or active knot hoof tissue diseases.
RD assessment and data collection
RD was evaluated using the late effects of normal tissue-subjective objective management analytical (LENT/SOMA) scale, which ranges from 1 to 4 [26]. The symptoms to RD were recorded by a physician and researchers. The maximum grade across the subjective measures and objective measures was used as a single value to represent the highest-grade late toxicity. The clinical characteristics and blood test results of patients prior to RT were gathered from institution’s electronic health record system. Measurements of hematological indices before RT required participants to fast for more than 8 h.
Mouse models
Female C57BL/6J mice aged 6–8 weeks from Beijing Sibeifu Experimental Animal Co., Ltd., were acclimated for one week in a controlled environment (22 ± 2 ℃, 12-hour light/dark cycle). A control group and a radiation group were formed by randomly dividing the animals. The RD mouse models were subsequently constructed. The radiation group of mice was exposed to 9 Gy of radiation for two consecutive days (9 Gy×2 F) using a Rad Source RS2000pro biological X-ray irradiator (Rad Source Technologies, GA, USA) at a dose rate of 1.63 Gy/min. Mouse body weight was recorded at three-day intervals. At the end of the experiments, all of the mice were sacrificed by cervical dislocation. Tissues were harvested at 1 month after radiation and bimonthly thereafter to determine the effect of injury.
Hematoxylin-eosin staining and Masson’s trichrome staining
Mouse skin tissues were kept in 4% paraformaldehyde at 4 ℃ for 48 h. The tissues were dried out with xylene and a series of ethanol solutions of varying concentrations, embedded in paraffin, sliced into 4 μm sections, and stained with hematoxylin-eosin and Masson’s trichrome solutions. Epidermal thickness was computationally defined as the distance between the distal border and the dermis-epidermis interface for the skin injury assessment. Collagen deposition was measured in Masson’s trichrome-stained sections by calculating the ratio of the blue-stained area to the total stained area. The sections were viewed under a light microscope at 400× magnification. Two investigators independently reviewed each section in a blinded manner. ImageJ software was used for the statistical analyses.
Immunohistochemical staining
The paraffin slices of skin tissues were dehydrated twice with xylene, hydrated using a gradient series of ethanol solutions (100%, 100%, 95%, 80%, 75%), and underwent high-pressure antigen retrieval in a citrate buffer with a concentration of 10 mM (pH 6.0). Following the cooling of the glass slides to room temperature, they were blocked with 10% goat serum for an hour. They were then incubated overnight at 4 °C using primary antibodies against vimentin (1:200; 5741T; Cell Signaling Technology, Boston, USA) and α-smooth muscle actin (α-SMA; 1:640; 19245 S; Cell Signaling Technology, Boston, USA). Next, the samples were incubated for two hours at room temperature with goat anti-rabbit IgG secondary antibodies bound to horseradish peroxidase (HRP; 1:1000; ab6721, Abcam, Cambridge, UK). After diaminobenzidine (DAB) staining, the slices were thoroughly rinsed for 2–5 min, dehydrated, and counterstained with hematoxylin for 30 s. Dehydration was performed using an ethanol gradient (75%, 80%, 95%, 100%, 100%). After dehydration, the tissue was cleared with xylene and sealed with neutral gum. Images were captured with a light microscope and quantitatively assessed using ImageJ software.
Cell culture and experimental treatment
HaCaT human keratinocytes were acquired from the National Biomedical Experimental Cell Resource Bank located in Beijing, China, and maintained in DMEM enriched with 10% fetal bovine serum and 1% antibiotics at 37 °C in a humidified atmosphere with 5% CO2. HSF (SV40) immortalization of human dermal fibroblasts was acquired from the Cellverse Co., Ltd located in Shanghai, China, and maintained in HSF cell basal medium enriched with 1% HSF cell culture additives, 10% fetal bovine serum and 1% antibiotics at 37 °C in a humidified atmosphere with 5% CO2. Cells were incubated with palmitic acid (PA; SYSJ-KJ001/KC001; Kunchuang Biotechnology, Xi’an, China) for 36 h and collected for detection. The autophagy inhibitor 3-methyladenine (3-MA, 5 mM; HY-19312, MCE, Dallas, TX, USA) was utilized to examine the role of autophagy in RD development.
Cell proliferation and apoptosis
For the evaluation of proliferation, each well of a 96-well plate was seeded with 6 × 103 HaCaT cells or 8 × 103 HSF cells. After 24 h, each well received 10 µL of Cell Counting Kit-8 (CCK-8, K1018, Apexbio, Houston, USA) solution and incubated for 2 h at 37 °C with 5% CO2. The absorbance at 450 nm was then measured using a SpectraMax i3x microplate reader (San Jose, CA, USA). For the apoptosis analysis, each well in a 6-well plate was seeded with 2 × 105 HaCaT cells or 2 × 105 HSF cells. The cells were collected, rinsed with cold PBS, and stained using the Annexin V-APC and 7-AAD Apoptosis Kit (E-CK-A218, Elabscience, Wuhan, China) according to the manufacturer’s instructions. Apoptotic cell percentages were measured using a BD Accuri C6 Plus flow cytometer. FlowJo software (v10.8.1) was used to analyze apoptotic cells.
TG levels assay and lipid droplet fluorescence assay
The levels of TG in human serum and mouse serum were measured with an automatic biochemical analyzer. HaCaT cells were kept at 37 °C in an environment with 5% CO2 for 24 h. If the aim was to detect TG levels, 1 × 107 cells were inoculated into 10 cm dishes; if the aim was to detect lipid droplet fluorescence, 1 × 104 cells were inoculated on a mu-slide 6-well plate. The supernatant was taken off, followed by two washes of the cells using a serum-free medium. DMEM containing 100, 200, 300, or 400 µmol/L PA or its solvent control was added to each well and incubated overnight at 37 °C in a 5% CO2 atmosphere. After 36 h, following the removal of the supernatant, the cells underwent two washes with a medium lacking serum. The TG levels in HaCaT cells were measured using a TG assay kit (BC0625, Solarbio, Beijing, China). A Lipid Droplet Fluorescence Assay Kit (LD02, Dojindo, Kyushu Island, Japan) was used to stain the intracellular lipid droplets. After a 30-minute incubation with the working liquid prepared with serum-free medium, the fluorescent lipid droplets were immediately viewed using a confocal microscope (Leica TCS SP8, Germany).
Western blot analysis
For the Western blot analysis, HaCaT cells (6 × 105 per well) were incubated in 6-well plates for 24 h and subjected to the following treatments: untreated, 2 Gy of radiation, 200 µmol/L PA, and 2 Gy of radiation with 200 µmol/L PA. The assay was carried out as previously detailed [27] using antibodies against PI3K (1:1000; 4249T, Cell Signaling Technology, Boston, USA), p-PI3K (1:1000; 17366T, Cell Signaling Technology, Boston, USA), Akt (1:1000; AF0016, Affinity Biosciences, Jiangsu, China), p-Akt (1:1000; AP0140, ABclonal, Wuhan, China), mTOR (1:1000; ab32028, Abcam, Cambridge, UK), p-mTOR (1:1000; ab137133, Abcam, Cambridge, UK), LC3 (1:1000; 3868 S, Cell Signaling Technology, Boston, USA), p62 (1:1000; A11250, ABclonal, Wuhan, China), p16 (1:1000; ab51243, Abcam, Cambridge, UK), p21 (1:1000; A19094, ABclonal, Wuhan, China), γH2AX (1:1000; 7631T, Cell Signaling Technology, Boston, USA), and horseradish peroxidase (HRP)-labeled secondary antibodies (1:1000; AS014, ABclonal, Wuhan, China). A ChemiDoc™ Touch imaging system (Bio-Rad, CA, USA) facilitated hypersensitivity-enhanced chemiluminescence. Western blot bands were quantified with ImageJ software.
Statistical analysis
Each experiment was repeated in triplicate at least three times. Chi-square or Fisher’s exact tests were applied to evaluate categorical variables in the clinical data, characterizing the research group. Quantitative data are shown as medians (Q1, Q3) and were evaluated using the Mann-Whitney U test. A binary logistic regression analysis was conducted to assess the relationships between the variables and the grade 3 + RD predictors. For the multivariate analyses, factors with a P value lower than 0.2 from the univariate analysis were included. The strength of these relationships was represented using odds ratios (ORs) and 95% confidence intervals (CIs). All the statistical analyses were performed with SPSS (version 26) software. Charts were created with GraphPad Prism (v10.1.2) and the R programming language (v3.5.3). The Mann-Whitney U test was applied in every experiment to determine significant differences, with significance defined as a P value under 0.05 divided by the number of relevant comparisons. For all figures, the following symbols denote significance levels: * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Results
TG levels are an independent predictor of RD in HNC patients
The study included 248 patients with locally advanced HNC, comprising 87.90% males and 12.10% females. The median age was 56.00 (49.00, 63.00) years. Eighteen (7.26%) participants were diagnosed with grade 3 RD, and no patients were diagnosed with grade 4 RD. Interestingly, all patients whose RD grade was 3 were male. Patients were divided into a grade 1–2 RD group and a grade 3 + RD group, which is due to the severe impact of an RD of grade three or higher on patients’ quality of life in clinical settings. The baseline characteristics are displayed in Table 1. A logistic regression analysis was conducted to determine risk factors for a grade 3 + RD. The univariate analysis indicated that higher TG and TC contents in the blood were significantly linked to an increased likelihood of having grade 3 + RD (Fig. 1A). The multivariate analysis revealed that TG contents were the sole independent risk factor for grade 3 + RD (OR = 1.010, 95% CI: 1.004–1.016, P = 0.002). TG levels showed a skewed distribution, ranging from 33.67 to 392.50 mg/dL. The median level, along with the first and third quartiles, was 105.88 (80.85, 142.53), as depicted in Fig. 1B. The violin plot (Fig. 1C) shows that TG contents were elevated in the grade 3 + group relative to the grade 1–2 group. Receiver operating characteristic (ROC) curves indicated that TG levels effectively predicted grade 3 + RD (Fig. 1D). Restricted cubic splines (RCSs) analyses were conducted to identify the linear relationship with the RD grade (Fig. 1E).
Table 1.
Patients characteristics
| Characteristics | Total (N = 248) |
|---|---|
| Age | |
| ≥ 60 | 88 (35.48%) |
| < 60 | 160 (64.52%) |
| KPS | |
| ≥ 90 | 180 (72.58%) |
| < 90 | 68 (27.42%) |
| Smoking | |
| No | 126 (50.81%) |
| Yes | 122 (49.19%) |
| Alcohol | |
| No | 138 (55.65%) |
| Yes | 110 (44.35%) |
| Hypertension | |
| No | 198 (79.84%) |
| Yes | 50 (20.16%) |
| Diabetes | |
| No | 236 (95.16%) |
| Yes | 12 (4.84%) |
| T stage | |
| 1–2 | 97 (39.11%) |
| 3–4 | 151 (60.89%) |
| N stage | |
| 0–1 | 57 (22.98%) |
| 2–3 | 191 (77.02%) |
| TNM stage | |
| III | 95 (38.31%) |
| IV | 153 (61.69%) |
Fig. 1.
TG levels are closely associated with RD grades in patients with locally advanced HNC. (A) Forest plot of grade 3 + RD. (B) TG levels exhibit a skewed distribution. (C) The TG contents in the grade 3 + RD group were higher than those in the grade 1–2 RD group. (D) The ROC curve showed that TG levels could predict grade 3 + RD events. (E) RCS curve indicating that TG levels were linearly associated with the RD grade
TG levels increase in mouse blood and HaCaT cells after radiation treatment
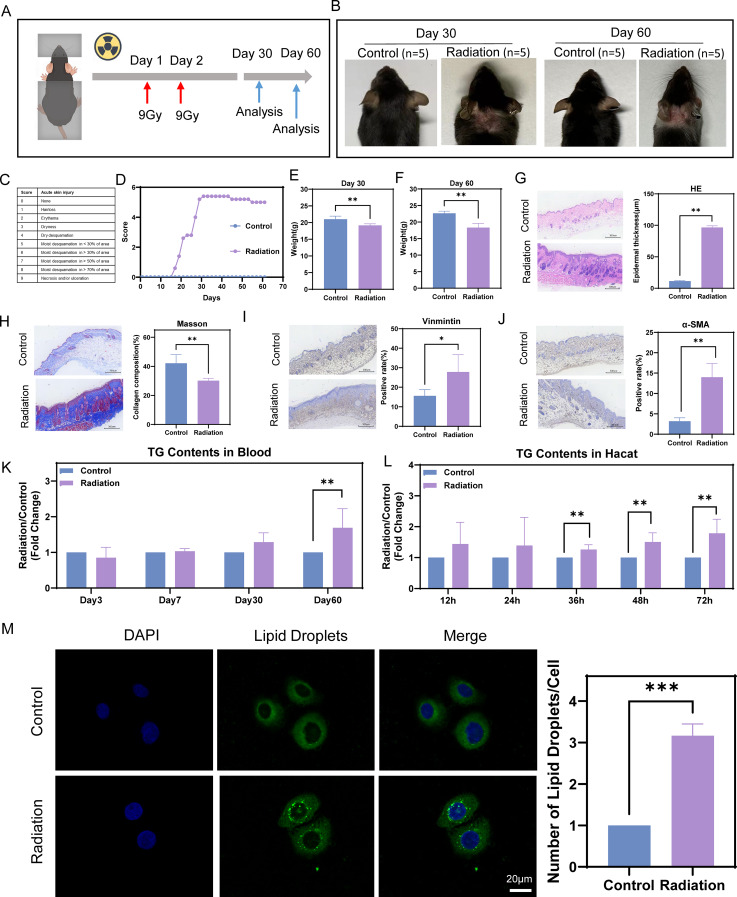
RD model mice were established according to the methods shown in Fig. 2A. The mice started to experience hair loss beginning on Day 17 after exposure to radiation. By Day 31, the skin damage in the radiated mice was the worst, and the damage did not subside after that point (Fig. 2B-D). Additionally, the irradiated group presented a decrease in weight compared with the control group by Days 30 and 60 (Fig. 2E-F). These findings indicate that the model effectively simulates the reduction in weight experienced by patients with HNC following radiotherapy. The skin tissue of the mice in the radiation group exhibited significant histopathological alterations at both Day 30 and Day 60. HE staining revealed that epidermal thickness was markedly increased in the radiation group (Supplementary Fig. 1A, Fig. 2G). Masson’s trichrome staining showed a substantial loss of collagen from the skin of irradiated mice (Supplementary Fig. 1B, Fig. 2H). Immunohistochemical staining revealed that the percentages of vimentin- and α-SMA- positive cells were obviously increased, which indicated that the extent of skin fibrosis was significantly more severe (Supplementary Fig. 1C-D; Fig. 2I-J). Mouse serum TG levels were measured to prove that elevated TG levels were induced by radiation. At 60 days, a significant statistical difference in TG levels was found between the two groups (Fig. 2K). TG levels in HaCaT keratinocytes were measured to explore the underlying mechanisms more conveniently, and the same trend was detected (Fig. 2L). Lipid droplets were visualized using confocal microscopy to further confirm the results (Fig. 2M).
Fig. 2.
TG levels increase in mouse blood and HaCaT cells after radiation treatment. (A) Scheme of the radiation treatment. (B) Images of the control group (n = 5) and radiation group (n = 5) at Days 30 and 60. (C-D) Scoring of skin lesion severity. (E-F) Differences of mouse weights between the control group (n = 5) and radiation group (n = 5) at Days 30 and 60. (G) HE staining results revealed that epidermal thickness was markedly increased in the radiation group (n = 5) on Day 60. (H) Masson’s trichrome staining revealed that a large amount of collagen was lost from the irradiated mouse skin on Day 60. (I-J) Immunohistochemical staining revealed that the percentage of vimentin- and α-SMA-positive cells was obviously increased on Day 60. (K) Mouse serum TG levels were increased by radiation on Day 60. (L) TG levels in skin cells were increased by radiation at 36 h, 48 h and 72 h. (M) The number of lipid droplets in skin cells was increased by radiation at 36 h. Mann-Whitney U tests were used for intergroup comparisons
High TG contents can aggravate radiation-induced skin damage
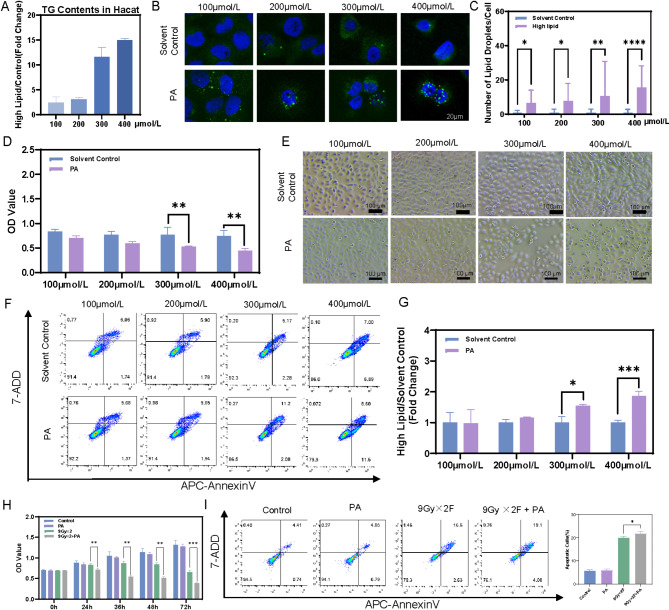
HaCaT cells were incubated with PA at concentrations of 100, 200, 300, or 400 µmol/L for 36 h to establish the high-TG cell model. The results revealed that both TG levels and lipid droplet contents increased in cells treated with the four varying concentrations of PA (Fig. 3A-C). The effects of TG levels on skin cell viability were investigated at 36 h via CCK-8 assays. Figure 3D shows that cell viability remained largely unchanged in the low-concentration groups (100 µmol/L and 200 µmol/L) after 36 h of incubation. This finding aligns with the results from optical microscopy and CCK-8 assays, as depicted in Fig. 3E. The flow cytometry analysis also confirmed the lack of toxic effects of low concentrations of PA on skin cells (Fig. 3F-G). Compared with those in the high-concentration groups (300 µmol/L and 400 µmol/L), apoptotic cells in the low-concentration groups did not increase significantly. Thus, a concentration of 200 µmol/L was used and an incubation time of 36 h were used in the following experiments. The amplified damage caused by increasing TG levels in irradiated skin cells was next identified. The radiation regimen of 9 Gy×2 F, known to induce RD, was selected. CCK-8 assays revealed that, compared with radiation alone, high TG concentrations (200 µmol/L PA) potently decreased the viability of irradiated skin cells (Fig. 3H). The flow cytometry analysis of cell apoptosis showed that 9 Gy×2 F of radiation plus 200 µmol/L PA markedly increased cell apoptosis (Fig. 3I).
Fig. 3.
High TG contents can aggravate are more susceptible to radiation-induced skin damage. (A-C) TG contents and numbers of lipid droplets in skin cells incubated with different concentrations of PA for 36 h. (D-G) CCK-8 assay, microscopy and flow cytometry images show the effects of different concentrations of PA on the viability, morphology and apoptotic rate of skin cells. (H-I) CCK-8 and flow cytometry assays revealed that high-TG-content skin cells subjected to 9 Gy×2 F of radiation presented a significant decrease in cell viability and an increased apoptosis rate
High TG contents can induce radiation-induced skin damage
In order to further clarify the important role of high TG levels on RD, we explored the radiotherapy dose that did not affect HaCaT cell proliferation or apoptosis rates. The effects of various single radiation doses (1, 2, 4, 6, and 9 Gy) on cell viability were assessed. Then, 2 Gy was chosen as a suitable dose that did not impact cell viability (Fig. 4A-B). CCK-8 assays and flow cytometry analyses of cell apoptosis revealed no significant difference in cell viability or the apoptosis rate between the 200 µmol/L PA alone group and the 2 Gy radiation alone group compared with the control group. Interestingly, 200 µmol/L PA plus 2 Gy radiation significantly suppressed cell proliferation and increased the cell apoptosis rate (Fig. 4C-D). To validate these findings, HSF cell line was used and consistent conclusion was obtained (Fig. 4E-F). These results indicate that high TG levels play a significant role in RD.
Fig. 4.
High TG contents can induce radiation-induced skin damage. A single dose of 2 Gy of radiation, which did not significantly affect cell viability (A) or the apoptosis rate (B), was chosen. CCK-8 and flow cytometry assays indicated that a high TG content in HaCaT cells (C-D) and HSF cells (E-F) subjected to 2 Gy of radiation significantly decreased cell viability and increased the percentage of apoptotic cells
High TG levels in skin cells can cause RD by inducing excessive autophagy
Autophagy and senescence are common causes of injury. Detection of the autophagy-related proteins LC3 and p62 was performed through Western blotting to confirm whether autophagy was related to RD. These findings indicated a significant decrease in p62 levels and a significant increase in LC3 II levels, suggesting that 2 Gy of radiation combined with 200 µmol/L PA induced excessive autophagy (Fig. 5A-C). The contents of senescence-associated proteins such as p16, p21, and γH2AX showed no significant differences across the four groups (Fig. 5D-G). The inhibitor 3-MA was employed to elucidate the function of autophagy in RD. These findings indicated that 3-MA decreased LC3 II expression while increasing p62 protein levels. These findings suggested that 3-MA successfully suppressed autophagy (Fig. 5H). 3-MA reversed the inhibitory effect of 2 Gy of radiation plus 200 µmol/L PA on cell proliferation (Fig. 5I). The flow cytometry analysis showed a notable reduction in the apoptosis rate following the introduction of the autophagy inhibitor (Fig. 5J-K). These findings revealed that high TG contents in skin cells can trigger RD by inducing excessive autophagy.
Fig. 5.
High TG levels in skin cells can cause RD by inducing excessive autophagy. (A-C) Significant changes in the protein expression of LC3 and p62 in HaCaT cells were measured by Western blotting and quantitatively analyzed. (D-G) Western blot analysis revealed that the protein expression levels of p21, p16, and γH2AX did not differ among the four groups. (H) The autophagy inhibitor 3-methyladenine (3-MA) inhibited autophagy. (I-K) CCK-8 and flow cytometry assays revealed that inhibiting autophagy led to a reduction in RD
Elevated TG levels in skin cells can trigger autophagy by suppressing the PI3K/Akt/mTOR signaling pathway
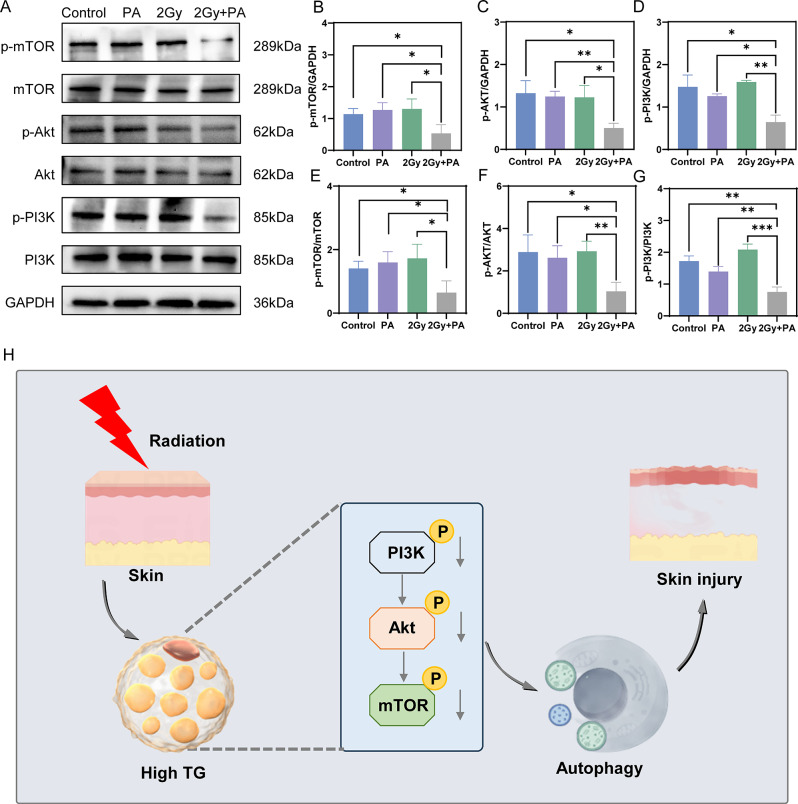
Given that the PI3K/Akt/mTOR pathway negatively regulates autophagy, Western blot analyses were conducted to assess alterations in protein levels in this pathway across the four groups. Compared with those in the other three groups, the concentrations of PI3K, Akt, and mTOR were stable, while the levels of their phosphorylated forms, p-PI3K, p-Akt, and p-mTOR, were lower in the group treated with 2 Gy of radiation and 200 µmol/L PA (Fig. 6A-G). High TG levels in skin cells may trigger autophagy by suppressing the PI3K/Akt/mTOR pathway, leading to RD (Fig. 6H).
Fig. 6.
High TG levels in skin cells can inhibit the PI3K/Akt/mTOR signaling pathway. (A-G) The levels of the PI3K, Akt, and mTOR proteins and their phosphorylation in HaCaT cells were measured by Western blotting and quantitatively analyzed. (H) Schematic illustration
Discussion
RD is a significant issue for HNC patients after RT [28]. Research indicates that lipid metabolism is crucial in RD development, and the underlying mechanisms need to be further explored [29]. While previous research has indicated that reducing TG levels can alleviate RD in both cellular models and patients [15, 16], to our knowledge, no studies have elucidated the underlying mechanisms by which TG levels influence RD. In the present study, the TG contents were found as an independent predictor of RD in HNC patients. Given that HNC has a higher incidence rate in male than in female [30], the study population included a greater proportion of male participants. This is further supported by the fact that male skin is characterized by more vigorous sebaceous gland secretion compared to female skin [31]. The observations indicate that radiation induces RD by increasing TG levels, which triggers autophagy and suppresses the PI3K/Akt/mTOR pathway.
Many studies have identified the strong associations between TG levels and injury, including kidney injury, osseous or articular injury, and spinal cord injury [32–34]. The research revealed a link between TG levels and the severity of RD in HNC patients, indicating a notable correlation where a higher TG level corresponds to more severe RD. TG levels, as a risk factor for RD, are advantageous to measure because of their convenience, speed, practicality, and ease of implementation in clinical settings.
At 60 days, mice exposed to radiation presented significant histopathological alterations in their skin tissue and elevated TG levels. The damage caused by high TG contents in HaCaT cells was confirmed via a 9 Gy×2 F radiation scheme. A single dose of 2 Gy of radiation, which had no effect on the proliferation or apoptosis of HaCaT cells, was subsequently used to elucidate the role of elevated TG levels in skin cells in relation to the occurrence of RD. These results suggest that the skin cells with high TG contents are more susceptible to radiation-induced injury. Similarly, Cui et al. found that elevated levels of PA can trigger an inflammatory response in epidermal keratinocytes [35]. Researchers have shown that activated autophagy is crucial for increasing the survival of keratinocytes under various stresses. Autophagy can modulate inflammatory pathways by clearing potential proinflammatory agents within the cell and directly degrading inflammasome components [36]. In addition, some studies have demonstrated that high fat stress or PA can induce autophagy [37–39]. Hence, it could be inferred that autophagy may be activated to limit excessive PA-induced inflammatory responses, which is similar to radiation induced injury. These findings indicate that 2 Gy of radiation combined with PA triggers autophagy, a process that can be inhibited by 3-MA. The inhibition of autophagy rescued apoptosis and promoted cell proliferation. Cellular senescence is a key indicator of aging [40] and a significant contributor to RD [41]. Senescent cells negatively affect nearby healthy cells by releasing cytokines, chemokines, growth factors, and signaling molecules [40, 42]. Previous studies have indicated that radiation and high-fat conditions can induce senescence in a variety of cells [43–45]. Regrettably, the study revealed no substantial difference in the level of the senescence-related proteins p16, p21, and γH2AX between the 2 Gy plus PA group and the other groups. This discrepancy may be due to the radiation doses and PA induction concentrations that previous studies reported inherently can cause damage, whereas the study chose radiation doses and PA concentrations that do not induce damage. Previous studies have shown that both radiation and PA can induce autophagy and downregulate the PI3K/Akt/mTOR pathway [46–48]. In addition, mTOR inhibitors can also induce autophagy [49, 50]. The PI3K/Akt/mTOR pathway is crucial for autophagy in epidermal cells. Inhibiting the PI3K/Akt/mTOR pathway can enhance autophagy by modulating autophagy-related gene expression or activating associated signaling pathways [51–53]. The results indicate that radiation combined with PA triggers autophagy by suppressing the PI3K/Akt/mTOR pathway, resulting in RD.
Strengths and limitations
The study not only verified the association between TG levels in blood before RT and the RD grade in HNC patients but also revealed one of the TG-related mechanisms underlying the occurrence of RD. This research provides fresh perspectives on innovative approaches for preventing and treating RD.
However, it is essential to acknowledge the limitations of this study. First, PA was used to induce an increase in TG levels. However, the process inevitably induced changes of other lipids. Additionally, the analysis in this study was limited to cell samples; however, analyzing skin samples in future research would provide a more detailed and accurate assessment of the effects of TG on RD.
Conclusions
In conclusion, the TGs are crucial in the process of RD. Elevated TG levels after radiation suppress the PI3K/Akt/mTOR pathway, promote autophagy, and initiate RD (Fig. 5H). This research offers a fresh perspective on preventing and managing RD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary Fig. 1 (A) HE staining results showing that the epidermal thickness was markedly increased in the radiation group on Day 30. (B) Masson’s trichrome staining results revealed that a large amount of collagen was lost from the irradiated mouse skin on Day 30. (C-D) Immunohistochemical staining revealed that the percentages of vimentin- and α-SMA-positive cells were obviously increased on Day 30.
Acknowledgements
The authors express their gratitude to Dr. Xu Dong Zhuang from the Medical Research Center, Fujian Maternity and Child Health Hospital, for the assistance with confocal imaging.
Author contributions
Y. H., H. G., and Y. C. performed the study. Y. H., H. G., Y. C., and Y. W. wrote manuscript. R. Z., X. D., Z. Z., and B. X. revised the manuscript. J. L., Y. W., and Y. Z. curated data. Y. H., H. G., Y. C., and Y. W. formed analysis. X. D., Z. Z., and B. X. administrated project. X. D. and B. X. provided funding. Z. Z. designed study. The final manuscript version has been approved by all the authors.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82202958), Fujian Provincial Natural Science Foundation of China (No. 2022J02037), Science Technology Program of Jinan (No. 202225013), and China Postdoctoral Science Foundation (No. 2023M734299).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent for participation
The Institutional Review Board of Shandong Cancer Hospital and Institute approved the study protocol. All participants provided informed consent. The local animal care committee approved the animal experiments (approval number SDTHEC2024004013). Animal testing meets the requirements of animal ethics and animal welfare.
Consent for publication
All the authors read the final version and agreed to publish it.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yafang Hong, Hongdan Guan and Yunhao Chen contributed equally to this work.
Contributor Information
Rong Zheng, Email: zhengrrong@outlook.com.
Xingchen Ding, Email: SDdingxingchen@126.com.
Zihan Zhou, Email: zihanzhou123@163.com.
Benhua Xu, Email: benhuaxu123@126.com.
References
- 1.Schoenfeld GO, Amdur RJ, Morris CG, Li JG, Hinerman RW, Mendenhall WM. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):377–85. [DOI] [PubMed] [Google Scholar]
- 2.Grégoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2015;33(29):3277–84. [DOI] [PubMed] [Google Scholar]
- 3.Hwa C, Bauer EA, Cohen DE. Skin biology. Dermatol Ther. 2011;24(5):464–70. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(2 Suppl Understanding):S41–62. [DOI] [PubMed] [Google Scholar]
- 5.Boothe PF, Kumar VP, Kong Y, Wang K, Levinson H, Mu D et al. Radiation induced skin fibrosis (RISF): opportunity for angiotensin II-Dependent intervention. Int J Mol Sci. 2024;25(15). [DOI] [PMC free article] [PubMed]
- 6.Haftek M, Roy DC, Liao IC. ARTICLE: evolution of skin barrier science for healthy and compromised skin. J Drugs Dermatology: JDD. 2021;20(4):s3–9. [DOI] [PubMed] [Google Scholar]
- 7.Bontempo PSM, Ciol MA, Menêses AG, Simino GPR, Ferreira EB, Reis P. Acute radiodermatitis in cancer patients: incidence and severity estimates. Volume 55. Revista da Escola de Enfermagem da U S P; 2021. p. e03676. [DOI] [PubMed]
- 8.Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC skin toxicity study group. Supportive Care Cancer: Official J Multinational Association Supportive Care Cancer. 2013;21(10):2933–48. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal J, Young MR, Radiation Dermatitis. Recognition, Prevention, and Management. Oncology (Williston Park, NY). 2017;31(12):885-7, 94– 9. [PubMed]
- 10.Seité S, Bensadoun RJ, Mazer JM. Prevention and treatment of acute and chronic radiodermatitis. Breast cancer (Dove Med Press). 2017;9:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang TL, Hsu HC, Chen HC, Lin HC, Chien CY, Fang FM, et al. Long-term effects on carotid intima-media thickness after radiotherapy in patients with nasopharyngeal carcinoma. Radiation Oncol (London England). 2013;8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H, Zhou Q, Zheng F, Wu T, Tang YD, Jiang J. The causal effects of lipid profiles on sleep apnea. Front Nutr. 2022;9:910690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson MD, Ballinger KR, Khetani SR. Long-term exposure to abnormal glucose levels alters drug metabolism pathways and insulin sensitivity in primary human hepatocytes. Sci Rep. 2016;6:28178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Lin J, Wang Y, Chen Y, Zhang Y, Ding X, et al. Acute radiation skin injury in stage III-IV head and neck cancer: scale correlates and predictive model. World J Surg Oncol. 2024;22(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgier C, Auperin A, Rivera S, Boisselier P, Petit B, Lang P, et al. Pravastatin reverses established Radiation-Induced cutaneous and subcutaneous fibrosis in patients with head and neck cancer: results of the Biology-Driven phase 2 clinical trial Pravacur. Int J Radiat Oncol Biol Phys. 2019;104(2):365–73. [DOI] [PubMed] [Google Scholar]
- 16.Sun C, Song B, Sheng W, Yu D, Yang T, Geng F, et al. Fenofibrate attenuates Radiation-Induced oxidative damage to the skin through fatty acid binding protein 4 (FABP4). Front Bioscience (Landmark edition). 2022;27(7):214. [DOI] [PubMed] [Google Scholar]
- 17.Ye H, Chen M, Cao F, Huang H, Zhan R, Zheng X. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol. 2016;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KH, Lee MS. Autophagy–a key player in cellular and body metabolism. Nat Reviews Endocrinol. 2014;10(6):322–37. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Zhang M, Wu Y, Jiang H, Fu H, Cai Y, et al. Aberrant activation of Notch-1 signaling inhibits podocyte restoration after islet transplantation in a rat model of diabetic nephropathy. Cell Death Dis. 2018;9(10):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones SA, Mills KH, Harris J. Autophagy and inflammatory diseases. Immunol Cell Biol. 2013;91(3):250–8. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Lei X, Zhao G, Guo R, Cui N. mTOR in programmed cell death and its therapeutic implications. Cytokine Growth Factor Rev. 2023;71–72:66–81. [DOI] [PubMed] [Google Scholar]
- 22.Yin Y, Qu H, Yang Q, Fang Z, Gao R. Astragaloside IV alleviates Schwann cell injury in diabetic peripheral neuropathy by regulating microRNA-155-mediated autophagy. Phytomedicine: Int J Phytotherapy Phytopharmacology. 2021;92:153749. [DOI] [PubMed] [Google Scholar]
- 23.Xie H, Zhou L, Liu F, Long J, Yan S, Xie Y, et al. Autophagy induction regulates Aquaporin 3-mediated skin fibroblast ageing. Br J Dermatol. 2022;186(2):318–33. [DOI] [PubMed] [Google Scholar]
- 24.Mostafa DK, Omar SI, Abdellatif AA, Sorour OA, Nayel OA, Abod Al Obaidi MR. Differential modulation of autophagy contributes to the protective effects of Resveratrol and Co-Enzyme Q10 in photoaged mice. Curr Mol Pharmacol. 2021;14(3):458–68. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Qin W, Wang L, Jin Y, Tu J, Yuan X. Autophagy gene Atg7 regulates the development of radiation-induced skin injury and fibrosis of skin. Skin Res Technology: Official J Int Soc Bioeng Skin (ISBS) [and] Int Soc Digit Imaging Skin (ISDIS) [and] Int Soc Skin Imaging (ISSI). 2023;29(6):e13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao MH, Zhang J, Zhang JG. Comparing the RTOG/EORTC and LENT-SOMA scoring systems for the evaluation of late skin toxicity after (125)I seed brachytherapy for Parotid gland cancer. Brachytherapy. 2017;16(4):877–83. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Liu P, Shang Y, Kerndl H, Kumstel S, Gong P, et al. Metformin and LW6 impairs pancreatic cancer cells and reduces nuclear localization of YAP1. J Cancer. 2020;11(2):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda H, Ueda Y, Ikawa T, Ohira S, Miyazaki M, Enomoto K, et al. Effect of topical agents on skin surface dose in volumetric modulated Arc therapy for head and neck cancer. J Radiat Res. 2023;64(4):644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Yan T, Mo W, Song B, Zhang Y, Geng F, et al. Altered bile acid metabolism in skin tissues in response to ionizing radiation: deoxycholic acid (DCA) as a novel treatment for radiogenic skin injury. Int J Radiat Biol. 2024;100(1):87–98. [DOI] [PubMed] [Google Scholar]
- 30.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 31.Rahrovan S, Fanian F, Mehryan P, Humbert P, Firooz A. Male versus female skin: what dermatologists and cosmeticians should know. Int J Women’s Dermatology. 2018;4(3):122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si M, Li D, Liu T, Cai Y, Yang J, Jiang L et al. Triglycerides as biomarker for predicting systemic lupus erythematosus related kidney injury of negative proteinuria. Biomolecules. 2022;12(7). [DOI] [PMC free article] [PubMed]
- 33.Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, Herzog W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XH, Graham ZA, Harlow L, Pan J, Azulai D, Bauman WA, et al. Spinal cord injury reduces serum levels of fibroblast growth Factor-21 and impairs its signaling pathways in liver and adipose tissue in mice. Front Endocrinol. 2021;12:668984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui L, Wu Y, Chen Z, Li B, Cai J, Chang Z, et al. N(6)-methyladenosine modification-tuned lipid metabolism controls skin immune homeostasis via regulating neutrophil chemotaxis. Sci Adv. 2024;10(40):eadp5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi A, Shabani M, Alizadeh S, Meshkani R. Interplay between oxidative stress and autophagy function and its role in inflammatory cytokine expression induced by palmitate in skeletal muscle cells. Cytokine. 2020;125:154835. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Peng S, Xiong S, Niu A, Xia M, Xiong X, et al. Naringin inhibits autophagy mediated by PI3K-Akt-mTOR pathway to ameliorate endothelial cell dysfunction induced by high glucose/high fat stress. Eur J Pharmacol. 2020;874:173003. [DOI] [PubMed] [Google Scholar]
- 38.Li T, Wei Y, Jiao B, Hao R, Zhou B, Bian X, et al. Bushen Huoxue formula attenuates lipid accumulation evoking excessive autophagy in premature ovarian insufficiency rats and palmitic acid-challenged KGN cells by modulating lipid metabolism. Front Pharmacol. 2024;15:1425844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shu Z, Li X, Zhang W, Huyan Z, Cheng D, Xie S, et al. MG-132 activates sodium palmitate-induced autophagy in human vascular smooth muscle cells and inhibits senescence via the PI3K/AKT/mTOR axis. Lipids Health Dis. 2024;23(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78. [DOI] [PubMed] [Google Scholar]
- 41.Paldor M, Levkovitch-Siany O, Eidelshtein D, Adar R, Enk CD, Marmary Y, et al. Single-cell transcriptomics reveals a senescence-associated IL-6/CCR6 axis driving radiodermatitis. EMBO Mol Med. 2022;14(8):e15653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanton C, Bernard E, Abbosh C, André F, Auwerx J, Balmain A, et al. Embracing cancer complexity: hallmarks of systemic disease. Cell. 2024;187(7):1589–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JH, Brown SL, Gordon MN. Radiation-induced senescence: therapeutic opportunities. Radiation Oncol (London England). 2023;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balasubramanian P, Kiss T, Gulej R, Nyul Toth A, Tarantini S, Yabluchanskiy A et al. Accelerated Aging Induced by an Unhealthy High-Fat Diet: Initial Evidence for the Role of Nrf2 Deficiency and Impaired Stress Resilience in Cellular Senescence. Nutrients. 2024;16(7). [DOI] [PMC free article] [PubMed]
- 45.Zhang CY, Tan XH, Yang HH, Jin L, Hong JR, Zhou Y et al. COX-2/sEH dual inhibitor alleviates hepatocyte senescence in NAFLD mice by restoring autophagy through Sirt1/PI3K/AKT/mTOR. Int J Mol Sci. 2022;23(15). [DOI] [PMC free article] [PubMed]
- 46.Xu Q, Zhang H, Liu H, Han Y, Qiu W, Li Z. Inhibiting autophagy flux and DNA repair of tumor cells to boost radiotherapy of orthotopic glioblastoma. Biomaterials. 2022;280:121287. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Liu B, Tong R, Ding S, Wu J, Lei Q, et al. Improved stability and targeted cytotoxicity of Epigallocatechin-3-Gallate palmitate for anticancer therapy. Langmuir. 2021;37(2):969–77. [DOI] [PubMed] [Google Scholar]
- 48.Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D et al. Metformin attenuates UVA-Induced skin Photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int J Mol Sci. 2022;23(13). [DOI] [PMC free article] [PubMed]
- 49.Xia Y, Chen J, Yu Y, Wu F, Shen X, Qiu C, et al. Compensatory combination of mTOR and TrxR inhibitors to cause oxidative stress and regression of tumors. Theranostics. 2021;11(9):4335–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed pharmacotherapy = Biomedecine Pharmacotherapie. 2018;103:699–707. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y, Zhang Y, Fan Y. Eupafolin ameliorates lipopolysaccharide-induced cardiomyocyte autophagy via PI3K/AKT/mTOR signaling pathway. Iran J Basic Med Sci. 2019;22(11):1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, et al. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Q, Xu M, Zhang P, Liu L, Meng X, Dong L. Therapeutic potential of autophagy in glioblastoma treatment with phosphoinositide 3-Kinase/Protein kinase B/Mammalian target of Rapamycin signaling pathway inhibitors. Front Oncol. 2020;10:572904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Fig. 1 (A) HE staining results showing that the epidermal thickness was markedly increased in the radiation group on Day 30. (B) Masson’s trichrome staining results revealed that a large amount of collagen was lost from the irradiated mouse skin on Day 30. (C-D) Immunohistochemical staining revealed that the percentages of vimentin- and α-SMA-positive cells were obviously increased on Day 30.
Data Availability Statement
No datasets were generated or analysed during the current study.