Abstract
Background:
Physical activity is an important outcome in oncology trials. Physical activity is commonly assessed using self-reported questionnaires, which are limited by recall and response biases. Recent advancements in wearable technology have provided oncologists with new opportunities to obtain real-time, objective physical activity data. The purpose of this review was to describe current uses of wearable activity monitors in oncology trials.
Methods:
We searched Pubmed, Embase, and the Cochrane Central Register of Controlled Trials for oncology trials involving wearable activity monitors published between 2005 and 2016. We extracted details on study design, types of activity monitors used, and purpose for their use. We summarized activity monitor metrics including step counts, sleep and sedentary time, and time spent in moderate-to-vigorous activity.
Results:
We identified 41 trials of which 26 (63%) involved cancer survivors (post-treatment) and 15 trials (37%) involved patients with active cancer. Most trials (65%) involved breast cancer patients. Wearable activity monitors were commonly used in exercise (54%) or behavioral (29%) trials. Cancer survivors take between 4660 and 11,000 steps/day and those undergoing treatment take 2885 to 8300 steps/day.
Conclusion:
Wearable activity monitors are increasingly being used to obtain objective measures of physical activity in oncology trials. There is potential for their use to expand to evaluate and predict clinical outcomes such as survival, quality of life, and treatment tolerance in future studies. Currently, there remains a lack of standardization in the types of monitors being used and how their data are being collected, analyzed, and interpreted.
Precis:
Recent advancements in wearable activity monitor technology have provided oncologists with new opportunities to monitor their patients’ daily activity in real-world settings. The integration of wearable activity monitors into cancer care will help increase our understanding of the associations between physical activity and the prevention and management of the disease, in addition to other important cancer outcomes.
Keywords: Cancer, Oncology trials, Physical activity, Wearable activity monitors, Wearable technology, Outcome assessment, Cancer survivorship
1. Introduction
Physical activity is associated with improved outcomes and quality of life in cancer survivors [1,2]. Given the importance of physical activity to health and recovery, the majority of oncology trials involve tools to capture activity-related measures including exercise, sleep, energy expenditure, and functional performance. While a number of validated questionnaires have been used extensively to estimate physical activity and sleep, they are based on self-report and limited by recall and response biases [2,3]. Thus, physical activity tends to be overestimated with regards to activity frequency, duration, and intensity [4].
Recent technological advances in wearable activity monitors, have created new opportunities to collect continuous, objective patient data in a non-obtrusive manner. Wearable activity monitors measure movement to estimate the number of steps taken each day, distance travelled, energy expenditure, sleep parameters, and heart rate, among other activity metrics. There are different types of wearable devices that can be used to monitor components of daily activity. This review focuses on wearable activity monitors used for monitoring and tracking fitness-related metrics. Devices commonly used are: (1) Pedometers: “estimate the number of steps taken through mechanical or digital measurements in only the vertical plane”; (2) Accelerometers: “Detect acceleration in one, two or three directions to determine the frequency, quantity and intensity of movements”; (3) Integrated multisensor systems: “Combine accelerometry with other sensors that capture body responses to exercise (e.g. heart rate) in an attempt to optimize physical activity assessments.” [5–7].
Although pedometers have been used to record step counts for decades, the emergence of contemporary activity monitors into oncology trials is relatively new. With the rapid technological advancements of wearable activity monitors and complex systems in place to measure different components of activity, the use and applications of activity monitors in healthcare has broadened. Currently, there is a lack of knowledge on how wearable activity monitors are being integrated into the design and conduct of clinical trials. Furthermore, there is a need to better understand physical activity levels as measured using wearable activity monitors in cancer survivors or patients undergoing cancer treatment, as most studies have focused on summarizing physical activity levels in healthier populations. Thus, we conducted a review of the literature: (1) Describe current uses of wearable activity monitors in oncology trials; (2) Summarize physical activity patterns in cancer patients; (3) Identify opportunities for future applications. Findings from this review will provide information on the use of this emerging technology and on current physical activity levels of cancer survivors.
2. Methods
2.1. Eligibility criteria
Eligible studies were limited to randomized controlled cancer trials that used activity monitors to gain understanding of their specific use in controlled settings. Study participants could be either newly diagnosed, undergoing active treatment, or survivors of any cancer type. All activity monitors were considered eligible if they could be worn (e.g., on the wrist, arm, waist, hip, or ankle) and were used to track any form of physical activity. Activity metrics of interest included step count, activity count, energy expenditure, sleep, heart rate, duration of activity or any other form of physical activity that can be tracked and monitored by a wearable device. Published clinical trial protocols were reviewed and summarized for trials that are ongoing or not yet complete. Validation studies and other non-randomized or quasi-randomized studies were not included in the review. The search was limited to RCTs published between 2005 and 2016, to capture the more contemporary applications of devices. No language restrictions were applied.
2.2. Search strategy
While this was not intended to be a formal systematic review of the literature, we followed similar methods when screening and reviewing articles. Search terms for the population and devices were combined to identify studies for inclusion in Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and conference abstracts. Study screening, selection and data extraction were completed in Covidence- a Cochrane tool specifically used for systematic reviews. Two independent reviewers (GG & LG) screened titles and abstracts. Discrepancies were discussed and a third reviewer (AS) was consulted if needed. Full-text review was conducted in a similar manner.
2.3. Data extraction
Details on study design, interventions and outcomes of interest were extracted. Study details including study title, authors, year, country and registration number; study design (e.g., parallel, crossover, adaptive…); number of arms; number of participants; study duration and follow-up time were recorded. Primary and secondary outcomes were recorded and further categorized as “Physical activity”; “Behavioral/Cognitive”; “Quality of Life/Functional status”; and “Treatment/Survival” outcomes. Information on the study population including the cancer type (s), age groups, gender, and other baseline characteristics were extracted. Details of the devices used were recorded including the name and manufacturer of device, type of device (pedometer/accelerometer/multi-sensor system), placement, total wear-time, definition of valid wear-time and device output were recorded. If available, adherence rates and information on valid wear-days were extracted. Study results were extracted if they provided a step count or total time spent active and sedentary/asleep. Data from the baseline visit of the study population or control group (if overall data not reported) were used for the quantitative analysis.
2.4. Statistical analysis
Summary statistics were generated for trial characteristics. Where applicable, the average daily step count, sleep duration, sedentary time, and active minutes were summarized and plotted. Studies that did not report these activity data were excluded from the quantitative analysis. STATA version 13.0 was used for all analyses.
3. Results
The initial search identified 508 individual studies. Further screening of titles and abstracts yielded 71 trials to be included for full-text review. Excluded were multiple reports of the same trial, non-cancer trials, and trials that were not fully randomized. After full-text review, a total of 41 randomized clinical trials involving wearable activity monitors in cancer populations were included (Fig. 1). Trials were published between January 1st, 2005 and December 31st, 2016. Most trials were conducted in North America (USA/Canada) (68%). Additional study locations included Europe (15%), Asia (7%) and other countries (10%). Characteristics of the included trials are displayed in Supplementary Table 1.
Fig. 1.
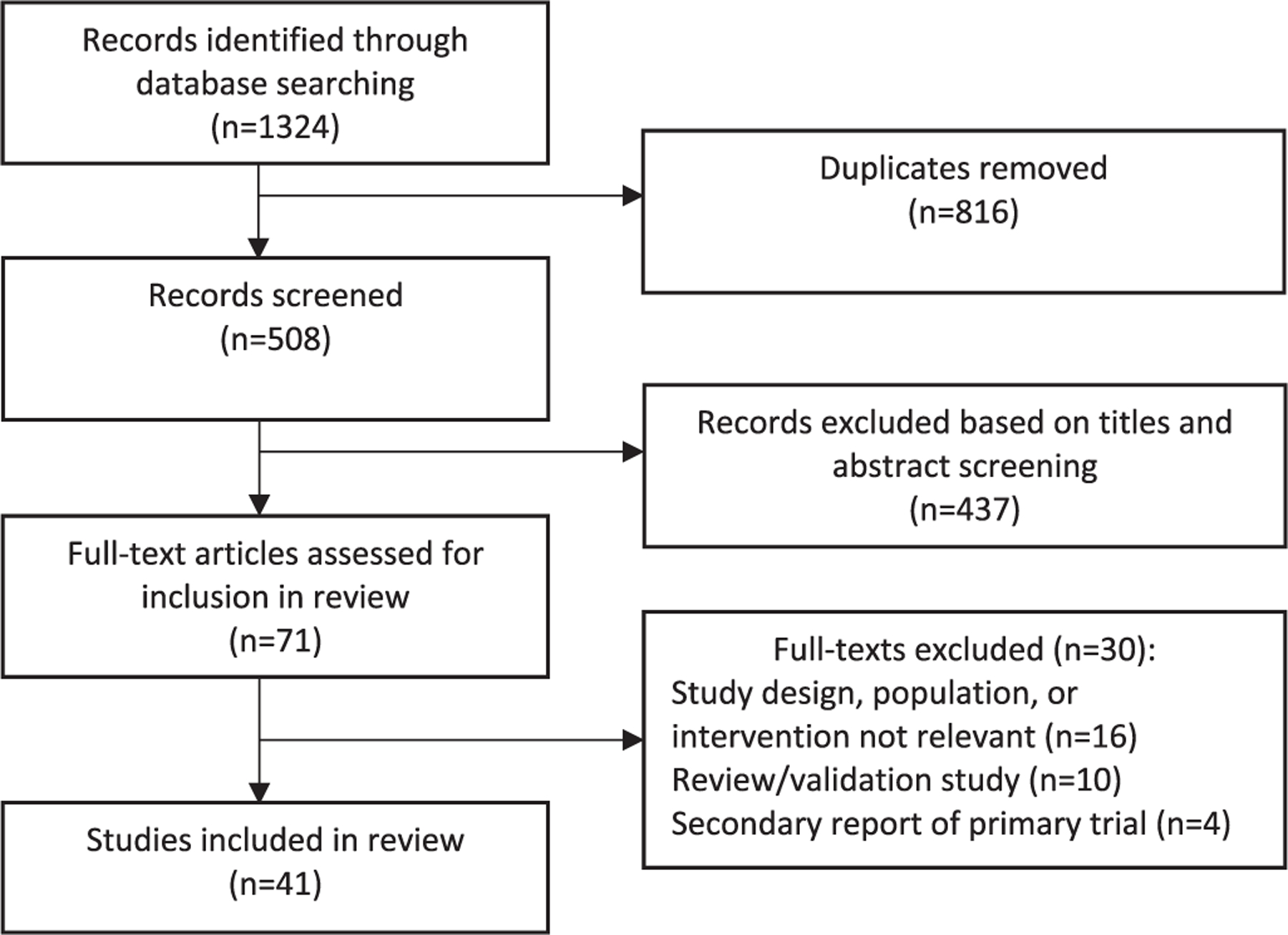
Study selection flowchart.
3.1. Population
Over half of the trials were done in individuals who had completed treatment or were considered by their oncologist to have no evidence of disease (survivors) (n = 26, 63%). The majority of these were breast cancer patients (n = 17, 65%), followed by multiple cancer types (n = 7, 27%), lung cancer (n = 1, 4%) and colorectal cancer (n = 1, 4%). Most survivorship trials involved only females (72%), as a result of the large number of breast cancer studies. The mean age of survivors was 56 years, ranging from 44 to 67 years.
There were 15 trials (37%) that included patients with active cancer. Patients were on average 56 years old (range 46–62). Within this subset, 7 trials (47%) included non-metastatic or early-stage cancer patients, 5 trials (33.3%) included a mix of early and advanced stages (stages 1–4) and 3 trials (20%) involved only advanced or metastatic cancer patients. Cancer patients were either currently receiving treatment (radiation, chemotherapy, chemoradiation, or other multimodality treatment) in 9 trials (60%), had completed treatment in 3 trials (20%), were receiving only palliative care in 1 trial (6.7%), or had surgery only in 1 trial (6.7%). Therapy was received in the adjuvant setting in 3 of the 15 trials (27.3%) (Supplementary Table 1).
3.2. Wearable activity monitors
The number of cancer trials involving activity monitors has increased over the last decade (Fig. 2). There has also been a shift in the type of activity monitor used over time. While the use of pedometers has remained relatively constant over time, an increase in the use of accelerometers is observed most notably between 2009 and 2013. A transition from the use of single axis accelerometers to three-axis accelerometers (triaxial accelerometers) occurred between 2011 and 2016, mirroring the advancement of technology. A list of wearable activity monitor types, manufacturers, placement, and output is outlined in Table 1.
Fig. 2.

Number of cancer trials involving wearable activity monitors, by year published.
Table 1:
Characteristics of devices reported in the literature.
| Author (year) | Cancer type | Device type(s) | Device placement(s) | Device outcome(s) |
|---|---|---|---|---|
| Pinto [44] | Breast, post-treatment | (1) Caltrac accelerometer | Hip | (1) Minutes of moderate-intensity physical activity; Total weekly Energy Expediture (kcal) |
| (2) Digiwalker Pedometer | (2) Step count | |||
| Epstein [45] | Breast, post-treatment | Actigraph | Wrist | Sleep duration; Sleep efficiency |
| Matthews [18] | Breast, post-treatment | (1) Actigraph | Waist | (1) Activity Counts (ct/min/day); Activity Duration; Steps per day; METs |
| (2) Pedometer | (2) Steps per day | |||
| Nikander [46] | Breast, during treatment | (1) Newtest Oy triaxial accelerometer | Not reported | (1) Relative ground reaction force |
| (2) Digiwalker Pedometer | (2) Steps per day | |||
| Vallance [47] | Breast, post-treatment | Digiwalker SW-200 Pedometer | Not reported | (1) Steps per day |
| Espie [50] | Multiple cancer types, post-treatment | (1) Actigraph (Cambridge) | Non-dominant wrist | Sleep onset latency; total sleep time; wake time after sleep onsent; sleep efficiency |
| Irwin [17] | Breast, post-treatment | (1) Pedometer | Not reported | (1) Steps per day |
| (2) Heart rate monitor | (2) Heart rate reserve | |||
| Demark-Wahnefried [48] [49] | Breast + prostate, post-treatment | RT3 triaxial accelerometer | Not reported | Activity counts per minute; Minutes of moderate, high and very high exercise |
| Berger [51] | Breast, post-treatment | Motionlogger Actigraph | Wrist | Wrist movement per minute |
| Innominato [15] | Colorectal, during treatment | (1) Mini-Motionlogger | Non-dominant wrist | Rest-activity circadian rhythm parameters (I < 0, r24, meanAct) |
| (2) Actigraph | ||||
| Maddocks [52] | Lung, during treatment | ActivPAL | Thigh | Step count per day |
| Rogers [53] | Breast, during treatment | GT1M accelerometer (Actigraph) | Wrist | Activity counts |
| Haines [13] | Breast, during treatment | Pedometer | Unclear | Unclear |
| Barsevick [54] | Multiple cancer types, during treatment | Motionloggor Actigraph | Wrist | Sleep duration; Sleep efficiency |
| Djuric [83] | Breast, during treatment | Pedometer (Omron HJ112) | Hip | Sleep duration |
| Hacker [55] | Malignancy requiring hematopoietic stem cell, during treatment | Actiwatch-Scorel | Wrist | Physical activity |
| Reeves [56] | Breast, post-treatment | Actigraph GT3X+ | Not reported | Change in accelerometer-derived moderate-to-vigorous activity |
| van der Meij [14] | Lung, during treatment | Physical activity monitor (PAM) accelerometer (model AM101, PAM BV) | Hip | Physical activity score; minutes of low/moderate-intense activity |
| Ancoli-Israel [57] | Breast, during treatment | Actiwatch-Light (MiniMitter/Phillips/ Respironics) | Wrist | Sleep, circadian rhythm |
| Gielissen [58] | Multiple cancer types, post-treatment | Actometer | Ankle | Physical activity (unspecified) |
| Coleman [82] | Multiple Myeloma, during treatment | ActiGraph | Not reported | Minutes of daytime/nighttime sleep |
| Bruera [59] | Multiple cancer types, palliative | Actiwatch | Wrist | Sleep onset; sleep efficacy; wake after sleep onset; total sleep time |
| Takiguchi [11] | Gastric, during treatment | Activtracer AC-301A | Waist | Recovery of physical activity |
| Guinan [16] | Breast, post-treatment | (1) RT3 accelerometer (Stayhealthy Inc) | (1) Hip | (1) Physical activity (METs, time sedentary, intensity) |
| (2) Heart rate monitor (Polar FT4F) | (2) Chest | (2) Aerobic intensity zone | ||
| Pinto [60] | Colorectal, post-treatment | CSA monitor | Waist | Active minutes |
| Prinsen [61] | Multiple cancer types, post-treatment | Actigraph | Ankle | Activity counts |
| Jones [62] | Breast, post-treatment | Pedometer | Not reported | Step count per day |
| Clark [9] | Breast, post-treatment | (1) ActivPAL inclinometer | (1) Waist | Sedentary time |
| (2) Actigraph GT3X + accelerometer | (2) Adhesive | |||
| Mustian [63] | Multiple cancer types, post-treatment | Actiwatch 64 (Mini Mitter) | Wrist | Sleep efficiency, sleep latency, and wake after sleep onset |
| Garland [64] | Multiple cancer types, post-treatment | GT1M Actigraph | Wrist | Sleep duration/efficiency |
| Mayo [65] | Multiple cancer types, during treatment | Pedometer | Wrist | Step count per day |
| Backman [12] | Breast + colon, during treatment | Pedometer | Wrist | Step count per day |
| Savard [66] | Breast, post-treatment | Actiwatch-64 | Wrist | Sleep onset latency, wakening after sleep onset, number awakenings, number/duration naps, total sleep time, sleep efficiency) |
| Rogers [67] | Breast, post-treatment | ActiGraph | Wrist | Sleep efficiency |
| Lengacher [68] | Breast, post-treatment | Actiwatch Score | Non-dominant wrist | Sleep duration/ efficiency |
| Short [69] | Breast, post-treatment | Pedometer | Not reported | Steps |
| Pinto [70] | Breast, post-treatment | ActiGraph | Not reported | Active minutes |
| Rogers Courneya [71] | Breast, post-treatment | ActiGraph | Wrist | Activity counts |
| James [72] | Multiple cancer types, post-treatment | Pedometer | Hip | Steps |
| Roveda [73] | Breast, post-treatment | Actigraph Actiwatch | Non-dominant wrist | Actual sleep time; actual wake time; sleep efficiency; sleep latency; immobility time; mean activity score; movement and gramentation index |
| Ungar [84] | Multiple cancer types, post-treatment | ActiGraph | Wrist | Active minutes |
Activity monitor manufacturers were reported in 38 articles, representing 16 different manufacturers. The majority of activity monitors were manufactured by ActiGraph (Actigraph LLC., Pensacola, FL) (n = 15, 34%), followed by Philips Respironics (Philips, Koninklijke Philips LLC., N·V) (n = 4, 9%), Ambulatory Monitoring, Inc. (Ambulatory Monitoring, Inc., Ardsley, NY) (n = 3, 7%), Cambridge Neurotechnology (Cambridge Neurotechnology, LTD, Cambridge UK) (n = 2, 5%), and Mini-Mitter (Mini-Mitter Co. Inc. Bend, OR) (n = 2, 5%). The remaining devices were manufactured by different companies.
Activity monitor placement can affect how movement is recorded, thus affecting how activity data are collected [8]. Device placement included the wrist/arm (n = 25, 57%), waist or hip (n = 15, 34%), or leg/ankle (n = 4, 9%). Placement was dictated by type of activity monitor, since pedometers are most often worn at the hip, and accelerometers on the wrist. Multi-sensor monitors were placed on the leg, arm or wrist. One device was adhesive and taped to thigh (ActivPal, Pal technologies) [9]. Device output included step counts, activity counts, metabolic equivalents, time active, time spent in moderate-to-vigorous activity, sedentary time, sleep duration, sleep efficiency, total sleep time/sleep duration, sleep onset latency, and wrist movement per minute.
3.3. Clinical trial experimental and control groups
Trial interventions were categorized into physical activity/exercise (n = 22, 54%), behavioral (n = 12, 29%), and other (e.g., therapeutic, palliative care, or surgical interventions) (n = 7, 16%). Details of study interventions are provided in Supplementary Table 1. Physical activity interventions included home-based exercise programs, walking programs, strength-training, physical activity counselling, and other exercise programs. The majority of the physical activity/exercise trials involved cancer survivors (post-treatment) (68%) although there were seven oncology trials evaluated exercise programs in patients currently undergoing treatment [8,40,46,47,49,60]. These trials involved breast cancer patients (n = 3)[9,40,47], advanced lung cancer (n = 1)[46], and a mix of other malignancies (n = 3) [8,49,60]. Experimental interventions in active cancer patients included walking programs[8,60], aerobic exercise programs[9,40,47], strength-training[49], and neuromuscular stimulation of quadricepts[46].
Behavioral interventions included cognitive behavior therapy, behavioral strategies, and lifestyle counselling targeted to improve nutrition/diet, sleep quality, insomnia, stress reduction, weight loss or cognitive behavior. In the above categories, activity monitors were used to measure adherence to the exercise or behavioral programs or as objective measures of physical activity. Experimental behavioral interventions tested in cancer survivors included cognitive behavioral therapy [39,42,51,57,59,61], nutritional supplements, lifestyle intervention and weight-loss plan[5,52], and mindful-ness stress reduction [68]. Half of these involved breast cancer survivors and the remaining involved a mix of cancer types. Behavioral interventions used in cancer patients undergoing active treatment included behavior therapy [45], nutritional supplements and programs[53], and a program to manage fatigue/sleep [48]. Trials categorized as using “other” interventions (n = 7) included cancer therapy trials [11,15,58] (n = 3), light therapy [50] (n = 1), or other therapeutic and surgical interventions [7,9,10].
The control groups consisted of usual care or “wait-list control” in 24 trials (58%), active controls (e.g., informational pamphlets, single-component educational programs, sham activity programs, etc.) in 14 trials (34%) and other controls in 6 trials (15%). Other controls included standard treatments (surgical; chemotherapy; psychiatric), dim light for sleep therapy, or subjective method for tracking moderate-to-vigorous activity.
3.4. Clinical trial outcomes
Physical activity measures were primary outcomes in the majority of trials (n = 16, 39%). Change in physical activity levels pre and post intervention were evaluated either through self-report physical activity questionnaires, in the lab using treadmill tests to assess maximum oxygen uptake (V02), maximum heart rate, and intensity, or with activity monitors (step count; minutes of intense activity). In two trials, the number of steps per day as measured using a wearable activity monitor was reported as the primary outcome. Three studies reported the mean daily steps, as measured using a wearable accelerometer, as a primary outcome [10,11]. A third trial reported the proportion of participants who completed 10,000 pedometer steps per day as a primary outcome [12]. Sleep was the second most commonly reported outcome, including sleep duration, sleep quality, or sleep behavior (n = 6). Related to sleep outcomes were fatigue, insomnia or sedentary outcomes (n = 8). Other reported outcomes included cognitive change or cognitive behavior towards physical activity and sleep (n = 2).
Quality of Life as a primary outcome was reported in two studies [13,14]. Quality of life was assessed using the EORTC QLQ-30 scale and functional status was evaluated based on the Karnofsky Performance Scale [14]. In this trial, an accelerometer was used to obtain objective measurements of physical activity. A second trial, which included a pedometer to measure steps, used EQ-5D & VAS, EORTC C30, and BR23 as quality of life instruments [13]. Another example includes a randomized therapeutic trial that evaluated activity data from the accelerometer as a predictor of survival outcomes and functional outcomes [15]. The primary outcomes of the trial were overall survival and progression free survival and secondary outcomes included quality of life and performance status, measured using the EORTC Quality of Life Questionannaire-C30 and the WHO PS scale, respectively. Quality of life as a secondary outcome was evaluated or mentioned in a total of 19 articles (46%).
3.5. Wearable activity monitor metrics
Mean daily step counts were reported in 12 trials, of which 10 involved cancer survivors (Fig. 3). In survivors, mean step counts ranged from 4,660 to 11,000 steps per day. Daily step counts were slightly lower in patients undergoing active treatment for their cancer, reported in 2 articles (2,885 to 8,300 steps/day). Active minutes per week were reported in 7 trials and ranged from 109 to 336 active minutes per week Supplementary Figure 1). Sleep duration was reported in seven trials articles and ranged from 402 to 498 min of sleep per night. Sedentary time was reported in six trials and ranged from 413 to 556 min sedentary per day. Heart rate was assessed in two trials and one protocol. Two trials used the heart rate monitors to evaluate mean heart rate and max heart rate during exercise sessions,[16,74] and one trial measured average heart rate and maximal heart rate to estimate performance intensity during 30 min of moderate-to-vigorous aerobic exercise using a heart-rate monitor during treadmill sessions [17]. Overall, the wearable activity monitor output depended on the cancer population, types of interventions and outcomes specified in each trial (Table 1, Supplementary Table 1).
Fig. 3.

Reported baseline step counts* from clinical trials using wearable activity monitors.
*Steps per day are the average number of steps taken each day. Horizontal line indicates the recommended 10,000 steps per day average. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Adherence to wearable activity monitor
The percentage of participants adhering to wearing the device for the specified period of time was reported in nine trials. Adherence ranged from 73% to 97%, with a median value of 82%. The number of days of activity data required to be considered adherent or evaluable was reported in 17 trials, ranging from 3 to 7 consecutive days of activity data. The most commonly reported number was 3 consecutive days, reported in 5 trials. Valid wear time was defined based on the minimum number of hours of wear per day, ranging from 5 h per day to 10 h per day, in 6 trials. A detailed description of how missing actigraphy data were handled was provided in only one trial [18]. In most trials, methods for handling nonwear time and missing data were unclear.
3.7. Current protocols
Published protocols of clinical trials [74–81] involving wearable activity monitors in cancer populations are listed in Supplementary Table 2. The most commonly reported device among protocols is the triaxial GT3X+ accelerometer (n = 3) [76,79,81]. Other trial designs include the use multisensor systems paired with smart phone applications (n = 2) [79,80], pedometers/step counters (n = 2) [74,77], and other accelerometers (n = 2) [75,78], and two trials plan to use more than one device [77,79].
4. Discussion
This review focused on characterizing and understanding applications of wearable activity monitors in oncology trials. We identified 41 randomized controlled trials published between 2005 and 2016 that used wearable activity monitors in cancer survivors. We found that wearable activity monitors are increasingly being used for the objective measurement of daily physical activity in clinical trials involving cancer survivors. More studies are using triaxial accelerometers and multisensor systems as they have become more abundant at decreased costs in the last five years. The rapid increase in the use of activity monitors is apparent when comparing our findings to those in a recent literature review conducted in 2010, where only 10 oncology trials that used activity monitors were identified [19]. Overall, there remains substantial heterogeneity in the types of devices used, the outcomes measured, duration of time they were worn and how the data were analyzed and reported in this review. A summary of the features of each of the three primary types of wearable activity monitors identified in this review and recommendations for their use in future clinical trials is provided in Table 2. The primary purposes for activity monitors are also described in the paragraphs that follow.
Table 2:
Comparison of wearable activity monitors used in oncology triales.
| Pedometer | Accelerometers (Uni or triaxial) | Consumer-based multi-sensor systems | |
|---|---|---|---|
| Examples of activity monitors | • Digiwalker • Fitbit Zip |
• ActiGraph GT3X+ • RT3 Research Tracker |
• Fitbit Charge HR • Apple Watch |
| Average cost | ~$10.00 to $60.00 | ~$200 to $1000.00 + software | ~$150.00 to $400.00 |
| Most common placement | • Hip/Waist • Wrist |
• Hip/Waist • Wrist |
• Wrist |
| Battery life | Up to 6 months | Up to 30 days | Up to 5 days |
| Syncing | • Not available for simple models (e.g., Digiwalker) | • Additional software required (e.g., ActiSync) | • Wireless sync with smartphones or tablets through Wifi or Bluetooth |
| • Wireless sync for more contemporary models | |||
| Compatibility | • Not available for simple models | • Windows | • iOS |
| • Android | |||
| • Windows | |||
| Primary activity output | • Steps | • Activity counts | • Steps |
| • Heart Rate | • Heart Rate | ||
| • Acceleration | |||
| Activity export (derived metrics from primary activity output) | • Distance | • Steps | • Stairs |
| • Distance | • Distance | ||
| • Activity intensity | • Active minutes | ||
| • Calories | • Calories | ||
| • Sleep | • Sleep | ||
| • Metabolic Equivalents | • Metabolic Equivalents | ||
| Additional Features | • Clock display | • Clock display | • Clock display |
| • Water resistant | • Water resistant | • Notifications | |
| • GPS tracking | • GPS tracking | ||
| • Exercise type | |||
| • Water resistant (some models water proof) | |||
| • Food and weight logs | |||
| Pros (+) | +Inexpensive | + Validated for clinical and sleep research (some models FDA-approved) | + Real-time accessible data on personal devices |
| +Longer battery life | + Export of raw acceleration available | + Inexpensive | |
| +Easy to use | + More sensitive to movement and sleep activity | + User friendly | |
| + Comfortable | |||
| + Aesthetically pleasing | |||
| Cons (−) | − Limited activity output | − Expensive | − Validity still being explored in cancer populations |
| − Lack of real-time data | − Additional syncing software required | − Shorter Battery life | |
| − Less accurate than triaxial accelerometers or multi-sensor systems | − Less user friendly and comfortable | − Proprietary algorithms | |
| − May not sync with personal devices (e.g., phones, tablets) | − Cannot be blinded | ||
| Recommendations | Recommend for low-budget clinical trials if primary purpose is to log steps and accuracy of step data less important. More sophisticated models with syncing capabilities recommended. | Recommended for studies of shorter durations where accurate estimates of activity and sleep are desired. Given cost of devices and software, a larger study budget is needed. | Recommended for studies of longer duration if it is of interest to obtain real-time activity data. Useful for studies integrating devices with other web-based applications and interventions. |
4.1. Wearable activity monitors allow for objective measurement of physical activity
We found that the most common purpose for wearable activity monitors was for the objective measurement of physical activity, either as step counts, duration of activity, or activity intensity. Most trials involved exercise and behavioral interventions to increase daily activity. The emphasis on physical activity after cancer diagnosis emerges from evidence that physical activity is associated with improved outcomes in cancer survivors [20,21]. While there is more evidence available regarding the benefits of exercise in cancer survivors, some trials have also shown benefits for physical activity during cancer treatment [1,22]. Current physical activity guidelines for cancer patients recommend 150 min of exercise per week [23]. However, few cancer survivors meet the recommended guidelines [23,24]. Consequently, exercise and behavioral programs are being developed and tested in randomized controlled settings to determine their effectiveness at increasing physical activity among cancer populations and encouraging lifestyle changes. The use of wearable activity monitors will allow for the objective monitoring of physical activity in cancer while reducing the biases and patient burden associated with self-report physical activity questionnaires and diaries [25].
4.2. Wearable activity monitors are used to motivate physical activity goals
The most simple and oldest activity monitor is the pedometer, with the specific function of counting steps. Investigators that used pedometers in the reviewed trials defined a per day step goal and measured a participant’s adherence to achieving that goal. Some studies evaluated the proportion of participants achieving a step goal after a specified period of day. A cut-point of 10,000 steps per day has been traditionally used, although there is increasing evidence that this cut-off is not accurate in people living with chronic conditions [26]. Such people take fewer steps, and have been shown to be more active in household chores that may not be accurately captured by the pedometer [15]. While pedometers are simple and inexpensive, they are limited in tracking activity other than step counts.
The accelerometer records additional activity outcomes such as activity counts and intensity, obtained from the raw acceleration data of the device. The studies identified in this review used accelerometers to measure the amount of time spent in different levels of activity such as sedentary, light or moderate-to-vigorous activity. Sedentary time is an important outcome as increased sedentary behavior is associated with poor health outcomes [27]. Interventions focused at decreasing sedentary behavior were evaluated in six trials where accelerometers were used as objective measures of sedentary time. Other interventions were developed to increase time spent in moderate-to-vigorous activity. Moderate-to-vigorous physical activity has also been shown to be associated with improved outcomes. In the cancer survivor guidelines, approximately 150 min per week of moderate intensity or 75 min per week of vigorous activity [23,28]. Additional limitations and precautions, however, are listed for survivors who are currently on radiation treatment, or experiencing side effects from the cancer or treatment [23].
4.3. Wearable activity monitor data are correlated with sleep
Sleep and fatigue are considered important patient-reported outcomes in oncology trials. There were fourteen oncology trials identified in this review that used accelerometry to obtain objective measures of sleep. The ActiGraph was the most commonly used device in these studies. Cancer patients report high levels of sleep-related problems including insomnia, excessive fatigue, leg restlessness, and sleepiness. The association between sleep and cancer has been explained by disruption of circadian rhythms in the brain and periphery, thus making sleep tracking an important feature of wrist-worn activity monitors [29]. Fatigue is an important symptom in cancer patients, characterized by feelings of exhaustion, weariness, malaise, lack of energy, motivation and inability to concentrate [30]. Given the biochemical evidence, many clinical trials have focused on measuring and reporting sleep variables and fatigue. Sleep has been found to be a correlate of autonomic dysfunction, which is prevalent in cancer patients [31,32]. More recent evidence has also suggested an association between telomere length, sleep and cancer [33]. Self-reported sleep duration and quality are often collected through quality of life questionnaires or sleep scales, but are also of limited value due to recall biases, as observed in self-reported physical activity. Objective monitoring of sleep, using actigraphy, has allowed for investigators to objectively measure sleep duration, sleep latency, number of awakenings, and sleep efficiency within their own home environment.
4.4. Potential for wearable activity monitor data to estimate quality of life
Quality of life (QOL) is an outcome that has been increasingly measured as a primary outcome in oncology trials. In oncology, QOL is considered a patient-centered outcome measure often collected using self-reported questionnaires such as the EORTC QLQ-30 [34]. The assessment of QOL in cancer patients is increasing as a result of a growing recognition for the importance of QOL in healthcare and cancer settings [35,36]. A recent Cochrane review reported on the association between physical activity on quality of life in cancer patients, and demonstrated beneficial effects of physical activity on QOL domains including physical functioning, role function, social functioning, and fatigue [37]. Thus, there is a growing interest in correlating objective activity metrics, as measured using wearable activity monitors, with subjective scales and overall quality of life. As it is a relatively new concept, only three trial reports focused on correlating objective activity with quality of life in this review [13,14,29]. However, there are several ongoing trials that are evaluating the relationships between objectively measured physical activity and quality of life. Further, it is anticipated that more oncology trials will use wearable activity monitors to measure and predict quality of life in the next few years [38]. Further correlations with Patient-Reported Outcome measures such as the NIH PROMIS® scales are also expected to increase in the next few years [39–41].
4.5. Summary of physical activity in cancer patients
Based on the available baseline data, we found that survivors (post-treatment) walk between 4660 and 11,000 steps per day while cancer patients undergoing treatment walk between 2885 and 8300 steps per day. These findings are similar to other reports including a review that looked at step counts in elderly and special populations (Range: 6500–8500 steps/day) [22]. The broad range reported may be explained by the substantial heterogeneity in oncology trials with regards to the cancer severity, treatment type, and duration. Thus, it is difficult to apply findings from our own review to a general cancer population. Given the substantial differences between activity patterns in cancer survivors who have completed treatment and those who are currently undergoing therapy for their cancer, it is recommended that activity levels are reported separately for these two groups. There is also a need for more trials involving cancer patients currently undergoing treatment in order to better understand how different therapies may impact their physical activity levels. Despite the observed differences across populations and broad range in physical activity, however, the average daily step count was consistently below the average recommended 10,000 steps per day for healthy adults in the trials included in this review.
4.6. Limitations associated with the use of wearable activity monitors in clinical settings
These activity estimates were based on data from monitors worn either on the wrist, hip or other parts of the body, manufactured by 16 different companies and worn for different durations of time. These findings highlight the challenge investigators face with the integration of activity monitors into clinical research and practice. There is a lack of standardization across the types, placement and purpose for device use in oncology trials, affecting the accuracy of the data. Furthermore, there is uncertainty with regards to how the activity data are managed and analyzed, which is echoed in several reports [26,32–42]. Other methodological issues discussed include the challenge with the identification of wear-time of the accelerometer, defining the minimal number of hours of wear for a valid day, identifying artefactual movement, computing summary variables and aggregating days of data, and extracting patterns of activity [3]. In this review, the majority of reports did not include methods for handling missing data and did not elaborate on the different issues surrounding the use of wearable activity monitors. Thus, it is unclear what data are being used to derive such estimates, and accurate summaries of physical activity may be difficult to achieve. The lack of discussion of missing data in clinical trial reports seriously limits the ability to determine the overall validity of the study.
A final limitation associated with the use of activity monitors in clinical settings is that not all monitors being used are validated for clinical purposes or for the population under study. While many devices that have been validated in healthy populations, only few have been validated in chronically ill or cancer populations [6,43]. A previously published systematic review identified 134 validation studies of activity monitors where 118 were conducted in healthy adults and the remaining 16 were conducted in chronically ill populations [6]. Validation of these devices will continue to be a challenge as the technological platform grows and new and improved devices emerge with greater frequency.
Despite the new challenges faced with the onset and growth of an emerging technology, the use of wearable activity monitors presents new and promising opportunities in cancer care. As wearable activity monitors continue to be integrated into future clinical trials, applications for their use will broaden. For instance, trials may involve more sophisticated technology including multi-sensor systems or biosensors to capture multiple physical components. Furthermore, wearable activity monitors can be used in therapeutic clinical trials to measure a patient’s improvement or decline over the course of treatment and assess their function and performance status. Third, the use of wearable activity monitor data for long-term, real-time monitoring of a patient’s well-being and activity during and after treatment will be important as the cancer survivor population continues to grow. In conclusion, the use of wearable activity monitors in oncology trials as well as other clinical settings demonstrates strong potential towards the improvement in healthcare that we are only beginning to understand.
Supplementary Material
Footnotes
Conflicts of interest
All authors of the manuscript report no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2017.11.002.
References
- [1].Warburton DE, Nicol CW, Bredin SS, Health benefits of physical activity: the evidence, Can. Med. Assoc. J 174 (6) (2006) 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schrack JA, Gresham G, Wanigatunga AA, Understanding physical activity in cancer patients and survivors: new methodology, new challenges, and new opportunities, Mol. Case Stud (2017) mcs. a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matthews CE, Hagstromer M, Pober DM, Bowles HR, Best practices for using physical activity monitors in population-based research, Med. Sci. Sports Exerc 44 (1 Suppl 1) (2012) S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Hebert JR, The effect of social desirability and social approval on self-reports of physical activity, Am. J. Epidemiol 161 (4) (2005) 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dobkin BH, Wearable motion sensors to continuously measure real-world physical activities, Curr. Opin. Neurol 26 (6) (2013) 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Van Remoortel H, Giavedoni S, Raste Y, et al. , Validity of activity monitors in health and chronic disease: a systematic review, Int. J. Behav. Nutr. Phys. Act 9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Plasqui G, Westerterp KR, Physical activity assessment with accelerometers: an evaluation against doubly labeled water, Obesity 15 (10) (2007) 2371–2379. [DOI] [PubMed] [Google Scholar]
- [8].Murphy SL, Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct, Prev. Med 48 (2) (2009) 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clark BK, Winkler E, Healy GN, Gardiner PG, Dunstan DW, Owen N, Reeves MM, Adults’ past-day recall of sedentary time: reliability, validity, and responsiveness, Med. Sci. Sports Exerc 45 (6) (2013) 1198–1207. [DOI] [PubMed] [Google Scholar]
- [10].James EL, Stacey F, Chapman K, Lubans DR, Asprey G, Sundquist K, Boyes A, Girgis A, Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers, BMC Public Health 11 (236) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, Mori M, Doki Y, Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study, World J. Surg 37 (10) (2013) 2379–2386. [DOI] [PubMed] [Google Scholar]
- [12].Backman M, Wengstrom Y, Johansson B, Skoldengen I, Borjesson S, Tarnbro S, Berglund AA, Randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer, Acta oncologica. (Stockholm, Sweden) 53 (4) (2014) 510–520. [DOI] [PubMed] [Google Scholar]
- [13].Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, Smith A, Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation, Breast Cancer Res. Treat (2010). [DOI] [PubMed] [Google Scholar]
- [14].Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, Smit EF, Leeuwen PA, Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT, Eur. J. Clin. Nutr 66 (3) (2012) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Innominato PF, Focan C, Gorlia T, et al. , Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer, Cancer Res 69 (11) (2009) 4700–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guinan E, Hussey J, Broderick JM, Lithander FE, O’Donnell D, Kennedy MJ, Connolly EM, The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—a pilot study, Supportive care in cancer 21 (7) (2013) 1983–1992. [DOI] [PubMed] [Google Scholar]
- [17].Irwin ML, Cadmus L, Alvarez-Reeves M, et al. , Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study, Cancer 112 (11 Suppl) (2008) 2593–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matthews CE, Wilcox S, Hanby CL, Der Ananian C, Heiney SP, Gebretsadik T, Shintani A, Evaluation of a 12-week home-based walking intervention for breast cancer survivors, Supportive care in cancer 15 (2) (2007) 203–211. [DOI] [PubMed] [Google Scholar]
- [19].Rogers LQ, Objective monitoring of physical activity after a cancer diagnosis: challenges and opportunities for enhancing cancer control, Phys. Ther. Rev 15 (3) (2010) 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM, Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review, J. Natl. Cancer Inst 104 (11) (2012) 815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH, An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis, J. Cancer Surviv 4 (2) (2010) 87–100. [DOI] [PubMed] [Google Scholar]
- [22].Tudor-Locke C, Craig CL, Aoyagi Y, et al. , How many steps/day are enough? For older adults and special populations, Int. J. Behav. Nutr. Phys. Act 8 (2011) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rock CL, Doyle C, Demark-Wahnefried W, et al. , Nutrition and physical activity guidelines for cancer survivors, CA Cancer J. Clin 62 (4) (2012) 242–274. [DOI] [PubMed] [Google Scholar]
- [24].Blanchard CM, Courneya KS, Stein K, Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II, J. Clin. Oncol 26 (13) (2008) 2198–2204. [DOI] [PubMed] [Google Scholar]
- [25].Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay MA, Comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review, Int. J. Behav. Nutr. Phys. Act 5 (1) (2008) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schrack JA, Zipunnikov V, Goldsmith J, Bai J, Simonsick EM, Crainiceanu C, Ferrucci L, Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity, J. Gerontol. A Biol. Sci. Med. Sci 69 (8) (2014) 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim Y, Wilkens LR, Park S-Y, Goodman MT, Monroe KR, Kolonel LN, Association between various sedentary behaviours and all-cause, cardiovascular disease and cancer mortality: the Multiethnic Cohort Study, Int. J. Epidemiol 42 (4) (2013) 1040–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].PANEL E, American College of Sports Medicine roundtable on exercise guidelines for cancer survivors, J. ACSM (2010) 1409–1426. [DOI] [PubMed] [Google Scholar]
- [29].Gery S, Koeffler HP, Circadian rhythms and cancer, Cell Cycle 9 (6) (2010) 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE, The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review, Brain Behav. Immun 21 (4) (2007) 413–427. [DOI] [PubMed] [Google Scholar]
- [31].Lakoski SG, Jones LW, Krone RJ, Stein PK, Scott JM, Autonomic dysfunction in early breast cancer: incidence, clinical importance, and underlying mechanisms, Am. Heart J 170 (2) (2015) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stone CA, Kenny RA, Nolan B, Lawlor PG, Autonomic dysfunction in patients with advanced cancer; prevalence, clinical correlates and challenges in assessment, BMC palliative care 11 (1) (2012) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cribbet MR, Carlisle M, Cawthon RM, Uchino BN, Williams PG, Smith TW, Gunn HE, Light KC, Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults, Sleep 37 (1) (2014) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aaronson NK, Ahmedzai S, Bergman B, et al. , The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology, J. Natl. Cancer Inst 85 (5) (1993) 365–376. [DOI] [PubMed] [Google Scholar]
- [35].Ferriolli E, Skipworth RJ, Hendry P, et al. , Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J. Pain Symptom Manag 43 (6) (2012) 1025–1035. [DOI] [PubMed] [Google Scholar]
- [36].Dahele M, Skipworth RJ, Wall L, Voss A, Preston T, Fearon KC, Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy, J. Pain Symptom Manag 33 (6) (2007) 676–685. [DOI] [PubMed] [Google Scholar]
- [37].Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O, Exercise interventions on health-related quality of life for people with cancer during active treatment, Cochrane Database Syst. Rev 8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pirl WF, Fujisawa D, Stagl J, Eusebio J, Traeger L, El-Jawahri A, Greer JA, Temel JS, Actigraphy as an objective measure of performance status in patients with advanced cancer, Proc. Am. Soc. Clin. Oncol (2015). [Google Scholar]
- [39].Cella D, Yount S, Rothrock N, et al. , The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years, Med. Care 45 (5 Suppl 1) (2007) S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shinde AM, Gresham GK, Hendifar AE, et al. , Correlating wearable activity monitor data with PROMIS detected distress and physical functioning in advanced cancer patients, Proc. Am. Soc. Clin. Oncol 35 (15 Suppl) (2017) e21689. [Google Scholar]
- [41].Gresham GK, Neeman E, Hendifar AE, et al. , Assessing performance status and clinical outcomes with wearable activity monitors, Proc. Am. Soc. Clin. Oncol 35 (15 Suppl) (2017) 6571. [Google Scholar]
- [42].Keadle SK, Shiroma EJ, Kamada M, Matthews CE, Harris TB, Lee IM, Reproducibility of accelerometer-assessed physical activity and sedentary time, Am. J. Prev. Med 49 (5) (2017) 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vooijs M, Alpay LL, Snoeck-Stroband JB, Beerthuizen T, Siemonsma PC, Abbink JJ, Sont JK, Rovekamp TA, Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease, Interact. J. Med. Res 3 (4) (2014) e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH, Home-based physical activity intervention for breast cancer patients, J. Clin. Oncol 23 (15) (2005) 3577–3587. [DOI] [PubMed] [Google Scholar]
- [45].Epstein DR, Dirksen SR, Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors, Oncol. Nurs. Forum 34 (5) (2007) E51–9. [DOI] [PubMed] [Google Scholar]
- [46].Nikander R, Sievanen H, Ojala K, Oivanen T, Kellokumpu-Lehtinen PL, Saarto T, Effect of a vigorous aerobic regimen on physical performance in breast cancer patients - a randomized controlled pilot trial, Acta oncol. (Stockholm, Sweden) 46 (2) (2007) 181–186. [DOI] [PubMed] [Google Scholar]
- [47].Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR, Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors, J. Clin. Oncol 25 (17) (2007) 2352–2359. [DOI] [PubMed] [Google Scholar]
- [48].Sloane R, Snyder DC, Demark-Wahnefried W, Lobach D, Kraus WE, Comparing the 7-day physical activity recall with a triaxial accelerometer for measuring time in exercise, Med. Sci. Sports Exerc 41 (6) (2009) 1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, Snyder DC, Giguere JK, Shaw E, Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy, Clin. breast cancer 8 (1) (2008) 70–79. [DOI] [PubMed] [Google Scholar]
- [50].Espie CA, Fleming L, Cassidy J, et al. , Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer, J. Clin. Oncol (2008). [DOI] [PubMed] [Google Scholar]
- [51].Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, Agrawal S, One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue, J. Clin. Oncol 27 (35) (2009) 6033–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Maddocks M, Lewis M, Chauhan A, Manderson C, Hocknell J, Wilcock A, Randomized controlled pilot study of neuromuscular electrical stimulation of the quadriceps in patients with non-small cell lung cancer, J. Pain Symptom Manag 38 (6) (2009) 950–956. [DOI] [PubMed] [Google Scholar]
- [53].Rogers LQ, Hopkins-Price P, Vicari S, et al. , A randomized trial to increase physical activity in breast cancer survivors, Med. Sci. Sports Exerc 41 (4) (2009) 935–946. [DOI] [PubMed] [Google Scholar]
- [54].Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, Newhall T, Brown S, Stewart K, Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy, J. Pain Symptom Manag 40 (2) (2010) 200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hacker ED, Larson J, Kujath A, Peace D, Rondelli D, Gaston L, Strength training following hematopoietic stem cell transplantation, Cancer Nurs 34 (3) (2011) 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Reeves M, Winkler E, McCarthy N, Lawler S, Eakin E, Healy G, Living well after breast cancer: changes in objectively-measured physical activity in a weight loss trial, J. Sci. Med. Sport 15 (Suppl 1) (2012) S334. [Google Scholar]
- [57].Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, Lawton S, Desan P, Liu L, Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer, Support. Care Cancer 20 (6) (2012) 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gielissen MF, Wiborg JF, Verhagen CA, Knoop H, Bleijenberg G, Examining the role of physical activity in reducing postcancer fatigue, Support. Care Cancer 20 (7) (2012) 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bruera E, Yennurajalingam S, Balachandran D, Padhye NS, Williams JL, Frisbee-Hume S, Actigraphy findings and cancer-related fatigue (CRF) in advanced cancer patients treated with methylphenidate (MP) and nursing telephone intervention (NTI), J. Clin. Oncol 31 (Suppl 15) (2013) e20528. [Google Scholar]
- [60].Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N, Home-based physical activity intervention for colorectal cancer survivors, Psycho-Oncology 22 (1) (2013) 54–64. [DOI] [PubMed] [Google Scholar]
- [61].Prinsen H, Bleijenberg G, Heijmen L, Zwarts MJ, Leer JW, Heerschap A, Hopman MT, van Laarhoven HW, The role of physical activity and physical fitness in postcancer fatigue: a randomized controlled trial, Support. Care Cancer 21 (8) (2013) 2279–2288. [DOI] [PubMed] [Google Scholar]
- [62].Jones SB, Thomas GA, Hesselsweet SD, Alvarez-Reeves M, Yu H, Irwin ML, Effect of exercise on markers of inflammation in breast cancer survivors: The Yale exercise and survivorship study, Cancer Prevention Research (Phila) 6 (2) (2013) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mustian KM, Sprod LK, Janelsins M, et al. , Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors, J. Clin. Oncol 31 (26) (2013) 3233–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS, Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial, J. Clin. Oncol 32 (5) (2014) 449–457. [DOI] [PubMed] [Google Scholar]
- [65].Mayo NE, Moriello C, Scott SC, Dawes D, Auais M, Chasen M, Pedometer-facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial, Clin. Rehabil 28 (12) (2014) 1198–1209. [DOI] [PubMed] [Google Scholar]
- [66].Savard J, Ivers H, Savard MH, Morin CM, Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial, Sleep 37 (8) (2014) 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rogers LQ, Fogleman A, Trammell R, et al. , Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial, Psycho-Oncology 24 (3) (2015) 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lengacher CA, Reich RR, Paterson CL, et al. , The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial, Psycho-Oncology 24 (4) (2015) 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Short CE, James EL, Girgis A, D’Souza MI, Plotnikoff RC, Main outcomes of the move more for life trial: a randomised controlled trial examining the effects of tailored-print and targeted-print materials for promoting physical activity among post-treatment breast cancer survivors, Psycho-Oncology 24 (7) (2015) 771–778. [DOI] [PubMed] [Google Scholar]
- [70].Pinto BM, Stein K, Dunsiger S, Peers promoting physical activity among breast cancer survivors: a randomized controlled trial, Health Psychol 34 (5) (2015) 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rogers LQ, Courneya KS, Anton PM, Hopkins-Price P, Verhulst S, Vicari SK, Robbs RS, Mocharnuk R, McAuley E, Effects of the BEAT cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial, Breast Cancer Res. Treat 149 (1) (2015) 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].James EL, Stacey FG, Chapman K, Boyes AW, Burrows T, Girgis A, Asprey G, Bisquera A, Lubans DR, Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial, BMC Cancer 15 (2015) 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Roveda E, Vitale JA, Bruno E, et al. , Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors, Integr. Cancer Ther 16 (1) (2016) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Velthuis MJ, May AM, Koppejan-Rensenbrink RA, et al. , Physical activity during cancer treatment (PACT) study: design of a randomised clinical trial, BMC Cancer 10 (2010) 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zick SM, Wyatt GK, Murphy SL, Arnedt JT, Sen A, Harris RE, Acupressure for persistent cancer-related fatigue in breast cancer survivors (AcuCrft): a study protocol for a randomized controlled trial, BMC Complement. Altern. Med (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Galiano-Castillo N, Ariza-Garcia A, Cantarero-Villanueva I, Fernandez-Lao C, Diaz-Rodriguez L, Legeren-Alvarez M, Sanchez-Salado C, Del-Moral-Avila R, Arroyo-Morales M, Telehealth system (e-CUIDATE) to improve quality of life in breast cancer survivors: rationale and study protocol for a randomized clinical trial, Trials 14 (Jun 22 2013) 187, 10.1186/1745-6215-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jarden M, Moller T, Kjeldsen L, Birgens H, Christensen JF, Bang Christensen K, Diderichsen F, Hendriksen C, Adamsen L, Patient activation through counseling and exercise–acute leukemia (PACE-AL)–a randomized controlled trial, BMC Cancer 13 (2013) 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Munro J, Adams R, Campbell A, et al. , CRIB - the use of cardiac rehabilitation services to aid the recovery of patients with bowel cancer: a pilot randomised controlled trial (RCT) with embedded feasibility study, BMJ Open 4 (2) (Feb 18 2014) e004684, 10.1136/bmjopen-2013-004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hartman SJ, Natarajan L, Palmer BW, Parker B, Patterson RE, Sears DD, Impact of increasing physical activity on cognitive functioning in breast cancer survivors: rationale and study design of memory & motion, Contemp. Clin. Trials 45 (Pt B) (2015) 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wolvers M, Bruggeman-Everts FZ, Effectiveness, mediators, and effect predictors of internet interventions for chronic cancer-related fatigue: the design and an analysis plan of a 3-armed randomized controlled trial, 4 (2) (2015) e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wiskemann J, Kuehl R, Dreger P, Huber G, Kleindienst N, Ulrich CM, Bohus M, Physical exercise training versus relaxation in allogeneic stem cell transplantation (PETRA study)–rationale and design of a randomized trial to evaluate a yearlong exercise intervention on overall survival and side-effects after allogeneic stem cell transplantation, BMC Cancer 15 (1) (2015) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Coleman EA, Goodwin JA, Kennedy R, Coon SK, Richards K, Enderlin C, Stewart CB, McNatt P, Lockhart K, Anaissie EJ, Effects of exercise on fatigue, sleep, and performance: a randomized trial, Oncol. Nurs. Forum 39 (5) (2012. Sep) 468–477, 10.1188/12.ONF.468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Djuric Z, Ellsworth JS, Weldon AL, Ren J, Richardson CR, Resnicow K, Newman LA, Hayes DF, Sen A, A diet and exercise intervention during chemotherapy for breast cancer, Open Obes J 3 (2011) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ungar N, Sieverding M, Weidner G, Ulrich CM, Wiskemann J, A self-regulation-based intervention to increase physical activity in cancer patients, Psychol. Health Med 21 (2) (2016) 163–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


