Abstract
Protein synthesis is an essential and highly regulated cellular process. Here, we demonstrate the versatility of polysome profiling—a methodology traditionally used to assess levels of protein synthesis—to monitor ribosomal integrity and modulation of specific steps in mRNA translation. Using expanded polysome profiling methodologies, we systematically illustrate defects in ribosome biogenesis, translation initiation, and translational elongation in different cellular conditions. We additionally provide instruction for how a modified polysome profiling protocol can be leveraged to identify and characterize the function of factors that regulate protein synthesis. These methodologies are broadly applicable to a range of physiological conditions and human diseases in which ribosome biogenesis or the phases of protein synthesis are distinctly regulated or dysregulated.
The steps in protein synthesis (i.e., ribosome biogenesis, translation initiation, translation elongation, and translation termination) are highly-coordinated, but we do not fully understand how these processes are distinctly regulated or dysregulated in different physiological or disease states.
This work illustrates the extensibility of polysome profiling and provides examples of impairments in ribosome biogenesis and distinct phases of translation.
The protocols detailed here will enable the interrogation of translation regulation in various contexts, as well as the identification and characterization of the factors that contribute to this highly-orchestrated process.
INTRODUCTION
As the final step in the central dogma, translation control plays critical roles in cell homeostasis, proliferation, and growth (Gebauer and Hentze, 2004; Sonenberg and Hinnebusch, 2009; Hershey et al., 2012). As such, mutations in the mRNA translational machinery, including ribosomal proteins and associated translation factors, cause a dysregulation in protein synthesis that underlies a wide range of human diseases (Tahmasebi et al., 2018; Farley-Barnes et al., 2019; Costa-Mattioli and Walter, 2020; Young-Baird et al., 2020; Doty et al., 2022). Addressing how translation is regulated or dysregulated in various contexts has largely relied on molecular approaches that assess bulk protein synthesis levels (Schmidt et al., 2009; He and Green, 2013). For instance, polysome profiling, is a popular method that employs sucrose gradient ultracentrifugation to separate the 40S and 60S ribosomal subunits from mRNAs translated by a single 80S ribosome or multiple ribosomes, termed polysomes (Figure 1).
FIGURE 1:
Overview of polysome profiling. Cell lysates are layered on top of a 10–50% sucrose gradient and subjected to ultracentrifugation. mRNAs distribute throughout the gradient based upon their ribosomal occupancy, and RNA distribution is monitored at an absorbance of A260. Sucrose fractions can be collected from top-to-bottom or bottom-to-top of the gradient depending on the type of fractionation equipment that is utilized.
The traditional polysome profiling approach has been a critical tool to assess bulk protein synthesis levels in multiple cellular contexts and model systems. This methodology has also been combined with downstream analyses of sucrose gradient fractions to reveal more detailed information about ribosomal occupancy on specific RNAs and the function of ribosome- and RNA-binding proteins that play important roles translational control (Teske et al., 2011; Bunnik et al., 2013; He and Green, 2013; Hu and Coller, 2013; Faye et al., 2014; Gandin et al., 2014; Kudla and Karginov, 2016; Lecampion et al., 2016; Chasse et al., 2017; Jin and Xiao, 2018; Pereira et al., 2018; Pringle et al., 2019; Seimetz et al., 2019; Kedia et al., 2021; Han et al., 2022; Lokdarshi and Von Arnim, 2023). Importantly, all of the polysome profiling approaches highlighted in the methods manuscripts above rely upon ribosome stabilizing agents such as magnesium and cycloheximide (CHX) that can mask the regulation of or defects in ribosome biogenesis and the specific steps in protein synthesis (i.e., translation initiation, elongation, and termination). This study provides a cohesive catalogue of adaptations to the polysome profiling methodology with varying or no reliance upon ribosome stabilizing agents to systematically examine these individual processes. These modifications emphasize the extensibility of polysome profiling, enable in-depth assessments of ribosome biogenesis, and delineate changes in the steps of protein synthesis that are often distinctly regulated or dysregulated in different physiological or disease states (Hinnebusch and Lorsch, 2012; Woolford and Baserga, 2013; Dever et al., 2018; Hellen, 2018; Tahmasebi et al., 2018; Farley-Barnes et al., 2019; Costa-Mattioli and Walter, 2020; Young-Baird et al., 2020; Doty et al., 2022). Here, we demonstrate defects in ribosome biogenesis and specific steps in translation by targeting the expression of key translation factors RPS19, RPL35A, and eEF2K, and subjecting cells to a cellular stress–inducing agent. Last, we emphasize an alternative cell lysis approach to further leverage polysome profiling in the identification and characterization of factors that interact with the translational machinery to regulate protein synthesis.
RESULTS
RPS19 or RPL35A depletion impairs ribosome biogenesis
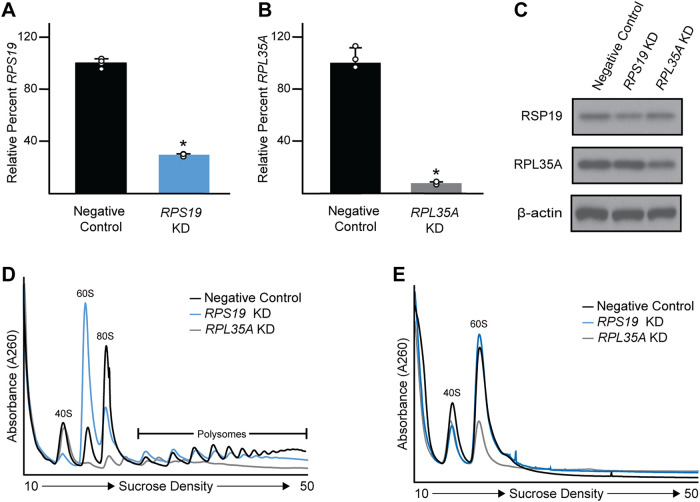
To illustrate the utility of a modified polysome profiling protocol in the assessment of ribosome biogenesis, cells were transfected with siRNA for RPS19 or RPL35A, that encode 40S or 60S ribosomal subunit proteins, respectively. Roughly 70% of the RPS19 and 95% of the RPL35A mRNAs were depleted (KD) compared with controls (Figure 2, A and B), which led to a modest reduction in RPS19 and RPL35A protein levels (Figure 2C; Supplemental Figure S1) likely owing to their long protein half-lives of ∼4 d (Dorrbaum et al., 2018). As an assessment of overall protein synthesis, cells were lysed with standard buffer containing CHX to lock elongating ribosomes onto actively translated mRNAs. Depletion of either ribosomal subunit protein resulted in a decrease in the height of the 80S monosome and polysome peaks, consistent with a reduction in overall protein synthesis (Figure 2D).
FIGURE 2:
RPS19 or RPL35A depletion impairs ribosome biogenesis. (A) qPCR of RPS19 and (B) RPS35A in KD cells relative to controls (n = 3; mean value depicted with S.D.; asterisks indicate statistical significance between groups using Student's t test, p < 0.05). (C) Immunoblots of the indicated proteins in RPS19 and RPL35A KD cells and negative controls. (D) Polysome profiling of cells transfected with the indicated siRNAs and lysed in standard buffer with CHX and Mg2+. (E) Polysome profiling of cells transfected with the indicated siRNAs and lysed in ribosome biogenesis lysis buffer that lacks CHX and Mg2+.
Using either standard buffer or buffer lacking magnesium (Mg2+), required for ribosomal subunit joining, we observed a decrease in the 40S subunit peak in the RPS19 KD as expected (Figure 2, D and E). Furthermore, depletion of RPS19 also resulted in an increase in the 60S peak in the profile from cells lysed in standard buffer (Figure 2D), but not buffer lacking Mg2+ (Figure 2E). These data indicate that reduced 40S subunit biogenesis in the RPS19 KD results in increased “free” (i.e., unbound to the 40S) 60S ribosomal subunits without a change in 60S biogenesis. Applying this approach, we recently demonstrated that a RPS19 mutation underlying Diamond–Blackfan Anemia (DBA) drives adverse phenotypes through specifically diminishing 40S subunit biogenesis in DBA patient cells (Doty et al., 2022).
As anticipated, we also observed a decrease in the 60S subunit peak upon RPL35A KD when cells were lysed in either standard lysis buffer or buffer lacking Mg2+ (Figure 2, D and E). Intriguingly, depletion of RPL35A resulted in a decrease in the height of the 40S subunit peak as well in both polysome profiles (Figure 2, D and E). Prior work has shown that an interaction between the mature 60S subunit and the immature pre-40S is required for 20S pre-rRNA processing into the 18S rRNA and complete 40S subunit biogenesis (Strunk et al., 2012; Ferreira-Cerca et al., 2014; Belhabich-Baumas et al., 2017; Ghalei et al., 2017). Consistent with these previous studies, our data suggest that decreased 60S subunit formation in the RPL35A KD cells may also cause a secondary, downstream defect in 40S subunit biogenesis.
Specific stress-responsive factors are translationally up-regulated while global translation initiation is suppressed during cellular stress
We also utilized specific lysis protocols to monitor changes in the distinct phases of protein synthesis. To interrogate translation initiation, we treated cells with thapsigargin (TG), a stress-inducing drug known to cause phosphorylation of the α subunit of the eukaryotic translation initiation factor 2 (eIF2α-P) and decrease overall protein synthesis (Walter and Ron, 2011). Compared with controls (NT), TG resulted in decreased polysomes with a corresponding increase in the 80S peak when cells were lysed in the presence of CHX (Figure 3A). This shift in mRNA distribution from heavy polysome fractions to the 80S monosome is indicative of decreased overall translation initiation (Holmes et al., 2022). Intriguingly, specific mRNAs are also translationally up-regulated during eIF2α-P through a pathway known as the integrated stress response (ISR) (Harding et al., 2003; Young and Wek, 2016). To monitor ribosome occupancy on a canonical ISR factor subject to preferential translation during stress (Vattem and Wek, 2004), we specifically assessed ATF4 mRNA distribution in sucrose gradients. As expected, we observed a shift in ATF4 distribution toward heavy polysomes in the TG-treated cells, consistent with the translational up-regulation of ATF4 (Figure 3, B and C). Together, these data demonstrate the versatility of polysome profiling to assess changes in global translation initiation and gene-specific protein synthesis.
FIGURE 3:

Specific stress-responsive factors are translationally up-regulated while global translation initiation is suppressed during cellular stress. (A) Polysome profiles of controls (NT) or cells treated with TG and lysed in standard buffer containing CHX and Mg2+. (B) ATF4 mRNA distribution in NT and TG-treated cells (n = 3; mean values depicted with S.D.). Total RNA was isolated from each sucrose fraction and the amount of ATF4 mRNA in each fraction relative to total ATF4 mRNA in the cell lysate was determined by qPCR. Data are graphed as the percentage of ATF4 mRNA in each fraction. (C) Percentage of ATF4 associated with polysome fractions 8–15 from NT or TG-treated cells (n = 3; mean values depicted with S.D.; asterisks indicate statistical significance between groups using Student's t test, p < 0.05).
eEF2K overexpression impairs ribosome translocation
Expanding our capacity to interrogate specific steps in translation, we examined defects in translation elongation by targeting the activity of eukaryotic elongation factor 2 (eEF2) that promotes ribosome translocation (Liu and Proud, 2016; Dever et al., 2018). The eEF2 kinase (eEF2K) is activated in response to nutrient depletion and blocks elongation by phosphorylating eEF2 (eEF2-P) and inhibiting its recruitment to the ribosome (Carlberg et al., 1990; Proud, 2019). By overexpressing eEF2K to inhibit eEF2 activity, we demonstrated an anticipated increase in eEF2-P as compared with cells transfected with a control vector (Figure 4A). For our polysome profiling analysis, we lysed cells in the absence of CHX to allow for ribosome run-off. Cells overexpressing eEF2K exhibited reproducibly sustained heavy polysomes as compared with controls (Figure 4, B and C; Supplemental Figure S2). These data are consistent with increased ribosome stalling resulting from impaired translocation during eEF2-P. Surprisingly, eEF2K overexpression also resulted in a consistent increase in the free 60S subunit peak (Figure 4B; Supplemental Figure S2), suggesting a potential role for eEF2-P in modulating 60S subunit biogenesis or stability, in addition to its well-documented impact on 80S ribosome translocation (Proud, 2019). As high levels of eEF2K expression have been suggested to promote cancer and tumor progression (Liu and Proud, 2016), eEF2-P and ribosome translocation is likely modulated in both physiological and disease contexts.
FIGURE 4:
eEF2K overexpression impairs ribosome translocation. (A) Immunoblots of the indicated proteins in cells transfected with a control or HA-eEF2K overexpression plasmid. (B) Polysome profiling of cells transfected with a control or HA-eEF2K overexpression plasmid and lysed in translation elongation buffer that lacks CHX. Inset displays zoom-in of polysomes. (C) Average polysome/monosome (P/M) ratio from cells transfected with a control or HA-eEF2K overexpression plasmid (n = 3; mean values depicted with S.D.; asterisks indicate statistical significance between groups using Student's t test, p < 0.05).
Polysome profiling has significant utility in the identification and characterization of ribosome- and RNA-binding factors
Polysome profiling can be adapted to identify and characterize ribosome- and RNA-binding factors that modulate protein synthesis. In this adaptation, the addition of 10% formaldehyde (CH2O) to cells prior to lysis cross-links ribosomes and mRNAs to associated factors that are typically dislodged during sucrose ultracentrifugation. Polysome profiles of cells lysed after treatment with CH2O presented with halfmers (i.e., “shoulders”) after each polysome peak (Figure 5). Previous characterization of halfmers has indicated that they are composed of mRNA, one or more translating 80S ribosomes, and a 40S subunit with associated preinitiation complex factors including eIF2 and eIF3 (Helser et al., 1981; Rotenberg et al., 1988; Nielsen et al., 2004; Valasek et al., 2007). As preinitiation complexes are sensitive to the forces of ultracentrifugation (Nielsen et al., 2004; Valasek et al., 2007), and halfmers are not typically observed under standard polysome profiling conditions (Figure 5), these data highlight the potential utility of CH2O as a stabilization agent to characterize intrinsically unstable complexes and identify ribosome- and RNA-binding factors that may associate transiently. For instance, collection of sucrose gradient fractions followed by RNA isolation and qPCR, northern blotting, or RNA-based deep sequencing may be used to examine the association of noncoding RNAs with specific ribosomal subunit and ribosome fractions under various conditions (Valencia-Sanchez et al., 2006; Statello et al., 2021). Similarly, identifying and characterizing the contributions of RNA- and ribosome-binding proteins to translation regulation (Harvey et al., 2018) may be implemented by subjecting sucrose fractions to targeted protein blotting, mass spectrometry, and advanced cryogenic electron microscopy (cryo-EM) approaches.
FIGURE 5:

Polysome profiling can be leveraged to identify and characterize ribosome- and RNA-binding factors. Polysome profile of cells treated with formaldehyde prior to lysis in buffer lacking CHX. Halfmers representing mRNA, one or more translating 80S ribosomes, and a 40S subunit with associated preinitiation complex factors are indicated by arrows.
DISCUSSION
With the work presented in this manuscript, we aim to illustrate the scope of protein synthesis assessments that can be conducted with polysome profiling. By modulating expression of RPS19, RPL35A, and eEF2K, and treating cells with a cellular stress–inducing agent, we systematically show that in addition to being a reliable tool to monitor bulk protein synthesis levels, polysome profiling can also be leveraged to interrogate ribosome biogenesis and specific steps in protein synthesis that are often distinctly regulated or impaired in different physiological conditions or disease states (Hinnebusch and Lorsch, 2012; Woolford and Baserga, 2013; Dever et al., 2018; Hellen, 2018; Tahmasebi et al., 2018; Farley-Barnes et al., 2019; Costa-Mattioli and Walter, 2020; Young-Baird et al., 2020; Doty et al., 2022).
As with any experimental methodology, it is important to note a few constraints and potential pitfalls that accompany polysome profiling approaches and data interpretation. Importantly, a lacking component to polysome profiling is the visualization of where ribosomes are located on a given mRNA at nucleotide resolution. For instance, in addition to the main open reading frame (ORF), many mRNAs contain small internal ORFs or ORFs in the 5′- and 3′-UTRs that can also be translated (Young and Wek, 2016; Orr et al., 2020). Changes to the translation of these small ORFs can impact how a particular mRNA may be distributed in the polysome fractions, complicating the interpretation of how robustly a given mRNA is translated in polysome profiling experiments like the one conducted in Figure 3B. Additionally, defects in translation termination can also cause stalled or queued ribosomes that inhibit ribosome run-off (Young et al., 2018; Turnbull et al., 2024), resulting in polysome profiles that mimic those observed under translation elongation defects (e.g., Figure 4B). Thus, complementary molecular and biochemical techniques may also be employed alongside polysome profiling to provide additional insight into how global and gene-specific translation is regulated.
Addressing precisely how translation is modulated during ribosome biogenesis and the steps in protein synthesis is becoming increasingly important as drugs that target the protein synthesis machinery are entering clinical trials for a broad range of diseases (National Library of Medicine, 2015; EU Medicines Agency, 2018; Dalla Bella et al., 2021; National Library of Medicine, 2021a, 2021b, 2021c; 2022a, 2022b; 2023). Thus, the modifications to polysome profiling described here will be instrumental in future efforts to explore translation regulation in various contexts; to identify and characterize the factors that contribute to this fine-tuned process; and to ultimately develop and validate targeted therapeutics for the myriad diseases accompanied by dysregulated protein synthesis.
MATERIALS AND METHODS
Request a protocol through Bio-protocol
Cell culture
HEK293T cells were cultured in DMEM high glucose media (Life Technologies, Catalogue No.11965-092) supplemented with 10% FBS (R&D Systems, Catalogue No. S11150) and 1% penicillin-streptomycin (Life Technologies, Catalogue No. 15140-122) in a 10 cm standard tissue culture dish at 37°C. Cells were split when they reached 60–70% confluency using 1 ml of 1X Trypsin-EDTA (Life Technologies, Catalogue No. 25200-0560) per plate. Following a 5-min incubation at 37°C, the cells were resuspended in the desired volume of culture media and plated. For freezing, cells were trypsinized as described above, collected, and then centrifuged for 5 min at 300 rpm to isolate the cell pellet that was resuspended in the desired volume of freezing media (∼ 1 ml freezing media per 10 cm dish) for storage in liquid nitrogen. For experiments that required TG (Thermo Fisher Scientific, Catalogue No. T7459) treatment, cells were treated with 1 µM of TG (final concentration) for 6 h and lysed as described below.
Transfections
Cells were seeded at 5.5 × 105 cells per 10 cm dish for the scramble negative control siRNA (Thermo Fisher Scientific, Catalogue No. 4390846) and 2.2 × 106 cells per plate for the RPS19 (Thermo Fisher Scientific, Catalogue No. 4392420 siRNA ID: s194768) and RPL35A (Thermo Fisher Scientific, Catalogue No. 4392420 siRNA ID: s12225) siRNA transfections. All plates were transfected using the Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific, Catalogue No.1377810) 24 h after cells were plated following the manufacturer's suggested protocol and with a final siRNA concentration of 0.035 µM. The cell culture media in all siRNA depletion experiments was supplemented with 20% FBS with a media change 6–8 h after transfection. For eEF2K overexpression experiments, cells were seeded at a density of 2.2 × 106 cells per plate and were transfected 24 h after plating using the Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific, Catalogue No. L30000001) per the manufacturer's instructions. A total of 3.25 µg of empty vector or eEF2K-HA pcDNA was transfected into each plate and cell culture media was exchanged for fresh media 6 h after transfection.
Polysome profiling: Cell lysis
Unless otherwise specified, cells were plated at a seeding density of 2.2 × 106 cells per plate in a 10 cm dish and cultured following standard tissue culture techniques until reaching 60–70% confluency. For assessments of overall protein synthesis and modulation of translation initiation, cells were pretreated and incubated at 37°C with 100 mg/ml CHX (Sigma, Catalogue No. C1988-1G) for 10 min prior to lysis. For formaldehyde cross-linking experiments, cells were cultured in 15 cm plates and pretreated with 0.1% formaldehyde (final concentration; Sigma, Catalogue No. F8775) for 5 min on ice. While maintaining the plates on ice, a final concentration of 75 mM glycine (VWR, Catalogue No. 76177-956) was added to the media and mixed by gently tilting the plates. The plates were incubated on ice for an additional 5 min prior to lysis. To harvest the cells in all conditions, tissue culture plates were placed on ice and the culture media was promptly aspirated. Cells were then rinsed with 5 ml of ice-cold 1X PBS without Ca+2 and MgCl2 (PBS; Quality Biological, Catalogue No. 119-069-131). PBS was aspirated, and the plates were tilted on the side of the ice bucket to allow pooling of the remaining liquid for aspiration. Following aspiration, the plates were placed flat on ice and 300 µl of ice-cold lysis buffer was added to each plate. Lysis buffer composition to assess the different phases of protein synthesis is included below.
Standard polysome profiling lysis buffer for assessments of overall protein synthesis and translation initiation
A total of 20 mM Tris-HCl (Sigma, Catalogue No. T2319), 100 mM NaCl (Sigma, Catalogue No. S5150), 10 mM MgCl2 (Sigma, Catalogue No. M1028), 0.4% NP-40 (Sigma, Catalogue No. 492016), 50 µg/ml CHX, and 1 cOmplete EDTA-free protease inhibitor cocktail tablet (Sigma, Catalogue No. 11873580001).
Ribosome biogenesis lysis buffer
A total of 20 mM Tris-HCl, 100 mM NaCl, 0.4% NP-40, EDTA (EDTA, Sigma, Catalogue No. E7889), and 1 cOmplete EDTA-free protease inhibitor cocktail tablet.
Translation elongation lysis buffer
A total of 20 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, 0.4% NP-40, and 1 cOmplete EDTA-free protease inhibitor cocktail tablet.
Formaldehyde cross-linking lysis buffer
A total of 20 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, 0.4% NP-40, and 1 cOmplete EDTA-free protease inhibitor cocktail tablet.
Cells were detached with a cell scrapper, and cell lysates were collected and transferred to a microcentrifuge tube on ice. The lysate was then passed through a 1 ml syringe with 26-gauge needle 10 times. Following a 10-min incubation on ice, the lysates were clarified by centrifugation for 10 min at 13,000 rpm and 4°C. The supernatant was recovered and placed in a new microcentrifuge tube on ice. The samples were measured at an absorbance wavelength of A260 and lysate concentrations were equilibrated in a total volume of 350 µl in a new microcentrifuge tube. In most cases, samples were normalized to 20 A260 units in 350 µl final buffer volume. In RPS19 and RPL35A KD experiments, samples were normalized to 15 A260 units in 350 µl final buffer volume. Fresh lysates were immediately subjected to the sucrose gradient ultracentrifugation protocol described below.
Polysome profiling: Sucrose gradient formation
A total of 10 and 50% sucrose solutions were prepared the day of cell lysis with either 5 or 25 g ultrapure sucrose (Thermo Fisher Scientific, Catalogue No. 15503-022), 20 mM Tris-HCl pH 7.5 (Sigma, Catalogue No. T2319), 100 mM NaCl (Sigma, Catalogue No. S5150), and 10 mM MgCl2 (Sigma, Catalogue No. M1028), and then sterile filtered. For ribosome biogenesis experiments, MgCl2 was left out of the sucrose solutions and 0.5 M EDTA (Sigma, Catalogue No. E7889) was added instead. For overall protein synthesis and translation initiation experiments, 100 µg/ml CHX (Sigma, Catalogue No. C1988-1G) was also added to each solution. The 10 and 50% sucrose solutions were then layered in an open top polyclear centrifuge tube (14 × 89 mm; Seton Part No. 7030) following the Biocomp Gradient Master protocol. A 10–50% gradient was generated using the 14 steps preprogram gradient settings on the Biocomp Gradient Master. Prepared gradients were stored at 4°C until use (i.e., ideally within 2 h of ultracentrifugation).
Polysome profiling: Sucrose gradient ultracentrifugation, fractionation, and polysome profile collection
First, 150 µl was removed from the top of freshly prepared 10–50% sucrose gradients. A total of 300 µl of freshly prepared cell lysate (see “polysome profiling: Cell lysis” for preparation of lysate) was then gently layered on top of each polyclear centrifuge tube without disturbing the sucrose gradient. Samples were centrifuged in a Beckman Coulter Optima L-90K Ultracentrifuge using a SW41 Ti rotor at 40,000 rpm for 2 h at 4°C. Following centrifugation, all sample tubes were stored at 4°C, while individual polysome profiles were obtained. A Biocomp Gradient Fractionator and Triax flow cell were used to collected polysome profile traces, following the manufacturer's instructions. To monitor the distribution of specific mRNAs in the sucrose gradient, the Biocomp Instrument outfitted with a Gilson Fractionator was used to collect 15 fractions from the top to the bottom of the 10–50% sucrose gradients. Collected sucrose gradient fractions were stored at −80°C until further processing as described below.
RNA isolation from whole cell lysates
RNA was isolated following the manufacturer's protocol from the RNAeasy Mini Kit (Qiagen, Catalogue No. 74104). In brief, cells were plated at a seeding density of 2.2 × 106 in a 10-cm plate and cultured following standard tissue culture techniques until reaching 60–70% confluency. Cells were washed with 5 ml of cold PBS, and all liquid was aspirated prior to cell lysis with 600 µl of Buffer RTL. Lysates were mixed with 600 µl of 70% ethanol and samples were further processed following the manufacturer's guidelines. RNA was eluted with 30 µl of molecular biology grade water and stored at −80°C.
RNA isolation from polysome fractions
RNA was isolated from polysome fractions using the TRIzolTM LS reagent (Invitrogen, Catalogue No. 10296010) and associated protocol (Doc. Part No. 10296010.PPS). In brief, 0.25 ml from each polysome fraction was transferred to a new microcentrifuge tube and 12 ng/ml (final concentration) of Firefly luciferase control RNA (Promega, Catalogue No. E1960) was spiked into each sample for normalization during qPCR. A total of 750 µl of TRIzol LS reagent was added and mixed five times using a micropipette to homogenize. The samples were then centrifuged for 5 m at 12,000 × g and 4°C, followed by a 5-min room temperature incubation. A total of 0.2 ml of chloroform was added and the samples were mixed well by inverting the tubes. Samples were incubated for 3 min at room temperature and centrifuged for 15 min at 12,000 × g and 4°C. A total of 350 µl of the clear, aqueous phase was transferred to a new tube containing 1 µl of GlycoBlue Coprecipitant (Catalogue No. AM9515). A total of 0.5 ml of isopropanol was added to each tube and samples were mixed by inversion. Samples were incubated at room temperature for 10 min and centrifuged for 10 min at 12,000 × g and 4°C. The supernatant was discarded and the RNA pellet was washed with 1 ml of 75% ethanol followed by centrifugation for 5 min at 7500 × g and 4°C. After discarding the supernatant, samples were centrifuged for 10 s to collect any remaining liquid and a P10 was used to remove as much liquid as possible without disturbing the RNA pellet. The pellet was air dried at room temperature for 5 min and solubilized in 10 µl of molecular biology grade water prior to storage at −80°C.
Reverse transcription
RNA concentrations were measured using a NanoDrop 2000 (Thermo Fisher Scientific, Catalogue No. ND2000USCAN) and diluted to 25 ng/µl with molecular grade water. A total of 3 µl of RNA was used in SuperScript III First Strand Synthesis Supermix (Thermo Fisher Scientific, Catalogue No. 18080400) reactions as per manufacturer's guidelines. For the annealing step, 2 µl of annealing master mix (random hexamers and annealing buffer) was added to each PCR tube, followed by a short vortex and brief centrifugation. The annealing reaction was incubated at 65°C for 5 min using a thermal cycler (Analytics Jena, Catalogue No. 846-2-070-724). The PCR tubes were transferred to ice and 12 µl of the enzyme master mix (2x First-Strand Reaction Mix and SuperScript III Enzyme) was added to each tube. Reverse transcription was carried out in a thermal cycler with the following program: 25°C for 10 min, 50°C for 50 min, 85°C for 5 min, followed by a 4°C hold. Samples were stored at −20°C.
mRNA measurement by qPCR
qPCR was performed following the manufacturer's protocol from the PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Catalogue No. A25742). MicroAmp 96-well reaction plates (Thermo Fisher Scientific, Catalogue No. 4306737) were used on a QuantStudio 7 Pro real-time PCR system for all experiments. Primers for qPCR analyses include:
RPS19 forward: ACTTCAGCCGAGGCTCCAAGAG,
RPS19 reverse: CCAGATCTCTTTGTCCCTGAGGTGTCAG,
RPL35A forward: GAACAACACAGTCACTCCTGGCG,
RPL35A reverse: CTGTGTCCAATGGCCTTAGCAGG,
ACTB forward: CACCATTGGCAATGAGCGGTTCC,
ACTB reverse: AGGTCTTTGCGGATGTCCACGT,
ATF4 forward: CTGAAGGAGATAGGAAGCCAGACTACACTG,
ATF4 reverse: CTCTGGGCTCATACAGATGCCACTATC,
Firefly forward: GTGTTGGGCGCGTTATTTATCGGAG,
Firefly reverse: CACTACGGTAGGCTGCGAAATGTTCATAC.
The ∆∆CT method was used in fold-change calculations for all experiments. ACTB was used as a normalization control for RPS19 and RPL35A knockdown experiments, and Firefly control RNA was used as a normalization control in polysome profiling experiments.
Immunoblot assays
Cells were seeded at 2.2 × 106 cells per 10 cm plate and maintained until reaching 60–70% confluency. To harvest the cells, tissue culture plates were placed on ice and the culture media was promptly aspirated. Cells were rinsed with 5 ml of ice-cold 1X PBS without Ca+2 and MgCl2 (PBS; Quality Biological, Catalogue No. 119-069-131). PBS was then aspirated off, and the plates were tilted on the side of the ice bucket to allow pooling of the remaining liquid for aspiration. A total of 25 µl of RIPA buffer (1.5 ml 150 mM NaCl, 500 µl of 5 mM EDTA, 2.5 ml of 50 mM Tris-HCl, 500 µl 1% Triton X-100 [Sigma 93443], 50 µl of 0.5% sodium deoxycholate, 0.1% SDS [Sigma L3771], and 1 cOmplete EDTA-free protease inhibitor cocktail tablet) was added to each plate. Cells were detached with a cell scrapper and transferred to a microcentrifuge tube. Lysates were sonicated for a total of 30 s with pulsar intervals of 5 s on and 5 s off, followed by centrifugation for 10 min at 13,000 rpm and 4°C. The supernatant was transferred to a new microcentrifuge tube and placed on ice. Protein concentrations were measured using 1X Protein Assay Dye Reagent (Bio-Rad, Catalogue No. 5000006) following the Bio-Rad Protein Assay Instruction Manual, with some modifications. Eight dilutions of 0, 0.5, 1, 2, 4, 8, 16, and 32 µg BSA (Sigma, Catalogue No. A3059) protein standards were prepared. A total of 1 ml of 1X Protein Assay Dye Reagent was added to the protein standards or 1 µl of each cell lysate, vortexed, and incubated at room temperature for 10 min with shaking. After incubation, standard and sample absorbances were measure at 595 nm and sample protein concentrations were calculated. Sample concentrations were equilibrated to 15 µg total protein in a 30 µl volume. SDS–PAGE was performed using the Criterion Cell and Blotter Systems (Bio-Rad, Catalogue No. 1704071) and Criterion TGX Stain-Free gels (Bio-Rad, Catalogue No. 5578034). Proteins were transferred to a 0.45-µm nitrocellulose membrane (Bio-Rad, Catalogue No. 1620115) and the membrane was blocked with 5% skim milk in 1X TBS (KD Medical, Catalogue No. RGF-3388) with 0.1% Tween 20 (Promega, Catalogue No. H5152) for 1 h. Membranes were incubated at 4°C with rocking overnight in primary antibody diluted in TBST (i.e., anti-HA [1:1000, Gene Tex, Catalogue No. GTX115044], eEF2-P [1:1000, Cell Signaling Technology, Catalogue No. 2331S], eEF2 [1:1000, Bethyl, Catalogue No. A301-688A] and β-Actin [1:20,000, Invitrogen, Catalogue No. BA3R]). Membranes were developed using rabbit and mouse secondary antibodies (1:5000) and 5 ml ECL reagent (Cytiva, Catalogue No. RPM2236).
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (S.D.) derived from three biological replicates. Statistical significance was calculated using a two-tailed Student's t test with a p value threshold of p = 0.05. Statistically significant differences are indicated by an asterisk (*).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Biochemistry and Molecular Biology Department at Uniformed Services University for helpful comments on the data presented in this manuscript. The authors would also like to thank the anonymous reviewers for their valuable feedback, which improved the quality of this Methods and Resources manuscript. This work was supported by the National Institutes of Health (NIH/NICHD) grant R00 HD099520 and Department of Defense grants OSD(HA).2022ICD.WBH-1 and R.0036258.4.171 to S.K.Y.-B. The opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, the Department of Defense, or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Abbreviations used:
- CHX
cycloheximide
- cryo-EM
cryogenic electron microscopy
- DBA
Diamond-Blackfan Anemia
- eEF2
eukaryotic elongation factor 2
- eEF2K
eukaryotic elongation factor 2 kinase
- eEF2-P
eukaryotic elongation factor 2 phosphorylation
- eIF2
eukaryotic initiation factor 2
- eIF2α-P
eukaryotic initiation factor 2 α phosphorylation
- CH2O
formaldehyde
- ISR
integrated stress response
- KD
knockdown
- Mg2+
magnesium
- NT
no treatment
- ORF
open reading frame
- P/M
polysome/monosome
- TG
thapsigargin.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E24-08-0341) on March 5, 2025.
REFERENCES
- Belhabich-Baumas K, Joret C, Jady BE, Plisson-Chastang C, Shayan R, Klopp C, Henras AK, Henry Y, Mougin A (2017). The Rio1p ATPase hinders premature entry into translation of late pre-40S pre-ribosomal particles. Nucleic Acids Res 45, 10824–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnik EM, Chung DW, Hamilton M, Ponts N, Saraf A, Prudhomme J, Florens L, Le Roch KG (2013). Polysome profiling reveals translational control of gene expression in the human malaria parasite Plasmodium falciparum. Genome Biol 14, R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O (1990). Functional properties of phosphorylated elongation factor 2. Eur J Biochem 191, 639–645. [DOI] [PubMed] [Google Scholar]
- Chasse H, Boulben S, Costache V, Cormier P, Morales J (2017). Analysis of translation using polysome profiling. Nucleic Acids Res 45, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Walter P (2020). The integrated stress response: From mechanism to disease. Science 368, eaat5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Bella E, Bersano E, Antonini G, Borghero G, Capasso M, Caponnetto C, Chio A, Corbo M, Filosto M, Giannini F, et al. (2021). The unfolded protein response in amyotrophic later sclerosis: Results of a phase 2 trial. Brain 144, 2635–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Dinman JD, Green R (2018). Translation elongation and recoding in eukaryotes. Cold Spring Harb Perspect Biol 10, a032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrbaum AR, Kochen L, Langer JD, Schuman EM (2018). Local and global influences on protein turnover in neurons and glia. Elife 7, e34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RT, Fan X, Young DJ, Liang J, Singh K, Pakbaz Z, Desmond R, Young-Baird SK, Chandrasekharappa SC, Donovan FX, et al. (2022). Studies of a mosaic patient with DBA and chimeric mice reveal erythroid cell-extrinsic contributions to erythropoiesis. Blood 139, 3439–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (2018). A study to explore the safety, tolerability, pharmacokinetic profile, and potential efficacy of Guanabenz in patients with early childhood onset Vanishing White Matter. EU Clinical Trials Register.
- Farley-Barnes KI, Ogawa LM, Baserga SJ (2019). Ribosomopathies: Old concepts, new controversies. Trends Genet 35, 754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye MD, Graber TE, Holcik M (2014). Assessment of selective mRNA translation in mammalian cells by polysome profiling. J Vis Exp e52295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Cerca S, Kiburu I, Thomson E, LaRonde N, Hurt E (2014). Dominant Rio1 kinase/ATPase catalytic mutant induces trapping of late pre-40S biogenesis factors in 80S-like ribosomes. Nucleic Acids Res 42, 8635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin V, Sikstrom K, Alain T, Morita M, McLaughlan S, Larsson O, Topisirovic I (2014). Polysome fractionation and analysis of mammalian translatomes on a genome-wide scale. J Vis Exp 51455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW (2004). Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5, 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalei H, Trepreau J, Collins JC, Bhaskaran H, Strunk BS, Karbstein K (2017). The ATPase Fap7 tests the ability to carry out translocation-like conformational changes and releases Dim1 during 40S ribosome maturation. Mol Cell 68, 1155. [DOI] [PubMed] [Google Scholar]
- Han C, Sun L, Pan Q, Sun Y, Wang W, Chen Y (2022). Polysome profiling followed by quantitative PCR for identifying potential micropeptide encoding long non-coding RNAs in suspension cell lines. STAR Protoc 3, 101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11, 619–633. [DOI] [PubMed] [Google Scholar]
- Harvey RF, Smith TS, Mulroney T, Queiroz RML, Pizzinga M, Dezi V, Villenueva E, Ramakrishna M, Lilley KS, Willis AE (2018). Trans-acting translational regulatory RNA binding proteins. Wiley Interdiscip Rev RNA 9, e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SL, Green R (2013). Polysome analysis of mammalian cells. Methods Enzymol 530, 183–192. [DOI] [PubMed] [Google Scholar]
- Hellen CUT (2018). Translation termination and ribosome recycling in eukaryotes. Cold Spring Harb Perspect Biol 10, a032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser TL, Baan RA, Dahlberg AE (1981). Characterization of a 40S ribosomal subunit complex in polyribosomes of Saccharomyces cerevisiae treated with cycloheximide. Mol Cell Biol 1, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JW, Sonenberg N, Mathews MB (2012). Principles of translational control: An overview. Cold Spring Harb Perspect Biol 4, a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Lorsch JR (2012). The mechanism of eukaryotic translation initiation: New insights and challenges. Cold Spring Harb Perspect Biol 4, a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MJ, Misra J, Wek RC (2022). Analysis of translational control in the integrated stress response by polysome profiling. Methods Mol Biol 2428, 157–171. [DOI] [PubMed] [Google Scholar]
- Hu W, Coller J (2013). Polysome analysis for determining mRNA and ribosome association in Saccharomyces cerevisiae. Methods Enzymol 530, 193–206. [DOI] [PubMed] [Google Scholar]
- Jin HY, Xiao C (2018). An integrated polysome profiling and ribosome profiling method to investigate in vivo translatome. Methods Mol Biol 1712, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S, Erickson SL, Yang G (2021). Analysis of translation in the developing mouse brain using polysome profiling. J Vis Exp. [DOI] [PubMed] [Google Scholar]
- Kudla M, Karginov FV (2016). Measuring mRNA translation by polysome profiling. Methods Mol Biol 1421, 127–135. [DOI] [PubMed] [Google Scholar]
- Lecampion C, Floris M, Fantino JR, Robaglia C, Laloi C (2016). An easy method for plant polysome profiling. J Vis Exp 54231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Proud CG (2016). Eukaryotic elongation factor 2 kinase as a drug target in cancer, and in cardiovascular and neurodegenerative diseases. Acta Pharmacol Sin 37, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokdarshi A, Von Arnim AG. (2023). A miniature sucrose gradient for polysome profiling. Bio Protoc 13, e4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Library of Medicine (2023). An open-label exploratory study of ABBV-CLS-7262 subjects with vanishing white matter disease. ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2015). Oral guanabenz for multiple sclerosis. ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2021a). A phase 1 study to investigate the safety and pharmacokinetics of ABBV-CLS-7262 in patients with amyotrophic lateral sclerosis. ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2021b). A study of HC-5404-FU to establish the maximum tolerated dose (MTD). ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2022a). A study of HC-7366 to establish the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D). ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2021c). A study to determine the safety, pharmacokinetics, and pharmacodynamics of DNL343 in participants with amyotrophic lateral sclerosis. ClinicalTrials.gov. [Google Scholar]
- National Library of Medicine (2022b). Treatment Combining Riluzole and IFB-088 in Bulbar Amyotrophic Lateral Sclerosis (TRIALS Protocol). ClinicalTrials.gov. [Google Scholar]
- Nielsen KH, Szamecz B, Valasek L, Jivotovskaya A, Shin BS, Hinnebusch AG (2004). Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J 23, 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MW, Mao Y, Storz G, Qian SB (2020). Alternative ORFs and small ORFs: Shedding light on the dark proteome. Nucleic Acids Res 48, 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira IT, Spangenberg L, Robert AW, Amorin R, Stimamiglio MA, Naya H, Dallagiovanna B (2018). Polysome profiling followed by RNA-seq of cardiac differentiation stages in hESCs. Sci Data 5, 180287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle ES, McCormick C, Cheng Z (2019). Polysome profiling analysis of mRNA and associated proteins engaged in translation. Curr Protoc Mol Biol 125, e79. [DOI] [PubMed] [Google Scholar]
- Proud CG (2019). Phosphorylation and signal transduction pathways in translational control. Cold Spring Harb Perspect Biol 11, a033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg MO, Moritz M, Woolford JL. (1988). Depletion of Saccharomyces cerevisiae ribosomal protein L16 causes a decrease in 60S ribosomal subunits and formation of half-mer polyribosomes. Genes Dev 2, 160–172. [DOI] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P (2009). SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6, 275–277. [DOI] [PubMed] [Google Scholar]
- Seimetz J, Arif W, Bangru S, Hernaez M, Kalsotra A (2019). Cell-type specific polysome profiling from mammalian tissues. Methods 155, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG (2009). Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 136, 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L, Guo CJ, Chen LL, Huarte M (2021). Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 22, 96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Novak MN, Young CL, Karbstein K (2012). A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 150, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S, Khoutorsky A, Mathews MB, Sonenberg N (2018). Translation deregulation in human disease. Nat Rev Mol Cell Biol 19, 791–807. [DOI] [PubMed] [Google Scholar]
- Teske BF, Baird TD, Wek RC (2011). Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol 490, 333–356. [DOI] [PubMed] [Google Scholar]
- Turnbull K, Paternoga H, von der Weth E, Egorov AA, Pochopien AA, Zhang Y, Nersisyan L, Margus T, Johansson MJO, Pelechano V, et al. (2024). The ABCF ATPase New1 resolves translation termination defects associated with specific tRNAArg and tRNALys isoacceptors in the P site. Nucleic Acids Res 52, 12005–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasek L, Szamecz B, Hinnebusch AG, Nielsen KH (2007). In vivo stabilization of preinitiation complexes by formaldehyde cross-linking. Methods Enzymol 429, 163–183. [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006). Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20, 515–524. [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC (2004). Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 101, 11269–11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Woolford JL, Baserga SJ (2013). Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195, 643–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Baird SK, Lourenco MB, Elder MK, Klann E, Liebau S, Dever TE (2020). Suppression of MEHMO syndrome mutation in eIF2 by small molecule ISRIB. Mol Cell 77, 875–886.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DJ, Makeeva DS, Zhang F, Anisimova AS, Stolboushkina EA, Ghobakhlou F, Shatsky IN, Dmitriev SE, Hinnebusch AG, Guydosh NR (2018). Tma64/eIF2D, Tma20/MCT-1, and Tma22/DENR recycle post-termination 40S subunits in vivo. Mol Cell 71, 761–774.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Wek RC (2016). Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J Biol Chem 291, 16927–16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.