Abstract
Work in many systems has shown large-scale changes in gene expression during aging. However, many studies employ just 2 arbitrarily chosen timepoints to measure expression and can only observe an increase or a decrease in expression between “young” and “old” animals, failing to capture any dynamic, nonlinear changes that occur throughout the aging process. We used RNA sequencing to measure expression in male head tissue at 15 timepoints through the lifespan of an inbred Drosophila melanogaster strain. We detected >6,000 significant, age-related genes, nearly all of which have been seen in previous Drosophila aging expression studies and that include several known to harbor lifespan-altering mutations. We grouped our gene set into 28 clusters via their temporal expression change, observing a diversity of trajectories; some clusters show a linear change over time, while others show more complex, nonlinear patterns. Notably, reanalysis of our dataset comparing the earliest and latest timepoints—mimicking a 2-timepoint design—revealed fewer differentially expressed genes (around 4,500). Additionally, those genes exhibiting complex expression trajectories in our multitimepoint analysis were most impacted in this reanalysis; their identification, and the inferred change in gene expression with age, was often dependent on the timepoints chosen. Informed by our trajectory-based clusters, we executed a series of gene enrichment analyses, identifying enriched functions/pathways in all clusters, including the commonly seen increase in stress- and immune-related gene expression with age. Finally, we developed a pair of accessible Shiny apps to enable exploration of our differential expression and gene enrichment results.
Keywords: gene expression, lifespan, aging, gene ontology enrichment, FlyBase
Introduction
Aging is marked by both a decline in organismal function and an increased risk for disease. As complex traits, both environmental and genetic factors contribute to variation in lifespan and health at old age. Identifying these factors, and their impact on lifespan and healthspan, enables understanding of the negative effects of aging, which is increasingly important as populations throughout the world age. In humans, the estimated heritability of longevity is 7–30% (Mayer 1991; Herskind et al. 1996; Kaplanis et al. 2018; Ruby et al. 2018), and genome-wide association studies (GWASs) have been used to study the genetic basis of aging (Newman et al. 2010; Deelen et al. 2011, 2014; Sebastiani et al. 2012, 2017; Broer et al. 2015; Joshi et al. 2016, 2017; Pilling et al. 2016, 2017; Zeng et al. 2016; Timmers et al. 2019; Melzer et al. 2020). GWAS often rely on examining sets of individuals who are >90 years old (Newman et al. 2010; Deelen et al. 2011, 2014; Sebastiani et al. 2012, 2017; Zeng et al. 2016), but this can constrain the sample size and power of such studies (Tan et al. 2008; Newman and Murabito 2013). Creative approaches have enabled markedly increased sample size—for instance, Joshi et al. (2016) associate offspring genotype with parental lifespan phenotype—yet human lifespan GWAS have only discovered a handful of replicable associations, for instance near APOE, FOXO3, and CHRNA3/5 (Deelen et al. 2011; Broer et al. 2015; Joshi et al. 2016, 2017; Pilling et al. 2016; Timmers et al. 2019).
Outside of a deficit of power in genetic studies, studying aging directly in humans can be difficult due to uncontrollable environmental variables, many of which can impact lifespan. For example, calorie restriction has been shown to increase lifespan and health in model organisms (McCay et al. 1939; Pletcher et al. 2002; Cypser et al. 2013; Mattison et al. 2017), and in humans a 2-year calorie restriction study showed a reduction in cardiometabolic risk factors including cholesterol and blood pressure (Kraus et al. 2019). However, effectively studying the impact of diet on lifespan in humans is challenging due to the difficulty accurately measuring calorie intake, the lack of adherence by participants, and several other concerns (Hu 2024). Ideally, studies investigating the genetic and molecular contributions to aging would limit all other sources of variation, something that is not feasible or practical in human studies.
Model organisms have proven useful conduits to study aging due to their shorter lifespans, ease of testing many individuals, and the ability to control both environmental and genetic variation. Both genetic and environmental contributors to aging have been successfully identified in vertebrate model systems (McCay et al. 1939; Gocmez et al. 2020; Cai et al. 2022), in invertebrates such as Drosophila melanogaster and Caenorhabditis elegans (Pletcher et al. 2002; McCarroll et al. 2004; Kenyon 2010; Highfill et al. 2017), and in the fungi Saccharomyces cerevisiae and Podospora anserina (Philipp et al. 2013; Hu et al. 2014). These models have helped identify and understand lifespan-associated genes and systems that have relevance for human populations. For instance, FOXO3—a member of the forkhead box transcription factor O (FOXO) family—has been implicated in human aging via GWAS (Willcox et al. 2008; Deelen et al. 2014; Broer et al. 2015), and studies in C. elegans and D. melanogaster have also identified and characterized FOXO family genes that impact lifespan (Kimura et al. 1997; Lin et al. 1997; Giannakou et al. 2007; Alic et al. 2014; Taormina et al. 2019).
Cellular processes do not remain static over an individual's lifetime, and instead change, adapt, and sometimes break down over time. By measuring these changes, we can understand the cellular and physiological phenomena that are affected by aging. A common strategy to assess such age-related changes is with the use of gene expression analyses. These analyses often compare the genome-wide gene expression profile between “young” and “old” individuals, and identify genes that are differentially expressed with age. This approach has been successfully employed in multiple systems (Lu et al. 2004; Wilson et al. 2015; Bordet et al. 2021; Wang et al. 2022), and has resulted in expression-based hallmarks of aging (Frenk and Houseley 2018), including increased expression of stress response and immunity genes (Lu et al. 2004; Bajgiran et al. 2021; Bordet et al. 2021; Wang et al. 2022), and decreased expression of genes associated with mitochondria and the electron transport chain (ETC) (Lu et al. 2004; Bordet et al. 2021).
A challenge with 2-timepoint, young vs old comparisons is that there is little consistency in the definitions of “young” and “old”, making comparisons across studies difficult. For instance, various studies in D. melanogaster have used “young” flies sampled between 1 and 10 days old, and “old” flies that were 35 to 61 days old (Landis et al. 2004; Girardot et al. 2006; Carnes et al. 2015; Bajgiran et al. 2021; Bordet et al. 2021). While in some cases the “old” timepoint is determined by the survivorship of the aging cohort, rather than by chronological age (Landis et al. 2004; Doroszuk et al. 2012; Highfill et al. 2017). Additionally, 2-timepoint expression analyses are limited to making binary conclusions about expression change; expression either increases or decreases with age. Aging is a complex process, and this simplification may often fail to yield an accurate picture of age-related expression for most genes. Indeed, work in several systems assaying various molecular phenotypes at multiple timepoints throughout lifespan have identified many genes and gene products with nonlinear trajectories (Lund et al. 2002; Pletcher et al. 2002; Remondini et al. 2010; Gheorghe et al. 2014; Haustead et al. 2016; Schaum et al. 2020; Xie et al. 2022; Olecka et al. 2024; Shen et al. 2024 Aug 14).
Here we set out to robustly measure expression trajectories throughout the adult lifespan of an inbred D. melanogaster strain, focusing on head tissue from males. Flies were aged for 59 days, and sampled for RNAseq at 15 timepoints. We recorded the number of deaths in our aging cohort daily, allowing us to calculate a survival-based “physiological age” in addition to the “chronological age” for each time point, and endeavored to sample roughly evenly through both metrics. Subsequently, using multiple analyses we identified genes whose expression changed with aging, and clustered differentially expressed genes based on their expression trajectories. With the idea that genes with similar expression trajectories may share similar functional roles (e.g. Zhang et al. 2004), we did a series of enrichment analyses for each of our clusters, identifying numerous enriched pathways. To better understand the value of a multitimepoint expression study, we subsequently reanalyzed our data in a 2-timepoint, young vs old analysis framework. Finally, we compared the results of our work with a series of prior expression-based D. melanogaster aging studies.
Materials and methods
Fly rearing, maintenance, and aging
We employed a single inbred D. melanogaster strain, A4, which is one of the founders of the Drosophila Synthetic Population Resource (King et al. 2012; Chakraborty et al. 2018), a panel of recombinant strains enabling the genetic characterization of complex traits. Following several generations of expansion, we generated 200 replicate vials of the A4 strain, clearing adults to maintain roughly even egg/larval densities over vials. In the following generation we harvested 0–2-day-old A4 males over CO2 anesthesia, collecting 132 vials of 40 males (for a subsequent collection of aged animals for RNAseq) and 5 vials of 10 males (for a collection of 3-day-old flies for RNAseq). The aging cohort was maintained in vials, flies were tipped to new vials every 2–3 days without anesthesia, and periodically the entire cohort was anesthetized and rearrayed into groups of 40 animals in vials. Dead animals were counted daily. Supplementary Table 1 provides further description of how the aging cohort was treated. We elected to employ males for our experiment for practical reasons; flies can be transferred to fresh vials less often since media is not degraded by egg laying and larval development.
Flies were raised and maintained in standard narrow fly vials (Fisher Scientific, AS515) containing ∼10 mL of a cornmeal-yeast-molasses media (see Supplementary Text 1) and kept in an incubator under the following environmental conditions: 25°C, 50% relative humidity, and a 12:12 light:dark cycle.
Fly/tissue sampling
Multiple groups of flies were sampled from the population through the aging process, with animals for the first—day 3—timepoint coming from those vials initially holding 10 flies (see above). Flies were sampled without anesthesia via manual aspiration. Sampled flies were always given at least 24 h between CO2 anesthesia and sampling, and sampling always occurred 3–4.5 h after lights on. See Supplementary Table 1 for details on when flies were sampled. We note that since flies were initially collected over a 2-day window (see above), on day 3 of the experiment, flies are actually 1–3 days old (on day 17, flies are 15–17 days old and so on). To simplify presentation below, we refer to sampling points solely as the day of the experiment (which is equivalent to the maximum age of the flies sampled that day).
On collection, groups of 10 male flies were moved into screw-top tubes, flash frozen in liquid nitrogen, and kept at −80°C. Subsequently—after the entire cohort had died and flies from all timepoints had been sampled—tubes were removed from the freezer into aluminum dry bath blocks held on dry ice. We then went through the tubes one by one, subjected each to liquid nitrogen, briefly vortexed to separate heads/bodies, poured the body parts into the flat surface of an aluminum dry bath block placed on dry ice, and used a paint brush to manually collect heads into a fresh screw-top tube. These destination tubes were prefilled with 4–6 glass beads (BioSpec Products, 11079127) that had been previously washed in bleach and thoroughly rinsed with distilled water. We elected to focus on head tissue since heads can be removed easily in Drosophila without dissection, and heads are enriched for neurological cells, which are of particular interest in aging.
RNA isolation, sequencing library preparation, and sequencing
RNA was isolated using the Zymo Direct-zol MicroPrep kit (Zymo, R2062), largely following the manufacturer's protocol (see Supplementary Text 2). Samples were isolated over 6 batches, replicate samples from a given timepoint were isolated in different batches, and RNA quantity was measured using a NanoDrop ND-1000.
Forty-eight RNA samples were used to generate poly-A selected mRNA sequencing libraries; 14 timepoints had 3 replicates each, while 1 (day 59, the final timepoint) had 6 replicates. We used 200–500 ng of total RNA from these samples to initiate half-reaction volume mRNA sequencing library construction (Illumina TruSeq stranded HT kit using dual indexing), generating libraries across 2 batches of 24 samples each. Libraries were quantified using a Qubit fluorometer, and a subset of 8 libraries from each batch was run on an Agilent TapeStation. Each of these libraries showed a single library peak, no evidence of adapter dimers, and estimated average fragment sizes of 277–294 bp. See Supplementary Table 2 for details on RNA isolation/library preparation batching and quantification. Given the relatively uniform fragment sizes, equal quantities of all 48 libraries were pooled together. The final 48-plex pool had an average fragment size of 289 bp and was run over 2 Illumina NextSeq550 PE75 flow cells, yielding a total of over 635 million read pairs, with an average of 13.2 million per sample (range = 9.0–17.4 million).
Quantifying expression level and identifying expression changes during aging
Reads from each of the 48 samples were processed using Salmon (Patro et al. 2017), employing release BDGP6.32 of the D. melanogaster transcriptome/annotation from Ensembl. Salmon quantifications were then summarized to gene level using R/tximeta (Love et al. 2020). To identify genes whose expression significantly changed with aging (adjusted P-value < 0.05), we used R/DESeq2 (Love et al. 2014), executing 3 different analyses, in each case associating expression with a different variable. First, we identified genes associated with chronological age (the “Day analysis”) by treating the age of the flies in each RNAseq sample as a continuous variable. Second, we identified genes associated with physiological age (the “Survival analysis”) by using the fraction of dead animals in the entire cohort at the point flies were sampled for RNAseq as a continuous variable (Supplementary Table 3). Third, we identified genes showing expression variation through aging by considering the 15 sampling points as levels of a categorical variable (the “Sampling Point analysis”). This final analysis sought to identify genes with expression patterns not easily captured by the 2 continuous variables (Day and Survival).
Clustering genes by their expression trajectories
Our 3 differential expression analyses (see above) collectively identified 6,142 unique genes, and we sought to cluster these genes into groups based on their expression trajectories through aging. To focus on expression trajectories and avoid confounding with varying expression levels, we standardized the expression counts of each gene via Z-scores. Briefly, for each gene, we calculated the mean expression across replicates for each sampling point, along with the overall mean expression across all sampling points and replicates. We then subtracted the overall mean from the mean of each sampling point and divided by the overall standard deviation.
The calculated Z-scores for the 6,142 genes were used to create a dissimilarity matrix using the Pearson correlation method in R/factoextra (Kassambara and Mundt 2020). We used this dissimilarity matrix for hierarchical clustering of our identified genes and then “cut” the resulting dendrogram into a designated number of gene groups/clusters. This was done using the hclust and cutree functions from the R/stats package (R Core Team 2021).
There are a variety of ways to cut a gene dissimilarity matrix into clusters, and variation in the approach and parameters will yield different numbers and sizes of clusters. Our goal was to examine whether clusters of genes with similar expression trajectories were enriched for particular properties [e.g. gene ontology (GO) terms]. To facilitate this, we sought to avoid clusters with either very small or very large numbers of genes, so targeted clusters with between 50 and 500 genes. After exploring several methods, we grouped our 6,142 differentially expressed genes into 28 clusters (see Supplementary Table 4 and Fig. 2 for more information).
Summarizing and classifying cluster expression trajectories
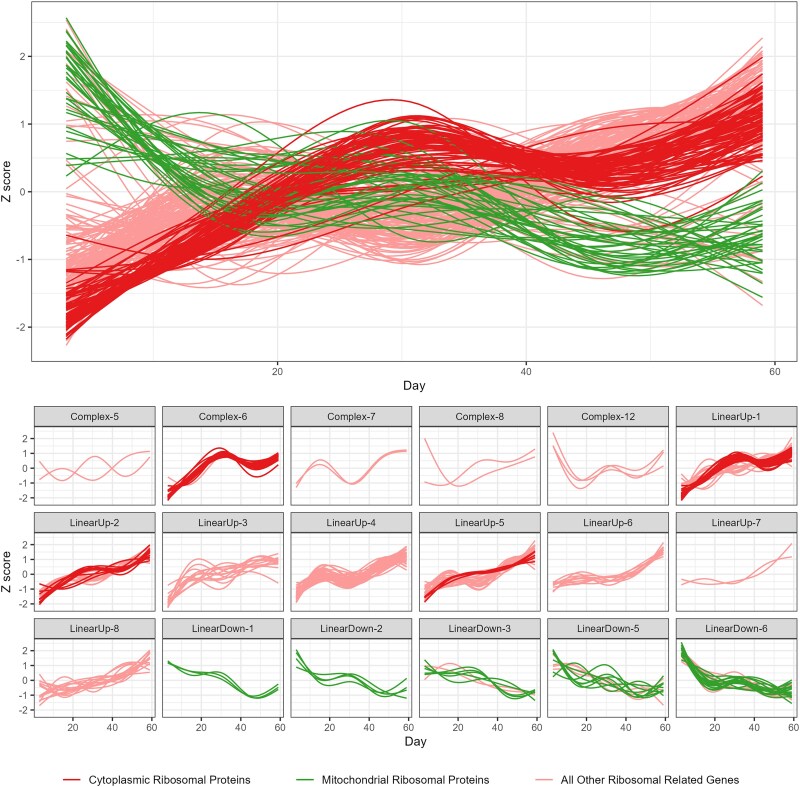
For each of the 28 clusters, we created a representative expression curve by smoothing the mean Z-score from all genes in the cluster for each sampling point (Fig. 4). The smoothing was executed using geom_smooth from R/ggplot2 (Wickham 2016) and spline modeling with rcs from R/rms (Harrell 2023). The resulting 28 curves show a diversity of trajectories, with some being generally linear, while others show a more complex pattern. To classify the cluster trajectories, we ran a linear regression between the mean Z-scores and the age of the sampled flies. A trajectory was designated as “Linear” if the P-value was <0.002 (0.05/28) or “Complex” otherwise. (Repeating this analysis using survival, or the numbered sampling points, SP1–SP15, yields the same designations.) Linear trajectories were further designated as “Up” (gene expression increases with aging) or “Down” (gene expression decreases with aging) based on the sign of the linear regression coefficient. To simplify subsequent discussion, clusters are named with these classifications (i.e. Complex, LinearUp, or LinearDown) and given with numeric codes based on the cluster position within the dendrogram (bottom to top in Fig. 3 and left to right in Supplementary Fig. 3). See Supplementary Table 5 for details on the trajectory-designating linear regression analyses.
Fig. 4.
Representative expression trajectories for all 28 clusters. Within a cluster, we calculated the average Z-score (y-axis) over genes for each timepoint and present a smoothed curve through those points highlighting the cluster-specific changes in gene expression over time in days (x-axis). We determined whether each cluster-specific set of mean Z-scores was statistically associated with age and used this information to designate each cluster as LinearUp or LinearDown (a significant association and expression either increases or decreases over time) or as Complex (there was no significant association between expression and age). This led to 14 Complex, 8 LinearUp, and 6 LinearDown clusters.
Fig. 3.
Clustering all 6,142 age-related genes into 28 clusters via their expression trajectories through lifespan. A simplified dendrogram representing the hierarchical clustering of our gene expression trajectory data. Each horizontal colored line represents all those genes in each of our 28 clusters; the closer clusters are to each other on the plot, the more similar their expression trajectories (see Fig. 4). Each cluster is named based on their expression trajectory (LinearUp, LinearDown, and Complex). Complex-1 to Complex-5 clusters are not individually labeled since they are very close together in the plot. Supplementary Fig. 3 shows the full dendrogram highlighting the relationships among all 6,142 genes.
Cluster-specific enrichment analyses
To understand whether genes with similar expression trajectories share similar properties/functions, we used PANGEA (Version 1.1 beta December 2022) (Hu et al. 2023), an online gene set enrichment tool that can perform GO analysis, identify enrichment of particular gene groups or pathways, and—importantly for our needs—can execute analyses on multiple gene lists simultaneously. For each cluster, we ran 6 separate enrichment analyses, examining 3 Drosophila GO subsets (SLIM2 GO BP—biological process, SLIM2 GO CC—cellular component, and SLIM2 GO MF—molecular function), 2 collections of gene groups (DRSC GLAD and FlyBase), and the REACTOME pathway set. For each analysis, we used a custom background set of 13,303 genes that included only those with at least one mapped read in our dataset. We identified terms significantly enriched in each cluster using a Benjamini–Hochberg false discovery rate of 0.05. Each of the PANGEA tables is available as Supplementary Tables 6–11, and our enrichment analysis code is available at https://github.com/Hanson19/RNAseq-Aging.
Comparing trajectory-based, multitimepoint analysis results to analyze contrasting groups of young and old animals
Our design differs from some previous examinations of age-related expression in that we generated expression data from many points through the aging process. To examine what might be gained from our approach, we reanalyzed our data after dropping the bulk of the timepoints. The samples from day 3 and day 6 (n = 6) collectively made up our “young” sample, while our “old” sample came from the last collection day, day 59 (n = 6). Using R/DESeq2 (Love et al. 2014), we identified genes whose expression changed significantly between these age groups and determined if gene expression increased or decreased over time. We subsequently repeated this analysis, comparing the days 3 + 6 “young” timepoint against every sequential pair of older timepoints (e.g. we compared days 3 + 6 with days 10 + 14, days 3 + 6 with days 14 + 17, and so on).
Comparison with previous Drosophila aging genome-wide expression studies
We compared our set of 6,142 multitimepoint significant genes with those identified in 8 previously published 2-timepoint aging expression studies (Supplementary Table 13). These papers vary in the strains/populations employed, the sex of the animals targeted, the tissue that was employed, and the actual timepoints during aging that were sampled. We validated all gene IDs using FlyBase (FB2024_01) (Jenkins et al. 2022), identified genes shared between our study and these previous works, and compared the expression change reported in the previous studies (up or down in expression with age) with the expression trajectories that these genes were grouped into in our study (LinearUp, LinearDown, and Complex; Supplementary Fig. 11).
Shiny apps to enable data exploration
Our analyses generated a considerable amount of data, and to make our results more accessible, we developed 2 interactive apps using R/shiny (version 1.8.0) (Chang et al. 2023). The Gene and Cluster app allows users to look up specific genes, receive information about whether the gene was identified in our analysis, and if so in which cluster it was found, and what its expression trajectory is over time. The Cluster Enrichment app allows users to select specific clusters, or sets of clusters, and identify any enriched terms. For more information on how to run and use these apps, see Supplementary Text 3 and 4.
Results and discussion
Over 6,000 genes show expression change during aging
We aged a cohort of 5,330 D. melanogaster males from a single inbred strain, recorded the number of fly deaths each day, and collected several replicates of 10 flies at 15 timepoints throughout the adult lifespan. Samples were collected every ∼4 days between day 3 (99.9% flies alive) and day 59 (1.92% alive) to roughly evenly sample flies throughout lifespan (see Kaplan–Meier survivorship curve in Fig. 1 and details on the timing of the sampling in Supplementary Table 3). RNA was isolated from the heads of collected flies, converted into RNAseq libraries and sequenced, and subsequently reads were assembled to the transcriptome to quantify gene expression.
Fig. 1.
Kaplan–Meier survivorship curve for over 5,000 males from the inbred A4 strain. Each point represents 1 day of the experiment, with black points denoting days when only the number of dead flies were counted, and red points denoting those days when flies were also sampled for subsequent RNA isolation (SP1–SP15).
Three separate analyses were used to identify genes with significant expression changes through aging. For each of 2 analyses, we associate gene expression with a different continuous variable, either the chronological age of the flies on the day samples were collected (Day analysis) or the survivorship of the aging cohort upon sampling (Survival analysis). In the 3rd analysis, we associated expression with a single categorical variable with 15 levels (SP1–SP15) that reflect the sampling points when flies were collected (Sampling Point analysis). This 3rd analysis has the potential to identify genes missed by the pair of continuous variable analyses, since expression variation of some genes may not follow a simple, continuous temporal pattern.
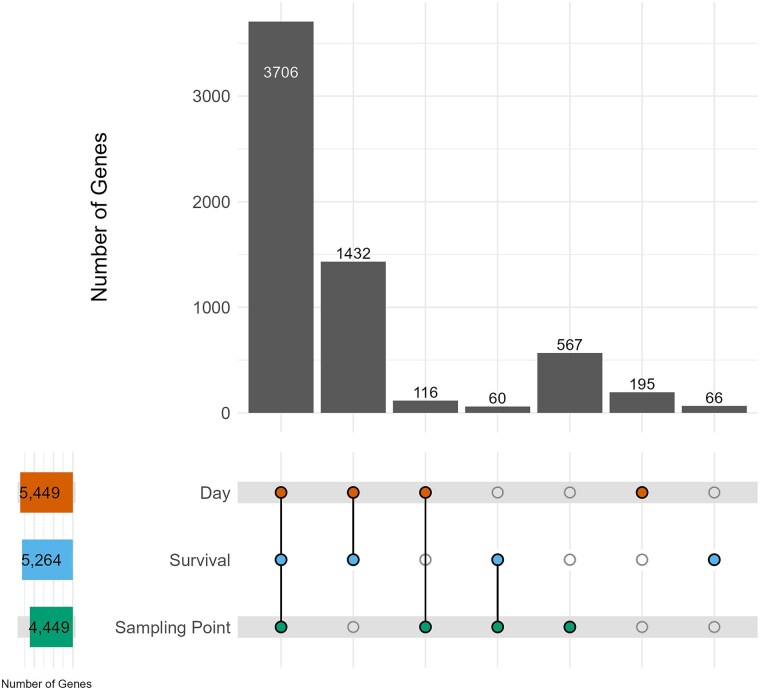
The 3 analyses collectively identified 6,142 unique genes that were differentially expressed through lifespan (Fig. 2), representing a little under half of the genes with detectable expression in our study (n = 13,303). The analyses individually identified 5,449 (Day), 5,264 (Survival), and 4,449 (Sampling Point) genes. Around 60% of the genes (3,706) were identified by all 3 analyses, and an additional 28% (1,432) were identified by both continuous variable analyses, Day and Survival. The large overlap of genes identified in both the Day and Survival analyses—well over 90% of the genes identified in each analysis are shared among the 2—is expected since age in days and survivorship are strongly correlated (r = 0.98, P < 10−9; Supplementary Fig. 1). The Sampling Point analysis identified the most genes unique to one analysis—567 (9% of the total number of unique genes identified)—demonstrating its utility in capturing genes not easily found by either continuous variable analysis.
Fig. 2.
Identification of 6,142 genes with age-related gene expression changes. We identified genes whose expression was significantly associated with Day of life (n = 5,449), with Survival (n = 5,264), and with Sampling Point (n = 4,449). The upper bar chart shows the number of significant genes identified, with colored circles below showing which analysis the genes were identified in (3,706 genes were identified in all 3 analyses, 1,432 genes were identified in both the Day and Survival analyses, and so on).
A diversity of gene expression trajectories through aging
Genes with similar functions, or that function in the same pathways, might be expected to share similar expression trends over time (e.g. Eisen et al. 1998; Zhang et al. 2004). To enable investigation of this, we normalized the expression trajectories of all 6,142 identified genes via Z-scores and clustered them into 28 groups (Fig. 3; Supplementary Fig. 3). Based on the results of linear regressions of cluster-specific expression patterns (Fig. 4) against time in Days, each cluster was assigned a trajectory designation of LinearUp or LinearDown (the mean cluster expression is significantly associated with time and either goes up or down over time, respectively) or Complex (there is no significant association with time after correcting for multiple testing). See the Materials and methods section for further details on this process and Supplementary Table 5 for statistical information. Over 80% of genes fall into the 6 LinearDown (2,596) and 8 LinearUp (2,536) clusters, with the remaining 1,010 genes split among 14 Complex clusters (Fig. 4). In general, the Complex clusters harbor considerably fewer genes than the more linear clusters.
Clearly, the LinearDown and LinearUp clusters do not show perfect linear patterns of expression; for instance, genes in LinearDown-1 (Fig. 4) show a slight increase in expression in midlife, before decreasing in expression toward late life. However, the curves for the linear clusters typically appear more linear than those of the Complex clusters (compare LinearUp-8 with Complex-1) and primarily show monotonic increases/decreases in expression over time (see LinearUp-7). Furthermore, among the Complex class of clusters, we see a great deal of variation in expression trajectory; many are decidedly nonlinear and show wave-like patterns (e.g. Complex-5) or are curved with the highest expression in midlife (e.g. Complex-10). However, some Complex cluster are somewhat linear and exhibit patterns not dissimilar to those of LinearUp/Down clusters (e.g. compare Complex-6 with LinearUp-4). We recognize that our “linear” clusters are not perfectly linear and do vary in their trajectories over time and that our “complex” clusters exhibit a wide spectrum of trajectories. However, to simplify presentation, we elected to employ a straightforward trajectory-based naming scheme for the clusters we identify (i.e. LinearUp, LinearDown, and Complex).
Genes with complex expression trajectories are often identified via the sampling point analysis
We examined the relationship between the statistical analyses a gene was identified in (Day, Survival, and Sampling Point), the cluster in which it resides, and the trajectory it was assigned (LinearUp, LinearDown, and Complex). More than 75% of the genes identified solely in the continuous variable analyses (i.e. Day only, Survival only, or both) reside in the LinearUp/Down clusters, whereas ∼43% of those genes found in Complex clusters were uniquely identified in the Sampling Point analysis (Supplementary Fig. 4). This result does not appear to be driven by specific clusters (Supplementary Fig. 5). This again implies that the Sampling Point analysis has the potential to identify genes whose age-related changes in expression are difficult to capture with linear analyses based on chronological time or survivorship.
Identification of known aging-relevant genes
Our analyses identified many genes previously associated with aging in Drosophila. We identified 107/176 genes associated with the GO term “determination of adult lifespan” (GO:0008340), with at least one such gene being present in 24 of the 28 clusters. For instance, we identified I’m not dead yet (Complex-6, FBgn0036816), a transporter of Krebs cycle intermediates, which when mutated increases lifespan via a mechanism resembling the effect of caloric restriction (Rogina et al. 2000). We found insulin signaling genes, including chico (LinearUp-8, FBgn0024248) and Insulin-like peptide 2 (Complex-11, FBgn0036046), for which loss-of-function mutations increase lifespan (Clancy et al. 2001; Grönke et al. 2010). We also identified the heat shock proteins Hsp22 (LinearUp-6, FBgn0001223), Hsp26 (LinearUp-8, FBgn0001225), and Hsp68 (LinearUp-5, FBgn0001230), which when overexpressed can increase lifespan (Wang et al. 2003, 2004; Morrow et al. 2004).
Exploring the biological functions of expression trajectory clusters
To understand if genes with similar expression trajectories share similar functions, we executed a series of enrichment analyses using the software PANGEA (Hu et al. 2023), identifying enriched GO terms, gene groups, and pathways within each of our 28 clusters. GO terms are derived from the Drosophila GO subsets available within PANGEA (terms are indicated below by the “GO” stem), the gene groups are from DRSC GLAD (“GLAD”) and FlyBase (“FBgg”), and pathways come from Reactome information (“R-DME”). In total, we identified 732 unique enriched terms, 595 of which are specific to just one of our expression trajectory designations (i.e. LinearUp, LinearDown, and Complex), suggesting our 3 designations represent largely distinct sets of biological processes. Furthermore, most of the unique enrichment terms are found in just a single cluster, with only 67 terms being shared by multiple clusters. (The PANGEA output tables are available in Supplementary Tables 6–11.)
Confirming common aging-related gene expression patterns
In Drosophila aging expression studies, it is commonly observed that both general stress response genes and immunity genes increase in expression with age (Pletcher et al. 2002; Landis et al. 2004; Girardot et al. 2006; Carlson et al. 2015; Highfill et al. 2017; Bordet et al. 2021; Zane et al. 2023), and we recapitulate this finding. Five of our clusters are enriched for genes that respond to stress (GO:0006950), all of which are designated LinearUp (LinearUp-2, 3, 5, 7, and 8; Fig. 4). Previous studies have seen that heat shock proteins increase in expression with age (King and Tower 1999; Landis et al. 2004; Manière et al. 2014), and we see these genes (FBgg0000501) are enriched in 2 LinearUp clusters (LinearUp-5 and 7). Of the 5 clusters that are enriched for stress response genes, 3 are more specifically enriched for immune response genes (GO: 0006955; LinearUp-2, 5, and 8). LinearUp-5 and 8 are enriched for antimicrobial peptides (FBgg0001101), including Attacin-A (LinearUp-8, FBgn0012042), Listericin (LinearUp-8, FBgn0033593), and Drosocin (LinearUp-5, FBgn0013088), which are commonly found to increase in expression with age in Drosophila expression studies (Landis et al. 2004; Lai et al. 2007; Carnes et al. 2015; Highfill et al. 2017; Bajgiran et al. 2021; Bordet et al. 2021; Zane et al. 2023).
A decrease in cognitive ability with advanced age has been reported in D. melanogaster (Tamura et al. 2003; Haddadi et al. 2014; Pacifico et al. 2018), mice (Kubanis et al. 1981; Lamberty and Gower 1990; Healy et al. 2024), and rats (Rowe et al. 1998; Sagheddu et al. 2024). Similarly, age-related neurodegeneration is commonly observed, with decreased neurogenesis with aging being reported in both mice (Maslov et al. 2004) and rats (Kuhn et al. 1996), and synaptic deterioration is seen in the motor neurons of aged C. elegans (Liu et al. 2013). Three of our clusters—LinearDown-3, 4, and 6—are enriched for genes that are found in synapses (GO:0045202) and are involved in synapse organization (GO:0050808), as well as those associated with cognition (GO:0050890) and nervous system development (GO:0007399). LinearDown-4 and 6 are also enriched for genes encoding voltage-gated potassium and sodium channel subunits (FBgg0000506, FBgg0000595). Notably, LinearDown-6 harbors Atpα (FBgn0002921), a gene encoding a subunit of the NA+/K+ exchanging ATPase pump, and which has been shown to affect lifespan (Palladino et al. 2003).
A decrease in the expression of genes involved with the ETC is a common finding in samples of aged individuals (Pletcher et al. 2002; Landis et al. 2004; Girardot et al. 2006; Highfill et al. 2017; Bordet et al. 2021; Zane et al. 2023). Such a decrease could lead to reduced ATP production, disruption of the NAD+/NADH ratio, and cellular senescence (Lenaz et al. 1997; Miwa et al. 2014; Miwa et al. 2022). Our study supports a decrease in ETC-related gene expression; genes in Complex-12 and LinearDown-6 show an overall decrease in expression across lifespan (Fig. 4), and these clusters are adjacent to each other in the dendrogram resulting from hierarchical clustering (Fig. 3; Supplementary Fig. 3). These 2 clusters are enriched for genes encoding mitochondrial ETC complexes I and V (FBgg001836, FBgg0001849). LinearDown-6 is also enriched for genes that are part of mitochondrial complexes III and IV (FBgg0001850, FBgg0001847). A series of ETC-related genes shown to influence lifespan are also present in these clusters; these include ND-20 (Complex-12, FBgn0030718) and ND-SGDH (LinearDown-6, FBgn0011455) (Copeland et al. 2009), levy (LinearDown-6, FBgn0034877) (Liu et al. 2007), and ATPsynD (LinearDown-6, FBgn0016120) (Sun et al. 2014). Additionally, LinearDown-6 harbors stress-sensitive B (FBgn0003360), which is involved in the transport of ADP and ATP in and out of the mitochondrial matrix and when mutated shortens lifespan (Celotto et al. 2006; Reynolds 2018).
A distinction between cytosolic and mitochondrial ribosomal gene expression responses
A decrease in the expression of ribosomal proteins and ribosomal biogenesis genes with age has been documented in a number of yeast studies (Yiu et al. 2008; Philipp et al. 2013; Kamei et al. 2014; Janssens et al. 2015; Choi et al. 2018), with 3 of these showing a reduction in cytosolic ribosome (GO: 0022626) gene expression over time (Yiu et al. 2008; Philipp et al. 2013; Choi et al. 2018). One fly study we identified (Doroszuk et al. 2012) observed a similar response, with enrichment of ribosome-related ontology terms in genes showing a reduction in expression with age in a given strain, including genes involved in ribosome biogenesis (GO:0042254) and mitochondrial ribosome (GO:0005761).
In the analyses of our dataset, we saw enrichment of ribosomal-related ontology terms in 6 clusters. Five of these clusters show a general increase in gene expression with age (LinearUp-1, 2, 4, 6, and Complex-6; see Fig. 4) and include genes associated with the terms ribosome biogenesis (GO:0042254), rRNA processing (R-DME-72312), cytoplasmic ribosomal proteins (FBgg0000141), and structural constituent of ribosomes (GO:0003735). The 6th (LinearDown-6) shows reduced expression with age and shows enrichment of mitochondrial ribosomal proteins (FBgg0000059). Thus, in an apparent contrast with prior results, it appears that in our data many ribosome-associated genes increase in expression with age, while only mitochondrial ribosomal protein genes decrease with advanced age.
To examine this further, we extracted from our complete set of 6,142 differentially expressed genes all those affiliated with any ribosome-related term (n = 270, see Supplementary Table 12 for list of terms). We plotted the age-related expression of these 270 protein-coding genes, separating out mitochondrial ribosomal proteins (n = 36, FBgg0000059), and can clearly see that while all mitochondrial ribosomal protein genes go down in expression with age, nearly all other ribosomal genes (220/234) increase in expression with age (Fig. 5). Furthermore, just contrasting cytoplasmic (n = 80, FBgg0000141) and mitochondrial (n = 36, FBgg0000059) ribosomal proteins, we see the former all go up, and the latter all go down in expression with age (Fig. 5).
Fig. 5.
Expression trajectories of ribosome-related protein-coding genes. We examined the expression patterns of mitochondrial ribosomal protein genes (n = 36, green), cytoplasmic ribosomal protein genes (n = 80, red), and all other ribosomal-related genes (n = 154, pink). All mitochondrial ribosomal genes are within LinearDown clusters, all cytoplasmic ribosomal genes are found within LinearUp cluster or Complex-6 (which has an overall increase in expression), and 140/154 of the remaining genes are found within LinearUp or Complex clusters.
A D. melanogaster brain-specific, single-cell RNAseq study (Davie et al. 2018) appears to support our finding that—outside of mitochondrial ribosomes—ribosomal genes increase in expression with age. First, Davie et al. report that the cytosolic small ribosomal subunit (GO: 0022627) gene stubarista (FBgn0003517) is statistically upregulated during aging; we also identified this gene in our LinearUp-5 cluster. Second, while Davie et al. see a reduction in the overall levels of RNA and transcription through aging—a result seen previously (Tahoe et al. 2004) and which we also observed (Supplementary Table 2 and Fig. 6)—on average, ribosomal protein genes show a lower decline in expression than other genes (see Fig. 5c in Davie et al. 2018). In a bulk RNAseq analysis framework, this result would be expected to translate to a relative increase in the expression of ribosomal proteins over time. Nonetheless, that we see a result that contrasts with some of the prior work on gene expression changes through aging is intriguing and worthy of future examination.
Various metabolic processes are enriched in some complex clusters
Many of our Complex clusters have small gene counts and relatively few enriched terms. However, 8/14 Complex clusters (2, 3, 4, 6, 7, 9, 10, and 12) are enriched for genes associated with metabolism (R-DME-1430728 and GLAD:24593). While this term is broad, when we focus on individual clusters more specific metabolic functions are evident. As described earlier, Complex-12 is enriched for genes involved with the ETC. Complex-2, 6, and 10 are specifically enriched for genes involved in the pentose phosphate pathway (R-DME-71336), a glucose catabolism pathway that produces NADPH and ribose sugars for nucleotide synthesis (Stincone et al. 2015). Notably, Complex-2 harbors Pgd (FBgn0004654) and G6pd (FBgn0004057), which are the 2 reducing enzymes involved in the pentose phosphate pathway (Geer et al. 1974; Gvozdev et al. 1976). Complex-3 is uniquely enriched for genes involved in galactose catabolism (R-DME-70370) and glycogen synthesis (R-DME-3322077), one of which—Agbe (FBgn0053138)—is involved in lifespan (Paik et al. 2012). That all of these metabolic pathways/genes emerged from our Complex clusters suggests that nonlinear changes in metabolic activity occur throughout lifespan, as has been suggested in a large, multiomic study in humans (Shen et al. 2024).
Enrichment for protein folding, modification, and transport in the LinearUp-7 cluster
LinearUp-7—which shows only limited change in expression for the first third of life, followed by increasing expression until end of life—is enriched for multiple terms involved with protein folding and modification and transporting proteins from the endoplasmic reticulum to the golgi apparatus. We see enrichment for chaperones and cochaperones (FBgg0001643), and heatshock proteins (see above), which can bind onto unfolded proteins (GO:0051082) and help correctly fold them (GO:0006457). Additionally, LinearUp-7 is enriched for genes involved in posttranslation protein modification pathways (R-DME-597592). LinearUp-7 is the only cluster to be enriched for genes associated with both the endoplasmic reticulum (GO:0005783) and the golgi apparatus (GO:0005794). The cluster also includes genes that are involved in transport between these 2 organelles and is enriched for both coat protein complex I (FBgg0000087) and II (FBgg0000116) genes and genes involved in ER-to-Golgi anterograde transport (R-DME-199977) and Golgi-to-ER retrograde transport (R-DME-8856688). These enrichment patterns further demonstrate that genes with similar roles can have very similar temporal expression patterns.
Multitimepoint trajectory-based datasets offer more detail than 2-timepoint studies
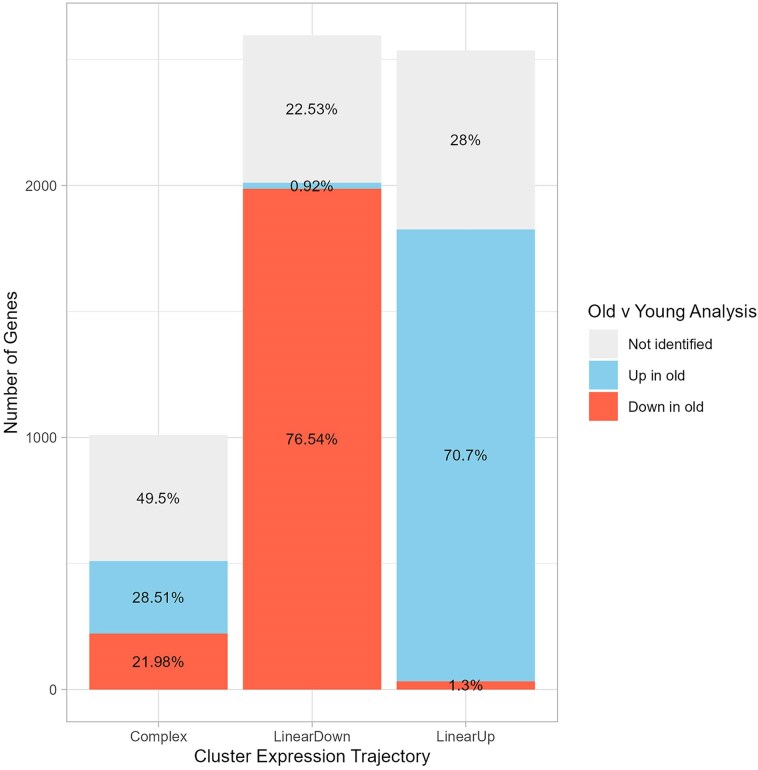
Often aging expression studies compare “young” and “old” samples, and we sought to reanalyze our data in this framework to discover what is gained from a multitimepoint approach. Our “young” timepoint combined all the day 3 and day 6 samples (to achieve a sample size of 6), and our “old” timepoint used the six day 59 samples. Contrasting these 2 sets of samples yielded 4,533 differentially expressed genes. Of the 6,142 genes identified in our multitimepoint analysis, 4,347 (∼71%) were reidentified in this 2-timepoint analysis. The reduction in the number of genes is likely a combination of the switch in analytical approach and a simple loss of power (since we have gone from 48 to 12 samples). Considering the assigned expression trajectories from our multitimepoint analysis, the 2-timepoint analysis recovered 77.5% of the LinearDown cluster genes and 72% of the LinearUp genes, but only 50.5% of the Complex genes (Fig. 6). As anticipated, genes that do not exhibit a straightforward, monotonic increase/decrease in expression through lifespan are much less likely to be identified when sampling is restricted to very young and very old animals.
Fig. 6.
Differences between a multitimepoint and a 2-timepoint analysis. Our 3 trajectory-based analyses revealed >6,000 differentially expressed genes that were grouped into 3 trajectories (Complex, LinearDown, and LinearUp). We compared these results with a reanalysis of a subset of the same data, where we directly contrasted expression between young (days 3 + 6) and old (day 59) samples. Each vertical bar depicts the fraction (in the figure) and the number (y-axis) of genes in each of our expression trajectories that are absent in the young vs old test (gray), are significantly upregulated in old animals (blue), or are significantly downregulated in old animals (red). Most genes with linear trajectories are reidentified, and ∼99% of those show the expected direction of change with age. However, only ∼50% of the Complex trajectory genes are reidentified in the 2-timepoint analysis, and these are split between those that appear to increase or to decrease in expression with age.
A challenge with a 2-timepoint analysis is that the only conclusion one can draw about the expression change is that it goes up or down with age. For those genes present in LinearUp/LinearDown clusters that were replicated in the 2-timepoint analysis, the inferred direction of the expression change matched expectations 98–99% of the time (Fig. 6), and this trend is consistent across the different LinearUp/LinearDown clusters (90–100% matched) (Supplementary Fig. 7). However, for the 50.5% of Complex genes that were reidentified in the 2-timepoint analysis, 56% of them showed an increase in expression in old samples, and 44% showed a decrease (Fig. 6). Examining the 2-timepoint expression change direction calls across Complex clusters reveals cluster-to-cluster variation (see Supplementary Fig. 7), and in most cases, reidentified genes within a given Complex cluster exhibit the same direction of expression change in the 2-timepoint analysis (see Supplementary Fig. 7). This likely reflects the cluster-specific nature of each Complex expression trajectory and the precise expression levels at the start and end of our experiment. For instance, Complex-7 shows a general increase in expression over time (Fig. 4), and all genes reidentified in the 2-timepoint analysis are marked as increasing in expression (Supplementary Fig. 7).
In our initial analysis we chose days 3 + 6 and day 59 to represent our “young” and “old” samples. However, studies have used flies of quite different ages to represent young and old animals (Landis et al. 2004; Girardot et al. 2006; Carnes et al. 2015; Bajgiran et al. 2021; Bordet et al. 2021). To understand the impact of varying the “old” timepoint, we repeated the 2-timepoint analysis several times. In each case, we fixed the young timepoint using our days 3 + 6 data and derived the old timepoint from 2 sequential sampling days (e.g. days 10 + 14, days 14 + 17, days 17 + 23, and so on) such that each analysis compared 2 sets of 6 samples. We then compared the differentially expressed genes that emerged from these analyses, along with the inferred expression change, with our initial days 3 + 6 vs day 59 analysis. As might be expected, using old sampling points that occur earlier in life results in fewer significant differentially expressed genes (Supplementary Fig. 8); there has simply been less time for change. As the old timepoint moves later in life, there is increasingly greater overlap with the days 3 + 6 vs day 59 analysis, and 97–99% of the shared genes show the same change in expression (Supplementary Fig. 8). Nonetheless, every alternative analysis with a different old timepoint identified genes we initially identified in our multitimepoint trajectory analysis, but that were missed in our initial 2-timepoint analysis (Supplementary Fig. 8). Particularly in those analyses using old timepoints that occur earlier in life (up to day 36, a little over halfway through the lifespan of our cohort), such genes are often associated with our multitimepoint Complex trajectory clusters (Supplementary Fig. 9). Between 9% and 17% of Complex genes found using the earlier alternative old timepoints were not found in our initial days 3 + 6 vs day 59 test. It is likely that the observed change in the expression of these genes over time is highly dependent on the exact timepoints chosen. In general, our analyses clearly demonstrate that the age when individuals are sampled impacts the genes identified.
Comparison with other aging expression studies in flies
We compared the outcome of our multitimepoint analysis with 9 datasets from 8 previously published expression analyses in D. melanogaster (Landis et al. 2004; Girardot et al. 2006; Lai et al. 2007; Carnes et al. 2015; Highfill et al. 2016; Bajgiran et al. 2021; Bordet et al. 2021; Zane et al. 2023). These were all 2-timepoint studies that varied in the sex of the flies, the target tissue, and the sampling points employed (Supplementary Table 13). Around 95% of the genes resulting from our multitimepoint analysis were identified in at least one of these datasets, and the number of previous studies that identified a given gene was not clearly associated with the trajectory designation (LinearUp, LinearDown, and Complex) we assigned (Supplementary Fig. 10). The high rate of gene reidentification across studies is notable given the diversity of the study designs in terms of fly sex, the tissue targeted, the sampling points used (Supplementary Table 13), and the likely many differences in the precise rearing/maintenance conditions employed. That the same sets of genes are regularly identified suggests some similarity across genotype/sex/tissue in the age-related expression profile (Izgi et al. 2022).
Similar to the comparisons between multitimepoint and 2-timepoint analyses within our own dataset (above), when we look at genes in our LinearUp/LinearDown clusters that were identified in prior studies, they broadly show the direction of expression change we would predict (Supplementary Fig. 11). However, as might also be expected, the fraction of such genes showing the predicted expression change in a prior study is often lower than it is in our within-study methodological comparison (compare Fig. 6 with Supplementary Fig. 11). The highest fraction of LinearUp/LinearDown genes showing the expected expression change in a prior study—95–98%—is with the study by Highfill et al. (2016; Supplementary Fig. 11), a study published by our group that used the same tissue type, employed a very similar fly maintenance environment, but targeted females rather than males. The other prior studies we examined varied more in their design, and these differences likely contribute to shared genes more often showing mis-matched expression changes (see Supplementary Fig. 11).
Benefits of a multitimepoint, trajectory-based transcriptomics approach to exploring dynamic biological processes
It is increasingly clear that a complete view of the gene regulatory changes underlying a range of dynamic processes—from the response to infection (Schlamp et al. 2021) to cellular differentiation (Strober et al. 2019) to aging (Pletcher et al. 2002; Gheorghe et al. 2014; Shen et al. 2024)—requires a timecourse experimental design, interrogating samples taken throughout the process of interest. Here, we showed that a 2-timepoint, young vs old analysis can successfully identify and correctly infer the direction of expression change of many genes that have largely linear expression trajectories through aging. But, we also showed that the set of genes identified depends on the pair of timepoints chosen. Furthermore, we showed that by limiting the sampling to just 2 timepoints, we would have failed to identify almost half of the genes with more complex, nonlinear expression trajectories and would not have captured the nature of the trajectories for genes that were identified. Even for those genes in the clusters we consider to be “linear,” there is variation in rate of expression change with time, which can only be captured with a multitimepoint approach (Lu et al. 2004; Gheorghe et al. 2014; Haustead et al. 2016; Schaum et al. 2020; Shen et al. 2024).
Another major benefit of being able to consider the trajectory of gene expression is that it enables the identification of genes with similar longitudinal expression patterns and allows assessment of whether genes with similar patterns have similar functional/molecular properties. Our enrichment analyses support the contention that genes with similar expression trajectories share similar functions. Due to the multiple points we sampled throughout the aging process, around 70% of all significant, enriched GO terms we identified were unique to a single cluster. This specificity allowed us to more precisely characterize the expression patterns of various sets of genes with related functional roles, rather than only—with a 2-timepoint framework—being able to state that particular groups of genes are upregulated or downregulated with age. Better understanding of the dynamic molecular changes underling aging will facilitate a deeper understanding of the cellular and physiological changes that occur as organisms age.
Caveats
We recognize that the scope of our results may be somewhat limited due to the use of only males from one inbred strain and the use of a single tissue type. Additionally, while our target tissue—the fly head—is enriched for brain/neuronal cells, since it also includes a mixture of other cell types, we lack true tissue specificity. This said, we were able to demonstrate some consistency over studies in the genes and expression patterns identified, despite these studies varying in multiple ways, making our results a useful resource for future aging investigations.
Accessible data exploration via interactive Shiny apps
Our expression, gene enrichment, and comparative analyses generated a significant amount of data. Above we have only discussed a subset of our observations. To make the results more accessible—in addition to sharing our raw data, summary data, and analytical code—we have developed 2 interactive R/shiny apps that enable individuals to explore our gene identification, clustering, and cluster enrichment results. The apps allow users to look up specific genes, examine their expression trajectory through time in our cohort of flies, the cluster they belong to, and to explore enriched terms within and across clusters. See the Materials and Methods section, along with Supplementary Text 3 and 4 for more information on the development of the apps and how to access and use them.
Acknowledgments
We thank the KU Genome Sequencing Core (supported by NIH P30 GM145499) for library construction and sequencing and the Kansas INBRE Data Science Core (supported by NIH P20 GM103418) for computational infrastructure.
Contributor Information
Katherine M Hanson, Department of Molecular Biosciences and Center for Genomics, University of Kansas, 1200 Sunnyside Avenue, Lawrence, KS 66045, USA.
Stuart J Macdonald, Department of Molecular Biosciences and Center for Genomics, University of Kansas, 1200 Sunnyside Avenue, Lawrence, KS 66045, USA.
Data availability
The A4 strain is available on request from the corresponding author. Raw FASTQ sequencing data are available from the NCBI SRA under BioProject accession number PRJNA1194574. All summary data and results are presented in supplementary files (available at GSA FigShare: https://doi.org/10.25387/g3.28439153), and all analysis code is available via GitHub (https://github.com/Hanson19/RNAseq-Aging).
Funding
This work was supported by National Institutes of Health (NIH) R21 AG086734 and by NIH R01 OD034064.
Literature cited
- Alic N, Giannakou ME, Papatheodorou I, Hoddinott MP, Andrews TD, Bolukbasi E, Partridge L. 2014. Interplay of dFOXO and two ETS-family transcription factors determines lifespan in Drosophila melanogaster. PLoS Genet. 10(9):e1004619. 10.1371/journal.pgen.1004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgiran M, Azlan A, Shamsuddin S, Azzam G, Halim MA. 2021. Data on RNA-seq analysis of Drosophila melanogaster during ageing. Data Brief. 38:107413. 10.1016/j.dib.2021.107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet G, Lodhi N, Kossenkov A, Tulin A. 2021. Age-related changes of gene expression profiles in Drosophila. Genes (Basel). 12(12):1982. 10.3390/genes12121982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer L, Buchman AS, Deelen J, Evans DS, Faul JD, Lunetta KL, Sebastiani P, Smith JA, Smith AV, Tanaka T, et al. 2015. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J Gerontol A Biol Sci Med Sci. 70(1):110–118. 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Wu Y, Huang Y. 2022. Induction of accelerated aging in a mouse model. Cells. 11(9):1418. 10.3390/cells11091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KA, Gardner K, Pashaj A, Carlson DJ, Yu F, Eudy JD, Zhang C, Harshman LG. 2015. Genome-wide gene expression in relation to age in large laboratory cohorts of Drosophila melanogaster. Genet Res Int. 2015:835624. 10.1155/2015/835624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes MU, Campbell T, Huang W, Butler DG, Carbone MA, Duncan LH, Harbajan SV, King EM, Peterson KR, Weitzel A, et al. 2015. The genomic basis of postponed senescence in Drosophila melanogaster. PLoS One. 10(9):e0138569. 10.1371/journal.pone.0138569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotto AM, Frank AC, McGrath SW, Fergestad T, Van Voorhies WA, Buttle KF, Mannella CA, Palladino MJ. 2006. Mitochondrial encephalomyopathy in Drosophila. J Neurosci. 26(3):810–820. 10.1523/JNEUROSCI.4162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, VanKuren NW, Zhao R, Zhang X, Kalsow S, Emerson JJ. 2018. Hidden genetic variation shapes the structure of functional elements in Drosophila. Nat Genet. 50(1):20–25. 10.1038/s41588-017-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Cheng J, Allaire J, Sievert C, Schloerke B, Xie Y, Allen J, McPherson J, Dipert A, Borges B. 2023. shiny: web application framework for R. [cited 2024 Mar 18]. Available from https://CRAN.R-project.org/package=shiny.
- Choi K-M, Hong S-J, van Deursen JM, Kim S, Kim KH, Lee C-K. 2018. Caloric restriction and rapamycin differentially alter energy metabolism in yeast. J Gerontol A Biol Sci Med Sci. 73(1):29–38. 10.1093/gerona/glx024. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. 2001. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 292(5514):104–106. 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Copeland JM, Cho J, Lo T Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. 2009. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 19(19):1591–1598. 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Kitzenberg D, Park S-K. 2013. Dietary restriction in C. elegans: recent advances. Exp Gerontol. 48(10):1014–1017. 10.1016/j.exger.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Davie K, Janssens J, Koldere D, De Waegeneer M, Pech U, Kreft Ł, Aibar S, Makhzami S, Christiaens V, Bravo González-Blas C, et al. 2018. A single-cell transcriptome atlas of the aging Drosophila brain. Cell. 174(4):982–998.e20. 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh H-W, Broer L, Ayers KL, Tan Q, Kamatani Y, Bennet AM, Tamm R, Trompet S, et al. 2014. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum Mol Genet. 23(16):4420–4432. 10.1093/hmg/ddu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh H-W, Helmer Q, Kuningas M, Christiansen L, Kremer D, van der Breggen R, Suchiman HED, Lakenberg N, et al. 2011. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 10(4):686–698. 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroszuk A, Jonker MJ, Pul N, Breit TM, Zwaan BJ. 2012. Transcriptome analysis of a long-lived natural Drosophila variant: a prominent role of stress- and reproduction-genes in lifespan extension. BMC Genomics. 13(1):167. 10.1186/1471-2164-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 95(25):14863–14868. 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk S, Houseley J. 2018. Gene expression hallmarks of cellular ageing. Biogerontology. 19(6):547–566. 10.1007/s10522-018-9750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer BW, Bowman JT, Simmons JR. 1974. The pentose shunt in wild-type and glucose-6-phosphate dehydrogenase deficient Drosophila melanogaster. J Exp Zool. 187(1):77–86. 10.1002/jez.1401870110. [DOI] [PubMed] [Google Scholar]
- Gheorghe M, Snoeck M, Emmerich M, Bäck T, Goeman JJ, Raz V. 2014. Major aging-associated RNA expressions change at two distinct age-positions. BMC Genomics. 15(1):132. 10.1186/1471-2164-15-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. 2007. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 6(4):429–438. 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Girardot F, Lasbleiz C, Monnier V, Tricoire H. 2006. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics. 7(1):69. 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocmez SS, Yazir Y, Gacar G, Demirtaş Şahin T, Arkan S, Karson A, Utkan T. 2020. Etanercept improves aging-induced cognitive deficits by reducing inflammation and vascular dysfunction in rats. Physiol Behav. 224:113019. 10.1016/j.physbeh.2020.113019. [DOI] [PubMed] [Google Scholar]
- Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. 2010. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6(2):e1000857. 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvozdev VA, Gerasimova TI, Kogan GL, Braslavskaya OYu. 1976. Role of the pentose phosphate pathway in metabolism of Drosophila melanogaster elucidated by mutations affecting glucose 6-phosphate and 6-phosphogluconate dehydrogenases. FEBS Lett. 64(1):85–88. 10.1016/0014-5793(76)80255-4. [DOI] [PubMed] [Google Scholar]
- Haddadi M, Jahromi SR, Sagar BKC, Patil RK, Shivanandappa T, Ramesh SR. 2014. Brain aging, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav Brain Res. 259:60–69. 10.1016/j.bbr.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Harrell FE Jr. 2023. rms: regression modeling strategies. [cited 2024 Mar 4]. Available from https://CRAN.R-project.org/package=rms.
- Haustead DJ, Stevenson A, Saxena V, Marriage F, Firth M, Silla R, Martin L, Adcroft KF, Rea S, Day PJ, et al. 2016. Transcriptome analysis of human ageing in male skin shows mid-life period of variability and central role of NF-κB. Sci Rep. 6(1):26846. 10.1038/srep26846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D, Murray C, McAdams C, Power R, Hollier P-L, Lambe J, Tortorelli L, Lopez-Rodriguez AB, Cunningham C. 2024. Susceptibility to acute cognitive dysfunction in aged mice is underpinned by reduced white matter integrity and microgliosis. Commun Biol. 7(1):105. 10.1038/s42003-023-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. 1996. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 97(3):319–323. 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Highfill CA, Reeves GA, Macdonald SJ. 2016. Genetic analysis of variation in lifespan using a multiparental advanced intercross Drosophila mapping population. BMC Genet. 17(1):113. 10.1186/s12863-016-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfill CA, Tran JH, Nguyen SKT, Moldenhauer TR, Wang X, Macdonald SJ. 2017. Naturally segregating variation at Ugt86Dd contributes to nicotine resistance in Drosophila melanogaster. Genetics. 207(1):311–325. 10.1534/genetics.117.300058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB. 2024. Diet strategies for promoting healthy aging and longevity: an epidemiological perspective. J Intern Med. 295(4):508–531. 10.1111/joim.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Chen K, Xia Z, Chavez M, Pal S, Seol J-H, Chen C-C, Li W, Tyler JK. 2014. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes Dev. 28(4):396–408. 10.1101/gad.233221.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Comjean A, Attrill H, Antonazzo G, Thurmond J, Chen W, Li F, Chao T, Mohr SE, Brown NH, et al. 2023. PANGEA: a new gene set enrichment tool for Drosophila and common research organisms. Nucleic Acids Res. 51(W1):W419–W426. 10.1093/nar/gkad331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izgi H, Han D, Isildak U, Huang S, Kocabiyik E, Khaitovich P, Somel M, Dönertaş HM. 2022. Inter-tissue convergence of gene expression during ageing suggests age-related loss of tissue and cellular identity. Elife. 11:e68048. 10.7554/eLife.68048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens GE, Meinema AC, González J, Wolters JC, Schmidt A, Guryev V, Bischoff R, Wit EC, Veenhoff LM, Heinemann M. 2015. Protein biogenesis machinery is a driver of replicative aging in yeast. Elife. 4:e08527. 10.7554/eLife.08527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins VK, Larkin A, Thurmond J; FlyBase Consortium . 2022. Using FlyBase: a database of Drosophila genes and genetics. Methods Mol Biol. 2540:1–34. 10.1007/978-1-0716-2541-5_1. [DOI] [PubMed] [Google Scholar]
- Joshi PK, Fischer K, Schraut KE, Campbell H, Esko T, Wilson JF. 2016. Variants near CHRNA3/5 and APOE have age- and sex-related effects on human lifespan. Nat Commun. 7(1):11174. 10.1038/ncomms11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schraut KE, Clark DW, Nutile T, Barnes CLK, Timmers PRHJ, et al. 2017. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat Commun. 8(1):910. 10.1038/s41467-017-00934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Tamada Y, Nakayama Y, Fukusaki E, Mukai Y. 2014. Changes in transcription and metabolism during the early stage of replicative cellular senescence in budding yeast. J Biol Chem. 289(46):32081–32093. 10.1074/jbc.M114.600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanis J, Gordon A, Shor T, Weissbrod O, Geiger D, Wahl M, Gershovits M, Markus B, Sheikh M, Gymrek M, et al. 2018. Quantitative analysis of population-scale family trees with millions of relatives. Science. 360(6385):171–175. 10.1126/science.aam9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A, Mundt F. 2020. factoextra: extract and visualize the results of multivariate data analyses. [cited 2024 May 31]. Available from https://cran.r-project.org/web/packages/factoextra/index.html.
- Kenyon CJ. 2010. The genetics of ageing. Nature. 464(7288):504–512. 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 277(5328):942–946. 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- King EG, Merkes CM, McNeil CL, Hoofer SR, Sen S, Broman KW, Long AD, Macdonald SJ. 2012. Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 22(8):1558–1566. 10.1101/gr.134031.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V, Tower J. 1999. Aging-specific expression of Drosophila hsp22. Dev Biol. 207(1):107–118. 10.1006/dbio.1998.9147. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E, et al. 2019. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7(9):673–683. 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanis P, Gobbel G, Zornetzer SF. 1981. Age-related memory deficits in Swiss mice. Behav Neural Biol. 32(2):241–247. 10.1016/S0163-1047(81)90555-0. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. 1996. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 16(6):2027–2033. 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-Q, Parnell LD, Lyman RF, Ordovas JM, Mackay TFC. 2007. Candidate genes affecting Drosophila life span identified by integrating microarray gene expression analysis and QTL mapping. Mech Ageing Dev. 128(3):237–249. 10.1016/j.mad.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ. 1990. Age-related changes in spontaneous behavior and learning in NMRI mice from maturity to middle age. Physiol Behav. 47(6):1137–1144. 10.1016/0031-9384(90)90364-a. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavaré S, Tower J. 2004. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 101(20):7663–7668. 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova ML, Marchetti M, Pich MM, Pallotti F, Parenti Castelli G, et al. 1997. Mitochondrial complex I defects in aging. Mol Cell Biochem. 174(1/2):329–333. 10.1023/A:1006854619336. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 278(5341):1319–1322. 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Liu W, Gnanasambandam R, Benjamin J, Kaur G, Getman PB, Siegel AJ, Shortridge RD, Singh S. 2007. Mutations in cytochrome c oxidase subunit VIa cause neurodegeneration and motor dysfunction in Drosophila. Genetics. 176(2):937–946. 10.1534/genetics.107.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu A-L, Xu XZS. 2013. Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metab. 18(3):392–402. 10.1016/j.cmet.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 15(12):550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Soneson C, Hickey PF, Johnson LK, Pierce NT, Shepherd L, Morgan M, Patro R. 2020. Tximeta: reference sequence checksums for provenance identification in RNA-seq. PLoS Comput Biol. 16(2):e1007664. 10.1371/journal.pcbi.1007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao S-Y, Li C, Kohane I, Chan J, Yankner BA. 2004. Gene regulation and DNA damage in the ageing human brain. Nature. 429(6994):883–891. 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. 2002. Transcriptional profile of aging in C. elegans. Curr Biol. 12(18):1566–1573. 10.1016/S0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Manière X, Krisko A, Pellay FX, Di Meglio J-M, Hersen P, Matic I. 2014. High transcript levels of heat-shock genes are associated with shorter lifespan of Caenorhabditis elegans. Exp Gerontol. 60:12–17. 10.1016/j.exger.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 24(7):1726–1733. 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 8(1):14063. 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer PJ. 1991. Inheritance of longevity evinces no secular trend among members of six New England families born 1650–1874. Am J Hum Biol. 3(1):49–58. 10.1002/ajhb.1310030109. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin C-S, Jan YN, Kenyon C, Bargmann CI, Li H. 2004. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat Genet. 36(2):197–204. 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- McCay CM, Maynard LA, Sperling G, Barnes LL. 1939. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories: four figures. J Nutr. 18(1):1–13. 10.1093/jn/18.1.1. [DOI] [PubMed] [Google Scholar]
- Melzer D, Pilling LC, Ferrucci L. 2020. The genetics of human ageing. Nat Rev Genet. 21(2):88–101. 10.1038/s41576-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, Treumann A, von Zglinicki T. 2014. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. 5(1):3837. 10.1038/ncomms4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Kashyap S, Chini E, von Zglinicki T. 2022. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 132(13):e158447. 10.1172/JCI158447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Battistini S, Zhang P, Tanguay RM. 2004. Decreased lifespan in the absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila. J Biol Chem. 279(42):43382–43385. 10.1074/jbc.C400357200. [DOI] [PubMed] [Google Scholar]
- Newman AB, Murabito JM. 2013. The epidemiology of longevity and exceptional survival. Epidemiol Rev. 35(1):181–197. 10.1093/epirev/mxs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Walter S, Lunetta KL, Garcia ME, Slagboom PE, Christensen K, Arnold AM, Aspelund T, Aulchenko YS, Benjamin EJ, et al. 2010. A meta-analysis of four genome-wide association studies of survival to age 90 years or older: the cohorts for heart and aging research in genomic epidemiology consortium. J Gerontol A Biol Sci Med Sci. 65(5):478–487. 10.1093/gerona/glq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olecka M, van Bömmel A, Best L, Haase M, Foerste S, Riege K, Dost T, Flor S, Witte OW, Franzenburg S, et al. 2024. Nonlinear DNA methylation trajectories in aging male mice. Nat Commun. 15(1):3074. 10.1038/s41467-024-47316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico R, MacMullen CM, Walkinshaw E, Zhang X, Davis RL. 2018. Brain transcriptome changes in the aging Drosophila melanogaster accompany olfactory memory performance deficits. PLoS One. 13(12):e0209405. 10.1371/journal.pone.0209405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik D, Jang YG, Lee YE, Lee YN, Yamamoto R, Gee HY, Yoo S, Bae E, Min K-J, Tatar M, et al. 2012. Misexpression screen delineates novel genes controlling Drosophila lifespan. Mech Ageing Dev. 133(5):234–245. 10.1016/j.mad.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Bower JE, Kreber R, Ganetzky B. 2003. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J Neurosci. 23(4):1276–1286. 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 14(4):417–419. 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp O, Hamann A, Servos J, Werner A, Koch I, Osiewacz HD. 2013. A genome-wide longitudinal transcriptome analysis of the aging model podospora anserine. PLoS One. 8(12):e83109. 10.1371/journal.pone.0083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling LC, Atkins JL, Bowman K, Jones SE, Tyrrell J, Beaumont RN, Ruth KS, Tuke MA, Yaghootkar H, Wood AR, et al. 2016. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging (Albany NY). 8(3):547–560. 10.18632/aging.100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling LC, Kuo C-L, Sicinski K, Tamosauskaite J, Kuchel GA, Harries LW, Herd P, Wallace R, Ferrucci L, Melzer D. 2017. Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging (Albany NY). 9(12):2504–2520. 10.18632/aging.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. 2002. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 12(9):712–723. 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2021. R: a language and environment for statistical computing. [cited 2022 Mar 31]. Available from https://www.R-project.org/.
- Remondini D, Salvioli S, Francesconi M, Pierini M, Mazzatti DJ, Powell JR, Zironi I, Bersani F, Castellani G, Franceschi C. 2010. Complex patterns of gene expression in human T cells during in vivo aging. Mol Biosyst. 6(10):1983–1992. 10.1039/C004635C. [DOI] [PubMed] [Google Scholar]
- Reynolds ER. 2018. Shortened lifespan and other age-related defects in bang sensitive mutants of Drosophila melanogaster. G3 (Bethesda). 8(12):3953–3960. 10.1534/g3.118.200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Reenan RA, Nilsen SP, Helfand SL. 2000. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 290(5499):2137–2140. 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Spreekmeester E, Meaney MJ, Quirion R, Rochford J. 1998. Reactivity to novelty in cognitively-impaired and cognitively-unimpaired aged rats and young rats. Neuroscience. 83(3):669–680. 10.1016/s0306-4522(97)00464-8. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Wright KM, Rand KA, Kermany A, Noto K, Curtis D, Varner N, Garrigan D, Slinkov D, Dorfman I, et al. 2018. Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics. 210(3):1109–1124. 10.1534/genetics.118.301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu C, Stojanovic T, Kouhnavardi S, Savchenko A, Hussein AM, Pistis M, Monje FJ, Plasenzotti R, Aufy M, Studenik CR, et al. 2024. Cognitive performance in aged rats is associated with differences in distinctive neuronal populations in the ventral tegmental area and altered synaptic plasticity in the hippocampus. Front Aging Neurosci. 16:1357347. 10.3389/fnagi.2024.1357347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, et al. 2020. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 583(7817):596–602. 10.1038/s41586-020-2499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp F, Delbare SYN, Early AM, Wells MT, Basu S, Clark AG. 2021. Dense time-course gene expression profiling of the Drosophila melanogaster innate immune response. BMC Genomics. 22(1):304. 10.1186/s12864-021-07593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Gurinovich A, Bae H, Andersen S, Malovini A, Atzmon G, Villa F, Kraja AT, Ben-Avraham D, Barzilai N, et al. 2017. Four genome-wide association studies identify new extreme longevity variants. J Gerontol A Biol Sci Med Sci. 72(11):1453–1464. 10.1093/gerona/glx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Solovieff N, DeWan AT, Walsh KM, Puca A, Hartley SW, Melista E, Andersen S, Dworkis DA, Wilk JB, et al. 2012. Genetic signatures of exceptional longevity in humans. PLoS One. 7(1):e29848. 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Wang C, Zhou X, Zhou W, Hornburg D, Wu S, Snyder MP. 2024. Nonlinear dynamics of multi-omics profiles during human aging. Nat Aging. 4(11):1619–1634. 10.1038/s43587-024-00692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stincone A, Prigione A, Cramer T, Wamelink MMC, Campbell K, Cheung E, Olin-Sandoval V, Grüning N-M, Krüger A, Tauqeer Alam M, et al. 2015. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 90(3):927–963. 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober BJ, Elorbany R, Rhodes K, Krishnan N, Tayeb K, Battle A, Gilad Y. 2019. Dynamic genetic regulation of gene expression during cellular differentiation. Science. 364(6447):1287–1290. 10.1126/science.aaw0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wheeler CT, Yolitz J, Laslo M, Alberico T, Sun Y, Song Q, Zou S. 2014. A mitochondrial ATP synthase subunit interacts with TOR signaling to modulate protein homeostasis and lifespan in Drosophila. Cell Rep. 8(6):1781–1792. 10.1016/j.celrep.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahoe NMA, Mokhtarzadeh A, Curtsinger JW. 2004. Age-related RNA decline in adult Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 59(9):B896–B901. 10.1093/gerona/59.9.B896. [DOI] [PubMed] [Google Scholar]
- Tamura T, Chiang A-S, Ito N, Liu H-P, Horiuchi J, Tully T, Saitoe M. 2003. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 40(5):1003–1011. 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- Tan Q, Zhao JH, Zhang D, Kruse TA, Christensen K. 2008. Power for genetic association study of human longevity using the case-control design. Am J Epidemiol. 168(8):890–896. 10.1093/aje/kwn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taormina G, Ferrante F, Vieni S, Grassi N, Russo A, Mirisola MG. 2019. Longevity: lesson from model organisms. Genes (Basel). 10(7):518. 10.3390/genes10070518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers PR, Mounier N, Lall K, Fischer K, Ning Z, Feng X, Bretherick AD, Clark DW; eQTLGen Consortium; Shen X, et al. 2019. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. Elife. 8:e39856. 10.7554/eLife.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. 2003. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 5(5):811–816. 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang Q, Song Y, He Z, Zhang H, Song M, Zhang X, Dai Y, Karalay O, Dieterich C, et al. 2022. Ageing induces tissue-specific transcriptomic changes in Caenorhabditis elegans. EMBO J. 41(8):e109633. 10.15252/embj.2021109633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-D, Kazemi-Esfarjani P, Benzer S. 2004. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 101(34):12610–12615. 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. [cited 2024 Dec 30]. Available from https://cran.r-project.org/package=ggplot2.
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. 2008. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 105(37):13987–13992. 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CH, Shalini S, Filipovska A, Richman TR, Davies S, Martin SD, McGee SL, Puccini J, Nikolic A, Dorstyn L, et al. 2015. Age-related proteostasis and metabolic alterations in Caspase-2-deficient mice. Cell Death Dis. 6(1):e1615. 10.1038/cddis.2014.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Fuchs H, Scifo E, Liu D, Aziz A, Aguilar-Pimentel JA, Amarie OV, Becker L, da Silva-Buttkus P, Calzada-Wack J, et al. 2022. Deep phenotyping and lifetime trajectories reveal limited effects of longevity regulators on the aging process in C57BL/6J mice. Nat Commun. 13(1):6830. 10.1038/s41467-022-34515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, McCord A, Wise A, Jindal R, Hardee J, Kuo A, Shimogawa MY, Cahoon L, Wu M, Kloke J, et al. 2008. Pathways change in expression during replicative aging in Saccharomyces cerevisiae. J Gerontol A Biol Sci Med Sci. 63(1):21–34. 10.1093/gerona/63.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zane F, Bouzid H, Sosa Marmol S, Brazane M, Besse S, Molina JL, Cansell C, Aprahamian F, Durand S, Ayache J, et al. 2023. Smurfness-based two-phase model of ageing helps deconvolve the ageing transcriptional signature. Aging Cell. 22(11):e13946. 10.1111/acel.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Nie C, Min J, Liu X, Li M, Chen H, Xu H, Wang M, Ni T, Li Y, et al. 2016. Novel loci and pathways significantly associated with longevity. Sci Rep. 6(1):21243. 10.1038/srep21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Morris QD, Chang R, Shai O, Bakowski MA, Mitsakakis N, Mohammad N, Robinson MD, Zirngibl R, Somogyi E, et al. 2004. The functional landscape of mouse gene expression. J Biol. 3(5):21. 10.1186/jbiol16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The A4 strain is available on request from the corresponding author. Raw FASTQ sequencing data are available from the NCBI SRA under BioProject accession number PRJNA1194574. All summary data and results are presented in supplementary files (available at GSA FigShare: https://doi.org/10.25387/g3.28439153), and all analysis code is available via GitHub (https://github.com/Hanson19/RNAseq-Aging).