Abstract
Open replacement of multifocal aortic aneurysms is an operative and clinical challenge. Thoughtful consideration of both surgical technique and timing is important to reduce risk of major cardiovascular and neurologic complications. We discuss the management of a 34-year-old male with suspected connective tissue disease admitted with multifocal, giant aneurysms of his right subclavian artery, ascending and aortic arch, descending and abdominal aorta, and right iliac artery. A complex, open three-stage repair was undertaken. Genetic testing revealed a missense MYH11 mutation. We describe our approach and patient outcome.
Keywords: Aortic aneurysm, Connective tissue disease, Mega aortic syndrome, Multifocal giant aneurysms, MYH11 mutation
Mega aorta syndrome (MAS) is a rare disease characterized by aneurysmal transformation of the thoracic and abdominal aorta. Optimal surgical approach is unknown as each patient differs in extent and location of disease and surgical risk.1,2 Thoughtful consideration of surgical technique and timing is important to reduce the risk of major cardiovascular and neurologic complications.
Case report
A 34-year-old male with hypertension and no personal or familial history of aortopathy presented to the emergency department with a three-month history of cough and chest pain. Computed tomography (CT) of the head, neck, chest, abdomen, and pelvis demonstrated multifocal aneurysms of his right subclavian artery (SCA), ascending, aortic arch, descending thoracic (DTA) and abdominal aorta, and right common iliac artery (Fig 1). Cardiac workup was negative. Given the his young age, connective tissue disease (CTD) was suspected. Decision-making was guided by a multidisciplinary aortic team. We performed a complex, three-stage open surgical approach with excellent results.
Fig 1.
Preoperative computed tomography (CT) angiogram of chest, abdomen, and pelvis. (A) Three-dimensional reconstruction of thoracic and abdominal aorta. (B) Coronal view. Imaging studies demonstrate multifocal aneurysms including right subclavian artery (SCA) (6 cm), ascending aorta (7.7 cm), aortic arch (7.1 cm), descending thoracoabdominal aorta (DTA) (7 cm), and right common iliac artery (5.7 cm). Proximal descending aorta (zone 3) was 2.2 cm.
Operative technique
First stage
The ascending aortic aneurysm (7.7 cm) posed the greatest risk of rupture. Stage one was performed via median sternotomy with right supraclavicular extension. Cardiopulmonary bypass (CPB) was performed through central venous and arterial cannulation. Total arch replacement with right subclavian bypass using a 26 mm Terumo graft was performed under cardioplegic and circulatory arrest at 24 °C. Elephant trunk or frozen elephant trunk was deferred, given the small caliber of the accessible proximal DTA and the risk of sacrificing intercostals at a second-stage procedure. After head vessel reconstruction, the patient was rewarmed and the right SCA was controlled. The SCA aneurysm was opened, and the right vertebral artery was identified. The distal right SCA was anastomosed to the 8-mm graft sidearm previously used for CPB. Subsequently, the right vertebral artery was transposed onto the right SCA graft with the assistance of vascular surgery (Fig 2, A). The chest was packed open for planned delayed closure the following day. He ultimately discharged on postoperative day (POD) 10 without complication. As an inpatient, Invitae Aortopathy Gene Panel was obtained, demonstrating a missense variant of MYH11 gene with unknown significance. He was referred to cardiogenomics clinic.
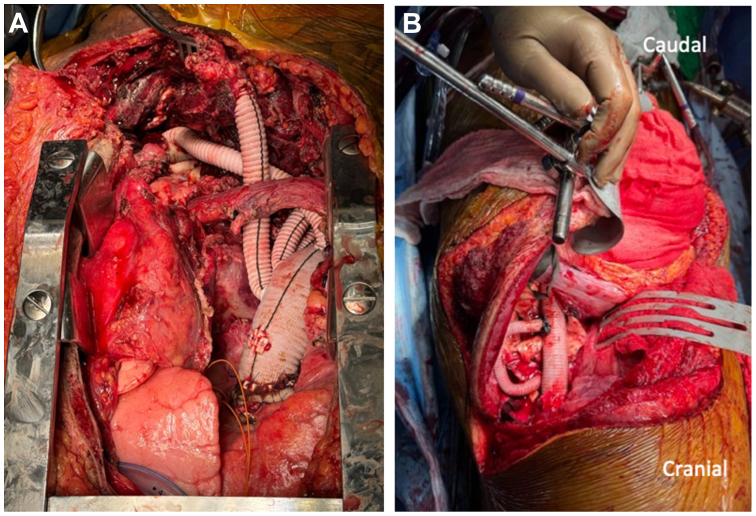
Fig 2.
Intraoperative views from Stage I and Stage II operations. (A) Stage I repair with exposure through sternotomy with right supraclavicular extension. (B) Stage II repair through standard thoracoabdominal incision.
Second stage
Postoperative CTA at 4 weeks demonstrated growth of the thoracoabdominal aorta (TAA) by 5 mm. The second-stage operation was expedited to 6 weeks in conjunction with the vascular surgery team. An extent II thoracoabdominal aneurysm repair with a 24-mm Coselli graft was performed with left heart bypass at 34 °C. A preoperatively placed lumbar drain, sequential clamping, moderate hypothermia, and intercostal artery bypass were used for neuroprotection. The proximal anastomosis was sewn to the distal aspect of the previous aortic segment left in situ (15 cm) with a felt buttress. The distal anastomosis was sewn to normal caliber infrarenal aorta with felt while perfusing the abdominal viscera. Individual visceral artery reimplantation was sequentially performed. Two large spinal arteries were reimplanted in C-configuration using a 10-mm sidearm of graft that was sewn to our Coselli graft (Fig 2, B). Patient was discharged home on POD 10 without complication. Surveillance CT at 5 weeks demonstrated normal postoperative changes and a thrombosed c-loop with no associated clinical deficits.
Third stage
Repair of the infrarenal aortic and right iliac aneurysms was performed by vascular surgery 6 weeks later. Preoperative lumbar drain and clamp-sew technique were used to optimize spinal cord perfusion. A modified aorto-biiliac grafting was performed. The proximal clamp was placed above the inferior mesenteric artery. The proximal anastomosis was sewn just below the inferior mesenteric artery. A 7.0-cm segment of native aorta containing several large, patent lumbar arteries was left in situ. Perfusion was re-established to optimize spinal perfusion. Distal anastomoses were completed sequentially, with single limbs connected to the left common iliac artery and right internal and external iliac arteries. Patient was discharged home on POD 9 without complication. Following three-stage reconstruction, the proximal and distal landing zones surrounding native aortic arch segment were 2.5 cm and 11.5 cm, and 4.0 cm and 3.0 cm surrounding the native abdominal aortic segment.
Discussion
MAS in the setting of CTD treated with staged, open surgery underlines the complexity in decision-making and emphasizes consideration of both present and future risks of complication and aneurysmal degeneration. Two segments of native aorta containing large intercostals or lumbar arteries were left in situ to decrease risk of spinal cord ischemia; the resultant configuration facilitates future endovascular repair as tortuosity and major aortic size discrepancies were mitigated. There are no published guidelines on the preferred treatment of MAS. Operative approaches include open surgery or hybrid repair, which utilizes both open surgical and endovascular techniques with varying degrees of staging. An open approach was selected due to the small caliber of the proximal DTA, suspected CTD, significant tortuosity, and extensive variation in aortic diameter. Staging reduces the risk of major complications (eg, death, stroke, and spinal cord ischemia), decreases CPB and circulatory arrest times, and allows for a period of clinical convalescence. However, inter-stage mortality can be as high as 13%, and frequently patients are unwilling to proceed with a second operation.3,4 Single-stage open approach, although technically feasible and associated with acceptable outcomes at experienced centers,5 was deemed too high risk, given the patient’s age and extent of disease and variation in aortic size.
Multi-stage hybrid repair is the preferred approach in elderly patients or those at higher operative risk, although studies show improved long-term outcomes for staged open surgery.6 A hybrid approach, including open ascending aortic replacement with arch debranching and endovascular TAA repair, may carry higher risk of neurologic insult and spinal cord ischemia.6 The extent of open and endovascular surgery in hybrid repair can also vary. The Lupiae technique entails proximal aortic debranching and infra-renal abdominal aneurysm replacement with diversion of visceral vessels, followed by endovascular repair of the thoracic segment; it has been associated with decreased risk of endoleak and precludes a third operation.7 Ten-year follow-up data from their cohort of 27 patients demonstrated a late survival rate of 51.7% and no spinal cord ischemia.8 This technique would have been significantly hindered by the tortuosity and large size discrepancy within the DTA.
Twenty percent of patients who undergo repair of thoracic aortic aneurysm or dissection have familial clustering of the disease.9 Non-syndromic thoracic aortic aneurysms are commonly associated with mutations in smooth muscle cell (SMC) contractile proteins such as MYH11, ACTA2, MYLK, and PRKG1.10 Mutations in MHY11 (myosin heavy chain 11), located on chromosome 16, cause failure of correct myosin filament assembly and histologically are associated with loss of SMCs and elastic and collagen fibers in addition to SMC disarray and focal areas of hyperplasia.11 Genetic testing of our patient showed a MYH11 missense variant (c.5648 A>G [p.Asn1883Ser]), which has not been previously described and is currently deemed a variant of unknown significance. Genetic testing is recommended for patients with syndromic features, family history of thoracic aortic disease, or early age of disease onset (<60 years old).12
Conclusions
MAS poses unique challenges for cardiovascular surgeons. Thoughtful approach to patient anatomy, perioperative risk, and the lifetime management of aortic disease through multi-disciplinary discussion mitigates perioperative and postoperative complications. At 4-week follow-up, our patient was progressing well and neurologically normal with a near-normal appearing CT scan (Fig 3). At 1 year, he is alive and well. CT surveillance will continue yearly to monitor the two segments of native aorta which are suitable for endovascular repair.
Fig 3.
Computed tomography angiography (CTA) of chest, abdomen, and pelvis demonstrating final result of three-stage open repair.
Funding
None.
Disclosures
None.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Belov Y.V., Charchyan E.R., Breshenkov D.G., et al. Surgical treatment of mega aorta syndrome: a single-center experience. Khirurgiia (Mosk) 2021;6. Vyp. 2:15–25. doi: 10.17116/hirurgia202106215. [DOI] [PubMed] [Google Scholar]
- 2.Gkremoutis A., Zierer A., Schmitz-Rixen T., et al. Staged treatment of mega aortic syndrome using the frozen elephant trunk and hybrid thoracoabdominal repair. J Thorac Cardiovasc Surg. 2017;154:1842–1849. doi: 10.1016/j.jtcvs.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Ius F., Hagl C., Haverich A., Pichlmaier M. Elephant trunk procedure 27 years after Borst: what remains and what is new? Eur J Cardiothorac Surg. 2011;40:1–11. doi: 10.1016/j.ejcts.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 4.LeMaire S.A., Carter S.A., Coselli J.S. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg. 2006;81:1561–1569. doi: 10.1016/j.athoracsur.2005.11.038. https://doi.org/10.1016/j.athoracsur.2005.11.038 discussion: 1569. [DOI] [PubMed] [Google Scholar]
- 5.Kouchoukos N.T., Masetti P., Mauney M.C., Murphy M.C., Castner C.F. One-stage repair of extensive chronic aortic dissection using the arch-first technique and bilateral anterior thoracotomy. Ann Thorac Surg. 2008;86:1502–1509. doi: 10.1016/j.athoracsur.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro T.S., Gadelha H.P.J., Santos M.A.D. Hybrid repair versus conventional open repair approaches for aortic arch disease: a comprehensive review. Braz J Cardiovasc Surg. 2021;36:244–252. doi: 10.21470/1678-9741-2020-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito G., Pennesi M., Bichi S., et al. Hybrid multistep approach to mega-aortic syndrome: the Lupiae technique. Eur J Cardiothorac Surg. 2015;47:126–133. doi: 10.1093/ejcts/ezu102. discussion: 133. [DOI] [PubMed] [Google Scholar]
- 8.Esposito G., Cappabianca G., Beghi C., et al. Hybrid three-stage repair of mega-aortic syndrome with the Lupiae technique: 10-year results. Ann Cardiothorac Surg. 2018;7:357–365. doi: 10.21037/acs.2018.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albornoz G., Coady M.A., Roberts M., et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–1405. doi: 10.1016/j.athoracsur.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 10.Salmasi M.Y., Alwis S., Cyclewala S., et al. Members of the London Aortic Mechanobiology Working Group The genetic basis of thoracic aortic disease: the future of aneurysm classification? Hellenic J Cardiol. 2023;69:41–50. doi: 10.1016/j.hjc.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Pannu H., Tran-Fadulu V., Papke C.L., et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. Erratum in: Hum Mol Genet. 2008 Jan 1;17(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isselbacher E.M., Preventza O., Hamilton Black J., III, et al. Peer Review Committee Members 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical Practice guidelines. Circulation. 2022;146:e334–e482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]