Abstract
The 1,670-bp nucleotide sequence of the heat shock operon groESL and the 1,236-bp sequence of the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys were determined. The topology of the groEL- and gltA-based phylogenetic tree was similar to that derived from 16S rRNA gene analyses with distances. Both groESL- and gltA-based PCRs specific to A. platys were also developed based upon the alignment data.
Anaplasma (Ehrlichia) platys is a bacterial parasite of dog platelets that causes infectious cyclic thrombocytopenia (8). A. platys has been shown to be closely related to Anaplasma marginale, Anaplasma centrale, and Anaplasma phagocytophila, including the former human granulocytic ehrlichia (HGE) agents Ehrlichia equi and Ehrlichia phagocytophila, based on 16S rRNA gene sequences (7); however, little information is available regarding the natural history of the pathogen. The 16S rRNA gene had been the only known gene sequence of A. platys before the heat shock protein gene (groEL) was sequenced recently (21). A groEL sequence analysis supported the phylogenetic relationship between A. platys and related species. The groESL operon contains a spacer region between groES and groEL which is thought to be more divergent than the coding regions (18, 19). However, the nucleotide sequence of the spacer region of A. platys has not been studied yet. Thus, the nucleotide sequences of groES and the spacer region between groES and groEL were analyzed for additional phylogenetic characterization of A. platys. More recently, we sequenced the citrate synthase gene (gltA) of 13 species, including Ehrlichia, Anaplasma, and Neorickettsia, for phylogenetic analyses and found higher variation than for the 16S rRNA gene (10). The topology of the gltA-based phylogenetic tree confirmed the reorganization of genera in the families Rickettsiaceae and Anaplsmataceae reported recently (7). However, the gltA sequence of A. platys has yet to be determined. Thus, the nucleotide sequences of A. platys gltA were also analyzed to support the phylogenetic relationship of A. platys among related species. We also propose to use sequence data from groESL and gltA with greater differences among species to develop an A. platys-specific PCR method. New PCR primers to specifically detect A. platys fragments were developed based on the alignment data of these two genes for a diagnostic assay.
The A. platys DNA analyzed in this study was supplied from a dog infected in Somieres, France (2). The dog had a history of a clotting disorder. At the time of bleeding, the platelet count was 256,000/μl, which was in the normal range, and A. platys was observed within 58% of platelets on a Giemsa-stained peripheral blood smear. To evaluate the species-specific PCR designed in this study, DNA from other strains of A. platys were used. DNA from a dog infected with A. platys in Venezuela was kindly provided by E. B. Breitschwerdt, North Carolina State University (17). DNA from a dog infected with A. platys in Okinawa, Japan, was also used (9).
For amplification of the groESL operon of A. platys, EEgro1F and EEgro2R were used to amplify an approximately 1,700-bp fragment with an annealing temperature of 55°C (3) (Table 1). The amplification products were purified using the QIAquick PCR purification kit (Qiagen GmbH, Hilden, Germany) and sequenced. Five primers shown in Table 1 were used to complete the sequence of groESL. Fluorescence-labeled dideoxynucleotide technology was used for DNA sequencing reactions (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.). Samples were then sequenced using a Perkin-Elmer ABI Prism 377 automated DNA sequencer at the DNA Core Facility of the Center for Gene Research, Yamaguchi University.
TABLE 1.
Oligonucleotide sequences of primers used in this study
| Primer name | Oligonucleotide sequence (5′→3′)a | Reference |
|---|---|---|
| PCR amplification for groESL | ||
| EEgro1F | 5-GAG-AGA-TGC-TTA-TGG-TAA-GAC-3 | 1 |
| EEgro2R | 5-CAG-CGT-CGT-TCT-TAC-TAG-GAA-C-3 | 1 |
| Sequence primers for groESL | ||
| SQ3F | 5-ATT-AGC-AAG-CCT-TAT-GGG-TC-3 | This study |
| SQ4R | 5-CTT-TAG-GCT-ATC-AAG-AGA-TG-3 | This study |
| SQ4F | 5-CAT-CTC-TTG-ATA-GCC-TAA-AG-3 | This study |
| SQ5F | 5-TCA-GTG-TGT-GAA-GGA-AGT-TG-3 | This study |
| SQ6R | 5-TGC-TTC-CTA-TGT-TCT-TAT-CG-3 | This study |
| PCR amplification of gltA fragments | ||
| F4b | 5-CCG-GGT-TTT-ATG-TCT-ACT-GC-3 | 8 |
| F1b | 5-GAT-CAT-GAR-CAR-AAT-GCT-TC-3 | This study |
| EHR-CS779R | 5-GCN-CCM-CCA-TGM-GCT-CG-3 | 14 |
| HG-1085R | 5-ACT-ATA-CCK-GAG-TAA-AAG-TC-3 | 8 |
| gltA-specific primer for the Genome Walker method | ||
| PLATYS-SPF | 5-CTG-CCG-GAA-CAG-AGC-TAT-TC-3 | This study |
| PLATYS-SPR1 | 5-TCG-GAT-GAC-AGA-GCA-TAG-TG-3 | This study |
| PLATYS-SPR2 | 5-TTG-CTC-GAA-CAC-TGT-GTC-TG-3 | This study |
| A. platys-specific primers | ||
| PLA-HS475F | 5-AAG-GCG-AAA-GAA-GCA-GTC-TTA-3 | This study |
| PLAT-HS1198R | 5-CAT-AGT-CTG-AAG-TGG-AGG-AC-3 | This study |
| PLA-CSM136F | 5-TTG-CAA-AAA-GTA-AGC-GGA-GC-3 | This study |
| PLA-CS1359R | 5-AAC-CAC-AGG-CTT-ATG-ACA-AC-3 | This study |
K, G or T; R, G or A; M, C or A; N, G, C, A, or T.
The strategy for determining the gltA sequence was similar to that used in our previous report (10). A partial sequence of A. platys gltA was first determined using two sets of degenerate primers, F1b and EHR-778R, and F1b and HG1085R (10, 15) (Table 1). These primers were designed based upon the sequence of gltA of A. phagocytophila, A. marginale, and A. centrale. The amplification conditions were the same as in the previous study (10), with an annealing temperature of 53°C. The amplification products were purified and sequenced as described above. After a partial determination of the sequence, the unknown areas of the 3′ and 5′ ends of the gene were determined using the Universal Genome Walker kit (Clontech Laboratories, Palo Alto, Calif.). Briefly, genomic DNA was digested with EcoRV, DraI, PvuII, StuI, and ScaI. DNA fragments were ligated with a Genome Walker adaptor, which had one blunt end and one end with a 5′ overhang. A ligation mixture of the adaptor and ehrlichial genomic DNA fragments was used as a template for PCR. This PCR was performed using an adaptor primer supplied by the manufacturer and A. platys gltA-specific primers to walk downstream on the DNA sequence (Table 1). For the amplification, the conditions were as in our previous report (10).
The sequences of A. platys and the registered sequences of other related species deposited in GenBank were analyzed for phylogenetic relationships. Multiple alignment analysis, the calculation of distance matrices, and the construction of phylogenetic trees were performed with the ClustalW program (20) version 1.8 in the DNA Data Bank of Japan (Mishima, Japan; http://www.ddbj.nig.ac.jp/htmls/E-mail/clustalw-e.html). The distance matrices for the aligned sequences with all gaps ignored were calculated using the Kimura two-parameter method (11), and the neighbor-joining method was used for constructing a phylogenetic tree (16). The stability of the tree obtained was estimated by bootstrap analysis for 100 replications using the same program. Tree figures were generated using the TreeView program, version 1.61 (14).
A primer set, forward primer PLA-HS475F and reverse primer PLA-HS1198R, was designed based upon the alignment data to specifically amplify an A. platys groESL fragment. Another set of primers, PLA-CSM136F and PLA-CS1359R, was also designed based upon the alignment data of gltA. PCR conditions were the same as described above but with an annealing temperature of 58°C and the use of 40 cycles. The specificity of the reaction was tested with DNA extracted from the three strains of A. platys and related species, including A. phagocytophila (formerly the HGE strain Webster) (J. S. Dumler), E. equi strain California (J. E. Madigan), E. phagocytophila strain 1602 (A. Garcia-Perez), A. marginale strain Florida (G. H. Palmer), E. canis strain Oklahoma (J. Dawson), Wolbachia pipientis (M. Taylor), and Neorickettsia helminthoeca (Y. Rikihisa). The sensitivities of both PCR systems were also examined using DNA from A. platys strain France. The DNA was diluted 10-fold from 1:1 to 1:10,000 with distilled water.
A 1,670-bp groESL fragment of A. platys was determined to contain 41 bp of the partial groES, 51 bp of the spacer region, and 1,577 bp of the groEL coding region. The groESL operon was compared with that of other Anaplasma and Ehrlichia bacteria reported previously and was found to be closely related to the operon of A. phagocytophila and A. marginale, with 81.4 and 78.8% identity, respectively. The level of similarity among groESL sequences was much lower than that for the 16S rRNA gene sequence in the same species (98.6% with A. phagocytophila and 96.1% with A. marginale). The spacer length of 51 nucleotides was similar to that for related Anaplasma species: 52 bp for A. phagocytophila and 47 bp for A. marginale. The percent identities of the nucleotide sequence in the spacer region of A. platys compared to that of A. phagocytophila and to that of A. marginale were 74.5 and 72.3%, respectively, revealing a greater degree of divergence than for the entire groEL coding region.
After the initial identification of the 955-bp partial sequence of A. platys gltA using the degenerate PCR strategy, the full-length open reading frame extending from the ATG start codon to the TAA stop codon was determined using the Genome Walker PCR method. The length of the gltA open reading frame was 1,236 bp and encoded a protein of 411 amino acids. The complete gltA sequence was compared with that of other Anaplasma and Ehrlichia bacteria reported previously (10) and found to be closely related to those of A. phagocytophila and A. marginale, with 62.7 and 63.2% identity, respectively. The level of similarity among ehrlichial gltA was much lower than that for the 16S rRNA gene sequence in the same species. Thus, the sequence of gltA is much more variable among these species than is groESL. The length of the gltA sequence of A. platys (1,236 bp) is the same as that of A. phagocytophila but slightly shorter than that of A. marginale and A. centrale (1,254 bp).
In topology, the gltA-based phylogenetic tree (Fig. 1a) was very similar to the tree derived from analysis of the 16S rRNA gene analyses (3) and the groEL-based tree reported previously (21). However, the trees constructed from gltA and groESL nucleotide sequences showed more distance than the 16S rRNA-based trees. These findings also support the use of gltA-based and groESL-based comparisons in determining the phylogeny of Anaplasma, Ehrlichia, and Neorickettsia agents and strengthen the 16S rRNA- and groESL-based phylogeny reported recently (7). There have been reports of A. platys-like organisms identified based on 16S rRNA analysis, including bacteria from white-tailed deer in North America (6) and from a cow in South Africa (7). It would be interest to determine the phylogenetic position of these agents by analyzing the gltA and groESL gene sequences.
FIG. 1.
Phylogenetic relationship of various Anaplasma, Ehrlichia, and Neorickettsia spp. based on the nucleotide sequences of gltA (a) and groESL (b) genes. The neighbor-joining method was used to construct the phylogenetic tree with the ClustalW program. The scale bar represents 10% divergence. The numbers at nodes are the proportions of 100 bootstrap resamplings that support the topology shown. The GenBank accession numbers of the groESL sequences used to construct the phylogenetic tree and aligned data are as follows: A. phagocytophila (strain HGE agent), AF165812; E. equi, AF172162; E. phagocytophila, U96729; A. marginale, AF165812; E. chaffeensis, L1 0917; E. canis, U96731; E. muris, AF210459; Ehrlichia sp. detected from I. ovatus, AB032711; Ehrlichia (Cowdria) ruminantium, U13638; Neorickettsia (Ehrlichia) risticii, U96732; Neorickettsia (Ehrlichia) sennetsu, U88092; Rickettsia prowazekii, Y15783; Bartonella henselae, U96734. The GenBank accession numbers of the gltA sequences used for comparative analysis are as follows: A. phagocytophila (strain HGE agent), AF304136; E. equi, AF304137; E. phagocytophila, AF304138; A. marginale, AF304139; A. centrale, AF304141; E. chaffeensis, AF304142; E. canis, AF304143; E. muris, AF304144; Ehrlichia sp. detected from I. ovatus, AF304145; E. (C.) ruminantium, AF304146; N. (E.) risticii, AF304147; N. (E.) sennetsu, AF304148: N. helminthoeca, AF304149; R. prowazekii, M17149; B. henselae, U78514.
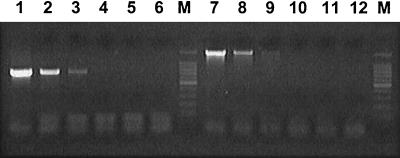
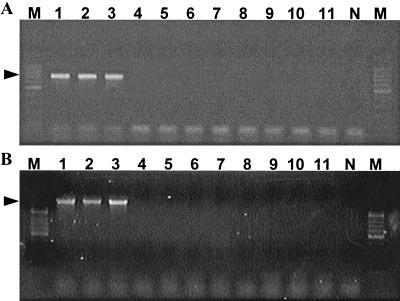
PCR is a powerful tool for epidemiological or diagnostic purposes because of its high sensitivity and specificity; however, there are few molecular tools available for A. platys. All PCR assays reported previously were developed based upon 16S rRNA gene sequences (4, 5, 9, 12, 13). In the present study, new PCR primers were designed to amplify A. platys DNA specifically and were based upon the alignment data of groESL and gltA of A. platys and closely related Anaplasma and Ehrlichia species. The PCR produced a fragment of 724 bp from groESL and 1,459 bp from gltA with the DNA from A. platys strain France (Fig. 2). The sensitivities of the PCR systems were examined by using diluted DNA. Both groESL- and gltA-based PCRs detected DNA diluted 1:100 (Fig. 2), similar to the 16S rRNA-based method (9). As 5 μl of the original DNA solution contained genomic DNA from approximately 375 platelets infected with A. platys, both PCR systems can detect DNA from 3.75 infected platelets in a reaction mixture. The specificity of both systems was also examined using DNA of three A. platys strains from different geographic locations, France, Japan, and Venezuela, and using DNA from related species, including A. phagocytophila and A. marginale. Figure 3 shows that both PCR systems were specific for A. platys. Furthermore, the gltA-based PCR amplifies the whole gltA sequence of A. platys, which contains both start and stop codons. As the sequences of gltA and groESL have greater variation than the sequence of 16S rRNA, the sequence analysis of PCR products may supply useful information for phylogenic studies of the agents. Our findings suggest that both groESL- and gltA-based PCRs are useful for the specific detection of A. platys DNA, and they would be additional molecular tools for both phylogenetic study and diagnosis in veterinary medicine.
FIG. 2.
The sensitivities of the A. platys-specific PCR based upon the groESL (lanes 1 to 6) and gltA (lanes 7 to 12) genes were evaluated. DNA equivalent to that from 375, 37.5, 3.75, 0.375, and 0.0375 infected platelets was used as a template for the amplicons demonstrated in lanes 1 and 7, 2 and 8, 3 and 9, 4 and 10, and 5 and 11, respectively. Distilled water was used in lanes 6 and 12 as a negative control. Lane M, 100-bp DNA ladder.
FIG. 3.
A. platys-specific PCRs based upon the groESL (A) and gltA (B) genes were evaluated for their specificity with DNA from A. platys strains from Somieres, France (lane 1), Okinawa, Japan (lane 2), and Venezuela (lane 3), and from A. marginale (lane 4), A. centrale (lane 5), A. phagocytophila strain HGE agent (lane 6), E. equi (lane 7), E. phagocytophila (lane 8), W. pipientis (lane 9), E. canis (lane 10), and N. helminthoeca (lane 11), with distilled water as a negative control (lane N). Positive bands (arrows) were observed only with DNA of the three strains of A. platys in both groESL- and gltA-based PCRs.
Nucleotide sequence accession numbers.
The nucleotide sequences of groESL and gltA of A. platys strain France determined herein have been deposited in the GenBank database under the accession numbers AY044161 and AB058782, respectively.
Acknowledgments
We acknowledge the technical expertise of the DNA Core Facility of the Center for Gene Research, Yamaguchi University, which is supported by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan. This work was supported in part by a grant for an international joint research project from Institut National de la Sante et de la Recherche Medicale in France and the Japan Society for the Promotion of Science and a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (grant no. 14360190).
We also thank E. B. Breitschwerdt for the A. platys DNA from a dog in Venezuela and J. S. Dumler for scientific suggestions and English correction.
REFERENCES
- 1.Allsopp, M. T. E. P., E. S. Visser, J. L. du Plessis, S. W. Vogel, and B. A. Allsopp. 1997. Different organisms associated with heartwater as shown by analysis of 16S ribosomal RNA gene sequences. Vet. Parasitol. 71:283-300. [DOI] [PubMed] [Google Scholar]
- 2.Beaufils, J.-P., H. Inokuma, J. Martin-Granel, P. Jumelle, M. Barbault-Jumelle, and P. Brouqui. 2002. Anaplasma platys (Ehrlichia platys) infection in a dog in France: description of the case, and characterization of the agent. Rev. Med. Vet. 153:85-90. [Google Scholar]
- 3.Chae, J.-S., J. E. Foley, J. S. Dumler, and J. E. Madigan. 2000. Comparison of the nucleotide sequence of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from northern California. J. Clin. Microbiol. 38:1364-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, A. C. H., W. L. Chang, C. T. Lin, M. J. Pan, and S. C. Lee. 1996. Canine infectious cyclic thrombocytopenia found in Taiwan. J. Vet. Med. Sci. 58:473-476. [DOI] [PubMed] [Google Scholar]
- 5.Chang, W. L., and M. J. Pan. 1996. Specific amplification of Ehrlichia platys DNA from blood specimen by two-step PCR. J. Clin. Microbiol. 34:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olsen. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odoceileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 7.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Pulmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia, descriptions of six new species combination and designation of Ehrlichia equi and ′HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 8.Harvey, J. W., C. F. Simpson, and J. M. Gaskin. 1978. Cyclic thrombocytopenia induced by a rickettsia-like agent in dogs. J. Infect. Dis. 137:182-188. [DOI] [PubMed] [Google Scholar]
- 9.Inokuma, H., K. Ohno, T. Onishi, D. Raoult, and P. Brouqui. 2001. Detection of ehrlichial infection by PCR in dogs from Yamaguchi and Okinawa Prefecture. Jpn. J. Vet. Med. Sci. 63:815-817. [DOI] [PubMed] [Google Scholar]
- 10.Inokuma, H., P. Brouqui, M. Drancourt, and D. Raoult. 2001. Citrate synthase gene sequences: a new tool for phylogenetic and identification of Ehrlichia. J. Clin. Microbiol. 39:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura, M. 1980. A simple method for estimating evolutional rates of base substitutions through comparative studies of nucleotide sequence. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 12.Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. McPherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 37:2631-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew, J. S., S. A. Ewing, G. L. Murphy, K. M. Kocan, R. E. Corstvet, and J. C. Fox. 1997. Characterization of a new isolate of Ehrlichia platys (Order Rickettsiales) using electron microscopy and polymerase chain reaction. Vet. Parasitol. 68:1-10. [DOI] [PubMed] [Google Scholar]
- 14.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 15.Parola, P., H. Inokuma, J.-L. Camicas, P. Brouqui, and D. Raoult. 2001. Detection of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7:1014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Med. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 17.Suksawat, J., C. Pitulle, C. Arraga-Alvarado, K. Madridal, S. I. Hancock, and E. B. Breitschwerdt. 2001. Coinfection with three Ehrlichia species in dogs from Thailand and Venezuela with emphasis on consideration of 16S ribosomal DNA secondary structure. J. Clin. Microbiol. 39:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner, J. W., G. A. Storch, R. S. Buller, A. M. Liddell, S. L. Stockham, Y. Rikihisa, S. Messenger, and C. D. Paddock. 2000. PCR amplification and phylogenetic analysis of groESL operon sequences from Ehrlichia ewingii and Ehrlichia muris. J. Clin. Microbiol. 38:2746-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acid Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu, X. J., X.-F. Zhang, J. W. McBride, Y. Zhang, and D. H. Walker. 2001. Phylogenetic relationship of Anaplasma marginale and "Ehrlichia platys' to other Ehrlichia species determined by groEL amino acid sequences. Int. J. Syst. Evol. Microbiol. 51:1143-1146. [DOI] [PubMed] [Google Scholar]