Abstract
All-trans retinoic acid (ATRA) suppresses growth of cervical dysplasias in vivo, although the sensitivity to retinoids is frequently lost during cervical carcinogenesis. It has been suggested that prolonged treatment or use of higher doses of retinoids might offer favorable response rates. We found SiHa cervical squamous carcinoma cells that were virtually resistant to ATRA-induced growth-inhibitory effects at physiological doses (10−7 to 10−6 M) to be more responsive at pharmacological doses (10−5 to 10−4 M). The growth inhibition by high-dose (10−4 M) ATRA was associated with a sustained activation of interferon regulatory factor 1 (IRF-1), while a low dose (10−6 M) of ATRA activated IRF-1 only transiently. Antisense IRF-1 inhibited the high-dose (10−4 M), ATRA-mediated growth arrest; forced expression of IRF-1 caused a significant reduction in cell growth. High-dose (10−4 M) ATRA increased binding of NF-κB and STAT1 proteins to sequences that originated from the IRF-1 promoter region, while low-dose (10−6 M) ATRA induced only NF-κB binding. A delayed tyrosine phosphorylation of the signal transducer and activator of transcription-1 (STAT1) was observed after high-dose (10−4 M) but not low-dose (10−6 M) ATRA treatment. In agreement with this, induction of IRF-1 mRNA by ATRA was only modest and transient in a STAT1 knockout cell line, suggesting the importance of STAT1 in sustained IRF-1 expression. Our data showed that ATRA is capable of inducing dose-dependent cellular changes, which might be appropriate to overcome resistance to retinoids that frequently develops during cervical carcinogenesis.
Vitamin A and its natural or synthetic derivatives (collectively known as retinoids) (29) are potent regulators of growth of various malignancies, including cervical cancer (14).
In clinical trials, ATRA could reverse or suppress low-grade or moderate- to high-grade cervical dysplasias (8, 17). However, retinoids were not effective in patients with more advanced dysplasias (17); this finding is similar to the resistance to ATRA observed with HPV16-transformed cervical keratinocytes in vitro (25). It has been suggested that prolonged treatment or use of higher doses of retinoids might offer a favorable response rate (19).
Retinoids are potent modulators of cellular proliferation and differentiation. In cervical carcinoma cells, retinoic acid induces interferon regulatory factor (IRF-1) (28), which is responsible for growth arrest (11, 21, 28). In growth-arrested cells, IRF-1 mRNA expression is markedly elevated, but its expression declines prior to and during DNA synthesis: in this context, IRF-1 is a tumor suppressor (10). IRF-1 expression can be stimulated by ATRA at the level of transcription through a gamma interferon-activated site (GAS) (20), via an NF-κB site (21), or directly via a retinoid-responsive element (30) found in the promoter of the IRF-1 gene.
Accordingly, our aim was to determine the effects of different doses of ATRA on the expression and regulation of IRF-1 and on the subsequent inhibition of growth in cervical squamous carcinoma (SiHa) cells.
MATERIALS AND METHODS
Cell lines.
The HPV16-positive cervical squamous carcinoma cell line (SiHa) was purchased from ATCC and maintained at 37°C in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum in a 5% CO2 atmosphere. 2fTGH and U3A fibroblast cell lines were gifts from George Stark (The Cleveland Clinic Foundation Research Center, Cleveland, Ohio), and they were maintained at 37°C in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum plus 250 μg of hygromycin/ml in a 5% CO2 atmosphere.
Plasmids.
The expression vector that contains IRF-1 cDNA (pHuIRF3-1) was kindly provided by Tadatsugu Taniguchi (Department of Immunology, Graduate School of Medicine and Faculty of Medicine, University of Tokyo, Tokyo, Japan). The IRF-1 cDNA was PCR amplified and subcloned into the pCR3.1 bidirectional eukaryotic TA cloning vector (Invitrogen, Carlsbad, Calif.) in both sense and antisense orientations, according to the manufacturer's instructions.
Antibodies.
Anti-STAT1 and anti-phospho-STAT1 antibodies were purchased from Upstate Biochemical, Inc. (Lake Placid, N.Y.). The anti-IRF-1 antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
MTT assay.
The growth rates of cells were measured with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 2 × 103 cells in 0.2 ml of culture medium were plated in each well of a 96-well culture plate. A total of 10 wells per time point were used for treatment. Cells were analyzed at regular intervals of 2, 4, 6, 8, and 10 days by the addition of 40 μl of MTT, 1.25 μg of MTT/ml of phosphate-buffered saline (PBS), to each well of the plate. The cells were incubated at 37°C for 2.5 h, the medium was aspirated, and the cells were then lysed in 100 μl of dimethyl sulfoxide. Conversion of MTT to formazan by metabolically viable cells was monitored at 570 nm in an enzyme-linked immunosorbent assay reader, and the results were analyzed by regression analysis from triplicate experiments.
Western blot analysis.
Cells were washed, scraped in PBS, and then centrifuged. The pellet was resuspended in a RIPA buffer containing protease inhibitors and kept on ice for 60 min. After centrifugation the protein content of the supernatant (whole-cell extract) was determined by a Bio-Rad DC (Hercules, Calif.) method. Fifty micrograms of whole-cell extract was analyzed by standard methods on a sodium dodecyl sulfate-polyacrylamide gel as described earlier (3). Proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) and incubated with the primary antibody at 4°C overnight. Protein bands were detected by an enhanced chemiluminescent method (ECL; Amersham Pharmacia, Piscataway, N.J.). Bands were analyzed on an AlphaImager system (Alpha Innotech, San Leandro, Calif.). Blots were stripped and reprobed several times. Equal protein loads were checked by rehybridizing the blots with a glyceraldehyde-3-phosphate dehydrogenase (G3PDH) antibody (Advanced Immunochemical, Long Beach, Calif.) that served as a constitutively expressed internal control.
RNA isolation and semiquantitative reverse transcription-PCR.
Cells were washed with PBS and directly lysed in TriReagent-LS (Molecular Research Center, Inc., Cincinnati, Ohio) and precipitated according to the manufacturer's recommendations. One microgram of RNA was reverse transcribed (SuperScript II; Gibco/BRL, Grand Island, N.Y.) and subjected to PCR amplification as described earlier (2). Primers for IRF-1 were custom designed and synthesized (Genosys, The Woodlands, Tex.). Primers for G3PDH were purchased from Clontech (Palo Alto, Calif.). PCR fragments were resolved by agarose gel electrophoresis and transferred to a nylon membrane (Amersham Pharmacia, Piscataway, N.J.) and hybridized with end-labeled oligonucleotide probes. Oligonucleotide probes were custom designed and synthesized. Autoradiograms were analyzed by densitometry (AlphaImager; Alpha Innotech, San Leandro, Calif.).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared by lysing cells in a buffer containing 10 mM HEPES (pH 7.9), 60 mM KCl, 1 mM dithiothreitol, 1 mM EDTA, 0.5% NP-40, and 1 mM phenylmethylsulfonyl difluoride. Five to ten micrograms of nuclear extracts were incubated in a binding buffer (10 mM Tris-HCl [pH 7.6], 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 6% glycerol, 1 mM dithiothreitol, 1 μg of polydIC) in the presence or absence of a 100-fold molar excess of unlabeled competitor DNA and/or appropriate antibodies on ice for 10 min. Following incubation, 10,000 cpm of 32P-end-labeled oligonucleotide probe was added and the reaction was incubated at room temperature for an additional 30 min. The DNA-protein complexes were separated from free probe by electrophoresis on a 4 to 6% polyacrylamide gel. The gel was dried and subjected to autoradiography. Oligonucleotides were custom designed and synthesized (Genosys, Inc.). Annealing of individual oligonucleotides was done according to standard protocols.
EMSA oligonucleotides.
For gel shift competitions and experiments, the following double-stranded oligonucleotides were used. (The sense strand is shown. The oligonucleotides were synthesized by Genosys, Inc., and annealed according to a standard protocol.) IRF1-NF-κB (21), TTAGCGGGATTCCCCAGCCCT; IRF1-GAS (23), AGCCTGATTTCCCCGAAATGAC.
Plasmid transfection. Cells were transfected with the recombinant IRF-1 plasmid using a GenePORTER 2 transfection reagent (GTS Inc., San Diego, Calif.). Cells were plated at 5 × 105 cells/60-mm dish density in 1 ml of culture medium 24 h before the transfection so that they will be 60 to 90% confluent on the day of transfection. Then, 4 μg of plasmid DNA was diluted in 80 μl of serum-free medium plus 20 μl of GenePorter 2, which gives a final volume of 100 μl. This mixture was incubated at room temperature for 10 min and then was added to the cells. After 24 h, fresh medium was added. Transfections were made in triplicate. Controls such as mock-transfected and vector-transfected cells were included.
RESULTS
ATRA inhibits growth of SiHa cells in a dose-dependent fashion.
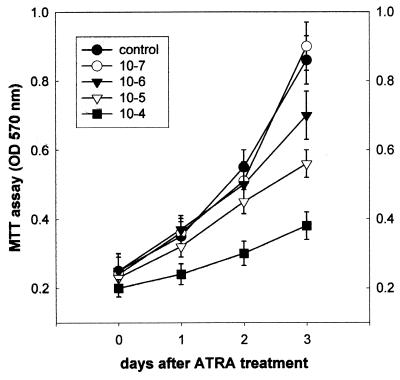
SiHa cells were grown in the presence or absence of ATRA for various time points (Fig. 1). Cell growth was determined by an MTT assay. Apparently, physiological (low) doses (10−7 and 10−6 M) of ATRA did not inhibit cell growth significantly, while administration with pharmacological (high) doses (10−5 and 10−4 M) of ATRA caused significant growth inhibition.
FIG. 1.
Effects of ATRA on cell growth. SiHa cells were seeded in a multiwell plate and treated with either low-dose (10−7 and 10−6 M) or high-dose (10−5 and 10−4 M) ATRA for the times indicated. An MTT assay was employed to determine the amounts of viable cells. Results are shown as means ± standard deviations (n = 3).
IRF-1 participates in ATRA-induced growth inhibition.
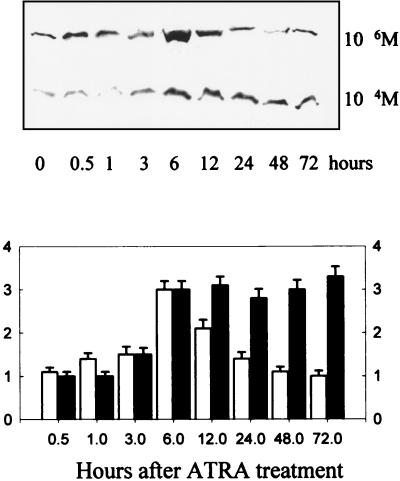
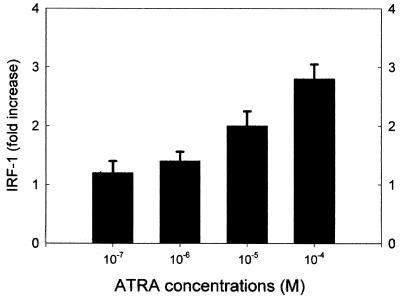
IRF-1 is an important regulator of cell proliferation (27), so its role in ATRA-induced growth inhibition was investigated. Accordingly, protein lysates from SiHa cells treated with low-dose (10−6 M) or high-dose (10−4 M) ATRA were evaluated by Western immunoblotting. ATRA at a concentration of 10−6 M increased protein levels of IRF-1 at a peak of fourfold 6 h after treatment (Fig. 2), while induction of IRF-1 protein by 10−4 M ATRA was sustained. We also determined IRF-1 protein levels 24 h after treatment with various concentrations of ATRA (Fig. 3). Apparently, induction of IRF-1 at 24 h was dose dependent; significant induction occurred at high doses. In another set of experiments, SiHa cells were transiently transfected with an IRF-1 expression vector. Using a trypan blue exclusion test, we determined the cell number 48 h posttransfection. The results (representing the mean of three independent measurements in which the standard deviation did not exceed 4%) showed that IRF-1 transfection significantly reduced cell numbers, to 65% of the level of untreated control cells. Also, transient transfection of an antisense IRF-1 construct abrogated ATRA-induced growth arrest.
FIG. 2.
Effects of ATRA on IRF-1 protein levels in SiHa cells. Confluent cultures of SiHa cells were treated with either low-dose (10−6 M) or high-dose (10−4 M) ATRA for the times indicated. Top, Western immunoblotting was performed on the total cell lysate as described in Materials and Methods. Bottom, Densitometric results are expressed as fold change to the untreated controls. Values are shown as means ± standard deviations (n = 3).
FIG. 3.
Dose-dependent induction of IRF-1 by ATRA. Confluent cultures of SiHa cells were treated for 24 h with various doses of ATRA as indicated. Western immunoblotting was performed on the total cell lysate as described in Materials and Methods. Densitometric results are expressed as fold change to the untreated controls. Values are shown as means ± standard deviations (n = 3).
Prolonged induction of IRF-1 requires STAT1.
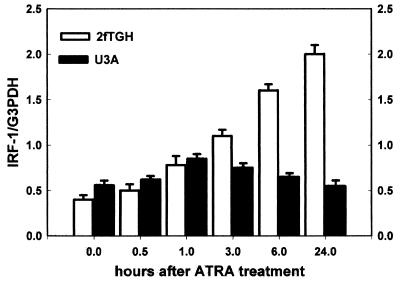
IRF-1 mRNA levels were increased only moderately and transiently after treatment with ATRA in a STAT1 knockout cell line (U3A), while treatment of the parental (STAT1 wild-type) line (2fTGH) with ATRA increased it significantly and for a longer time (Fig. 4).
FIG. 4.
Prolonged induction of IRF-1 requires STAT1. STAT1 knockout (U3A) or STAT1 wild-type (2fTGH) cells were treated with ATRA (10−6 M) for different time points. IRF-1 mRNA levels were determined by reverse transcription-PCR together with the constitutively expressed G3PDH. IRF-1 levels are given as ratios of IRF-1/G3PDH. Values are shown as means ± standard deviations (n = 3).
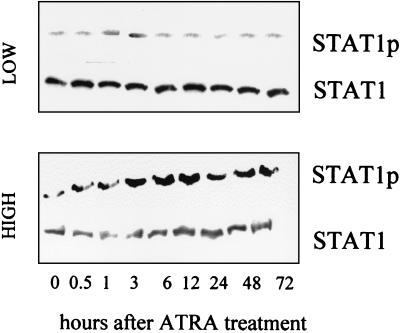
Only high-dose ATRA induces phosphorylation of STAT1.
Considering the role of STAT1 in regulation of IRF-1 expression, we determined the phosphorylation status of STAT1 after ATRA treatment. Treatment with 10−6 M ATRA did not affect STAT1 tyrosine phosphorylation (Fig. 5). In contrast, 10−4 M ATRA treatment significantly elevated STAT1 tyrosine phosphorylation (Fig. 5) between 3 to 12 h posttreatment. STAT1 levels, however, remained unchanged during ATRA treatment.
FIG. 5.
Effects of different doses of ATRA on tyrosine phosphorylation of STAT1 in SiHa cells. Confluent cultures of SiHa cells were treated with low-dose (10−6 M) or high-dose (10−4 M) ATRA for the times indicated. Western immunoblotting was performed using anti-phosphoSTAT1 (STAT1p) as well as anti-STAT1 antibodies. Experimental data shown are representative of three independent experiments.
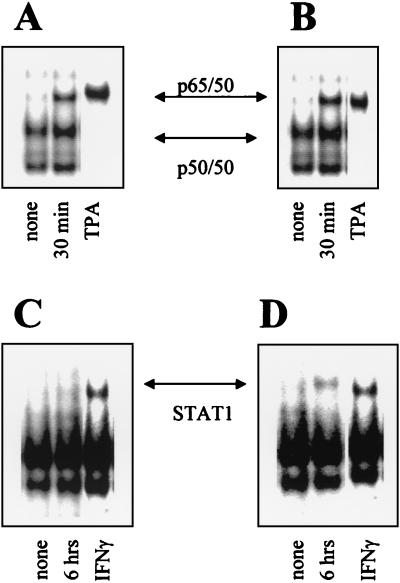
NF-κB binding to an oligonucleotide from the IRF-1 promoter is independent of ATRA concentrations.
IRF-1 transcription is regulated via NF-κB sites in the IRF-1 gene promoter (26). Accordingly, we tested NF-κB binding to oligonucleotides corresponding to NF-κB-binding sites in the IRF-1 promoter by a gel-shift assay. As shown, NF-κB (mostly the p65-p50 complex) binding to the IRF-1 promoter was increased after 30 min by both low- and high-dose ATRA treatment (Fig. 6A and B).
FIG. 6.
Effects of different doses of ATRA treatment on binding of cellular proteins to oligonucleotides representing NF-κB or GAS sites of the IRF-1 promoter. Nuclear extracts from SiHa cells treated with low-dose (A and C) or high-dose (B and D) ATRA were incubated with labeled oligonucleotides representing the NF-κB (A and B) or GAS (C and D) binding sites of the IRF-1 promoter, and an EMSA was performed as described in Materials and Methods. In panels A and B we showed only the 30-min time point that represented the first appearance of NF-κB binding. STAT1 binding to the GAS oligomer (C and D) appeared after 6 h of treatment. Next, 100× cold ligand was used to demonstrate specificity of both probes (data not shown). Tetradecanoyl phorbol acetate (TPA)- or gamma interferon (IFNγ)- treated cell extracts were used as positive controls. Preincubations with specific anti-NF-κB p65 and p50 or STAT1 antibodies were performed to demonstrate the presence of these proteins in the observed complexes (data not shown).
STAT1 binding to an oligonucleotide from the IRF-1 promoter depends on ATRA concentration.
IRF-1 transcription could be also regulated via GAS/SIE sites in the IRF-1 gene promoter (9). Accordingly, we tested STAT1-binding oligonucleotides corresponding to a GAS site in the IRF-1 promoter by a gel-shift assay. In the extracts of ATRA-treated cells, binding of STAT1 to a GAS site was observed only after high-dose, but not low-dose, ATRA treatment (Fig. 6C and D).
DISCUSSION
Induction of IRF-1 mRNA and the consequent growth inhibition in cervical squamous carcinoma cells by ATRA have been demonstrated (7). Clinically, retinoids reversed or suppressed low-grade or moderate- to high-grade cervical dysplasias (7, 8, 17) but were not effective in patients with more advanced dysplasias (16). Although immortalized keratinocytes are more sensitive to growth control by retinoids than their normal counterparts (24), at later stages transformed keratinocytes frequently become resistant to retinoids (4, 25).
SiHa cervical squamous carcinoma cells have been reported to be unresponsive to retinoids (1, 6). In our experiments, SiHa cells exhibited resistance to 10−7 to 10-6 M ATRA-induced growth inhibition, but 10−5 M and especially 10−4 M ATRA significantly inhibited the growth of SiHa cells (Fig. 1).
A hallmark of growth inhibition is the high expression of the IRF-1 gene (27). Ultimately, this induction of IRF-1 is responsible for the observed growth inhibition of target cells. Introduction of an IRF-1 expression vector into SiHa cells caused growth arrest(see above). Similarly, in the presence of an antisense IRF-1 expression plasmid, high-dose (10−4 M) ATRA did not inhibit cell growth (viable cells were at 95% of control level, compared to 60% with ATRA but without the IRF-1 expression plasmid). IRF-1 expression was elevated in ATRA-treated cells in a dose-dependent fashion: low-dose (10−6 M) ATRA increased it temporarily, while high-dose (10−4M) ATRA caused a sustained expression of IRF-1 (Fig. 2 and 3). However, only high-dose (10−5 to 10−4 M) but not low-dose (10−7 to 10−6 M) ATRA could significantly inhibit cell growth in vitro. These data strongly suggested that duration of IRF-1 expression is critical for cell growth inhibition in this system.
Our next set of experiments aimed to determine the molecular events behind this dose-dependent induction of IRF-1 by ATRA. In STAT1 knockout cells (15), ATRA moderately and temporarily induced IRF-1 mRNA (Fig. 4), while IRF-1 induction in the parental cell line (2fTGH) was long lasting under the same conditions. Activation (tyrosine phosphorylation) of STAT1 occurred only after high-dose (10−4 M), but not low-dose (10−6 M), ATRA treatment in SiHa (Fig. 5), suggesting that the capability of high-dose ATRA to activate STAT1 was responsible for the long-lasting activation of IRF-1 expression and consequent inhibition of cell growth. Interestingly, the kinetics of this tyrosine phosphorylation was delayed (3 to 13 h) compared to the immediate-early (5 to 15 min) phosphorylation by cytokines (31). Similarly, binding of the activated STAT1 to the GAS site was a late event (Fig. 6) (6 h).
We observed that ATRA treatment—at both 10−6 and 10−4 M concentrations—induced binding of NF-κB to a double-stranded oligonucleotide originating from the IRF-1 promoter (Fig. 6A and B), similar to that described by others (21). However, binding of STAT1 to its cognate GAS elements in the IRF-1 promoter was observed only after high-dose (10−4 M) ATRA treatment (Fig. 6C to D), suggesting that STAT1 activation was responsible for the sustained IRF-1 expression. Phosphorylation of STAT1 and its binding to the IRF-1 promoter occurred with delayed kinetics compared to IRF-1 activation via NF-κB binding. The effects of GAS and NF-κB sites on the activity of the IRF-1 promoter might be synergistic (12, 18, 22) and could be accountable for the long-lasting activation of IRF-1 expression.
It has been postulated that retinoids directly increase the expression of transcription factors (STAT1 or IRF-1) that play key roles in JAK-STAT signaling, thereby restoring interferon (IFN) sensitivity (5). Our data suggest that ATRA could restore sensitivity to itself by a dose-dependent induction of STAT1, resulting in a sustained activation of the IRF-1 signal that might be critical in determining downstream cellular responses. The downstream targets might include the activation of apoptotic events associated with the IRF-1/CAS pathway (13) or NF-kB induction (21) or both. The role of these pathways in high-dose ATRA-induced inhibition of cell growth, however, needs further evaluation.
Acknowledgments
We acknowledge Tadatsugu Taniguchi (Department of Immunology, Graduate School of Medicine and Faculty of Medicine, University of Tokyo, Tokyo, Japan) for the IRF-1 expression plasmid. U3A and 2fTGH cells were a generous gift from George Stark (The Cleveland Clinic Foundation Research Center, Cleveland, Ohio).
REFERENCES
- 1.Agarwal, C., R. A. S. Chandraratna, M. Teng, S. Nagpal, E. A. Rorke, and R. L. Eckert. 1996. Differential regulation of human ectocervical epithelial cell line proliferation and differentiation by retinoid X receptor- and retinoic acid receptor-specific retinoids. Cell Growth Differ. 7:521-530. [PubMed] [Google Scholar]
- 2.Arany, I., M. M. Brysk, H. Brysk, and S. K. Tyring. 1996. Response to interferon treatment decreases with epidermal dedifferentiation in condylomas. Antivir. Res. 32:19-26. [DOI] [PubMed] [Google Scholar]
- 3.Arany, I., P. Rady, and S. K. Tyring. 1994. Interferon treatment enhances the expression of underphosphorylated (biologically active) retinoblastoma protein in human papilloma virus-infected cells through the inhibitory TGF beta 1/IFN beta cytokine pathway. Antivir. Res. 23:131-141. [DOI] [PubMed] [Google Scholar]
- 4.Borger, D. R., Y. Mi, G. Geslani, L. L. Zyzak, A. Batova, T. S. Engin, L. Pirisi, and K. E. Creek. 2000. Retinoic acid resistance at late stages of human papillomavirus type 16-mediated transformation of human keratinocytes arises despite intact retinoid signaling and is due to a loss of sensitivity to transforming growth factor-beta. Virology 270:397-407. [DOI] [PubMed] [Google Scholar]
- 5.Chelbi-Alix, M. K., and L. Pelicano. 1999. Retinoic acid and interferon signaling cross talk in normal and RA-resistant APL cells. Leukemia 13:1167-1174. [DOI] [PubMed] [Google Scholar]
- 6.Giandomenico, V., F. Lancillotti, G. Fiorucci, Z. A. Percario, R. Rivabene, W. Malorni, E. Affabris, and G. Romeo. 1997. Retinoic acid and IFN inhibition of cell proliferation is associated with apoptosis in squamous carcinoma cell lines: role of IRF-1 and TGase II-dependent pathways. Cell Growth Differ. 8:91-100. [PubMed] [Google Scholar]
- 7.Giandomenico, V., G. Vaccari, G. Fiorucci, Z. Percario, S. Vanucchi, P. Matarrese, W. Malorni, G. Romeo, and E. Affabris. 1998. Apoptosis and growth inhibition of squamous carcinoma cells treated with interferon-α, interferon-β and retinoic acid are associated with induction of the cyclin-dependent kinase inhibitor p21. Eur. Cytokine Netw. 9:619-631. [PubMed] [Google Scholar]
- 8.Graham, V., E. S. Surwit, S. Weiner, and F. L. Meyskens, Jr. 1986. Phase II trial of beta-all-trans-retinoic acid for cervical intraepithelial neoplasia delivered via a collagen sponge and cervical cap. West. J. Med. 145:192-195. [PMC free article] [PubMed] [Google Scholar]
- 9.Harada, H., T. Fujita, M. Miyamoto, Y. Kimura, M. Maruyama, A. Furia, T. Miyata, and T. Taniguchi. 1989. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58:729-739. [DOI] [PubMed] [Google Scholar]
- 10.Harada, H., M. Kitagawa, N. Tanaka, H. Yamamoto, K. Harada, M. Ishihara, and T. Taniguchi. 1993. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science 259:971-974. [DOI] [PubMed] [Google Scholar]
- 11.Harada, H., T. Taniguchi, and N. Tanaka. 1998. The role of interferon regulatory factors in the interferon system and cell growth. Biochimie 80:641-650. [DOI] [PubMed] [Google Scholar]
- 12.Imanishi, D., K. Yamamoto, H. Tsushima, Y. Miyazaki, K. Kuriyama, M. Tomonaga, and T. Matsuyama. 2000. Identification of a novel cytokine response element in the human IFN regulatory factor-1 gene promoter. J. Immunol. 165:3907-3916. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, M. C., T. L. Lin, T. L. Lee, H. T. Huang, C. L. Lin, and C. F. Liao. 2001. IRF-1-mediated CAS expression enhances interferon-gamma-induced apoptosis of HT-29 colon adenocarcinoma cells. Mol. Cell Biol. Res. Commun. 4:353-358. [DOI] [PubMed] [Google Scholar]
- 14.Lippman, S. M., S. E. Benner, and W. K. Hong. 1994. Cancer chemoprevention. J. Clin. Oncol. 12:851-873. [DOI] [PubMed] [Google Scholar]
- 15.McKendry, R., J. John, D. Flavell, M. Muller, I. M. Kerr, and G. R. Stark. 1991. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc. Natl. Acad. Sci. USA 88:11455-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyskens, F. L., Jr., and A. Manetta. 1995. Prevention of cervical intraepithelial neoplasia and cervical cancer. Am. J. Clin. Nutr. 62:1417S-1419S. [DOI] [PubMed] [Google Scholar]
- 17.Meyskens, F. L., Jr., E. Surwit, T. E. Moon, J. M. Childers, J. R. Davis, R. T. Dorr, C. S. Johnson, and D. S. Alberts. 1994. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J. Natl. Cancer Inst. 86:539-543. [DOI] [PubMed] [Google Scholar]
- 18.Ohmori, Y., R. D. Schreiber, and T. A. Hamilton. 1997. Synergy between interferon-γ and tumor necrosis factor-α in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor κB. J. Biol. Chem. 272:14899-14907. [DOI] [PubMed] [Google Scholar]
- 19.Oridate, N., D. Lotan, M. F. Mitchell, W. K. Hong, and R. Lotan. 1995. Inhibition of proliferation and induction of apoptosis in cervical carcinoma cells by retinoids: implications for chemoprevention. J. Cell. Biochem. Suppl. 13:50-86. [DOI] [PubMed] [Google Scholar]
- 20.Pelicano, L., F. Li, C. Schindler, and M. K. Chelbi-Alix. 1997. Retinoic acid enhances the expression of interferon-induced proteins: evidence for multiple mechanisms of action. Oncogene 15:2349-2359. [DOI] [PubMed] [Google Scholar]
- 21.Percario, Z. A., V. Giandomenico, G. Fiorucci, M. V. Chiantore, S. Vannucchi, J. Hiscott, E. Affabris, and G. Romeo. 1999. Retinoic acid is able to induce interferon regulatory factor 1 in squamous carcinoma cells via a STAT-1 independent signalling pathway. Cell Growth Differ. 10:263-270. [PubMed] [Google Scholar]
- 22.Pine, R. 1997. Convergence of TNFα and IFNγ signalling pathways through synergistic induction of IRF-1/ISGF-2 is mediated by a composite GAS/NF-κB promoter element. Nucleic Acids Res. 25:4346-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pine, R., A. Canova, and C. Schindler. 1994. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFNα and IFNγ, and is likely to autoregulate the p91 gene. EMBO J. 13:158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirisi, L., A. Batova, G. R. Jenkins, J. R. Hodam, and K. E. Creek. 1992. Increased sensitivity of human keratinocytes immortalized by human papillomavirus type 16 DNA to growth control by retinoids. Cancer Res. 52:187-193. [PubMed] [Google Scholar]
- 25.Sarma, D., X. Yang, G. Jin, M. Shindoh, M. M. Pater, and A. Pater. 1996. Resistance to retinoic acid and altered cytokeratin expression of human papillomavirus type 16-immortalized endocervical cells after tumorigenesis. Int. J. Cancer 65:345-350. [DOI] [PubMed] [Google Scholar]
- 26.Sims, S. H., Y. Cha, M. F. Romine, P.-Q. Gao, K. Gottlieb, and A. B. Deisseroth. 1993. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol. Cell. Biol. 13:690-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi, T., M. S. Lamphier, and N. Tanaka. 1997. IRF-1: the transcription factor linking the interferon response and oncogenesis. Biochim. Biophys. Acta 1333:M9-M17. [DOI] [PubMed] [Google Scholar]
- 28.Um, S. J., E. J. Kim, E. S. Hwang, S. J. Kim, S. E. Namkoong, and J. S. Park. 2000. Antiproliferative effects of retinoic acid/interferon in cervical carcinoma cell lines: cooperative growth suppression of IRF-1 and p53. Int. J. Cancer 85:416-423. [PubMed] [Google Scholar]
- 29.Vahlquist, A. 1999. What are natural retinoids? Dermatology 199(Suppl 1):3-11. [DOI] [PubMed] [Google Scholar]
- 30.Weihua, X., V. Kolla, and D. V. Kalvakolanu. 1997. Modulation of interferon action by retinoids. Induction of murine STAT1 gene expression by retinoic acid. J. Biol. Chem. 272:9742-9748. [DOI] [PubMed] [Google Scholar]
- 31.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]