Abstract
Background
Self-reported health problems following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are common and often include relatively non-specific complaints such as fatigue, exertional dyspnoea, concentration or memory disturbance and sleep problems. The long-term prognosis of such post-acute sequelae of COVID-19/post-COVID-19 syndrome (PCS) is unknown, and data finding and correlating organ dysfunction and pathology with self-reported symptoms in patients with non-recovery from PCS is scarce. We wanted to describe clinical characteristics and diagnostic findings among patients with PCS persisting for >1 year and assessed risk factors for PCS persistence versus improvement.
Methods and findings
This nested population-based case-control study included subjects with PCS aged 18–65 years with (n = 982) and age- and sex-matched control subjects without PCS (n = 576) according to an earlier population-based questionnaire study (6–12 months after acute infection, phase 1) consenting to provide follow-up information and to undergo comprehensive outpatient assessment, including neurocognitive, cardiopulmonary exercise, and laboratory testing in four university health centres in southwestern Germany (phase 2, another 8.5 months [median, range 3–14 months] after phase 1). The mean age of the participants was 48 years, and 65% were female. At phase 2, 67.6% of the patients with PCS at phase 1 developed persistent PCS, whereas 78.5% of the recovered participants remained free of health problems related to PCS. Improvement among patients with earlier PCS was associated with mild acute index infection, previous full-time employment, educational status, and no specialist consultation and not attending a rehabilitation programme. The development of new symptoms related to PCS among participants initially recovered was associated with an intercurrent secondary SARS-CoV-2 infection and educational status. Patients with persistent PCS were less frequently never smokers (61.2% versus 75.7%), more often obese (30.2% versus 12.4%) with higher mean values for body mass index (BMI) and body fat, and had lower educational status (university entrance qualification 38.7% versus 61.5%) than participants with continued recovery. Fatigue/exhaustion, neurocognitive disturbance, chest symptoms/breathlessness and anxiety/depression/sleep problems remained the predominant symptom clusters. Exercise intolerance with post-exertional malaise (PEM) for >14 h and symptoms compatible with myalgic encephalomyelitis/chronic fatigue syndrome were reported by 35.6% and 11.6% of participants with persistent PCS patients, respectively. In analyses adjusted for sex-age class combinations, study centre and university entrance qualification, significant differences between participants with persistent PCS versus those with continued recovery were observed for performance in three different neurocognitive tests, scores for perceived stress, subjective cognitive disturbances, dysautonomia, depression and anxiety, sleep quality, fatigue and quality of life. In persistent PCS, handgrip strength (40.2 [95% confidence interval (CI) [39.4, 41.1]] versus 42.5 [95% CI [41.5, 43.6]] kg), maximal oxygen consumption (27.9 [95% CI [27.3, 28.4]] versus 31.0 [95% CI [30.3, 31.6]] ml/min/kg body weight) and ventilatory efficiency (minute ventilation/carbon dioxide production slope, 28.8 [95% CI [28.3, 29.2]] versus 27.1 [95% CI [26.6, 27.7]]) were significantly reduced relative to the control group of participants with continued recovery after adjustment for sex-age class combinations, study centre, education, BMI, smoking status and use of beta blocking agents. There were no differences in measures of systolic and diastolic cardiac function at rest, in the level of N-terminal brain natriuretic peptide blood levels or other laboratory measurements (including complement activity, markers of Epstein–Barr virus [EBV] reactivation, inflammatory and coagulation markers, serum levels of cortisol, adrenocorticotropic hormone and dehydroepiandrosterone sulfate). Screening for viral persistence (PCR in stool samples and SARS-CoV-2 spike antigen levels in plasma) in a subgroup of the patients with persistent PCS was negative. Sensitivity analyses (pre-existing illness/comorbidity, obesity, medical care of the index acute infection) revealed similar findings. Patients with persistent PCS and PEM reported more pain symptoms and had worse results in almost all tests. A limitation was that we had no objective information on exercise capacity and cognition before acute infection. In addition, we did not include patients unable to attend the outpatient clinic for whatever reason including severe illness, immobility or social deprivation or exclusion.
Conclusions
In this study, we observed that the majority of working age patients with PCS did not recover in the second year of their illness. Patterns of reported symptoms remained essentially similar, non-specific and dominated by fatigue, exercise intolerance and cognitive complaints. Despite objective signs of cognitive deficits and reduced exercise capacity, there was no major pathology in laboratory investigations, and our findings do not support viral persistence, EBV reactivation, adrenal insufficiency or increased complement turnover as pathophysiologically relevant for persistent PCS. A history of PEM was associated with more severe symptoms and more objective signs of disease and might help stratify cases for disease severity.
Author summary
Why was this study done?
Self-reported health problems following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have commonly been described and may persist for months. They typically include relatively non-specific complaints such as fatigue, exertional dyspnoea, concentration or memory disturbance and sleep problems.
The long-term prognosis of this post-COVID-19 syndrome (PCS) is unknown. To the best of our knowledge, measurable single or multiple organ dysfunction and pathology and their correlation with self-reported symptoms in patients with non-recovery from PCS for more than a year have not been well described.
What did the researchers do and find?
We invited individuals who had participated in a previous population-based survey of post-acute complaints and symptoms after acute SARS-CoV-2 infection to undergo a follow-up investigation that included a comprehensive medical evaluation. Results were compared between patients with persistent PCS (cases) and those study participants who had not developed PCS (controls).
We found that two-thirds of the individuals with PCS had persisting disease for more than a year with no major changes in symptom clusters.
Objective signs of organ dysfunction and pathology among individuals with persistent PCS correlated with self-reported symptoms, were detected more often among PCS patients with longer lasting post-exertional malaise, and included both reduced physical exercise capacity and reduced cognitive test performances while we did not find differences in the results of multiple laboratory investigations after adjustment for possible confounders such as body mass index and educational status.
The severity of the index infection, lower educational status, no previous full-time employment and (need for) specialist consultation or a rehabilitation programme (the latter probably due to reverse causation) were factors for non-recovery from PCS.
What do these findings mean?
In the majority of patients, PCS symptoms did not improve in the second year of their illness and typically continued to include fatigue and measurable exercise intolerance and cognition deficits, but there seems to be no major pathology in laboratory investigations. Sociodemographic variables appear to play a role not only for the development, but also for the non-recovery from PCS.
Limitations include the missing information on pathology before acute infection, response and recall biases.
In a population-based case-control study, Raphael Peter and colleagues investigate the clinical characteristics and diagnostic findings in patients with post-COVID-19 syndrome.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic resulted in over 750 million confirmed cases worldwide [1]. Besides morbidity and mortality in the acute phase of the infection, considerable post-acute health problems and sequelae are reported [2–5]. The WHO defined post-coronavirus disease 2019 [COVID-19] condition as the continuation or development of new symptoms after acute SARS-CoV-2 infection, lasting for at least 2 months, and being unexplained by an alternative diagnosis [6]. Slightly different definitions and alternative wording (such as long COVID-19), post-acute sequelae of SARS-CoV-2 infection or post-COVID-19 syndrome [PCS]) have been used [7,8], and are in part relevant for the widely differing prevalence estimates in previous studies [9]. Furthermore, some prevalence estimates may have been biased since many of the early studies focussed on hospitalised or healthcare-seeking patients only [10–12], although most COVID-19 patients do not require medical treatment for the acute infection. Further limitations have been the difficulty of including an uninfected control group to estimate background prevalence of symptoms. In fact, many studies have assessed PCS prevalence and trajectories by using various questionnaires asking for self-reported health problems. Although many of the symptoms may impact everyday functioning, health-related quality of life and work ability [3,4], they lack specificity (i.e., they can have many other causes and overlap with other conditions), are usually not well evaluable in claims data studies and have often not been validated through systematic protocol-prespecified diagnostic studies.
More recently, several diagnostic studies have been able to confirm some impaired neurocognitive functions in patients with PCS [13–17], while the results for cardiac and pulmonary function tests have been variable and less consistent [18,19]. Laboratory studies have suggested a number of altered blood biomarkers (such as various cytokines/chemokines, immune cell markers, plasma metabolites and cortisol) with potential pathophysiologic and diagnostic relevance in PCS patients [20–23]. Many of the clinical or laboratory diagnostic studies; however, were small, lacked appropriate controls, adjustments (e.g., for age and sex, smoking and body composition, educational or socioeconomic status, severity of the acute infection and pre-existing or concomitant disease), or showed only subtle changes compared to controls. Higher body mass index (BMI), for example, has been predictive for persisting dyspnoea in COVID-19 patients [24]. Obesity has been reported as a risk factor for PCS [10,25,26], and mechanistic evidence of why obesity could make people more susceptible to PCS has been provided [27]. Outside the COVID-19 context, BMI in association with sex has been found to be a major confounder in studies of proinflammatory markers [28], and obesity itself has also been associated with cognitive dysfunction [29]. Cognitive dysfunction, interestingly, has been measurable after COVID-19 in individuals who were asymptomatic or had no more symptoms than age- and sex-matched uninfected controls [30,31]. Symptom-based phenotypic stratification of PCS, although attractive and intriguing, thus, may be misleading in diagnostic studies if not evaluated against adequate controls and if not adjusted for potential confounders.
The aim of the present study was to medically validate PCS among individuals having participated in our previous population-based study of SARS-CoV-2 infected adults (6–12 months after infection) and having been considered to have the syndrome based on self-reported new symptoms with at least moderate impairment in daily life plus either impaired general health or work ability [32]. From this population, we invited a number of individuals with PCS (as cases) and of symptom-free individuals after recovery (as controls) to undergo a comprehensive outpatient medical examination and clinical evaluation, including standardised and validated questionnaires, neurocognitive and cardiopulmonary testing and laboratory investigations. Based on estimates from experience in our and other PCS care centres and the literature available at the time of planning the study [33,34], we hypothesised that roughly half of the cases following the invitation would be persistent cases at the time of medical examination and expected that our clinical evaluation of patients with persistent PCS would result in an appreciable proportion of subjects with measurable organ dysfunction and pathology and show significant differences in at least one of the medical tests compared to the control group of individuals with continued recovery. We were also interested in markers and risk factors for more severe disease and its possible underlying pathophysiology.
Materials and methods
Study design and selection of participants
This study was a prospective, multi-centre, observational, nested case-control study. Participants with (cases) and without PCS (controls) were recruited from the Epidemiology of Long Covid (EPILOC) phase 1 non-interventional, population-based questionnaire study that included subjects aged 18–65 years who had tested positive for SARS-CoV-2 by PCR between October 1st, 2020 and April 1st, 2021, and whose infection had been notified (compulsory according to the German Infection Protection Act) to the responsible local public health authority (in four administratively and geographically defined regions in the Federal State of Baden-Württemberg in southwestern Germany). We estimated that most participants were infected with the wild type of SARS-CoV-2, that less than 15% of the cohort with B.1.1.7 (alpha) and less than 1% with B.1.351 (beta) [32].
The PCS case definition used was “general health or working capacity recovered to a level no more than 80% (compared to pre-COVID-19), and any new symptom (a list of 30 symptoms was provided, three additional symptoms could be added) of moderate to strong degree regarding impairment in daily life and not already present before the acute infection (excluding vomiting, nausea, stomach ache, diarrhoea, chills, fever)”. Study participants who had recovered to 100% (of general health and work ability perceived in the time before acute infection) and reported no new symptoms of grade moderate-to-strong qualified as controls. Using these definitions, EPILOC phase 1 had categorised 28.5% of the 11,710 evaluable respondents as suffering from PCS (cases), whereas 38% of the respondents were considered as (PCS-free) recovered controls [32].
From these two groups, we invited participants into the phase 2 nested case-control study. Individuals qualifying as neither PCS case nor control were not invited. A total of 982 patients with PCS and 576 frequency-matched symptom-free recovered (control) participants (matched by sex- and age-group with a target sampling ratio of 1,000 cases to 600 controls) followed the invitation and underwent a comprehensive clinical evaluation at one of the four study sites (Fig 1). The unequal sampling ratio was based on the assumption that a significant number of phase 1 patients with PCS might have had recovered until presentation in phase 2, while we expected that only a small number of symptom-free recovered participants might have developed new symptoms compatible with PCS at the time of the clinical evaluation in phase 2.
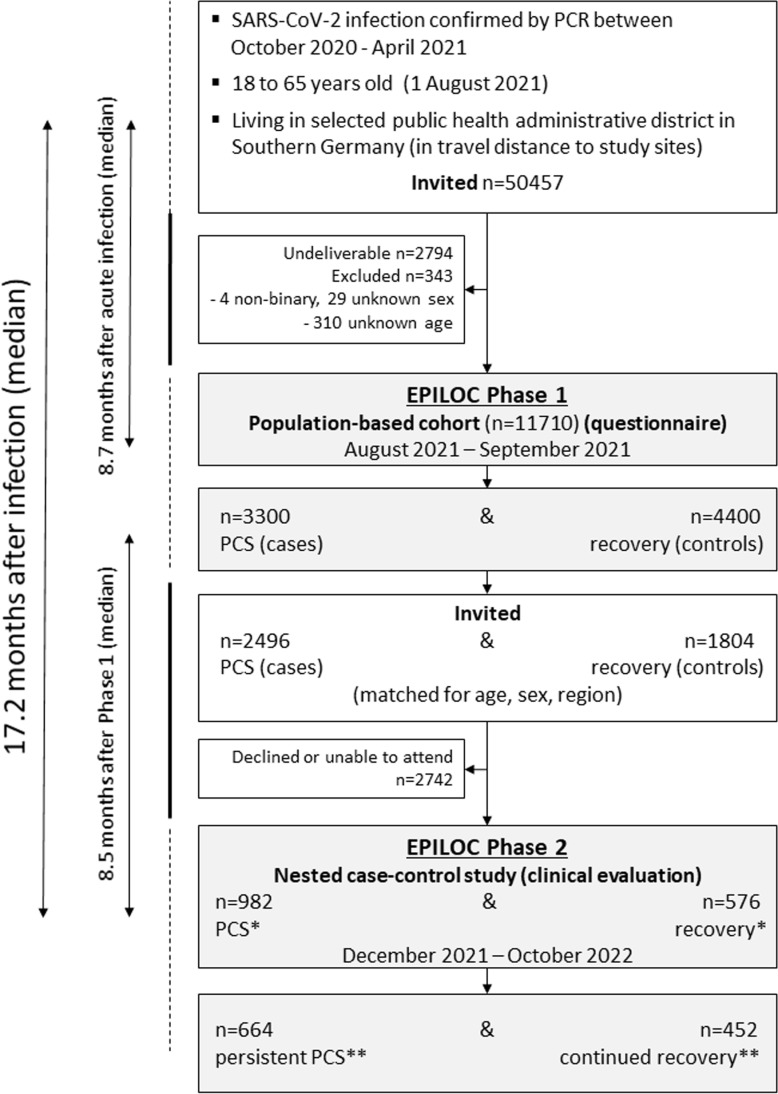
Fig 1. Flow-chart covering EPILOC study phases 1 and 2.
NB * before (at phase 1) and **after clinical examination (in phase 2). The time from PCR-confirmed acute SARS-CoV-2 infection to phase 1 was 8.7 months (median), the time from phase 1 participation until clinical examination in phase 2 was 8.5 months (median), and the median time between acute infection and phase 2 was 17.2 months, ranging from 9.2 to 24.4 months.
All study procedures and analyses were pre-planned and listed in a study handbook (only available in German language) with the exception of un-planned interim analyses of the tests for spike antigen in serum and for SARS-CoV-2 RNA in faecal samples; after negative results in both tests with samples from >100 patients with persistent PCS, the investigation of further samples (as initially planned) was discontinued (see below).
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (S1 STROBE Checklist).
Data sources and measurements
Besides the information collected during the phase 1 study (see [32]), we again used data from a number of standardised questionnaires that included sociodemographic characteristics, lifestyle factors, SARS-CoV-2 vaccines received, medical history and current symptoms. The symptom questionnaire (see S1 Appendix) contained the same items as in phase 1 and asked for medical treatment of current symptoms, for the grade to which each symptom impaired daily life and activities (“how much do you feel impaired by this at the moment?”) using a 4-point Likert scale (none, light, moderate or strong) and for the degree of general health and working capacity regained (compared with the time before the index infection). Based on this information, we defined participants either as having persistent (or improved) PCS or as individuals with continued recovery (or as recovered individual with worsening), using the same definition as in phase 1.
We evaluated individual symptoms, but also symptom clusters composed of highly interrelated individual symptoms as defined earlier after analysis of the phase 1 study results [32]. Details of the approach to define symptom clusters have previously been described [32].
Clinical assessments.
Apart from taking the medical history, the study physician completed a modified-Medical Research Council Dyspnoea Scale (mMRC), asked for post-exertional malaise (PEM) and its duration [35], and clarified questions and responses to the questionnaires. The participants underwent a complete physical examination, including measurements of height, weight, heart rate (HR) at rest and blood pressure.
The maximal grip strength was recorded after three measurements of both hands with a digital hand dynamometer. Whole body composition was measured using a multi-frequency bioelectrical impedance analysis device and expressed as % body fat. Methodological details are included in S2 Appendix.
Validated questionnaires.
Study participants were asked to fill validated questionnaires on sleep quality (Pittsburgh Sleep Quality Index [PSQI], Insomnia Severity Index [ISI], Epworth Sleepiness Scale [ESS]), fatigue (Chalder Fatigue Scale [CFQ-11]), health-related quality of life (Short Form-12 Health Survey [SF-12], assessing both physical and mental components), symptoms of depression (Patient Health Questionnaire 9 [PHQ-9]), anxiety (Generalised Anxiety Disorder 7 [GAD-7]), perceived stress (10-item Perceived Stress Scale [PSS-10]), subjective cognition (“Fragebogen zur geistigen Leistungsfähigkeit” [FLei]), and dysautonomia symptoms (Composite Autonomic Symptom Score 31 [COMPASS-31]). More details and references are given in S2 Appendix.
Neurocognitive tests.
All participants were asked to undergo neuropsychological tests administered by trained clinical staff. The test battery included the Montreal Cognitive Assessment (MoCA), the Trail making test part B (TMT-B), and the Symbol Digit Modalities Test (SDMT) (S2 Appendix).
Cardiopulmonary function tests.
We recorded resting 12-lead electrocardiograms (ECGs) and pulse oximeter measurements of peripheral oxygen saturation (SpO2). Resting echocardiograms were performed according to current guidelines, with determination of the left ventricular volume and ejection fraction (LV-EF), the ratio between early mitral inflow and mitral annular early diastolic velocities (LV-E/e’), the ratio of maximal early to late diastolic transmitral flow velocity (LV-E/A), and grading of diastolic dysfunction (for details see S3 Appendix).
Participants underwent cardiopulmonary exercise testing (CPET) using a ramp protocol on the cycle ergometer. Before CPET, spirometry was conducted to assess lung function with recording of the forced expiratory volume in one second (FEV1), and the forced vital capacity (FVC). During CPET, blood pressure, SpO2 and ECG with HR were monitored. We evaluated the following CPET parameters: HR, oxygen uptake (VO2max), breathing reserve (BR), respiratory exchange ratio (RER) and the slope of minute ventilation to carbon dioxide production (VE/VCO2 slope). More details are included in S3 Appendix.
Laboratory investigations.
Routine laboratory investigations included a rapid chromatographic immunoassay (for SARS-CoV-2 antigen in nasopharyngeal samples), blood cell counts, coagulation, clinical chemistry, levels of C-reactive protein (CRP), thyroid-stimulating hormone (TSH), glycated haemoglobin (HbA1c), N-terminal pro-brain natriuretic peptide (pro-BNP), classical pathway complement haemolytic activity (CH50) (determined for participants at two centres), immunoglobulin (Ig) G and IgM antibodies against cytomegalovirus (CMV), antibodies against SARS-CoV-2 nucleocapsid (N) protein and the S1 receptor binding domain of the viral spike glycoprotein, and others (see S4 Appendix for analytes and methods).
Cortisol, adrenocorticotropic hormone (ACTH) and dehydroepiandrosterone sulfate (DHEA-S) levels in frozen morning blood samples were measured centrally using standard methods (see S4 Appendix for details). Additional laboratory investigations in our central virology laboratory included the measurement of antibodies to Epstein–Barr virus (EBV) antigens, of spike antigen in serum (in a subgroup of individuals with persistent PCS and continued recovery), and SARS-CoV-2 RNA by reverse transcription polymerase chain reaction (RT-PCR) in faecal samples (see S4 Appendix for detailed methodologies).
Statistical methods
Participant characteristics were analysed descriptively. Predictors of case-control status change from phase 1 to phase 2 were evaluated using logistic regression. Regression models were run separately for phase 1 cases and controls, and mutually adjusted odds ratios were calculated for improvement in cases (no longer fulfilling the case definition) and worsening in recovered individuals (no longer fulfilling the control definition).
Results of standardised questionnaires, neurocognitive tests, laboratory measurements, electrocardiographic, echocardiographic and spiroergometric parameters were presented as least square means. Due to a high correlation between PSQI, ISI and ESS, we present only the results for the PSQI instrument (see S2 Appendix).
We used analysis of covariance with adjustment for sex-age class combinations and university entrance qualification. Additional adjustments were made as indicated. Geometric instead of natural means are reported where appropriate. The area under the curve (AUC) for discrimination of persistent cases versus stable controls (excluding improved cases and worsened controls), based on logistic regression, is also reported. We did not use imputation, but missing observations were excluded in the specific analyses. Statistical procedures were performed with the SAS statistical software package (release 9.4 SAS Institute) or R version 4.3.2.
Ethical approval
The study was registered with “Deutsches Register Klinischer Studien” (DRKS 00027362). All participants provided written informed consent. Ethical approval was obtained from the respective ethical review boards of the study centres in Freiburg (21/1484_1), Heidelberg (S-846/2021), Tübingen (845/2021BO2), and Ulm (337/21).
Results
Baseline characteristics of the study participants
The study included 982 participants who were phase 1 PCS patients (cases) and 576 age- and sex-matched recovered subjects (phase 1 controls). As shown in S1 Table, the sex and age distributions were (as expected by design) similar in cases and controls. Most (65.8%) participants were female, and the mean age was 48 years. The mean time between phases 1 and 2 was 9.1 months for patients with PCS (range 3.0–14.2 months) and 8.4 months for recovered individuals (range 2.9–14.0 months). A similar proportion of patients with PCS versus recovered individuals experienced a secondary SARS-CoV-2 infection (23%) and almost all had been vaccinated against SARS-CoV-2 once or more times before phase 2 (S1 Table).
Differences between patients with PCS and recovered participants already known from the analysis of phase 1 data included the proportion of obese participants, smokers, pre-existing diseases, medical care (outpatient or inpatient versus none) for the earlier index acute SARS-CoV-2 infection (each higher among patients with PCS) and educational level (less frequent university entrance qualification among patients with PCS). Healthcare utilisation in the last 6 months prior to phase 2 examination (in particular regarding specialist physician consultation) and attending a rehabilitation programme were also much more frequent among patients with PCS. S1 Fig describes the probability of participation in the two groups of cases and controls by selected baseline characteristics.
Risk for PCS persistence
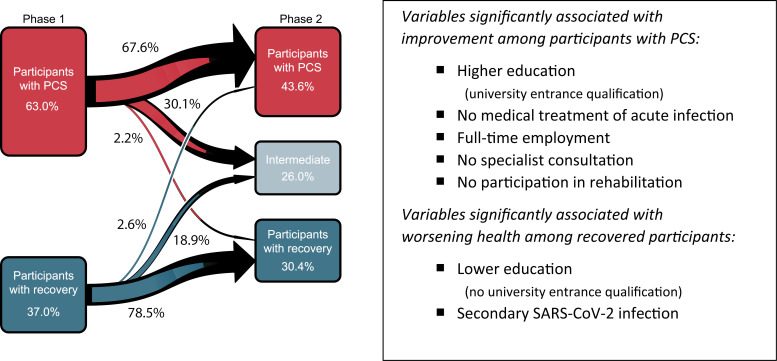
Roughly, two-thirds (67.6%) of the 982 participants with PCS in phase 1 were considered having persistent PCS (according to our working definition) after the phase 2 clinical assessment. Most of the remaining participants with PCS phase 1 (30.1%) had improved until phase 2, but only very few (2.2%) were classified as completely clinically recovered (Fig 2). Conversely, the majority (78.5%) of symptom-free participants from phase 1 who participated in phase 2 were classified as having continued recovery, but almost one-fifth (18.9%) reported new symptoms (without fulfilling the PCS case definition), and 2.6% were classified as (new-onset) PCS cases (Fig 2). S2 Fig displays changes in the prevalence of the five main symptom clusters among the patients with PCS between phases 1 and 2. In the overall population, the net prevalence of all symptom clusters, except anxiety, depression or sleep disorder decreased, most prominent for smell and taste disorders (S2 Fig).
Fig 2. Change in case-control status of study participants (N = 1,558) between initial questionnaire survey (phase 1) and clinical examination (phase 2).
The time from phase 1 participation until clinical examination in phase 2 was 8.5 months (median). Factors associated with improvement of patients with PCS in phase 1 and with worsening among recovered participants in phase 1 were assessed for significance after calculation of ORs with mutual adjustment for the following variables: sex, age, university entrance qualification, marital status, medical treatment of acute infection, obesity (BMI ≥ 30 kg/m²), full-time employment (phase 1), time between phases 1 and 2 (per month), secondary SARS-CoV-2 infection since phase 1, two or more vaccine doses, (any) specialist consultation in the last 6 months, participation in a post-COVID-rehabilitation program (see S2 Table).
As summarised in Fig 2 (and detailed in S2 Table), factors associated with improvement (either to intermediate or control status) of PCS in an adjusted analysis were educational status (university entrance qualification), full-time employment (at phase 1), no medical care/treatment of the acute index infection (as a proxy for milder acute infection) and no (need for) specialist consultation within the last 6 months or participation in a post-COVID-19 rehabilitation program (the latter two probably a result of reverse causation). For recovered individuals, the odds of worsening until phase 2 were higher with lower educational status and after a secondary SARS-CoV-2 infection since phase 1. SARS-CoV-2 vaccination had no measurable association with improvement or worsening. Also, age, sex or the time between phases 1 and 2 was not statistically significantly associated with case-control status changes (S2 Table).
Clinical evaluation of persistent PCS cases
In comparison of the characteristics of the four groups (persistent PCS, PCS improved, continued recovery, recovery with worsening) (Table 1), we found differences in educational status, smoking, BMI (as well as obesity prevalence and body fat), medical care/treatment of the acute SARS-CoV-2 index infection and prevalence of comorbidities. The proportion of participants with obesity was highest in persistent PCS (30.2% compared with 12.4% in stable controls), and many more participants with continued recovery than with persistent PCS have had no medical care for their acute index infection, had obtained university entrance qualification and were never smokers (Table 1). We found a much higher current use of medication in patients with persistent PCS versus participants with continued recovery across all anatomical-therapeutic-chemical groups (S3 Table).
Table 1. Characteristics of the phase 2 study participants by case-control status.
| Persistent PCS | PCS with improvement | Recovery with worsening | Continued recovery | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean or frequency | N | Mean or frequency | N | Mean or frequency | N | Mean or frequency | |
| Male, n (%) | 664 | 227 (34.2) | 318 | 122 (38.4) | 124 | 44 (35.5) | 452 | 153 (33.9) |
| Female, n (%) | 437 (65.8) | 196 (61.6) | 80 (64.5) | 299 (66.2) | ||||
| Age at phase 1 (years), mean (SD) | 664 | 48.9 (12.1) | 318 | 46.3 (12.5) | 124 | 48.4 (11.9) | 452 | 48.5 (12.4) |
| Age class at phase 1 (years), n (%) | ||||||||
| 18–29 | 74 (11.1) | 49 (15.4) | 14 (11.3) | 55 (12.2) | ||||
| 30–39 | 76 (11.5) | 50 (15.7) | 14 (11.3) | 55 (12.2) | ||||
| 40–49 | 128 (19.3) | 60 (18.9) | 27 (21.8) | 91 (20.1) | ||||
| 50–59 | 267 (40.2) | 116 (36.5) | 49 (39.5) | 159 (35.2) | ||||
| 60+ | 119 (17.9) | 43 (13.5) | 20 (16.1) | 92 (20.4) | ||||
| University entrance qualification, n (%) | 664 | 257 (38.7) | 318 | 163 (51.3) | 124 | 60 (48.4) | 452 | 278 (61.5) |
| Full-time employment at phase 1, n (%) | 663 | 306 (46.2) | 318 | 194 (61.0) | 124 | 66 (53.2) | 451 | 223 (49.5) |
| Smoking status, n (%) | 662 | 317 | 124 | 452 | ||||
| Current | 52 (7.9) | 20 (6.3) | 10 (8.1) | 17 (3.8) | ||||
| Former | 205 (31.0) | 78 (24.6) | 36 (29.0) | 93 (20.6) | ||||
| Never | 405 (61.2) | 219 (69.1) | 78 (62.9) | 342 (75.7) | ||||
| BMI at phase 2 (kg/m²), mean (SD) | 662 | 28.0 (6.1) | 318 | 26.6 (5.5) | 124 | 26.1 (4.5) | 452 | 25.0 (4.5) |
| Obese (≥30 kg/m²), n (%) | 200 (30.2) | 64 (20.1) | 25 (20.2) | 56 (12.4) | ||||
| Body fat (per cent), mean (SD) | 659 | 32.2 (10.6) | 316 | 29.3 (9.4) | 123 | 28.5 (9.0) | 452 | 27.4 (8.9) |
| >25% in men, >35% in women, n (%) | 344 (52.2) | 122 (38.6) | 45 (36.6) | 126 (27.9) | ||||
| Treatment of acute SARS-CoV-2 infection, n (%) | ||||||||
| No medical care | 655 | 341 (52.1) | 313 | 200 (63.9) | 123 | 108 (87.8) | 450 | 408 (90.7) |
| Outpatient care | 258 (39.4) | 92 (29.4) | 12 (9.8) | 37 (8.2) | ||||
| Inpatient care (without ICU) | 45 (6.9) | 17 (5.4) | 3 (2.4) | 3 (0.7) | ||||
| Intensive care | 11 (1.7) | 4 (1.3) | 0 (0.0) | 2 (0.4) | ||||
| Comorbidities, n (%) | 664 | 318 | 124 | 452 | ||||
| Cardiovascular disease | 29 (4.4) | 2 (0.6) | 1 (0.8) | 3 (0.7) | ||||
| Chronic pulmonary disease | 62 (9.3) | 34 (10.7) | 6 (4.8) | 23 (5.1) | ||||
| Diabetes mellitus | 33 (5.0) | 8 (2.5) | 2 (1.6) | 5 (1.1) | ||||
| Cancer | 13 (2.0) | 3 (0.9) | 1 (0.8) | 4 (0.9) | ||||
Note: BMI, body mass index; SD, standard deviation.
Predominant symptoms, symptom clusters and symptom severity.
An analysis of the frequency of all reported symptoms with all degrees of impairment among participants with persistent PCS (S3 Fig) showed the predominance of individual complaints and symptoms that we summarise in the symptom clusters “fatigue”, “neurocognitive disturbance”, “chest symptoms”, “smell or taste disorder” and “anxiety/depression/sleep disorder”. As shown in S3 Fig, there were some differences in individual symptom prevalence and severity between female and male participants (with females being more affected—similar to findings in phase 1), and several individual symptoms were scored comparatively low regarding their grade of daily life impairment (e.g., dizziness, paraesthesia, confusion and chest pain). Abdominal symptoms, fever and chills, and skin problems were rare, similar to what we found in phase 1.
We next displayed the distribution of (case-defining, i.e., moderate-or-severe) predominant symptoms and symptom clusters among patients with persistent PCS versus the other subgroups, together with the scoring results from corresponding validated questionnaires either as proportions at relevant cut-offs (Table 2) or as adjusted average ratings (Fig 2). As shown in Table 2, fatigue, neurocognitive disturbance and chest symptoms were among the predominant symptom clusters in persistent PCS. We observed a large overlap of these three clusters, with a substantial proportion of patients with persistent PCS (26.8%) reporting moderate or severe symptoms in all three main symptom clusters (S4 Fig). The second largest overlap was the combination of fatigue and neurocognitive disturbance (prevalence, 20.1%). One or more of these three main symptom clusters affected the vast majority (90.4%) of participants with persistent PCS.
Table 2. Prevalence of major symptom new clusters/symptoms and associated severity ratings according to validated questionnaires by case-control status at clinical examination in phase 2.
| Persistent PCS | PCS with improvement | Recovery with worsening | Continued recovery | |||||
|---|---|---|---|---|---|---|---|---|
| N | Frequency | N | Frequency | N | Frequency | N | Frequency | |
| Fatigue/exhaustion/exertion intolerance, n (%) | ||||||||
| Chronic fatigue and/or rapid physical exhaustion as moderate/severe symptom cluster | 661 | 449 (67.9) | 318 | 51 (16.0) | 124 | 15 (12.1) | 452 | 0 (0.0) |
| CFQ-11 bimodal score >3 | 649 | 598 (92.1) | 311 | 200 (64.3) | 122 | 44 (36.1) | 441 | 35 (7.9) |
| CFQ-11 total score >19 | 453 (69.8) | 76 (24.4) | 15 (12.3) | 6 (1.4) | ||||
| CFQ-11 total score >29 | 60 (9.2) | 3 (1.0) | 0 (0.0) | 0 (0.0) | ||||
| Fatigue with PEM lasting >14 h | 612 | 218 (35.6) | 300 | 15 (5.0) | 122 | 3 (2.5) | 450 | 0 (0.0) |
| ME/CFS-like (according to Canadian consensus criteria) | 649 | 75 (11.6) | 317 | 3 (1.0) | 124 | 2 (1.6) | 452 | 0 (0.0) |
| Neurocognitive disturbance, n (%) | ||||||||
| Concentration difficulties as moderate/severe symptom | 663 | 416 (62.8) | 317 | 44 (13.9) | 124 | 15 (12.1) | 451 | 3 (0.7) |
| Memory difficulties as moderate/severe symptom | 664 | 360 (54.2) | 317 | 40 (12.6) | 124 | 11 (8.9) | 451 | 1 (0.2) |
| FLei memory subscore >19 | 662 | 360 (54.4) | 317 | 73 (23.0) | 122 | 11 (9.0) | 451 | 16 (3.6) |
| FLei attention subscore >19 | 643 | 281 (43.7) | 310 | 46 (14.8) | 123 | 9 (7.3) | 448 | 7 (1.6) |
| FLei total score >45 | 629 | 396 (63.0) | 309 | 80 (25.9) | 121 | 20 (16.5) | 445 | 18 (4.0) |
| Chest symptoms, n (%) | ||||||||
| Chest pain, shortness of breath and/or wheezing as moderate/severe symptom cluster | 664 | 315 (47.4) | 318 | 42 (13.2) | 124 | 15 (12.1) | 452 | 0 (0.0) |
| Dyspnoea mMRC grade 1 | 656 | 274 (41.8) | 317 | 72 (22.7) | 124 | 18 (14.5) | 452 | 10 (2.2) |
| Dyspnoea mMRC grade 2 | 48 (7.3) | 5 (1.6) | 2 (1.6) | 0 (0.0) | ||||
| Dyspnoea mMRC grade 3–4 | 21 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Anxiety/depression/sleep disorder, n (%) | ||||||||
| Anxiety as moderate/severe symptom | 663 | 121 (18.3) | 318 | 18 (5.7) | 124 | 3 (2.4) | 452 | 0 (0.0) |
| GAD-7 score >9 | 658 | 244 (37.1) | 316 | 40 (12.7) | 123 | 8 (6.5) | 447 | 11 (2.5) |
| Depression as moderate/severe symptom | 664 | 176 (26.5) | 318 | 19 (6.0) | 124 | 10 (8.1) | 451 | 3 (0.7) |
| PHQ-9 score >14 | 646 | 148 (22.9) | 308 | 17 (5.5) | 122 | 6 (4.9) | 446 | 2 (0.5) |
| Sleep disorder as moderate/severe symptom | 664 | 327 (49.3) | 318 | 57 (17.9) | 123 | 33 (26.8) | 452 | 12 (2.7) |
| PSQI score >10 | 625 | 224 (35.8) | 307 | 40 (13.0) | 120 | 8 (6.7) | 439 | 8 (1.8) |
| ISI score >14 | 644 | 296 (46.0) | 313 | 55 (17.6) | 122 | 17 (14.0) | 443 | 11 (2.5) |
| ESS score >10 | 636 | 259 (40.7) | 310 | 76 (24.5) | 119 | 23 (19.3) | 443 | 31 (7.0) |
Note: CFQ-11 total score >19 or bimodal score >3: fatigue, CFQ-11 total score >29: extreme fatigue. FLei total score >45: subjectively impaired mental performance, FLei memory subscore >19: subjectively impaired memory, FLei attention subscore >19: subjectively impaired attention. mMRC grade 1: dyspnoea when hurrying or walking up a slight hill, mMRC grade 2: walks slower than people of the same age because of dyspnoea or has to stop for breath when walking at own pace, mMRC grade 3–4: stops for breath after walking 100 m or after a few minutes, or too dyspneic to leave house or breathless when dressing. GAD-7 score >9: moderate-to-severe anxiety. PHQ-9 score >14: moderate-to-severe depression. PSQI score >10: poor sleep quality. ISI score >14: insomnia; ESS score >10: excessive daytime sleepiness. For abbreviations and methods see text and S2 Appendix).
The frequency estimates for a given symptom or symptom cluster varied with more detailed questioning or rating, allowing a more valid estimation of severity. Fatigue as the most prevalent self-reported symptom cluster (based on reporting chronic fatigue or rapid physical exhaustion of moderate or strong grade in the symptom questionnaire), for example, had a prevalence among patients with persistent PCS of 67.6%, while the prevalence assessed with the CFQ-11 scale at a bimodal score >3 (earlier defined as a “fatigue case”) or at a total score >19 was 92.1% and 69.8%, respectively. The prevalence of extreme fatigue (CFQ-11 total score >29) was relatively low among patients with persistent PCS (9.2%) (Table 2).
We also assessed the prevalence of fatigue with PEM lasting >14 h (35.6%) and of symptoms compatible with a myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome (ME/CFS)-like condition (11.6%). Interestingly, the frequency of individual symptoms (of any degree) among patients with PEM (lasting >14 hours) differed from those who had no PEM. Patients with persistent PCS and PEM had more symptoms than patients with persistent PCS without PEM. In particular, pain syndromes (chest pain, myalgia, joint pain, melalgia and headache), confusion and dizziness were more often reported by case patients with PEM (apart from fatigue and exhaustion) (S3 Fig). PEM was highly prevalent (>50%) among patients with persistent PCS who reported symptoms from all three dominant clusters (fatigue, neurocognitive disturbances and chest symptoms) (S5 Fig).
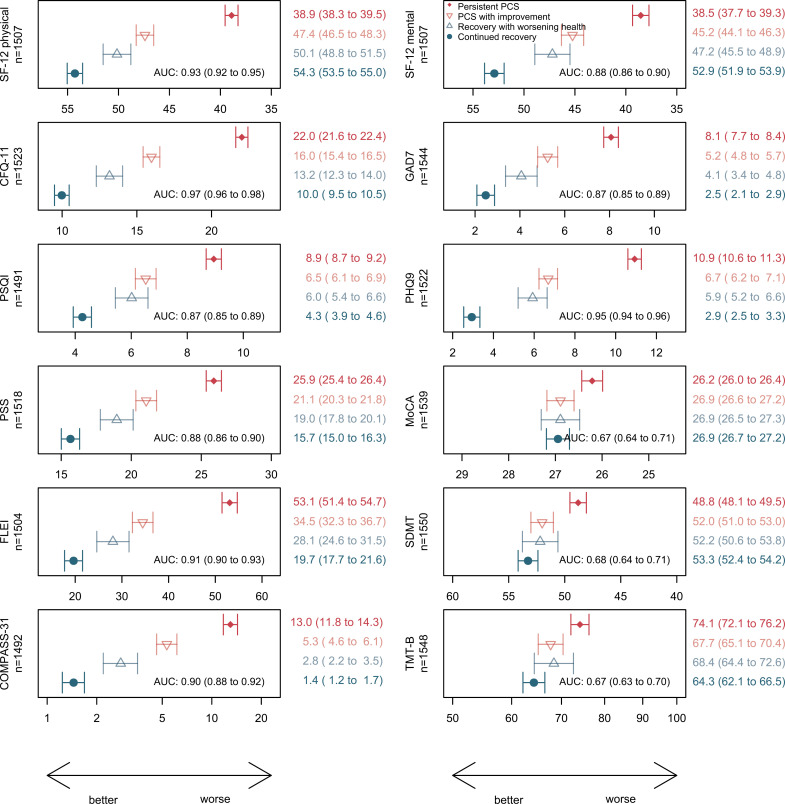
Neurocognitive impairment remained the second most frequent symptom cluster (per symptom questionnaire) after fatigue in patients with persistent PCS, which correlated well with the FLei questionnaire results (Table 2). Dyspnoea was most often non-severe when assessed with mMRC grading (Table 2). The prevalence of mMRC grade 1 dyspnoea among patients with persistent PCS was 41.8%, and dyspnoea of grade 2 or more was seen in 10.5%. Symptoms of anxiety, depression and sleep disorders (that had earlier been classified as a single cluster of highly interrelated symptoms) were also much more prevalent among patients with persistent PCS than among participants with continued recovery. The average scores of CFQ-11, FLei, GAD-7, PHQ-9 and PSQI differed substantially and consistently between the subgroups, and all these instruments discriminated participants with versus those without persistent PCS very well, with the CFQ-11 having the highest AUC (>0.90) (Fig 3).
Fig 3. Means (geometric mean for COMPASS-31 and TMT-B) of self-reported health outcomes and neurocognitive tests (with 95% CI) by case-control status at clinical examination in phase 2, adjusted for sex-age class combinations, study centre and university entrance qualification.
The reported area under the curve (AUC) for persistent PCS vs. continued recovery by the respective instrument also includes sex-age class combinations and university entrance qualification. The AUC for sex-age class combinations, study centre and university entrance qualification alone was 0.64. For comparability, the x-axis is scaled from mean −1 SD to mean + 1 SD for all panels. Abbreviations: PSQI, Pittsburgh Sleep Quality Index; CFQ-11, Chalder Fatigue Scale; SF-12, Short Form-12 Health Survey; PHQ-9, Patient Health Questionnaire 9; GAD-7, Generalised Anxiety Disorder 7; PSS-10, Perceived Stress Scale; Flei, “Fragebogen zur geistigen Leistungsfähigkeit” (subjective mental performance questionnaire); COMPASS-31, Composite Autonomic Symptom Score 31; MoCA, Montreal cognitive assessment scale (points); SDMT, Symbol Digit Modalities Test (number of correct symbols); TMT-B, Trail making test part B (time in seconds).
Symptoms of dysautonomia.
As shown in Fig 3, the average COMPASS-31 score among patients with persistent PCS was 13 compared with <2 among individuals with continued recovery, and the proportion of patients with persistent PCS with a score >19 (suggesting moderate or severe dysautonomia) was 40.7%. Almost half of the individuals with persistent PCS (49.7% compared with 7.5% of individuals with continued recovery) indicated that they experienced weakness, dizziness, light-headedness or difficulty thinking after standing up from sitting or lying down, suggesting orthostatic problems.
Perceived stress and health-related quality of life.
As a measure of stress and health-related quality of life, we used the PSS-10 instrument (scoring from 0 to 40) and the commonly used SF-12 questionnaire with its physical and mental component summary scores, assessing general health and well-being, including the perceived impact of any illnesses or adverse condition on a broad range of functional domains. As shown in Fig 3, all three scores discriminated well between individuals with persistent PCS and those with continued recovery and had similarly high AUCs >0.8. The differences in the average scores between patients with persistent versus improved PCS and between individuals with continued recovery versus initial recovery with worsening showed a similar pattern as the other instruments. A direct comparison of the current SF-12 results for both components among patients with PCS with the results obtained earlier in the same individuals (at phase 1) indicated no improvement in health-related quality of life, with mean changes in the physical subscale of −0.51 (95% confidence interval [CI] [−1.13, 0.10]), and in the mental subscale of −0.92 (95% CI [−1.68, −0.16]), respectively.
Neurocognitive testing.
The results of the three neurocognitive tests are depicted in Fig 3. In adjusted analysis, the mean MoCA score was significantly lower among patients with persistent PCS compared with the other groups, and the proportion of participants with a MoCA score below 26 (suggesting mild-to-moderate cognitive impairment) was 33.3% among patients with persistent PCS versus 18.9% among participants with continued recovery, respectively. Similar patterns were seen with the two other tests, SDMT (assessing impaired attention, concentration and speed of information processing) and TMT-B (to screen executive dysfunction). Although the mean differences between cases and controls were large, the discrimination in adjusted analysis between the two groups; however, was relatively poor for each test (AUCs 0.67 compared to 0.63 without neurocognitive testing). Further adjustment for CFQ-11 and PHQ-9 attenuated the association with MoCA to some degree, with differences for participants with persistent PCS versus participants with continued recovery losing statistical significance (p = 0.0672). However, the additional adjustment had little effect on the association with SDMT and TMT-B (p = 0.0086 and 0.0008).
Grip strength and cardiopulmonary function tests.
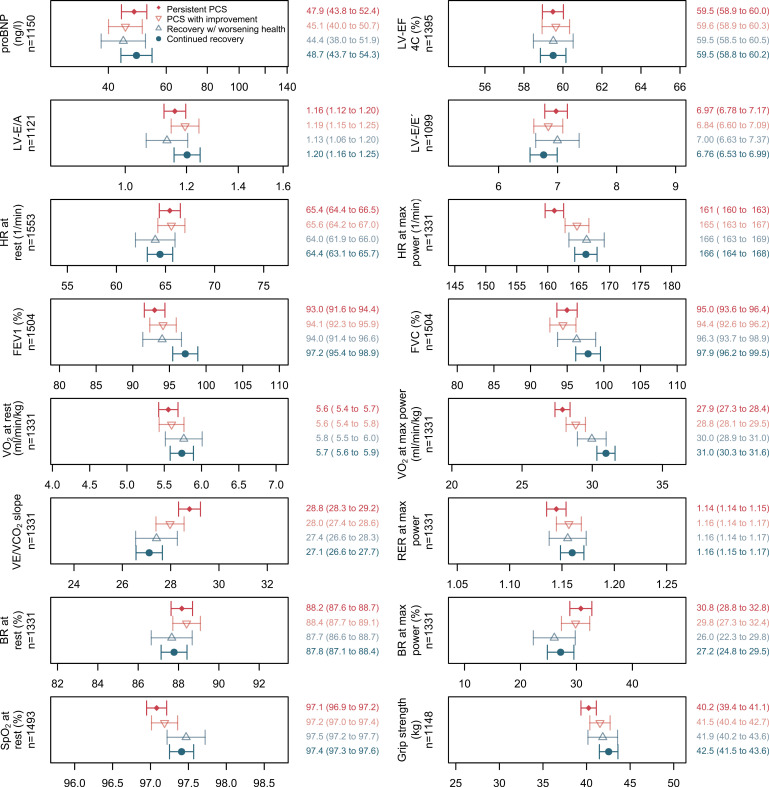
The mean maximal handgrip strength was 40.2 kg among patients with persistent PCS, significantly lower than among participants with continued recovery (42.5 kg) (Fig 4). As expected, grip strength was lower in women than men (30.9 versus 50.7 kg) and associated inversely with body fat and BMI (r = −0.06, 95% CI [−0.12, −0.01] and r = −0.07, 95% CI [−0.12, −0.01]).
Fig 4. Cardiopulmonary function indicators and grip strength (means with 95% CI) by case-control status at the clinical examination in phase 2, adjusted for sex-age class combinations, study centre, university entrance qualification, BMI, smoking status and use of beta blocking agents.
Cardiopulmonary exercise testing could be completed in 1,331 participants (87.2% of participants with continued recovery; 83.7% of patients with persistent PCS). For comparability, the x-axis is scaled from mean −1 SD to mean + 1 SD for all panels. Abbreviations: pro-BNP, N-terminal brain natriuretic peptide; LV-EF, left ventricular volume and ejection fraction; LV-E/eʹ, ratio between early mitral inflow and mitral annular early diastolic velocities; LV-E/A, ratio of maximal early to late diastolic transmitral flow velocity; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SpO2, peripheral oxygen saturation; HR, heart rate; VO2max, oxygen uptake; BR, breathing reserve; RER, respiratory exchange ratio; VE/VCO2 slope, slope of minute ventilation to carbon dioxide production.
As depicted in Fig 4, left ventricular function (including LV-EF, LV-E/eʹ and LV-E/A) and pro-BNP blood levels were not different between the groups. We observed a higher prevalence of diastolic dysfunction grades 1 and 2 among patients with persistent PCS compared with participants with continued recovery (30.9% versus 21.9%) (S4 Table). The difference; however, was not statistically significant after adjustment for sex-age class combinations, study centre, university entrance qualification, BMI and smoking status. Also, we did not observe differences between the subgroups in the mean values for resting HR and BR (Fig 4), respiratory rate (adjusted mean: 16.2–16.7 per minute for all subgroups, p = 0.71) and systolic and diastolic blood pressure (adjusted systolic mean: 128–131 mm Hg for all subgroups, p = 0.88; diastolic mean: 80–81 mm Hg for all subgroups, p = 0.21).
Differences were observed for FEV1 and FVC, SpO2 at rest, and several CPET derived variables (Fig 4). Values for FEV1 (p < 0.0001), FVC (p = 0.0011) and SpO2 (p = 0.0001) were lower among subjects with persistent PCS (versus participants with continued recovery), but the differences were small, and the proportion of persons with FEV1/FVC <0.70 was similar in both participants with or without PCS (10.3% versus 9.6%) (S4 Table).
In CPET, patients with persistent PCS achieved a lower maximal power with lower HR than the participants of the other subgroups, but RER values at the end of CPET were similar and well above 1.05, indicating exhaustion and attaining VO2max. Also, the median values of the Borg CR10 scale were similar for persistent case patients and the control group (median = 8). The most relevant and significant CPET differences between patients with persistent PCS and participants with continued recovery were observed for VE/VCO2 slope (higher values in persistent PCS) and VO2max (lower values in persistent PCS) (Fig 4). The proportion of patients with persistent PCS with VO2max < 85% of target value (suggesting reduced exercise capacity possibly due to deconditioning or peripheral muscle limitations) was significantly greater than that of stable control subjects in adjusted analyses (35.3% versus 8.4%) (S4 Table). Similarly, the differences in the proportion of participants with VO2max below defined thresholds for males and females were substantial and highly significant between persistent case patients and participants with continued recovery S4 Table). Furthermore, the significant difference in the mean VE/VCO2 slope (28.8 versus 27.1) (Fig 4) between patients with persistent PCS and participants with continued recovery corresponded to higher proportions of the participants with persistent PCS (versus the participants with continued recovery) with VE/VCO2 slope values >30 (34.9% versus 18.5%) or >34 (13.5% versus 4.1%) (S4 Table).
We explored a possible overlap of objective signs of cognition deficits and reduced cardiorespiratory capacity within the persistent PCS case-patient population (S5 Table). The proportion of participants with MoCA ≤25 and SDMT <36 increased with increasing VE/VCO2 slope, and there were more participants with SDMT <36 among patients with persistent PCS and poor VO2max (<85% predicted), but there were no such results for TMT-B, and patients with persistent PCS with differences in the Tiffenau test did not differ in their cognitive test performances (S5 Table).
Laboratory investigations.
Besides pro-BNP (see above and Fig 4), we measured complete blood counts, several blood levels including CRP, lactate dehydrogenase, ferritin, liver and renal function and coagulation markers (D-dimer, von Willebrand factor antigen and activity), TSH, cortisol, ACTH, DHEA-S, HbA1c, 25-hydroxy-vitamin D3, CH50 and others. After adjustment for sex-age class combinations, study centre, university entrance qualification, BMI and smoking status, we found no significant differences between patients with persistent PCS and individuals with continued recovery in any of these laboratory investigations (S6 and S7 Figs and S6 Table and S4 Appendix). Notably, levels of CRP, HbA1c and D-dimers were significantly higher in patients with persistent PCS than in the other subgroups (p = 0.004, p = 0.001, p = 0.01, respectively) before adjustment for BMI and smoking.
We did not observe significant differences in the prevalence of N SARS-CoV-2 antibodies (p = 0.82) or in the level of antibodies against SARS-CoV-2 S1 antigen (S8 Fig). Also, positivity rates for antibodies against CMV and several EBV antigens (viral capsid antigen [VCA], EBV nuclear antigen [EBNA] and early antigen D [EA-D]) did not differ significantly between groups (S7 Table). The proportion of study participants with EBV serology indicative of reactivation was 13% (194 of 1,468 seropositive participants). However, we detected no elevated risk for EBV reactivation among patients with persistent PCS or recovered individuals reporting new symptoms between phases 1 and 2 (S7 Table). We additionally looked at EA-D and EBNA IgG antibody levels in participants with evidence for EBV reactivation but did not observe differences between patients with persistent PCS with or without PEM and individuals with continued recovery (S9 Fig).
All study participants were negative for SARS-CoV-2 antigen in oropharyngeal swabs by a rapid antigen assay at presentation. Using an ultrasensitive antigen ECL assay, we could not detect SARS-CoV-2 spike antigen in plasma samples from a subgroup of 100 participants with persistent PCS and 100 persons in the control group. Also, RT-PCR for SARS-CoV-2 RNA was negative in all tested stool samples from a similar subgroup of 156 patients with persistent PCS and 103 participants with continued recovery (see also S4 Appendix), allowing to state with a certainty of 95% that the true PCR positivity prevalence in patients with persistent PCS 17 months after infection is less than or equal to 1.9%.
Sensitivity analyses
The results of several sensitivity analyses (pre-existing illness/comorbidity, obesity, PEM, medical care of the index acute infection) are presented in the Supporting information figures in S1 Sensitivity Analyses. The general patterns persisted as described above, and the differences in the validated questionnaire scores, in neurocognitive as well as in cardiopulmonary tests that were significant in the full analysis set, remained significant. The odds of finding abnormal neurocognitive and cardiopulmonary test results were higher for female than for male participants with persistent PCS, but the differences were significant only for the TMT-B test (S10 Fig).
We also show that in the subpopulation of participants without pre-existing diseases and comorbidity, the changes between phases 1 and 2 in the prevalence of main symptom clusters were similar to those observed in the full analysis (S2 Fig). When participants with persistent PCS were stratified according to PEM (lasting >14 h), the burden of symptoms and complaints as reported and as assessed by validated questionnaires was much higher among patients with versus those without PEM symptoms, including sleep problems, depression and anxiety, perceived stress and subjective cognition impairment, fatigue and dysautonomia (S3 Fig and Fig G in S1 Sensitivity Analyses). The analysis of neurocognitive testing also showed PEM to be associated with substantially worse results (Fig G in S1 Sensitivity Analyses), particularly in the SDMT which assesses cognitive processing speed. However, participants with persistent PCS without PEM still had significantly worse results in all three tests than participants in the control group. Patients with persistent PCS and PEM also showed reduced handgrip strength, lower SpO2, lower peak HR, higher values for VE/VCO2 slope and reduced VO2max when compared with patients without PEM (Fig H in S1 Sensitivity Analyses), and the proportion with VO2max < 85% of target value was higher (41.0% versus 32.5% in persistent PCS with versus without PEM). Several other variables of cardiopulmonary function differed between the two subgroups (Fig H in S1 Sensitivity Analyses), although some showed only small clinically non-relevant differences (e.g., LV-E/A).
Discussion
In this nested population-based case-control study, we found persistence of symptoms and impairments in two-thirds of patients with PCS after more than 1 year following acute SARS-CoV-2 infection. The comprehensive medical evaluation and comparison of individuals with persistent PCS with a control group of age- and sex-matched symptom-free convalesced persons demonstrated that many of the patients with persistent PCS had objective signs of cognitive deficits and reduced exercise capacity. Apart from observing large and discriminant differences in standardised measures of fatigue, neurocognitive disturbance, sleep quality, perceived stress, depression, anxiety, dysautonomia and quality of life, we detected significant differences between participants with persistent PCS and participants with continued recovery in MoCA, SDMT and TMT-B tests, in grip strength, VO2max, VE/VCO2 slope and a few other exercise capacity measures, and this finding was independent of age, sex, BMI and education (as probably the most significant potential confounding factors) and other variables. In contrast, laboratory tests (including inflammatory and coagulation markers) or resting echocardiographic results were not different after adjustment for covariates and were unable to discriminate cases from controls. These observations appear important since, unlike in many other studies, we included only adults in working age, and most study participants did not have medical treatment and were not hospitalised for their acute SARS-CoV-2 infection. Also, the initial population-based survey from which the participant population for the present study was retrieved had been performed 6–12 months following acute infection and thereby excluded persons with post-acute symptoms in the sense of delayed convalescence.
In the majority of participants who had developed PCS 6–12 months after COVID-19, symptoms and complaints persisted, and most of the 32% of the patients who reported an improvement at follow-up did not fully recover. In a recent Swiss study [36], the proportion of persons returning to a normal health status between 6 and 24 months after acute infection was roughly 25%, while the rate of improvement of symptoms associated with PCS was 37%. In another Swiss study [37], the proportion of patients with PCS and improvement between 7 and 15 months after acute infection was 48%. In both studies as well as in other work [38–40], there was a tendency of disease chronification beyond 6–12 months after acute infection, and our current findings support these observations. We saw some differential evolution of the predominant symptom clusters between phases 1 and 2. Fatigue, chest symptoms and smell/taste disorders showed a net decrease in prevalence over time. In contrast, the rate of improvement of the cognition and the depression/anxiety/sleep disorder clusters was similar to the rate of aggravation, resulting in only minor changes in the net prevalence. Others have also observed a tendency for more persistence of neurocognitive disturbances rather than other symptom clusters [16,41–47]. Stratified longitudinal analyses with objective measures are needed to better evaluate chronicity and prognosis of cognition deficits or other organic impairments, and such studies may benefit from advanced methods for defining different recovery clusters and multi-parameter modelling with validation across different cohorts [7,48–51].
Interestingly, risk factors for non-improvement of case status in the present study included lower educational status, and this was complemented by the finding of lower educational status as a risk factor for worsening health among initially recovered persons—besides secondary SARS-CoV infection. In the study reported by Hartung and colleagues [52], lower education was associated with cognitive non-recovery but not with persisting fatigue. In a large online survey [47], lower educational status was associated with worse symptom scores at all-time points post-infection. In our previous phase 1 study, lower educational status was already found to be associated with symptomatic disease at 6–12 months post-infection, and a similar association has been reported from two large US-cohorts [53]. We cannot exclude that sampling bias accounts for these observations. Educational status, in general, is strongly associated with many underlying social, economic, lifestyle and behavioural factors. Which factors behind the educational status variable accounts most for the improvement/worsening effects is not known. Employment, obviously, was an independent factor for case-control status change between the two phases. The fact that we found cases without recent specialist consultation and without participation in rehabilitation between phases 1 and 2 to be more likely to improve, probably reflects a less severe acute and post-acute illness with a better prognosis (i.e., reverse causation).
An important finding was that post-acute vaccination against SARS-CoV-2 did not appear to be associated with PCS improvement. Several studies have shown a decreased PCS prevalence after vaccination, but it was often unclear whether one or more of the vaccine doses were in fact administered after illness onset [11]. Also, many studies were retrospective and did not adjust for confounders. In the study reported by Tran and colleagues [54], in which vaccine recipients with PCS were propensity score matched to non-vaccinated individuals with PCS and observed for 4 months, there were positive associations of (a first) vaccination with fewer symptoms, less severity and remission of PCS. In our study, the proportion of post-infection vaccine recipients was large. Almost all participants had already received their first vaccine before phase 1 (without measurable effects on symptom prevalence and severity), and many had received their second or booster doses between phases 1 and 2. As almost all had been vaccinated, it is difficult to ascertain a relationship between vaccination and recovery from PCS.
Symptom ratings and questionnaire data consistently showed that fatigue and cognitive disturbance were the most prevalent health problems (>60% for each cluster) among individuals with persistent PCS, a finding confirming the results of other studies with a similar follow-up time [43]. Of note were the large overlap between self-reported fatigue, cognition problems and chest symptoms and the strong correlation of various symptom ratings with health-related quality of life scores. Extreme fatigue and symptoms compatible with ME/CFS affected approximately one-tenth of the patients with persistent PCS, while PEM lasting >14 h was reported by 36% and was associated with worse scores in all questionnaires, but also in cognitive and cardiopulmonary exercise tests. This underscores the usefulness of including the history and duration of PEM when exploring patients with possible PCS [55,56]. Using the full set of DePaul questionnaire items, estimates for PEM might have been higher. In a Swiss cohort, PEM was observed in 48% of PCS patients, but in that study, fewer subjects (6%) fulfilled the criteria for ME/CFS [57]. A prevalence of 45% for PEM was observed in a Dutch cohort of PCS patients [58].
Cognitive disturbance was the second most frequent symptom cluster, with concentration problems being slightly more often reported than memory problems. A similar observation independent of the time after acute infection has also been made in a large online survey among subjects with complaints for at least 3 months after infection [47]. In a large claims data network analysis of neurologic and psychiatric sequelae, Taquet and colleagues [59] found that risks of cognitive deficits, dementia, psychotic disorders and epilepsy/seizures remained increased over a 2-year follow-up period after SARS-CoV-2 infection, which was unlike the risks of (other) common psychiatric disorders that rapidly returned to baseline. Other studies also reported persisting or increasing cognition or concentration problems with generally decreasing rates of other symptoms and physical health over time [16,41–47]. A memory questionnaire study found worse memory problems up to 3 years after acute infection (when compared to uninfected controls) [60], and a recent elegant study showed reaction time slowing with increasing time after acute SARS-CoV-2 infection [61]. Taken together, these findings and the results of the present study indicate that cognition problems might, in fact, tend more to chronicity than other health problems of PCS patients. Reports of lower prevalence (22%–32%) of cognitive disturbances in meta-analyses may be due to differences in sample composition (more patients hospitalised during acute infection) and shorter follow-up times.
Sleep disorder, in particular insomnia, was another frequent complaint among cases. Pooled data of previous studies on >15,000 participants revealed a prevalence of 40%–50% for sleep disorder among individuals with PCS [62], which is comparable to our data. The importance of pre-pandemic healthy sleep to prevent PCS has been demonstrated by us and others [63,64]. It will be interesting to explore whether poor sleep quality remains a risk factor for continued non-recovery from PCS. Symptom reports and rating data on depressive and anxiety symptoms generally fit in the meta-analyses on neuropsychiatric manifestations in PCS [62,65].
We note that most of the routine clinical examination results and laboratory measurements did not discriminate between persistent cases and controls, including resting left ventricular systolic and diastolic function as well as the Tiffeneau test. These findings are essentially in line with the results of many other groups [48,66–70]. Small differences in values after crude analyses were no longer statistically significant after adjustment, in particular for BMI, smoking status and study site. D-dimer levels, for example, were slightly elevated among individuals with persistent PCS, but the differences were not significant in adjusted analyses, a result similar to those seen in earlier reports [67,71,72]. Because several studies suggested hypocortisolism as a possible explanation for PCS in at least some patients [20,73,74], we included blood levels of cortisol, ACTH and DHEA-S in our analysis. However, we could not find significant differences between persistent PCS and controls, suggesting a low likelihood of subacute or chronic adrenal insufficiency as a major contributing factor for PCS symptoms. Other recent studies also failed to identify differences in cortisol levels between PCS patients and several control groups [23,75,76]. Furthermore, we were not able to detect differences between persistent PCS and controls in complement turnover, a hypothesis recently raised in a number of studies [77,78]. We did; however, screen only for differences in CH50, but not for individual complement component blood levels.
Serological investigations indicated that the SARS-CoV-2 spike S1 antibody levels in our cohort were essentially driven by vaccination rather than being associated with PCS (as reported by Klein and colleagues),[20] and we did not find a significant association between elevated EA-D IgG antibodies (suggesting EBV reactivation) and PCS in an adjusted analysis. Previous data on this issue have been conflicting, with studies reporting [20,79,80] or failing to find [81,82] EBV reactivation markers associated with PCS. It has to be kept in mind that EA-D IgG antibody levels rise early after active viral replication and typically remain positive for only 3–6 months, while our samples were collected >12 months after acute SARS-CoV-2 infection which does not exclude a role of acute or early post-acute reactivation. However, we also did not observe increased levels of IgG antibodies against EBNA, which has been suggested as a longer-lasting surrogate for EBV reactivation and have previously been associated with neurocognitive disturbances in patients with PCS [83].
SARS-CoV-2 persistence has been proposed as another mechanism in non-recovery and PCS development. However, in our analysis, we did not observe antigen positivity in nasopharyngeal specimens, PCR positivity in stool samples, or viral antigen in plasma, which argues against persistent virus replication as a driver of PCS. The prevalence of viral persistence in non-invasive biospecimens from individuals with PCS as measured by a variety of methods has also been low in previous studies [82,84–86], with the exception of two small studies that showed spike antigenemia in >60% of patients with PCS some of whom were also PCR-positive in plasma samples [87,88], and a study reporting S1 protein persistence in monocyte populations of patients with PCS up to 15 months post-infection [89]. A recent large study demonstrated that throat swab samples in a subgroup of patients with PCS and repeated PCR positivity in the early post-acute phase became negative beyond 3 months after acute infection [90]. Both spike and N protein were detected in plasma samples of 10 out of 100 patients with severe illness for at least 3 months (exact times not stated) after COVID-19 [82], but there was no apparent link between detectable antigen and symptoms. No viral RNA was detected in stool samples taken >300 days after acute infection, while prolonged shedding was associated with gastrointestinal symptoms but not PCS. In an exploratory study [91], four out of five subjects with a variety of symptoms had positive SARS-CoV-2 RNA detected in rectal biopsies obtained between days 158 and 676 after acute infection [92]. So far, very few patients with PCS and symptoms >12 months have been investigated for viral antigen/protein and/or RNA persistence [93], and an association between viral persistence and PCS remains an unproven hypothesis.
Neurocognitive testing showed significant group differences, indicating cognition deficits concerning attention and executive functioning, with problems in divided attention (TMT-B) and lower processing speed (SDMT) in patients with persistent PCS, and this finding appeared to be independent of pre-existing illnesses. One-third of the participants with persistent PCS (versus 18.9% among recovered participants) showed MoCA values < 26, which is slightly higher than observed in previous studies [61,94]. The mean value among participants with persistent PCS was 26.2 (25.8 in cases with PEM) compared with 26.9 among participants with continued recovery (and similar values in the other two groups). This small albeit significant difference may at least partly be related to the fact that the MoCA has limited specificity as a test originally designed to detect mild cognitive impairment among the elderly.
Impaired executive functioning and reduced processing speed, as observed here in persistent PCS is in agreement with a report of similar deficits observed in a large registry cohort [15] of COVID-19 patients followed up with multi-domain cognitive assessment, with pronounced cognitive slowing in 270 patients from two PCS cohorts [15,61], and with attention and executive function deficits in a comprehensive cognitive assessment of patients with PCS after mostly mild initial disease [95]. Although the cognitive findings described in the present study may be insufficient as a diagnostic aid to differentiate cases from controls because of the small-to-medium effect sizes, the data can help to better understand the nature of cognitive impairments in PCS. Controlling the group differences in cognitive test results for fatigue or depressive symptoms attenuated the association of the case status with the MoCA to some degree, but had little effect on the SDMT and TMT-B group differences, indicating that depressive mood and fatigue alone cannot explain the reduced performance in cognitive tests. This is in accordance with previous data [96]. Taken together, the information so far supports the concept of different pathomechanisms with regard to depression and cognitive disorders in PCS.
An impaired physical exercise capacity with reduced handgrip strength (or 6-min walk test) and reduced VO2max appear to be hallmark signs of PCS. Both measures were significantly different between patients with persistent PCS and participants with continued recovery in the present study. A reduced VO2max (<85% predicted) was observed in 35% of the persistent PCS patients, which is comparable to the prevalence found recently in other studies [97]. Similar to earlier observations [97–101], we also found a lower peak HR among patients with persistent PCS, while RERmax and the rate of perceived exertion were similar. Taken together, these findings are compatible with deconditioning as a contributor to the impaired performance capacity [102], but muscular dysfunction/myopathy possibly due to mitochondrial lesions, may be an alternative explanation and additional mechanism. Ventilatory inefficiency is likely to be another contributing factor. Breathlessness as a moderate-to-severe symptom was reported by almost 50% of patients with persistent PCS who also had significantly higher VE/VCO2 slope values than stable control subjects. Other investigators have also found such differences in VE/VCO2 slope between cases and controls [70,103,104]. The prevalence among PCS patients of a VE/VCO2 slope >30 (increased) or >34 (abnormal) in our study was substantial (35% and 14%, respectively), greater than among recovered persons and similar to the proportions reported by Sørensen and colleagues. Even subtle differences in VE/VCO2 slope may impact cardiorespiratory symptom severity after exercising [99,101]. Besides hyperventilation, erratic breathing with high variability in tidal volume and breathing frequency was described in quite a number of patients with PCS [105–109]. However, there is no universal gold standard for diagnosing dysfunctional breathing, and the present study did not include systematic screening for erratic breathing. Again, dysfunctional breathing would also be compatible with respiratory muscular dysfunction.
In accordance with previous data [98,103,110], the normal systolic function in the resting echocardiography in patients with persistent PCS described in the present study suggests that the reduced performance capacity is not caused by central cardiac limitation. Also, bronchial obstruction does not seem to be a cause for the hyperventilatory response to exercise since Tiffeneau tests were similar across all subgroups and BR was not exhausted. The (slightly) reduced FVC among cases (95.9% versus 99.1% for controls) is small but noteworthy. Longitudinal studies assessing FVC changes over time after SARS-CoV-2 infection produced conflicting results [70,111,112], while several cross-sectional studies have shown reduced lung volume associated with persistent symptoms [70,98,113,114]. In a study with patients hospitalised for acute infection [115], reduced FVC at 4 months correlated with increased findings in chest tomographs, reduced lung diffusion capacity, lower SpO2, reduced exercise capacity, more fatigue and lower quality of life. The reason for the lower lung volume in our patients with PCS who had typically not been hospitalised may be respiratory muscle weakness [109,116,117], which remains to be further elucidated. There has been no clear evidence for an impairment of lung diffusion capacity among patients with initially mild acute infection [99,118]. Lung diffusion capacity was not measured in the present study. However, SpO2 at cessation of exercise was not different between groups, making such a hypothesis in our study participants unlikely. Finally, we cannot exclude that the CPET results were affected by a lower level of physical fitness already existing prior to infection. The persistent impaired exercise capacity shown here might best be explained by multi-system dysfunction with a peripheral limitation, for example, impaired oxygen extraction due to mitochondrial dysfunction [119–121], and/or a low preceding fitness level [122], rather than a central cardiac or pulmonary limitation, but the roles of dysfunctional breathing and chronotropic incompetence need to be further investigated. In addition, it is not clear what the relatively frequent orthostatic complaints (measured via the COMPASS-31 instrument) contribute to reduced exercise capacity and how this correlates with dysfunctional breathing and chronotropic incompetence.
One of the strengths of the present study is the nested, population-based approach in defined geographic regions with a large number of individuals with PCR-confirmed earlier infection, regardless of the need for medical treatment. We focussed on adults in the working age. We avoided an overrepresentation of hospitalised elderly patients who are likely to show more SARS-CoV-2-non-specific adverse health sequelae due to more severe acute infection, comorbidities and ageing. We used within-participant comparisons considering symptom frequency before acute SARS-CoV-2 infection and considered only new symptoms not present before the acute infection. In addition, we included at least moderate severity of symptoms and considered impaired activities of daily living or work ability in our working definition of PCS. Another strength is the comprehensive clinical diagnostic work-up of both symptomatic and symptom-free study participants, which included medical history and physical examination, laboratory investigations, CPET and a neuropsychiatric characterisation with cognitive assessment. The study allowed us to provide comparative analyses with adjustment for important confounders such as BMI, smoking and educational level and to stratify the population of persistent PCS cases by the presence of PEM (lasting >14 h) as a probably important as well as pragmatic and simple surrogate for severity.
An important limitation is that we had no objective information on exercise capacity and cognition before acute infection. We did not perform lung diffusion capacity measurements, neuroimaging or more valid measures of dysautonomia that may provide a more comprehensive understanding of the pathophysiology of PCS. Virological analyses were performed only in a subgroup and only on serum and—for a representative part of the cohort—on stool samples, but did not include the analysis of biopsy material. Furthermore, the time of sample collection >1 year post-SARS-CoV-2 infection may have precluded detection of any transient changes induced in the course of acute infection. Recall bias may be particularly relevant in individuals with more severe neurocognitive deficits. Study participation was higher by cases than by controls from phase 1, and study participants with risk factors (e.g., smoking, obesity) were less likely to respond. Another limitation is the lack of opportunities to include patients with PCS with difficulties attending the study centres because of disease severity and who would have needed admission or more support by accompanying relatives or nurses during travelling and outpatient assessment with medical tests. This might also have caused an underestimation of the prevalence of both ME/CFS and longer-lasting PEM. In addition, our screening did not include all DePaul questionnaire item scorings, which may yield PEM prevalence estimates among subjects with PCS of up to 50% or even higher [69,123–127]. We note that the selection of patients fulfilling specific PCS criteria as cases and participants with full recovery after COVID-19 and without complaints and any moderate or severe symptoms as controls (i.e., extreme phenotype selection) may lead to higher AUCs of the questionnaires when compared to representative populations. Furthermore, the population is not representative of Germany since we derived our study participants from a population of medium-sized university cities in the southwestern part of the country with substantial sociocultural and socioeconomic differences from other regions in the country. Finally, we did not include subjects from phase 1 who had symptoms compatible with PCS but did not meet the working definition criteria.
As a conclusion, we report that two-thirds of patients with PCS 6–12 months after acute SARS-CoV-2 infection continue to report persistent symptoms interfering with daily living and associated with reduced quality of life and/or work ability. The symptoms appear to change slightly but the predominant symptoms, often clustering together, remain fatigue, cognitive disturbance and chest symptoms, including breathlessness, with sleep disorder and anxiety as additional complaints in a substantial proportion of cases. In a thorough medical examination, many patients with persistent PCS show findings that significantly differ from controls and are in part abnormal/out of reference; these include impaired executive functioning, reduced cognitive processing speed and reduced physical exercise capacity only in part explained by deconditioning and typically unrelated to central cardiac or pulmonary limitations. Patients with PCS reporting PEM lasting longer than 14 h complained about more severe symptoms and showed worse findings in both cognition and exercise capacity testing. Our findings do not support hypotheses of viral persistence, EBV reactivation, adrenal insufficiency or increased complement turnover as pathophysiologically relevant for persistent PCS.
The results call for the inclusion of cognitive and exercise testing in the clinical evaluation and monitoring of patients with suspected PCS. Together with other research findings, they suggest that further studies should be undertaken to assess the role of skeletal muscle metabolism and dysfunctional breathing as well as neurometabolic and neuroinflammatory disorders and dysautonomia for an advanced understanding of PCS development and prognosis [128,129]. Observational studies with longer follow-up are urgently needed to evaluate factors for improvement and non-recovery from PCS.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank all study participants with their care-givers and the following key collaborators (in alphabetic order) on this work: Julian Böhm, Stefan Brockmann, Stefanie Bröer, Christof Burgstahler, Katharina Caesar, Bettina Deibert, Xiaohong Du, Nelli Edel, Sabine Gerbersdorf, Jennifer Hermann, Katja Hirth, Achim Jerg, Johannes Kirsten, Manuela Licka, Jennifer Müller, Hasema Persch, Patrick Roling, Stephan Rusch, Michaela Schmid, Patrick Schneeweiß, Katarina Stete, Elisabeth Stoll, Adrian Tassoni, Hanna Tschischka, Shirin Vollrath, Vanessa Walz, Dietrich Walzer. We acknowledge the participating local laboratories and biobanking facilities for their technical support. Additional members of the EPILOC Phase 2 Study Group (in alphabetic order): Parwez Aidery (Tübingen), Daniel Bizjak (Ulm), Stefanie Bunk (Tübingen), Nadine Conzelmann (Tübingen), Stefanie Döbele (Tübingen), Lisamaria Eble (Ulm), Melanie Greibich (Heidelberg), Beate Grüner (Ulm), Lucas John (Ulm), Gerhard Kindle (Freiburg), Oliver Krumnau (Freiburg), Jessica Langel (Heidelberg), Nisar Malek (Tübingen), Moritz Munk (Ulm), Stefanie Pfau (Freiburg), Stephan Prettin (Freiburg), Hardy Richter (Tübingen), Siegbert Rieg (Freiburg), Cynthia Stapornwongkul (Freiburg), Sabine Tuma-Kellner (Heidelberg), Kay Winkert (Ulm).
Abbreviations
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- BMI
body mass index
- BR
breathing reserve
- CFQ-11
Chalder Fatigue Scale
- CH50
complement haemolytic activity
- CI
confidence interval
- CMV
cytomegalovirus
- COMPASS-31
Composite Autonomic Symptom Score 31
- CPET
cardiopulmonary exercise testing
- CRP
C-reactive protein
- DHEA-S
dehydroepiandrosterone sulfate
- EA-D
early antigen D
- EBNA
EBV nuclear antigen
- EBV
Epstein–Barr virus
- ECGs
electrocardiograms
- EPILOC
Epidemiology of Long Covid
- ESS
Epworth Sleepiness Scale
- FEV1
forced expiratory volume in one second
- FLei
“Fragebogen zur geistigen Leistungsfähigkeit”
- FVC
forced vital capacity
- GAD-7
Generalised Anxiety Disorder 7
- HbA1c
glycated haemoglobin
- HR
heart rate
- ISI
Insomnia Severity Index
- LV-E/A
ratio of maximal early to late diastolic transmitral flow velocity
- LV-E/e’
ratio between early mitral inflow and mitral annular early diastolic velocities
- LV-EF
left ventricular volume and ejection fraction
- ME/CFS
myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome
- mMRC
modified-Medical Research Council Dyspnoea Scale
- MoCA
Montreal Cognitive Assessment
- N
nucleocapsid
- PCS
post-COVID-19 syndrome
- PEM
post-exertional malaise
- PHQ-9
Patient Health Questionnaire 9
- pro-BNP
pro-brain natriuretic peptide
- PSQI
Pittsburgh Sleep Quality Index
- PSS-10
10-item Perceived Stress Scale
- RER
respiratory exchange ratio
- RT-PCR
reverse transcription polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SDMT
Symbol Digit Modalities Test
- SF-12
Short Form-12 Health Survey
- SpO2
peripheral oxygen saturation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TMT-B
Trail making test part B
- TSH
thyroid-stimulating hormone
- VCA
viral capsid antigen
- VE/VCO2 slope
slope of minute ventilation to carbon dioxide production
- VO2max
oxygen uptake
Data Availability
Data can be made available from the University of Ulm (via: duac.EPILOC@uni-ulm.de) for researchers who meet the criteria for access to confidential data.
Funding Statement
This work was funded by a Baden-Württemberg Federal State Ministry of Science and Art (https://mwk.baden-wuerttemberg.de) grant (number MR/S028188/1) to WVK, HGK, UM, DR, SG and JS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. [cited 18 Oct 2023]. Available from: https://covid19.who.int [Google Scholar]
- 2.Wise J. Covid-19: WHO urges action as 17 million long COVID cases are estimated in Europe. BMJ. 2022;378:o2232. doi: 10.1136/bmj.o2232 [DOI] [PubMed] [Google Scholar]
- 3.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 6.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–7. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentilotti E, Górska A, Tami A, Gusinow R, Mirandola M, Rodríguez Baño J, et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62:102107. doi: 10.1016/j.eclinm.2023.102107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934–46. doi: 10.1001/jama.2023.8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagen DME, van Bilsen CJA, Brinkhues S, Van Herck M, Konings K, den Heijer CDJ, et al. Prevalence of long-term symptoms varies when using different post-COVID-19 definitions in positively and negatively tested adults: the PRIME post-COVID study. Open Forum Infect Dis. 2023;10(10):ofad471. doi: 10.1093/ofid/ofad471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566–80. doi: 10.1001/jamainternmed.2023.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceban F, Kulzhabayeva D, Rodrigues NB, Di Vincenzo JD, Gill H, Subramaniapillai M, et al. COVID-19 vaccination for the prevention and treatment of long COVID: a systematic review and meta-analysis. Brain Behav Immun. 2023;111:211–29. doi: 10.1016/j.bbi.2023.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozik V, Reuken P, Utech I, Gramlich J, Stallmach Z, Demeyere N, et al. Characterization of neurocognitive deficits in patients with post-COVID-19 syndrome: persistence, patients’ complaints, and clinical predictors. Front Psychol. 2023;14:1233144. doi: 10.3389/fpsyg.2023.1233144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver SF, Lazoff SA, Popovich J, Enfield KB, Quigg M, Davis EM, et al. Chronic neurocognitive, neuropsychological, and pulmonary symptoms in outpatient and inpatient cohorts after COVID-19 infection. Neurosci Insights. 2023;18:26331055231186998. doi: 10.1177/26331055231186998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker JH, Lin JJ, Doernberg M, Stone K, Navis A, Festa JR, et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open. 2021;4(10):e2130645. doi: 10.1001/jamanetworkopen.2021.30645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheetham NJ, Penfold R, Giunchiglia V, Bowyer V, Sudre CH, Canas LS, et al. The effects of COVID-19 on cognitive performance in a community-based cohort: a COVID symptom study biobank prospective cohort study. EClinicalMedicine. 2023;62:102086. doi: 10.1016/j.eclinm.2023.102086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobrino-Relaño S, Balboa-Bandeira Y, Peña J, Ibarretxe-Bilbao N, Zubiaurre-Elorza L, Ojeda N. Neuropsychological deficits in patients with persistent COVID-19 symptoms: a systematic review and meta-analysis. Sci Rep. 2023;13(1):10309. doi: 10.1038/s41598-023-37420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommen SL, Havdal LB, Selvakumar J, Einvik G, Leegaard TM, Lund-Johansen F, et al. Inflammatory markers and pulmonary function in adolescents and young adults 6 months after mild COVID-19. Front Immunol. 2023;13:1081718. doi: 10.3389/fimmu.2022.1081718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmig LM, Rako ZA, Ziegler S, Richter MJ, G S AT, Roller F, et al. Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir Res. 2022;23(1):263. doi: 10.1186/s12931-022-02173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139–48. doi: 10.1038/s41586-023-06651-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espín E, Yang C, Shannon CP, Assadian S, He D, Tebbutt SJ. Cellular and molecular biomarkers of long COVID: a scoping review. EBioMedicine. 2023;91:104552. doi: 10.1016/j.ebiom.2023.104552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai Y-J, Liu S-H, Manachevakul S, Lee T-A, Kuo C-T, Bello D. Biomarkers in long COVID-19: a systematic review. Front Med (Lausanne). 2023;10:1085988. doi: 10.3389/fmed.2023.1085988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mourelatos P, Vrettou CS, Diamantopoulos A, Vassiliou AG, Jahaj E, Angelousi A, et al. A prospective study on endocrine function in patients with long-COVID symptoms. Hormones (Athens). 2024;23(1):59–67. doi: 10.1007/s42000-023-00511-0 [DOI] [PubMed] [Google Scholar]
- 24.Cecchetto A, Guarnieri G, Torreggiani G, Vianello A, Baroni G, Palermo C, et al. Dyspnea in post-acute COVID-19: a multi-parametric cardiopulmonary evaluation. J Clin Med. 2023;12(14):4658. doi: 10.3390/jcm12144658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):3528. doi: 10.1038/s41467-022-30836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–14. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang M, Wu X, Jing H, Novakovic VA, Shi J. The intersection of obesity and (long) COVID-19: hypoxia, thrombotic inflammation, and vascular endothelial injury. Front Cardiovasc Med. 2023;10:1062491. doi: 10.3389/fcvm.2023.1062491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Munckhof ICL, Bahrar H, Schraa K, Brand T, Ter Horst R, van der Graaf M, et al. Sex-specific association of visceral and subcutaneous adipose tissue volumes with systemic inflammation and innate immune cells in people living with obesity. Int J Obes (Lond). 2024;48(4):523–32. doi: 10.1038/s41366-023-01444-9 [DOI] [PubMed] [Google Scholar]
- 29.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. doi: 10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 30.Hampshire A, Trender W, Chamberlain SR, Jolly AE, Grant JE, Patrick F, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39:101044. doi: 10.1016/j.eclinm.2021.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampshire A, Azor A, Atchison C, Trender W, Hellyer PJ, Giunchiglia V, et al. Cognition and memory after Covid-19 in a large community sample. N Engl J Med. 2024;390(9):806–18. doi: 10.1056/NEJMoa2311330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter RS, Nieters A, Kräusslich H-G, Brockmann SO, Göpel S, Kindle G, et al. Post-acute sequelae of COVID-19 six to 12 months after infection: population based study. BMJ. 2022;379:e071050. doi: 10.1136/bmj-2022-071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I; CoviCare Study Team. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021;174(9):1252–60. doi: 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015–6. doi: 10.1001/jama.2021.5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotler J, Holtzman C, Dudun C, Jason LA. A brief questionnaire to assess post-exertional malaise. Diagnostics (Basel). 2018;8(3):66. doi: 10.3390/diagnostics8030066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballouz T, Menges D, Anagnostopoulos A, Domenghino A, Aschmann HE, Frei A, et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ. 2023;381:e074425. doi: 10.1136/bmj-2022-074425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nehme M, Braillard O, Chappuis F; CoviCare Study Team, Guessous I. The chronification of post-COVID condition associated with neurocognitive symptoms, functional impairment and increased healthcare utilization. Sci Rep. 2022;12(1):14505. doi: 10.1038/s41598-022-18673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiyama A, Takafuta T, Sato T, Kitahara Y, Yoshinaga Y, Abe K, et al. Natural course of post-COVID symptoms in adults and children. Sci Rep. 2024;14(1):3884. doi: 10.1038/s41598-024-54397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon D, Slama D, Linard F, Dumesges N, Le Baut V, Hakim F, et al. Patients with Long COVID continue to experience significant symptoms at 12 months and factors associated with improvement: a prospective cohort study in France (PERSICOR). Int J Infect Dis. 2024;140:9–16. doi: 10.1016/j.ijid.2023.11.038 [DOI] [PubMed] [Google Scholar]
- 40.Wynberg E, Verveen A, van Willigen HDG, Nieuwkerk P, Davidovich U, Lok A, et al. Two-year trajectories of COVID-19 symptoms and their association with illness perception: a prospective cohort study in Amsterdam, the Netherlands. Influenza Other Respir Viruses. 2023;17(10):e13190. doi: 10.1111/irv.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latifi A, Flegr J. Is recovery just the beginning? Persistent symptoms and health and performance deterioration in post-COVID-19, non-hospitalized university students-a cross-sectional study. Biol Methods Protoc. 2023;8(1):bpad037. doi: 10.1093/biomethods/bpad037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fjelltveit EB, Blomberg B, Kuwelker K, Zhou F, Onyango TB, Brokstad KA, et al. Symptom burden and immune dynamics 6 to 18 months following mild severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2): a case-control study. Clin Infect Dis. 2023;76(3):e60–70. doi: 10.1093/cid/ciac655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahlgren C, Forsberg G, Divanoglou A, Östholm Balkhed Å, Niward K, Berg S, et al. Two-year follow-up of patients with post-COVID-19 condition in Sweden: a prospective cohort study. Lancet Reg Health Eur. 2023;28:100595. doi: 10.1016/j.lanepe.2023.100595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bek LM, Berentschot JC, Heijenbrok-Kal MH, Huijts S, van Genderen ME, Vlake JH, et al. Symptoms persisting after hospitalisation for COVID-19: 12 months interim results of the CO-FLOW study. ERJ Open Res. 2022;8(4):00355–2022. doi: 10.1183/23120541.00355-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Cancela-Cilleruelo I, Moro-López-Menchero P, Rodríguez-Jiménez J, Pellicer-Valero OJ. Exploring the trajectory recovery curve of the number of post-COVID symptoms: the LONG-COVID-EXP-CM multicenter study. Int J Infect Dis. 2022;117:201–3. doi: 10.1016/j.ijid.2022.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yelin D, Margalit I, Nehme M, Bordas-Martínez J, Pistelli F, Yahav D, et al. Patterns of long COVID symptoms: a multi-center cross sectional study. J Clin Med. 2022;11(4):898. doi: 10.3390/jcm11040898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brus IM, Spronk I, Haagsma JA, de Groot A, Tieleman P, Biere-Rafi S, et al. The prolonged impact of COVID-19 on symptoms, health-related quality of life, fatigue and mental well-being: a cross-sectional study. Front Epidemiol. 2023;3:1144707. doi: 10.3389/fepid.2023.1144707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahanic S, Tymoszuk P, Luger AK, Hüfner K, Boehm A, Pizzini A, et al. COVID-19 and its continuing burden after 12 months: a longitudinal observational prospective multicentre trial. ERJ Open Res. 2023;9(2):00317–2022. doi: 10.1183/23120541.00317-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prabhakaran D, Day GS, Munipalli B, Rush BK, Pudalov L, Niazi SK, et al. Neurophenotypes of COVID-19: Risk factors and recovery outcomes. Brain Behav Immun Health. 2023;30:100648. doi: 10.1016/j.bbih.2023.100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong AW, Tran KC, Binka M, Janjua NZ, Sbihi H, Russell JA, et al. Use of latent class analysis and patient reported outcome measures to identify distinct long COVID phenotypes: a longitudinal cohort study. PLoS One. 2023;18(6):e0286588. doi: 10.1371/journal.pone.0286588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torrell G, Puente D, Jacques-Aviñó C, Carrasco-Ribelles LA, Violán C, López-Jiménez T, et al. Characterisation, symptom pattern and symptom clusters from a retrospective cohort of Long COVID patients in primary care in Catalonia. BMC Infect Dis. 2024;24(1):82. doi: 10.1186/s12879-023-08954-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartung TJ, Bahmer T, Chaplinskaya-Sobol I, Deckert J, Endres M, Franzpötter K, et al. Predictors of non-recovery from fatigue and cognitive deficits after COVID-19: a prospective, longitudinal, population-based study. EClinicalMedicine. 2024;69:102456. doi: 10.1016/j.eclinm.2024.102456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Sawano M, Wu Y, Shah RM, Bishop P, Iwasaki A, et al. Risk factors for experiencing Long-COVID symptoms: Insights from two nationally representative surveys. 2024. doi: 10.1101/2024.01.12.24301170 [DOI] [Google Scholar]
- 54.Tran V-T, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of first dose of COVID-19 vaccine versus no vaccination on symptoms of patients with long COVID: target trial emulation based on ComPaRe e-cohort. BMJ Med. 2023;2(1):e000229. doi: 10.1136/bmjmed-2022-000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu L, Valencia IJ, Garvert DW, Montoya JG. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: a patient-centered, cross-sectional survey. PLoS One. 2018;13(6):e0197811. doi: 10.1371/journal.pone.0197811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jason LA, Evans M, So S, Scott J, Brown A. Problems in defining post-exertional malaise. J Prev Interv Commun. 2015;43(1):20–31. doi: 10.1080/10852352.2014.973239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nehme M, Chappuis F, Kaiser L, Assal F, Guessous I. The prevalence, severity, and impact of post-COVID persistent fatigue, post-exertional malaise, and chronic fatigue syndrome. J Gen Intern Med. 2023;38(3):835–9. doi: 10.1007/s11606-022-07882-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagen DME, Van Herck M, van Bilsen CJA, Brinkhues S, Konings K, den Heijer CDJ, et al. High proportions of post-exertional malaise and orthostatic intolerance in people living with post-COVID-19 condition: the PRIME post-COVID study. Front Med. 2023;10. Available from: https://www.frontiersin.org/articles/10.3389/fmed.2023.1292446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815–27. doi: 10.1016/S2215-0366(22)00260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellingjord-Dale M, Brunvoll SH, Søraas A. Prospective memory assessment before and after Covid-19. N Engl J Med. 2024;390(9):863–5. doi: 10.1056/NEJMc2311200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Martin EM, Reuken PA, Scholcz A, Ganse-Dumrath A, Srowig A, et al. Long COVID is associated with severe cognitive slowing: a multicentre cross-sectional study. EClinicalMedicine. 2024;68:102434. doi: 10.1016/j.eclinm.2024.102434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seighali N, Abdollahi A, Shafiee A, Amini MJ, Teymouri Athar MM, Safari O, et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): a systematic review and meta-analysis. BMC Psychiatry. 2024;24(1):105. doi: 10.1186/s12888-023-05481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schilling C, Nieters A, Schredl M, Peter RS, Rothenbacher D, Brockmann SO, et al. Pre-existing sleep problems as a predictor of post-acute sequelae of COVID-19. J Sleep Res. 2024;33(2):e13949. doi: 10.1111/jsr.13949 [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Huang T, Weisskopf MG, Kang JH, Chavarro JE, Roberts AL. Multidimensional sleep health prior to SARS-CoV-2 infection and risk of post-COVID-19 condition. JAMA Netw Open. 2023;6(5):e2315885. doi: 10.1001/jamanetworkopen.2023.15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chudzik M, Lewek J, Kapusta J, Banach M, Jankowski P, Bielecka-Dabrowa A. Predictors of long COVID in patients without comorbidities: data from the polish long-COVID cardiovascular (PoLoCOV-CVD) study. J Clin Med. 2022;11(17):4980. doi: 10.3390/jcm11174980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022;175(7):969–79. doi: 10.7326/M21-4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bahmer T, Borzikowsky C, Lieb W, Horn A, Krist L, Fricke J, et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51:101549. doi: 10.1016/j.eclinm.2022.101549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demko ZO, Yu T, Mullapudi SK, Varela Heslin MG, Dorsey CA, Payton CB, et al. Two-year longitudinal study reveals that long COVID symptoms peak and quality of life nadirs at 6-12 months postinfection. Open Forum Infect Dis. 2024;11(3):ofae027. doi: 10.1093/ofid/ofae027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladlow P, Holdsworth DA, O’Sullivan O, Barker-Davies RM, Houston A, Chamley R, et al. Exercise tolerance, fatigue, mental health, and employment status at 5 and 12 months following COVID-19 illness in a physically trained population. J Appl Physiol (1985). 2023;134(3):622–37. doi: 10.1152/japplphysiol.00370.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Larragoiti N, Cano-Mendez A, Jimenez-Vega Y, Trujillo M, Guzman-Cancino P, Ambriz-Murillo Y, et al. Inflammatory and prothrombotic biomarkers contribute to the persistence of sequelae in recovered COVID-19 patients. Int J Mol Sci. 2023;24(24):17468. doi: 10.3390/ijms242417468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranucci M, Baryshnikova E, Anguissola M, Pugliese S, Falco M, Menicanti L. The long term residual effects of COVID-associated coagulopathy. Int J Mol Sci. 2023;24(6):5514. doi: 10.3390/ijms24065514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ach T, Ben Haj Slama N, Gorchane A, Ben Abdelkrim A, Garma M, Ben Lasfar N, et al. Explaining long COVID: a pioneer cross-sectional study supporting the endocrine hypothesis. J Endocr Soc. 2024;8(3):bvae003. doi: 10.1210/jendso/bvae003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urhan E, Karaca Z, Unuvar GK, Gundogan K, Unluhizarci K. Investigation of pituitary functions after acute coronavirus disease 2019. Endocr J. 2022;69(6):649–58. doi: 10.1507/endocrj.EJ21-0531 [DOI] [PubMed] [Google Scholar]
- 75.Fleischer M, Szepanowski F, Mausberg AK, Asan L, Uslar E, Zwanziger D, et al. Cytokines (IL1β, IL6, TNFα) and serum cortisol levels may not constitute reliable biomarkers to identify individuals with post-acute sequelae of COVID-19. Ther Adv Neurol Disord. 2024;17:17562864241229567. doi: 10.1177/17562864241229567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devine K, Russell CD, Blanco GR, Walker BR, Homer NZM, Denham SG, et al. Plasma steroid concentrations reflect acute disease severity and normalise during recovery in people hospitalised with COVID-19. Clin Endocrinol (Oxf). 2024;100(4):317–27. doi: 10.1111/cen.15012 [DOI] [PubMed] [Google Scholar]
- 77.Baillie K, Davies HE, Keat SBK, Ladell K, Miners KL, Jones SA, et al. Complement dysregulation is a prevalent and therapeutically amenable feature of long COVID. Med. 2024;5(3):239–53.e5. doi: 10.1016/j.medj.2024.01.011 [DOI] [PubMed] [Google Scholar]
- 78.Cervia-Hasler C, Brüningk SC, Hoch T, Fan B, Muzio G, Thompson RC, et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science. 2024;383(6680):eadg7942. doi: 10.1126/science.adg7942 [DOI] [PubMed] [Google Scholar]
- 79.Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 2021;10(6):763. doi: 10.3390/pathogens10060763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durstenfeld MS, Peluso MJ, Kaveti P, Hill C, Li D, Sander E, et al. Reduced exercise capacity, chronotropic incompetence, and early systemic inflammation in cardiopulmonary phenotype long coronavirus disease 2019. J Infect Dis. 2023;228(5):542–54. doi: 10.1093/infdis/jiad131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoeggerl AD, Nunhofer V, Lauth W, Badstuber N, Held N, Zimmermann G, et al. Epstein-Barr virus reactivation is not causative for post-COVID-19-syndrome in individuals with asymptomatic or mild SARS-CoV-2 disease course. BMC Infect Dis. 2023;23(1):800. doi: 10.1186/s12879-023-08820-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez L, Tan Z, Lakshmikanth T, Wang J, Barcenilla H, Swank Z, et al. Restrained memory CD8+T cell responses favors viral persistence and elevated IgG responses in patients with severe Long COVID. 2024. doi: 10.1101/2024.02.11.24302636 [DOI] [Google Scholar]
- 83.Peluso MJ, Deveau T-M, Munter SE, Ryder D, Buck A, Beck-Engeser G, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023;133(3):e163669. doi: 10.1172/JCI163669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitsios GD, Blacka S, Jacobs JJ, Mirza T, Naqvi A, Gentry H, et al. Subphenotypes of self-reported symptoms and outcomes in long COVID: a prospective cohort study with latent class analysis. BMJ Open. 2024;14(3):e077869. doi: 10.1136/bmjopen-2023-077869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76(3):e487–90. doi: 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peluso MJ, Swank ZN, Goldberg SA, Lu S, Dalhuisen T, Borberg E, et al. Plasma-based antigen persistence in the post-acute phase of SARS-CoV-2 infection. medRxiv. 2023;2023.10.24.23297114. doi: 10.1101/2023.10.24.23297114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Bosurgi L, et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J Med Virol. 2023;95(1):e28364. doi: 10.1002/jmv.28364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Craddock V, Mahajan A, Spikes L, Krishnamachary B, Ram AK, Kumar A, et al. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J Med Virol. 2023;95(2):e28568. doi: 10.1002/jmv.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. 2022;12:746021. doi: 10.3389/fimmu.2021.746021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghafari M, Hall M, Golubchik T, Ayoubkhani D, House T, MacIntyre-Cockett G, et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature. 2024;626(8001):1094–101. doi: 10.1038/s41586-024-07029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peluso MJ, Ryder D, Flavell R, Wang Y, Levi J, LaFranchi BH, et al. Multimodal molecular imaging reveals tissue-based T cell activation and viral RNA persistence for up to 2 years following COVID-19. medRxiv. 2023;2023.07.27.23293177. doi: 10.1101/2023.07.27.23293177 [DOI] [Google Scholar]
- 92.Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med. 2022;3(6):371–87.e9. doi: 10.1016/j.medj.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fernández-de-Las-Peñas C, Torres-Macho J, Macasaet R, Velasco JV, Ver AT, Culasino Carandang THD, et al. Presence of SARS-CoV-2 RNA in COVID-19 survivors with post-COVID symptoms: a systematic review of the literature. Clin Chem Lab Med. 2024;62(6):1044–52. doi: 10.1515/cclm-2024-0036 [DOI] [PubMed] [Google Scholar]
- 94.Reuken PA, Besteher B, Finke K, Fischer A, Holl A, Katzer K, et al. Longterm course of neuropsychological symptoms and ME/CFS after SARS-CoV-2-infection: a prospective registry study. Eur Arch Psychiatry Clin Neurosci. 2024;274(8):1903–10. doi: 10.1007/s00406-023-01661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delgado-Alonso C, Valles-Salgado M, Delgado-Álvarez A, Yus M, Gómez-Ruiz N, Jorquera M, et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J Psychiatr Res. 2022;150:40–6. doi: 10.1016/j.jpsychires.2022.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldstein FC, Hajjar I, Summers A, Truong AD, Lee FFE-H, Han JE, et al. Frequency and correlates of subjective cognitive complaints and objective cognitive screening results in African American adults following COVID-19 infection. Brain Behav Immun Health. 2023;34:100691. doi: 10.1016/j.bbih.2023.100691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sørensen L, Pedersen CL, Andersen MJ, Schmid JM, Oestergaard LG, Schiøttz-Christensen B, et al. Cardiopulmonary exercise testing in patients with long COVID. CHEST Pulmonary. 2024;2(2):100036. doi: 10.1016/j.chpulm.2024.100036 [DOI] [Google Scholar]
- 98.Jamieson A, Al Saikhan L, Alghamdi L, Hamill Howes L, Purcell H, Hillman T, et al. Mechanisms underlying exercise intolerance in long COVID: an accumulation of multisystem dysfunction. Physiol Rep. 2024;12(3):e15940. doi: 10.14814/phy2.15940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agostoni P, Mapelli M, Salvioni E, Mattavelli I, Banfi C, Bonomi A, et al. Symptomatic post COVID patients have impaired alveolar capillary membrane function and high VE/VCO2. Respir Res. 2024;25(1):82. doi: 10.1186/s12931-023-02602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baranauskas MN, Carter SJ. Evidence for impaired chronotropic responses to and recovery from 6-minute walk test in women with post-acute COVID-19 syndrome. Exp Physiol. 2022;107(7):722–32. doi: 10.1113/EP089965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aparisi Á, Ybarra-Falcón C, García-Gómez M, Tobar J, Iglesias-Echeverría C, Jaurrieta-Largo S, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10(12):2591. doi: 10.3390/jcm10122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J. 2021;58(2):2101763. doi: 10.1183/13993003.01763-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161(1):54–63. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beaudry RI, Brotto AR, Varughese RA, de Waal S, Fuhr DP, Damant RW, et al. Persistent dyspnea after COVID-19 is not related to cardiopulmonary impairment; a cross-sectional study of persistently dyspneic COVID-19, non-dyspneic COVID-19 and controls. Front Physiol. 2022;13:917886. doi: 10.3389/fphys.2022.917886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holley AB, Fabyan KD, Haynes ZA, Holtzclaw AW, Huprikar NA, Shumar JN, et al. Cardiopulmonary exercise testing in younger patients with persistent dyspnea following acute, outpatient COVID-19 infection. Physiol Rep. 2024;12(3):e15934. doi: 10.14814/phy2.15934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frésard I, Genecand L, Altarelli M, Gex G, Vremaroiu P, Vremaroiu-Coman A, et al. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in “long COVID” patients with persistent dyspnoea. BMJ Open Respir Res. 2022;9(1):e001126. doi: 10.1136/bmjresp-2021-001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Genecand L, Altarelli M, Binkova A, Loew S, Vaudan S, Gex G, et al. Dysfunctional breathing symptoms, functional impact and quality of life in patients with long COVID-19: a prospective case series. BMJ Open Respir Res. 2023;10(1):e001770. doi: 10.1136/bmjresp-2023-001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Voorthuizen EL, van Helvoort HAC, Peters JB, van den Heuvel MM, van den Borst B. Persistent exertional dyspnea and perceived exercise intolerance after mild COVID-19: A critical role for breathing dysregulation? Phys Ther. 2022;102(10):pzac105. doi: 10.1093/ptj/pzac105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Altmann CH, Zvonova E, Richter L, Schüller PO. Pulmonary recovery directly after COVID-19 and in long-COVID. Respir Physiol Neurobiol. 2023;315:104112. doi: 10.1016/j.resp.2023.104112 [DOI] [PubMed] [Google Scholar]
- 110.Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985). 2021;130(5):1470–8. doi: 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ippoliti L, Coppeta L, Somma G, Bizzarro G, Borelli F, Crispino T, et al. Pulmonary function assessment after COVID-19 in vaccinated healthcare workers. J Occup Med Toxicol. 2023;18(1):31. doi: 10.1186/s12995-023-00400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mogensen I, Hallberg J, Björkander S, Du L, Zuo F, Hammarström L, et al. Lung function before and after COVID-19 in young adults: a population-based study. J Allergy Clin Immunol Glob. 2022;1:37–42. doi: 10.1016/j.jacig.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lehmann A, Gysan M, Bernitzky D, Bal C, Prosch H, Zehetmayer S, et al. Comparison of pulmonary function test, diffusion capacity, blood gas analysis and CT scan in patients with and without persistent respiratory symptoms following COVID-19. BMC Pulm Med. 2022;22(1):196. doi: 10.1186/s12890-022-01987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chamley RR, Holland JL, Collins J, Pierce K, Watson WD, Green PG, et al. Exercise capacity following SARS-CoV-2 infection is related to changes in cardiovascular and lung function in military personnel. Int J Cardiol. 2024;395:131594. doi: 10.1016/j.ijcard.2023.131594 [DOI] [PubMed] [Google Scholar]
- 115.Sanhueza S, Vidal MA, Hernandez MA, Henriquez-Beltran ME, Cabrera C, Quiroga R, et al. Clinical and pulmonary function analysis in long-COVID revealed that long-term pulmonary dysfunction is associated with vascular inflammation pathways and metabolic syndrome. Front Med (Lausanne). 2023;10:1271863. doi: 10.3389/fmed.2023.1271863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Severin R, Franz CK, Farr E, Meirelles C, Arena R, Phillips SA, et al. The effects of COVID-19 on respiratory muscle performance: making the case for respiratory muscle testing and training. Eur Respir Rev. 2022;31(166):220006. doi: 10.1183/16000617.0006-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vonbank K, Nics H, Zwick RH, Maasz J, Sabic B, Potzmann M, et al. Decreased phrenic nerve compound muscle action potential, inspiratory muscle strength, and exercise capacity after COVID-19. Front Neurol. 2024;14:1308443. doi: 10.3389/fneur.2023.1308443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patton MJ, Benson D, Robison SW, Dhaval R, Locy ML, Patel K, et al. Characteristics and Determinants of Pulmonary Long COVID. medRxiv. 2024;2024.02.13.24302781. doi: 10.1101/2024.02.13.24302781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colosio M, Brocca L, Gatti MF, Neri M, Crea E, Cadile F, et al. Structural and functional impairments of skeletal muscle in patients with postacute sequelae of SARS-CoV-2 infection. J Appl Physiol (1985). 2023;135(4):902–17. doi: 10.1152/japplphysiol.00158.2023 [DOI] [PubMed] [Google Scholar]
- 120.Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 2024;15(1):17. doi: 10.1038/s41467-023-44432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bizjak DA, Ohmayer B, Buhl JL, Schneider EM, Walther P, Calzia E, et al. Functional and morphological differences of muscle mitochondria in chronic fatigue syndrome and post-COVID syndrome. Int J Mol Sci. 2024;25(3):1675. doi: 10.3390/ijms25031675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kisiel MA, Janols H, Nordqvist T, Bergquist J, Hagfeldt S, Malinovschi A, et al. Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Ups J Med Sci. 2022;127:10.48101/ujms.v127.8794. doi: 10.48101/ujms.v127.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cornelissen MEB, Bloemsma LD, Vaes AW, Baalbaki N, Deng Q, Beijers RJHCG, et al. Fatigue and symptom-based clusters in post COVID-19 patients: a multicentre, prospective, observational cohort study. J Transl Med. 2024;22(1):191. doi: 10.1186/s12967-024-04979-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Twomey R, DeMars J, Franklin K, Culos-Reed SN, Weatherald J, Wrightson JG. Chronic fatigue and Postexertional Malaise in people living with long COVID: an observational study. Phys Ther. 2022;102(4):pzac005. doi: 10.1093/ptj/pzac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vernon SD, Hartle M, Sullivan K, Bell J, Abbaszadeh S, Unutmaz D, et al. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work. 2023;74(4):1179–86. doi: 10.3233/WOR-220581 [DOI] [PubMed] [Google Scholar]
- 126.Bonilla H, Quach TC, Tiwari A, Bonilla AE, Miglis M, Yang PC, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol. 2023;14:1090747. doi: 10.3389/fneur.2023.1090747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jason LA, Dorri JA. ME/CFS and post-exertional malaise among patients with long COVID. Neurol Int. 2022;15(1):1–11. doi: 10.3390/neurolint15010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greene C, Connolly R, Brennan D, Laffan A, O’Keeffe E, Zaporojan L, et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat Neurosci. 2024;27(3):421–32. doi: 10.1038/s41593-024-01576-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Azcue N, Del Pino R, Acera M, Fernández-Valle T, Ayo-Mentxakatorre N, Pérez-Concha T, et al. Dysautonomia and small fiber neuropathy in post-COVID condition and chronic fatigue syndrome. J Transl Med. 2023;21(1):814. doi: 10.1186/s12967-023-04678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]