Abstract
Mice minute virus (MMV) and mouse parvovirus (MPV) type 1 are the two parvoviruses known to naturally infect laboratory mice and are among the most prevalent infectious agents found in contemporary laboratory mouse colonies. Serologic assays are commonly used to diagnose MMV and MPV infections in laboratory mice; however, highly accurate, high-throughput serologic assays for the detection of MMV- and MPV-infected mice are needed. To this end, the major capsid viral protein (VP2) genes of MMV and MPV were cloned and MMV recombinant VP2 (rVP2) and MPV rVP2 proteins were expressed by using a baculovirus system. MMV rVP2 and MPV rVP2 spontaneously formed virus-like particles that were morphologically similar to empty parvovirus capsids. These proteins were used as antigens in enzyme-linked immunosorbent assays (ELISAs) to detect anti-MMV or anti-MPV antibodies in the sera of infected mice. Sera from mice experimentally infected with MMV (n = 43) or MPV (n = 35) and sera from uninfected mice (n = 30) were used to evaluate the ELISAs. The MMV ELISA was 100% sensitive and 100% specific in detecting MMV-infected mice, and the MPV ELISA was 100% sensitive and 98.6% specific in detecting MPV-infected mice. Both assays outperformed a parvovirus ELISA that uses a recombinant nonstructural protein (NS1) of MMV as antigen. The MMV rVP2 and MPV rVP2 proteins provide a ready source of easily produced antigen, and the ELISAs developed provide highly accurate, high-throughput assays for the serodiagnosis of MMV and MPV infections in laboratory mice.
Mice minute virus (MMV) (formerly minute virus of mice) and mouse parvovirus (MPV) type 1 are among the most prevalent infectious agents found in contemporary laboratory mouse colonies (15). Although the immunosuppressive strain of MMV (MMVi) can induce a potentially lethal renal hemorrhagic disease when experimentally inoculated into neonatal mice (7), clinical disease and histologic lesions have not been reported for mice naturally infected with MMV. Similarly, clinical disease and histologic lesions have not been observed in mice naturally or experimentally infected with MPV (14, 29). Despite the absence of clinical disease and histopathology, murine parvoviruses can have significant deleterious effects on research due to their immunomodulatory effects both in vivo and in vitro (5, 10, 19-22). In addition, there is significant potential for MMV and MPV to be transmitted among animals within a facility due to the high degree of environmental stability of these agents (2, 11). Therefore, identification of infected laboratory mice is critical to minimize the impact of murine parvovirus infections on research.
Serologic evaluation for the presence of antiparvovirus antibodies has typically been used to diagnose MMV and MPV infections in mice (13), although the type of immunoassay and the source of diagnostic antigen vary significantly among rodent diagnostic laboratories. The most common methods used for the diagnosis parvovirus infections in mice include the enzyme-linked immunosorbent assay (ELISA), the indirect fluorescent-antibody assay (IFA), and the hemagglutination inhibition (HAI) assay, with the ELISA being preferred due to its high-throughput capability. More important than methodology is the choice of parvovirus antigen. MMV antigens have been generated from cell lines experimentally infected with the prototype strain of MMV (MMVp), which grows very well in vitro, yielding highly concentrated preparations of MMV antigen. This antigen works well for the detection of MMV infections in mice by the ELISA, IFA, and HAI assay formats (17, 28), but MMVp antigen also displays some cross-reactivity with antibodies directed against MPV (22). This cross-reactivity is primarily the result of antibodies generated to the nonstructural (NS) proteins of MPV binding to the NS proteins of MMVp in the antigen preparation, reflecting the high degree of homology among the NS proteins of the rodent parvoviruses (3). Cell culture-propagated MPV can be used as antigen to detect anti-MPV antibodies (29). However, high-titer stocks of MPV are extremely difficult and expensive to obtain via cell culture propagation, and this has largely precluded the use of MPV antigens in ELISAs. For the past few years, a recombinant nonstructural protein of MMV (rNS1), which is highly conserved among rodent parvoviruses, has been used as antigen in serologic ELISAs to detect MMV and MPV infections (23). However, recent findings indicate that this assay lacks sensitivity in detecting infections, particularly MPV infections (4). Thus, serodiagnostic assays for MMV and MPV that use capsid proteins as antigens are needed.
The goals of this study were to develop sensitive and specific recombinant antigen-based ELISAs to detect parvovirus-infected mice. Here we report on the cloning of the genes that encode the major capsid proteins (VP2) of MMV and MPV, the expression of recombinant VP2 (rVP2) of MMV and MPV with baculovirus vectors, and the use of these recombinant proteins as antigens in ELISAs to detect anti-MMV or anti-MPV antibodies in sera from experimentally infected mice. Our results show that the newly developed ELISAs are highly sensitive and specific for detecting parvovirus infections in mice.
MATERIALS AND METHODS
Viruses.
MPV-1b was propagated in CTLL-2 murine cytotoxic T cells as described previously (4). MMVi was a kind gift from Dave Pintel of the University of Missouri.
Construction of recombinant baculoviruses.
Standard molecular biology techniques were performed as described previously (25). The MMV VP2 and MPV VP2 genes were amplified by PCR. The MMV VP2 gene was amplified with platinum Pfx DNA polymerase (Invitrogen, San Diego, Calif.) according to the protocol of the manufacturer. Each 50-μl reaction mixture contained 1.25 U of polymerase, 290 ng of MMVp pML-n plasmid (an MMVp clone that was a kind gift from Dave Pintel, University of Missouri) as template, and primers MMV VP2 2664-2682 forward (5′-gcggatccgtcgacATGAGTGATGGCACCAGCC-3′) and MMV VP2 4436-4413 reverse (5′-gcggtaccgcggccgcTTAGTAAGTATTTCTAGCAACAGG-3′). The uppercase letters of the primers correspond to MMV genome sequences, while the lowercase letters of the forward primer represent engineered restriction endonuclease sites for BamHI and SalI and the lowercase letters of the reverse primer represent engineered restriction endonuclease sites for KpnI and NotI. Thermocycling parameters consisted of an initial denaturation step (94°C, 2 min), followed by 35 cycles of denaturation (94°C, 15 s), annealing (55°C, 30 s), and extension (68°C, 2 min).
The MPV VP2 gene was amplified by a nested PCR strategy. A region of the MPV genome was amplified with platinum Pfx DNA polymerase (Invitrogen) according to the protocol of the manufacturer with the primers MPV 2634-2653 forward (5′-GCACAGCAAAGAACTCAGAC-3′) and MPV 4440-4419 reverse (5′-CAGAAAGAAAGAACATGGTTGG-3′) and an annealing temperature of 55°C. Each 50-μl reaction mixture contained 1.25 U of polymerase and 370 ng of template DNA from MPV-infected CTLL-2 murine cytotoxic T cells that was purified by the DNeasy (Qiagen Inc., Valencia, Calif.) protocol. An aliquot (0.5 μl) of the amplification product from the first PCR was used as template to amplify the MPV VP2 gene by using the Expand High Fidelity PCR system (Roche, Indianapolis, Ind.) and internal primers MPV VP2 2664-2682 forward (5′-gcgaattcggatccgcATGAGTGATGGCGCCGAGC-3′) and MMV VP2 4436-4413 reverse (see above) with an annealing temperature of 55°C. The uppercase letters of the primers correspond to regions of the MPV genome, and the lowercase letters of the forward primer represent engineered restriction endonuclease sites for EcoRI and BamHI.
The amplified MMV VP2 and MPV VP2 genes were electrophoresed for 1 h at 80 V on a 1% SeaPlaque GTG agarose gel (FMC BioProducts, Rockland, Maine) that contained 0.5% ethidium bromide. Amplicons were visualized under UV light, excised from the gel, and purified with the Zymoclean Gel DNA recovery kit (Zymo Research, Orange, Calif.); and DNA concentrations were determined by measuring the A260 with a spectrophotometer (Gene Quant; Amersham Biosciences Corp., Piscataway, N.J.).
The purified MMV and MPV VP2 PCR products were digested with BamHI and NotI. The MMV VP2 gene was directionally cloned, in frame, into the His-tagged fusion vector pFastBac HTb (Invitrogen) to create the plasmid MMV-VP2-pFastBac HTb. The MPV VP2 PCR product was directionally cloned, in frame, into the His-tagged fusion vector pFastBac HTc (Invitrogen) to create the plasmid MPV-VP2-pFastBac HTc. Plasmid MMV-VP2-pFastBac HTb or MPV-VP2-pFastBac HTc was used to construct independent recombinant baculovirus expression vectors MMV-VP2 Bac and MPV-VP2 Bac, respectively, by using the Bac-to-Bac Baculovirus expression system (Invitrogen). High-titer stocks of recombinant baculoviruses were generated in Spodoptera frugiperda (Sf-9) insect cells (Invitrogen), and viral titers were determined by plaque assay (1).
Production of recombinant proteins and purification of ELISA antigens.
Standard insect cell and baculovirus propagation protocols were followed (1). Pilot experiments were performed to determine the kinetics for maximal production of each recombinant protein (1). To generate large quantities of MMV rVP2 or MPV rVP2 protein for use as antigens, 1-liter pendulum spinner flasks (Wheaton Science Products, Millville, N.J.) were seeded with 600 ml of High Five insect cells (Invitrogen) at a density of 9 × 105 cells per ml in complete insect cell medium (Insecta-FIVE medium; Mediatech, Inc., Herndon, Va.) supplemented with 1.36 mg of l-glutamine per ml and 5% fetal bovine serum. Cells were infected with recombinant baculovirus at a multiplicity of infection of 1.0 for MMV-VP2 Bac or 0.1 for MPV-VP2 Bac. At 72 h postinoculation the cells were pelleted by centrifugation at 500 × g for 15 min at 4°C, resuspended in 50 ml of phosphate-buffered saline (PBS), and disrupted by six 30-s cycles of sonication (Sonifer Cell Disrupter 185; Branson Ultrasonics Corp., Danbury, Conn.) with a cup sonicator (Heat Systems Ultrasonics, Farmingdale, N.Y.) on ice. Insoluble material was pelleted by centrifugation at 20,000 × g for 1 h at 4°C. The clarified supernatant of the MMV rVP2 preparation (semipurified protein) was harvested and used as antigen in the MMV rVP2 ELISA. Uninfected High Five insect cells cultivated and processed in the same manner were used as controls in the MMV ELISA. To further purify the MPV rVP2 for use as antigen in the MPV ELISA and to purify the MMV rVP2 and MPV rVP2 proteins for electron microscopy, clarified supernatants of vector-infected insect cells were loaded onto 10 to 40% cesium chloride gradients and centrifuged at 26,500 rpm in an SW48 rotor (Beckman Coulter, Inc., Fullerton, Calif.) for 16 h at 4°C in an ultracentrifuge (Beckman Coulter, Inc.). Following centrifugation, the area of the gradient containing the rVP2 proteins was visualized as a blue band at a density of 1.31 g/cm3. This fraction was harvested, and the recovered proteins were dialyzed overnight at 4°C against PBS through a 10,000-Da-cutoff membrane (Pierce Endogen, Rockford, Ill.). The concentrations of the recovered proteins were determined by a bicinchoninic acid procedure (30), and the proteins were stored at −80°C until use.
Western blot analysis.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18) and transferred to Immobilon-P transfer membranes (Millipore, Bedford, Mass.) (31). Molecular weight markers were included on each gel to facilitate the calculation of the sizes of the protein bands. Membranes were blocked with 5% nonfat dry milk in PBS (1.4 mM NaH2PO4, 10.8 mM Na2HPO4, 137.9 mM NaCl [pH 7.4]) and probed with diluted sera (1:200) from mice experimentally infected with MMV, mice naturally infected with MPV, or parvovirus-free mice. The membranes were incubated sequentially in solutions of biotinylated goat anti-mouse antibody diluted 1:1,000 (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) and avidin DH (Vector Laboratories, Inc.) and were developed with peroxidase substrate solution (Kirkegaard & Perry, Gaithersburg, Md.).
Parvovirus serology.
For the MMV ELISA, semipurified MMV rVP2 protein was diluted to 1.0 μg/ml in coating buffer (0.1 M NaHCO3 [pH 9.6]) and insect cell control protein was diluted to 0.5 μg/ml in coating buffer; 200 μl of each was coated onto separate wells of an Immulon 2 96-well plate (Dynex Technologies, Inc., Chantilly, Va). For the MPV ELISA, CsCl-purified MPV rVP2 protein was diluted to 0.1 μg/ml in coating buffer, and 200 μl was loaded into test wells of an Immulon 2 96-well plate. Control wells were uncoated. Proteins were allowed to bind to the plates for 48 h at 4°C. The wells were emptied and washed three times in PBS with 0.05% Tween 20 (PBS-Tween). Blocking buffer (PBS with 0.5% nonfat dry milk and 0.05% Tween 20) (300 μl) was added to each well, the mixture was incubated for 30 min at room temperature, and the buffer was discarded; 200 μl of serum diluted 1:100 in blocking buffer was added to experimental and control wells. After incubation at 37°C for 2 h, the plates were washed three times in PBS-Tween. Two hundred microliters of alkaline phosphatase-labeled secondary antibody [F(ab′)2 fragment goat anti-mouse immunoglobulin G (heavy and light chains); Jackson Immunoresearch Laboratories, Inc.]) diluted 1:7,000 in PBS-Tween was added to each well, and the plate was incubated at 37°C for 2 h. The plates were washed five times in PBS-Tween. One hundred microliters of phosphatase substrate solution (1 mg of Sigma 104 phosphatase substrate [Sigma Chemical Company, St. Louis, Mo.] per 1 ml of substrate buffer [2 mM MgCl2, 27.5 mM NaCO3, 22.5 mM NaHCO3 {pH 9.8}]) was added to each well, and the plate was incubated in the dark at room temperature for 45 min. The optical density (OD) of each well was measured (Kinetic Reader EL312E; Bio-Tek Instruments, Inc., Winooski, Vt.) and automatically calculated as the OD at 405 nm (OD405) minus the OD490 to correct for optical interference. For each serum sample, the final OD was calculated as the OD of the test well minus the OD of the control well. The cutoff OD value for each ELISA was set at 3 standard deviations above the mean for 30 serum samples from mice negative for parvovirus infections. The MMV rVP2 ELISA and the MPV rVP2 ELISA were compared to a parvovirus ELISA that uses a recombinant nonstructural protein (rNS1) of MMV as antigen. The rNS1 ELISA was performed as described previously (23).
Performance of ELISAs.
The following were calculated for each ELISA: sensitivity = TP/(TP + FN) × 100, specificity = TN/(TN + FP) × 100, positive predictive value = TP/(TP + FP) × 100, negative predictive value = TN/(TN + FN) × 100, and accuracy = (TP + TN)/(TP + FP + TN + FN), where TP is the number of samples with true-positive results, TN is the number of samples with true-negative results, FP is the number of samples with false-positive results, and FN is the number of samples with false-negative results. For each ELISA, a Kruskal-Wallis one-way analysis of variance on ranks was performed to test for differences between groups of mice. Statistical significance was set at a P value of ≤0.01.
Mice, experimental infections, and sera.
Four-, 8-, and 12-week-old male Hsd:ICR(CD-1) mice and 12-week-old male BALB/cAnNHsd, C3H/HeNHsd, C57BL/6NHsd, and DBA/2NHsd mice were obtained (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.). The mice were separated into experimental and control groups of three to five animals each according to strain, age, and dose of viral inoculum. Mice were inoculated with 5 × 104 to 1 × 106 50% tissue culture infective doses of MPV-1b diluted in TE (50 mM Tris base, 10 mM EDTA [pH 8.7]) or with TE by intragastric gavage under isofluorane anesthesia. At 4 weeks postinoculation, the mice were euthanized with an inhaled overdose of carbon dioxide, blood was collected by cardiocentesis, and the sera were collected. For the MMV experimental infections, pregnant female C3H/HeNHsd mice were obtained (Harlan Sprague-Dawley), and neonatal male and female pups were inoculated oronasally with 5 × 104 50% tissue culture infective doses of MMVi. Mice were euthanized with an inhaled overdose of carbon dioxide at 8 weeks postinoculation, blood was collected by cardiocentesis, and the sera were collected. Sera from all mice were diluted 1:5 in PBS, heat inactivated at 56°C for 30 min, and stored at −20°C until use. The source colonies for all mice were documented to be free of known mouse pathogens, including MPV and MMV. Each experimental and control group was housed separately in microisolation cages, and all animal manipulations were performed in a class II biological safety cabinet by standard microisolation technique. All animal studies were approved by the University of Missouri or the University of Arizona Institutional Animal Care and Use Committee.
Sera from MPV-inoculated mice that were positive for anti-MPV antibodies by an MPV IFA, sera from MMV-inoculated mice that were positive for anti-MMV antibodies by an MMV IFA, and sera from uninfected mice were used in the ELISAs. The MMV and MPV IFAs were performed as described previously (4). In Western blot experiments, sera from MMV-inoculated mice that were positive for anti-MMV antibodies by IFA were used as MMV-positive sera, sera from FVB/NCr mice that were naturally infected with MPV were used as MPV-positive sera, and sera from uninfected mice were used as negative sera. The mice naturally infected with MPV were positive for anti-MPV antibodies by MPV IFA, positive for MPV DNA by MPV PCR of mesenteric lymph nodes, negative for anti-MMV antibodies by MMV IFA, and negative for MMV DNA by PCR of mesenteric lymph nodes.
Electron microscopy.
The MPV rVP2 and MMV rVP2 proteins were expressed and purified by CsCl gradient centrifugation as described above, with the exception that the recovered fractions were diluted in PBS and centrifuged at 41,000 rpm for 5 h in an SW 41 rotor (Beckman) at 4°C to pellet the proteins. The pellets of each protein were resuspended in 1% ammonium acetate. A small droplet of each sample in 4% phosphotungstate was deposited on a carbon-coated grid and examined with a Hitachi H-6700 transmission electron microscope.
RESULTS
MMV rVP2 and MPV rVP2 self-assemble into VLPs.
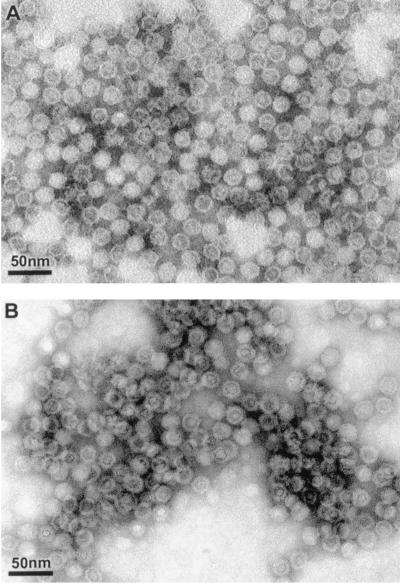
The VP2 genes of MMV and MPV were cloned to create the baculovirus vectors MMV-rVP2 Bac and MPV-rVP2 Bac. These vectors were used to express the recombinant capsid proteins MMV rVP2 and MPV rVP2 in insect cells. It is known that many parvovirus recombinant VP2 proteins, including MMV rVP2, assemble into virus-like particles (VLPs) when expressed in baculovirus expression systems (6, 8, 16, 24, 27). To determine if baculovirus-expressed MMV rVP2 or MPV rVP2 proteins self-assembled into VLPs, we subjected clarified supernatants of insect cells infected with the MMV-rVP2 Bac vector or the MPV-rVP2 Bac vector to CsCl gradient ultracentrifugation. Each gradient yielded a visible blue band at a density of 1.31 g/cm3 of CsCl, which is slightly less dense than previously reported densities of MMV VLPs and empty parvovirus capsids (1.32 g/cm3 of CsCl) (12, 26). Transmission electron microscopic examination of negative stained preparations of the collected bands revealed the presence of VLPs morphologically similar to empty parvovirus capsids (Fig. 1) (9). The MMV VLPs and MPV VLPs were isometric and approximately 20 nm in diameter.
FIG. 1.
Transmission electron micrographs of negative stained preparations of MPV VLPs produced from baculovirus-expressed MPV rVP2 protein (A) and MMV VLPs produced from baculovirus-expressed MMV rVP2 protein (B). The VLPs were purified by CsCl gradient ultracentrifugation.
Western blots of MMV rVP2 and MPV rVP2.
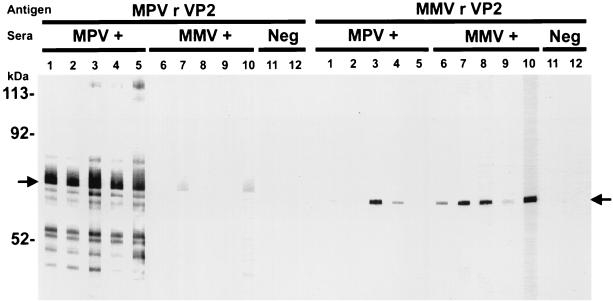
To determine if MMV rVP2 and MPV rVP2 reacted with natural antibodies to MMV or MPV and if the recombinant proteins shared cross-reactive epitopes, CsCl-purified MMV rVP2 and MPV rVP2 were subjected to SDS-PAGE and Western blot analysis. The Western blots were probed with MMV-positive sera, MPV-positive sera, or parvovirus-negative sera. MMV rVP2 was visualized as a 68-kDa immunoreactive protein band, and MPV rVP2 was visualized as a 69-kDa immunoreactive protein band (Fig. 2). The molecular masses of the recombinant proteins represent the molecular mass of the VP2 protein plus the molecular mass of the leader peptide of the fusion protein. The MMV rVP2 protein reacted strongly with four of five MMV-positive serum samples, reacted weakly with one of five MMV-positive serum samples, and displayed cross-reactivity with two of five MPV-positive serum samples. The MPV rVP2 protein reacted strongly with all five MPV-positive serum samples and cross-reacted weakly with two of five MMV-positive serum samples. The MMV rVP2 and MPV rVP2 proteins did not react with sera from uninfected mice. In an ELISA format, MMV-positive sera reacted only with MMV rVP2, MPV-positive sera reacted only with MPV rVP2, and parvovirus-negative sera did not react with either antigen.
FIG. 2.
Western blot analysis of MPV rVP2 VLPs or MMV rVP2 VLPs separated by SDS-PAGE. The blot was probed with sera from MPV-infected mice (lanes 1 to 5), MMV-infected mice (lanes 6 to 10), and uninfected mice (lanes 11 and 12). Each lane contains 5 μg of protein; test sera were run at a 1:200 dilution. The positions of the MPV rVP2 and the MMV rVP2 are indicated by arrows on the left and right, respectively. The positions of the molecular mass standards are indicated on the left.
MMV- and MPV-specific ELISAs.
The baculovirus-expressed MMV rVP2 and MPV rVP2 proteins yielded large amounts of readily produced antigens to develop the MMV- and MPV-specific ELISAs. From 600-ml suspension cultures of vector-infected insect cells, 250 mg of semipurified MMV rVP2 antigen or 1.2 mg of CsCl gradient-purified MPV rVP2 was produced. The semipurified preparation of MMV rVP2 was used as antigen for the MMV ELISA, and the CsCl-purified MPV rVP2 was used as antigen for the MPV ELISA. These antigen preparations were chosen on the basis of preliminary ELISA experiments that showed the marked immunoreactivity of semipurified MMV antigen with MMV-positive sera and the poor immunoreactivity of semipurified MPV antigen with MPV-positive sera. Strong immunoreactivity of MPV rVP2 in an ELISA was obtained only when CsCl-purified MPV rVP2 was used as antigen (data not shown). The baseline cutoff OD405 values used to designate positive sera were calculated to be 0.160 for the MMV ELISA and 0.093 for the MPV ELISA.
To compare the performances of the MMV ELISA and the MPV ELISA to that of a previously described generic parvovirus ELISA that detects anti-NS1 antibodies, sera from mice experimentally infected with MMV or MPV and sera from uninfected mice were tested by the MMV ELISA, the MPV ELISA, and the rNS1 ELISA (Table 1). The MMV ELISA was 100% sensitive and 100% specific in detecting MMV-infected mice and had a 100% positive predictive value, a 100% negative predictive value, and an accuracy of 100%. The MPV ELISA was 100% sensitive and 98.6% specific in detecting MPV-infected mice and had a 97.2% positive predictive value, a 100% negative predictive value, and an accuracy of 99%. The rNS1 ELISA was 21% sensitive and 100% specific in detecting MPV-infected mice, was 0% sensitive and 100% specific in detecting MMV-infected mice, and had a 100% positive predictive value, a 28% negative predictive value, and an accuracy of 76% for detecting mice infected with MMV or MPV. These results demonstrate that the MMV ELISA and the MPV ELISA are sensitive and specific and markedly outperform the rNS1 ELISA for detecting antibodies to MMV or MPV.
TABLE 1.
Comparison of serologic test results for uninfected mice and mice experimentally infected with MPV or MMV
| Infection status | No. of serum samples positive/no. of serum samples tested (%)
|
||
|---|---|---|---|
| MPV ELISA | MMV ELISA | Parvovirus ELISA | |
| Uninfected | 0/30 (0) | 0/30 (0) | 0/30 (0) |
| MPV infected | 35/35 (100) | 0/35 (0) | 9/35 (26) |
| MMV infected | 1/43 (2) | 43/43 (100) | 0/43 (0) |
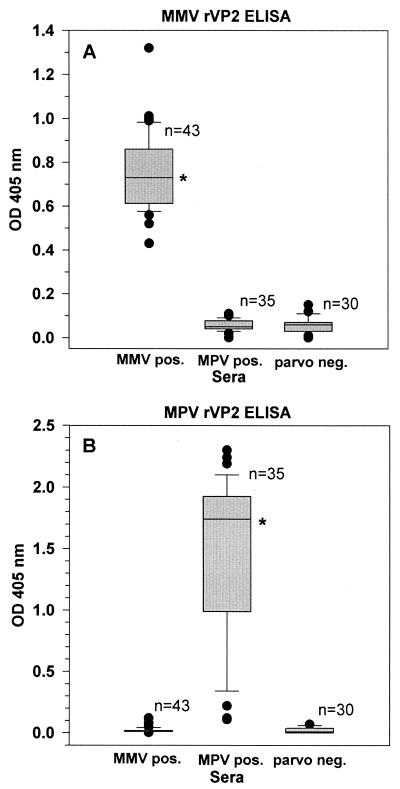
The performances of the MMV ELISA and the MPV ELISA were further characterized by examining the intensities of the OD values generated when sera from MMV-infected mice, MPV-infected mice, and parvovirus-negative mice were evaluated (Fig. 3). For the MMV ELISA, the median absorbance value for sera from MMV-infected mice was statistically higher than the median absorbance values for sera from mice infected with MPV or sera from parvovirus-free mice (P < 0.01). For the MPV ELISA, the median absorbance value for sera from MPV-infected mice was significantly higher than the median absorbance values for sera from mice infected with MMV or sera from parvovirus-free mice (P < 0.01).
FIG. 3.
Serum antibody responses of mice experimentally infected with MMV or MPV or of uninfected mice tested by MMV ELISA (A) or MPV ELISA (B). The datum points are presented as box plots; for each experimental group the lower boundary of the box indicates the 25th percentile, the upper boundary of the box indicates the 75th percentile, and the line within the box marks the median value. The line below the box marks the 10th percentile, the line above the box marks the 90th percentile, and datum points outside this range are indicated (•). ∗, P < 0.01 compared to each other experimental group.
Prevalence of MMV and MPV infections in research mice.
In addition to evaluating sera from experimentally infected mice, we also evaluated all mouse serum samples that were submitted for routine serologic evaluation over a 4-week-period to the University of Missouri Research Animal Diagnostic Laboratory (Columbia, Mo.). These sera were from 83 research institutions throughout the United States and Canada and were obtained from a variety of immunocompetent mouse strains and stocks. Of 2,473 mouse serum samples tested, 205 serum samples (8.3%) were positive for MPV by ELISA, 38 serum samples (1.5%) were positive for MMV by ELISA, and 32 serum samples (1.3%) were positive for both MMV and MPV by ELISA. Seventeen institutions had sera positive for MPV, three institutions had sera positive for MMV, and five institutions had sera positive for both agents. A group of 22 mice that were serologically positive for MMV and MPV by ELISA were also evaluated for the presence of MMV and MPV DNA by PCR analysis of the mesenteric lymph nodes. Of these 22 mice, 19 were positive for MMV and MPV by PCR. Collectively, these data suggest that MPV and MMV infections are present in research mouse colonies, MPV infections are more prevalent than MMV infections, and dual infections with MPV and MMV occur in some mice.
DISCUSSION
In this report, we describe the development of ELISAs that detect anti-MMV or anti-MPV antibodies in the sera of parvovirus-infected mice. The MMV ELISA and the MPV ELISA displayed high sensitivities and high specificities for the detection of species-specific parvovirus antibodies in MMV- and MPV-infected mice, and each ELISA had high positive and negative predictive values. These assays are ideally suited for use as primary serologic tests to screen mouse colonies for MMV and MPV infections because the assays were highly accurate and because ELISAs are readily automated, making it possible to test large numbers of samples quickly and at relatively little expense. In addition, large quantities of the MMV rVP2 and the MPV rVP2 antigens used in these assays were readily produced with the baculovirus expression system, providing ample antigens for high-volume testing. This is particularly significant for MPV because it is difficult and expensive to produce large quantities of MPV-specific ELISA antigens in vitro from MPV-infected cells.
The MMV and MPV ELISAs performed with high sensitivities in detecting MMV and MPV infections in mice, respectively, and these two assays were much more sensitive in detecting parvovirus infections in mice than a generic parvovirus ELISA that uses a recombinant nonstructural protein (rNS1) of MMV as antigen. This is consistent with previous work that shows that mice experimentally inoculated with MPV, especially mice infected after 12 weeks of age, develop anticapsid antibodies more so than anti-NS1 antibodies (4). The VP2 protein is the major capsid protein of the parvovirus virion and can be presented to the immune system upon exposure to the virus or during viral replication. However, nonstructural proteins, including NS1, are not present in the parvovirus virion and are produced more transiently upon infection. Thus, an undetectable anti-NS1 humoral immune response could result from a nonproductive parvovirus infection or from a productive infection that produces an insufficient amount of NS1 to induce an immune response (4). The MMV and MPV ELISAs also performed with high specificities in detecting MMV and MPV infections in mice, respectively. Previously described ELISAs that use whole-virus preparations of MMV or rNS1 proteins as antigens are not specific for the identification of MMV- and MPV-infected mice and require the use of secondary testing with MMV- or MPV-specific HAI assays (22) to elucidate the true infection status of the animal. Thus, the high specificities of the newly developed MMV and MPV ELISAs eliminate the need for secondary testing by the HAI assay.
An interesting feature of the baculovirus-expressed MMV and MPV rVP2 proteins was that they self-assembled into VLPs that were morphologically similar to empty parvovirus capsids. Self-assembly of baculovirus-expressed recombinant parvovirus VP2 proteins has been reported for many parvoviruses including MMV (6, 8, 16, 24, 27); however, this is the first report of self-assembly of MPV rVP2 into VLPs. MMV rVP2 and MPV rVP2 were expressed as fusion proteins that contained an N-terminal polyhistidine tag with the intent of purifying these proteins by metal-affinity chromatography. In preliminary experiments, MMV rVP2 and MPV rVP2 could not be successfully purified by affinity chromatography (data not shown). However, this proved to be unnecessary because the semipurified preparation of MMV rVP2 was highly immunoreactive and therefore required no further purification. While the semipurified preparation of MPV was weakly immunoreactive, highly immunoreactive MPV rVP2 in the form of MPV VLPs was easily purified by CsCl ultracentrifugation. The N-terminal polyhistidine tag and leader sequences added an estimated 3.6 kDa to each rVP2. While the leader peptides did not alter the ability of the rVP2 proteins to assemble into VLPs, they may have caused a slight decrease in the densities of the VLPs (1.31 g/cm3 of CsCl) compared to previously reported densities of MMV VLPs and empty parvovirus capsids (1.32 g/cm3 of CsCl) (12, 26).
The formation of VLPs by the MMV and MPV rVP2 proteins may have contributed to the high sensitivities of the MMV and MPV ELISAs. Interestingly, the MMV rVP2 protein that was subjected to Western blotting reacted strongly with only four of five MMV-positive serum samples. The one MMV-positive serum sample that reacted weakly with MMV rVP2 by Western blotting was strongly reactive with MMV rVP2 by ELISA. It is possible that conformational epitopes present on the exterior of the MMV VLPs were not present when MMV rVP2 was disrupted or denatured for Western blot analysis. Our data also suggest that formation of MMV and MPV VLPs likely enhanced the specificities of the MMV and MPV ELISAs over those which would be attained with unassembled MMV rVP2 or MPV rVP2 proteins as antigens. Sera from two MPV-infected mice strongly cross-reacted with MMV rVP2 proteins on Western blots. These sera did not cross-react with MMV rVP2 in an ELISA format. In addition, two serum samples from MMV-infected mice displayed weak cross-reactivity with MPV rVP2. These two serum samples were not cross-reactive with the MPV rVP2 protein in an ELISA format. A possible explanation for these observations is that in the ELISA format, the MMV rVP2 and MPV rVP2 VLPs behave antigenically like whole virions and anticapsid antibodies are able to associate only with antigenic epitopes present on the exterior of the VLPs. However, when the VLPs are subjected to SDS-PAGE and Western blotting, the VLPs are disrupted and the proteins are denatured, possibly exposing cross-reactive epitopes.
The MMV and MPV ELISAs were used to survey sera obtained from numerous mouse colonies from geographically disparate locations in North America. The prevalence of sera positive for a parvovirus was nearly 10%, with the majority of these sera being positive for MPV. This is consistent with a recent survey that showed that in the United States the prevalence of parvovirus infections among laboratory mouse colonies is high (14). Given the potential for parvovirus infections to alter or invalidate studies that use infected mice (5, 9, 18-21), there is a need for highly accurate, high-throughput assays to detect parvovirus infections in mice. Baculovirus-expressed MMV rVP2 and MPV rVP2 proved to be excellent antigens for the development of such assays. The MMV and MPV ELISAs performed with high sensitivities and specificities and markedly outperformed another widely used parvovirus ELISA that detects antibodies to NS1 proteins in infected mice. The two newly developed serologic assays offer rapid, inexpensive, and accurate methods for the screening of research mouse colonies for MMV and MPV infections.
Acknowledgments
We thank Greg Purdy, Beth Talken, Mark Gifford, Scott Korte, Ted McSheehy, and Ruguang Ou for technical assistance; Cheryl Jensen and Randy Tindall for electron microscopy; and Howard Wilson for photography.
REFERENCES
- 1.Bernard, A., M. Payton, and K. R. Radford. 2000. Protein expression in the baculovirus system, p. 5.5.1-5.5.13. In B. M. Dunn, J. E. Coligan, H. L. Ploegh, D. W. Speicher and P. T. Wingfield (ed.), Current protocols in protein science. John Wiley & Sons, Inc., New York, N.Y.
- 2.Berns, K. I., M. Bergoin, M. Bloom, M. Lederman, N. Muzyczka, G. Siegl, J. Tal, and P. Tattersall. 2000. Parvoviridae, p. 311-323. In M. H. V. van Rgenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Academic Press, Inc., San Diego, Calif.
- 3.Besselsen, D. G., D. J. Pintel, G. A. Purdy, C. L. Besch-Williford, C. L. Franklin, R. R. Hook, Jr., and L. K. Riley. 1996. Molecular characterization of newly recognized rodent parvoviruses. J. Gen. Virol. 77:899-911. [DOI] [PubMed] [Google Scholar]
- 4.Besselsen, D. G., A. M. Wagner, and J. K. Loganbill. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp. Med. 50:498-502. [PubMed] [Google Scholar]
- 5.Bonnard, G. D., E. K. Manders, D. A. Campbell, Jr., R. B. Herberman, and M. J. Collins, Jr. 1976. Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. Evidence for minute virus of mice causing the inhibition. J. Exp. Med. 143:187-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, C. S., J. W. Van Lent, J. M. Vlak, and W. J. Spaan. 1991. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J. Virol. 65:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownstein, D. G., A. L. Smith, R. O. Jacoby, E. A. Johnson, G. Hansen, and P. Tattersall. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Investig. 65:357-364. [PubMed] [Google Scholar]
- 8.Christensen, J., T. Storgaard, B. Bloch, S. Alexandersen, and B. Aasted. 1993. Expression of Aleutian mink disease parvovirus proteins in a baculovirus vector system. J. Virol. 67:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doane, F. W., and N. Anderson. 1987. Parvoviridae, p. 56-61. In Electron microscopy in diagnostic virology: a practical guide and atlas. Cambridge University Press, New York, N.Y.
- 10.Engers, H. D., J. A. Louis, R. H. Zubler, and B. Hirt. 1981. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J. Immunol. 127:2280-2285. [PubMed] [Google Scholar]
- 11.Harris, R. E., P. H. Coleman, and P. S. Morahan. 1974. Stability of minute virus of mice to chemical and physical agents. Appl. Microbiol. 28:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernando, E., A. L. Llamas-Saiz, C. Foces-Foces, R. McKenna, I. Portman, M. Agbandje-McKenna, and J. M. Almendral. 2000. Biochemical and physical characterization of parvovirus minute virus of mice virus-like particles. Virology 267:299-309. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby, R. O., L. J. Ball-Goodrich, D. G. Besselsen, M. D. McKisic, L. K. Riley, and A. L. Smith. 1996. Rodent parvovirus infections. Lab. Anim. Sci. 46:370-380. [PubMed] [Google Scholar]
- 14.Jacoby, R. O., E. A. Johnson, L. Ball-Goodrich, A. L. Smith, and M. D. McKisic. 1995. Characterization of mouse parvovirus infection by in situ hybridization. J. Virol. 69:3915-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacoby, R. O., and J. R. Lindsey. 1998. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J. 39:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajigaya, S., H. Fujii, A. Field, S. Anderson, S. Rosenfeld, L. J. Anderson, T. Shimada, and N. S. Young. 1991. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc. Natl. Acad. Sci. USA 88:4646-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft, V., and B. Meyer. 1986. Diagnosis of murine infections in relation to test methods employed. Lab. Anim. Sci. 36:271-276. [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.McKisic, M. D., D. W. Lancki, G. Otto, P. Padrid, S. Snook, D. C. Cronin II, P. D. Lohmar, T. Wong, and F. W. Fitch. 1993. Identification and propagation of a putative immunosuppressive orphan parvovirus in cloned T cells. J. Immunol. 150:419-428. [PubMed] [Google Scholar]
- 20.McKisic, M. D., J. D. Macy, Jr., M. L. Delano, R. O. Jacoby, F. X. Paturzo, and A. L. Smith. 1998. Mouse parvovirus infection potentiates allogeneic skin graft rejection and induces syngeneic graft rejection. Transplantation 65:1436-1446. [DOI] [PubMed] [Google Scholar]
- 21.McKisic, M. D., F. X. Paturzo, and A. L. Smith. 1996. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T cell effector functions. Transplantation 61:292-299. [DOI] [PubMed] [Google Scholar]
- 22.McMaster, G. K., P. Beard, H. D. Engers, and B. Hirt. 1981. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J. Virol. 38:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley, L. K., R. Knowles, G. Purdy, N. Salome, D. Pintel, R. R. Hook, Jr., C. L. Franklin, and C. L. Besch-Williford. 1996. Expression of recombinant parvovirus NS1 protein by a baculovirus and application to serologic testing of rodents. J. Clin. Microbiol. 34:440-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saliki, J. T., B. Mizak, H. P. Flore, R. R. Gettig, J. P. Burand, L. E. Carmichael, H. A. Wood, and C. R. Parrish. 1992. Canine parvovirus empty capsids produced by expression in a baculovirus vector: use in analysis of viral properties and immunization of dogs. J. Gen. Virol. 73:369-374. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Santaren, J. F., J. C. Ramirez, and J. M. Almendral. 1993. Protein species of the parvovirus minute virus of mice strain MVMp: involvement of phosphorylated VP-2 subtypes in viral morphogenesis. J. Virol. 67:5126-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedlik, C., J. Sarraseca, P. Rueda, C. Leclerc, and I. Casal. 1995. Immunogenicity of poliovirus B and T cell epitopes presented by hybrid porcine parvovirus particles. J. Gen. Virol. 76:2361-2368. [DOI] [PubMed] [Google Scholar]
- 28.Smith, A. L. 1983. Response of weanling random-bred mice to inoculation with minute virus of mice. Lab. Anim. Sci. 33:37-39. [PubMed] [Google Scholar]
- 29.Smith, A. L., R. O. Jacoby, E. A. Johnson, F. Paturzo, and P. N. Bhatt. 1993. In vivo studies with an “orphan” parvovirus of mice. Lab. Anim. Sci. 43:175-182. [PubMed] [Google Scholar]
- 30.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]