Abstract
Objective
This study aims to summarize the clinicopathological characteristics, treatment methods, and prognosis of these patients. The goal is to enhance our understanding of the disease and provide insights for the standardized diagnosis and treatment of abdominal inflammatory myofibroblastic tumors(IMT).
Methods
This retrospective cohort study included clinical data of 26 patients with abdominal IMT admitted to the First Hospital of Jilin University between January 2015 and December 2023. The clinical manifestations, pathological features, treatment methods, and prognoses were analyzed.
Results
Among 26 patients, 6 had hepatic IMT, 2 splenic IMT, and 1 abdominal wall IMT, all detected incidentally as painless masses during routine exams. Six patients with mesenteric IMT reported abdominal distension, pain, nausea, vomiting, and low-grade fever. Of five patients with gastric IMT, three had gastrointestinal bleeding, one had distension and fever, and one had dysphagia. Four small intestine cases included one asymptomatic and three with obstruction symptoms. The colon and rectal cases presented with intermittent hematochezia. Surgery was performed in 24 patients, and 2 with metastases received palliative therapy. During follow-up, five patients relapsed; three received palliative therapy, and two had surgery. At last follow-up, 20 patients were disease-free, 3 were living with tumors, and 3 had died.
Conclusions
Abdominal IMTs are rare, low-grade tumors with favorable prognoses. Pathological examination is essential for diagnosis, and surgery is the primary treatment. Adjuvant therapy depends on tumor location and risk factors. Close follow-up is necessary due to the potential for recurrence and metastasis.
Keywords: Inflammatory myofibroblastic tumor, Abdominal tumors, Surgery, Diagnosis, Pathology, Adjuvant therapy, Tumor location
Introduction
Inflammatory myofibroblastic tumors (IMT) are rare mesenchymal soft tissue tumors exhibiting both neoplastic and inflammatory characteristics, involving myofibroblasts and inflammatory cells. Due to its potential for recurrence and metastasis, IMT is classified as a low-grade malignant or borderline tumor [1, 2]. Although IMT predominantly occurs in children and adolescents, it can also affect adults [3]. However, the exact etiology and pathogenesis remain unclear [4]. IMT can arise in various body parts, with the lungs being the most common site, followed by the abdominopelvic organs, head and neck, trunk, and limbs [5, 6]. Abdominal IMTs account for approximately 75% of extrapulmonary IMTs and have been reported in the mesentery, omentum, liver, spleen, stomach, and retroperitoneum [6–9]. The clinical presentation of abdominal IMT is diverse, lacking specific symptoms and signs, and with no characteristic systemic manifestations or imaging features, making them challenging to differentiate from other tumors in clinical practice [10–12].
Given the rarity of abdominal IMT, current research primarily consists of case reports or small series, with limited studies on its clinicopathological features, treatment, and prognosis. Through a retrospective analysis of clinical data from 26 patients with abdominal IMT, this study aims to summarize the clinicopathological characteristics, treatment methods, and prognosis of these patients. The goal is to enhance our understanding of the disease and provide insights for the standardized diagnosis and treatment of abdominal IMT.
Materials and methods
Clinical data
This study retrospectively included patients diagnosed with abdominal IMT at the First Hospital of Jilin University from January 2015 to December 2023. We identified patients with a pathological diagnosis of abdominal IMT through the hospital's electronic medical record system. The initial cohort was reviewed to exclude patients who did not meet the inclusion criteria or who met the exclusion criteria. Detailed records were maintained for each patient, including clinical manifestations, imaging features, pathological results, treatment plans, and follow-up status. All methods in this study were carried out in accordance with the relevant guidelines and regulations, including the ethical standards of the institutional and national research committee and the Helsinki Declaration of 1964 and its later amendments. The study was approved by the Ethics Committee of the First Hospital of Jilin University (Approval number: 2024-1067), and informed consent was obtained from all participants.
Inclusion and exclusion criteria
Inclusion Criteria:
Patients with a histopathological diagnosis of abdominal IMT.
Complete medical records.
The follow-up period was > 6 months.
Exclusion Criteria:
Patients with suspected but unconfirmed diagnoses
Patients with other concurrent malignant tumors.
After pathological confirmation of the biopsy results, the patient or their families refused therapy.
Death due to complications within 30 days after surgery.
Patients with incomplete clinical records or missing follow-up data.
Treatment methods
In this study, all treatment plans were formulated by a multidisciplinary team (MDT) comprising surgeons, medical oncologists, pathologists, radiologists, and radiation oncologists. The MDT first conducted a comprehensive assessment of each patient’s clinical data, including medical history, physical examination, imaging results, and pathological diagnosis. For patients suspected of having distant metastasis, additional whole-body positron emission tomography computed tomography (PET-CT) scans were performed. For resectable tumors, minimally invasive surgery was prioritized. For larger tumors or those with significant adhesions, combined organ resection was considered to ensure negative margins. For patients with positive surgical margins, recurrence, or high-risk factors (e.g., high Ki-67 proliferation index, large tumor size), the MDT developed adjuvant treatment plans. Additionally, the MDT established detailed follow-up plans to detect recurrence or metastasis and adjust treatment strategies accordingly.
Follow-up
During the first postoperative year, patients returned to the outpatient clinic of our hospital for follow-up every 3 months. Beginning in the second year, follow-ups were conducted every 6 months. For patients unable to visit the clinic, follow-ups were carried out via phone calls.
Statistical analysis
In this study, all data were entered into an Excel database and underwent preliminary organization and cleaning to ensure data integrity and accuracy. Continuous variables was described using the median and range. Categorical variables, including gender, tumor location, clinical manifestations, pathological characteristics, and outcomes, were presented as numbers (percentages).
Results
Clinical features
This study included 26 patients (12 males and 14 females) aged 4 months to 79 years (median age: 50 years). The locations of the lesions and their clinical presentations were as follows: six cases in the liver, two in the spleen, and one in the abdominal wall, all of which presented as localized, painless masses; six cases in the mesentery, with symptoms including abdominal distension, pain, nausea, vomiting, and low-grade fever; five cases in the stomach, of which three presented with upper gastrointestinal bleeding (hematemesis and melena), one with upper abdominal distension and fever (with a peak temperature of 39.5 °C), and one with dysphagia; four cases in the small intestine, with one asymptomatic case detected during a routine examination and three others presenting with symptoms of intestinal obstruction, including abdominal distension, nausea, and vomiting; and one case each in the ascending colon and rectum, both presenting with intermittent bleeding and blood-streaked stool. After IMT diagnosis, full-body scans revealed distant metastasis in two cases. The patients’ general clinical data are presented in Table 1.
Table 1.
Clinicopathologic characteristics of abdominal IMT patients (n = 26)
| Basic characteristic | n = 26 |
|---|---|
| Median age (years,range) | 50(4 m-79y) |
| Gender (male:female) | 12:14 |
| Tumor location (n, %) | |
| Hepatic | 6 (23.08) |
| Mesentery | 6 (23.08) |
| Stomach | 5 (19.23) |
| Small intestine | 4 (15.38) |
| Spleen | 2 (7.69) |
| Ascending colon | 1 (3.85) |
| Rectum | 1 (3.85) |
| Abdominal wall | 1 (3.85) |
| Clinical manifestation (n, %) | |
| Abdominal distension/pain, nausea and vomiting | 10(38.46) |
| Painless masses | 10(38.46) |
| Hematemesis and melena | 3 (11.55) |
| Bloody stool | 2 (7.69) |
| Fever | 1 (3.85) |
| Dysphagia | 1 (3.85) |
| IHC(n,%) | |
| SMA(+) | 19 (19/23) |
| Vimentin(+) | 16 (16/21) |
| Desmin (+) | 14(14/22) |
| ALK(+) | 11 (11/21) |
| S-100(-) | 19 (19/22) |
| Dog-1(-) | 12 (12/15) |
| CD117(-) | 16 (16/19) |
| Ki-67 | 10 (3–40%) |
| Status at last follow-up (n, %) | |
| Non-neoplastic death | 1 (3.85) |
| Neoplastic-related deaths | 2 (7.69) |
| With disease survival | 3 (11.54) |
| Disease-free survival | 20 (76.92) |
SMA, smooth muscle actin; ALK, anaplastic lymphoma kinase; Dog-1, discovered on gist-1; CD117,cluster of differentiation 117; Ki-67, Kiel-67
Imaging features
Ultrasonographically, an abdominal IMT often appears as a heterogeneous, hypoechoic mass with well-defined or infiltrative borders. Most lesions are solid; however, a few have cystic areas that manifest as mixed solid and cystic nodules. Color Doppler flow imaging (CDFI) shows sparse blood flow signals within lesions. In this study, 11 patients underwent abdominal ultrasonography. Hepatic IMT appears as a hypoechoic solid mass with heterogeneous internal echoes, relatively clear margins, and sparse blood flow signals. Splenic IMT presents as hypoechoic masses in the spleen, with clear boundaries, calcified foci within the interior, and blood flow signals at the margins. Abdominal wall IMT revealed a hypoechoic subcutaneous mass with unclear margins, indistinct separation from the adjacent anterior abdominal wall muscle layers, and a few small punctate blood flow signals within the lesion. The small intestinal IMT appeared intraperitoneally as a solid hypoechoic mass with heterogeneous internal echoes and a few punctate blood flow signals. The ultrasound findings of hepatic IMT are shown in Fig. 1.
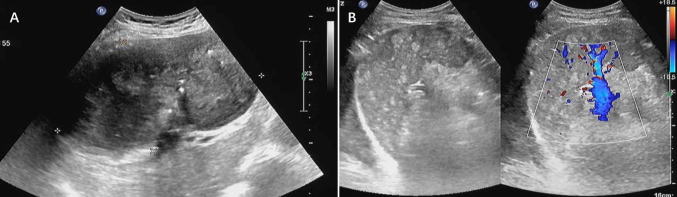
Fig. 1.
Ultrasound findings of hepatic IMT. A A heterogeneous mass in the liver, approximately 15.0 × 8.9 cm, with clear boundaries; B CDFI shows no obvious blood flow signal
On computed tomography [13] scans, most abdominal IMTs appear as low-density masses with unclear boundaries. Contrast-enhanced CT scans show various enhancement patterns, including homogeneous or heterogeneous enhancement, peripheral enhancement, and delayed enhancement, depending on the composition of the lesion. In our study, 23 patients underwent CT, of whom 17 underwent an enhanced scan. CT imaging showed that most lesions had unclear boundaries and heterogeneous density, with some displaying calcification or cystic changes. On contrast-enhanced scans, most lesions exhibited heterogeneous enhancement, with some continuing to show enhancement during the delayed phase. There were two cases of hepatic IMT; one case showed a slightly low-density nodule in the upper segment of the right anterior lobe, with heterogeneous mild hypodense enhancement and unclear margins. Thoracic and cranial CT revealed multiple space-occupying lesions, suggesting metastasis. In the other case, CT showed three nodular low-density lesions in hepatic segments S2 and S6, with ring-like enhancement on contrast-enhanced scanning and multiple enlarged lymph nodes in the abdominal cavity, suggesting metastasis. The CT manifestations of mesenteric IMT are shown in Fig. 2.
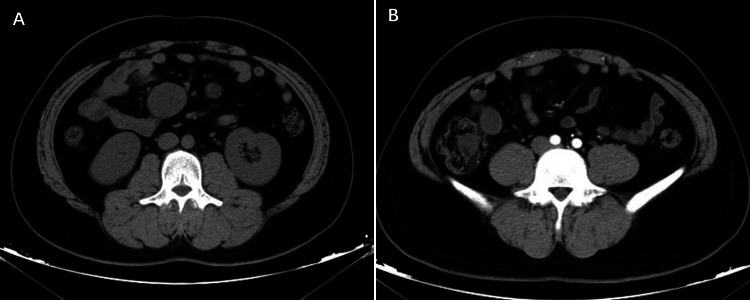
Fig. 2.
CT manifestations of mesenteric IMT. A A round soft tissue shadow is seen in the mesenteric region of the right mid-abdomen, with homogeneous density; B Enhancement scan shows moderate arterial phase enhancement
On magnetic resonance imaging (MRI), abdominal IMTs typically present as nodular lesions with irregular shapes and unclear boundaries. The lesions exhibit slightly low or isointense signals on T1-weighted images (T1 WI) and a variable range of high, slightly high, isointense, or slightly low signal intensities on T2-weighted images (T2 WI). The enhancement pattern on contrast-enhanced MRI is similar to that on CT and reflects the lesion’s internal components. In our study, seven patients underwent MRI, five with enhanced scanning. Hepatic IMT appeared as a slightly low signal on T1 WI, a mildly high signal on T2 WI, and a high on diffusion-weighted imaging (DWI). Arterial-phase scans showed nodular peripheral enhancement, with no obvious expansion in venous and equilibrium phases, and hepatocyte-specific phases showed low uptake. In one case of mesenteric IMT (invading the colon), MRI revealed a cystic-solid abnormal signal lesion in the posterior wall of the mid-abdomen, with heterogeneous enhancement of the cyst wall on contrast. DW1 detected a low or slightly high signal inside the capsule, with unclear boundaries between the adjacent mesentery and small intestine. One case of rectal IMT presented as a round mass approximately 4.8 cm from the anal verge, measuring 5.1 cm × 4.4 cm × 4.2 cm, with a long T1 and slightly long T2 signal. The center of the lesion showed patchy long T1 and T2 signals, while contrast-enhanced images displayed marked heterogeneous enhancement. In the MRI scan of hepatic IMT, a roundish abnormal signal was observed in the left lobe of the liver. The lesion measured approximately 1.9 cm × 1.8 cm. On T1 WI, the signal was slightly hypointense, while on T2 WI, it appeared slightly hyperintense. DWI showed high signal intensity. During the arterial phase of the contrast-enhanced scan, the lesion exhibited marginal nodular enhancement. No significant expansion of the enhancement was observed in the portal venous or equilibrium phases. In the hepatobiliary phase, the lesion showed low uptake. The detailed MRI findings for hepatic IMT are shown in Fig. 3.
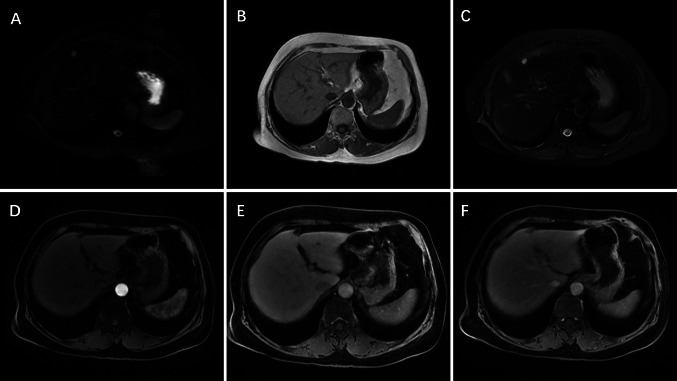
Fig. 3.
MRI findings of hepatic IMT. A A round abnormal signal is observed in the left lobe of the liver; DWI shows a high signal; B T1 WI shows a slightly hypointense signal; C T2 WI shows a slightly hyperintense signal; D Enhancement scan shows marginal nodular enhancement in the arterial phase; E, F No expanded enhancement in the venous and delay phase
Endoscopic examination primarily detects diseases in the esophagus, stomach, and other parts of the digestive system. IMT typically appears as a raised, smooth-surfaced mass during endoscopy. Larger masses can disrupt the mucosa, causing erosion and ulceration. In our study, seven patients underwent gastrointestinal endoscopy. One case of gastric IMT presented as a 4 cm mucosal protrusion with surface erosion at the junction of the gastric body and fundus, along with blackish-red bloody mucus in the stomach. Another case of gastric IMT appeared as a large raised lesion on the anterior wall of the lower gastric body, breaching the mucosa and measuring approximately 4 × 5 cm. The surface tissue was tough to touch and prone to bleeding. The endoscopic presentation of the gastric IMT is shown in Fig. 4.
Fig. 4.
Endoscopic presentation of gastric IMT. A Esophagus—smooth mucosa with normal color, no erosions, ulcers or protuberant lesions; B Cardia—smooth mucosa with good opening and closing, dentate line visible; C Antrum—smooth mucosa with normal color, no erosions, ulcers or protuberant lesions; D, E Duodenum (bulb and descending)—smooth mucosa with normal color, no erosions, ulcers or protuberant lesions; F Gastric angle—smooth mucosa with normal color, no erosions, ulcers or protuberant lesions; G Fundus—localized mucosal thickening; H Gastric body—localized mucosal thickening, with poor expansion and contractility, and visible erosion
Pathological features
Macroscopically, the lesions were mostly solid with a firm texture, and the cut surfaces appeared gray, white, or light brown. Microscopically, the tumor showed loose proliferation of spindle- or short spindle-shaped myofibroblasts with nuclear pleomorphism and mild atypia, while mitosis was rare. The stroma showed varying degrees of myxoid degeneration, hyaline changes, and collagen formation, accompanied by infiltration of lymphocytes and plasma cells. Immunohistochemical (IHC) staining revealed that anaplastic lymphoma kinase [14] was positive in 11 cases, smooth muscle actin (SMA) in 13 cases (partially or focally positive in 6 cases), Vimentin in 16 cases, and Desmin in 11 cases (partially or focally positive in 3 cases), whereas S-100, cluster of differentiation 117(CD117), and discovered on gist-1(Dog-1) were typically negative. The Kiel-67(Ki-67) positivity rate ranged from 3 to 40%. Representative hematoxylin and eosin (H&E) and IHC staining results are shown in Fig. 5, and detailed IHC results are summarized in Table 2.
Fig. 5.
Pathological examination of a patient with mesenteric IMT. A HE × 20; B HE × 40; C ALK (+) × 40; D CD117 (−) × 40; E Desmin (+) × 40; F DOG-1 (−) × 40; G S100 (-) × 40; H SMA (+) × 40; I Vimentin (+) × 40; J Ki-67 approximately 5% × 4
Table 2.
IHC characteristics of 26 abdominal IMT cases
| Case | ALK | Vimentin | Desmin | SMA | CD117 | S-100 | Dog-1 | Ki-67 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | NP | partly + | NP | weakly + | - | 10 |
| 2 | – | – | NP | + | NP | – | weakly + | 15 |
| 3 | – | + | – | + | NP | – | NP | 10 |
| 4 | + | – | – | – | – | – | NP | 10 |
| 5 | + | + | – | NP | – | – | – | 10 |
| 6 | + | NP | + | + | – | – | – | 30 |
| 7 | - | + | + | partly + | – | – | NP | 5 |
| 8 | + | NP | + | – | – | – | – | 15 |
| 9 | NP | + | partly + | + | – | – | NP | 3 |
| 10 | + | - | + | + | weakly + | – | NP | 30 |
| 11 | – | + | – | + | – | NP | NP | 5 |
| 12 | + | + | – | + | – | – | NP | 35 |
| 13 | NP | NP | + | partly + | weakly + | – | – | 5 |
| 14 | NP | + | partly + | – | NP | – | – | 5 |
| 15 | + | + | NP | partly + | NP | – | – | 10 |
| 16 | – | + | – | + | NP | – | NP | 8 |
| 17 | NP | + | partly + | NP | weakly + | – | – | 5 |
| 18 | – | + | – | + | – | NP | NP | 3 |
| 19 | – | + | + | NP | – | NP | – | 10 |
| 20 | + | – | + | – | – | – | NP | 5 |
| 21 | + | – | – | + | – | weakly + | – | 10 |
| 22 | NP | + | + | + | – | NP | – | 15 |
| 23 | – | + | + | partly + | – | – | NP | 5 |
| 24 | – | NP | + | partly + | – | – | weakly + | 8 |
| 25 | – | + | NP | + | NP | – | weakly + | 35 |
| 26 | + | NP | + | + | – | weakly + | – | 40 |
NP,Not performed; ALK, anaplastic lymphoma kinase; SMA, smooth muscle actin; Dog-1, discovered on gist-1; CD117,cluster of differentiation 117; Ki-67, Kiel-67
Treatment and survival status
Among the 26 patients included in our study, 2 did not undergo surgery due to distant metastasis and were treated with palliative chemotherapy and an ALK inhibitor. Surgical treatment was performed in 24 cases, including 1 case of endoscopic surgery, 8 cases of open surgery, and 15 cases of laparoscopic surgery. All patients were followed up as of August 2024. During follow-up, five patients experienced recurrence,the recurrence rate was 20.83%. Among the five patients with recurrence, two underwent surgical resection again. Of these, one received adjuvant therapy after surgery, while the other did not receive adjuvant therapy but was monitored with regular follow-ups. The remaining three recurrent cases were managed with palliative antitumor therapy. As of the end of the study, three patients died. The mortality rate was 11.54%. Among the remaining patients, 20 were disease-free, while three continued to live with the disease. Detailed treatment methods, follow-up, and prognostic information are presented in Table 3.
Table 3.
Treatment and follow-up results of abdominal IMT patients (n = 26)
| Case | Site | Surgical methods | Adjuvant therapy | Recurrence/metastasis | Treatment after recurrence | Follow-up time |
|---|---|---|---|---|---|---|
| 1 | Hepatic | Laparoscopic hepatic left lateral lobectomy | No | No | No | 66 months |
| 2 | Hepatic | Partial hepatectomy | No | Yes | Chemotherapy (methotrexate and vinorelbine) and corticosteroids (dexamethasone) | 15 months |
| 3 | Hepatic | Laparoscopic partial hepatectomy | No | No | No | 96 months |
| 4 | Hepatic | Laparoscopic hepatic left lateral lobectomy | No | No | No | 18 months |
| 5 | Gastric | Laparoscopic partial gastrectomy | No | No | No | 36 months |
| 6 | Gastric | Partial gastrectomy, distal pancreatic resection, and splenectomy | No | Yes | Surgery + ALK inhibitor (crizotinib) + nonsteroidal anti-inflammatory drugs (celecoxib) | 32 months |
| 7 | Gastric | Laparoscopic partial gastrectomy | No | No | No | 54 months |
| 8 | Gastric | Laparoscopic subtotal gastrectomy, side-to-side gastrojejunal anastomosis, and perigastric lymph node dissection | No | No | No | 34 months |
| 9 | Gastric | Laparoscopic partial gastrectomy | No | No | No | 30 months |
| 10 | Mesenteric | Laparoscopic exploration and resection of the mesenteric mass | No | Yes | ALK inhibitor (crizotinib) + corticosteroids (dexamethasone) | 36 months |
| 11 | Mesenteric | Resection of mesenteric mass + partial resection and anastomosis of small intestine | No | No | No | 90 months |
| 12 | Mesenteric | Partial small intestine resection + biopsy of peritoneal nodules + ileostomy + placement of nose-intestine nutritional tube | No | Yes | ALK inhibitor (ensartinib) | 20 months |
| 13 | Mesenteric | Laparoscopic exploration + right hemicolectomy + ileum transverse colon end-to-side anastomosis + periintestinal lymph node dissection | No | No | No | 42 months |
| 14 | Mesenteric | Resection of mesenteric mass | No | No | No | 38 months |
| 15 | Mesenteric | Laparoscopic exploration + resection of mesenteric mass | No | No | No | 48 months |
| 16 | Small intestine | Laparoscopic exploration + partial resection and anastomosis of small intestine + periintestinal lymph node dissection | No | No | No | 10 months |
| 17 | Small intestine | Laparoscopic exploration + partial resection and anastomosis of small intestine | No | No | No | 30 months |
| 18 | Small intestine | Resection of duodenal mass | No | No | No | 28 months |
| 19 | Small intestine | Exploratory laparotomy + partial resection and anastomosis of small intestine | No | No | No | 38 months |
| 20 | Splenic | Laparoscopic splenectomy | No | No | No | 24 months |
| 21 | Splenic | Laparoscopic splenectomy | No | No | No | 29 months |
| 22 | Ascending colon | Colonoscopy assisted polypectomy | No | Yes | Surgery(Laparoscopic exploration + right hemicolectomy + end-to-side anastomosis of ileum and transverse colon + periintestinal lymph node dissection) | 18 months |
| 23 | Abdominal wall | Resection of abdominal wall mass | No | No | No | 60 months |
| 24 | Rectum | Laparoscopic radical resection of rectal tumor + retroperitoneal lymph node dissection + prophylactic terminal ileostomy | No | No | No | 12 months |
| 25 | Hepatic | Percutaneous needle biopsy of liver through ultrasonic induction | Chemotherapy (methotrexate and vinorelbine) + apatinib | – | – | 14 months |
| 26 | Hepatic | Percutaneous needle biopsy of liver through ultrasonic induction | ALK inhibitor (ensartinib) + corticosteroids (dexamethasone) | – | – | 21 months |
Case 2: Hepatic IMT. After completing relevant examinations, the patient underwent partial hepatectomy. Postoperative pathology showed a tumor measuring 13.0 cm × 7.0 cm × 5.5 cm, with negative margins and no vascular or nerve invasion. Adjuvant therapy was not administered. Seventeen months postoperatively, CT revealed thickening of the peritoneum and omentum with nodular changes and heterogeneous enhancement on contrast-enhanced CT, suggesting metastasis. Multiple small nodules were also found in both lungs, raising the suspicion of metastasis. Following the MDT discussion, the patient received palliative chemotherapy with methotrexate (40 mg/m2, intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest) and vinorelbine (intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest), along with oral dexamethasone (6 mg/day for 5 consecutive days, with a 3-week cycle). The patient is currently living with the disease.
Case 6: Gastric IMT. The patient underwent two surgeries over the course of the disease. The first surgery included resection of the gastric tumor, partial gastrectomy, distal pancreatectomy, and splenectomy. Postoperative pathology revealed a gastric tumor measuring 9.0 cm × 9.0 cm × 5.0 cm, involving the muscular and serosal layers of the stomach and the splenic parenchyma. Tumor invasion was observed in the nerves but not in the vasculature. Adjuvant therapy was not administered. Twelve months later, a CT scan showed multiple masses around the anastomosis, anterior to the remnant pancreas, and on the left side of the abdominal cavity, with the largest mass measuring 4.0 cm × 10.3 cm × 7.5 cm. The masses had lobulated edges and heterogeneous enhancement on contrast-enhanced CT tomography. The boundary between the lesion around the anastomotic stoma, the adjacent gastric wall, and the left lobe of the liver was unclear. Multiple nodules measuring 0.4 cm to 1.7 cm were seen around the lesion, with marked enhancement on imaging. The examination suggested tumor recurrence or metastasis without invasion of the adjacent gastric wall and liver. Following the MDT discussion, the patient underwent a second surgery, including resection of the abdominal masses, left hemicolectomy, partial gastrectomy, partial hepatectomy, and lymph node dissection. Postoperative pathological examination revealed negative margins and no lymph node metastases. The patient received an ALK inhibitor (crizotinib, 250 mg orally, twice daily) and a nonsteroidal anti-inflammatory drugs (NSAIDs)(celecoxib, 100 mg orally, twice daily). Nine months after the second surgery, metastatic omental and peritoneal lesions were detected. The patient was then treated with chemotherapy, including methotrexate (40 mg/m2, intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest) and vinorelbine (intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest). Concurrently, the patient received oral prednisone (60 mg once daily) and oral ensartinib (225 mg once daily). The patient is currently living with the disease.
Case 10: Mesenteric IMT. The patient underwent laparoscopic exploration, mesenteric mass resection, and lymph node dissection around the intestine. Postoperative pathology revealed a tumor measuring 10.5 cm × 8.0 cm × 3.5 cm, with negative surgical margins and no vascular or nerve invasion. No metastasis was found in the mesenteric lymph nodes, and adjuvant therapy was not administered. Six months after surgery, an abdominal CT scan showed multiple nodules around the intestinal anastomosis, posterior to the bladder, in the right anterior bladder wall, between the intestinal loops, and in the lower abdominal wall, with heterogeneous enhancement on contrast imaging, suggesting metastasis. Following the MDT discussion, the patient was treated with an ALK inhibitor (crizotinib, 250 mg orally, twice daily) and oral dexamethasone (6 mg/day for 5 consecutive days, with a 3-week cycle). Currently, the patient is living with the disease.
Case 12: Mesenteric IMT involving the small intestine. The patient underwent partial small intestine resection, ileostomy, and biopsy of the peritoneal nodules, along with placement of a naso-intestinal feeding tube. The surgical margin was positive, and the patient refused further treatment; therefore, no adjuvant therapy was administered. After 15 months, CT imaging revealed multiple nodules in the liver and lungs, suggesting metastasis. Given the patient’s history of hepatocellular carcinoma, the MDT recommended treatment with oral ensartinib (225 mg once daily). To date, the patient has died.
Case 22: Ascending colon IMT. The patient underwent two surgeries over the course of the disease. The first surgery involved resection of the colonic mass via colonoscopy. Pathological examination confirmed the diagnosis of IMT, and no adjuvant therapy was administered. Four years after the operation, the patient underwent a CT examination due to abdominal distension and pain, which revealed a cystic-solid mass in the mid-abdomen, measuring approximately 7.0 cm × 5.0 cm × 4.0 cm. The cystic portion was non-enhanced, while the solid portion showed heterogeneous enhancement, with an unclear boundary adjacent to the ascending colon. Following the MDT discussion, the patient underwent laparoscopic exploration, right hemicolectomy, ileocolic anastomosis, and lymph node dissection. Postoperative pathology revealed negative margins and no lymph node metastases. The patient recovered well and did not receive adjuvant therapy. Eleven months after the second surgery, the patient remains disease-free.
Two cases (Case 25 and Case 26) of hepatic IMT with distant metastasis were diagnosed via biopsy. Case 25 received chemotherapy with methotrexate (40 mg/m2, intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest) and vinorelbine (intravenous infusion, weekly for 3 consecutive weeks followed by a 1-week rest), along with oral apatinib. Case 26 was treated with an ALK inhibitor (ensartinib, 225 mg orally, once daily) and corticosteroids (dexamethasone, oral dosage not specified). Both patients have since passed away.
During treatment, we closely monitored patients for adverse reactions. Common adverse reactions included hematologic toxicities (leukopenia, anemia, and thrombocytopenia). For Grade II or higher hematologic toxicities, chemotherapy was temporarily discontinued, and supportive care (e.g., colony-stimulating factors) was provided. Gastrointestinal symptoms (nausea, vomiting, and anorexia) were managed with antiemetics administered prior to chemotherapy, and some patients also received gastric mucosal protectants or acid-suppressing agents. Other adverse reactions, such as rash, pruritus, and musculoskeletal pain, were treated symptomatically, and relevant chemotherapy drugs were discontinued if necessary.
Discussion
IMTs are rare mesenchymal tumors primarily composed of differentiated myofibroblastic spindle cells, accompanied by significant infiltration of inflammatory cells, including plasma cells, lymphocytes, and eosinophils [5, 12]. According to the 2020 WHO classification of soft tissue and bone tumors, IMTs are categorized as low-grade malignant or borderline tumors with the potential for recurrence and metastasis [1, 15]. Prior to being formally recognized as IMTs, these tumors were often referred to by various names, including inflammatory pseudotumor, pseudosarcoma, myxoid hamartoma, plasma cell granuloma, benign myofibroblastoma, xanthogranuloma, fibrohistiocytoma, and inflammatory myofibroblastic proliferation [16, 17]. As true neoplasms, most IMTs exhibit an indolent course; however, a small proportion may show malignant potential or low-grade malignancy, especially in those with poor prognosis, often arising in the abdominal cavity [18]. To date, the etiology and pathogenesis of IMTs remain unclear. Some scholars suggest that factors such as trauma, inflammation, autoimmune diseases, surgery, human herpesvirus or Epstein-Barr virus infection, and abnormal expression or mutation of the ALK gene may be related to the occurrence and development of IMT [19, 20]. The pathophysiological mechanism of IMT formation due to these factors may involve uncontrolled proliferation of myofibroblasts, leading to the formation of tumor lesions [21, 22]. Additionally, some researchers have reported cases of IMT discovered during follow-up after treatment of an initial primary tumor[23]. In our study, one patient with mesenteric IMT had previously undergone surgery for hepatocellular carcinoma four years earlier. Although the hepatocellular carcinoma was well-controlled, mesenteric IMT was diagnosed during follow-up. Whether a causal relationship exists between prior hepatocellular carcinoma surgery and the development of mesenteric IMT remains uncertain and requires further investigation.
Abdominal IMT can occur at any age, with the first incidence peak occurring before the age of 20 years and the second between 50 and 60 years of age, with no significant sex preference [24, 25]. In our study, the ages of the patients ranged from 4 months to 79 years, with a median age of 50 years and a male-to-female ratio of approximately 1:1.2, consistent with previous studies [6]. IMT can develop throughout the body but most commonly occurs in the lungs, followed by the abdominopelvic organs, trunk, limbs, retroperitoneal spaces, and other locations [6, 26]. As the most common extrapulmonary site, abdominal IMT affects multiple organs, and the clinical presentation varies based on the tumor’s location but lacks specific symptoms and signs. Hepatic and splenic IMTs often present as slow-growing painless masses that can cause local compression and distension [18, 27]. IMTs in the gastrointestinal tract, mesentery, and omentum may present with non-specific symptoms such as abdominal pain, distension, nausea, vomiting, gastrointestinal bleeding (hematemesis or melena), and symptoms of bowel obstruction[28, 29]. Approximately 15–30% of abdominal IMT patients may exhibit systemic symptoms such as fever of unknown cause, weight loss, anemia, thrombocytosis, hypergammaglobulinemia, elevated erythrocyte sedimentation rate (ESR), and elevated C-reactive protein (CRP) levels, etc. [12, 20, 30]. In our study, the tumors were located in the liver (6 cases), spleen (2 cases), and abdominal wall (1 case), all of which presented as local, painless masses. Patients with mesenteric IMTs (6 cases), gastric IMTs (5 cases), small intestine IMTs (4 cases), ascending colon IMT (1 case), and rectal IMT (1 case) presented with symptoms such as abdominal distension, pain, nausea, vomiting, dysphagia, hematemesis, and intermittent bleeding, resembling other gastrointestinal diseases without specific symptoms or signs. Elevated white blood cell counts and CRP levels were observed in eight patients, and six patients had fever, all of which returned to normal after surgery.
Ultrasonography, endoscopy, CT and MRI are commonly used to screen for abdominal tumors and are widely applied in clinical practice [31, 32]. Ultrasound is a convenient and cost-effective preliminary screening tool capable of identifying tumor location, size, blood flow, and internal echoes [10, 33]. CT and MRI provide better evaluations of tumor size, extent, and relationship with surrounding structures, with MRI offering superior differentiation of tumor margins from adjacent soft tissues, which is crucial for treatment planning and prognostic evaluation [34]. Endoscopy plays a unique role in diagnosing and treating digestive system diseases by providing direct visualization of lesions and utilizing techniques such as chromoendoscopy to detect small lesions. Endoscopic ultrasound can further assess tumor infiltration depth, relationships with adjacent organs and assist in diagnosis, staging, and minimally invasive treatment when necessary. The imaging manifestations of abdominal IMT are diverse and relate to the proportion of fibrosis in the lesion, inflammatory cell infiltration, and disease stage. However, due to the lack of specific imaging features, abdominal IMTs often appear as solid masses, making clinical diagnosis based on imaging alone challenging w signals [11]. On CT scans, abdominal IMTs often present as round, oval, or irregularly shaped low-density lesions with various degrees of enhancement during contrast scanning, including homogeneous or heterogeneous enhancement, peripheral enhancement, and progressive enhancement, depending on the composition of the lesion [35]. Larger lesions may show areas of necrosis and calcification without enhancement [35]. Compared with CT, MRI better delineates the relationship between the IMT and surrounding soft tissue structures. Abdominal IMTs often appear as irregular nodular signals with unclear boundaries on MRI, displaying mixed low signals on T1 WI and heterogeneous high signals on T2 WI. The enhancement patterns observed on MRI contrast scans are similar to those observed on CT [36]. Endoscopic examination can provide a histopathological diagnosis and if necessary, treatment during the procedure [37]. Endoscopically, IMTs often present as smooth, elevated masses, and larger tumors may breach the mucosa, causing erosion or ulceration [28, 38]. Given that IMTs have both neoplastic and inflammatory histological characteristics,the role of PET-CT in diagnosing IMT is limited [39]. FDG uptake in abdominal IMTs shows high variability, possibly related to the proportion of tumor and inflammatory cells and their activities. Both malignant tumors and IMTs exhibit increased fluorodeoxyglucose(FDG) uptake [40]. Some reports have indicated that IMTs can be misdiagnosed as lymphomas on PET-CT [41]. Nonetheless, PET-CT is valuable for detecting primary tumors, assessing local recurrence, evaluating distant metastases, and monitoring treatment responses [42, 43]. In our study, 11 patients underwent abdominal ultrasonography, all of whom exhibited hypoechoic solid masses with relatively clear boundaries and sparse blood flow, suggesting that they may be low-grade tumors. CT was performed on 23 patients, of whom 17 underwent contrast-enhanced scans. CT revealed that most lesions had unclear boundaries and heterogeneous density, with some showing calcification or cystic changes. Most lesions displayed heterogeneous enhancement during contrast scanning, with some showing persistent enhancement during the delayed phase. MRI was performed on seven patients, with five undergoing contrast-enhanced scans, but no specific imaging features were noted. Therefore, based on our study, we can conclude that while imaging findings may suggest malignancy, it remains difficult to definitively diagnose abdominal IMT based on imaging alone.
With advances in pathological detection methods, clinicians and pathologists have deepened their understanding of IMT. Since its discovery, the nomenclature and definitions of the disease have significantly evolved. The term “IMT” has gradually gained recognition among experts and scholars, and it is now classified as a true neoplasm [20]. Currently, the diagnosis of abdominal IMT relies on histopathological examination and IHC staining [6]. Microscopically, IMT is primarily composed of spindle-shaped myofibroblastic cells and infiltrating inflammatory cells and is often accompanied by fibrosis, hyaline degeneration, calcification, or necrosis. The inflammatory component can vary and usually includes plasma cells, lymphocytes, eosinophils, and neutrophils [12, 44]. Based on the proportion and distribution of these cells, IMT can be classified into three subtypes: myxoid/vascular, spindle cell-dense, and fibrous [45]. Although these subtypes can coexist within the same lesion, one subtype typically dominates [46]. IHC staining plays a crucial role in diagnosing abdominal IMT, helping to identify the immunophenotype of myofibroblasts and exclude other diagnoses [47]. In abdominal IMT, SMA is expressed in approximately 80–90% of spindle cells, whereas muscle-specific actin (MSA) and desmin are seen in 60–70% of cases, usually with focal or diffuse positive expression [48]. Vimentin is typically diffusely positive [49], while markers such as S-100, CD34, CD117, CD21, and CD23 are generally negative. This suggests that the expression of vimentin, SMA, and desmin, along with the absence of S-100, provides relatively high specificity for diagnosing IMT [50]. Among the various IHC markers, ALK has unique significance in diagnosing abdominal IMT. It is now recognized as a specific diagnostic marker and driver gene for IMT[51]. Recent studies have shown that approximately 50%–70% of patients with IMT exhibit ALK gene rearrangements on chromosome 2p23, leading to ALK overexpression [6]. These ALK rearrangements can fuse with different partner genes, including TPM3/4, CARS, GCC2, EML4, TRAF3, TNS1, THBS1, and DCTN1, which may contribute to IMT [52–55]. In ALK-negative IMT, ROS1 and NTRK3 gene rearrangements are the most common mutations, occurring in 5%–15% of cases, whereas RET and PDGFRB rearrangements are relatively rare [56–58]. Some researchers recommend fluorescence in situ hybridization(FISH) analysis to assess ALK fusion status if ALK is positive on IHC testing [59]. In addition to IHC and FISH, more comprehensive molecular analyses, such as next-generation sequencing (NGS), are important diagnostic tools that can provide deeper insights into the molecular mechanisms and genetic characteristics of IMT [60]. In our study, H&E staining showed loose proliferation of spindle and short spindle myofibroblasts, with mild atypia and stromal changes, such as myxoid degeneration, hyalinization, and collagen formation, accompanied by varying degrees of lymphocyte and plasma cell infiltration. IHC staining revealed positivity rates of 82.61%, 76.19%, 63.64%, and 52.38% for SMA, vimentin, desmin, and ALK, respectively. Based on typical histological and IHC features, diagnosing IMT is not difficult.
During the progression of abdominal IMT, a small percentage of patients may experience malignant transformation, in which the tumor loses its original differentiation and progresses toward higher malignancy. When high-grade transformation occurs, typical spindle cells change into polygonal or round cells with increased mitotic activity, cellular atypia, vesicular nuclei with prominent nucleoli, more frequent mitoses, and sometimes necrosis [61]. As borderline tumors, approximately 8%–18% of IMT cases may undergo a malignant transformation during the disease course, and, in some cases, multiple biopsies may be necessary, particularly for recurrent or metastatic IMTs [62, 63]. In our study, five patients experienced postoperative recurrence, two of whom underwent further surgical treatment. Pathological examination of both specimens showed no significant evidence of malignant transformation, which may be due to the small sample size in our study. The average Ki-67 index of patients with recurrence was approximately 20%, which was higher than that of patients without recurrence. Additionally, two patients with recurrence had initial pathology reports indicating nerve invasion and positive margins, which may have contributed to their recurrence, although further studies are needed to confirm these findings.
As a borderline tumor, abdominal IMT has the potential for recurrence and distant metastasis. Therefore, it should neither be treated with extensive resection, as with a malignant tumor, which can cause significant trauma, nor resected in situ as a benign lesion, which could increase postoperative complications [5, 64]. According to the guidelines of the European Society for Medical Oncology (ESMO) and other studies, surgery remains the mainstay treatment for abdominal IMT, as it not only provides a definitive pathological diagnosis and symptom relief but also effectively treats the tumor [65]. An appropriate surgical approach should be selected based on the location, size, and extent of the tumor to ensure radical operation (RO) [12]. Performing frozen pathological examinations during surgery is also recommended. Based on the frozen results, the specific scope of tumor resection can be determined[66]. Most patients are cured after RO resection [6]. Studies by Sagar [67] and Iwai [68] indicate that the prognosis is favorable after RO resection, with a 5-year survival rate of approximately 91%. However, the recurrence rate after RO resection varies depending on the anatomical location, with recurrence rates of approximately 2% for pulmonary IMT and 25% for extrapulmonary IMT [24]. The efficacy of conventional chemotherapy or radiotherapy for abdominal IMT is uncertain, particularly in determining whether adjuvant therapy is needed after RO resection [69, 70]. Comprehensive treatments such as chemotherapy, radiotherapy, or ALK inhibitors should be considered for patients with incomplete surgical resection, postoperative recurrence, or histological evidence of malignant transformation [24]. Some researchers suggest that abdominal IMT, especially those arising from the mesentery or peritoneum, have a high potential for local infiltration or distant metastasis, and chemotherapy may be considered for these patients [69]. However, no standardized chemotherapy regimen for abdominal IMTs has been established. Studies on different chemotherapy regimens, including anthracyclines and vinorelbine/vinblastine, show an overall response rate (ORR) of 50%–64%, with some cases achieving long-term disease control [24, 69]. Radiotherapy is less commonly used for IMT and is primarily reported in case studies, often involving tumors located in anatomically complex areas, such as the head and neck region, with limited large-scale studies confirming its efficacy [21, 71]. With an increasing understanding of IMT, neoadjuvant therapy for abdominal IMT has also been reported and shows promise as a feasible approach. Neoadjuvant therapy may offer inoperable patients a chance for surgical resection by reducing the tumor size and facilitating RO resection while preserving organ function, particularly in patients with complex anatomical structures [14, 72].
Genetic abnormalities serve not only as a basis for diagnosing IMT but also as key therapeutic targets. ALK expression in abdominal IMT has paved the way for personalized molecular-targeted therapies [73, 74]. Crizotinib, a tyrosine kinase inhibitor targeting ALK, MET, ROS1, and RON, is recommended by the National Comprehensive Cancer Network (NCCN) as one of the treatment options for ALK-positive IMT [75, 76]. Targeted therapy has become a key treatment method for advanced, unresectable, recurrent, or metastatic ALK-positive IMTs [6, 77], with favorable outcomes and manageable side effects [21, 78]. In cases where resistance to crizotinib develops, second-generation ALK inhibitors such as ceritinib and alectinib [79, 80] or third-generation ALK inhibitors such as lorlatinib may be considered [72]. ALK-negative patients tend to have a higher risk of distant metastasis and a poorer prognosis. Some case reports suggest that NSAIDs [81] and small-molecule tyrosine kinase inhibitors [82] may be effective in these patients, although the evidence is limited. Additionally, mutations in ROS1, NTRK, ETV6, RET, and PDGFR-β have been identified in ALK-negative IMTs [56, 59, 83]. For ALK-positive IMTs, neoadjuvant therapy with ALK inhibitors before surgery may reduce the surgical risk associated with larger, multifocal, or difficult-to-resect tumors, ultimately benefiting patients [84].
After radical resection, the overall prognosis for IMT is generally favorable, with recurrence rates varying by tumor location, while distant metastases are relatively rare [24, 85]. The local recurrence rate for abdominal IMT is approximately 14.2%–35.0% and may be influenced by factors such as surgical margins, tumor location, and size [8, 12, 30]. IMTs occurring in the mesentery, omentum, or retroperitoneum tend to be more aggressive, with a higher likelihood of local recurrence and distant metastasis [18]. Previous studies have identified surgical margin status as a crucial predictor of local recurrence and overall survival [3, 42]. Additionally, factors such as tumor size, pseudocapsule, necrosis within the lesion, Ki-67 labeling index, ALK expression, and specific gene fusions may also impact prognosis [86, 87]. However, the relationship between ALK expression and the prognosis of IMT remains unclear. Some studies suggest that ALK expression is associated with a better prognosis as ALK over-expression may contribute to tumorigenesis while also presenting an opportunity for targeted therapy [88, 89]. Other studies indicate that ALK-positive IMTs may have a higher risk of local recurrence, while ALK-negative IMTs tend to be more aggressive and prone to distant metastasis [6, 87]. However, the optimal treatment strategy for recurrent disease remains unclear. If only local recurrence occurs and the patient is in good physical condition, reoperation is still recommended [90]. For advanced abdominal IMTs, where surgery is no longer an option, individualized comprehensive treatment should be considered [87]. In our study, 24 patients underwent surgical treatment, including 1 endoscopic procedure, 8 traditional open surgeries, and 15 laparoscopic surgeries. Postoperatively, 23 patients had negative margins, and none received adjuvant therapy after the initial surgery. During follow-up, five cases recurred: two of whom underwent surgical resection again, one received adjuvant therapy postoperatively, one was monitored with regular follow-ups without adjuvant therapy, and the remaining three were treated with palliative antitumor therapy. Of the recurrent cases, two were located in the mesentery, one had positive surgical margins during the first surgery, one had nerve invasion, and one involved endoscopic surgery. Additionally, three of the recurrent tumors had diameters greater than 10 cm, suggesting that these factors may be associated with recurrence [91, 92].
However, this study has some limitations. The primary limitation of our study is the relatively small sample size, which is inherent to the rarity of abdominal IMT. This limited sample size may reduce the statistical power of our analyses and the generalizability of our findings. Furthermore, our study is retrospective in nature, which may introduce selection bias and limit the robustness of our conclusions. Future prospective studies with larger cohorts are needed to validate our findings and further explore the optimal management strategies for abdominal IMT.
Conclusion
In summary, abdominal IMTs are rare, low-grade malignant tumors with a relatively favorable prognosis. Preoperative diagnosis is challenging due to the lack of specific symptoms and imaging features. Histopathology and immunohistochemistry are key to the diagnosis of abdominal IMT. Surgical resection remains the primary treatment method, and the need for adjuvant therapy should be assessed based on tumor location, surgical conditions, and the presence of high-risk factors. Although abdominal IMTs are generally considered low-grade or borderline tumors, their potential for recurrence and metastasis necessitates close follow-up after treatment.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
All authors contributed to the study conception and design. Writing—original draft preparation: [Qiang Zhang, Zhiwei Zhang, Jing Fan]; Writing—review and editing: [Qiang Zhang,Zhiwei Zhang, Zhuoma Ji, Jing Fan]; Conceptualization: [Feng Liu, Qiang Zhang, Chunyan Wang]; Methodology: [Zhiwei Zhang, Zhuoma Ji, Jing Fan]; Formal analysis and investigation: [Feng Liu, Chunyan Wang, Zhuoma Ji]; Resources: [Feng Liu, Chunyan Wang]; Supervision: [Feng Liu, Chunyan Wang, Qiang Zhang], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The data used during the current study are available from the corresponding author(Feng Liu) in case of any queries or requirement of data.
Declarations
Ethics approval and consent to participate
This study has received approval from the Ethics Committee of the First Hospital of Jilin University (Approval number: 2024-1067) and informed consent has been duly obtained from both the patients and their families.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-Yan Wang, Email: wang_chuny@jlu.edu.cn.
Feng Liu, Email: liufeng@sxmu.edu.cn.
References
- 1.Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: news and perspectives. Pathologica. 2021;113:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal A, Goyal S, Goyal A, Jana M. WHO classification of soft tissue tumours 2020: an update and simplified approach for radiologists. Eur J Radiol. 2021;143: 109937. [DOI] [PubMed] [Google Scholar]
- 3.Al Shenawi H, Al-Shaibani SA, Al Saad SK, Al-Sindi F, Al-Sindi K, Al Shenawi N, Naguib Y, Yaghan R. An extremely rare case of malignant jejunal mesenteric inflammatory myofibroblastic tumor in a 61-year-old male patient: a case report and literature review. Front Med. 2022;9:1042262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camela F, Gallucci M, di Palmo E, Cazzato S, Lima M, Ricci G, Pession A. Pulmonary inflammatory myofibroblastic tumor in children: a case report and brief review of literature. Front Pediatr. 2018;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan P, Casanova M, Ferrari A, Fordham A, Trahair T, Venkatramani R. Inflammatory myofibroblastic tumor: molecular landscape, targeted therapeutics, and remaining challenges. Curr Probl Cancer. 2021;45: 100768. [DOI] [PubMed] [Google Scholar]
- 6.Siemion K, Reszec-Gielazyn J, Kisluk J, Roszkowiak L, Zak J, Korzynska A. What do we know about inflammatory myofibroblastic tumors?—a systematic review. Adv Med Sci. 2022;67:129–38. [DOI] [PubMed] [Google Scholar]
- 7.Meng Y, Xie J, Liang Y, Wu M, Lu Y, Lu Q. Inflammatory myofibroblastic tumor in the liver: a case report. Front Oncol. 2024;14:1349692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da M, Qian B, Mo X, Xu C, Wu H, Jiang B, Peng W, Qi J, Sun J, Wu K. Inflammatory myofibroblastic tumors in children: a clinical retrospective study on 19 cases. Front Pediatr. 2021;9: 543078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Sharma S, Mittal A, Barwad A, Rastogi S. Recurrent infantile inflammatory myofibroblastic tumor of mesentery–case report and review of imaging findings. Radiol Case Rep. 2021;16:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao M, Wang C, Zhang B, Jiang Q, Liu J, Liao J. Distinguishing hepatocellular carcinoma from hepatic inflammatory pseudotumor using a nomogram based on contrast-enhanced ultrasound. Front Oncol. 2021;11: 737099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filips A, Maurer MH, Montani M, Beldi G, Lachenmayer A. Inflammatory myofibroblastic tumor of the liver: a case report and review of literature. World J Hepatol. 2020;12:170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory myofibroblastic tumour: state of the art. Cancers. 2022;14:3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nocturne G, Boudaoud S, Miceli-Richard C, Viengchareun S, Lazure T, Nititham J, Taylor KE, Ma A, Busato F, Melki J, et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren’s syndrome. Blood. 2013;122:4068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trahair T, Gifford AJ, Fordham A, Mayoh C, Fadia M, Lukeis R, Wood AC, Valvi S, Walker RD, Blackburn J, et al. Crizotinib and surgery for long-term disease control in children and adolescents with ALK-positive inflammatory myofibroblastic tumors. JCO Precis Oncol. 2019;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. 2021;28:44–58. [DOI] [PubMed] [Google Scholar]
- 16.Khatri A, Agrawal A, Sikachi RR, Mehta D, Sahni S, Meena N. Inflammatory myofibroblastic tumor of the lung. Adv Respir Med. 2018;86:27–35. [DOI] [PubMed] [Google Scholar]
- 17.Bashir MR, Al Sohaibani MO, Al-Rikabi AC. An unusual case of inflammatory myofibroblastic tumor of the appendix masquerading as acute appendicitis. Oman Med J. 2018;33:250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu GX, Xu CC, Yao NF, Gu JZ, Jiang HL, Han XF. Inflammatory myofibroblastic tumor: a demographic, clinical and therapeutic study of 92 cases. Math Biosci Eng. 2019;16:6794–804. [DOI] [PubMed] [Google Scholar]
- 19.Taiymi A, Meryem N, Bouziane M, Zazour A, Kharrasse G, Khannoussi W, Ismaili Z. Abdominal inflammatory myofibroblastic tumour presenting as a pancreatic mass: a case report. Cureus. 2023;15: e41213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W, Zhu Y. Clinical characteristics and outcomes of 17 cases of inflammatory myofibroblastic tumor at a university hospital in China. Oncol Lett. 2021;21:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Gong C, Zhang J, Feng W, Guo Y, Sang Y, Wang C, Chen Y, Wang J, Yu L, et al. Clinicopathological analysis and treatment of adult patients with inflammatory myofibroblastic tumor: a 15-year single- center study. Cancer Res Treat. 2023;55:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang E, Zhang L, Wang Y, Zhang M. Epstein-Barr virus-negative inflammatory pseudotumor-like variant of follicular dendritic cell sarcoma of the liver: a case report. Asian J Surg. 2023;46:1846–7. [DOI] [PubMed] [Google Scholar]
- 23.Shen Q, Liu X, Zhang L, Li T, Zhou J. Inflammatory myofibroblastic tumor of the liver after adrenal neuroblastoma surgery: a case report. Discov Oncol. 2024;15:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, Corradini N, Minard-Colin V, Zanetti I, Bisogno G, et al. Inflammatory myofibroblastic tumor: the experience of the European pediatric soft tissue sarcoma study group (EpSSG). Eur J Cancer. 2020;127:123–9. [DOI] [PubMed] [Google Scholar]
- 25.Nakano K. Inflammatory myofibroblastic tumors: recent progress and future of targeted therapy. Jpn J Clin Oncol. 2023;53:885–92. [DOI] [PubMed] [Google Scholar]
- 26.Ariafar A, Ahmed F, Khorshidi A, Torabi-Nezhad S, Hosseini SH. Inflammatory myofibroblastic tumor of the right kidney mimicking a locally advanced renal carcinoma: a case report. J Kidney Cancer VHL. 2022;9:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang EJ, Kim KW, Kang SH, Pak MG, Han SH. Inflammatory myofibroblastic tumors arising from pancreas head and peri-splenic area mimicking a malignancy. Ann Hepatobiliary Pancreat Surg. 2021;25:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai G, Parikh DM, Wagle PK. A giant solid-cystic gastric inflammatory myofibroblastic tumor: a case report and literature review. Cureus. 2023;15: e37167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li TH, Yang FF, Lee CT, Chan RH. Intra-abdominal multicentric inflammatory myofibroblastic tumors mimicking ruptured appendicitis. Int J Surg Case Rep. 2022;93: 106990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karaisli S, Kamer E, Ekinci N, Cengiz F, Er A, Peskersoy M. Inflammatory myofibroblastic tumour of the colon: 2 case reports and a comprehensive review of the literature. Int J Colorectal Dis. 2020;35:947–58. [DOI] [PubMed] [Google Scholar]
- 31.Sharma N, Kumar A, Sambhakar S, Bhatia D, Hussain S, Mursal M, Singh B, Narayan KP. Recent Advances in Nanotherapeutics and Theranostics for Squamous Cell Carcinoma: A Comprehensive Review. Curr Drug Deliv. 2025. 10.2174/0115672018342513241230061704. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Dhiman C, Narayan KP, Kumar M, Sharma M. Nanotherapeutics:Restoring Gut-Dybiosis and inducing cancer apoptosis.IGI Global 2025.28.
- 33.Qian J, Zhu K, Ye J. Ultrasonic manifestations of mesenteric inflammatory myofibroblastic tumors in children. Front Pediatr. 2019;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QA, Chen HW, Wu RC, Wu CE. Update of diagnosis and targeted therapy for alk(+) inflammation myofibroblastic tumor. Curr Treat Options Oncol. 2023;24:1683–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamura K, Beppu T, Oda E, Sato N, Yuki H, Motohara T, Miyamoto H, Miyamura S, Onishi K, Komohara Y, Akahoshi S. Hepatic inflammatory pseudotumor mimicking malignant tumor with rare onset of intra-abdominal hemorrhage. Anticancer Res. 2021;41:2727–32. [DOI] [PubMed] [Google Scholar]
- 36.Calistri L, Maraghelli D, Nardi C, Vidali S, Rastrelli V, Crocetti L, Grazioli L, Colagrande S. Magnetic resonance imaging of inflammatory pseudotumor of the liver: a 2021 systematic literature update and series presentation. Abdom Radiol. 2022;47:2795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda Y, Kanazawa Y, Goto O, Kakinuma D, Higuchi K, Koizumi E, Nakata R, Sakurazawa N, Ando F, Suzuki M, et al. Primary gastric inflammatory myofibroblastic tumor treated with non-exposed endoscopic wall-inversion surgery (NEWS): a case report and literature review. DEN Open. 2024;4: e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada H, Funasaka K, Nakagawa M, Hirayama Y, Horiguchi N, Nagasaka M, Nakagawa Y, Kuzuya T, Hashimoto S, Miyahara R, et al. Large inflammatory myofibroblastic tumor of the esophagus: a case report and literature review. Intern Med. 2023;62:3473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning MA, Paal EE, Srivastava A, Mortele KJ. Nonepithelial neoplasms of the pancreas, part 2: malignant tumors and tumors of uncertain malignant potential from the radiologic pathology archives. Radiographics. 2018;38:1047–72. [DOI] [PubMed] [Google Scholar]
- 40.Budylev A, Solar I, Kessner R, Aizic A. ROS1-positive inflammatory myofibroblastic tumor of the small bowel causing obstruction: a case report. J Radiol Case Rep. 2022;16:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma C, Lu J, Chen G, Wang W, Su F, Su X. Inflammatory myofibroblastic tumor mimicking lymphoma on (18)F-FDG PET/CT. Report of a case and review of the literature. Hell J Nucl Med. 2018;21:77–80. [DOI] [PubMed] [Google Scholar]
- 42.Parker NC, Singanallur P, Faiek S, Gao J, White P. Inflammatory myofibroblastic tumor after receiving treatment for non-small cell carcinoma. Cureus. 2024;16: e59359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vounckx M, Jansen YJL, Fadaei S, Geers C, De Pauw V, Smets D. Unraveling the spectrum of inflammatory myofibroblastic tumors in the lung: a comprehensive case series highlighting endobronchial, pleural, and lung parenchymal tumors. JTCVS Open. 2024;17:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Shen L, Yun T, Zhu C, Wang P, Wang S. Clinicopathological features of gastric inflammatory myofibroblastic tumor: report of five cases. Exp Ther Med. 2021;22:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strianese D, Tranfa F, Finelli M, Iuliano A, Staibano S, Mariniello G. Inflammatory myofibroblastic tumor of the orbit: a clinico-pathological study of 25 cases. Saudi J Ophthalmol. 2018;32:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol. 2021;28:119–38. [DOI] [PubMed] [Google Scholar]
- 47.Cerier E, Beal EW, Dillhoff ME. Inflammatory myofibroblastic tumour: an unusual presentation including small bowel obstruction and palpable abdominal mass. BMJ Case Rep. 2018. 10.1136/bcr-2018-224549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang F, Zhang W, Han C, Jiang H. A case of pulmonary inflammatory myofibroblastic tumor treated with bronchoscopic therapy plus lobectomy. J Cardiothorac Surg. 2021;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun JS, Song SY, Na KJ, Kim S, Choi YD. Inflammatory myofibroblastic tumor arising from the ascending aorta mimicking a thymoma. Gen Thorac Cardiovasc Surg. 2020;68:1193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K, Guo R, Siegal GP, Wei S. Inflammatory myofibroblastic tumor of bone harboring an ALK gene amplification. Pathol Res Pract. 2019;215: 152535. [DOI] [PubMed] [Google Scholar]
- 51.Mohammad N, Haimes JD, Mishkin S, Kudlow BA, Leong MY, Chew SH, Koay E, Whitehouse A, Cope N, Ali RH, et al. ALK is a specific diagnostic marker for inflammatory myofibroblastic tumor of the uterus. Am J Surg Pathol. 2018;42:1353–9. [DOI] [PubMed] [Google Scholar]
- 52.He W, Ji X, Song C, Song S, Liu L. Case report: efficacy of ensartinib treatment in pulmonary inflammatory myofibroblastic tumor with a rare GCC2-ALK fusion. Front Oncol. 2022;12: 934887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuisma H, Jokinen V, Pasanen A, Heikinheimo O, Karhu A, Välimäki N, Aaltonen L, Bützow R. Histopathologic and molecular characterization of uterine leiomyoma-like inflammatory myofibroblastic tumor: comparison to molecular subtypes of uterine leiomyoma. Am J Surg Pathol. 2022;46:1126–36. [DOI] [PubMed] [Google Scholar]
- 54.Collins K, Ramalingam P, Euscher ED, Reques Llanos A, García A, Malpica A. Uterine inflammatory myofibroblastic neoplasms with aggressive behavior, including an epithelioid inflammatory myofibroblastic sarcoma: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2022;46:105–17. [DOI] [PubMed] [Google Scholar]
- 55.Haimes JD, Stewart CJR, Kudlow BA, Culver BP, Meng B, Koay E, Whitehouse A, Cope N, Lee JC, Ng T, et al. Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am J Surg Pathol. 2017;41:773–80. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto H, Yoshida A, Taguchi K, Kohashi K, Hatanaka Y, Yamashita A, Mori D, Oda Y. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology. 2016;69:72–83. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi A, Kurosawa M, Uemura M, Kitazawa J, Hayashi Y. Anaplastic lymphoma kinase-negative uterine inflammatory myofibroblastic tumor containing the ETV6-NTRK3 fusion gene: a case report. J Int Med Res. 2018;46:3498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheek EH, Fadra N, Jackson RA, Davila JI, Sukov WR, Uckerman MT, Clayton A, Keeney GL, Halling KC, Torres-Mora J, Schoolmeester JK. Uterine inflammatory myofibroblastic tumors in pregnant women with and without involvement of the placenta: a study of 6 cases with identification of a novel TIMP3-RET fusion. Hum Pathol. 2020;97:29–39. [DOI] [PubMed] [Google Scholar]
- 59.Chang JC, Zhang L, Drilon AE, Chi P, Alaggio R, Borsu L, Benayed R, Travis WD, Ladanyi M, Antonescu CR. Expanding the molecular characterization of thoracic inflammatory myofibroblastic tumors beyond ALK gene rearrangements. J Thorac Oncol. 2019;14:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Racanelli D, Brenca M, Baldazzi D, Goeman F, Casini B, De Angelis B, Guercio M, Milano GM, Tamborini E, Busico A, et al. Next-generation sequencing approaches for the identification of pathognomonic fusion transcripts in sarcomas: the experience of the Italian ACC sarcoma working group. Front Oncol. 2020;10:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C, Huang M, He H, Wu S, Liu M, He J, Zang H, Xu R. Inflammatory myofibroblastic tumor of the urinary bladder: an 11-year retrospective study from a single center. Front Med. 2022;9: 831952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyal L, Rao S, Reddy GS, Agarwal P. Inflammatory myofibroblastic tumor of anterior maxillary gingiva: an unusual clinical presentation. J Oral Maxillofac Pathol. 2022;26:S73-s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao J, Zhou ML, Zhou SH. Inflammatory myofibroblastic tumors of the head and nec. Int J Clin Exp Med. 2015;8:1604–10. [PMC free article] [PubMed] [Google Scholar]
- 64.Imazu N, Shibata M, Koya Y, Morino K, Honma Y, Senju M, Watanabe T, Harada M. Hepatic inflammatory pseudotumor protruding from the liver surface and directly penetrating the colon. Intern Med. 2020;59:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong Y, Zahid KR, Han Y, Hu P, Zhang D. Treatment of pediatric inflammatory myofibroblastic tumor: the experience from China children’s medical center. Children. 2022;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan C, Fan J, Xu L. Inflammatory myofibroblastic tumor of the upper arm: a case report. Medicine (Baltimore). 2023;102: e36558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwai H, Yanagawa N, Deguchi H, Tomoyasu M, Shigeeda W, Kaneko Y, Yoshimura R, Kanno H, Sugai M, Shikanai S, et al. Surgical treatment for lung metastasis of inflammatory myofibroblastic tumor of the lung: a case report. Thorac Cancer. 2023;14:1644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sagar AES, Jimenez CA, Shannon VR. Clinical and histopathologic correlates and management strategies for inflammatory myofibroblastic tumor of the lung. A case series and review of the literature. Med Oncol. 2018;35:102. [DOI] [PubMed] [Google Scholar]
- 69.Baldi GG, Brahmi M, Lo Vullo S, Cojocaru E, Mir O, Casanova M, Vincenzi B, De Pas TM, Grignani G, Pantaleo MA, et al. The activity of chemotherapy in inflammatory myofibroblastic tumors: a multicenter. Eur Retrospect Case Series Anal Oncol. 2020;25:e1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang Z, Wang L, Tang G, Medeiros LJ. Fluorescence in situ hybridization (FISH) for detecting anaplastic lymphoma kinase (ALK) rearrangement in lung cancer: clinically relevant technical aspects. Int J Mol Sci. 2019;20:3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biswas R, Halder A, Gangopadhyay M, Biswas D. Inflammatory myofibroblastic tumor of maxillary sinus successfully treated with radiotherapy and corticosteroid: report of a rare case. J Egypt Natl Canc Inst. 2020;32:26. [DOI] [PubMed] [Google Scholar]
- 72.Comandini D, Catalano F, Grassi M, Pesola G, Bertulli R, Guadagno A, Spina B, Mascherini M, De Cian F, Pistoia F, Rebuzzi SE. Outstanding response in a patient with ros1-rearranged inflammatory myofibroblastic tumor of soft tissues treated with crizotinib: case report. Front Oncol. 2021;11: 658327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chmiel P, SłOwikowska A, Banaszek Ł, Szumera CA, Szostakowski B, SpałEk MJ, Świtaj T, Rutkowski P, Czarnecka AM. Inflammatory myofibroblastic tumor from molecular diagnostics to current treatment. Oncol Res. 2024;32:1141–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X, Li L, Zhang Y, Meng F, Xie H, Duan R. A recurrent inflammatory myofibroblastic tumor patient with two novel ALK fusions: a case report. Transl Cancer Res. 2022;11:3379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunt AL, Nutcharoen A, Randall J, Papazian A, Deeken J, Maxwell GL, Bateman NW, Petricoin EF, Benyounes A, Conrads TP, Cannon TL. Integration of multi-omic data in a molecular tumor board reveals EGFR-associated ALK-inhibitor resistance in a patient with inflammatory myofibroblastic cancer. Oncologist. 2023;28:730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:815–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, Klingebiel T, Lehrnbecher T. Crizotinib in ALK(+) inflammatory myofibroblastic tumors-Current experience and future perspectives. Pediatr Blood Cancer. 2018. 10.1002/pbc.26920. [DOI] [PubMed] [Google Scholar]
- 78.Fischer M, Moreno L, Ziegler DS, Marshall LV, Zwaan CM, Irwin MS, Casanova M, Sabado C, Wulff B, Stegert M, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1764–76. [DOI] [PubMed] [Google Scholar]
- 79.Mansfield AS, Murphy SJ, Harris FR, Robinson SI, Marks RS, Johnson SH, Smadbeck JB, Halling GC, Yi ES, Wigle D, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol. 2016;27:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saiki M, Ohyanagi F, Ariyasu R, Koyama J, Sonoda T, Nishikawa S, Kitazono S, Yanagitani N, Horiike A, Ninomiya H, et al. Dramatic response to alectinib in inflammatory myofibroblastic tumor with anaplastic lymphoma kinase fusion gene. Jpn J Clin Oncol. 2017;47:1189–92. [DOI] [PubMed] [Google Scholar]
- 81.Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: a case report and review of the literature. Oncol Lett. 2013;5:1672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Q, Wei J, Liu X, Wang J. Anaplastic lymphoma kinase-negative pulmonary inflammatory myofibroblastic tumor with multiple metastases and its treatment by Apatinib: a case report. Med. 2019;98: e18414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Debonis SA, Bongiovanni A, Pieri F, Fausti V, De Vita A, Riva N, Gurrieri L, Vanni S, Diano D, Mercatali L, Ibrahim T. ALK-negative lung inflammatory myofibroblastic tumor in a young adult: a case report and literature review of molecular alterations. Medicine. 2021;100: e25972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N, Zeng Q, Chen C, Yu J, Yan D, Xu C, Liu D, Zhang Q, Zhang X. Clinical characteristics and prognosis of pulmonary inflammatory myofibroblastic tumor: an over 10-year retrospective analysis. Pediatr Investig. 2020;4:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raitio A, Losty PD. Treatment and outcomes in pediatric inflammatory myofibroblastic tumors—a systematic review of published studies. Eur J Surg Oncol. 2024;50: 108388. [DOI] [PubMed] [Google Scholar]
- 86.Han Q, He X, Cui L, Qiu Y, Li Y, Chen H, Zhang H. Case report: early distant metastatic inflammatory myofibroblastic tumor harboring EML4-ALK fusion gene: study of two typical cases and review of literature. Front Med. 2022;9: 826705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domínguez-Massa C, Doñate-Bertolín L, Blanco-Herrera ÓR, Heredia-Cambra T, Pérez-Guillén M, Martínez-Cózar V, Mayordomo-Aranda E, Hornero-Sos F. Inflammatory myofibroblastic tumor: a rare entity with a complex diagnosis. Rev Port Cardiol. 2023;42:169.e161-169.e164. [DOI] [PubMed] [Google Scholar]
- 88.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–74. [DOI] [PubMed] [Google Scholar]
- 89.Yorke J, Solanki K, Theegala V, Georges T, Sinha AK, Asberry DE, El Abbassi A. A rare case of incidentally diagnosed pulmonary inflammatory myofibroblastic tumour with dramatic response to crizotinib in a postpartum woman. Eur J Case Rep Intern Med. 2023;10: 003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Preobrazhenskaya EV, Iyevleva AG, Suleymanova AM, Tiurin VI, Mitiushkina NV, Bizin IV, Ivanstov AO, Gorustovich OA, Shelekhova KV, Kachanov DY, et al. Gene rearrangements in consecutive series of pediatric inflammatory myofibroblastic tumors. Pediatr Blood Cancer. 2020;67: e28220. [DOI] [PubMed] [Google Scholar]
- 91.Kumar A, Sakhare K, Bhattacharya D, Chattopadhyay R, Parikh P, Narayan KP, Mukherjee A. Communication in non-communicable diseases (NCDs) and role of immunomodulatory nutraceuticals in their management. Front Nutr. 2022;9: 966152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma M, Satya Sri Kotipalli R, Siva Kumar N, Kumar A, Rawat M, Dhiman C, Kumar M. Innovations in drug delivery strategies for breast cancer. IntechOpen.2024.105772
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used during the current study are available from the corresponding author(Feng Liu) in case of any queries or requirement of data.