Lately, several emerging infectious diseases have had a great deal of play in the media, resulting in anxiety among health care workers and their patients. This article explores the epidemiology and clinical characteristics of some of these emerging infections.
BOVINE SPONGIFORM ENCEPHALOPATHY AND VARIANT CREUTZFELDT-JAKOB DISEASE
Bovine spongiform encephalopathy (BSE), also known as “mad cow disease,” and variant Creutzfeldt-Jakob disease (vCJD) are the cattle and human forms of a group of relentlessly progressive neurodegenerative diseases known as the transmissible spongiform encephalopathies. There are acquired and hereditary forms of the diseases, and the transmissible spongiform encephalopathies are grouped together due to similar clinical and pathological features.
The inherited forms of human spongiform encephalopathy include familial Creutzfeldt-Jakob disease, Gerstman-Straussler-Scheinker disease, and familial fatal insomnia. The acquired forms can be classified as either idiopathic or environmental. The idiopathic forms of the disease include “classic,” or “sporadic,” Creutzfeldt-Jakob disease (sCJD) and sporadic familial fatal insomnia. The environmental forms include vCJD and kuru in humans, as well as various veterinary forms such as BSE (cattle), scrapie (sheep), chronic wasting disease (deer and elk), and transmissible feline encephalopathy (domestic and wild cats).
The etiology of the spongiform encephalopathies remains controversial. The most prominent hypothesis was proposed by Stanley Prusiner, MD, of the University of California, San Francisco, in 1982 (1). He proposed the existence of “proteinaceous infectious particles,” or prions. Dr. Prusiner hypothesized that scrapie is caused by a transmissible infectious protein without an accompanying nucleic acid. All transmissible spongiform encephalopathies, whether affecting humans or animals, are characterized by the accumulation of an abnormal, transformed host glycoprotein, termed “prion protein,” in the central nervous system. The normal function of prion protein is unknown; however, it is chromosomally encoded by host DNA (in humans, on the short arm of chromosome 20) and normally expressed in an alpha-helical conformation that is susceptible to cleavage by proteinase K (2, 3). Aberrant prion protein is expressed in a beta-pleated sheet conformation that is resistant to cleavage by proteinase K; this aberrant protein eventually polymerizes and accumulates in the central nervous system as amyloid (4, 5).
The prion hypothesis postulates that transmissible spongiform encephalopathies such as BSE, kuru, and vCJD are caused by the introduction of an aberrant prion protein from a different subject into a susceptible host. The aberrant prion protein is thought to induce normally folded host prion protein to change conformation into the beta-pleated sheet form, resulting in resistance to cleavage and eventual polymerization and accumulation in the central nervous system. Hereditary forms of the spongiform encephalopathies are thought to result when aberrant host prion protein is encoded by mutant host chromosomal DNA and expressed in the abnormally folded state. Indeed, mutations in the prion protein gene at codons 117,129,178, and 200 have been described in the human familial spongiform encephalopathies (6).
Apparently, no nucleic acids are associated with the postulated infectious aberrant prion protein, although some scientists argue that a very small genome of <50 nucleotides could be present. Host RNA or other host proteins such as a complement may be involved in pathogenesis (6). Lymphoid tissue may serve as a reservoir, and it is thought that the infectious prion protein reaches the central nervous system by axonal transport (7, 8). Aberrantly folded prion protein is highly neurotoxic, with its accumulation causing neuronal cell apoptosis and cell death (9). Pathologic changes in the central nervous system include spongiform degeneration, neuron loss, proliferative astrocytosis and microgliosis, and accumulation of aberrantly folded prion protein demonstrated on special stains (10).
The epidemiology and clinical features of sCJD are quite distinct from those of vCJD. sCJD was first described in 1920 by Hans Gerhard Creutzfeldt and independently in 1921 by Alfons Maria Jakob. It is the most common of the human spongiform encephalopathies; even so, it is quite rare, with a global incidence of 1 case per 1 million persons per year. There is worldwide distribution of the disease. The mean age at onset of symptoms is 65 years; the incubation period for naturally occurring (as opposed to iatrogenically induced) disease is unknown. There are no clusters of incidence in naturally occurring disease, and there is apparently no naturally occurring human-to-human transmission, although iatrogenic transmission by corneal transplants, dural grafts, and human pituitary growth hormone is well described (6).
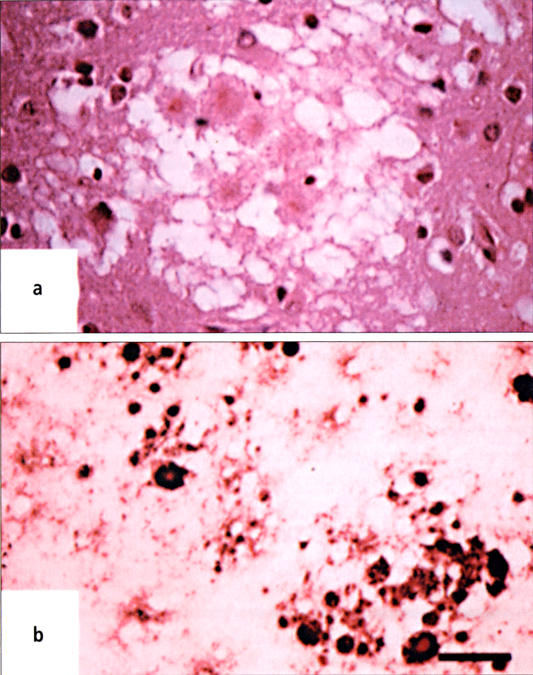
sCJD has a rapidly progressive course, with a mean time of 5 months between onset of symptoms and death. Cognitive changes predominate early in the course of the illness, with impairment of memory, concentration, and judgment. Myoclonus is present at some time in the course of the illness in >90% of patients, with hypokinesia, nystagmus, and ataxia in >70% and hyperreflexia, positive Babinski sign, and spasticity in 40% to 80% of cases. Cranial nerve and sensory symptoms occur rarely. There is no pleocytosis or abnormality of glucose or protein on analysis of the cerebrospinal fluid. The 14-3-3 protein is present in the cerebrospinal fluid in 53% to 95% of biopsy-proven cases of sCJD; however, false positives can occur with herpes simplex virus encephalitis, hypoxic/metabolic encephalopathy, and central nervous system metastasis. The electroencephalogram demonstrates characteristic periodic synchronous bi- or triphasic sharp wave complexes in 67% to 95% of patients at some point in the illness; these results may be modified with benzodiazepines or barbiturates. Neuroimaging by brain computed tomography shows nonspecific changes and atrophy as the disease progresses; however, magnetic resonance imaging may demonstrate characteristically increased T2-weighted signal in the anterior basal ganglia, most commonly in the putamen and head of the caudate (Figure 1). Definitive diagnosis is made by brain biopsy, with demonstration of widespread spongiform degeneration and reactive gliosis in the cerebral cortex (Figure 2). Rare amyloid plaques may be present (6, 11).
Figure 1.

Axial fluid-attenuated inversion recovery magnetic resonance image at the level of the basal ganglia in a patient with sporadic Creutzfeldt-Jakob disease. Image shows hyperintensity of the caudate head and anterior putamen (arrows). Image reproduced courtesy of Dr. D. Summers, National CJD Surveillance Unit, Western General Hospital, Edinburgh EH4 2XU.
Figure 2.
(a) Widespread spongiform degeneration in a specimen of cerebral cortex from a patient with sporadic Creutzfeldt-Jakob disease (hematoxylin and eosin, ×200). (b) Widespread reactive gliosis in a specimen of cerebral cortex from a patient with sporadic Creutzfeldt-Jakob disease; the specimen is immunostained with antibodies against glial fibrillary acid protein. Reprinted with permission from reference 10. Copyright © 2001 Massachusetts Medical Society. All rights reserved.
vCJD is thought to be associated with BSE. BSE was first documented in cattle in the United Kingdom (UK) in 1986 and is thought to have resulted as a cross-species “jump” by the agent of scrapie from sheep to cattle. The BSE epizootic in the UK was eventually traced to the use of sheep and cattle offal (brain and intestines) in the manufacture of ruminant feeds; the use of organic solvents in the rendering process had been discontinued in the UK in 1982. There were 180,000 confirmed cases of BSE in the UK, with an estimated 50,000 cattle entering the human food chain. The UK Department of Health began surveillance for human forms of the disease in 1990; the European Union followed suit in 1993. Tragically, the first case of human disease, vCJD, was described in the UK in 1995 (Figure 3). Aside from the epidemiologic association, further evidence that vCJD was associated with BSE came from the finding that the aberrant prion protein found in vCJD is identical in glycosylation and electrophoretic pattern to that of BSE and is distinct from the abnormal prion proteins of the other transmissible spongiform encephalopathies. In addition, inoculation of either vCJD or BSE prion protein into experimental animals produces identical clinical and neuropathic changes (6, 11).
Figure 3.
Total confirmed cases of bovine spongiform encephalopathy in cattle and of human variant Creutzfeldt-Jakob disease, UK, 1988–August 2003. From reference 6.
As of January 2004, 155 cases of vCJD have been reported worldwide: 145 in the UK, 6 in France, and 1 each in Italy, Ireland, Canada, and the USA. All of the cases outside the UK have been linked to travel or residence in the UK at the time of the epizootic's peak or to exposure to British beef through imports. The peak incidence of vCJD in the UK was 28 cases in 2000, confirmed cases of bovine spongiform encephalopathy in cattle variant Creutzfeldt-Jakob disease, UK, 1988-August 2003. From with a subsequent annual decline/plateau since 2001 (6, 11). In January 2004 there was a report of human-to-human transmission of vCJD in the UK via blood transfusion. The patient in question died in the fall of 2003; autopsy confirmed vCJD. This patient had received a blood transfusion in 1996 from an apparently healthy blood donor who later developed and died from vCJD in 1999. However, both persons lived in the UK at the time of the peak epizootic and could have been independently exposed through consumption of beef or beef products (12). vCJD predominantly affects younger persons, with the mean age at onset of symptoms being 29 years. The course of the disease is somewhat more prolonged than that of sCJD, with an average interval between onset of symptoms and death of 14 months. Psychiatric symptoms, particularly depression and delusions, are prominent early in the course of the disease. In contrast to sCJD, sensory symptoms are prominent in vCJD, with delayed onset of neurological signs, which may include ataxia, dysarthria, and paresis of upward gaze, as well as myoclonus. The patient eventually develops dementia, mutism, and finally coma.
As with sCJD, cerebrospinal fluid analysis is unremarkable with respect to cell count and chemistries; yet in contrast to sCJD, the cerebrospinal fluid 14-3-3 protein is a very insensitive means of diagnosing vCJD. The electroencephalogram shows nonspecific slowing without periodic sharp waves. On neuroirriaging, magnetic resonance imaging shows a characteristic intense T2-weighted signal in the pulvinar region of the thalamus (Figure 4). Aberrant prion protein can be demonstrated in lymphoid tissue antemortem and may serve as a diagnostic tool. Interestingly, although mutations in the normal human prion protein gene on chromosome 20 are not present, all patients with vCJD have been homozygous for methionine at codon 129. It is not known whether this is associated with genetic susceptibility to infection or is a marker for more rapid progression of disease. Again, definitive diagnosis can by made by brain biopsy (6, 11). vCJD has very distinctive neuropathology, characterized by the presence of “florid” fibrillary amyloid aberrant prion protein plaques, the accumulation of which is most marked in the cerebral and cerebellar cortex. In addition, posterior thalamic gliosis is usually present, along with the characteristic spongiform changes, which are most severe in the basal ganglia and cerebellar molecular layer (Figure 5) (10). The UK Department of Health has established criteria for the diagnosis of vCJD (Table).
Figure 4.

Axial fluid-attenuated inversion recovery magnetic resonance image at the level of the basal ganglia in a patient with variant Creutzfeldt-Jakob disease. Image shows high signal in the posterior nuclei (“pulvinar”′) of the thalamus (arrows). Image reproduced courtesy of Dr. D. Summers, National CJD Surveillance Unit, Western General Hospital, Edinburgh EH4 2XU.
Figure 5.
(a) A specimen of cerebral cortex obtained from a patient with new variant Creutzfeldt-Jakob disease shows amyloid deposits within vacuoles (hematoxylin and eosin, ×200). These deposits have been referred to as “florid plaques.” (b) A specimen of cerebral cortex obtained from the same location as in panel A but subjected to hydrolytic autoclaving and immunostaining for prion protein reveals numerous prion protein plaques, many of which are in clusters, as well as minute deposits of prion protein surrounding many cortical neurons and their proximal processes (×100). Reprinted from reference 10 with permission from Dr. James W. Ironside.
Table.
Case definition of variant Creutzfeldt-Jakob disease of the United Kingdom Department of Health, 2003*
| Diagnostic criteria for variant CJD | |
| I | A, Progressive neuropsychiatric disorder; 8, duration of illness of >6 months; C, routine investigations do not suggest an alternative diagnosis; D, no history of potential iatrogenic exposure |
| II | A, Early psychiatric symptoms†; B, persistent, painful sensory symptoms‡; C, ataxia; D, myoclonus or chorea or dystonia; E, dementia |
| III | A, EEG does not show the typical appearance of sporadic CJD§ (or no EEG performed); B, bilateral pulvinar high signal on MRI scan |
| IV | Positive tonsil biopsy |
| Definite | Criterion IA and neuropathological confirmation of variant CJD¶ |
| Probable | Criterion I plus 4 of the 5 criteria for II plus IIIA and IIIB, or I and IV |
*From reference 6.
†Depression, anxiety, apathy, withdrawal, and/or delusions.
‡This includes both frank pain and/or unpleasant dysaesthesia.
§Generalized triphasic periodic complexes at a rate of ˜1 per second.
¶Spongiform change and extensive prion protein deposition with florid plaques throughout the cerebrum and cerebellum.
CJD indicates Creutzfeldt-Jakob disease; EEG, electroencephalogram; MRI, magnetic resonance imaging.
The treatment of vCJD is primarily supportive, with isolated case reports of beneficial results with amantadine, vidarabine, methisoprinol, and hyperbaric oxygen. Human trials of chlorpromazine and quinacrine are ongoing in the UK. Additionally, Congo red, anthracyclines, dimethyl sulfoxide, glycerol, and polyene antibiotics have some degree of activity in vitro or in experimental animals (6).
On December 12, 2003, the first case of BSE was confirmed in the USA. The affected animal, a 6V2-year-old dairy cow, had been brought to slaughter in the state of Washington. The animal was tested because she could not stand or walk; such animals are termed “downers” and are routinely tested for BSE per policy of the US Department of Agriculture (USDA). The lymphoid and neural tissue was removed from the carcass, and the meat was released by the USDA for sale at market before results of testing were available. The affected meat was distributed in Washington, Oregon, Idaho, Montana, Alaska, Nevada, California, and Hawaii. After BSE was confirmed, the USDA recalled all meat processed the same day the affected animal was slaughtered. The cow's origin was ultimately determined to be Edmonton, Alberta, where BSE was first reported in Canada; the USDA is in the process of tracing the birth herd of this animal (11, 13). The immediate repercussions of this single documented case of BSE included a ban on the importation of US beef by several foreign governments and a drop of 20% in the US live cattle markets. In April 2004, a cow with central nervous system symptoms was slaughtered in Texas and shipped to a processor for rendering into animal feed. The carcass had been rendered by the time the Food and Drug Administration (FDA) traced the animal, leaving no suitable material for BSE testing; however, the FDA was able to impound the rendered material (14).
The UK was able to control the epizootic of BSE with a comprehensive prevention strategy in addition to widespread testing for BSE. In November 1990, the UK prohibited the use of ruminant-derived proteins in all animal and poultry feeds, including pet foods after domestic cats were diagnosed with feline spongiform encephalopathy after eating commercially produced cat food containing beef by-products contaminated with BSE. Additionally, various meat industry regulations were developed. As it is thought that neural tissue, such as brain and spinal cord, and bone represent higher-risk materials, the UK has banned the use and sale of brain and bone-in cuts of meat; muscle meat is thought to be of low risk for transmission of BSE. Milk is not thought to be infectious. Older animals are thought to be more infectious than younger animals; thus, as of March 1996 the UK has banned the slaughter of animals older than 30 months. In addition, because of the concern for possible transmission of vCJD by transfusion, the UK has required that all blood products be leukocyte depleted (15).
The USA has adopted various control measures regarding BSE and vCJD. In 1989, the USA instituted a ban on cattle and cattle products imported from countries with documented cases or at high risk of BSE. In 1990, “downer” cattle or cattle with central nervous system symptoms were targeted for BSE testing. In 1997, the use of ruminant-derived proteins in cattle feed was banned, excluding blood and blood products. In 2000, the FDA and the American Red Cross instituted guidelines for deferral of blood donors who had lived in the UK or other high-risk countries during the peak time of the epizootic. By 2001, the FDA recommended that bovine-derived vaccine components be replaced by those from countries certified to be free from BSE. Since the confirmation of the first US BSE case in December 2003, the USDA has banned the use of downer cattle for human consumption, increased BSE testing from approximately 20,000 animals in 2003 to more than 200,000 (anticipated) in 2004, held suspect cases until tests came back as negative, banned the use of high-risk meat products in the human food supply, and recommended changes in various slaughterhouse, meat processing, and feed mill procedures to minimize the risk of contamination with neural tissue or offal (16, 17). In 2001 and again in 2003, the Center for Risk Assessment at Harvard University affirmed that the USDA's prevention and control plan would minimize any possible spread of the disease and ultimately eliminate it from the US cattle population (18).
AVIAN INFLUENZA
Between December 2003 and February 2004, an outbreak of highly pathogenic avian influenza A (H5N1) occurred among poultry in Southeast Asia, including Cambodia, China, Japan, Indonesia, Laos, South Korea, Vietnam, and Thailand. By February 2004, there were 23 cases of laboratory-confirmed influenza A (H5N1) in humans in Vietnam and Thailand, resulting in 18 deaths, for a case-fatality rate of 78%. One hundred additional cases were under investigation. Based on isolates from 5 of the human cases, the influenza virus was confirmed to be of avian origin, with no evidence of genetic reassortment between avian and human viruses. This virus was noted to be resistant to amantadine and rimantadine and sensitive to oseltamivir and preliminarily to zanamavir (19–21).
The natural reservoir for influenza A virus is wild and domestic birds. Humans appear to be incidental hosts in avian influenza A outbreaks. Transmission occurs bird-to-bird and bird-to-human by nasal and respiratory secretions and feces. Human acquisition of the disease is thought to be due to direct contact with poultry or poultry feces, resulting in inoculation of mucous membranes.
Four prior avian influenza outbreaks have been documented in humans. In 1997, an outbreak was described in Hong Kong, with 18 human cases and 6 deaths. Poultry was documented as the source of the infection, identifying for the first time direct transmission from bird to human. Rare person-to-person spread was also noted. In 1998–1999, a similar outbreak was reported in mainland China and Hong Kong (H9N2). In 2003, H5N1 and H9N2 strains were reported in Hong Kong (4 cases, 2 deaths). Also in 2003, an outbreak of avian influenza (H7N7) occurred among poultry workers and their families in the Netherlands (80 cases, 1 death); conjunctivitis was the primary manifestation (22).
Why are such relatively few, isolated reports cause for such consternation? Some background information on influenza A is necessary for understanding the importance of these cases. As mentioned, wild and domestic birds, particularly waterfowl, are the natural reservoir of all influenza A, whether human or avian. Influenza A is a single-stranded RNA virus, the classification of which is based on 2 surface glycoproteins. The hemagglutinin glycoprotein is the major antigen to which vaccine-induced antibody is directed. The neuraminidase glycoprotein is a minor antigen, but its action is subject to inhibition by the drugs oseltamivir (Tamiflu) and zanamavir (Relenza).
Influenza A viruses undergo frequent mutations, resulting in what is termed “antigenic drift” or “antigenic shift.” Antigenic drift results from relatively minor changes in the viral genome. It is caused by point mutations in the surface glycopeptides, which result in new substrains of influenza A. Antigenic drift occurs continually and is the reason why a new influenza vaccine is needed each year. Antigenic shift, in contrast, is a major change in the virus and describes, for example, the emergence of an influenza A virus bearing a new hemagglutinin or a new hemagglutininneuraminidase combination. Antigenic shift is caused by a major reassortment of the genome, in which 2 different influenza A viruses, one human and one avian, each with a different hemagglutinin, infect a common host with resulting transfer of genetic material. If the common host is a human, the potential exists for introduction of a new, very efficiently transmitted influenza strain into a nonimmune population, resulting in a pandemic.
The influenza A strains to date that primarily infect humans are some combination of HI, H2, or H3 and Nl or N2. The wide introduction of an H5 hemagglutinin-containing human influenza A virus into the human population could have catastrophic consequences. For example, in 1918, influenza AH IN 1 was introduced into the population, resulting in 20 million to 50 million deaths worldwide and 500,000 deaths in the USA alone. Nearly half of the deaths occurred in young, otherwise healthy adults, resulting in major social disruption and economic loss.
It is for this reason that the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have developed guidelines relating to the diagnosis and management of avian influenza outbreaks. The CDC defines a suspect case as a patient with a documented fever >100.4°F and cough, sore throat, or dyspnea; a history of contact with poultry or domestic birds; clinical or radiographic features of influenza; and a history of travel within 10 days of onset of illness to a country with a documented H5N1 avian influenza outbreak in poultry or humans. These patients should be tested for influenza A by commercially available influenza A antigen detection kits or polymerase chain reaction (PCR) methodology. Any specimens from suspect cases that test positive by these methods should be sent to the CDC for further analysis.
The patient with a suspected case should be placed in respiratory isolation in a negative-pressure room with 6 to 12 air exchanges per hour. All health care workers and visitors must wear an N-95 respirator, similar to the guidelines issued for prevention of transmission of tuberculosis. Hand hygiene, gowns, gloves, and protective eyewear must be utilized. Outpatients and discharged patients, if otherwise stable, may be isolated at home for 14 days after the onset of symptoms; the CDC publishes guidelines for home isolation.
In February 2004, the CDC banned importation of birds from areas affected by avian influenza; the governments of these countries instituted widespread culling of affected flocks. The USA and the UK are in the process of developing an H5N1 human influenza A reference virus to be used in vaccine manufacture if warranted by widespread outbreaks (19). However, the mean time to commercial production of vaccine is at least 6 months, which is why infection control policies are so crucial.
SEVERE ACUTE RESPIRATORY SYNDROME
In February 2003 the Chinese Ministry of Health first notified the WHO of a cluster of severe respiratory illnesses in Guangdong Province that had occurred between November 16, 2002, and February 9, 2003. These illnesses were characterized by transmission to household contacts, health care workers, and other hospitalized patients. On February 22, 2003, a 64 year-old nephrologist from Guangdong Province was admitted to a hospital in Hong Kong with a similar type of illness and eventually died. Subsequently, 10 cases were described, including household contacts, health care workers, and guests at the same hotel at which the nephrologist had been staying; the hotel guests had not had direct contact with the index patient (23). In March, multiple cases were reported in Singapore, Taiwan, and Toronto, all in patients who had recently traveled to China, their household contacts, or health care workers involved in their treatment (24). At this point the term “severe acute respiratory syndrome” (SARS) was coined by the WHO. In May 2003, the CDC isolated a novel coronavirus from patients with SARS; this was ultimately termed SARS-related coronavirus, or SARS-coV (25). A worldwide epidemic followed from February 2003 to July 2003: 8098 cases from 29 countries were reported to the WHO, including 774 deaths, for a case fatality rate of 9.6% (26). For the first time in its history, the WHO issued travel alerts to countries affected by SARS.
In the USA, 418 cases were initially reported during the 2003 epidemic, 344 “suspected” cases and 74 “probable” cases. Subsequently, the case total was revised downward based on convalescent serology results. Ultimately, the total was revised to 211 cases, with 175 “suspected” cases, 28 “probable” cases, and 8 laboratory confirmed cases. No deaths occurred in the USA (26).
As mentioned, the etiology of SARS was ultimately determined to be a novel coronavirus, isolated in cell culture at the CDC and detectable by immunofluorescent assay, reverse transcriptase PCR, and electron microscopy. The sequence analysis indicated that the causative organism was a previously undescribed coronavirus with 2 distinct genotypes (25). The origin of the virus is unknown but is thought to be from an animal known as a civet.
The civet, or civet cat, is actually a relative of the mongoose. Several species are described throughout Africa and Southeast Asia. Many of the original SARS cases were in restaurant workers, and civet is considered a Chinese delicacy and is one of the major ingredients in dragon-tiger-phoenix soup (the civet is the tiger; chicken is the phoenix; and, interestingly, cobra is the dragon). The SARS-coV has 99.8% sequence homology with a coronavirus isolated from the gastrointestinal tract of the civet, and the civet coronavirus was isolated from cages in restaurants from which employees developed SARS. The CDC banned the importation of civets to the USA in January 2004 (27).
Transmission of SARS is known to be person-to-person via airborne droplet nuclei. There is also some question of environmental transmission, particularly with reference to the cluster of cases in the Hong Kong hotel in which several guests developed SARS without direct contact with the index case. Contamination of the potable water supply by sewage has been implicated. There is some question of direct transmission from civet to human, whether by environmental contamination with civet feces or by consumption of improperly prepared and cooked civet meat (27, 28).
The incubation period after exposure to SARS-coV is 2 to 10 days. SARS is a 2-stage illness, with an initial prodrome of low-grade fever, rigors, malaise, headache, myalgias, diarrhea, and possibly early mild upper respiratory symptoms followed by a respiratory phase. The respiratory phase occurs 3 to 7 days after the onset of initial symptoms and is characterized by a dry cough (80% to 100% of patients), dyspnea (60% to 80%), and frequent progression to respiratory failure requiring intubation. The white blood cell count may be normal or low, although lymphopenia was described in 98% of cases. Thrombocytopenia is prominent, as is elevation of the aspartate aminotransferase, alanine aminotransferase, and creatine phosphokinase early in the course of the illness. The lactate dehydrogenase is often elevated as well and portends a poor prognosis (along with older age and coexistent diabetes mellitus). The chest x-ray usually reveals bilateral interstitial infiltrates consistent with acute respiratory distress syndrome, although focal consolidation has been described. In 53% of the US cases, the chest x-ray was normal. Computed tomography scans of the chest may be more sensitive than radiographs (29).
The CDC recommends that SARS should be considered in the differential diagnosis of a case of radiographically confirmed pneumonia or acute respiratory distress syndrome of unknown etiology when the patient has one of the following risk factors in the 10 days prior to onset of symptoms: travel to mainland China, Hong Kong, or Taiwan, or close contact with an ill person who recently traveled to one of these areas; high-risk employment (health care or laboratory worker); or being part of a cluster of cases of atypical pneumonia without an alternative diagnosis. The diagnosis of SARS is a diagnosis of exclusion. Bacterial and treatable viral causes must be ruled out. SARS-coV antibodies should be obtained at presentation and then again in 28 days. Reverse-transcriptase PCR testing for SARS-coV on nasal and oropharyngeal swabs and serum or plasma may be done if the patient has been ill for less than a week; swabs are placed in viral transport media. Reverse-transcriptase PCR can be done on nasal and oropharyngeal swabs and stool after the first week of illness. Reverse-transcriptase PCR can also be done on nasopharyngeal wash, bronchoalveolar lavage, tracheal aspirate, pleural fluid, sputum, and tissue autopsy specimens (29).
The mainstay of SARS treatment is supportive care. Many of the patients in the 2003 epidemic were treated with combinations of oseltamivir and ribavirin or ribavirin and corticosteroids; the efficacy of these regimens is not established, and some patients actually worsened with therapy. Ribavirin has no in vitro activity against SARS-coV. Twenty-two Canadian patients were treated with interferon-alpha and corticosteroids, resulting in less impairment of oxygenation and faster radiographic resolution of infiltrates. Pegylated interferon-alpha has been demonstrated to be effective in macaques before and after exposure to SARScoV. Interestingly, glycyrrhizin, an ingredient of licorice, has been noted to have in vitro activity against SARS-coV (28).
The 2003 epidemic was controlled by a combination of modalities, including closure of schools, businesses, and other facilities; quarantine of patients and their contacts; and a series of travel advisories issued by the WHO and the CDC between March and August 2003. Again, hospital infection control is of tantamount importance, with emphasis on early case recognition, placement of the patient in a negative-pressure private room with 6 to 12 air exchanges per hour, the use of the N-95 respirator for health care workers and visitors, and meticulous attention to hand hygiene and the appropriate use of gowns, gloves, and eyewear. Patients are isolated in the hospital or at home, if otherwise stable, for 10 days after resolution of symptoms. The CDC recommends exclusion of a health care worker from duties if fever or respiratory symptoms develop within 10 days of exposure to SARS, continuing until 10 days after the symptoms resolve (29). A vaccine is in development and appears effective in the murine model, but again, as the lag time between development of a reference virus and commercial production of vaccine is at least 6 months, early case recognition and infection control policies are key (30).
SUMMARY
Despite tremendous advances made in antimicrobial therapy, emerging infectious diseases will continue to plague the global health care system for the foreseeable future. Several questions remain regarding the risks of vCJD, avian influenza, and SARS. What is the risk of vCJ D in the USA? What is the risk to humans from transmissible spongiform encephalopathies affecting other mammals, such as chronic wasting disease of deer and elk and feline spongiform encephalopathy? What is the risk of vCJD with transfusion and solid organ transplantation? Is there any etiologic relationship between the prion diseases and other neurodegenerative disorders, such as Alzheimer's and Parkinson's? What is the likelihood of a worldwide pandemic from avian influenza or SARS? No effective therapy has been definitively documented for any of these infections, with the possible exception of the neuraminidase inhibitors for avian influenza. As with many diseases, prevention is often the best medicine, with early recognition and reporting of illnesses; sound policies regarding the livestock, food supply, and transfusion industries; and effective isolation and infection control policies crucial to the successful containment of emerging infections.
Footnotes
Presented at internal medicine grand rounds, Baylor University Medical Center, April 20, 2004.
References
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Harris DA. Cellular biology of prion diseases. Clin Microbiol Rev. 1999;12:429–444. doi: 10.1128/cmr.12.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparkes RS, Simon M, Cohn VH, Fournier RE, Lem J, Klisak I, Heinzmann C, Blatt C, Lucero M, Mohandas T, et al. Assignment of the human and mouse prion protein genes to homologous chromosomes. Proc Nad Acad Sci USA. 1986;83:7358–7362. doi: 10.1073/pnas.83.19.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downing DT, Lazo ND. Molecular modelling indicates that the pathological conformations of prion proteins might be beta-helical. Biochem J. 1999;343:453–460. [PMC free article] [PubMed] [Google Scholar]
- 5.Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35:349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- 6.Beisel CE, Morens DM. Variant Creutzfeldt-Jakob disease and the acquired and transmissible spongiform encephalopathies. Clin Infect Dis. 2004;38:697–704. doi: 10.1086/381028. [DOI] [PubMed] [Google Scholar]
- 7.Klein MA, Frigg R, Flechsig E, Raeber AJ, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel RM, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 8.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: evidence for neural spread of infection to the CNS. J Gen Virol. 1980;51:183–187. doi: 10.1099/0022-1317-51-1-183. [DOI] [PubMed] [Google Scholar]
- 9.Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 10.Prusiner SB. Shattuck lecture—neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Bovine spongiform encephalopathy in a dairy cow—Washington state, 2003. MMWR Morb Mortal Wkly Rep. 2004;52:1280–1285. [PubMed] [Google Scholar]
- 12.Pincock S. Patient's death from vCJD may be linked to blood transfusion. Committee to discuss need for further precautions to prevent possible vCJD transmission through blood. Lancet. 2004;363:43. doi: 10.1016/s0140-6736(03)15247-6. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Statement of probable case of BSE in Washington State, December 24, 2003 [press release] Available at http://www.fda.gov/bbs/topics/NEWS/2003/NEW00999.html; accessed July 14, 2004.
- 14.US Food and Drug Administration. Statement on Texas cow with central nervous system symptoms, May 4, 2004 [press release] Available at http://www.fda.gov/bbs/topics/NEWS/2004/NEW01061.html; accessed July 14, 2004.
- 15.Department of the Environment, Food, and Rural Affairs [UK] Transmissible Spongiform Encephalopathies in Great Britain: A Progress Report. Available at http://www.defra.gov.uk/animalh/bse/bse-publications/progress/dec02/order.pdf; accessed July 14, 2004.
- 16.US Department of Health and Human Services. Expanded “mad cow” safeguards announced to strengthen existing firewalls against BSE transmission, January 26, 2004 [press release] Available at http://www.hhs.gov/news/press/2004pres/20040126.html; accessed July 14, 2004.
- 17.Statement by Lester M. Crawford, DVM, PhD, Deputy Commissioner of Food and Drugs, Department of Health and Human Services, before the Committee on Agriculture, Nutrition and Forestry, US Senate, January 27, 2004, Available at http://www.fda.gov/ola/2004/bse0127.html; accessed July 14, 2004.
- 18.Cohen JT, Duggar K, Gray GM, Kreindel S, Abdelrahman H, Habte Mariam T, Oryang D, Tameru B. Evaluation of the Potential for Bovine Spongiform Encephalopathy in the UnitedStates. Boston: Harvard Center for Risk Analysis, Harvard School of Public Health; 2003. Available at http://www.hcra.harvard.edu/pdf/madcow.pdf; accessed July 14, 2004. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Outbreaks of avian influenza A (H5N1) in Asia and interim recommendations for evaluation and reporting of suspected cases-United States, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:97–100. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Cases of influenza A (H5N1)- Thailand, 2004. MMWR Morb Mortal Wtdy Rep. 2004;53:100–103. [PubMed] [Google Scholar]
- 21.Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J World Health Organization International Avian Influenza Investigative Team. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Basic information about avian influenza (bird flu) Available at http://www.cdc.gov/fiu/avian/facts.htm; accessed July 14,2004.
- 23.Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1785. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 24.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ National Microbiology Laboratory, Canada, Canadian Severe Acute Respiratory Syndrome Study Team. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 25.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ SARS Working Group. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Revised US surveillance case definition for severe acute respiratory syndrome (SARS) and update on SARS cases—United States and worldwide, December 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1202–1206. [PubMed] [Google Scholar]
- 27.Order of the Centers for Disease Control and Prevention, Department of Health and Human Services: Notice of Embargo of Civets, January 13, 2004, Available at http://www.cdc.gov/ncidod/sars/civet_ban_exec_order.htm; accessed July 14, 2004.
- 28.Lapinsky SE, Granton JT. Critical care lessons from severe acute respiratory syndrome. Curr Opin Crit Care. 2004;10:53–58. doi: 10.1097/00075198-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. In the Absence of SARS-CoV Transmission Worldwide: Guidance for Surveillance, Clinical and Laboratory Evaluation, and Reporting, Version 2. Available at http://www.cdc.gov/ncidod/sars/absenceofsars.htm; accessed July 14, 2004.
- 30.Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, Nabel GJ. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]