Abstract
Control of scrapie, an ovine transmissible spongiform encephalopathy or prion disorder, has been hampered by the lack of conventional antemortem diagnostic tests. Currently, scrapie is diagnosed by postmortem examination of the brain and lymphoid tissues for PrPSc, the protein marker for this group of disorders. For live, asymptomatic sheep, diagnosis using tonsil or third-eyelid lymphoid tissue biopsy and PrPSc assay has been described. To evaluate the feasibility and efficacy of third-eyelid testing for identification of infected flocks and individual infected sheep, 690 sheep from 22 flocks were sampled by third-eyelid lymphoid tissue biopsy and immunohistochemistry. Sheep were further evaluated for relative genetic susceptibility and potential contact exposure to scrapie. Third-eyelid testing yielded suitable samples for 80% of the sheep tested, with a mean of 18.1 lymphoid follicles (germinal centers) per histologic section. Three hundred eleven of the sheep were sampled through passive surveillance programs, in which only sheep with potential contact with an infected sheep at a lambing event were tested, regardless of their scrapie susceptibility genotype. In addition, 141 genetically susceptible sheep with no record of contact with an infected animal at a lambing event were sampled through a targeted active surveillance program. Ten PrPSc-positive sheep were identified through the passive surveillance program, and an additional three PrPSc-positive sheep, including two from flocks with no history of scrapie, were identified through the active surveillance program. All PrPSc-positive sheep had the highly susceptible PrP genotype. Third-eyelid testing is a useful adjunct to flock monitoring programs, slaughter surveillance, and mandatory disease reporting in a comprehensive scrapie eradication and research program.
Scrapie is a fatal neurologic disease of sheep and goats, introduced into the United States in 1947 and now endemic in many states (32). Scrapie is a member of the heterogeneous group of prion diseases, or transmissible spongiform encephalopathies (TSE), which includes bovine spongiform encephalopathy (BSE), cervid chronic wasting disease, and transmissible mink encephalopathy. Although scrapie is not a zoonotic disease, the apparent transmission of BSE to humans in the United Kingdom (4, 31) has resulted in a call for eradication of all TSE in food-producing animals.
The transmissible agent in scrapie remains controversial because no conventional bacterial, viral, fungal, or toxic source has been identified. The major component in infectious tissue homogenates is a relatively denaturation-resistant conformational isoform (PrPSc) of the highly conserved mammalian PrP protein (PrPC) (13, 21). The prion model proposes that PrPSc and other less well characterized cofactors induce conversion of PrPC to the pathogenic isoform PrPSc through aggregation and posttranslational structural changes. Regardless of the etiology of scrapie, PrPSc is a reliable marker for infection.
Scrapie transmission is thought to occur by oral exposure to the causative agent, uptake through the intestinal wall, amplification of the agent in the gut-associated lymphoid and nervous tissue (1, 29), and transport to the brain through the autonomic fibers of the vagus nerve (11). In U.S. sheep, PrPSc detected by immunoassay (16, 22) or a transmissible agent detected by rodent bioassay (9) is found in some lymphoid tissue by the age of 14 months and in the brain by 2.5 years, approximately 6 months before onset of clinical signs. Early accumulation of PrPSc in lymphoid tissue is the basis for preclinical scrapie diagnosis by immunohistochemistry (IHC) assay of postmortem tonsil (28) and antemortem third-eyelid lymphoid tissue (16) or tonsil biopsy samples (23).
Disease transmission depends on both exposure to the infectious agent and susceptible genetics in the host. Sheep housed with an infected postparturient ewe (within 60 days of lambing or abortion) are considered to be at increased risk of scrapie, presumably because they are exposed to the infectious agent in placental and fetal tissues or fluids (20, 22, 26, 27). Following contact with an infected postparturient ewe, disease occurs almost exclusively in sheep with particular polymorphisms in the host PrP gene (2, 5, 10, 19, 24, 25). In the U.S. sheep population, scrapie (whether associated with clinical disease or with PrPSc accumulation in the absence of clinical signs) has been confirmed only in sheep homozygous for the PrP allele encoding glutamine at codon 171 (QQ171) (17, 18, 30), regardless of breed. In Europe and the United Kingdom, relative susceptibility of sheep with several other PrP genotypes is reported in various breeds, a finding useful in national scrapie control programs (6).
Scrapie is typically diagnosed by IHC assay of brain tissue (12) from sheep with clinical signs of scrapie, notably weight loss, wool loss, and ataxia. Following diagnosis of an index animal, control measures in the flock include culling or quarantine of sheep at risk due to susceptible genetics and/or possible contact with the infected postparturient ewe (8). However, many infected sheep die from intercurrent diseases or routine culling without diagnostic testing, and identification of infected flocks by surveillance for clinical scrapie alone may not be sufficient for disease eradication. Cooperative state-federal programs that meet or exceed the current control measures and that are designed to test or improve program procedures or facilitate research are allowed as pilot programs (8). The state of Wyoming has proposed a pilot program in which passive surveillance (quarantine, testing, or removal of sheep having potential contact with an infected ewe) is supplemented with active surveillance, including live-animal testing of genetically susceptible, clinically normal sheep with no reported contact with an infected sheep during a lambing event. The objective of this study was to investigate the feasibility and efficacy of including third-eyelid testing and susceptibility genetics in active and passive surveillance programs.
MATERIALS AND METHODS
Study flocks.
Twenty-two flocks in Wyoming were included in this study. Ninety sheep from the University of Wyoming Animal Science Department were sampled by third-eyelid biopsy to estimate the efficiency of eyelid biopsy sampling (percentage of sheep with suitable biopsy samples) for sheep of various ages and breeds. The sheep were aged 14 months to 7 years and represented four breeds (Suffolk, Columbia, Hampshire, and Rambouillet) (Table 1). In addition, 600 sheep from privately owned flocks in Wyoming were tested (Table 2). These sheep were of Suffolk and Hampshire origin. All sheep over the age of 14 months at the time of testing were sampled in flocks 1 to 5 and 7 to 13. Only sheep over the age of 14 months and considered at risk due to potential contact with a postparturient ewe subsequently diagnosed with scrapie (see “Relative risk designation” below) were sampled in flocks 6 and 14 to 16. The group designated flock 16 (Table 2) represents one sheep with potential contact risk from each of five flocks and two sheep with potential contact risk from a sixth flock. Flocks were included in the study following identification of an infected animal or an animal with potential contact exposure, either born into the flock (flocks 3 to 5) or purchased (flocks 6 to 16). Flocks 1 and 2 were volunteered by the producers for educational purposes or due to industry concerns about scrapie.
TABLE 1.
Suitabilitya of eyelid biopsy samples from sheep of four breeds, aged 1 to 7 years
| Age (yrs) | No. of sheep with at least one suitable sampleb/no. of sheep tested
|
Total (%) by age group | |||
|---|---|---|---|---|---|
| Hampshire | Columbia | Suffolk | Rambouillet | ||
| 1 | 2/2 | 18/18b | 8/8 | 0/0 | 28/28 (100) |
| 2 | 1/1 | 2/2 | 6/6b | 2/2 | 11/11 (100) |
| 3 | 1/1b | 7/12 | 1/2 | 0/0 | 9/15 (60) |
| 4 | 2/3 | 2/3b | 4/7b | 0/0 | 8/13 (62) |
| 5 | 2/4 | 1/4 | 2/2 | 0/0 | 5/10 (50) |
| 6 | 3/4 | 0/0 | 4/8 | 0/0 | 7/12 (58) |
| 7 | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 (0) |
| Total (%) by breed | 11/15 (73) | 30/39 (77) | 25/34 (74) | 2/2 (100) | 68/90 (76) |
Suitable biopsy samples contained more than six lymphoid follicles per section.
Both eyes were sampled on nine sheep in the age-breed groups indicated because the left eye did not appear to have adequate lymphoid tissue when examined grossly.
TABLE 2.
Flocks and sheep sampled by third-eyelid biopsy and PrP genotyping
| Flock no. | No. of sheep sampled per surveillance programa (no. of PrPSc-positive sheep)
|
% of sheep with suitable biopsy samples (mean follicle count per section) | ||
|---|---|---|---|---|
| Active | Passive | Neither | ||
| 1 | 33 (1) | 0 | 19 | 83 (9.5) |
| 2 | 12 (1) | 0 | 39 | 72 (34.4) |
| 3 | 0 | 32 (1) | 0 | 81 (26) |
| 4 | 0 | 117 | 0 | 73 (15.7) |
| 5 | 0 | 115 (6) | 0 | 74 (11.1) |
| 6 | 0 | 8 (1) | 0 | 75 (11.5) |
| 7 | 17 | 0 | 11 | 78.5 (13.5) |
| 8 | 15 | 2 | 15 | 74 (13.6) |
| 9 | 27 | 6 | 28 | 68 (14.9) |
| 10 | 31 (1) | 2 (1) | 47 | 87 (32.9) |
| 11 | 0 | 6 (1) | 2 | 87 (28.3) |
| 12 | 1 | 4 | 1 | 50 (6.7) |
| 13 | 4 | 4 | 8 | 75 (15.3) |
| 14 | 1 | 1 | 0 | 100 (15) |
| 15 | 0 | 7 | 0 | 57 (15.5) |
| 16 | 0 | 7 | 0 | 86 (13.7) |
| Total | 141 | 311 | 148 | 80 (18.1) |
Passive surveillance includes all sheep with potential contact risk, as defined in Materials and Methods, regardless of genotype. Active surveillance includes only genetically susceptible sheep with no reported contact risk. Sheep with neither potential contact risk nor high genetic susceptibility were sampled in some flocks.
Restraint.
One eye on each sheep, usually the left eye, was pretreated with topical 0.5% sterile ophthalmic proparacaine HCl (Bausch and Lomb, Tampa, Fla.) several minutes before the procedure was performed. The sheep was haltered and restrained manually. All sheep were sampled on the farm of origin, and equipment for restraint of the sheep varied among the facilities. Many farms were equipped with a fitting stand, in which the head was immobilized by resting the chin on a horizontal support and securing a restraint strap behind the head. Alternative restraint devices included a tilt table in which the sheep was restrained in a squeeze chute, or a head catch in which partial restraint was afforded by a stanchion. In all cases, immobilization of the head was achieved through traction on the halter strap. Optimally, one operator restrained the sheep's body, one operator maintained control of the halter, one person retracted the third eyelid, and a fourth person collected the biopsy specimen. If only two operators were available, equipment suitable for restraint of the sheep was needed; one operator maintained control of the halter and retracted the third eyelid, and one operator collected the sample.
Biopsy sample collection.
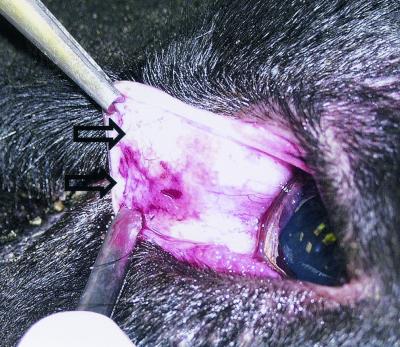
After adequate immobilization of the sheep, the third eyelid was retracted by using 1 by 2 toothed forceps (Econo Thumb Tissue forceps 21-762; Sklar Instruments, West Chester, Pa.) and treated again with proparacaine. The lymphoid tissue was visualized on the bulbar surface as a slightly raised pink tissue on either side of the midline (Fig. 1, lower arrow). The color, location, and size of the tissue varied slightly among individual sheep. Sheep were sampled even if there was no gross evidence of lymphoid tissue. The tissue was grasped with a second set of forceps, raised slightly from the underlying connective tissue, and excised with single-use curved Metzenbaum scissors (Econo Metzenbaum scissors 21-336; Sklar Instruments) by using a medial to lateral cut; typically, two or three sequential cuts with the scissors were required to acquire a single biopsy specimen of sufficient size containing the entire area of lymphoid tissue on one side of the midline (Fig. 1, upper arrow). The biopsy sample was fixed in 10% neutral buffered formalin for at least 24 h.
FIG. 1.
Bulbar surface, nictitating membrane of a Suffolk sheep. The lymphoid tissue is typically visualized as a slightly raised, red to pink area (lower arrow). Complete excision of the lymphoid tissue (upper arrow) is visible approximately 2 months after biopsy. The lymphoid tissue at this site does not regenerate.
Tissue processing.
Typically, fixed biopsy samples were observed to be folded in half with the lymphoid tissue facing outward, secured by the indentation formed by the forceps teeth. Biopsy samples were opened using iris scissors and placed flat between biopsy sponges in plastic histopathology cassettes. The lymphoid tissue was placed down in the cassettes. Cassettes with properly positioned biopsy samples were immersed in 95 to 98% formic acid for 1 h to decontaminate the tissue (3). Samples were rinsed in three changes of deionized water and for at least 10 min in running deionized water, then reequilibrated in 10% neutral buffered formalin. Tissues were processed conventionally and embedded in paraffin; care was taken to position the biopsy samples in the paraffin blocks so that the lymphoid tissue was facing and on the same plane as the surface of the block, to provide the largest possible section when cut.
Adhesion of formic acid-decontaminated tissue to glass slides required additional attention during preparation. Three- to five-micrometer-thick sections were cut with a microtome and applied to adhesive-coated aminosilane-treated slides (Newcomer Supply, Middleton, Wis.). Residual moisture under the section was removed with absorbent laboratory tissue paper, and slides were positioned vertically to dry for at least 1 h. Slides were baked at 57°C for approximately 15 h (overnight) to remove excess paraffin.
Immunostaining.
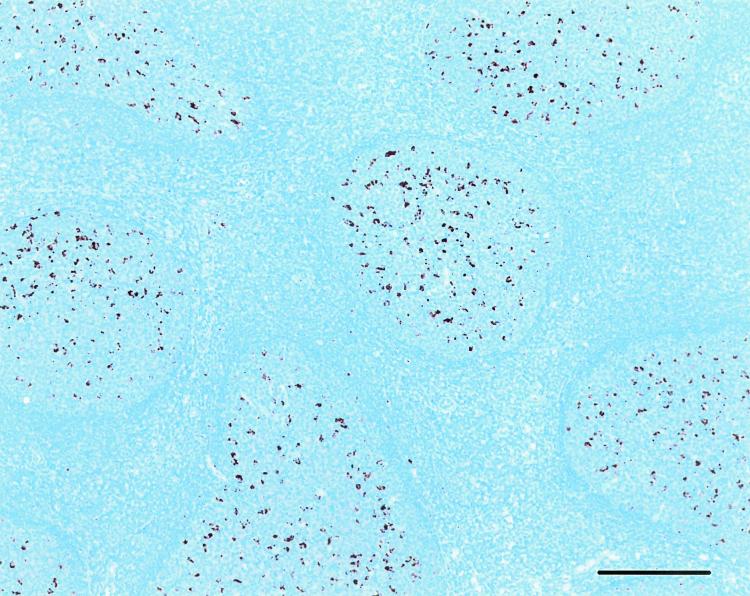
Sections were rehydrated through xylene substitutes and graded alcohols, then treated with 95% formic acid (7) for 5 min and neutralized by three changes of 0.1 M Tris-HCl, pH 7.6. Antigen retrieval was performed by incubation at 120°C in a medical pressure cooker (BioCare Medical, Walnut Creek, Calif.) for 20 min in a modified citrate buffer (Target Retrieval solution, pH 6.1; Dako Corporation, Carpinteria, Calif.). IHC was performed on an automated immunostainer (NexES; Ventana Medical Systems, Tucson, Ariz.) by using the manufacturer's detection reagents (antibody diluent, AEC detection kit, hematoxylin, bluing, and mouse immunoglobulin G1 [IgG1] negative control antibody). The primary antibody was a cocktail of two monoclonal antibodies (MAbs) to separate epitopes on the PrP molecule (MAb F99/97.6.1 [14] and MAb F89/160.1.5 [15]; Veterinary Medical Research Development, Inc., Pullman, Wash.) at 5 μg/ml each in antibody diluent (Ventana Medical Systems) filtered through a 0.22-μm-pore-size syringe top filter. The primary antibody was applied for the maximum time available with the automated system (32 min), followed by biotinylated goat anti-mouse IgG, streptavidin-conjugated horseradish peroxidase, and 3-amino-9-ethylcarbazole as a chromogen. The default settings for incubation time and temperature provided by the manufacturer were used for all detection steps. The slides were counterstained with hematoxylin, rinsed, and covered with coverslips by use of a compatible mounting medium (Faramount aqueous mounting medium; Dako). Lymphoid tissue (tonsil or retropharyngeal lymph node) from an infected sheep was used as a positive control. Negative controls included replacement of the primary anti-PrP antibodies with an isotype control antibody (Ventana) and assay of tissues from uninfected sheep. Lymphoid follicles appearing as discrete oval or round areas with a darker border (Fig. 2) were counted on each section. Sections were considered “PrPSc detected” if a granular red precipitate was noted in lymphoid follicles (germinal centers) (Fig. 2), regardless of the number of follicles in the section. Sections were considered “no PrPSc detected” if no precipitate was noted in a section. A minimum of six lymphoid follicles per section were needed for this designation. Sections with no immunostaining and fewer than six lymphoid follicles were scored “insufficient lymphoid tissue for determination.” All IHC assays were scored by trained observers with no knowledge of the potential contact or genetic susceptibility status of the sheep.
FIG. 2.
IHC detection of PrPSc in the nictitating membrane lymphoid tissue. A third-eyelid biopsy specimen from a live sheep with preclinical scrapie is shown. Lymphoid follicles (discrete round to oval accumulations of lymphocytes, macrophages, and dendritic cells with a dark border) show granular PrPSc immunoreactivity (red) when immunostained with a cocktail of MAbs F89/160.1. and F99/97.6.1. Bar, 200 μm.
Relative risk designation.
Sheep were scored for potential contact risk by using producer records and the definitions established in the Code of Federal Regulations 79.4 (8). Briefly, sheep were defined as high risk by potential contact if they were born in a flock during the same lambing season as a lambing event by a sheep subsequently diagnosed with scrapie. Lambing events include the birth of an infected sheep as well as parturition or abortion by an infected ewe in any year of her life. Sheep were considered to be at lower risk by contact if they were born on the farm at other times, in different lambing facilities on the same farm, or on farms without reported cases of scrapie. If an infected ewe had produced at least one lamb at the facility and no birth records were available for the flock, all sheep born in the flock during the years that the infected ewe lambed or aborted were designated high risk. Relative contact risk status was based on producer records, and no outside measure of the accuracy of those records is available. No producer could rule out a previously undetected scrapie case, because some of the clinical signs (particularly weight loss) occur frequently in these flocks and result in culling of the animal without diagnostic testing.
Sheep were scored for relative genetic susceptibility based on the deduced amino acid sequence at residue 171. Blood was collected by jugular venipuncture into EDTA-treated vacuum tubes and shipped to a commercial laboratory for scrapie susceptibility testing (GeneCheck, Fort Collins, Colo.). DNA was extracted from the buffy coat, amplified by PCR, and analyzed by a DNA mismatch binding assay. The diploid genotype was reported as QQ, QR, or RR. However, this assay does not distinguish between sequences encoding glutamine (Q) and sequences encoding histidine (H) (both are reported as 171Q), and samples scored QQ may actually be QQ, QH, or HH. Samples scored QR may be QR or HR. Samples scored RR are unequivocally homozygous for arginine (R) at position 171. Sheep were considered to have high genetic susceptibility if their genotype was 171QQ, 171QH, or 171HH and to have lower genetic susceptibility if their genotype was 171QR, 171HR, or 171RR.
Surveillance programs.
Under the current program, passive surveillance includes testing of all sheep in flocks in which an infected animal was born or produced a lamb and all sheep sold from those flocks if they were born in a year in which the infected ewe produced a lamb. The passive surveillance program therefore includes sheep with potential contact with the transmissible agent shed at parturition, even if those sheep are at low genetic risk. Active surveillance includes only genetically susceptible sheep with no reported contact risk. In some flocks, all sheep over the age of 14 months were sampled, even if they were not eligible for either program. The number of sheep sampled in each surveillance program (or sampled as whole-flock testing but enrolled in neither program) is shown in Table 2.
RESULTS
Third-eyelid biopsy samples were collected from a university-owned flock to determine if particular breeds of sheep or age groups were unsuitable for this procedure. Ninety sheep of four breeds were available for sampling (Table 1). Suitable samples were those with at least six lymphoid follicles per section. Nine sheep were sampled twice because the left eye did not appear to have adequate lymphoid tissue when examined grossly. Five of these nine animals had suitable biopsy specimens from both eyes. One animal, a 5-year-old Columbia, had unsuitable samples from both eyelids. Three animals had one suitable sample and one unsuitable sample; this group included a 3-year-old Hampshire, a 4-year-old Columbia, and a 2-year-old Suffolk. The overall efficiency of testing was 76% (68 of 90) when the results from either eyelid were used to score an individual sheep. There was no breed for which all samples were unsuitable. There was a trend toward a lower percentage of suitable samples from sheep older than 3 years, but a larger study population is needed before conclusions on the feasibility of testing older sheep can be drawn.
A total of 600 sheep from 21 producer-owned flocks (Table 2) were assessed for evidence of infection with the scrapie agent by IHC of third-eyelid biopsy samples and for relative genetic susceptibility to scrapie by DNA genotyping. Sampled sheep were over the age of 14 months and were primarily of the Suffolk or Hampshire breed. Sheep were housed in flocks with no reported cases of scrapie, flocks in which an infected ewe was born or had produced at least one lamb, and flocks with at least one purchased animal with potential contact exposure in the flock of origin (Table 3). The PrP genotypic ratio in this sample was 0.48:0.52 (290 sheep with the highly susceptible 171QQ, -QH, or -HH genotype and 310 sheep with the lower-susceptibility 171QR, -RH, or -RR genotype). This ratio is not significantly different from that reported for an independent sample of 1,000 U.S. Suffolk sheep in 1996 (18).
TABLE 3.
Flock status at the time of testing and risk status of PrPSc-positivea sheep
| Flocks | Pretest flock statusb | No. of PrPSc-positive sheep
|
||||
|---|---|---|---|---|---|---|
| Total | With potential contact risk
|
With genetic susceptibility
|
||||
| High | Lower | High | Lower | |||
| 1 and 2 | None | 2 | 0 | 2 | 2 | 0 |
| 3-5 | Birth | 7 | 1 | 6 | 7 | 0 |
| 6-16 | Purchase | 4 | 3 | 1 | 4 | 0 |
| Total | 13 | 4 | 9 | 13 | 0 | |
Sheep with PrPSc detected in lymphoid follicles of the third eyelid.
None, no reports of scrapie in the flock; Birth, lambing event by an infected sheep on the premises; Purchase, the only sheep with reported potential contact were purchased from other flocks.
Three hundred eleven of the 600 sheep in the study were tested under passive surveillance programs (Table 2), representing sheep with potential contact risk from exposure to an infected ewe at parturition. Eighty-five of 311 had the highly susceptible genotype. One hundred forty-one of the 600 sheep were tested under the Wyoming active surveillance program and represent genetically susceptible sheep with no record of contact with an infected sheep at a lambing event, whether they resided in flocks with a purchased animal with potential contact risk at a different facility (n = 96) or in flocks with no history of scrapie contact in either native or purchased animals (n = 45). One hundred forty-eight sheep in the lower-genetic-susceptibility group and with no reported contact with an infected postparturient ewe were tested because they were housed with sheep in the active surveillance trial.
Sheep were tested for PrPSc, a marker for scrapie, by third-eyelid lymphoid tissue biopsy and IHC analysis (14). Biopsy samples of the third eyelid from 481 (80%) of the sheep tested contained at least six lymphoid follicles (Table 2) and were considered suitable for diagnosis, although all biopsy samples were analyzed by IHC. The mean follicle count in suitable biopsy samples was 18.1 (standard deviation, 13.3).
PrPSc was detected in 13 of the biopsy samples, collected from sheep on seven farms (Table 3). All 13 sheep with PrPSc detected in lymphoid tissue had the highly susceptible PrP 171 genotype QQ, QH, or HH. Of the 13 sheep with positive eyelid biopsy samples, 4 were removed for immediate necropsy, and scrapie was confirmed by IHC of brain and/or lymphoid tissues. Nine sheep were moved to quarantine. Scrapie has been confirmed in seven of these nine sheep by postmortem analysis following development of clinical signs and in one of these nine sheep following acute loss (found dead with no clinical signs). The last of the nine sheep brought to quarantine is asymptomatic at the age of approximately 36 months.
Ten of the 13 PrPSc-positive sheep were identified by the passive surveillance program, and 3 were identified by the active surveillance program. The positive samples were collected from sheep in flocks in which an infected sheep had been born (n = 7), flocks with no reported cases of scrapie (n = 2), and flocks with at least one sheep purchased from an infected flock (n = 4), although in one case, the PrPSc-positive sheep was not the purchased sheep with contact risk but an unrelated animal purchased from a different flock. The efficiency of the surveillance programs was estimated as the number of PrPSc-positive sheep per number of sheep in each program (10 of 311, or 3.2%, in the passive surveillance program and 3 of 141, or 2.1%, in the active surveillance program).
DISCUSSION
Scrapie was reported as a clinical entity nearly 300 years ago and was introduced into the United States in 1947. In the United States, control programs have relied on total or partial flock depopulation following diagnosis of the disease in a sheep with clinical signs of scrapie. The insidious nature of the disease, the long preclinical incubation period, the lack of a preclinical test, loss of infected sheep from intercurrent disease before development of clinical signs, and underreporting of scrapie due to the negative economic effect of a diagnosis have contributed to the failure of these programs to control the prevalence of scrapie, which is estimated at 0.07% in the United States (based on a mailed producer survey conducted in 1996 by the National Animal Health Monitoring System of the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service). State and federal flock certification programs, in which producers of monitored flocks agree to purchase breeding stock from similarly monitored flocks, permanent identification of individual sheep and/or flock of origin, and restrictions on interstate movement of suspect and high-risk animals have been in place since 1992. The USDA Animal and Plant Health Inspection Service recently expanded the program to include identification of breeding and mature sheep and breeding goats in interstate commerce, uniform standards for state scrapie control programs, indemnity payments, slaughter surveillance, and third-eyelid testing of exposed animals and/or flocks. These programs are being implemented to eradicate scrapie from the United States.
The development of the third-eyelid test for antemortem diagnosis of scrapie and the identification of PrP genotypes associated with clinical and subclinical PrPSc accumulation have provided additional means for controlling scrapie in the U.S. sheep population. The estimated specificity of the third-eyelid test approaches 100% (14). The estimated sensitivity of the test is 85 to 90%. The false-negative results are due to a number of factors. Some infected sheep have PrPSc detectable in the brain but not in lymphoid tissues; in other cases, PrPSc is detectable in tonsil but not in antemortem eyelid biopsy specimens, due in part to inadequate biopsy sample size. Optimal sampling conditions include a restraint device such as a tilt table, fitting stand, or head catch and adequately trained personnel for immobilization of the head, retraction of the eyelid, and biopsy sample collection. Laboratory handling of the tissue to maximize the number of follicles available for inspection in a single histologic section requires laboratory personnel trained in recognizing the lymphoid side of the tissue and embedding a flat sample in the plane of the microtome cut. In other trials, techniques such as embedding strips of lymphoid tissue at right angles to the plane of the microtome cut (14) or embedding folded or rolled tissues typically resulted in small numbers of follicles available for inspection in each section. In the present study, sampling was performed by one of three veterinarians (J.V.D., J.R.L., and E.S.W.), and IHC of samples from producer flocks was performed by one person (A.K.A.). When sampling, tissue handling, and assay were performed under these circumstances, approximately 80% of the sheep in each flock could be assessed.
In this study, the third-eyelid test identified PrPSc-positive sheep in flocks with previous scrapie exposure, although in two cases the individual sheep identified by eyelid biopsy had no reported contact with an infected ewe at parturition. The efficiency of the programs (2 to 3% in this relatively small sample) exceeds the estimated scrapie prevalence level of 0.07% nationally and illustrates the potential value of preclinical surveillance. However, there are some limitations to the use of the eyelid test in a scrapie eradication program. One hundred to 150 sheep can be sampled per day in a single location with proficient personnel using restraint equipment available on most facilities; testing small numbers of sheep at multiple locations decreases the efficiency of the testing personnel. The costs of personnel, equipment, topical anesthetics, and sample testing are borne by the state and federal governments through a joint scrapie eradication program. Although the procedure is performed under local anesthesia, third-eyelid sampling may be perceived as objectionable by some producers. The third-eyelid test is most useful in animals between the ages of 14 and 36 months, because older animals tend to have smaller areas of lymphoid tissue. Further, the estimated test sensitivity is approximately 85 to 90%. Therefore, the third-eyelid test is not suitable as a stand-alone eradication tool. The test is more appropriately considered an adjunct surveillance tool to identify infected flocks which could then be managed through an integrated program of testing, necropsy or quarantine, genetic selection, and husbandry modifications. The test is also useful for identification of naturally infected sheep for transfer to research facilities. Although active surveillance is expensive, cost-effectiveness is increased by targeting the testing program to 14- to 24-month-old sheep of heavily impacted breeds in flocks with a history of purchasing breeding ewes from multiple sources. Active surveillance is a justifiable addition to live-animal passive surveillance, in which flocks are identified only after submission of a clinical suspect. One of the sheep quarantined after selection by this program was found dead with no history of clinical signs, and another remains clinically normal at the age of 36 months. Inapparently infected sheep such as these produce lambs annually and serve as a continuing source of the transmissible agent. Slaughter surveillance and active live-animal surveillance programs such as the Wyoming pilot program will be critical for identification of infected flocks and eventual disease eradication.
This study demonstrated that identification of scrapie-infected flocks by third-eyelid testing is technically feasible with proficient personnel and adequate field and laboratory resources. Third-eyelid testing and genotype analysis are useful in identifying infected flocks for regulatory intervention and infected animals suitable for research purposes. Active surveillance for scrapie is a useful component of an integrated scrapie control program based on permanent identification, flock monitoring, slaughter surveillance, and passive surveillance by diagnosis of clinical suspects with testing of their scrapie-exposed flockmates.
Acknowledgments
This research was supported in part by the Agricultural Research Service, USDA (grant CWU 5348-32000-015-00D).
We thank Amy Lyda and Linda Hamburg for excellent technical assistance, N. Wineland, D. Knowles, and W. Tuo for review of the manuscript, and P. Steiner for management of a breeding flock of infected ewes.
REFERENCES
- 1.Andréoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J. M. Elsen, and F. Lantier. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. [DOI] [PubMed] [Google Scholar]
- 2.Bossers, A., B. E. C. Schreuder, I. H. Muileman, P. B. G. M. Belt, and M. A. Smits. 1996. PrP genotype contributes to determining survival times of sheep with natural scrapie. J. Gen. Virol. 77:2669-2673. [DOI] [PubMed] [Google Scholar]
- 3.Brown, P., A. Wolff, and D. C. Gajdusek. 1990. A simple and effective method for inactivating virus infectivity in formalin-fixed tissue samples from patients with Creutzfeldt-Jakob disease. Neurology 40:887-890. [DOI] [PubMed] [Google Scholar]
- 4.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttle, L. McCardie, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 5.Clouscard, C., P. Beaudry, J. M. Elsen, D. Milan, M. Dussaucy, C. Bounneau, F. Schelcher, J. Chatelain, J. M. Launay, and J. L. Laplanche. 1995. Different allelic effects of the codon 136 and 171 of the prion protein gene in sheep with natural scrapie. J. Gen. Virol. 76:2097-2101. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, M., L. J. Hoinville, B. D. Hosie, and N. Hunter. 1998. Guidance on the use of PrP genotyping as an aid to the control of clinical scrapie. Vet. Rec. 142:623-625. [PubMed] [Google Scholar]
- 7.Diedrich, J. F., P. E. Bendheim, Y. S. Kim, R. I. Carp, and A. T. Haase. 1991. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. USA 88:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federal Register. 2001. CFR 79. Scrapie in sheep and goats. Fed. Regist. 66:43990-44003. [Google Scholar]
- 9.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 10.Hunter, N. 1993. Natural transmission and genetic control of susceptibility of sheep to scrapie. Curr. Top. Microbiol. Immunol. 172:165-179. [DOI] [PubMed] [Google Scholar]
- 11.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, J. M., A. L. Jenny, W. D. Taylor, R. E. Race, D. R. Ernst, J. B. Katz, and R. Rubenstein. 1994. Detection of prion protein in formalin-fixed brain by hydrated autoclaving immunohistochemistry for the diagnosis of scrapie in sheep. J. Vet. Diagn. Investig. 6:366-368. [DOI] [PubMed] [Google Scholar]
- 13.Oesch, B., D. Westaway, M. Walchi, M. P. McKinley, S. B. H. Kent, R. Aebersold, R. A. Barry, P. Tempst, D. B. Teplow, L. E. Hood, S. B. Prusiner, and C. Weissmann. 1985. A cellular gene encodes scrapie PrP27-30 protein. Cell 40:735-746. [DOI] [PubMed] [Google Scholar]
- 14.O'Rourke, K. I., T. V. Baszler, T. E. Besser, J. M. Miller, R. C. Cutlip, G. A. H. Wells, S. J. Ryder, S. M. Parish, A. N. Hamir, N. E. Cockett, A. Jenny, and D. P. Knowles. 2000. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J. Clin. Microbiol. 38:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Rourke, K. I., T. V. Baszler, J. M. Miller, T. R. Spraker, I. Sadler-Riggleman, and D. P. Knowles. 1998. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J. Clin. Microbiol. 36:1750-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Rourke, K. I., T. V. Baszler, S. M. Parish, and D. P. Knowles. 1998. Preclinical detection of PrP-Sc in nictitating membrane lymphoid tissue of sheep. Vet. Rec. 142:489-491. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke, K. I., G. R. Holyoak, W. W. Clark, J. R. Mickelson, S. Wang, R. P. Melco, T. E. Besser, and W. C. Foote. 1997. PrP genotypes and experimental scrapie in orally inoculated Suffolk sheep in the United States. J. Gen. Virol. 78:975-978. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke, K. I., R. P. Melco, and J. R. Mickelson. 1996. Allelic frequencies of an ovine scrapie susceptibility gene. Anim. Biotech. 7:155-162. [Google Scholar]
- 19.Parry, H. B. 1979. Elimination of natural scrapie in sheep by sire genotype selection. Nature 277:127-129. [DOI] [PubMed] [Google Scholar]
- 20.Pattison, I. H., M. N. Hoare, J. N. Jebbett, and W. A. Watson. 1972. Spread of scrapie to sheep and goats by oral dosing with foetal membranes from scrapie-affected sheep. Vet. Rec. 90:465-468. [DOI] [PubMed] [Google Scholar]
- 21.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 22.Race, R., and D. Sutton. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 178:949-953. [DOI] [PubMed] [Google Scholar]
- 23.Schreuder, B. E. C., L. J. M. van Keulen, M. E. W. Vromans, J. P. M. Langeveld, and M. A. Smits. 1998. Tonsillar biopsy and PrPSc detection in the preclinical diagnosis of scrapie. Vet. Rec. 142:564-568. [DOI] [PubMed] [Google Scholar]
- 24.Thorgeirsdottir, S., S. Sigurdarson, H. M. Thorisson, G. Georgsson, and A. Palsdottir. 1999. PrP gene polymorphism and natural scrapie in Icelandic sheep. J. Gen. Virol. 80:2527-2534. [DOI] [PubMed] [Google Scholar]
- 25.Tranulis, M. A., A. Osland, B. Bratberg, and M. J. Ulvund. 1999. Prion protein gene polymorphisms in sheep with natural scrapie and healthy controls in Norway. J. Gen. Virol. 80:1073-1077. [DOI] [PubMed] [Google Scholar]
- 26.Tuo, W., D. Zhuang, D. P. Knowles, W. P. Cheevers, M. S. Sy, and K. I. O'Rourke. 2001. Prp-c and Prp-Sc at the fetal-maternal interface. J. Biol. Chem. 276:18229-18234. [DOI] [PubMed] [Google Scholar]
- 27.Tuo, W. B., K. I. O'Rourke, D. Y. Zhuang, W. P. Cheevers, T. R. Spraker, and D. P. Knowles. 2002. Pregnancy status and fetal prion genetics determine PrPSc accumulation in placentomes of scrapie-infected sheep. Proc. Natl. Acad. Sci. USA 99:6310-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Keulen, L. J. M., B. E. C. Schreuder, R. H. Meloen, G. Mooij-Harkes, M. E. W. Vromans, and J. P. M. Langeveld. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J. Clin. Microbiol. 34:1228-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Keulen, L. J. M., B. E. C. Schreuder, M. E. W. Vromans, J. P. M. Langeveld, and M. A. Smits. 1999. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J. Comp. Pathol. 121:55-63. [DOI] [PubMed] [Google Scholar]
- 30.Westaway, D., V. Zuliani, C. M. Cooper, M. Da Costa, S. Neuman, A. L. Jenny, L. Detwiler, and S. B. Prusiner. 1994. Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes Dev. 8:959-969. [DOI] [PubMed] [Google Scholar]
- 31.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 32.Wineland, N. E., L. Detwiler, and D. Salmon. 1998. Epidemiologic analysis of reported scrapie in sheep in the United States: 1,117 cases (1947-1992). J. Am. Vet. Med. Assoc. 212:713-718. [PubMed] [Google Scholar]