Abstract
Chronic hepatitis C is often asymptomatic and undiagnosed yet can progress to liver failure or hepatocellular carcinoma. This study determined the prevalence of hepatitis C in Texas and estimated the progression of disease in this cohort. National Health and Nutrition Evaluation Survey III data on the national prevalence of an antibody to the hepatitis C virus were extrapolated to Texas using census data weighted for local characteristics. A Markov model estimated the progression of liver disease. Results showed that 387,395 Texans (1.79%) are infected with the hepatitis C virus. County prevalence varied from 1.25% to 2.63%, with higher rates concentrated along the US–Mexico border. However, most cases of infection were located near major Texas cities. The number of infected persons will decline in the future. However, the proportion of cases progressing to cirrhosis will increase, resulting in more complications such as liver failure and hepatocellular carcinoma. Thus, chronic hepatitis C is common in Texas and will result in an increase in complications of cirrhosis in coming years. The disease will tax health care facilities and transplant units in the state.
Hepatitis C virus (HCV) is a bloodborne agent transmitted predominantly by percutaneous exposure (1). Most acutely infected individuals become chronically infected. Chronic hepatitis C is common, with a worldwide prevalence of 2.2% (2). In the USA, the estimated prevalence is as high as 1.8%, or approximately 4 million persons (3, 4). However, most HCV-infected persons do not realize that they have the infection because they do not feel ill until late in the course of their disease. Nonetheless, chronic hepatitis C often progresses to cirrhosis and can result in late complications such as ascites, variceal bleeding, or hepatic encephalopathy (5, 6). Chronic hepatitis C is also a common cause of hepatocellular carcinoma (HCC) and is the most common indication for liver transplantation in the USA (7, 8).
The recently approved regimen of pegylated interferon and oral ribavirin provides effective treatment for many patients with chronic hepatitis C (9, 10). Approximately half of treated patients permanently clear detectable virus. However, the success rate is variable and depends upon the viral genotype, virus levels, stage of fibrosis on liver biopsy, and ability to tolerate therapy (9, 10). Furthermore, most patients are not treated because their infection has not been recognized or they have contraindications to this treatment regimen. Thus, most HCV-infected individuals remain untreated and serve as a persistent source of infection within the population. They remain at risk for disease progression, particularly after prolonged infection (4–6).
The current and future societal costs of chronic hepatitis C are considerable. Several studies have projected that complications of chronic hepatitis C, particularly HCC and hepatic failure requiring liver transplantation, will more than double in coming years (4, 11). Thus, estimations of local disease prevalence may be helpful in projecting future health care needs. In this report, we utilized a recent US population survey to estimate the prevalence of chronic hepatitis C in the state of Texas and examined the potential future impact of the disease in the state.
METHODS
We used data from the National Health and Nutrition Evaluation Survey (NHANES) III, conducted from 1988 through 1994, to project hepatitis C prevalence in the Texas population (12). NHANES is conducted periodically by the National Center for Health Statistics, Centers for Disease Control and Prevention, to obtain information on the health and nutritional status of the noninstitutionalized civilian population in the USA by means of household interviews, standardized physical examinations, and collection and testing of blood samples in special mobile examination centers. Alter et al (3) reported the prevalence of antibody to HCV according to age, gender, income level, and ethnic group based upon NHANES III. These data were then weighted to provide an estimate of the prevalence of HCV infection in the US population.
We utilized 2000 US Census data for all Texas counties to characterize the populations by age, gender, race/ethnicity, and poverty level (13). The NHANES III hepatitis C subgroup antibody prevalence data were adjusted for aging of the population by adding 6 years to each of the NHANES (1994) age categories to better approximate the 2000 US Census data. We assumed that 90% of antibody-infected cases were true positives, i.e., HCV infected. Finally, a weighted estimate of the HCV prevalence in Texas counties was calculated using the age-adjusted NHANES III anti-HCV prevalence rates and the characteristics of county populations. County data were summed to provide a statewide HCV prevalence.
We applied these estimates to a previously described Markov model that has been used to project progression of chronic hepatitis C in the US population (4, 14). This model has been validated by replicating the results of two large natural history studies through computer simulation (14–16). The model utilizes annual cycles during which patients can remain in the same state of health (referred to as transition or disease states) or progress to another disease state, for example from chronic hepatitis to cirrhosis. In a Markov process, an individual faces a constant probability of moving from one state to another within each of these annual cycles (4, 14). However, since progression of chronic hepatitis C is typically slow, most patients remain in the same disease state from year to year. In addition, during each annual transition period, patients are subject to normal age-adjusted all-cause death rates as described by the National Center for Health Statistics (www.cdc.gov/nchswww/). Thus, the computer model is designed to quantify the expected number of HCV-positive patients in various disease states across time. Of particular interest to these projections was the relatively small subset that developed cirrhosis and complications of liver failure, since these patients are most likely to consume health care resources such as hospital beds and transplant services. Projections assumed no treatment of the cohort of infected cases since currently new prescriptions for treatment occur in <1% of the estimated number of infected cases per year (Schering Plough, personal communication).
RESULTS
Using the NHANES III anti-HCV prevalence rates and weighting to the characteristics of the 2000 Texas population, we estimated that 387,395 Texans, or 1.79% of the state population, were infected. This corresponds to a prevalence rate of 1.38% among whites, 2.82% among non-Hispanic blacks, 2.00% among Hispanics, and 1.79% among others. The majority of infected persons were white (39.5%) or Hispanic (36.4%). Most infected individuals were male (66.8%), and 88.8% were between the ages of 18 and 64 years. In fact, 36.8% and 22.9% of those infected were men between the ages of 18 and 44 and between 45 and 64, respectively. Infections were uncommon in those <18 years (5.3% of total) and ≧65 years (5.9% of total).
The estimated prevalence of infection varied significantly from county to county, ranging from 1.25% to 2.63%. The major factors influencing the estimated prevalence of infection were racial mix and proportion of the population below the federal poverty threshold. Counties with higher prevalence rates were usually concentrated along the US–Mexico border (Figure 1). Although the geographic grouping of prevalence was notable, the figures did not reflect potential disease burden since the population of many of these high-prevalence counties is small. In fact, when the estimated number of infected cases per county was examined, most infections occurred in population centers around major metropolitan areas despite their generally lower prevalence rates (Figure 2). For example, Hartley County had the highest estimated prevalence at 2.63%, yet only 80 of the county's 3023 residents had infection. In contrast, Montgomery County, a suburban area 40 miles north of Houston, had one of the lowest prevalence rates (1.40%), but an estimated 4358 of its residents were HCV infected.
Figure 1.
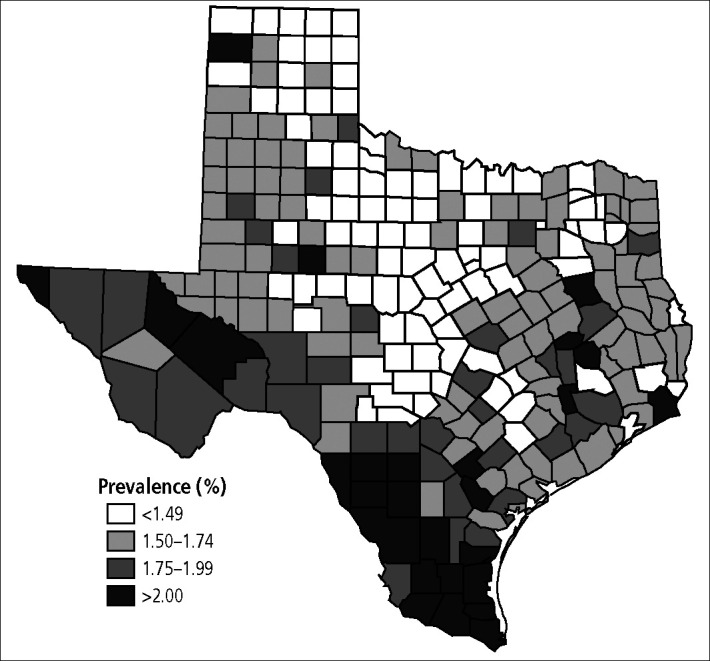
Prevalence rates of chronic hepatitis C virus infection by county in Texas.
Figure 2.
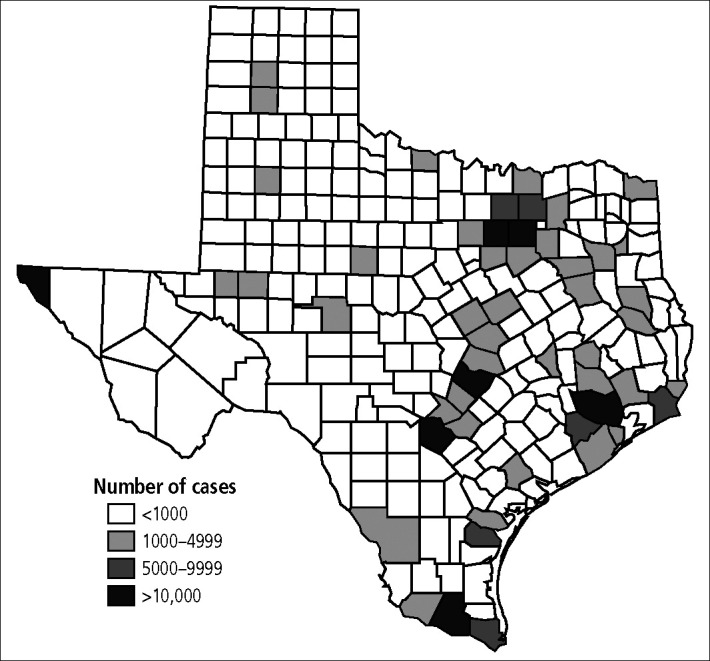
Estimated number of hepatitis C virus–infected cases by county in Texas.
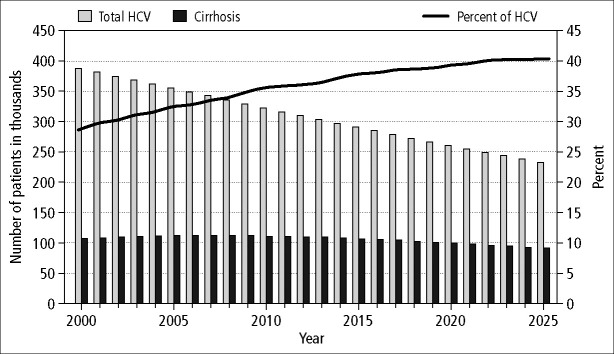
Estimates of the chronic hepatitis C disease–related health states were projected for future years using a previously developed and validated Markov model. The model anticipated a gradual decline in the total number of chronic HCV infections in coming years due to a decreasing number of acute infections and nonhepatic deaths of aging patients with chronic hepatitis C (Figure 3). The number of infected persons decreased from 387,359 in 2000 to 355,648 in 2005, 292,119 in 2015, and 234,858 in 2025. However, the duration of infection in the surviving cohort of patients with chronic hepatitis C increased, thereby resulting in an enlarging proportion with more advanced liver disease. Indeed, the number of cases with cirrhosis remained relatively stable, increasing slightly through 2007 and then gradually declining. However, cirrhosis as a proportion of HCV-infected patients progressively increased from 28.1% in 2000 to 40.6% in 2025 and remained over 40% thereafter (Figure 3).
Figure 3.
Projected number of all chronic hepatitis C virus (HCV) infections (light bars) and HCV-related cirrhosis (dark bars) in the state of Texas over the next two decades. The proportion of infected cases with cirrhosis is shown as a line.
As a result, the complications of cirrhosis also gradually increased. The number of patients with decompensated liver disease (ascites, encephalopathy, and variceal bleeding) continued to rise and peaked in 2012 at more than 12,495 cases (Figure 4). Furthermore, the proportion of patients with cirrhosis who decompensate continued to rise, reaching >12% by 2025. The number of cases of HCC peaked in the next few years, assuming that the annual risk of HCC among patients with chronic hepatitis C remained at the level observed prior to 2000 (Figure 4). However, recent data demonstrated that the annual risk of HCC is increasing, and therefore the model probably underestimated the risk of HCC. If the model were to assume an annual risk of 4% among cirrhotic patients based on recent European data (17) or 7% based on Japanese or the most recent US data (18, 19), then our current estimate of the number of cases of HCC in 2005 would have underestimated the true number of HCC cases by 29.6% or 50.0%, respectively. Finally, liver-related deaths increased in proportion to the observed increase in decompensated cirrhosis, though these estimates assume no advances in therapy or increases in the number of donors for liver transplantation.
Figure 4.
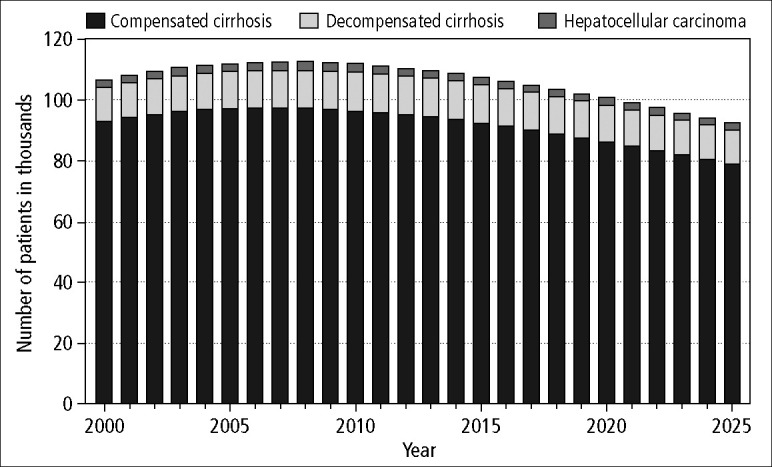
Projected total number of cases of HCV-related cirrhosis (total height of bar) in the state of Texas over the next two decades.
DISCUSSION
Chronic hepatitis C is a common infection, affecting approximately 1.8% of the population in the USA (3). There have been no surveys of the hepatitis C population prevalence in Texas. However, Baillargeon and colleagues reported that hepatitis C was present in 27% and 48% of men and women, respectively, who were incarcerated in state prisons (20). Incarcerated individuals may have a higher prevalence of infection than the general population. We utilized the recent NHANES III to estimate the prevalence of chronic HCV infection in the state of Texas. Age-, gender-, race- and income-specific prevalence rates from the NHANES III database were used to estimate a weighted prevalence for all counties in the state. Prevalence rates were generally highest in border counties, a result driven primarily by weighting for the high rate of poverty and the large number of Hispanics in those counties. The impact that the ongoing population migration will have on the prevalence of chronic hepatitis C is not known. The 2000 Census data found that more than 7 million Hispanics live in Texas, and the Migration Policy Institute reported that this number is growing rapidly due to both legal and illegal immigration, largely from Mexico (21). The prevalence of hepatitis C in this immigrant population is unknown. However, the prevalence is high in those who have already relocated to the USA (3), and it is reasonable to assume that the health care burden related to the infection will continue to increase in these border counties. Nonetheless, the number of infected cases is currently greatest in urban population centers, and therefore these areas will absorb most of the health care costs related to the future care of these patients.
We utilized a modification of a previously described and validated mathematical model of the natural history of chronic hepatitis C to project progression of liver disease in this pool of patients (4, 14). The model was adjusted to account for the estimate of HCV prevalence in the state in 2000 and cycled forward from that point. Thus, the model projected that the number of HCV-infected cases would gradually decrease in coming years as the incidence of new infection falls and as currently infected patients die from nonhepatic age-related causes. However, the pool of currently infected patients remains at risk of progressive liver disease. Indeed, the model predicted that an increasing proportion of the patients with chronic hepatitis C would develop cirrhosis over the next two decades, reaching 40% by the year 2025. As a result, there would be an increase in the number of cases with complications of liver failure, HCC, and death due to liver disease. In fact, recent observations confirm previously predicted increases in these complications (22–24).
Furthermore, our model used an extremely conservative estimate of risk for HCC. More recent estimates from the USA, Europe, and Japan suggest that we may have significantly underestimated the number of cases of hepatoma that will occur (17–19). Options for managing patients with HCC and advanced liver disease are limited. Liver transplantation is the best option and is considered in many patients. However, if every patient with decompensated cirrhosis or HCC in the state of Texas were considered for liver transplant, annual referrals would currently exceed 75% of the number of patients currently listed for liver transplant in the entire USA, and the number of cases would continue to increase for several more years.
Previously published models have examined whether antiviral treatment could decrease future complications of cirrhosis (4). In addition, some studies suggest that interferon therapy may reduce the risk of developing HCC (25). These reports and our prediction of HCV-related complications in Texas clearly emphasize the need for aggressive antiviral treatment of infected patients before complications of cirrhosis occur and for availability of liver transplantation once decompensation ensues. However, prescriptions for antiviral therapy in the state have averaged about 3000 per year since 1998, so we can safely assume that only a small percentage of patients are currently being offered such therapy.
While this report shows the likely increase in disease complications in patients infected with HCV, it is important to emphasize that most patients with chronic hepatitis C do not develop cirrhosis. Furthermore, most of those who demonstrate fibrosis progression never develop hepatic decompensation or HCC. Nonetheless, this should not detract from the urgency in identifying HCV-infected patients, developing more effective therapy, and increasing long-term recurrence-free posttransplantation survival.
The estimates and projections reported here are based on national prevalence data and therefore must be considered approximate. Furthermore, the accuracy of the model projections depends upon estimates and assumptions from published literature. The model is designed to utilize conservative estimates and is intentionally biased to underestimate disease complications whenever possible (4). To this end, it is of interest to note that the number of cases appears to have surpassed previous projections utilizing this and similar models (22–24).
Acknowledgments
We thank James E. Albright, MS, MBA, Outcomes Research Department, Parke-Davis, Ann Arbor, Michigan, for development of the disease model.
References
- 1.Alter MJ, Hadler SC, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Moyer LA, Fields HA, Bradley DW, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA. 1990;264:2231–2235. [PubMed] [Google Scholar]
- 2.Brown RS, Jr, Gaglio PJ. Scope of worldwide hepatitis C problem. Liver Transpl. 2003;9:S10–S13. doi: 10.1053/jlts.2003.50244. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting the future complications of hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 5.Koretz RL, Stone O, Mousa M, Gitnick GL. Non-A, non-B posttransfusion hepatitis—a decade later. Gastroenterology. 1985;88(5 Pt 1):1251–1254. doi: 10.1016/s0016-5085(85)80087-1. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Goodman ZD, Ishak KG, Hoofnagle JH, Melpolder JJ, Alter HJ. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(5 Suppl 1):S74–S83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 8.UNOS Scientific Registry. Primary liver disease of liver transplant recipients, 1991 and 1992. UNOS Update. 1993;9:27. [Google Scholar]
- 9.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–782. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital and Health Statistics. Series 1, No. 32 (DHHS Publication No. 94-1308) Washington, DC: Government Printing Office; July 1994. [PubMed] [Google Scholar]
- 13.US Census Bureau. State and county demographics. Available at http://www.census.gov/main/www/cen2000.html; accessed November 10, 2004.
- 14.Bennett WG, Inoue Y, Beck Jr, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, Hollinger FB, Gitnick G, Knodell RG, Perrillo RP, et al. The National Heart, Lung, and Blood Institute Study Group. Long-term mortality after transfusion-associated non-A, non-B hepatitis. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 16.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 17.Roffi L, Redaelli A, Colloredo G, Minola E, Donada C, Picciotto A, Riboli P, Del Poggio P, Rinaldi G, Paris B, Fornaciari G, Giusti M, Marin R, Morales R, Sangiovanni A, Belloni G, Pozzi M, Poli G, Mascoli N, Corradi C, Pioltelli P, Scalori A, Mancia G. Outcome of liver disease in a large cohort of histologically proven chronic hepatitis C: influence of HCV genotype. Eur J Gastroenterol Hepatol. 2001;13:501–506. doi: 10.1097/00042737-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Sakai H, Hashizume M, Hirohata T. A long-term follow-up study on risk factors for hepatocellular carcinoma among Japanese patients with liver cirrhosis. Jpn J Cancer Res. 1998;89:1241–1250. doi: 10.1111/j.1349-7006.1998.tb00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 20.Baillargeon J, Wu H, Kelley MJ, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 21.Grieco E. The foreign born from Mexico in the United States. Migration Information Source 2003 (October 1) Available at http://www.migrationinformation.org/feature/display.cfm?ID=163; accessed November 10, 2004.
- 22.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Kumar Shiv K, Thuluvath PJ, Shivakumar KS, Kumar Shiva K. Mortality from hepatocellular and biliary cancers: changing epidemiological trends. Am J Gastroenterol. 2002;97:167–171. doi: 10.1111/j.1572-0241.2002.05432.x. [DOI] [PubMed] [Google Scholar]
- 24.Darby SC, Ewart DW, Giangrande PL, Spooner RJ, Rizza CR, Dusheiko GM, Lee CA, Ludlam CA, Preston FE. UK Haemophilia Centre Directors' Organisation. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, Hino K, Okita K Osaka Liver Disease Study Group. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]