Abstract

We present the design of a helium liquefaction system tailored to efficiently recover helium vapor from either an individual or a small cluster of vibration-sensitive cryogenic instruments. This design prioritizes a compact footprint, mitigating potential contamination sources such as gas bags and oil-lubricated compressors while maximizing the recovery rate by capturing both the boil-offs during normal operation and the refilling process of the bath cryostat. We demonstrated its performance by applying it to a commercial low-temperature scanning probe microscope. It features a >94% recovery rate and induces negligible vibrational noise to the microscope. Due to its adaptability, affordability, compact size, and suitability for homemade setups, we foresee that our design can be utilized across a wide range of experimental measurements where liquid helium is used as the cryogen.
Keywords: liquid helium, helium recovery system, compact, low-vibration applications, scanning probe microscope
1. Introduction
Given the low density and chemical inertness,1 helium (He) is pivotal in various medical,2,3 industrial,4,5 and aerospace applications.6,7 It provides propulsion and cooling in aerospace contexts,8,9 facilitates deep-sea diving via mixed-gas formulations,10,11 and is integral to industrial processes like leak detection12,13 and semiconductor manufacturing.14,15 Its inertness helps create a chemically stable environment, which is critical in applications like zirconium16 or silicon production14,15 and gas chromatography.17,18 Its notable thermodynamic properties,1 especially the ability to approach near absolute zero temperatures either as the working gas19−21 or cryogen,22 make it essential in cryogenic applications such as cooling superconducting magnets23 and maintaining ultralow operational temperatures in particle colliders,24 spectrometers,25 microscopes,26,27 Mossbauer experiments,28 and quantum ion traps.29
The looming supply demand imbalance of He30−32 calls for efficient recovery technologies.24,33,34 Recent data show a 7.7% in production decrease, confronting an estimated 9.3% demand increase in the United States.35 Furthermore, He escapes into space due to its lightweight, making it impossible to recapture once released into the air.1 Thus, He recycling emerges not simply as a strategy to navigate the challenges of its scarcity but as a crucial approach, aligning with global sustainability and economic considerations and ensuring a stable supply amidst soaring market demand.30,32,36,37
Existing He recovery schemes for the research laboratories, mainly gasbag-style systems33,34 and closed-cycle designs,28,29,38−42 suffer from various drawbacks and very rarely meet both the need and financial situation of individual laboratories that run vibration-sensitive cryogenic instruments. On the one hand, while mature products exist,33,34 the gasbag-style recovery systems occupy substantial lab space that is not easily accessible to many research laboratories. Besides, the gas bag or balloon for temporarily storing the He boil-off together with the oil-lubricated compressor used to further compress He into the medium-pressure storage tanks introduces significant contamination and leakage,33,34 necessitating thorough purification processes. Normally, the gasbag-style systems take collaborative resources from multiple laboratories and are usually designed as shared facilities, making them economically unfavorable and even impractical to individual laboratories. On the other hand, closed-cycle28,29,38−42 designs offer another way to recover He vapor and avoid liquid He (LHe) replenishment. In these schemes, the cryocooler is usually placed in proximity to the cryogenic apparatus to ensure sufficient cooling power. As a result, the moving parts43 of the cryocooler very often transmit significant vibrations to the equipment, making these designs challenging for low-vibration applications. While solutions with vibrational decoupling mechanisms39,44 have been proposed to mitigate the mechanical noise, they typically require sophisticated engineering and special modifications to accommodate vibration-sensitive instruments.29,41−43,45 Therefore, it is either economically disadvantaged (as with gasbag-style system and closed-cycle design) or technically difficult (as with closed-cycle design) to implement the existing He recycling solutions to individual or a small cluster of cryostats that are sensitive to vibrations.
In response, our team has developed a simple and highly effective He recovery system that seamlessly integrates with a conventional bath cryostat (CRYOVAC) for low-temperature scanning probe microscopy (LT-SPM) (CreaTec). This setup is designed to optimize the cost-effectiveness and operational efficiency. It demonstrates an average daily LHe waste of less than 0.27 L in a 1-year duration, which translates to only 98.5 L loss after consuming 1825 L, including that from all major sources such as daily running, liquid transfer (cryostat refilling and initial cooldown), and servicing the cold head. Our design largely utilizes and repurposes commercially available components, avoiding complications from a custom design or development. This solution is shown to have a negligible impact on the operation and performance of the SPM and can easily be adapted for other vibration-sensitive apparatuses.
2. Experimental Methods and Designs
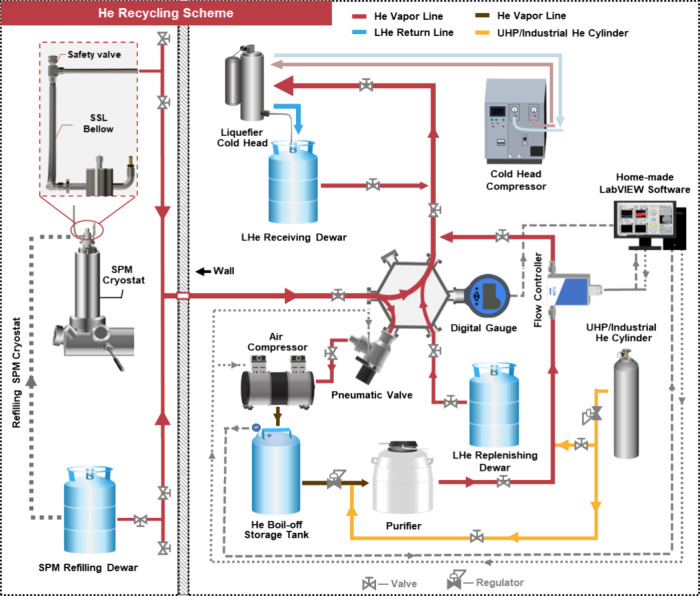
Our strategy involves the direct reliquefaction of daily He boil-off or vapor from both the SPM bath cryostat and storage dewars (CryoFab CMSH LH100), alongside capturing and storing any excess boil-off during liquid transfer into the medium-pressure tanks. Accordingly, the system mainly comprises two sections: He condensation and compression/storage (Figures 1 and 2a–c), which neatly fit into a small area of less than 4 m2. The condensation section, equipped with a commercial He liquefier cooled by a two-stage pulse tube cryocooler (Cryomech HeRL10 with the PT410 cold head), is rated to directly liquefy more than 10 L of LHe per day. The liquefier terminates with a liquid return line, which is directly inserted into a LHe dewar (receiving dewar, Figures 1 and 2b,c) via its refilling port. This setup allows for immediately (re)condensing the He boil-off into the receiving dewar. When close to full, the receiving dewar can be swapped with another container, the refilling dewar (Figures 1 and 2d), which replenishes the SPM cryostat (Figures 1 and 2e,f). In other words, LHe transfer from the receiving dewar to the refilling dewar is avoided, which usually induces extra He loss during evacuation and cooling of the transfer line in the gasbag-style recovery systems. Normally, the liquefier can capture the He boil-off from the cryostat together with the receiving and refilling dewars. However, when initially cooling or refilling the cryostat, the momentary boil-off rate will surpass the maximum liquefaction capacity. To maximize the recycling efficiency, the compression/storage section, mainly comprising a modified oil-free air compressor (California Air Tools 600040CAD) and 130-gallon gas storage tanks, is used to temporarily store this excess He boil-off and can hold up to 4677 L He gas (6.24 L LHe) at 125 psi (Figures 1 and 2a,b and Supporting Information Section A and B). A typical replenishment for our SPM cryostat adds less than 2100 L of He gas to the He boil-off storage tanks, which will be liquefied into the receiving dewar before the next refill (usually every 3 days). By recondensing the He boil-off from both daily machine running and cryostat initial cooldown/refilling, we manage to recover over 94% of the LHe that our lab consumes.
Figure 1.
Schematic layout of the He recovery system.
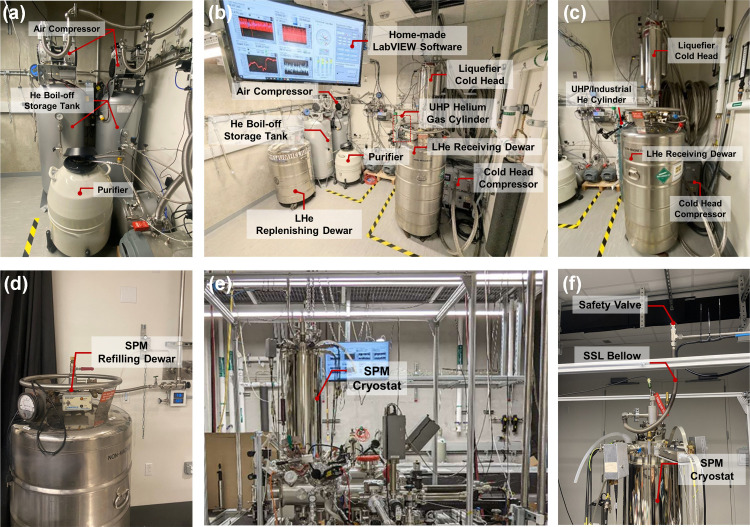
Figure 2.
Photos of the He recovery system. (a) Picture of the air compressor, He boil-off storage tanks, and purifier. (b) Overview of the He recovery room. (c) Picture of the liquefier, the LHe receiving dewar, and the cold head compressor. (d) View of the parking station of the refilling dewar. (e) Overview of He vapor line at SPM side. (f) Zoom-in image of the connection to SPM cryostat.
The successful implementation of our recycling system on the SPM relies on the ultrahigh purity of He boil-off, the long-term stable inline He vapor pressure, and the highly effective vibration reduction. To ensure the inline He purity, we mainly utilized stainless-steel (SSL) tubing with ConFlat (CF), vacuum coupling radiation (VCR), or Swagelok termination for the He vapor lines (Figures 1 and 2), with only few exceptions where Klein Flansche (KF), e.g., the outlet of SPM cryostat and the connection to air compressor, or national pipe tapered (NPT) connector, e.g., the safety valves and the connection to He boil-off storage tanks (Supporting Information Section A4), is adopted. Besides, the attachment of the LHe dewars to the He vapor lines is made contamination-free via the self-lock Swagelok quick connectors (Supporting Information Section A1). Consequently, the daily He boil-off from the SPM cryostat and two LHe dewars, accounting for more than 80% of the He recycled, is well isolated from the lab environment and does not need further purification before recondensation. Nevertheless, we found that the excess He boil-off that is compressed and stored in the He boil-off storage tanks during liquid transfer can clog the cold head, potentially due to a low amount of contamination induced by the air compressor. To improve the purity of this portion of He boil-off, we applied He-compatible sealants (Gasoila GE16) and Teflon tapes to the NPT connections on the air compressor motor and the storage tanks and remove the contaminants (primarily water, oil, and carbon dioxide as discussed in Sec 4) in the compressed He with a purifier (Figures 1 and 2a,b) before feeding this He to the liquefier to condense. It is worth noting that this purifier is simply built by immersing an SSL coil into liquid nitrogen (LN2) held in a commercial cryogenic freezer (MVE XC47/11-6) (Supporting Information Section C3). In this way, the He flow through the SSL coil is very effectively purified and will not compromise the cold head performance for an extended time.
Both the stable He vapor pressure and the low-vibration induction are critical to the performance of LT-SPM because the former elongates the low-temperature holding time and keeps the sample clean27 by maintaining an undisturbed scanner temperature while the latter guarantees a lower noise level for high-quality data acquisition.46 However, various operations can increase the daily boil-off rate of the SPM cryostat, such as transferring the tip and sample, and result in an elevated He vapor pressure. Accordingly, we took three measures to deal with the fluctuating He pressure without interfering with the run of SPM. First, the cold head compressor (Figures 1 and 2b,c), equipped with an optional inverter offered by the vendor, can increase/decrease the compressing frequency or liquefaction power to match a higher/lower He vapor load, thus targeting the He pressure (monitored by a pressure transducer) for a preset value. Second, when the total He boil-off rate is smaller than the minimal liquefaction rate, a heater inside the cryocooler supplies extra He vapor load by heating up the condensed He in the liquefier and hence always keeps the inline He pressure (monitored by a pressure transducer) around the set point. In addition, we wrote a LabVIEW program (Supporting Information Section B) to monitor the He pressure in both the vapor line and the He boil-off storage tanks via digital pressure gauges (Additel 681) (Figures 1 and 2b) and control a mass flow controller (MKS MFC GE50A) located between the purifier and He vapor line (Figure 1 and Supporting Information Section A3). This program automatically regulates the purified He flow into the vapor line through the flow controller according to the He vapor pressure and will cut off the flow when the storage tank pressure is below a set point, e.g., 20 psi, to prevent contamination. As a result, the He vapor pressure is maintained to be close to the set point pressure with a ± 0.01 psi variation most of the time. To reduce the vibration transmission from the recycling system to the SPM, we adopted three engineering designs. First of all, a pulse tube instead of a Gifford-McMahon cryocooler was used because the former19 generates smaller vibration than the latter20 without the need for a moving piston in the cold head. Besides, the cryocooler and other vibration-introducing instruments, including the air compressor, are placed in a remote room from the SPM (Figures 1 and 2a–c). Finally, the SPM cryostat is connected to the He vapor line via an SSL below to dampen any residual mechanical vibration from the recycling system further. With all of these measures taken, the SPM can run uninterruptedly except during the refilling of the cryostat or servicing of the cryocooler, as mentioned below.
3. System Operation
3.1. Initial Cooldown of the Liquefier Cold Head
To get started, we purge the whole He vapor line multiple times and fill it with ultrahigh purity (UHP) He gas. The receiving dewar is sequentially precooled to the LHe temperature with the refilling dewar via a direct liquid transfer since our cold head is not powerful enough to cool down directly at either room temperature or 77 K dewar. The He boil-off from cooling down the receiving dewar can be compressed into the He boil-off storage tanks to increase recycling efficiency by attaching the dewar vent port to the vapor line via self-lock Swagelok quick connectors (Supporting Information Section A1 and C1). Afterward, the liquefier return line is inserted into the cold receiving dewar while being purged with the UHP He. A UHP He cylinder (Figures 1 and 2b,c) is used to supply the He for cooling down the cold head, which normally takes ∼2 h to reach 4.2 K under the maximum power (Figure 3a).
Figure 3.

Temperature vs time curves recorded during (a) cooling down and (b) warming up the liquefier cold head and (c) swapping the receiving and refilling dewars. The temperature discontinuities in the warming-up curve, indicated by the arrows, correspond to the phase change temperatures of hydrogen (14 and 20 K), nitrogen (63 and 77 K), and water (273 K). The small bump in the cooling-down curve (indicated by the arrow) marks the moment when all He vapor loads are connected to the recovery system, signaling the system’s entering into its fully functional status.
3.2. Initial Cooldown of the SPM Cryostat
Once the cold head is functioning, we cooled and filled the SPM cryostat with LHe. Before filling it with LHe, the SPM cryostat is vented to the atmosphere through a bypassing valve (Supporting Information Section A2 and C2). Once the nitrogen in the cryostat is evacuated, we transfer LHe from the refilling dewar to it and connect it to the He vapor line to compress the excess boil-off into the He boil-off storage tanks. Generally, this process produces more He gas than the capacity of our 130-gallon tanks. Thus, we need to vent some He boil-off, but the portion is small according to our overall >94% recycling efficiency. This issue can be easily resolved by adding an extension storage tank (Supporting Information Section A4). When the cryostat is full, we isolate it from the vapor line and vent it to the air again before removing the LHe transfer line to avoid venting the whole recycling line. After the cryostat is capped, we reconnect it to the vapor line for recycling. The refilling dewar is also attached for recovery afterward.
3.3. Routine Maintenance
3.3.1. Refilling the SPM Cryostat
Refilling the SPM cryostat is similar to initially cooling it, but much less He boil-off is generated. Most of this gas can be compressed into the storage tanks except for the part used for evacuating and cooling the liquid transfer line. Besides, we have to isolate and vent the cryostat right before refilling to keep the rest sections of the recycling system unaffected (Supporting Information Section A2 and C2). It then follows the same procedures as those for the initial cooldown.
3.3.2. Regenerating the Purifier
The 3/8″ O.D. SSL coil immersed in LN2 in the purifier can be clogged by frozen contaminants over time. Hence, regeneration is necessary to resume the capabilities of the coil. Normally, the coil is blocked twice to three times a week and can be unclogged by lifting the top two loops up from the liquid level and blowing them with a hair dryer for several seconds since other gases like water and carbon dioxide usually freeze close to the liquid surface. Any potential contaminants escaping from the coil and heading toward the flow controller during this process will be purged via a Swagelok bellow valve (Supporting Information Section A3) before redirecting the purified He into the recycling line (Supporting Information Section C3). This method does not work as effectively after a month because the contaminants are eventually pushed to the lower loops of the coil. In that scenario, we replace the dirty coil with a clean one. This is made reproducible using Swagelok connections.
3.3.3. Swapping the Receiving Dewar and Refilling Dewar
When the receiving dewar is close to full, we swap it with the nearly empty refilling dewar with minimal interruption of the liquefier (Figure 3c). This is done by first isolating the liquefier from all tanks and then blowing the return line outlet with a heat gun while it is being purged with UHP He to avoid icing, and finally inserting the returning line into the empty dewar (Supporting Information Section C4). We have found it the most efficient to keep the liquefier running at its lowest power during this process.
3.3.4. Decontaminating the Liquefier Cold Head
The performance of our cold head is sometimes compromised by contaminants, which we suspect to be mostly nitrogen since our purifier does not effectively trap it. At this stage, we isolate the LHe dewars and SPM cryostat from the liquefier, warm the cold head up to 80 K, then cool it down and reconnect all tanks (Supporting Information Section C5). This proves to very efficiently remove the contaminants in most cases, usually once every several months. We also found it desirable to warm the cold head to room temperature once a year to fully clean it (Supporting Information Section C5). Decontaminating the cold head usually takes less than 6 h if only warming up to 80 K and can take nearly 1 day if warming up to room temperature (Figure 3a,b). This operation can be integrated with the dewar swapping introduced previously to reduce the reliquefication downtime further.
3.3.5. Replenishing LHe
Our setup has slight He loss during the initial cooldown, refilling, purging, and decontamination. Hence, LHe replenishment is necessary. This can be achieved by either providing gas to the recovery line using compressed gas cylinders or attaching the vent port of a commercial LHe dewar (LHe replenishing dewar in Figure 1) to the He vapor line. The former is more convenient for our purposes because of the low loss rate (Supporting Information Section C6). While utilizing UHP-grade gas is preferred, we have opted to use industrial-grade He cylinders to reduce costs. The impurities in industrial-grade He do not obviously compromise the performance of the liquefier, provided they are effectively removed through the purifier.
4. Performance
4.1. Purity of He in the Boil-Off Storage Tanks
The oil-free air compressor we used for compressing the excess He boil-off is low-cost and easy to maintain. However, it is designed for compressing air for pneumatic tools and is not optimized for He compression. Therefore, it unavoidably introduces contaminants into the He gas. To mitigate He leakage and prevent air contamination, we applied polytetrafluoroethylene seals to all connecting joints of the compressor and storage tanks. Figure 4 depicts the mass spectrum of the He gas compressed into the storage tanks, indicating a purity level above 99.5%. The main impurity detected is hydrogen (0.4%), likely permeating through the SSL tubing in the recovery line.47 As hydrogen solidifies around 14 K, we anticipate its accumulation close to the second stage of our pulse tube cryocooler, which is evident from the warming curve of the cold head (Figure 3b). However, no other adverse effects due to hydrogen contamination have been observed in our daily operation. The remaining impurities (<0.1%) consist of a mixture of water, nitrogen, oxygen, carbon dioxide, and a trace amount of organic compounds estimated to originate from the compressor lubricant. The majority of these impurities have condensation temperatures above 80 K and can be effectively removed with our cold-trap purifier. A low density of impurities, primarily nitrogen and oxygen, will need to be removed by warming up the cold head on a regular basis, as introduced previously.
Figure 4.

Mass spectrum of the sampled He vapor compressed into the He boil-off storage tanks.
4.2. Liquefier Performance
The liquefier can easily capture our 5 L daily LHe consumption. To optimize efficiency, we intentionally adjusted the cold head compressor frequency to its minimum level using the inverter. Operations like reading cryostat He level through the superconducting level meter or loading a new tip/sample from room temperature would increase the boil-off rate, causing a transient pressure change (Supporting Information Section D). As mentioned above, the inverter, together with the mass flow controller, is programmed to respond quickly to this pressure change. Consequently, both the cold head temperature and the inline He pressure are highly stable (Figure 5a,b). The average daily loss, accounting for all sources, is less than 0.27 L of liquid consumption out of 5 L of liquid consumption, where most of the loss is from the refilling and unclogging of the system. This loss rate can be further reduced by optimizing the refilling and decontamination procedures.
Figure 5.
Pressure and temperature stability of the He recovery system. (a) He vapor line pressure, (b) cold head temperature, and (c) SPM junction temperature during normal operation.
4.3. SPM Performance
The SPM we use can operate as either a scanning tunneling microscope (STM) or an atomic force microscope (AFM). Its junction temperature remains stable during prolonged normal operation (Figure 5c) due to the stable He vapor pressure. To evaluate the system’s noise level, we focused on the STM mode and analyzed the tunneling current with and without being connected to the recycling system (Figure 6). Upon connection to the reliquefying system, the measured noise level remained comparable to measurements taken without connection. The fast Fourier transform (FFT) analysis of the tunneling current revealed several noise peaks, including 60 Hz and its overtones from the AC power. Only a few additional noise peaks related to He liquefier can be identified.
Figure 6.

FFT spectra of the tunneling current without (a) and with (b) being connected to the He recovery system. Each spectrum is an average of 10 scans.
With no obvious interruption from the liquefier, the data quality of the STM is unaffected. In the STM community, detecting single-molecule vibrations using inelastic electron tunneling spectroscopy (IETS)48,49 is commonly regarded as one of the measurements requiring extremely high system stability. Here, we utilize the IETS of a surface-adsorbed CO molecule and topographic measurements of other materials as test systems to calibrate the performance of the SPM with the attached He recycling system. Atomic resolution and IETS measurements remain unaffected in our setup. Figure 7 displays an image of the Cu(100) single-crystal surface (Figure 7a), the NaCl thin film grown on Cu(100) (Figure 7b), and two CO molecules adsorbed on the Cu(100) surface (Figure 7c) when the He recycling system is active, along with the IETS spectrum of individual CO molecule (Figure 7d). The Cu and NaCl lattice structures are clearly shown under subnano resolution, and the hindered transitional (HT) and rotational (HR) modes of the CO are resolved by the STM-IETS.50
Figure 7.

Characterization of the SPM performance with the recovery system connected. STM topographic image of (a) a Cu lattice at 60 mV, 500 pA set points; (b) a NaCl lattice at −100 mV, 500 pA set points; and (c) two individual CO adsorbed on Cu(100) at −100 mV, 100 pA set points. (d) IETS of a single CO molecule. The energy of the HR and HT modes is marked by the dashed lines. The tunneling gap is set to 60 mV and 500 pA, and the spectrum is an average of 20 scans.
5. Conclusions
In summary, we have successfully designed and implemented an efficient and stable He recovery system, demonstrating its performance with a commercial LT-SPM. This setup exhibits an impressive recycling rate, maintaining a minimal He loss rate of less than 0.27 out of 5 L/day usage. A notable feature is its low vibration level, which, as confirmed by noise analysis and SPM performance, remains consistent regardless of the recovery system’s operational status. Our system primarily leverages commercially available components, such as the gas compressor commonly used in the construction and automotive industries, to minimize fabrication costs. This design can easily be adapted for other experimental measurements with a comparable or even higher (with a more powerful cold head) daily consumption rate and is a cost-efficient solution for individual research groups that usually cannot afford a large facility.
Acknowledgments
This research was supported by the United States National Science Foundation (NSF) under the grant nos. CHE-2303936 (to Shaowei Li) and DMR-2011924 (UC San Diego Materials Research Science and Engineering Center). The authors benefitted from the invaluable in-kind supports provided by Prof. Vicki Grassian, department of chemistry and biochemistry at UCSD, and Prof. Emeritus John Crowell.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmeasuresciau.4c00097.
Details of system connections, LabVIEW program, operation procedures, additional data (Sections A–D) (PDF)
Author Contributions
+ Zhiyuan Yin and Liya Bi contributed equally to this paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Hwang S. C.; Lein R. D.; Morgan D. A.. Noble Gases. In Kirk-Othmer Encyclopedia of Chemical Technology, Wiley; 2005 10.1002/0471238961.0701190508230114.a01.pub2. [DOI] [Google Scholar]
- Hashemian S. M.; Fallahian F. The use of heliox in critical care. Int. J. Crit. Illness Inj. Sci. 2014, 4 (2), 138–142. 10.4103/2229-5151.134153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berganza C. J.; Zhang J. H. The role of helium gas in medicine. Med. Gas Res. 2013, 3 (1), 18. 10.1186/2045-9912-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevey D.; Sallamand P.; Cicala E.; Ignat S. Gas protection optimization during Nd:YAG laser welding. Optics and Laser Technology 2005, 37 (8), 647–651. 10.1016/j.optlastec.2004.08.015. [DOI] [Google Scholar]
- Şahin S.; Wu Y. 3.14 Fission Energy Production. Compr. Energy Syst. 2018, 590–637. 10.1016/B978-0-12-809597-3.00331-X. [DOI] [Google Scholar]
- McLennan J. C. Helium: its production and uses. Journal of the Chemical Society, Transactions 1920, 117 (0), 923–947. 10.1039/ct9201700923. [DOI] [Google Scholar]
- Lloyd C. R. Helium and its relevance to the airship industry. Aeronautical Journal 1981, 85 (842), 91–93. 10.1017/S0001924000029444. [DOI] [Google Scholar]
- Hasenbein R.; Izenson M. G.; Swift W. L.; Sixsmith H.. High Efficiency Pump for Space Helium Transfer; NASA; 1991. https://ntrs.nasa.gov/citations/19920007101 (accessed Jan. 7, 2025).
- Walker M. L. R.; Russell R. P.; Singh L. A. Utilization of Residual Helium to Extend Satellite Lifetimes and Mitigate Space Debris. J. Propul. Power 2012, 1406. 10.2514/1.B34498. [DOI] [Google Scholar]; https://hpepl.ae.gatech.edu/papers/2012_JPP_Lake.pdf.
- Lee D. L.; Hsu C. W.; Lee H.; Chang H. W.; Huang Y. C. Beneficial effects of albuterol therapy driven by heliox versus by oxygen in severe asthma exacerbation. Acad. Emerg Med. 2005, 12 (9), 820–827. 10.1197/j.aem.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Barach A. L.; Eckman M. The Effects of Inhalation of Helium Mixed with Oxygen on the Mechanics of Respiration. J. Clin Invest 1936, 15 (1), 47–61. 10.1172/JCI100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerken A. History of Helium Leak Detection. Journal of Vacuum Science & Technology a-Vacuum Surfaces and Films 1991, 9 (3), 2036–2038. 10.1116/1.577450. [DOI] [Google Scholar]
- Kim H.; Chang Y. S.; Kim W.; Jo Y. W.; Kim H. J. Introduction to Helium Leak Detection Techniques for Cryogenic Systems. Applied Science and Convergence Technology 2015, 24 (4), 77–83. 10.5757/ASCT.2015.24.4.77. [DOI] [Google Scholar]
- Raineri V.; Campisano S. U. Silicon-on-insulator produced by helium implantation and thermal oxidation. Appl. Phys. Lett. 1995, 66 (26), 3654–3656. 10.1063/1.114130. [DOI] [Google Scholar]
- Cho T. S.; Park S.; Lubomirsky D.; Venkataraman S.. Dual Plasma Modes Operation of Hollow Cathode Electrode System for Remote Plasma Removals for Semiconductor Manufacturing. In 2016 IEEE International Conference on Plasma Science (Icops) 2016. 10.1109/PLASMA.2016.7534007. [DOI] [Google Scholar]
- Kroll W. J.; Stephens W. W. PILOT PLANTS.Production of Malleable Zirconium. Ind. Eng. Chem. 1950, 42 (2), 395–398. 10.1021/ie50482a046. [DOI] [Google Scholar]
- Hagen D. F.; Belisle J.; Johnson J. D.; Venkateswarlu P. Characterization of Fluorinated Metabolites by a Gas Chromatographic-Helium Microwave Plasma Detector - the Biotransformation of 1h,1h,2h,2h-Perfluorodecanol to Perfluorooctanoate. Anal. Biochem. 1981, 118 (2), 336–343. 10.1016/0003-2697(81)90591-1. [DOI] [PubMed] [Google Scholar]
- Woo J.-C.; Moon D.-M.; Kawaguchi H. Design and Characterization of a Helium-Discharge Ionization Detector for Gas Chromatography. Anal. Sci. 1996, 12 (2), 195–200. 10.2116/analsci.12.195. [DOI] [Google Scholar]
- Thummes G.; Wang C.; Heiden C. Small scale 4He liquefaction using a two-stage 4K pulse tube cooler. Cryogenics 1998, 38 (3), 337–342. 10.1016/S0011-2275(97)00169-0. [DOI] [Google Scholar]
- McMahon H. O.; Gifford W. E. A New Low-Temperature Gas Expansion Cycle. Adv. Cryog. Eng. 1960, 354. 10.1007/978-1-4757-0537-9_43. [DOI] [Google Scholar]
- Zu H.; Dai W.; de Waele A. T. A. M. Development of dilution refrigerators-A review. Cryogenics 2022, 121, 103390 10.1016/j.cryogenics.2021.103390. [DOI] [Google Scholar]
- Binnig G.; Rohrer H.; Gerber C.; Weibel E. Tunneling through a Controllable Vacuum Gap. Appl. Phys. Lett. 1982, 40 (2), 178–180. 10.1063/1.92999. [DOI] [Google Scholar]
- Teodorescu R.NMR Magnets: A Historical Overview. In Modern NMR Approaches To The Structure Elucidation of Natural Products, Volume 1: Instrumentation and Software, Williams A.; Martin G.; Rovnyak D.; Williams A.; Martin G.; Rovnyak D. Eds.; Vol. 1; The Royal Society of Chemistry, 2015; p 0. 10.1039/9781849735186-00026. [DOI] [Google Scholar]
- Claudet S.; Brodzinski K.; Darras V.; Delikaris D.; Duret-Bourgoz E.; Ferlin G.; Tavian L.. Helium inventory management and losses for LHC cryogenics: strategy and results for run 1. In Proceedings of the 25th International Cryogenic Engineering Conference and International Cryogenic Materials Conference 2014 2015, 67, 66–71 10.1016/j.phpro.2015.06.012. [DOI] [Google Scholar]
- Houck J. R.; Ward D. A LIQUID-HELIUM-COOLED GRATING SPECTROMETER FOR FAR INFRARED ASTRONOMICAL OBSERVATIONS. Publications of the Astronomical Society of the Pacific 1979, 91 (539), 140–142. 10.1086/130456. [DOI] [Google Scholar]; https://www.jstor.org/stable/40677459.
- Bi L. Y.; Liang K. K.; Czap G.; Wang H.; Yang K.; Li S. W. Recent progress in probing atomic and molecular quantum coherence with scanning tunneling microscopy. Prog. Surf. Sci. 2023, 98 (1), 100696 10.1016/j.progsurf.2022.100696. [DOI] [Google Scholar]
- Morgenstern M.; Schwarz A.; Schwarz U. D.. Low Temperature Scanning Probe Microscopy. In Springer Handbook of Nanotechnology, Bhushan B., Ed.; Springer Berlin Heidelberg, 2004; pp 413–447 10.1007/3-540-29838-X_14. [DOI] [Google Scholar]
- Wiggins J. W.; Oleson J. R.; Lee Y. K.; Walker J. C. Use of a Closed Cycle Helium Refrigerator for Mossbauer Studies. Rev. Sci. Instrum. 1968, 39 (7), 995. 10.1063/1.1683585. [DOI] [Google Scholar]
- Micke P.; Stark J.; King S. A.; Leopold T.; Pfeifer T.; Schmoger L.; Schwarz M.; Spiess L. J.; Schmidt P. O.; Crespo Lopez-Urrutia J. R. Closed-cycle, low-vibration 4 K cryostat for ion traps and other applications. Rev. Sci. Instrum. 2019, 90 (6), 065104 10.1063/1.5088593. [DOI] [PubMed] [Google Scholar]
- Olafsdottir A. H.; Sverdrup H. U. Assessing the Past and Future Sustainability of Global Helium Resources, Extraction, Supply and Use, Using the Integrated Assessment Model WORLD7. Biophysical Economics and Sustainability 2020, 5 (2), 6. 10.1007/s41247-020-00072-5. [DOI] [Google Scholar]
- Cao Q.; Li Y.; Fang C. H.; Liu R. H.; Xiao H. P.; Wang S. J. Status quo and utilization trend of global helium resources. Front. Environ. Sci. 2022, 10, 1028471 10.3389/fenvs.2022.1028471. [DOI] [Google Scholar]
- Siddhantakar A.; Santillán-Saldivar J.; Kippes T.; Sonnemann G.; Reller A.; Young S. B. Helium resource global supply and demand: Geopolitical supply risk analysis. Resour., Conserv. Recycl. 2023, 193, 106935 10.1016/j.resconrec.2023.106935. [DOI] [Google Scholar]
- Li J.; Meng Q.; Ouyang Z.; Shi L.; Ai X.; Chen X. Helium recovery and purification at CHMFL. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 171, 012012 10.1088/1757-899x/171/1/012012. [DOI] [Google Scholar]
- Barrios M.; Kynoch J. Helium recovery at the National High Magnetic Field Laboratory. Adv. Cryog. Eng. 2015, 101, 012103 10.1088/1757-899X/101/1/012103. [DOI] [Google Scholar]
- Goodin R. C.; U.S. Geological Survey, Mineral Commodity Summaries . Helium, 2024, https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-helium.pdf (accessed Jan. 7, 2025).
- Sunarso J.; Hashim S. S.; Lin Y. S.; Liu S. M. Membranes for helium recovery: An overview on the context, materials and future directions. Sep Purif Technol. 2017, 176, 335–383. 10.1016/j.seppur.2016.12.020. [DOI] [Google Scholar]
- Rufford T. E.; Chan K. I.; Huang S. H.; May E. F. A Review of Conventional and Emerging Process Technologies for the Recovery of Helium from Natural Gas. Adsorption Science & Technology 2014, 32 (1), 49–72. 10.1260/0263-6174.32.1.49. [DOI] [Google Scholar]
- Hackley J. D.; Kislitsyn D. A.; Beaman D. K.; Ulrich S.; Nazin G. V. High-stability cryogenic scanning tunneling microscope based on a closed-cycle cryostat. Rev. Sci. Instrum. 2014, 85 (10), 103704 10.1063/1.4897139. [DOI] [PubMed] [Google Scholar]
- Boolchand P.; Lemon G. H.; Bresser W. J.; Enzweiler R. N.; Harris R. A General-Purpose Cold Finger Using a Vibration-Free Mounted He Closed-Cycle Cryostat. Rev. Sci. Instrum. 1995, 66 (4), 3051–3057. 10.1063/1.1145528. [DOI] [Google Scholar]
- Ma R.; Li H.; Shi C.; Wang F.; Lei L.; Huang Y.; Liu Y.; Shan H.; Liu L.; Huang S.; et al. Development of a cryogen-free sub-3 K low-temperature scanning probe microscope by remote liquefaction scheme. Rev. Sci. Instrum. 2023, 94 (9), 093701 10.1063/5.0165089. [DOI] [PubMed] [Google Scholar]
- Eßer M.; Pratzer M.; Frömming M.; Duffhauß J.; Bhaskar P.; Krzyzowski M. A.; Morgenstern M. An ultra-high vacuum scanning tunneling microscope with pulse tube and Joule-Thomson cooling operating at sub-pm z-noise. Rev. Sci. Instrum. 2024, 95 (12), 123703 10.1063/5.0230892. [DOI] [PubMed] [Google Scholar]
- Coe A. M.; Li G. H.; Andrei E. Y. Cryogen-free modular scanning tunneling microscope operating at 4-K in high magnetic field on a compact ultra-high vacuum platform. Rev. Sci. Instrum. 2024, 95 (8), 083702 10.1063/5.0212244. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A.; Schmidt B.; Spagna S.; Dietzel D.; Falter J.; Thummes G.; Schirmeisen A. Low input power 4 K pulse tube cryocooler driven by an inverter helium compressor: Intrinsic temperature oscillations and mechanical vibrations. Cryogenics 2020, 108, 103085 10.1016/j.cryogenics.2020.103085. [DOI] [Google Scholar]
- Dhuley R. C.; Ruschman M.; Link J. T.; Eyre J. Thermal conductance characterization of a pressed copper rope strap between 0.13 and 10 K. Cryogenics 2017, 86, 17–21. 10.1016/j.cryogenics.2017.07.001. [DOI] [Google Scholar]
- Zhang S.; Huang D.; Wu S. A cryogen-free low temperature scanning tunneling microscope capable of inelastic electron tunneling spectroscopy. Rev. Sci. Instrum. 2016, 87 (6), 063701 10.1063/1.4952577. [DOI] [PubMed] [Google Scholar]
- Abraham D. W.; Williams C. C.; Wickramasinghe H. K. Noise-Reduction Technique for Scanning Tunneling Microscopy. Appl. Phys. Lett. 1988, 53 (16), 1503–1505. 10.1063/1.99940. [DOI] [Google Scholar]
- Herms E.; Olive J. M.; Puiggali M. Hydrogen embrittlement of 316L type stainless steel. Mat Sci. Eng. a-Struct 1999, 272 (2), 279–283. 10.1016/S0921-5093(99)00319-6. [DOI] [Google Scholar]
- Stipe B. C.; Rezaei M. A.; Ho W. Single-molecule vibrational spectroscopy and microscopy. Science 1998, 280 (5370), 1732–1735. 10.1126/science.280.5370.1732. [DOI] [PubMed] [Google Scholar]
- Bi L. Y.; Jamnuch S.; Chen A.; Do A.; Balto K. P.; Wang Z.; Zhu Q. Y.; Wang Y. F.; Zhang Y. N.; Tao A. R.; et al. Molecular-Scale Visualization of Steric Effects of Ligand Binding to Reconstructed Au(111) Surfaces. J. Am. Chem. Soc. 2024, 146 (17), 11764–11772. 10.1021/jacs.4c00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauhon L. J.; Ho W. Single-molecule vibrational spectroscopy and microscopy: CO on Cu(001) and Cu(110). Phys. Rev. B 1999, 60 (12), R8525–R8528. 10.1103/PhysRevB.60.R8525. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.