Probably no serious disease is so frequently overlooked by the practitioner. Post-mortem experience shows how often pericarditis is not recognized or goes on to resolution and adhesion without attracting notice.
—William Osler (1892) (1)
Clinically, pericardial heart disease is one of the more infrequent types of cardiac diseases, but morphologically, it is common. Nevertheless, many publications have appeared on clinical aspects of pericardial disease and surprisingly few have focused on its morphologic features. This report focuses on morphologic features of pericardial heart disease.
Although the term “pericarditis” is applied to many, indeed most, pericardial disorders, relatively few of the disorders actually are associated with inflammatory-cell reaction, and therefore probably most do not warrant the designation “pericarditis.” Fibrinous deposits without inflammatory cells, as occur in most patients with uremia, for example, do not justify a diagnosis of “pericarditis.” The presence of inflammatory cells in the pericardia rather than an acute clinical course or a pericardial friction rub should determine whether the term “pericarditis” is appropriate. “Pericardial heart disease” is a better general term.
CLINICAL AND MORPHOLOGIC SPECTRUM
Although its exact frequency from either clinical or necropsy standpoints is not certain, pericardial heart disease, as Osler emphasized, is observed more frequently at necropsy than during life. Pericardial heart disease has been found clinically in >1% of patients admitted to one large general hospital (2) and in about 5% of consecutive necropsies at another large general hospital (3). These figures were produced in the era before cardiac catheterization or operation, renal dialysis, or widespread use of antibiotics. The clinical and necropsy frequencies of pericardial heart disease in recent years are unknown. Better diagnostic tools, particularly echocardiography, and prolonged longevity in many systemic illnesses have almost certainly increased the clinical recognition of pericardial heart disease in recent years.
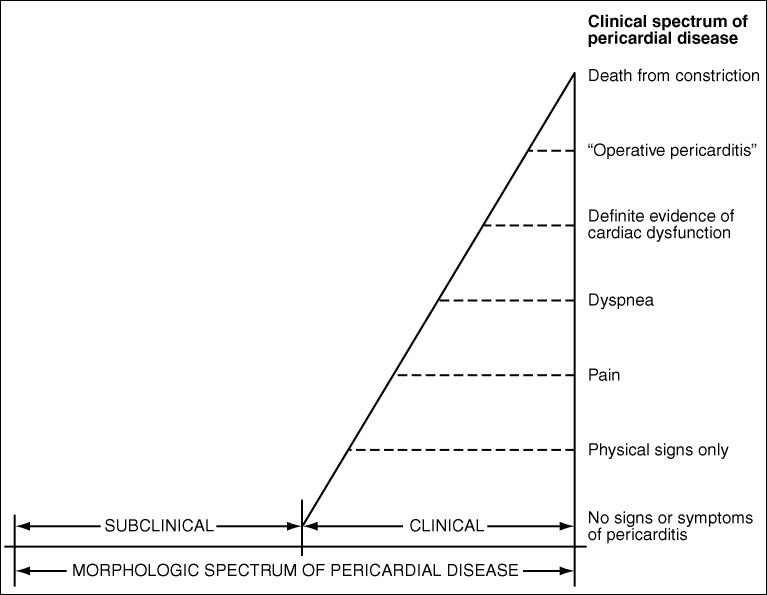
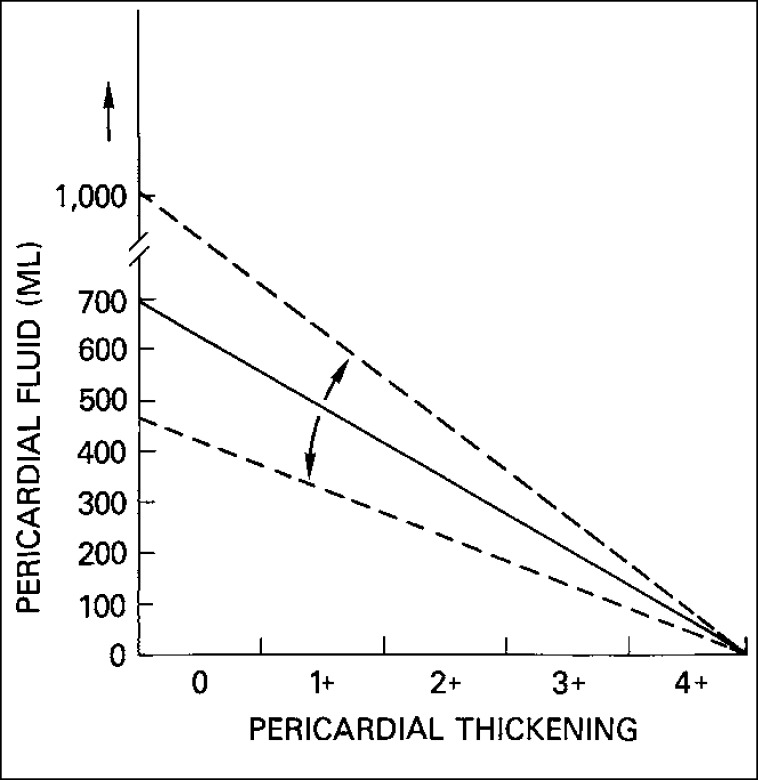
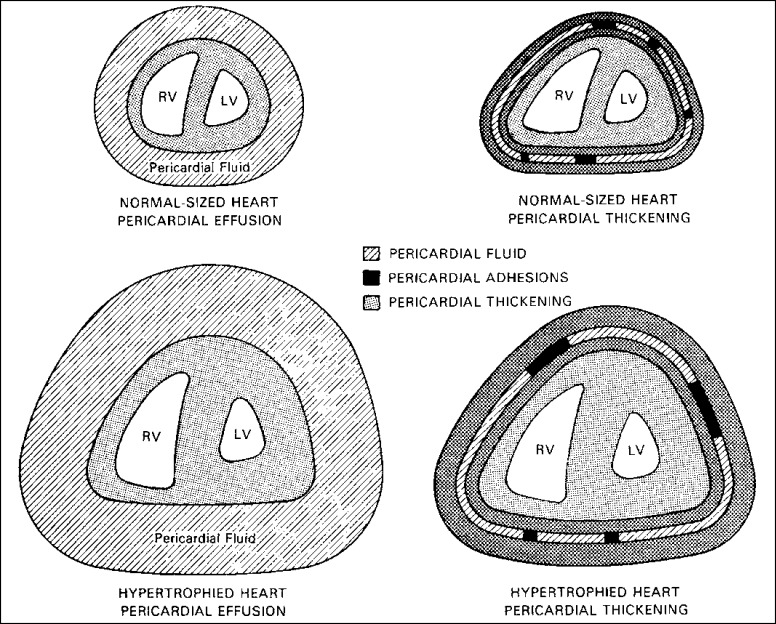
Nevertheless, as shown in Figure 1, pericardial heart disease is more often subclinical than clinical. Even when clinical manifestations are present, evidence of myocardial constriction is uncommon. Myocardial constriction from pericardial disease may result from pericardial effusion without pericardial thickening, from pericardial thickening without pericardial fluid, or from both effusion and thickening. As shown in Figure 2 and 3, among patients with “pericardial” constriction (in actuality, it is the myocardium that is constricted, not the pericardium), the larger the amount of pericardial fluid, the thinner the pericardia and, conversely, the thicker the pericardia, the smaller the amount of pericardial fluid.
Figure 1.
The clinical and morphologic spectrum of pericardial heart disease.
Figure 2.
Pericardial fluid accumulation is, in general, not proportional to pericardial thickening: the more the pericardial fluid, the less the pericardial thickening and vice versa. The dashed lines on either side of the uninterrupted line indicate that the latter may be shifted by an acute or chronic course. Less fluid is needed to produce tamponade if the fluid accumulation is rapid than if it is slow. Likewise, less pericardial thickening is needed to produce constriction if the thickening occurs rapidly as opposed to slowly.
Figure 3.
More pericardial fluid or thicker pericardia is necessary to constrict a hypertrophied heart than a normal-sized one. RV indicates right ventricle; LV, left ventricle.
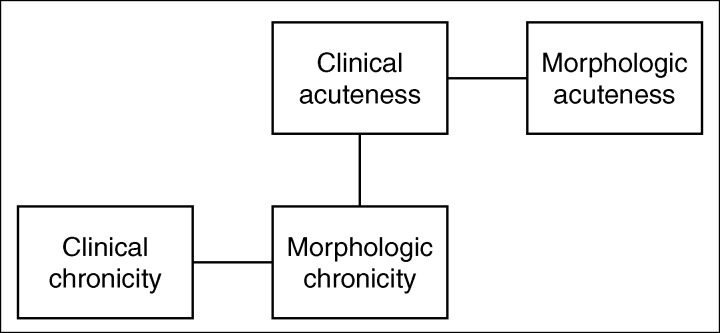
The type of clinical presentation of pericardial heart disease, i.e., acute or chronic, does not necessarily correlate with the type of morphologic derangement. The person with a thick constricting pericardium (obvious evidence of chronic disease) may present clinically with an acute picture (Figure 4). Probably more often than not, however, the clinical course follows the morphologic picture.
Figure 4.
Relationship between the clinical and morphologic features in pericardial heart disease. Although clinical and morphologic acuteness and clinical and morphologic chronicity often go together, a chronic morphologic process can present acutely.
NORMAL PERICARDIUM
The normal parietal pericardium (fibrous sac or fibrous pericardium), the bag that encloses the heart, consists of a 1-mm-thick layer of dense collagen (fibrous layer) with sparse interspersed elastic fibrils, and it is covered by a layer of mesothelial cells (serous layer) (Figure 5). The visceral pericardium (epicardium or serous pericardium) is the surface of the heart itself and consists of a thin (>1 mm) layer of loose fibrous tissue covered by mesothelial cells (serous layer). The serous layer of parietal pericardium is continuous with the serous layer of visceral pericardium at or near sites of attachment of the great vessels to the heart. The portion of epicardium that covers the vessels is arranged in the form of 2 tubes: the ascending aorta and pulmonary trunk are enclosed in the first (arterial mesocardium), and the superior and inferior venae cavae and the 4 pulmonary veins are enclosed in the second (venous mesocardium). The attachment of the latter to the parietal pericardium is in the shape of an inverted U, and the cul-de-sac enclosed between the limbs of the U lies behind the left atrium and is known as the oblique sinus. The passage between the venous and arterial mesocardia, i.e., that between the aorta and pulmonary trunk anteriorly and the atria posteriorly, is the transverse sinus.
Figure 5.
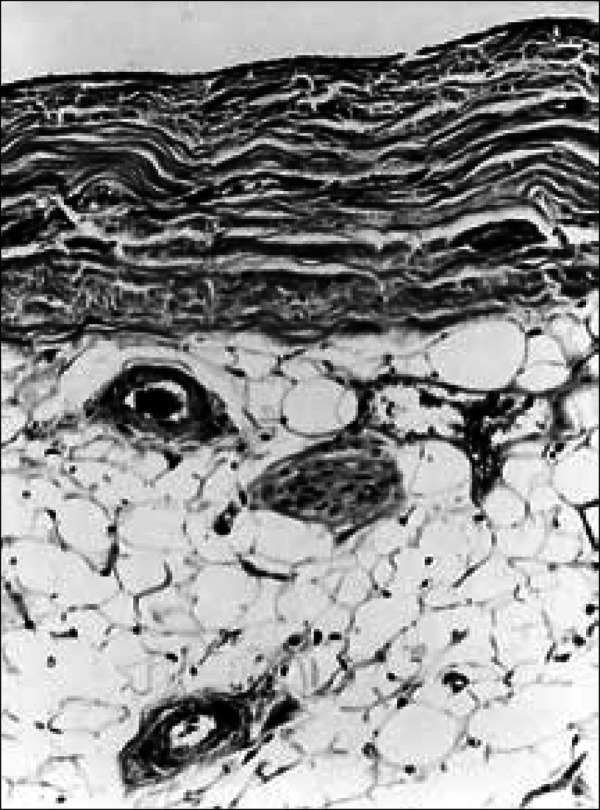
Normal parietal pericardium. A single layer of mesothelial cells covers the layer of dense fibrous tissue on the cardiac side. On the mediastinal side of the fibrous layer is adipose tissue, and in it are located vascular channels and nerves. Masson stain, ⋇185.
The flask-shaped bag of parietal pericardium is closed at its neck by fusion with the adventitia of the great vessels; its base is attached to the tendinous and muscular portions of the left-sided diaphragm. It is also attached to the posterior surface of the sternum by the superior (to manubrium) and inferior (to xiphoid process) pericardiosternal ligaments. Between the left main pulmonary artery and subjacent pulmonary vein is a triangular fold of visceral pericardium known as the ligament of the left vena cava, a remnant of the lower part of the left superior vena cava. The sinus node is located near the attachment of the parietal pericardium to the superior vena cava, and consequently it may be “irritated” by a pericardial process, producing an arrhythmia or a conduction disturbance.
Electron microscopic studies of the normal parietal pericardium show that the tissue is composed of 3 layers: 1) the serosa, consisting of a surface layer of mesothelial cells and a narrow submesothelial space, 2) the fibrosa, containing variously oriented layers of collagen fibrils and small elastic fibers, and 3) the epipericardial connective tissue, containing mainly large coarse bundles of collagen and forming part of the pericardiosternal ligament (4). Scanning electron microscopic studies show the mesothelial cells to contain single cilia covered with microvilli. The latter appear to bear friction and to increase the surface area for fluid transport.
Beneath the visceral pericardium is either myocardium or adipose tissue. The amount of subepicardial adipose tissue increases, up to a point, with age. Large quantities are rare in persons under 10 years of age and over 90 years of age. The deposits are largest in the atrioventricular sulci and, next, around the anterior and posterior descending coronary arteries. At times, the entire surface of the heart is covered by fat (Figure 6). In general, the amount of subepicardial adipose tissue is proportional to the amount of fat present in other body tissues (5, 6). At times, however, considerable quantities of subepicardial adipose tissue are present in persons of normal body weight, and the amount may not be excessively increased in persons of huge body weight (<135 kg [300 lb]). Corticosteroid therapy causes the amount of subepicardial tissue to increase (7, 8). In persons with debilitating diseases such as malignant neoplasms or chronic starvation, the subepicardial fat may atrophy, giving it a watery, gelatinous appearance.
Figure 6.
Massive increase in subepicardial adipose tissue in an 82-year-old man with idiopathic hemochromatosis. The myocardium was completely covered by fat. The heart weighed 500 gm. (a) Anterior view. (b) Posterior view. (c) Longitudinal section showing the thickness of fat compared with the thickness of the myocardial wall. (d) Close-up view of the right ventricular wall. The cause of the massive subepicardial adiposity in this patient is not certain.
When subepicardial adipose tissue is excessive, strands of fat cells also extend into adjacent myocardium. This occurrence is most frequent in the walls of the right ventricle and right atrium. The fat may extend through the myocardial wall and infiltrate the endocardium. Although they had no hemodynamic documentation, many writers in the 1800s believed that excessive subepicardial adipose tissue could cause cardiac dysfunction. Osler, writing in 1892, stated that excessive subepicardial fat “occasionally leads to dangerous or even fatal impairment of the contractile power of the heart” (1). Sudden nontraumatic death in the late 1800s was often attributed to a “fatty heart.”
Although normally the serous layer of parietal pericardium contacts the serous layer of visceral pericardium, up to 50 mL of fluid, which has a chemical composition similar to that of serum, normally is present in the pericardial sac (9). The pericardial fluid, in essence, serves as a “lubricating oil” to diminish the amount of friction between the moving heart and the adjacent tissues.
In the adipose tissue beneath the visceral pericardium and on the mediastinal aspect of the parietal pericardium are located vascular channels, including arteries, veins, and lymphatics, and also nerves. None of these structures is located within the fibrosa component of the parietal pericardium. The arteries of the pericardium arise from the internal mammary artery and its musculophrenic branch and from the descending thoracic aorta. Branches of the vagus and phrenic nerves and from the sympathetic trunk supply the pericardium.
Normally, the visceral pericardium is translucent and the underlying subepicardial tissue or myocardium is readily visible. In many individuals, the epicardium is focally white (Figure 7), and then the underlying structures are obscured. These foci (variously called milk spots, soldiers' spots, tendinous patches, and maculae tendineae) most commonly are present over the anterior surface of the right ventricle, often are multiple, and increase in incidence and size with age and with cardiac enlargement. Histologically, these foci consist of dense collagen, occasionally with underlying small collections of mononuclear cells (lymphocytes). Consequently, they may most appropriately be called “collagen plaques.” They are usually absent in the first decade of life and nearly always present by the sixth decade.
Figure 7.
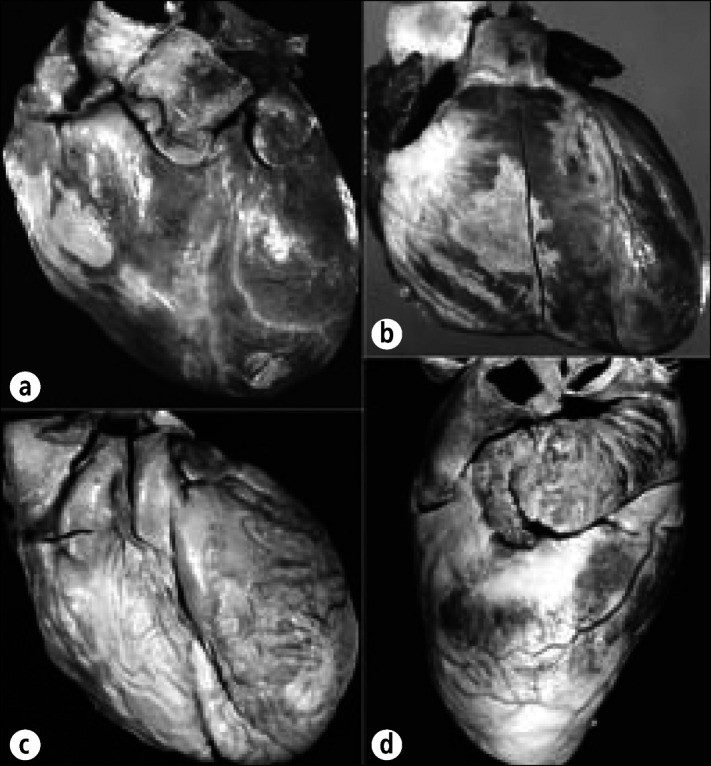
Epicardial collagen plaques (soldier's plaques, milk spots) in the hearts of 4 different patients. (a) Collagen plaques are present over the right ventricle and over the left ventricular apex. (b) A discrete collagen plaque is visible over the right ventricle. (c) Virtually the entire right ventricle and pulmonary trunk are covered by a collagen plaque. (d) The lateral view shows the collagen plaques just beneath the left atrial appendage and over the left ventricular apex. Note that the subepicardial adipose tissue is relatively sparse in the 4 patients. When more subepicardial fat is present, epicardial collagen plaques are less likely to form.
When the right ventricle is enlarged, the collagen plaques over its anterior surface may be quite prominent and presumably result from contact of the beating heart with the undersurface of the sternum. In conditions producing considerable enlargement of the right atrium—for example, atrial septal defect, chronic lung disease, or cardiomyopathy—the collagen plaques may occur over the right atrium (10). In kyphoscoliosis, the plaques often are found on the posterior surface of the heart, presumably due to contact with the vertebral bodies during cardiac motion. In conditions that cause left ventricular enlargement, particularly systemic hypertension and left ventricular outflow tract obstruction, collagen plaques often are present over the apex of the left ventricle. Plaques in this location may be anatomic expressions of the “point of maximal impulse” and result from contact of the left ventricular apex with the undersurface of the anterior ribs during ventricular systole. In patients with large amounts of subepicardial adipose tissue, collagen plaques may be less numerous, presumably since the epicardial fat acts as a cushion or absorbing vehicle for the underlying myocardium.
MORPHOLOGIC RESPONSES OF THE PERICARDIA TO INJURY
Although pericardial disease has many causes, the morphologic reaction of the pericardia to injury is rather limited. The pericardium reacts to acute injury by exuding fluid, fibrin, or cells or combinations of these three. The type of fluid and/or cells exuded is determined by the cause of the pericardial disease. There is no pericardial reaction to either serous fluid or to blood in the pericardial sac. If, however, the lipid component of pooled lysed erythrocytes is injected into the pericardial sac, a fibrinous reaction may result and fibrous or even cholesterol pericardial heart disease may develop (11). Blood in the pericardial space of injured pericardia, however, may lead to fibrous pericardial adhesions. Isolated injury to the serosal pericardia of rabbits without associated bleeding causes only a fibrinous reaction; if blood is injected after the serosal injury, pericardial adhesions may result (12). Deposits of fibrin alone on the pericardial surfaces apparently cause no permanent reaction.
If microorganisms enter the pericardial space or tissues, the reaction appears to depend on the type of infecting organism. Viruses, the presumed cause of “acute benign pericarditis,” generally produce only a transient pericardial reaction, which usually resolves (13). Acid-fast organisms produce a mononuclear cell reaction and generally go on to cause severe fibrous thickening of the pericardia. Pyogenic organisms cause a violent polymorphonuclear cell reaction that also may progress to fibrous thickening with or without constriction.
General morphologic responses to injury are listed in Tables 1 and 2. Although their occurrence in pure form is unusual, the recognition of the dominant morphologic component is useful in determining the cause of pericardial heart disease in any particular patient. The causes of each of the primary morphologic components are discussed more thoroughly in the remainder of this report.
Table 1.
Morphologic classification of pericardial heart disease
| Primary morphologic component | Potential for constriction | |
| 1. Fibrin | 0 | |
2. Fluid
|
+ | |
| 3. Purulent matter | + | |
| 4. Fibrous tissue | + | |
| 5. Neoplasm | + | |
| 6. Granuloma | + | |
| 7. Calcium | + | |
| 8. Cholesterol | + | |
Table 2.
Etiologic and morphologic classifications of pericardial heart disease
| Morphologic | ||||||||
|
|
||||||||
| Etiologic | Fibrinous | Effusion | Infective | Fibrous | Neoplastic | Granulomatous | Calcific | Cholesterol |
| 1. Idiopathic | ++ | + | 0 | ++ | 0 | 0 | ++ | ++ |
|
| ||||||||
| 2. Infective | ||||||||
| A. Pyogenic (purulent) | + | + | ++ | + | 0 | 0 | 0 | 0 |
| B. Tuberculous | + | + | + | ++ | 0 | ++ | + | + |
| C. Viral or “acute benign nonspecific” | ++ | 0 | + | + | 0 | 0 | 0 | 0 |
| D. Parasitic | + | + | ++ | + | 0 | + | + | 0 |
| E. Fungal | + | + | ++ | + | 0 | + | + | 0 |
|
| ||||||||
| 3. Associated with systemic disease | ||||||||
| A. Collagen disease | ||||||||
| 1. Rheumatic fever | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. Rheumatoid arthritis | + | 0 | 0 | ++ | 0 | + | 0 | + |
| 3. Systemic lupus erythematosus | + | + | 0 | ++ | 0 | 0 | 0 | 0 |
| 4. Scleroderma | + | 0 | 0 | ++ | 0 | 0 | 0 | 0 |
| B. Renal disease | ++ | + | 0 | + | 0 | 0 | 0 | 0 |
| C. Thyroid disease | 0 | + | 0 | 0 | 0 | + | + | ++ |
| D. Sarcoidosis | 0 | 0 | 0 | 0 | 0 | ++ | 0 | 0 |
|
| ||||||||
| 4. Associated with other diseases of the heart or aorta | ||||||||
| A. Acute myocardial infarction | ++ | 0 | 0 | + | 0 | 0 | 0 | 0 |
| B. Ascending aortic aneurysm | + | ++ | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| 5. Traumatic and iatrogenic | ||||||||
| A. Penetrating and nonpenetrating injury | + | ++ | 0 | ++ | 0 | 0 | 0 | + |
| B. Cardiac catheterization | ++ | + | 0 | 0 | 0 | 0 | 0 | 0 |
| C. Cardiac operation and postpericardiotomy syndrome | + | + | + | ++ | 0 | 0 | 0 | 0 |
| D. Resuscitation | + | + | 0 | ++ | 0 | 0 | 0 | 0 |
| E. Radiation | + | + | 0 | ++ | 0 | 0 | 0 | 0 |
| F. Drugs and hypersensitivity states | ++ | + | 0 | 0 | 0 | 0 | 0 | 0 |
| G. Talc | + | 0 | 0 | + | 0 | ++ | 0 | 0 |
|
| ||||||||
| 6. Neoplastic | + | + | 0 | 0 | ++ | 0 | 0 | 0 |
|
| ||||||||
| 7. Congenital | ||||||||
| A. True and false (diverticulae) cysts | – | – | – | – | – | – | – | – |
| B. Complete and partial absence | – | – | – | – | – | – | – | – |
FIBRINOUS PERICARDIAL HEART DISEASE
Fibrin deposits on the pericardial surfaces are observed commonly at necropsy. The deposits may be focal or diffuse, and they may or may not be associated with an increased quantity of pericardial fluid. Every type of pericardial disease probably is associated with fibrinous deposits at one time, and probably most cases resolve without residua. Fibrinous deposits are not capable by themselves of causing myocardial constriction. There are 6 general causes of fibrinous pericardial heart disease (Table 3).
Table 3.
Etiology of fibrinous pericardial heart disease
|
Acute myocardial infarction
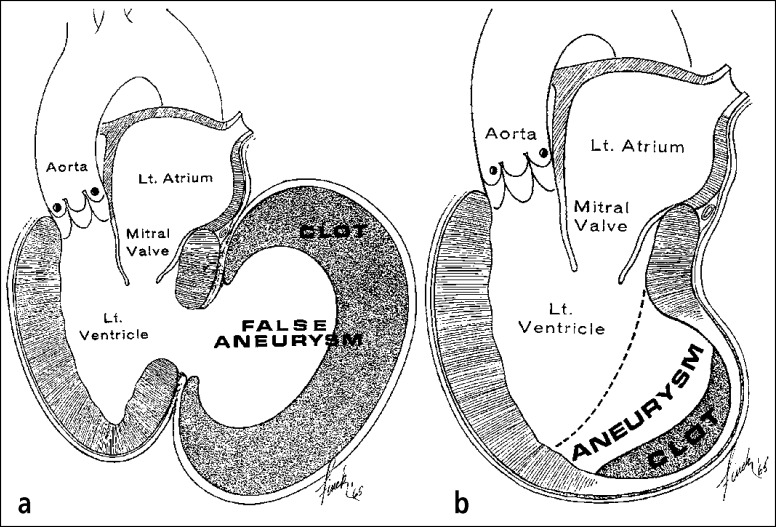
Fibrinous pericardial heart disease probably occurs in most patients with transmural acute myocardial infarction (Figure 8). The deposits may be limited to the visceral and parietal pericardium immediately overlying the area of transmural necrosis or may spread to involve the pericardial surfaces diffusely. The mechanism of development of diffuse fibrinous pericardial heart disease in these patients is not clear but may be related to the presence of an associated serosanguineous effusion. The parietal pericardium may adhere to the underlying visceral pericardium at the site of the transmural acute myocardial infarction. If the pericardial adherence occurs before the left ventricular free wall ruptures (as a consequence of the infarction), extravasation of blood into the pericardial space may be prevented and a false left ventricular aneurysm may result (14, 15) (Figure 9).
Figure 8.
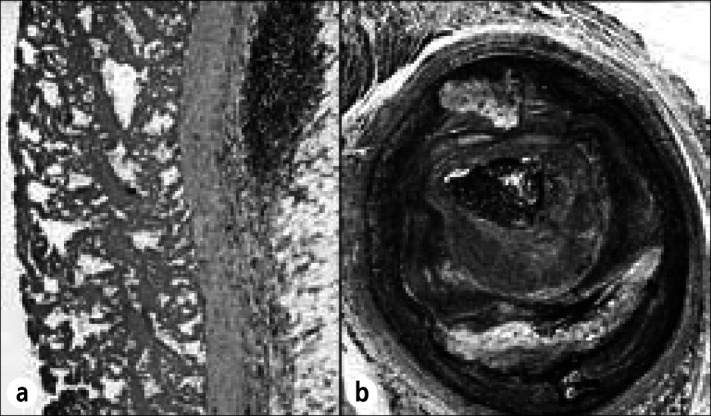
Diffuse fibrinous pericarditis from acute myocardial infarction. This 56-year-old man developed a precordial friction rub on day 6 following infarction and died on day 16. (a) A deposit of fibrin covers the mildly thickened epicardium. (b) The left circumflex coronary artery is completely occluded by both atherosclerotic plaque and thrombus. Hematoxylin-eosin stain, ⋇28 (a); Movat stain, ⋇14 (b).
Figure 9.
(a) False vs (b) true left ventricular aneurysms with adherent parietal pericardia. The wall of the false aneurysm is parietal pericardium, which had adhered to the epicardium before the left ventricle ruptured. In the true aneurysm, the parietal pericardium adheres to the scarred left ventricular myocardium, but the actual wall of the aneurysm had been myocardium at one time.
Iatrogenesis and trauma
Cardiac surgery probably is the most common cause of pericardial heart disease today. Whenever the mesothelial cells of the pericardia are “rubbed off,” a fibrinous reaction ensues. As the injured pericardia heal, the fibrin disappears and the 2 serous layers of pericardium remain adherent by fibrous adhesions. Despite this adherence, the parietal pericardium rarely is thickened by operative intervention. Obviously, blood and its products enter the pericardial space during any cardiac operation, and organization of the blood products may be more responsible for the eventual fibrous pericardial obliteration (12). Perforation of a cardiac wall by a catheter may lead to a pericardial reaction similar to that produced by cardiac surgery (16). Blunt or penetrating trauma also may cause a fibrinous pericardial reaction, which on rare occasion may progress to constriction (17).
Irradiation to the mediastinum and chemical agents initially cause a fibrinous pericardial reaction that also may heal by fibrous obliteration (18).
Infectious agents
Infectious agents are discussed under infective (purulent) pericardial heart disease.
Idiopathy
Although abundant clinical information is available on “acute benign pericarditis,” morphologic information on this entity is lacking because of its self-limited character and rare association with “pericardial constriction” (19). A viral etiology of this entity has been implicated on the basis of “viral shedding,” a rise in viral titers and the presence of lymphocytes and virus in aspirated pericardial fluid (20). However, a virus has never to my knowledge been observed ultrastructurally in pericardium, although viral antigen has been located in pericardium by immunofluorescence (21).
Neoplasm
A fibrinous pericardial reaction is common whenever the pericardium is infiltrated by a neoplasm, but the fibrin deposits are of minor consequence in this circumstance.
Association with systemic disease
“Uremic pericarditis,” at least initially, always is fibrinous and is found at necropsy in <50% of patients with fatal acute or chronic renal disease (22). Its cause remains unclear. The term “uremic pericarditis” is a misnomer because injection of neither urea nitrogen nor creatinine into the pericardial space results in a fibrinous pericardial reaction. Furthermore, “uremic pericarditis” may occur in patients with chronic renal disease whose levels of urea nitrogen and creatinine have been made normal for long periods by frequent dialysis (23, 24). Usually the amount of pericardial fluid is increased in patients with renal failure in addition to the fibrinous pericardial deposition. On occasion, the pericardia may become quite thick by fibrous proliferation, leading to constriction with or without associated serous or hemorrhagic effusion (25, 26).
Acute rheumatic fever usually is associated with a subclinical fibrinocellular cell reaction (27, 28). This pericardial reaction appears to resolve because evidence of previous pericardial disease is rarely observed at necropsy in patients with rheumatic heart disease. Calcific pericardial deposits also may occur in patients with rheumatic heart disease, but they too are rare and, when present, are focal and never associated with myocardial constriction.
In the precorticosteroid era, fibrinous pericardial heart disease occurred in about 60% of patients with systemic lupus erythematosus (7, 8). Today, a fibrous obliterative pericardial reaction is observed more commonly (8). A fibrinous pericardial reaction also is observed at necropsy in about 40% of patients with rheumatoid arthritis (29).
The morphologic features of pericardial disease resulting from hypersensitivity states and drug reactions are not certain, but it is presumed that the reaction is of a fibrinous type.
EFFUSION INTO PERICARDIAL SAC
Excessive (<50 mL) effusion into the pericardial sac is extremely common, probably far more common than generally appreciated, because the amounts rarely are measured accurately at necropsy. Serous effusions up to 200 mL, as occur commonly in patients with congestive cardiac failure or hypoalbuminemia, may be overlooked entirely at necropsy.
The amount of pericardial fluid necessary to cause ventricular constriction is highly variable and dependent on several factors: 1) the time period required to accumulate the fluid, 2) the amount of thickening of the underlying or overlying pericardia, 3) the amount of muscle mass (weight) of the cardiac ventricles, and 4) the blood volume. The more rapidly fluid accumulates, the greater the chance that myocardial dysfunction (constriction) will result. Fluid volumes many times the normal amount may be present without myocardial constriction if the accumulation is slow (Figure 2). The greater the thickness of the parietal or visceral pericardia or both, the smaller the amount of fluid needed to produce signs or symptoms of constriction. The thicker the ventricular walls, the greater the amount of fluid required for constriction. More fluid is required, for example, to constrict the hypertensive ventricle than the normotensive ventricle (see Figure 3).
The effusions can be serous, bloody, or lymphatic/chylous (Table 4).
Table 4.
Etiology of effusion (<50 mL) into pericardial space
Serous effusion
|
Bloody effusion (hematocrit >10%)
|
Lymphatic or chylous effusion
|
|
|
Serous effusions
Serous effusions are most common in patients with congestive cardiac failure and hypoalbuminemia (30). Irradiation (usually <4000 cGy) to the mediastinum may produce a large effusion with tamponade (31).
Bloody effusions
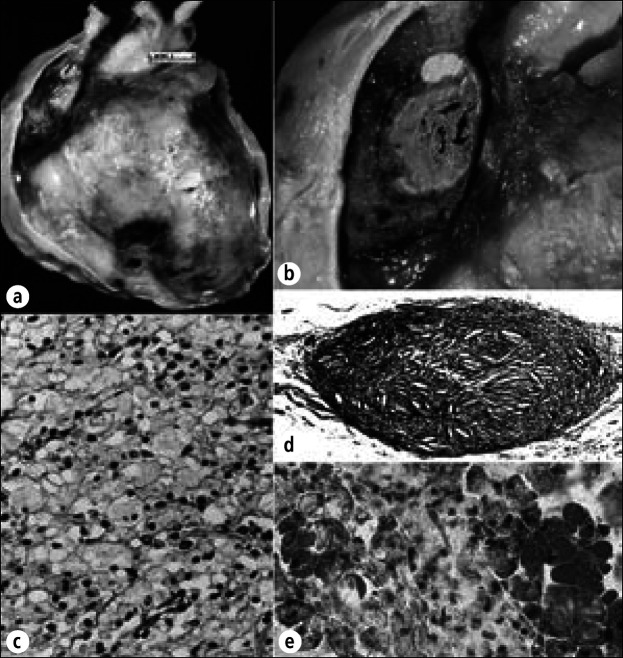
Bloody effusions most commonly result from acute myocardial infarction, rupture of the heart or aorta (32), neoplasms, cardiac operations or other procedures, drugs that alter clotting mechanisms (33), and chronic renal disease (34). That renal disease may be associated with large bloody effusions is largely unappreciated (35); this circumstance may occur in the natural history of renal disease (Figure 10) as well as that prolonged by chronic dialysis. Gaucher's disease also may be associated with large pericardial effusions (36, 37) (Figure 11). Hemopericardium always is an ominous sign because malignancy is one of its more common causes. Hemorrhages into the epicardium are common in patients with thrombocytopenia (Figure 12).
Figure 10.
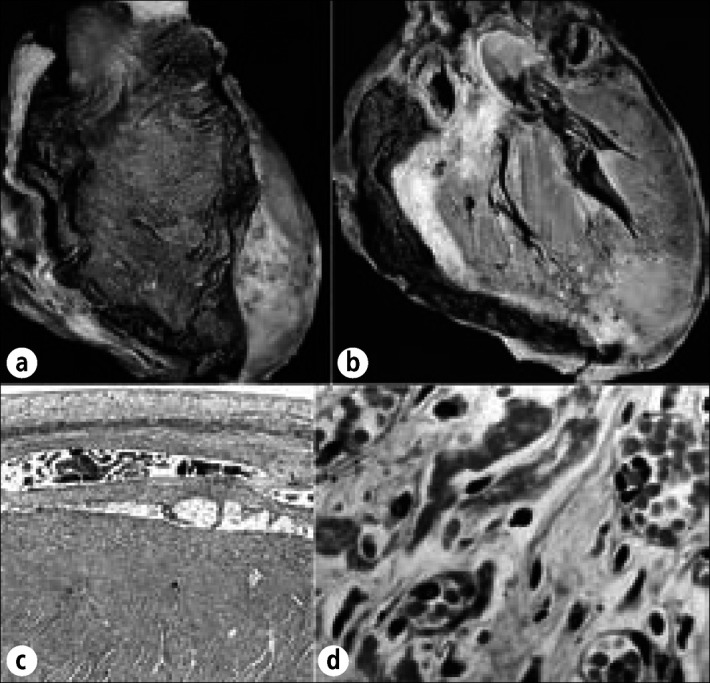
Hemorrhagic pericardial heart disease in chronic renal disease. This 26-year-old woman had renal failure and pericardial effusion on admission 3 weeks before death. Pericardiocentesis was performed twice, but cardiac tamponade progressed. At necropsy, 800 mL of hemorrhagic fluid was present and the pericardia were diffusely thickened and covered by fibrin deposits and blood clots. (a) Exterior view of the heart anteriorly. (b) Longitudinal section of the heart. The pericardial space over the left ventricle is obliterated. (c) Photomicrograph showing adherence of the parietal pericardium to underlying visceral pericardium over the left ventricle. (d) Close-up portion of the epicardium showing many vascular channels, the presumed source of the hemorrhagic pericardial effusion. Phosphotungstic acid hematoxylin, ⋇7.5 (c); hematoxylin-eosin stain; ⋇628 (d).
Figure 11.
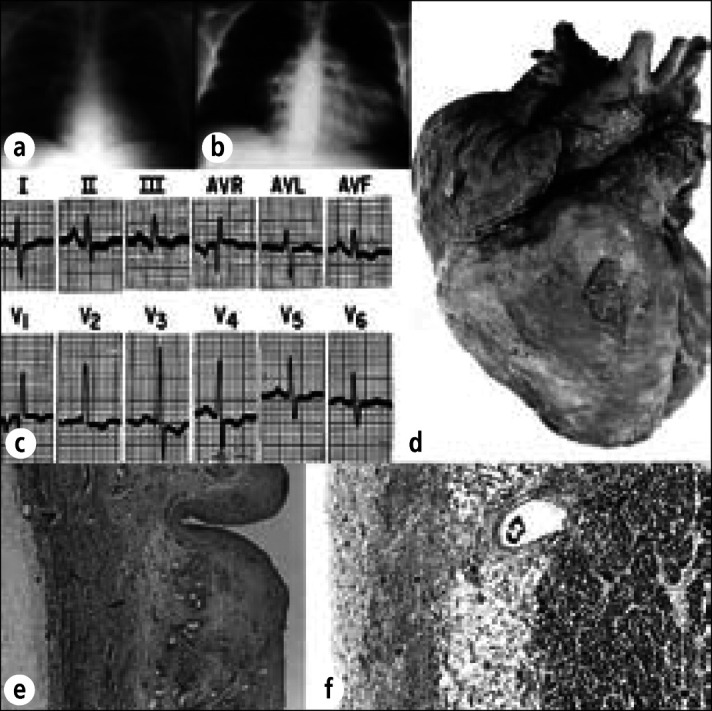
Hemorrhagic pericardial heart disease in a 30-year-old man with Gaucher's disease and pulmonary hypertension (from Gaucher cells plugging pulmonary capillaries). Chest radiographs (a) 2 months before death and (b) day of death, demonstrating marked enlargement of the cardiac silhouette. About 750 mL of blood was present in the pericardial sac. (c) Electrocardiogram recorded 1 hour before death. (d) Exterior of the heart showing diffuse fibrinous pericarditis and marked enlargement of the right atrium and ventricle. (e) Thickened parietal pericardium containing large vascular channels. (f) Thickened visceral pericardium. The cause of the hemorrhagic pericardial effusion was not determined. Periodic acid-Schiff stain, ⋇22 (e), ⋇33 (f).
Figure 12.
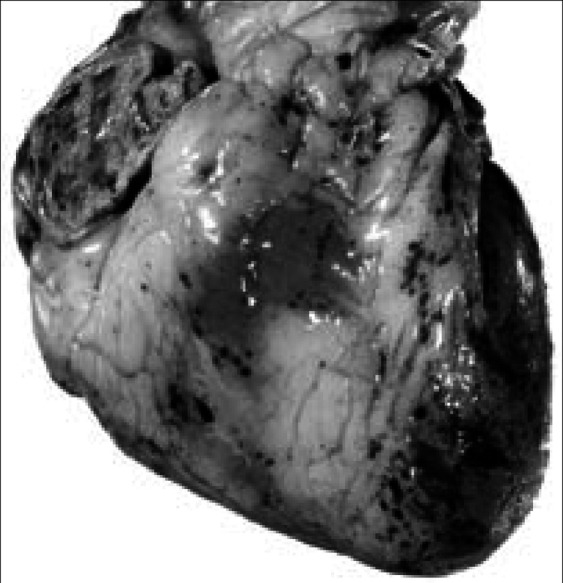
Focal epicardial hemorrhages in hypothrombocytopenia. The anterior surface of the heart shows focal hemorrhages in a 56-year-old man with acute myelogenous leukemia. The epicardial hemorrhages seen here are typical of those observed in many patients with severe hypothrombocytopenia from any cause. This man's platelet count 2 days before death was 500 per mm3.
Lymphatic and chylous effusion
Most reports concerning lymph or chyle within the pericardial space concern isolated patients because this condition is rare (38). Chylopericardium, i.e., milky effusion, most commonly results from obstruction or injury of the thoracic duct (39). At other times, its cause is not discernible but, nevertheless, hemipericardiectomy and ligation of pericardial lymphatics may result in disappearance of the chylous effusion (38). Lymphangiomatous hamartoma (cystic hygroma) of the mediastinum associated with a communication between the thoracic duct and pericardial space (40) also has caused chylopericardium. Chylous fluid may accumulate rapidly and even lead to tamponade (38, 41). Lymphopericardium, i.e., clear fluid, which is even less common than chylopericardium, usually results from pericardial lymphangiomas that may be part of generalized lymphangiectasia (42).
INFECTIVE (PURULENT) PERICARDIAL HEART DISEASE
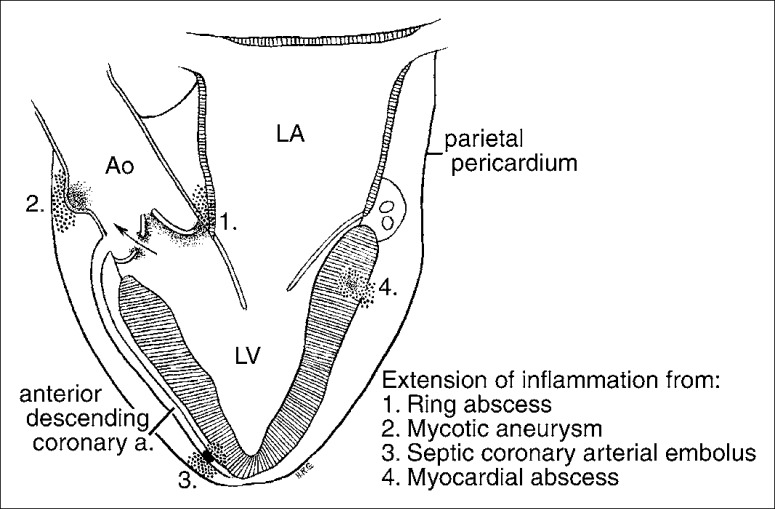
Infective pericarditis is the presence of pus, microorganisms, or both in the pericardial sac or tissues. Nontuberculous infective pericarditis is infrequent. In the preantibiotic and prethoracic surgical eras, the infection was usually spread from the lung, pleura, mediastinum, or abdomen or was a complication of a generalized septicemia. Today, the most common predisposing factors are cardiothoracic operations, immunosuppressive therapy, trauma, rupture of the esophagus into the pericardial sac secondary to neoplasm (Figure 13), and infective endocarditis with rupture of a ring abscess, septic coronary embolus, or rupture of a myocardial abscess (43) (Figure 14).
Figure 13.
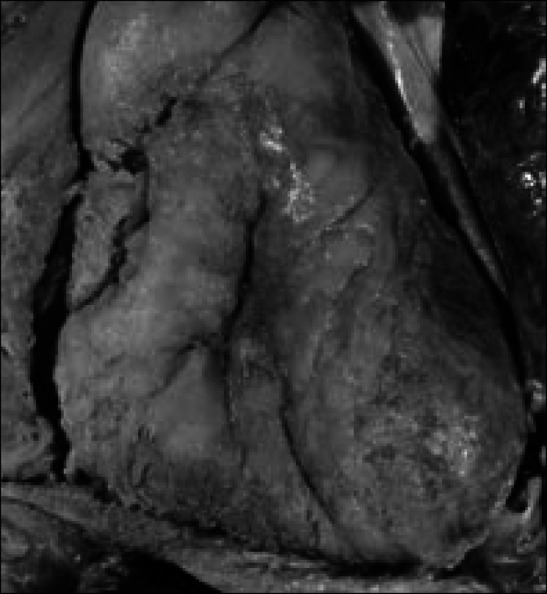
Purulent pericarditis in the exterior of the heart in a 56-year-old man with squamous cell carcinoma arising in the floor of the mouth. He developed a neoplastic abscess cavity in the left posterior mediastinum, and it opened into the esophagus, bronchus, left pleural cavity, and pericardial sac. Candida albicans was cultured from the purulent material in the pericardial sac.
Figure 14.
Schematic portrayal of the pathogenesis of pericarditis in infective endocarditis. Ao indicates aorta; LV, left ventricle; LA, left atrium.
The expected increase in frequency of purulent pericarditis with the advent of cardiothoracic operations has been offset by the widespread use of antibiotics and the decrease in bacterial infections in general. Indeed, purulent pericarditis is less frequent today than in the past (44). The occurrence of infective pericarditis as a consequence of infective endocarditis is not widely appreciated (45). In a review by Buchbinder and Roberts of 1284 patients with fatal infective endocarditis, 172 (13%) had pericarditis at necropsy. The pericarditis in this circumstance need not be purulent (45). Figure 14 shows several mechanisms of formation of pericarditis in patients with infective endocarditis.
The organisms responsible for nontuberculous infective pericarditis have changed considerably since the introduction of antibiotics, cardiothoracic surgery, and immunosuppression. In the preantibiotic era, a gram-positive bacterium was responsible in 80% and a gram-negative bacterium in 4% of cases (Table 5). These figures were from the study by Cabot of 186 necropsy patients with purulent pericarditis (28). In a later study from the same hospital (Massachusetts General) of 26 patients with purulent pericarditis, gram-positive bacteria were responsible in 42%, gram-negative bacteria in 39%, and fungi in 19% of cases (43) (Table 5). In none of Cabot's patients were fungi the causative agent. Furthermore, the types of gram-positive bacteria causing infective pericarditis have changed considerably. Streptococcus and pneumococcus were most common in the preantibiotic era and staphylococcus in the recent antibiotic era (Table 5).
Table 5.
Organisms responsible for nontuberculous infective pericardial heart disease
| Preantibiotic era Early (1896–1919)* | Early antibiotic era (1949–1959)† | Antibiotic era (1960–1974)‡ | |
| I. Gram-positive | |||
| Staphylococcus aureus | 14 | 7 | 8 |
| Staphylococcus epidermidis | 3 | 0 | 0 |
| Staphylococcus (species unspecified) | 4 | 0 | 0 |
| Streptococcus | 39 | 1 | 1 |
| Pneumococcus | 33 | 0 | 2 |
| II. Gram-negative | |||
| Escherichia coli | 3 | 1 | 0 |
| Klebsiella pneumoniae | 1 | 0 | 0 |
| Hemophilus influenzae | 0 | 0 | 2 |
| Neisseria meningitidis | 0 | 0 | 1 |
| III. Multiple bacteria | 7 | 0 | 0 |
| IV. Fungi | 0 | 0 | 5 |
| V. Unspecified or unproved | 9 | 2 | 7§ |
| VI. Negative cultures | 73 | 0 | 0 |
| Totals | 186 | 11 | 26 |
|
| |||
Purulent pericarditis with or without antibiotic treatment has a poor prognosis. It nearly always indicates a widespread infection of the mediastinum and lung. Although tamponade and constriction may occur, drainage of the pericardial pus usually is not lifesaving because the infective process is so extensive.
Although parasites can infect pericardium, all are rare. About 100 patients with amebic pericarditis have been reported, and in each spread was from a hepatic abscess (46). Before actual perforation into the pericardial space, amebic pericarditis is described classically as “anchovy-sauce” pus, but Entamoeba histolytica organisms are infrequently found in it (46). A rapid course to constriction may occur. Pericardial involvement by toxoplasmosis and echinococcosis is extremely rare, and cases of pericardial involvement by dracunculosis have been reported (47).
Fungal pericarditis has increased in frequency with the use of immunosuppressive agents. Although usually granulomatous, fungal pericarditis may be purulent. Coccidioides immitis has been found in pericardium, and clinical evidence of pericarditis occurs in about 15% of patients with pulmonary coccidioidomycosis (48). Pericarditis occurred in 2% of 470 necropsied patients with actinomycosis (49), but sulfur granules were rare. Pericardial calcific deposits may occur in patients who react to histoplasmosis but not to other fungal antigens, but acute pericarditis has not been reported in patients with histoplasmosis. Pericardial candidiasis has been observed in patients with leukemia receiving immunosuppressive drugs (Figure 15).
Figure 15.
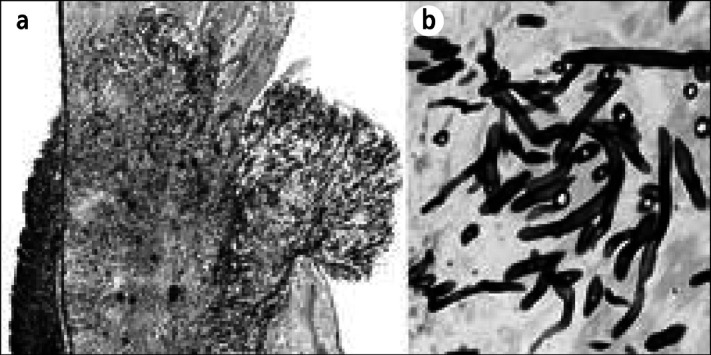
Candida tropicalis pancarditis in a 7-year-old girl with acute lymphocytic leukemia and a 21-month illness. The child never had signs or symptoms of cardiac disease but died of disseminated candidiasis. (a) Section of the right atrial wall with fungi extending from the epicardium through the myocardium into the endocardium. (b) Close-up of Candida organisms. Methenamine silver stain, ⋇12 (a), ⋇520 (b).
Pericarditis has been seen in association with several viral diseases, including infectious mononucleosis, atypical pneumonia, mumps, measles, smallpox, and influenza. Although viral titers may rise, morphologic confirmation of a viral etiology is lacking.
Pericarditis probably is most frequent from Coxsackie viruses (50). Biopsy of parietal pericardium, however, has not revealed intracellular virions, although viral antigens may be present. Indeed, almost no morphologic information is present on viral pericarditis in humans.
FIBROUS PERICARDIAL HEART DISEASE
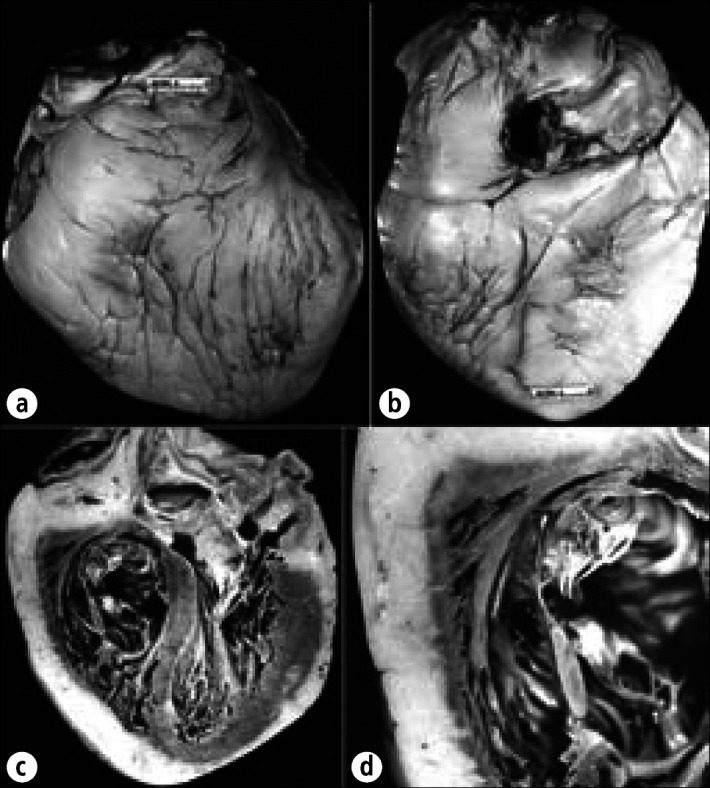
Fibrous pericardial heart disease is thickening of the pericardia or the presence of adhesions between the visceral and parietal pericardia or both. Either the thickening or the adhesions may be focal or diffuse. The thickening may involve both visceral and parietal pericardia or only one of them. The collagen plaque, for example, is a form of fibrous pericardial heart disease with focal thickening, usually limited to the epicardium. Pericardial thickening may occur in the absence of pericardial adhesions, adhesions may occur in the absence of pericardial thickening, or the two may occur together. When pericardial adhesions are diffuse, the pericardial space is obliterated, and this circumstance has been called “obliterative pericarditis.”
“Obliterative pericarditis” may occur in the absence of thickening of the parietal pericardium. Obviously, since its space no longer is present, pericardial effusion, by definition, cannot be present in the patient with “obliterative pericarditis.” A pericardial effusion may be present in patients with focal pericardial adhesions or in patients with diffuse pericardial thickening with either no adhesions or only focal adhesions.
There are many causes of pericardial thickening and pericardial adhesions. The most common are listed in Table 6.
Table 6.
Etiology of fibrous (adhesive) pericardial-heart disease
|
Healing of hemopericardium (of any cause)
As mentioned earlier, in and of itself the presence of blood within the pericardial sac leaves no residua. However, the presence of blood superimposed on injured pericardial surfaces (mesothelial layer “rubbed off,” for example) generally leads to adhesions between the pericardia with or without thickening of the pericardia. After cardiac operations, the pericardia adhere to each other by fibrous adhesions. In this circumstance, it is presumed that manipulation of the pericardia at operation damaged the pericardial surfaces. The healing of these damaged surfaces by organization of the blood products presumably accounts for the development of the pericardial adhesions. The adherence of the 2 layers therefore represents an “overreaction” of the healing process. After a cardiotomy, the parietal pericardium is not thickened. Blunt external trauma from, for example, resuscitation may produce a similar reaction. Nonpenetrating blunt trauma may also simply “bruise” the pericardial surfaces, producing a focal fibrinous reaction that may resolve or go on to fibrous adhesions if blood exudes into the pericardial space at the site of the contusion.
Irradiation
Radiation always produces injury, and repair of that injury may cause fibrous proliferation (18). Although the endocardium, myocardium, and pericardium may be injured by high-dose irradiation, the portion of the heart most frequently damaged is the pericardium. Experimentally, mediastinal irradiation to rabbits (in a dosage equivalent to 4000 cGy to the mediastinum of humans) initially produces a polymorphonuclear reaction in the pericardia followed in 1 or 2 days by a mononuclear reaction (51). Damage to the endothelial cells of both lymphatic and blood capillaries leads to luminal obstruction of these channels, which in turn leads to pericardial effusion. The pericardia also are thickened by fibrous tissue, but its mechanism of development is not certain.
Mediastinal irradiation in high doses in humans, with resulting pericardial injury, occurs primarily in patients with Hodgkin's disease (18, 52, 53), because it is the most common mediastinal tumor irradiated in sufficient doses to cause cardiac disease (Figure 16). Pericardial injury probably occurs in most patients receiving ≥4000 cGy to the mediastinum. Fibrous thickening of the pericardia combined with pericardial effusion (usually serous) has been the most frequent consequence of this irradiation. Clinical evidence of cardiac dysfunction resulting from pericardial disease occurs in about 5% of patients with Hodgkin's disease treated with high-dose mediastinal irradiation (31). Cardiac tamponade may occur, usually a result of combined pericardial effusion and fibrous thickening of the parietal pericardium (54). The coronary arteries lying in the subepicardial adipose tissue also may be damaged by irradiation, with resulting coronary arterial luminal narrowing and symptomatic or fatal ischemic heart disease (52, 53).
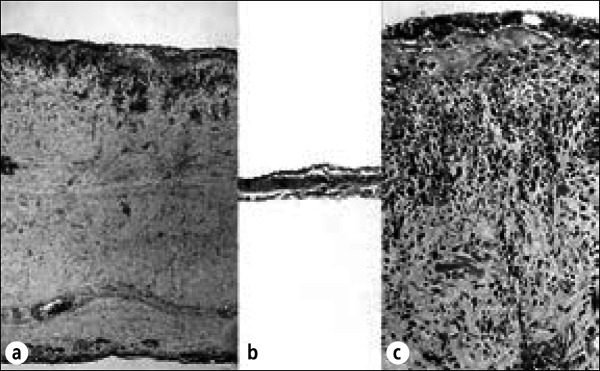
Figure 16.
Radiation pericarditis. Stage IIIB Hodgkin's disease was diagnosed in this 54-year-old man, and he then received radiation therapy to the mediastinum. Eighteen months later, he developed recurrent pericardial effusion and signs of pericardial constriction, including elevated venous pressure and congestive cardiac failure. He underwent pericardiectomy, and at operation the pericardial sac contained about 150 mL of fluid. The parietal and visceral pericardia were focally adherent by fibrinous adhesions. After excision of the anterior portion of parietal pericardium, which was up to 1.0 cm in thickness, the central venous pressure dropped from 15 to 8 cm of water. The patient had an uneventful recovery and no further symptoms of myocardial constriction. (a) Section of parietal pericardium showing diffuse fibrous thickening with fibroblast proliferation. Plasma cells and lymphocytes are present. (b) Section of normal pericardium for comparison. (c) Higher-power view of parietal pericardium in this patient showing fibroblasts, lymphocytes, and plasma cells. These changes are characteristic of radiation pericarditis. Hematoxylin-eosin stain, ⋇27 (a and b), ⋇160 (c).
A diagnostic dilemma occurs in patients who have mediastinal irradiation for a tumor and then develop evidence of pericardial effusion. Is the pericardial effusion radiation induced or is it due to contiguous spread of the neoplasm to the pericardium? It is of some value to know that pericardial effusion of sufficient magnitude to be detected by echocardiogram commonly is found after mediastinal irradiation, that it may lead to pericardial tamponade, and that the peak evidence of the pericardial reactions appears from 2 to 8 months after radiation therapy, although a wide range of time from irradiation to onset of pericardial symptoms has been reported.
Association with another disease
Although fibrinous pericarditis is the most common type of pericardial involvement in patients with chronic renal disease, fibrous thickening of the pericardia with or without associated fibrous adhesions or pericardial effusion also may occur in patients not treated with dialysis (Figure 10). Fibrous pericardial heart disease, however, appears to be more frequent in patients on chronic dialysis than in patients with chronic renal disease not treated in this fashion.
At necropsy, nearly 50% of patients with rheumatoid arthritis have fibrous obliterative pericardial heart disease and, rarely, calcific or cholesterol pericardial disease (55). Usually, no functional consequence results from the obliterative pericardial heart disease.
Among 36 patients with systemic lupus erythematosus (SLE) studied at necropsy (7, 8), 19 had pericardial heart disease: fibrinous in 4, purulent in 2, and fibrous in 13. Before corticosteroids were introduced, the fibrinous type was the most common seen in patients with SLE. Among patients with SLE treated with corticosteroids, the fibrous type is the most common. Among 19 SLE patients with pericardial heart disease at necropsy, none had evidence of myocardial dysfunction as a consequence of the pericardial involvement. Eleven of the 19 had pericardial friction rubs. In patients with SLE, pericardial heart disease serves as an excellent marker of the presence of associated endocarditis: endocarditis was present at necropsy in 18 of the 19 SLE patients with and in none of the 17 SLE patients without pericardial disease.
Although uncommon clinically, pericardial heart disease is common at necropsy in patients with scleroderma (56). The fibrous obliterative type is the most common form in scleroderma. The fibrinous type, seen less commonly, may result from associated renal failure rather than scleroderma per se (56). Among the scleroderma patients I studied at necropsy, a third had pericardial involvement, fibrous in each.
Infectious agent
See the section on infective (purulent) pericardial heart disease.
Idiopathy
Fibrous pericardial disease of unknown etiology is the most common “cause” today of “chronic constrictive pericarditis” (Table 7) (57). In a report of 137 patients undergoing pericardiectomy because of “constrictive pericarditis,” no cause was found on histologic study of the excised pericardia in 106 (77%) (58).
Table 7.
Etiology of constrictive pericardial heart disease in the USA treated by pericardiectomy
| Type of pericardial heart disease | Totals* |
| Idiopathic | 213 (68%) |
| Tuberculous | 63 (20%) |
| Traumatic | 6 (2%) |
| Rheumatic | 2 (1%) |
| Neoplastic | 3 (1%) |
| Pyogenic | 4 (1%) |
| Irradiation | 4 (1%) |
| Postcardiotomy | 2 (1%) |
| Rheumatoid arthritis | 2 (1%) |
| Viral | 1 (0%) |
| Acute nonspecific | 14 (4%) |
| Total | 314 (100%) |
Includes patients seen from 1930 to 1969 and included in 6 different studies.
An interesting entity called “occult constrictive pericardial disease” has been described. This syndrome consists of nonspecific symptoms of fatigue, dyspnea, and chest pain in patients, most of whom had a history of pericarditis in the past. They had abnormal hemodynamics at cardiac catheterization until they were challenged by an infusion of 1 L of warm saline. They then developed hemodynamic findings characteristic of constrictive pericarditis. These findings were absent in control patients with heart disease similarly challenged with volume expansion. Pericardiectomy relieved the symptoms and altered the hemodynamic findings in response to saline infusion in all patients. The pericardium was abnormal in all subjects.
Administration of procainamide, methysergide, or practolol has been well documented to result in constrictive pericarditis. One wonders whether a significant percentage of patients who have “idiopathic constrictive pericarditis” may have developed it as a complication of drug therapy.
NEOPLASTIC PERICARDIAL HEART DISEASE
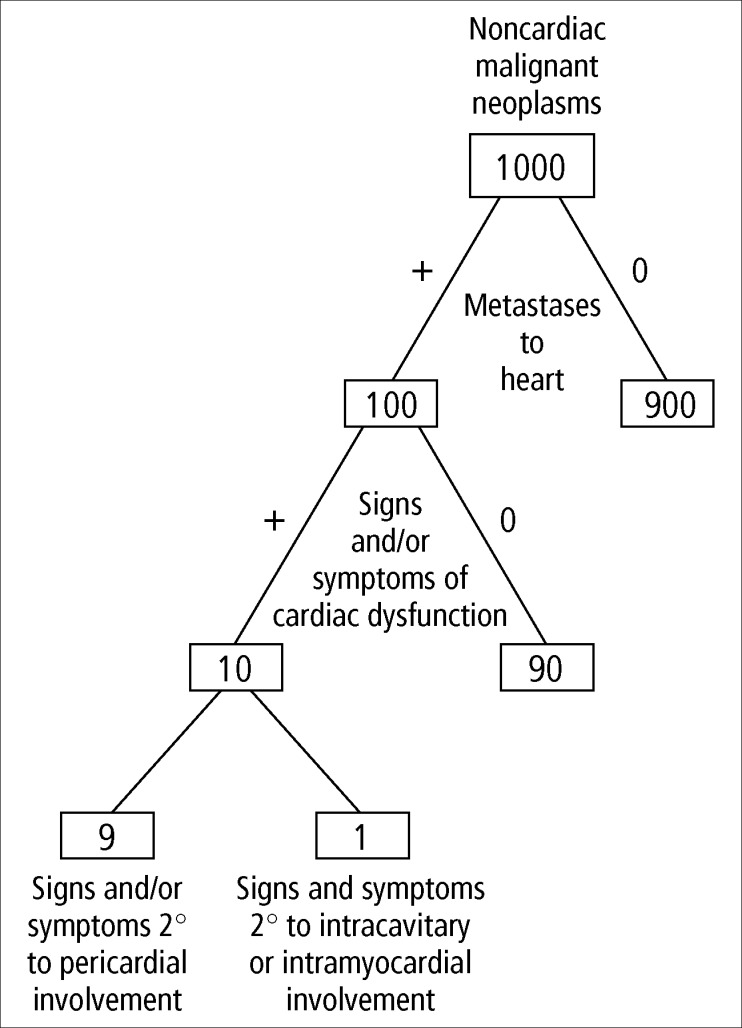
As seen in Figure 17, the heart is involved in approximately 10% of patients with malignant neoplasms, and 85% of the patients with cardiac involvement have neoplastic involvement of the pericardium (59). Furthermore, only about 10% of patients with cardiac involvement have clinical evidence of cardiac disease, and in 90% of those the clinical dysfunction is the result of pericardial involvement, usually constriction by either pericardial effusion or neoplastic thickening of pericardia or both. The most common neoplasms in absolute numbers with cardiac metastases are lung (in men) and breast (in women), followed by leukemia and lymphoma (Table 8). Nearly any tumor, however, may metastasize to the heart. Among patients with carcinoma of the breast or lung, about 10% have neoplastic involvement of the heart (59). Because spread of these 2 tumors to the heart appears to be by direct extension, the pericardia virtually always are involved.
Figure 17.
The frequency of pericardial involvement in malignant neoplasms.
Table 8.
Etiology of neoplastic pericardial heart disease
|
|
|
Among specific malignant neoplasms, the ones with the highest percentage of metastases to the heart are melanoma (70%) (60), leukemia (37%) (61), and lymphoma (24%) (62). As with the tumors that spread by direct extension, melanoma, leukemia, and lymphoma invade the pericardia in 85% of the patients with cardiac metastases, and spread of these neoplasms appears to be by the hematogenous routes.
Although pericardial effusions, often bloody, are frequent in patients with cardiac metastases, pericardial effusions also are common in patients with malignant neoplasms without cardiac metastases (59, 62). Pericardial effusions (<50 mL) were recorded in 17% of 48 patients with cardiac lymphoma and in 14% of 148 patients with lymphoma but without cardiac metastases (62). In both groups, the effusions were serous and probably the result of hypoalbuminemia (>3.5 gm/dL), which was present in 95% of the 196 necropsy patients with lymphoma (62).
Pericardial heart disease may occur in patients with malignant neoplasms who do not have involvement of the heart (including pericardia) by neoplasm. Varying types of the disease were present in 21% of 48 patients with cardiac lymphoma and in 7% of 148 patients with lymphoma but without metastases to the heart (62).
Symptoms and signs in patients with cardiac metastases often are attributed to cardiac involvement by the neoplasm, but this may not be justified. Dyspnea, for example, was present in 27% of 48 patients with and in 52% of 148 patients without cardiac lymphoma (62). Likewise, precordial or substernal chest pain (6%), precordial murmurs (35%), and ventricular gallops (5%) were present in the same frequencies among patients with lymphoma irrespective of the presence or absence of cardiac metastases (62). Surprisingly, electrocardiographic abnormalities were present in identical frequency among patients with lymphoma with and without cardiac tumor deposits: 62% in each of the 2 groups (62).
Primary pericardial neoplasms are extremely rare, and about half of them are benign (59). The most common is teratoma (63). Although usually histologically benign, teratoma can produce fatal compression of a cardiac chamber. Hemangioma, leiomyofibroma, lipoma, and fibroma are other “benign” pericardial neoplasms. The hemangioma may rupture to cause hemorrhagic pericardial effusion with or without tamponade.
Among the primary malignant pericardial neoplasms, mesothelioma is by far the most frequent. It may totally encase the heart, resulting in fatal myocardial constriction.
GRANULOMATOUS PERICARDIAL HEART DISEASE
Infectious agent
Among the causes of granulomatous pericarditis (Table 9), tuberculosis is by far the most important. Pericardial involvement by tuberculosis was described initially by Rokitansky in 1852, and it was he who noted the “association with, and dependence upon, an earlier tuberculous lesion” (64). Since that time there has been much debate over whether “primary” tuberculous pericarditis is a separate clinicopathologic entity or whether it is the recognizable result of an earlier resolved or inactive primary lesion.
Table 9.
Etiology of granulomatous pericardial heart disease
|
The frequency of tuberculous pericarditis has diminished in recent years, presumably paralleling the reduction in incidence of pulmonary tuberculosis. Osler observed tuberculosis in 215 (22%) of 1000 necropsies, and 7 (3%) of the 215 had pericardial involvement (65). Subsequent studies (66) have shown pericarditis to be present at necropsy in 8% of patients with pulmonary tuberculosis.
The pathogenesis of pericardial involvement by tuberculosis is not clear. Pericardial disease probably results from early dissemination following a primary infection. The infection appears to reach the pericardium either from the blood (miliary) or, more commonly, by retrograde lymphatic spread from infected mediastinal glands. The pericardial infection rarely arises from direct spread from the lung or pleura.
Four stages of tuberculous pericarditis have been described (67). The fibrinous stage is characterized by diffuse deposits of fibrin associated with a granulomatous reaction. This stage is observed rarely, and there is some debate as to whether it can resolve or whether it always goes on to the second stage, which is characterized by effusion into the pericardial sac. The effusions, which may be massive (<2000 mL), usually are serous or serosanguineous but may be bloody or turbid. Many lymphocytes may be present in the effusion, but tuberculous organisms are uncommonly observed morphologically in the fluid or cultured from it.
By stage 3, the parietal pericardium is considerably thickened by both fibrous tissue and granulomas, and most of the fluid has resolved. If the parietal pericardium becomes markedly thickened, myocardial constriction may occur, and this is considered stage 4. By this time, the pericardial space may be completely obliterated by diffuse fibrous adhesions. Eventually the granulomas apparently can be replaced entirely by fibrous tissue and, if observed morphologically at this point, the pericardial disease would have to be called idiopathic rather than granulomatous (tuberculosis). Calcific deposits with or without bone formation may occur in the markedly thickened pericardia. Progression from stage 3 to stage 4 may occur despite antituberculous therapy.
Tuberculous pericarditis by no means always progresses to the constrictive fibrous stage (Figure 18). Heimann and Binder (68) described 31 patients with granulomatous pericarditis; none had constriction and all had tuberculous hilar lymph nodes. Among patients with “chronic” constrictive pericarditis, however, tuberculosis remains an important cause, accounting for nearly 10% of the cases in the USA (Table 7). In some areas of the world, a tuberculous cause of constrictive pericarditis is more common than a nontuberculous cause. Among 61 patients with constrictive pericarditis undergoing pericardiectomy in India, the etiology was tuberculous in 37 (61%) and nontuberculous in 24 (39%) (69).
Figure 18.
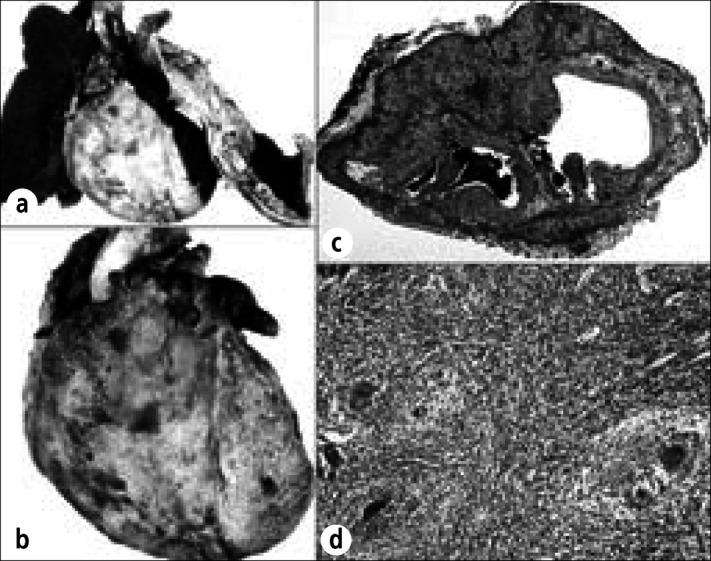
Tuberculous pericarditis in a 45-year-old dyspneic man whose chest radiograph showed complete loss of volume of the left lung and massive left pleural effusion. Congestive cardiac failure and respiratory insufficiency led to death. At necropsy, the entire left lung was collapsed, and the left hemithorax was filled with pus. The left pleura was markedly thickened and focally calcified. No granulomas or foci of caseation necrosis were observed in either lung or in the hilar lymph nodes. (a) Heart and lungs showing loss of left lung, cardiomegaly (600 gm), and diffuse pericarditis. (b) Closer view of the exterior of the heart showing fibrinous exudate covering the entire myocardial surface. (c) Section of the left atrial appendage showing complete involvement of the epicardium by caseating granulomas and giant cells. Fibrinous pericardial exudate also covers most of its surface. (d) High-power view of granulomas covering the left atrial appendage. No acid-fast organisms were found. Hematoxylin-eosin stain, ⋇5 (c); periodic acid-Schiff stain, ⋇80 (d).
Fungi and parasites are rare causes of granulomatous pericarditis.
Cholesterol
So-called cholesterol pericarditis always produces a granulomatous reaction; it is discussed separately below.
Iatrogenesis
The sprinkling of talc in the pericardial space, a procedure introduced in the early 1940s (70, 71) as a form of treatment for coronary heart disease, produces an extensive granulomatous reaction resulting in obliteration of the pericardial space (72) (Figure 19). A similar type of granulomatous reaction occurs in the lungs of persons who intravenously inject drugs intended for oral use because virtually every tablet contains talc (73). A foreign-body type of granulomatous reaction results from deposition of starch granules within the pericardial space, a minor complication of cardiac operations (74).
Figure 19.
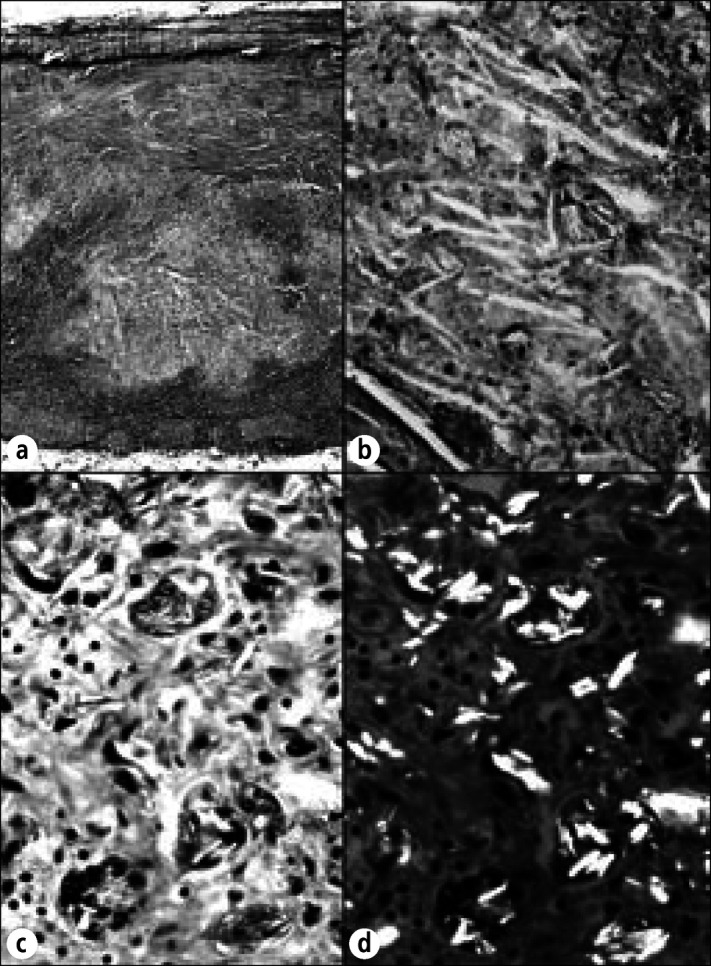
Talc-induced granulomatous pericarditis in a 92-year-old woman who was stated to have had angina pectoris beginning at age 63. At age 67, she underwent talcum-powder sprinkling on her pericardium (Beck or Thompson procedure). Immediately preoperatively, she was in congestive heart failure with atrial fibrillation and a slow ventricular response. At necropsy, an old healed anterolateral apical infarct was present, but no myocardial necrosis was observed. The striking finding, however, was a diffuse fibrous and granulomatous pericarditis secondary to talc with complete obliteration of the pericardial space. (a) Section of parietal pericardium showing large foci of acellular debris containing multiple cholesterol clefts. (b) Higher-power view showing cholesterol clefts and talc. (c) Multinucleated foreign-body giant cells with intracellular talc granules. A few mononuclear cells are present. (d) Same field as (c) with polarization showing highly refractile talc particles both intracellularly and extracellularly. Hematoxylin-eosin stain, ⋇23 (a), ⋇250 (b), ⋇480 (c, d).
Sarcoidosis
Not generally recognized as a cause of pericarditis because clinical evidence of pericardial involvement is rare and focal, noncaseating pericardial granulomas are observed at necropsy in patients with sarcoidosis (75). Among 35 patients with cardiac sarcoidosis studied at necropsy by Roberts and associates (75), 12 (34%) had granulomas involving epicardium (Figure 20). None, however, had clinical signs. In addition, another patient had obliterative fibrous pericardial heart disease causing no clinical signs. Only 3 patients had excessive pericardial fluid measured at necropsy, but it was >300 mL in each.
Figure 20.
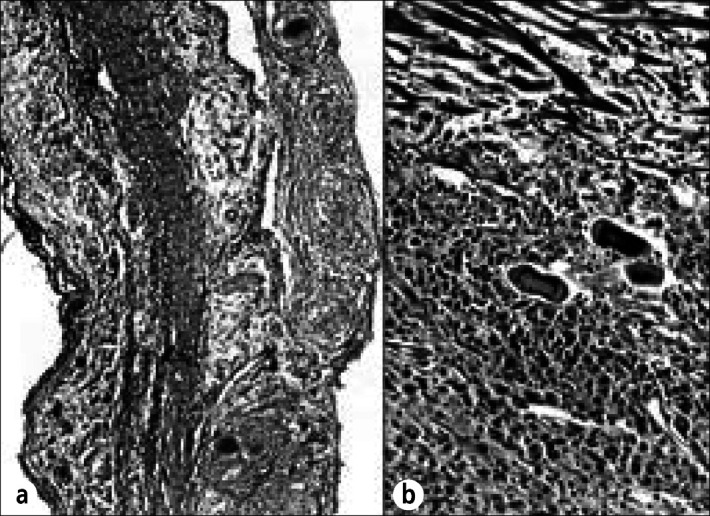
Sarcoidosis of the pericardium in a 47-year-old woman who was well until 16 months before death, when exertional dyspnea appeared. Thereafter, there was clinical evidence of recurring pleural and pericardial effusions. She died of progressive respiratory insufficiency. At necropsy, both lungs were extensively infiltrated by noncaseating granulomas devoid of microorganisms. Grossly, the pericardium and myocardium contained no focal lesions except in the left ventricular papillary muscles. On histologic examination, however, noncaseating granulomas were found in the (a) parietal pericardium and (b) visceral pericardium. In addition to the noncaseating granulomas in the papillary muscle, similar microscopic-sized granulomas were observed in the walls of all 4 cardiac chambers. Hematoxylin-eosin stain, ⋇80 (a), ⋇220 (b).
CALCIFIC PERICARDIAL HEART DISEASE
The occurrence of calcific deposits in pericardia constitutes calcific pericardial heart disease. The calcific deposits may vary in size from microscopic, i.e., visible only by histologic examination, to massive, i.e., encircling all or most of the heart and readily visible by radiographic examination. Calcific deposits appear to represent the end stage of organization of a pericardial process and in themselves are not indicative of any specific etiology. Histologic examination of calcified pericardia rarely provides specific diagnoses. Conditions predisposing to calcific pericardial heart disease are listed in Table 10. The etiology is based on historical data or an associated abnormality rather than on specific pericardial alteration. There is strong suggestive evidence that large calcific pericardial deposits represent “burnt out” pericardial tuberculosis.
Table 10.
Etiology of calcific pericardial heart disease
|
At times, huge calcific deposits may be present in the pericardia without evidence of myocardial constriction or other myocardial dysfunction (76) (Figure 21). For myocardial constriction to occur, the calcific deposits must encircle both ventricles (Figure 22). Localized bands of calcium, however, may cause cardiac dysfunction. The occurrence of a large band of calcium transversing the right ventricular conus has led to right ventricular outflow obstruction. An anular band of calcium in the right or left atrioventricular sulcus may delay or inhibit ventricular filling and consequently simulate tricuspid or mitral stenosis.
Figure 21.
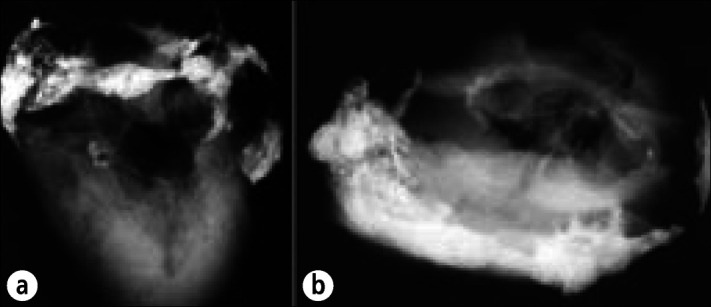
Calcific pericardial heart disease in a 78-year-old man, an alcoholic with cirrhosis, esophageal varices, and chronic pancreatitis, who died of a gastrointestinal hemorrhage. No evidence of cardiac dysfunction was ever present clinically. At necropsy the pericardial space was obliterated by fibrous adhesions. The adherence of the parietal to the visceral pericardium could not be separated over the right atrioventricular sulcus. The cardiac chambers, valves, and coronary arteries were normal. The right atrioventricular sulcus was extensively infiltrated by calcific deposits. Radiographs of the heart specimen at necropsy: (a) anteroposterior view; (b) cephalad caudal view. Calcific deposits also are present in the mitral anulus.
Figure 22.
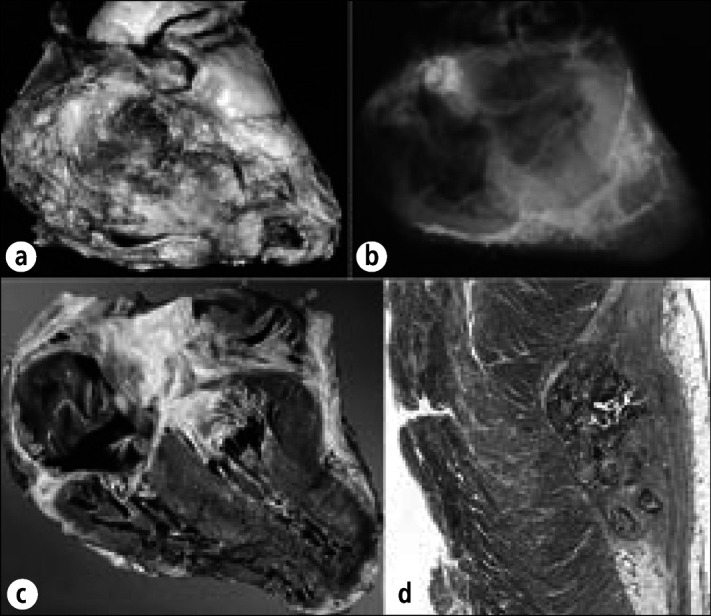
Calcific constrictive pericardial heart disease in a 68-year-old man who had had pericardiectomies for relief from constriction 15 and 7 years before death. Terminally, evidence of myocardial constriction reappeared. Chylous ascites secondary to obstruction of the thoracic duct by carcinoid tumor also were present. At necropsy, the heart weighed 530 gm. Diffuse obliterative constrictive pericarditis was present, and the remaining parietal pericardium was bound down to the epicardium by fibrous tissue. The inferior and superior venae cavae were enlarged, and the right atrium and pulmonary trunk were dilated. Yellow intimal streaks were seen in the pulmonary arteries, suggesting pulmonary arterial hypertension. Histologic sections showed fibrous thickening with calcific deposits of the pericardia with occasional collections of lymphocytes, plasma cells, and histiocytes, but no granulomas. The intrapulmonary arteries had thickened walls. No granulomas were found in the lungs. The cause of the pericardial constriction was never determined. (a) Exterior of the heart. (b) Radiograph of the heart showing residual calcific deposits over the ventricles. (c) Longitudinal section of the heart showing right atrial dilatation and right ventricular hypertrophy. (d) Section of left ventricle compressed by overlying calcific deposit. Hematoxylin-eosin stain, ⋇3.
CHOLESTEROL PERICARDIAL HEART DISEASE
Clinically, cholesterol pericardial heart disease most often is characterized by slowly developing, nonconstricting large effusions (77). Although the pericardial fluid may be turbid or clear, cholesterol crystals give the effusion a “gold paint” appearance. The parietal and visceral pericardia may become markedly thickened; histologic examination shows giant cells containing cholesterol clefts, mononuclear cells including foam cells, and fibrous tissue (Figure 23).
Figure 23.
Cholesterol pericardial heart disease in a 34-year-old woman with hypothyroidism. She was short and stocky and had enlarged earlobes, bilateral hypoplastic fourth toes, receding hairline, small tongue, and no menstruation. She had orthopnea, pedal edema, and hypoactive deep tendon reflexes. Chest radiograph showed a globular-shaped cardiac silhouette and clear lung fields, and angiogram showed an 18-mm-thick pericardial effusion. Despite diuresis, the pedal edema persisted. Pleuritic-type anterior chest pain appeared, and a 15-mm paradoxic pulse pressure was measured. Pericardiocentesis produced 500 mL of cloudy golden-colored fluid. The polyethylene catheter was left in place and over the next 5 days, 2 L of fluid was removed. Fluid cholesterol was 165 mg/dL; serum cholesterol was 355 mg/dL. The patient improved after pericardiocentesis but became anuric following aortography and died. (a) Exterior surface of the heart and pericardium showing thickening of the parietal pericardium and focal deposits of cholesterol (yellow) over the right ventricle. (b) View of thickened parietal pericardium over the right atrial appendage and an epicardial cholesterol deposit. (c) Foam cells in the cholesterol deposit shown in (b). (d) Cholesterol granuloma in the pericardium showing cholesterol clefts and a foreign body reaction. (e) Frozen section of epicardial deposits stained for lipid. The foam cells stain strongly for lipid. Hematoxylin-eosin stain, ⋇400 (c), ⋇50 (d); oil-red O stain, ⋇400 (e).
Cholesterol pericardial heart disease may be seen in many different conditions (Table 11) but is most common in patients with hypothyroidism, rheumatoid arthritis, and tuberculosis (77).
Table 11.
Etiology of cholesterol pericardial heart disease
|
The cause of the increased cholesterol content of the pericardial fluid in these patients is not clear, but several theories have been presented: 1) that necrosis of superficial cells of the pericardia liberates intracellular cholesterol, 2) that lysis of erythrocytes following hemopericardium yields the cholesterol, and 3) that pericardial inflammation decreases lymphatic drainage of pericardia, causing decreased reabsorption of cholesterol, with ultimate precipitation and crystallization (77). The cholesterol crystals then incite a vigorous cellular reaction, enhancing fluid production and pericardial thickening. Chronic exudation and diminished reabsorption of cholesterol might increase its concentration and allow crystallization. The total lipid content of the pericardial fluid, however, is approximately the same as that of serum.
In patients with hypothyroidism, the primary effusion may contain sufficient cholesterol to incite a local pericarditis, which then further increases the cholesterol concentration as less is reabsorbed. Large effusions with resulting high levels of cholesterol are produced. In these patients, the pericarditis appears to be a secondary phenomenon whereas cholesterol pericardial heart disease in euthyroid patients must be associated with an actual pericardial inflammation for the cholesterol to accumulate. Experimental studies have shown that small amounts of blood in the presence of pericardial damage from any cause will produce dense adhesions; this phenomenon probably accounts for cases of constriction in cholesterol pericardial disease.
CONGENITAL ANOMALIES OF THE PERICARDIUM
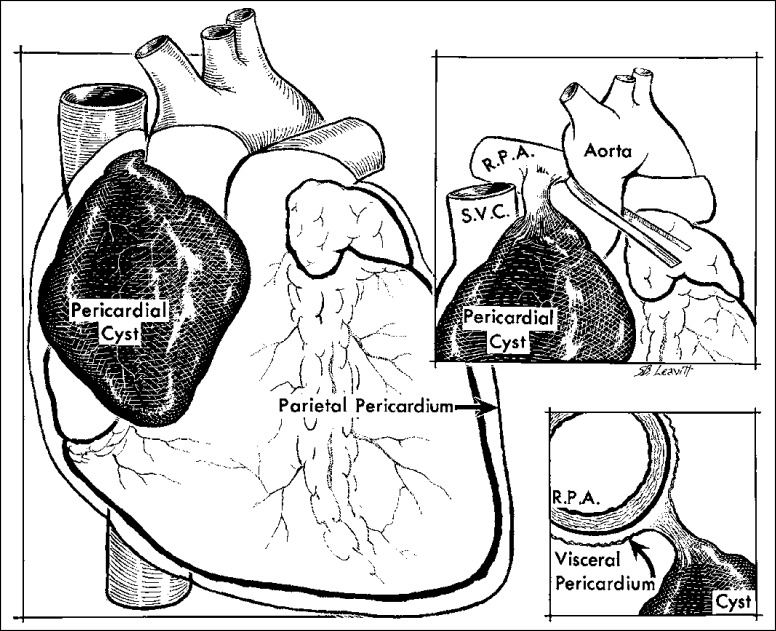
Congenital anomalies include cysts and absence of all or portions of parietal pericardia. These cases are rare. Cysts can be true or false. The true ones are located within the pericardial sac but have no communication with it; the false ones, or diverticulae, are protrusions of parietal pericardium and consequently have direct communication with it. Of 72 cysts reported by Wychulis and associates (78), 88% were true and 12% were false. The cysts are thin-walled and translucent, are lined by endothelial cells, contain watery yellow fluid, usually are attached by a thick fibrous pedicle, and most commonly are located in the right cardiophrenic angle (Figure 24). In one series, the cysts ranged in size from 2 to 16 cm in largest diameter. Although they can be multilobulated, 99% are unilobular. The fluid within the cysts is similar to normal pericardial fluid. The cysts produce signs or symptoms of cardiac dysfunction in 25% of the patients and no evidence of dysfunction in the other 75%. The larger the size, the more likely symptoms will result. The mechanism of formation of pericardial cysts is not clear. Failure of the pericardial mesenchymal lacunae to unite with the pericardial coelom has been suggested (79).
Figure 24.
Pericardial cyst, an incidental necropsy finding in a 75-year-old woman. The cyst, which contained serous fluid, overlay the right atrium and arose from a pedicle attached to the right main pulmonary artery (RPA). SVC indicates superior vena cava.
Absence of, or defects in, parietal pericardium may be classified into 3 major types (80): 1) heart and left lung in a common cavity (60%), 2) defect or foramen in parietal pericardium providing communication between pericardial sac and pleural sac (20%) (nearly always the left one) and 3) totally absent or rudimentary parietal pericardium (20%). These defects may be associated with anomalies of the heart (30%), lungs, pleura, peritoneum, or kidney and are more common in males. Cardiac enlargement occurs in about half of the patients, and commonly the heart is displaced to the left and abnormally mobile. Total absence of parietal pericardium usually produces no functional disturbance. Partial absence, however, may result in herniation of the entire heart or a part of the left atrium through the defect or allow direct extension of a lung infection to the pericardium via the defect.
Complete absence of the parietal left pericardium over the left side of the heart presents a clinical syndrome that is usually diagnostic. It consists of a physical finding of left ventricular enlargement and hypertrophy (apical impulse forceful and displaced to the left), a systolic ejection murmur at the base, an electrocardiogram that shows right axis deviation (as in the normal individual assuming the left lateral decubitus position), and a chest radiograph that shows levo position of the heart. This condition may be confused with a cardiomyopathy. Partial absence of the partial pericardium has been confused with a diagnosis of congenital heart disease with the prominent left atrial appendage misinterpreted as enlargement of the pulmonary trunk.
DIFFERENTIAL DIAGNOSIS
Constrictive pericardial heart disease—from pericardial effusion, thickened pericardia, or both—may be confused with a number of conditions (Table 12). An occasional patient with idiopathic dilated cardiomyopathy or extensive cardiac amyloidosis has been subjected in the past to thoracotomy because of suspected pericardial constriction. Echocardiography, of course, makes it easier to differentiate pericardial heart disease from these other conditions.
Table 12.
Differential diagnosis of constrictive pericardial heart disease
|
SUMMARY
An ideal classification of pericardial heart disease would take into account clinical, etiologic, and morphologic features of this condition, but a single classification combining these 3 components is lacking. Pericardial heart disease is relatively uncommon clinically. When present at necropsy, it usually had not been recognized during life. The term “pericarditis” is inaccurate because most pericardial diseases are noninflammatory. Morphologically, chronic pericardial heart disease symptoms are present; however, few patients develop evidence of cardiac dysfunction (constriction). When pericardial “constriction” occurs, it is the result of increased pericardial fluid or increased pericardial tissue or both. Increased fluid is treated by drainage; increased tissue is treated by excision. In most patients with chronic constrictive “pericarditis,” the etiology is not apparent even after histologic examination of pericardia.
References
- 1.Osler W. Designed for the Use of Practitioners and Students of Medicine. New York: Appleton Co; 1892. The Principles and Practice of Medicine; p. 1079. [Google Scholar]
- 2.Reeves RL. Cause of acute pericarditis. Am J Med Sci. 1953;225:34. [PubMed] [Google Scholar]
- 3.Griffith GC, Wallace L. The etiology of pericarditis. Am Heart J. 1949;37:636. [Google Scholar]
- 4.Ishihara T, Ferrans VJ, Jones M, Boyce SW, Kawanami O, Roberts WC. Histologic and ultrastructural features of normal parietal pericardium. Am J Cardiol. 1980;46:744–753. doi: 10.1016/0002-9149(80)90424-5. [DOI] [PubMed] [Google Scholar]
- 5.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76:414–418. doi: 10.1016/s0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 6.Shirani J, Roberts WC. Clinical, electrocardiographic and morphologic features of massive fatty deposits (“lipomatous hypertrophy”) in the atrial septum. J Am Coll Cardiol. 1993;22:226–238. doi: 10.1016/0735-1097(93)90839-s. [DOI] [PubMed] [Google Scholar]
- 7.Bulkley BH, Roberts WC. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy. A study of 36 necropsy patients. Am J Med. 1975;58:243–264. doi: 10.1016/0002-9343(75)90575-6. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WC, High ST. The heart in systemic lupus erythematosus. Curr Probl Cardiol. 1999;24:1–56. doi: 10.1016/s0146-2806(99)90019-1. [DOI] [PubMed] [Google Scholar]
- 9.Ferrons VJ, Roberts WC. Pathology of pericardial effusion. In: Reddy PS, Leon DF, Shaver JA, editors. Pericardial Disease. New York: Raven Press; 1982. pp. 77–92. [Google Scholar]
- 10.Just H, Mattingly TW. Interatrial septal defect and pericardial disease. Coincidence or causal relationship? Am Heart J. 1968;76:157–167. doi: 10.1016/0002-8703(68)90190-7. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenhaft JL, Taber RE. Hemopericardium and constrictive pericarditis. J Thorac Surg. 1952;24:355. [PubMed] [Google Scholar]
- 12.Cliff WJ, Grobéty J, Ryan GB. Postoperative pericardial adhesions. The role of mild serosal injury and spilled blood. J Thorac Cardiovasc Surg. 1973;65:744–750. [PubMed] [Google Scholar]
- 13.Swan WGA. Acute non-specific pericarditis. Br Heart J. 1960;22:651–659. doi: 10.1136/hrt.22.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts WC, Morrow AG. Pseudoaneurysm of the left ventricle. An unusual sequel of myocardial infarction and rupture of the heart. Am J Med. 1967;43:639–644. doi: 10.1016/0002-9343(67)90187-8. [DOI] [PubMed] [Google Scholar]
- 15.Gobel FL, Visudh-Arom K, Edwards JE. Pseudoaneurysm of the left ventricle leading to recurrent pericardial hemorrhage. Chest. 1971;59:23–27. doi: 10.1378/chest.59.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Peter R, Whalen R, Orgain E, McIntosh H. Postpericardiotomy syndrome as a complication of percutaneous left ventricular puncture. Am J Cardiol. 1966;17:86–90. doi: 10.1016/0002-9149(66)90265-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein S, Yu PN. Constrictive pericarditis after blunt chest trauma. Am Heart J. 1965;69:544–550. [PubMed] [Google Scholar]
- 18.Brosius FC, III, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–530. doi: 10.1016/0002-9343(81)90574-x. [DOI] [PubMed] [Google Scholar]
- 19.Rabiner SF, Spector LS, Gripstein CB, Schlecker AA. Chronic constrictive pericarditis as a sequel to acute benign pericarditis—report of a case. N Engl J Med. 1954;251:425–428. doi: 10.1056/NEJM195409092511104. [DOI] [PubMed] [Google Scholar]
- 20.Hirschman SZ, Hammer GS. Coxsackie virus myopericarditis. A microbiological and clinical review. Am J Cardiol. 1974;34:224–232. doi: 10.1016/0002-9149(74)90201-x. [DOI] [PubMed] [Google Scholar]
- 21.Burch GE, Colcolough HL. Progressive Coxsackie viral pancarditis and nephritis. Ann Intern Med. 1969;71:963–970. doi: 10.7326/0003-4819-71-5-963. [DOI] [PubMed] [Google Scholar]
- 22.Wacker W, Merrill JP. Uremic pericarditis in acute and chronic renal failure. JAMA. 1954;156:764–765. doi: 10.1001/jama.1954.02950080012005. [DOI] [PubMed] [Google Scholar]
- 23.Schupak E, Merrill JP. Experience with long-term intermittent hemodialysis. Ann Intern Med. 1965;62:509–518. doi: 10.7326/0003-4819-62-3-509. [DOI] [PubMed] [Google Scholar]
- 24.Abella R, Blondeel NJ, Roguska J, Walker C, Simon NM, Del Greco F. Periodic dialysis in terminal uremia. JAMA. 1967;199:362–368. [PubMed] [Google Scholar]
- 25.Reyman TA. Subacute constrictive uremic pericarditis. Am J Med. 1969;46:972–975. doi: 10.1016/0002-9343(69)90098-9. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay J, Jr, Crawley IS, Callaway GM., Jr Chronic constrictive pericarditis following uremic hemopericardium. Am Heart J. 1970;79:390–395. doi: 10.1016/0002-8703(70)90426-6. [DOI] [PubMed] [Google Scholar]
- 27.Moschcowitz E. Pathogenesis of constrictive pericardium. JAMA. 1953;153:194–198. doi: 10.1001/jama.1953.02940200016004. [DOI] [PubMed] [Google Scholar]
- 28.Cabot RC. Facts on the Heart. Philadelphia: WB Saunders Co; 1926. p. 781. [Google Scholar]
- 29.Cathcart ES, Spodick DH. Rheumatoid heart disease. A study of the incidence and nature of cardiac lesions in rheumatoid arthritis. N Engl J Med. 1962;266:959–964. doi: 10.1056/NEJM196205102661901. [DOI] [PubMed] [Google Scholar]
- 30.Sagrista-Sauleda J, Angel J, Sanchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med. 2004;350:469–475. doi: 10.1056/NEJMoa035630. [DOI] [PubMed] [Google Scholar]
- 31.Martin RG, Ruckdeschel JC, Chang P, Byhardt R, Bouchard RJ, Wiernik PH. Radiation-related pericarditis. Am J Cardiol. 1975;35:216–220. doi: 10.1016/0002-9149(75)90004-1. [DOI] [PubMed] [Google Scholar]
- 32.Roberts WC. Aortic dissection: anatomy, consequences, and causes. Am Heart J. 1981;101:195–214. doi: 10.1016/0002-8703(81)90666-9. [DOI] [PubMed] [Google Scholar]
- 33.Fell SC, Rubin IL, Enselberg CD, Hurwitt ES. Anticoagulant-induced hemopericardium with tamponade: its occurrence in the absence of myocardial infarction or pericarditis. N Engl J Med. 1965;272:670–674. doi: 10.1056/NEJM196504012721305. [DOI] [PubMed] [Google Scholar]
- 34.Beaudry CB, Nakamoto S, Kolff WJ. Uremic pericarditis and cardiac tamponade in chronic renal failure. Ann Intern Med. 1966;64:990–995. doi: 10.7326/0003-4819-64-5-990. [DOI] [PubMed] [Google Scholar]
- 35.Buja LM, Friedman CA, Roberts WC. Hemorrhagic pericarditis in uremia. Clinicopathologic studies in six patients. Arch Pathol. 1970;90:325–330. [PubMed] [Google Scholar]
- 36.Roberts WC, Fredrickson DS. Gaucher's disease of the lung causing severe pulmonary hypertension with associated acute recurrent pericarditis. Circulation. 1967;35:783–789. doi: 10.1161/01.cir.35.4.783. [DOI] [PubMed] [Google Scholar]
- 37.Harvey KPP, Jones MC, Anderson EG. Pericardial abnormalities in Gaucher's disease. Br Heart J. 1969;31:603–606. doi: 10.1136/hrt.31.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naef AP. Primary chylopericardium: its surgical treatment. Dis Chest. 1956;30:160–167. doi: 10.1378/chest.30.2.160. [DOI] [PubMed] [Google Scholar]
- 39.Hawker RE, Cartmill TB, Celermajer JM, Bowdler JD. Chylous pericardial effusion complicating aorta-right pulmonary artery anastomosis. J Thorac Cardiovasc Surg. 1972;63:491–494. [PubMed] [Google Scholar]
- 40.Pereira WM, Kalil RA, Prates PR, Nesralla IA. Cardiac tamponade due to chylopericardium after cardiac surgery. Ann Thorac Surg. 1988;46:572–573. doi: 10.1016/s0003-4975(10)64702-9. [DOI] [PubMed] [Google Scholar]
- 41.Miller SW, Pruett HJ, Long A. Fatal chylopericardium caused by hamartomatous lymphangiomatosis. Am J Med. 1959;24:951–956. doi: 10.1016/0002-9343(59)90217-7. [DOI] [PubMed] [Google Scholar]
- 42.Offerijns FGJ, van der Veen KJ, Durrer D, Ziedses des Plantes BG. Lymphopericardium with hypoproteinemia, intestinal loss of protein, and congenital defects of lymphatic system. Circulation. 1969;39:116–120. doi: 10.1161/01.cir.39.1.116. [DOI] [PubMed] [Google Scholar]
- 43.Rubin RH, Moellering RC., Jr Clinical, microbiologic and therapeutic aspects of purulent pericarditis. Am J Med. 1975;59:68–78. doi: 10.1016/0002-9343(75)90323-x. [DOI] [PubMed] [Google Scholar]
- 44.Boyle JD, Pearce JL, Guze LB. Purulent pericarditis: review of literature and report of 11 cases. Medicine. 1961;40:119–144. [Google Scholar]
- 45.Buchbinder NA, Roberts WC. Left-sided valvular active infective endocarditis. A study of forty-five necropsy patients. Am J Med. 1972;53:20–35. doi: 10.1016/0002-9343(72)90112-x. [DOI] [PubMed] [Google Scholar]
- 46.Ibarra-Pérez C, Green L, Calvillo-Juárez M, Vargas de la Cruz J. Diagnosis and treatment of rupture of amebic abscess of the liver into the pericardium. J Thorac Cardiovasc Surg. 1972;64:11–17. [PubMed] [Google Scholar]
- 47.Kinare SG, Parulkar GB, Sen PK. Constrictive pericarditis resulting from dracunculosis. Br Med J. 1962;1:845–846. doi: 10.1136/bmj.1.5281.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman MG, Kaplan L. Cardiac involvement in coccidioidomycosis. Am J Med. 1957;23:87–98. doi: 10.1016/0002-9343(57)90360-1. [DOI] [PubMed] [Google Scholar]
- 49.Louria DB, Gordon RE. Pericarditis and pleuritis cased by a recently discovered microorganism, Waksmania rosea. Am Rev Respir Dis. 1960;81:83–88. doi: 10.1164/arrd.1960.81.1P1.83. [DOI] [PubMed] [Google Scholar]
- 50.Gibbons JE, Goldbloom RB, Dobell ARC. Rapidly developing pericardial constriction in childhood following acute nonspecific pericarditis. Am J Cardiol. 1965;15:863–867. doi: 10.1016/0002-9149(65)90392-9. [DOI] [PubMed] [Google Scholar]
- 51.Fajardo LF, Stewart Jr. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 52.McReynolds RA, Gold GL, Roberts WC. Coronary heart disease after mediastinal irradiation for Hodgkin's disease. Am J Med. 1976;60:39–45. doi: 10.1016/0002-9343(76)90531-3. [DOI] [PubMed] [Google Scholar]
- 53.Harvey LAC, DeMaio SJ, Roberts WC. Radiation-induced cardiovascular disease including stenosis of coronary ostium, coronary and carotid arteries, and aortic valve. BUMC Proceedings. 1994;7(3):33–36. [Google Scholar]
- 54.Morton DL, Kagan AR, Roberts WC, O'Brien KP, Holmes EC, Adkins PC. Pericardiectomy for radiation-induced pericarditis with effusion. Ann Thorac Surg. 1969;8:195–208. doi: 10.1016/s0003-4975(10)66229-7. [DOI] [PubMed] [Google Scholar]
- 55.Arthur A, Oskvig R, Basta LL. Calcified rheumatoid constrictive pericarditis with cardiac failure treated by pericardiectomy. Chest. 1973;64:769–771. doi: 10.1378/chest.64.6.769. [DOI] [PubMed] [Google Scholar]
- 56.McWhorter JE, IV, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis) Am J Med. 1974;57:566–575. doi: 10.1016/0002-9343(74)90008-4. [DOI] [PubMed] [Google Scholar]
- 57.Conti CR, Friesinger GC. Chronic constrictive pericarditis. Clinical and laboratory findings in 11 cases. Johns Hopkins Med J. 1967;120:262–274. [PubMed] [Google Scholar]
- 58.Wychulis AR, Connolly DC, McGoon DC. Surgical treatment of pericarditis. J Thorac Cardiovasc Surg. 1971;62:608–617. [PubMed] [Google Scholar]
- 59.Roberts WC. Neoplasms involving the heart, their simulators, and adverse consequences of their therapy. BUMC Proceedings. 2001;14:358–376. doi: 10.1080/08998280.2001.11927789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glancy DL, Roberts WC. The heart in malignant melanoma: A study of 70 autopsy cases. Am J Cardiol. 1968;21:555–571. doi: 10.1016/0002-9149(68)90289-0. [DOI] [PubMed] [Google Scholar]
- 61.Roberts WC, Bodey GP, Wertlake PT. The heart in acute leukemia. A study of 420 autopsy cases. Am J Cardiol. 1968;21:388–412. doi: 10.1016/0002-9149(68)90143-4. [DOI] [PubMed] [Google Scholar]
- 62.Roberts WC, Glancy DL, De Vita VT., Jr Heart in malignant lymphoma (Hodgkin's disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides). A study of 196 autopsy cases. Am J Cardiol. 1968;22:85–107. doi: 10.1016/0002-9149(68)90250-6. [DOI] [PubMed] [Google Scholar]
- 63.Van Buren PC, Roberts WC. Cholesterol pericarditis and cardiac tamponade with congenital hypothyroidism in adulthood. Am Heart J. 1990;119:697–700. doi: 10.1016/s0002-8703(05)80305-9. [DOI] [PubMed] [Google Scholar]
- 64.Rokitansky CK. A Manual of Pathological Anatomy. 4th ed. London: The Sydenham Society; 1852. p. 137. [Google Scholar]
- 65.Osler W. Tuberculous pericarditis. Am J Med Sci. 1893;105:20–37. [Google Scholar]
- 66.Woods JA. Tuberculous pericarditis: a study of forty-one cases with special reference to prognosis. Am Heart J. 1951;42:737–745. doi: 10.1016/0002-8703(51)90179-2. [DOI] [PubMed] [Google Scholar]
- 67.Peel AAF. Tuberculous pericarditis. Br Heart J. 1948;10:195–207. doi: 10.1136/hrt.10.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heimann HL, Binder S. Tuberculous pericarditis. Br Heart J. 1940;2:165–176. doi: 10.1136/hrt.2.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Das PB, Gupta RP, Sukumar IP, Cherian G, John S. Pericardiectomy: indications and results. J Thorac Cardiovas Surg. 1973;66:58–70. [PubMed] [Google Scholar]
- 70.Thompson SA, Raisbeck MJ. Cardio-pericardiopexy. The surgical treatment of coronary arterial disease by the establishment of adhesive pericarditis. Ann Intern Med. 1942;16:495–520. [Google Scholar]
- 71.Schildt P, Stanton E, Beck CS. Communications between the coronary arteries produced by the application of inflammatory agents to the surface of the heart. Ann Surg. 1943;118:34–45. doi: 10.1097/00000658-194307000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eiseman B, Seeling MG, Womack NA. Talcum powder granuloma: a frequent and serious postoperative complication. Ann Surg. 1947;126:820–832. doi: 10.1097/00000658-194711000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnett EN, Battle WE, Russo JV, Roberts WC. Intravenous injection of talc-containing drugs intended for oral use: a cause of pulmonary granulomatosis and pulmonary hypertension. Am J Med. 1976;60:711–718. doi: 10.1016/0002-9343(76)90508-8. [DOI] [PubMed] [Google Scholar]
- 74.Osborne MP, Paneth M, Hinson KFW. Starch granules in the pericardium as a cause of the post-cardiotomy syndrome. Thorax. 1974;29:199–203. doi: 10.1136/thx.29.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II) Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 76.Mathewson FAL. Calcification of the pericardium in apparently healthy people. Electrocardiographic abnormalities found in tracings from apparently healthy persons with calcification of the pericardium. Circulation. 1955;12:44–51. doi: 10.1161/01.cir.12.1.44. [DOI] [PubMed] [Google Scholar]
- 77.Brawley RK, Vasko JS, Morrow AG. Cholesterol pericarditis. Considerations of its pathogenesis and treatment. Am J Med. 1966;41:235–248. doi: 10.1016/0002-9343(66)90019-2. [DOI] [PubMed] [Google Scholar]
- 78.Wychulis AR, Connolly DC, McGoon DC. Pericardial cysts, tumors, and fat necrosis. J Thorac Cardiovasc Surg. 1971;62:294–300. [PubMed] [Google Scholar]
- 79.Lillie WI, McDonald Jr, Clagett OT. Pericardial coelomic cysts and pericardial diverticula. A concept of etiology and report of cases. J Thorac Surg. 1950;20:494–504. [PubMed] [Google Scholar]
- 80.Moore RL. Congential deficiencies of the pericardium. Arch Surg. 1925;11:765–777. [Google Scholar]