A 48-year-old man was referred from Baylor College of Dentistry for evaluation of a 2-month history of large, flaccid, superficial bullae/erosions in the mouth, including the tongue, with subsequent development of blisters on the skin. Examination revealed shallow erosions on his oral mucosa (Figure 1) as well as discrete clusters of herpetiform lesions on his lower back (Figure 2). He was in good general health otherwise, with no new medications and no family history of similar lesions. A skin biopsy was performed (Figure 3).
Figure 1.
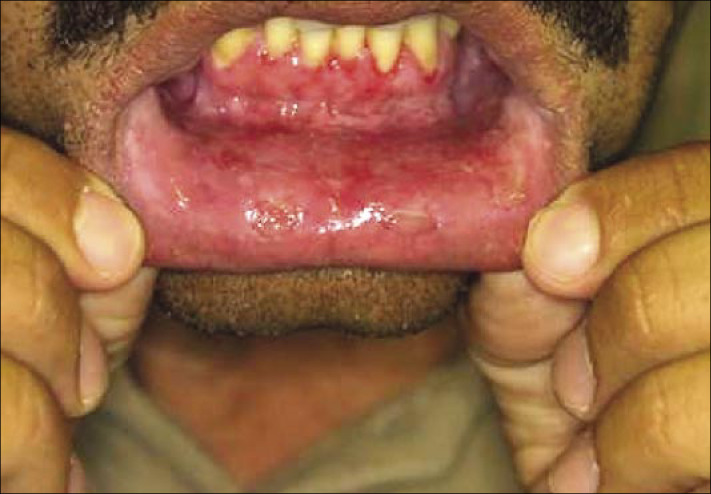
Desquamative gingivitis and superficial erosions on the oral mucosa.
Figure 2.
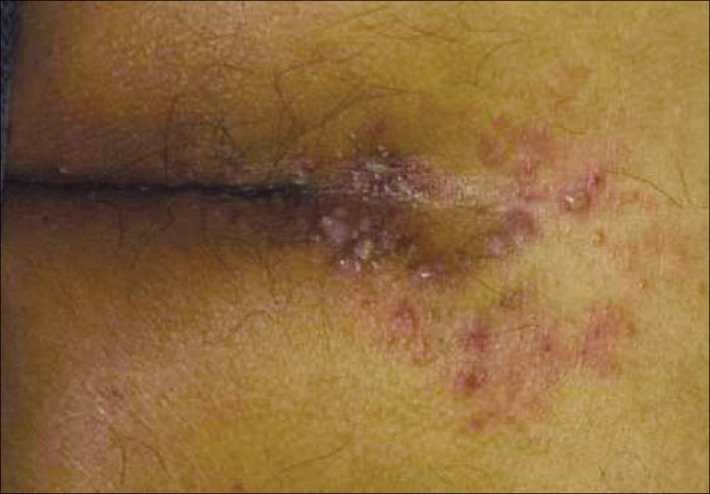
Small pruritic herpetiform bullae on the trunk.
Figure 3.
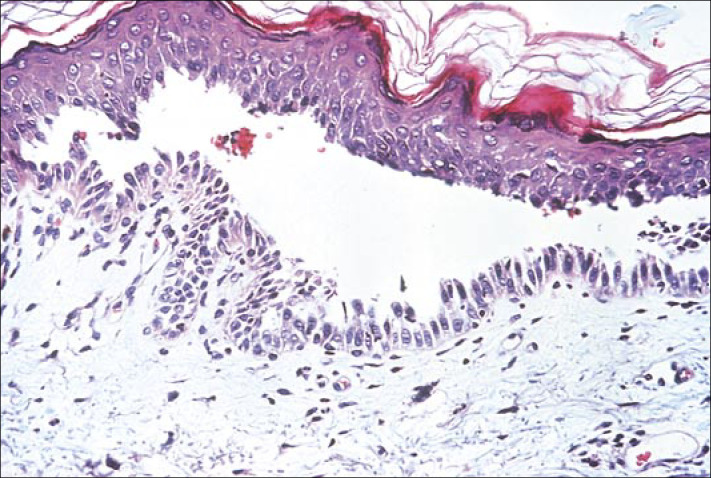
Skin biopsy demonstrating “tombstoning” of basal keratinocytes and an intraepidermal bulla. Hematoxylin-eosin stain.
What is the diagnosis? What treatment options exist?
DIAGNOSIS: Pemphigus vulgaris.
DISCUSSION
Pemphigus vulgaris is part of a group of chronic blistering skin diseases in which autoantibodies are directed against distinct antigens on the cell surface of keratinocytes, resulting in loss of cell adhesion (acantholysis) (1). Pemphigus has three major forms: pemphigus vulgaris, pemphigus foliaceus, and paraneoplastic pemphigus. Essentially all patients with pemphigus vulgaris have mucosal membrane erosions, and over half also have cutaneous blisters and erosions. Mucosal lesions usually predate skin lesions by many months. The bullae of pemphigus vulgaris develop in the deeper portion of the epidermis, just above the basal cell layer, while more superficial and flaccid subcorneal bullae are seen in pemphigus foliaceus.
Mucous membrane lesions usually appear as painful erosions. Intact blisters are rare; rupture occurs early since the mucous membranes have no keratin layer. Although scattered or extensive erosions may be seen anywhere in the oral cavity, the most common sites are the buccal and palatine mucosa. Extensive involvement may result in decreased intake of food and liquids.
The primary cutaneous lesions of pemphigus vulgaris are flaccid, thin-walled bullae, which may arise on normal-appearing or erythematous skin. The fluid within the bullae is initially clear but may become hemorrhagic, turbid, or even seropurulent. The blisters easily rupture to form painful crusted erosions that frequently become infected. Postinflammatory hyperpigmentation is common; however, true scarring is rare.
Two findings on clinical examination support the diagnosis of pemphigus: the Nikolsky and Asboe-Hansen signs. Both result from reduced keratinocyte adhesion within the epidermis. The Nikolsky sign is positive if slight pressure or rubbing of the skin produces lateral movement of the upper layers of the epidermis. The Asboe-Hansen sign, or “bulla-spread phenomenon,” is defined as gentle pressure on an intact bulla that forces the fluid to spread under the skin away from the site of pressure. Precipitating factors include echinacea (2), alga spirulina (2), enalapril (3), D-penicillamine (4), infection, neoplasms, and radiation (5), while pregnancy (6) may on occasion induce remissions. Numerous coexisting diseases have been reported with pemphigus, including psoriasis (3), vitiligo (7), lupus (8), renal disorders (9), and myasthenia gravis and thymoma (10). Paraneoplastic pemphigus is an extremely rare bullous eruption most often associated with an underlying lymphoreticular malignancy (non-Hodgkin's lymphoma, chronic lymphocytic leukemia, or Castleman's disease). Clinically, oral ulcerations are seen in conjunction with erythema multiforme–like (targetoid), psoriasiform, or lichenoid lesions. Persistent or progressive oral ulcerations should prompt immunofluorescence studies and a search for occult malignancy.
Diagnosis
The diagnosis of pemphigus vulgaris can be made based on clinical evaluation, biopsy results, immunofluorescence testing, and demonstration of circulating autoantibodies. A biopsy must be taken from an early lesion to establish the correct diagnosis. Oral mucosal biopsies are obtained from the active border of a denuded area, as intact blisters are rarely encountered. The characteristic histological finding is an intraepidermal bulla resulting from a loss of cell-cell adhesion of keratinocytes without any associated keratinocyte necrosis (Figure 3). Although the basal cells lose lateral desmosomal contact with their neighbors, the hemidesmosomes are unaffected. Therefore, attachment to the basement membrane is maintained, giving the appearance of a “row of tombstones.”
The hallmark of pemphigus is the finding of immunoglobulin G autoantibodies directed against the cell surface of keratinocytes (11). Pemphigus autoantibodies found in patients' sera play a primary pathogenic role in inducing the loss of desmosomal adhesions between keratinocytes and causing subsequent blister formation. Immunoelectron microscopy has localized both pemphigus vulgaris (desmoglein 3) and pemphigus foliaceus (desmoglein 1) antigens to the desmosomes, the most prominent cell-cell adhesion junctions in stratified squamous epithelia (12, 13). Serum antibody titers can be used to monitor disease activity. Demonstration of immunoglobulin G autoantibodies to desmoglein 3 and plakins on immunofluorescence is diagnostic of paraneoplastic pemphigus.
Diseases to consider in the differential diagnosis of pemphigus vulgaris are listed in Tables 1.
Table 1.
Differential diagnosis of pemphigus vulgaris
| Clinical diagnoses | ||
|
|
||
| Mucosal | Cutaneous | Histologic diagnoses |
|
| ||
| Aphthous stomatitis | Bullous drug eruptions | Hailey-Hailey disease |
| Erythema multiforme | Bullous pemphigoid | Darier's disease |
| Bullous lichen planus | Drug-induced pemphigus | Grover's disease |
| Cicatricial pemphigoid | Erythema multiforme | |
| Herpetic stomatitis | Hailey-Hailey disease | |
| Linear immunoglobulin A dermatosis | ||
| Paraneoplastic pemphigus | ||
| Pemphigus foliaceus | ||
| Transient acantholytic dermatosis (Grover's disease) | ||
Treatment and prognosis
Our patient had an excellent initial response to systemic prednisone at a dose of 60 mg/day plus a topical steroid gel (clobetasol propionate) to the mucosa. Without appropriate treatment, pemphigus vulgaris can be fatal: the epidermal barrier can become dysfunctional, resulting in loss of body fluids or secondary infections. The mainstay of therapy targets suppression of autoantibody production (Tables 2). Before the advent of systemic corticosteroids, many patients died within 5 years of disease onset. Systemic corticosteroids and immunosuppressive agents have greatly improved the prognosis of pemphigus; however, cumulative iatrogenic toxicities are a concern in patients with long-standing disease. While systemic corticosteroids are the mainstay of initial therapy for pemphigus, immunosuppressive agents are often added for their corticosteroid-sparing effect (19). The goal of therapy is to control the disease with systemic corticosteroids and then to taper the steroids while introducing a nonsteroidal immunosuppressant agent such as mycophenolate mofetil or azathioprine. Additionally, case series have demonstrated the excellent efficacy of intravenous immunoglobulin (20).
Table 2.
Drug therapies for pemphigus vulgaris
References
- 1.Stanley Jr. Pemphigus. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, Fitzpatrick TB, editors. Dermatology in General Medicine. New York: McGraw-Hill; 1998. pp. 654–666. [Google Scholar]
- 2.Lee AN, Werth VP. Activation of autoimmunity following use of immuno-stimulatory herbal supplements. Arch Dermatol. 2004;140:723–727. doi: 10.1001/archderm.140.6.723. [DOI] [PubMed] [Google Scholar]
- 3.Stavropoulos PG, Kostakis PG, Papakonstantinou AM, Panagiotopoulos A, Petridis AD. Coexistence of psoriasis and pemphigus after enalapril intake. Dermatology. 2003;207:336–337. doi: 10.1159/000073106. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro M, Jimenez S, Werth VP. Pemphigus vulgaris induced by D-penicillamine therapy in a patient with systemic sclerosis. J Am Acad Dermatol. 2000;42:297–299. doi: 10.1016/S0190-9622(00)90146-4. [DOI] [PubMed] [Google Scholar]
- 5.Orion E, Matz H, Wolf R. Pemphigus vulgaris induced by radiotherapy. J Eur Acad Dermatol Venereol. 2004;18:508–509. doi: 10.1111/j.1468-3083.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- 6.Shieh S, Fang YV, Becker JL, Holm A, Beutner EH, Helm TN. Pemphigus, pregnancy, and plasmapheresis. Cutis. 2004;73:327–329. [PubMed] [Google Scholar]
- 7.Jain R, Dogra S, Sandhu K, Handa S, Kumar B. Coexistence of vitiligo and pemphigus vulgaris in an Indian patient. Pediatr Dermatol. 2003;20:369–370. doi: 10.1046/j.1525-1470.2003.20326_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Nanda A, Kapoor MM, Dvorak R, Al-Sabah H, Alsaleh QA. Coexistence of pemphigus vulgaris with systemic lupus erythematosus. Int J Dermatol. 2004;43:393–394. doi: 10.1111/j.1365-4632.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- 9.Herron MD, Kohan DE, Hansen CD. Minimal change nephropathy associated with pemphigus vulgaris: a new relationship? J Am Acad Dermatol. 2004;50:645. doi: 10.1016/s0190-9622(02)61624-x. [DOI] [PubMed] [Google Scholar]
- 10.Patten SF, Dijkstra JW. Association of pemphigus and autoimmune disease with malignancy or thymoma. Int J Dermatol. 1994;33:836–842. doi: 10.1111/j.1365-4362.1994.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 11.Beuther EH, Jordan RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc Soc Exp Biol Med. 1964;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Li N, Diaz LA. Immunopathological mechanisms of acantholysis in pemphigus vulgaris: an explanation by ultrastructural observations. J Invest Dermatol. 2004;122:XIII–XIV. doi: 10.1111/j.0022-202X.2004.22438.x. [DOI] [PubMed] [Google Scholar]
- 13.Karpati S, Amagai M, Prussick R, Cehrs K, Stanley Jr. Pemphigus vulgaris antigen, a desmoglein type of cadherin, is located within keratinocyte desmosomes. J Cell Biol. 1993;122:409–415. doi: 10.1083/jcb.122.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain R, Kumar B. Immediate and delayed complications of dexamethasone cyclophosphamide pulse (DCP) therapy. J Dermatol. 2003;30:713–718. doi: 10.1111/j.1346-8138.2003.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu V, Mackool BT. Mycophenolate in dermatology. J Dermatolog Treat. 2003;14:203–211. doi: 10.1080/09546630310016826. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy A, Viguier M, Bedane C, Cordoliani F, Blaise S, Aucouturier F, Bonnetblanc JM, Morel P, Dubertret L, Bachelez H. Treatment of refractory pemphigus vulgaris with rituximab (anti-CD20 monoclonal antibody) Arch Dermatol. 2004;140:91–96. doi: 10.1001/archderm.140.1.91. [DOI] [PubMed] [Google Scholar]
- 17.Gach JE, Ilchyshyn A. Beneficial effects of topical tacrolimus on recalcitrant erosions of pemphigus vulgaris. Clin Exp Dermatol. 2004;29:271–272. doi: 10.1111/j.1365-2230.2004.01499.x. [DOI] [PubMed] [Google Scholar]
- 18.Hall VC, Liesegang TJ, Kostick DA, Lookingbill DP. Ocular mucous membrane pemphigoid and ocular pemphigus vulgaris treated topically with tacrolimus ointment. Arch Dermatol. 2003;139:1083–1084. doi: 10.1001/archderm.139.8.1083. [DOI] [PubMed] [Google Scholar]
- 19.Mimouni D, Nousari CH, Cummins DL, Kouba DJ, David M, Anhalt GJ. Differences and similarities among expert opinions on the diagnosis and treatment of pemphigus vulgaris. J Am Acad Dermatol. 2003;49:1059–1062. doi: 10.1016/s0190-9622(03)02738-5. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AR. Intravenous immunoglobulin therapy in the treatment of patients with pemphigus vulgaris unresponsive to conventional immunosuppressive treatment. J Am Acad Dermatol. 2001;45:679–690. doi: 10.1067/mjd.2001.116339. [DOI] [PubMed] [Google Scholar]


