Abstract
Yersinia pestis is the causative agent of plague, an enzootic vectorborne disease usually infecting rodents (rats) and fleas. Humans can become infected after being bitten by fleas that have fed on infected rodents. In humans, the disease usually occurs in the form of bubonic plague. In rare cases, the infection spreads to the lungs via the bloodstream and causes secondary pneumonic plague. Person-to-person transmission has been described for pneumonic plague but is rare in primary bubonic plague. Bubonic plague can usually be treated successfully with antibiotics; however, pneumonic plague develops rapidly and carries a high fatality rate despite immediate treatment with antibiotics. Plague is also recognized as a potential agent of bioterrorism. It has been used, or considered for use, as a biologic weapon on several occasions. It is important for the medical community to be familiar with the epidemiology, diagnosis, and symptoms of plague so it can deliver an appropriate and calm response should the unthinkable happen.
In recent years, the fear about terrorist attacks with biological weapons has grown. Plague has been identified by the Centers for Disease Control and Prevention (CDC) as a category A organism (1). This third article in a series of papers addressing issues related to biological warfare and bioterrorism gives a concise overview of the role that plague has played in the past and present as a biological weapon. As outlined in the historical review of biological warfare (1,2), plague has been one of the most devastating epidemic diseases to mankind, second only to smallpox. Given the presence and availability of plague around the world, the capacity for mass production and aerosol dissemination, the high fatality rate of pneumonic plague, and the potential for rapid secondary spread, the potential use of plague as a biological weapon is of great concern.
MEDLINE and OVID databases were searched to identify the pertinent articles and monographs related to plague, Yersinia pestis, and biological warfare/bioterrorism. A review of this bibliography led to subsequent identification of relevant references published prior to 1966. The consensus statement of the Working Group on Civilian Biodefense regarding plague was the basis of the final risk assessment and evaluation of the current threat (3). The final draft published in May 2000 provides a good basis for the development of strategies to counteract the potential threat posed by bioterrorism and the use of plague in particular. However, the conclusions and recommendations need to be regularly reassessed as new information and research become available.
HISTORICAL BACKGROUND AND EPIDEMIOLOGY
When the causative organism of plague was discovered in 1894, many of the new scientific concepts were subject to lengthy disputes. Naturally, these historic events are now seen retrospectively in light of concepts that are now considered proven. Because of the complexity of the historic background of the disease, this article can provide only a brief summary of the most important historic events.
The oldest account of plague is probably given in the Bible, in the First Book of Samuel. This book recounts that in approximately 1000 BC, the Philistines (people hostile to the Israelites in ancient Palestine), who had stolen the Ark of the Covenant from the Israelites, were afflicted with a dreadful disease. This disease, which probably was an epidemic of bubonic plague, had afflicted the people in the city of Ashdod, presently in Israel. Eventually, being overpowered by the pestilence, the Philistines were obliged to return the Ark of the Covenant with “five golden emerods and five golden mice.” The word “emerods” here may denote buboes, and the word “mice” may be translated as rats, both supporting the retrospective diagnosis of bubonic plague.
Another report of possible plague is given by Rufus of Ephesus in the first century AD. He describes a plague epidemic in the countries of Libya, Syria, and Egypt. In his account, additional earlier outbreaks of plague are noted, dating back to 300 BC. However, the original records are now lost (4, 5). More recent literature raises doubts about the true nature of these epidemics. Generally, it is very difficult and in some cases even impossible to render a clear diagnosis from the descriptions of ancient authors. Smallpox, typhus, and other infectious diseases could have accounted for some of the symptoms. The final states of some of these diseases are quite similar, making it even more difficult to differentiate them retrospectively based on scarce ancient texts.
The first undoubted report of bubonic plague is the “Great Plague of Justinian” (4, 6). The disease originated probably around AD 532 in Egypt and spread through the Middle East and the Mediterranean basin in the following years, reaching Turkey, Constantinople, and Greece in AD 541/542, Italy in AD 543, and the territories of France and Germany in AD 545/546. Procopius of Caesarea gives a detailed account of the outbreak of bubonic plague in Constantinople in his book De Bello Persico. The estimated population losses in North Africa, Europe, and central and southern Asia were between 50% and 60% (7). This great first pandemic was followed by many smaller outbreaks throughout the following two centuries, thus bridging the gap between the first and second great pandemics of plague.
In contrast to the first pandemic, the second or great medieval plague pandemic is well described by many authors and documents (5) (Figure 1). This second pandemic, also known as the Black Death or Great Pestilence, appeared in 1334 in China and then spread westward along the great trade routes in Tauris on the Black Sea and eventually to Constantinople. From India it reached the Crimea in 1347 and was then imported into Venice, Genoa, and Sicily (4, 8). The disease spread slowly and inevitably from village to village by infected rats and humans, or more quickly from country to country by ships, and eventually killed 20 to 30 million people in Europe: more than one third of the European population (9).
Figure 1.

The plague in Naples. Courtesy of the National Library of Medicine.
Despite the high mortality rate of the Black Death pandemic, the most devastating effects resulted from smaller, recurrent outbreaks that continued well into the 18th century, although with a lower frequency than in the 14th and 15th centuries. Between the years 1349 and 1665, only a few decades saw no plague epidemic. In most cases the epidemics originated from residual foci. In some other cases complete reintroduction of the disease occurred. In continental Europe, three major plague corridors were identified along which the plague epidemics expanded during the 16th to 18th centuries (10). The first route linked the Low Countries with the Rhineland; the second ran parallel to the Weser and Elbe rivers linking northwestern Germany to Bohemia. The third important corridor was along the coastal region of the Baltic Sea and the North Sea.
This second pandemic, which lasted more than 130 years, had major political, economic, cultural, and religious ramifications. While many doctors at that time reacted similarly to the Greek physician and philosopher Galen (AD 129–199), who fled when the disease reached Rome, others upheld the highest ideals of the medical profession and continued serving the sick even at their own risk (8). In the 16th and 17th centuries, many books and tracts were published on the plague and other “fevers.” However, few contributed significantly to medical progress. On the other hand, many devices and behavioral guidelines were established for those dedicated to the treatment of plague victims (Figure 2).
Figure 2.

The plague doctor (German woodcut, 1650s). Courtesy of the National Library of Medicine.
The first complete theory of infection was developed by Girolamo Fracastoro (1478–1553) and was published in 1546 (11, 12). He proposed that an infective agent of minute size, which he called “seminaria contagionis,” caused plague. In his theory, the seminaria caused spoiling and were transmitted by minute particles. Although this theory may appear similar to our modern concept of microorganisms, the two cannot be regarded as the same. Only with the invention of efficient microscopes by the Dutchman Antoni van Leeuwenhoek (1632–1723) were microorganisms finally discovered in 1674 and the old concepts of diseases slowly revised. Some 200 years later with the advent of modern microbiology by Louis Pasteur and Robert Koch, many pathogens were discovered, and with the conception of Koch's postulates the widespread theory of a miasmatic cause of disease was finally replaced by the foundation of a scientific bacteriology (10). However, it took an additional 20 years before the mystery of plague was lifted.
The third (and present) pandemic probably originated in the Chinese province of Yunnan around 1855 and spread to the southern coast of China, causing several smaller outbreaks. When the disease finally reached Canton and Hong Kong in 1894, large epidemics occurred, thus marking the beginning of the third pandemic. Plague spread rapidly throughout the world through all inhabited continents, except Australia. Rats aboard the faster steamships that had replaced slow-moving sailing vessels in merchant fleets carried the disease. Between the years 1894 and 1903, plague had entered 77 ports on 5 continents. Since then, smaller outbreaks have occurred around the world. During the early years of this third pandemic, the ultimate death toll in India and China alone was 12 million. In 1900, plague was introduced into North America (San Francisco), and between 1900 and 1924 most plague cases in the USA occurred in port cities along the Pacific and Gulf coasts (13). The disease spread slowly eastward with sporadic cases now being reported mainly in Arizona, New Mexico, Colorado, and Utah. The remainder of cases in the USA are reported in California; cases have been reported in Texas only rarely.
When the plague pandemic reached Hong Kong in 1894, the Japanese government dispatched a commission including the bacteriologist Shibasaburo Kitasato (1856–1931) to investigate this new epidemic, which at that time was spilling over to Japanese ports. At the same time, Alexandre Yersin (1863–1943) was dispatched by the French colonial minister on a similar mission (6) (Figure 3). Both arrived in Hong Kong in June 1894 and independently began their research, ultimately identifying a new bacterium from tissues obtained from dead rats and humans. It is possible that Kitasato was the first to describe the new organism, only a few days ahead of Yersin. A preliminary note appeared in The Lancet on August 25, 1894 (14). On the other hand, Yersin's description and explanations published only a few days later seem to be more accurate, with all striking characteristics of the disease emphasized (15). The literature has been quite inconsistent in crediting Yersin or Kitasato with the discovery of the plague bacillus (16). Since its discovery, the microorganism causing plague has undergone several nomenclature changes. Finally in 1970, it was named Yersinia pestis (7). For completeness of a historical review, I shall mention that in 1898 Paul-Louis Simond discovered that plague was transmitted by fleas (17, 18). In 1927, Ricardo Jorge found the explanation for the endemic occurrence of sporadic cases and outbreaks of plague. He explained that wild living rodents were the infection reservoir of endemic plague (19). This type of plague was subsequently termed sylvatic plague and has since been described in areas of Russia, South Africa, and South and North America.
Figure 3.

Dr. Alexandre Yersin in front of the National Quarantine Station, Shanghai Station, 1936. This was where Dr. Yersin first isolated and described Pasteurella pestis, the old term used for Yersinia pestis. Photo by Antoine Danchin; used courtesy of the Pasteur Research Centre and the Public Health Image Library.
The risk of importing plague to nonendemic regions may have increased over the past two decades. The worldwide extent of plague-endemic areas and the global incidence of reported cases have both increased (20), as have the volume and rapidity of national and international travel. In 1991, 1966 cases of human plague were reported; in 1997, the number was 4058. These numbers are the highest for the last 20 years (21). The recent increase in the number of cases of human plague together with the reappearance of epidemics in countries such as Malawi, Mozambique, and India in 2002 and 2003 led to its recognition as a reemerging infectious disease (22, 23).
MICROBIOLOGY
Y. pestis, the causative organism of plague, is a nonmotile, gram-negative bacillus that shows a bipolar staining pattern with Wright, Giemsa, or Wayson stains (Figure 4) The organism belongs to the Enterobacteriaceae family, is a lactose nonfermenter, and is urease and indole negative (24, 25). It grows optimally at 28°C on blood agar or MacConkey agar, typically requiring 48 hours for observable growth. The colonies are initially much smaller than those of other Enterobacteriaceae and can therefore be easily overlooked (Figure 5).
Figure 4.
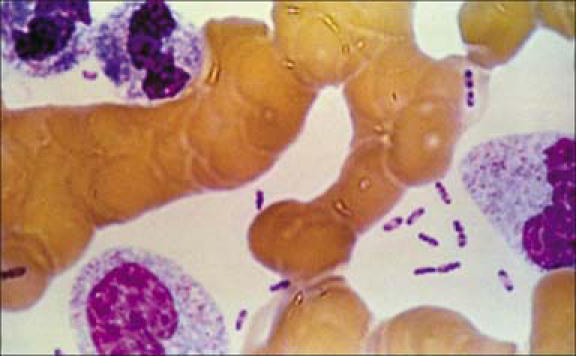
Dark stained bipolar ends of Yersinia pestis can clearly be seen in this Wright's stain of blood from a plague victim (1993). Photo from CDC; used courtesy of the Public Health Image Library.
Figure 5.
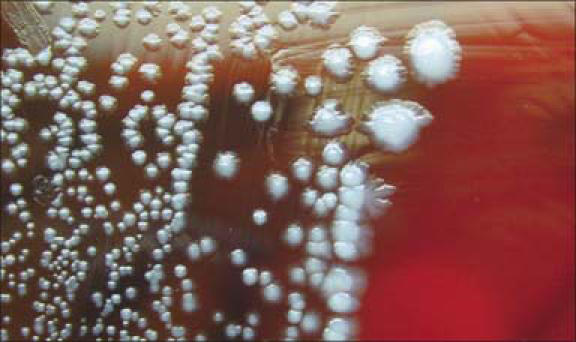
Yersinia pestis on sheep blood agar, 72 hours. Y. pestis grows well on most standard laboratory media. After 48 to 72 hours, it shows gray-white to slightly yellow opaque raised, irregular “fried egg” morphology; alternatively, colonies may have a “hammered copper” shiny surface. Photo by Larry Stauffer, Oregon State Public Health Laboratory; used courtesy of the CDC and the Public Health Image Library.
The main characteristic of Y. pestis is its homogeneity, considering the wide range of hosts and vectors: there is only one serotype, one phage type, and three biovars. Based on historical data and bacteriological characteristics of the strains isolated from remnant foci of ancient plague, Devignat described the Antiqua, Medievalis, and Orientalis biovars, which caused the first, second, and third pandemics, respectively (26, 27). Recent genetic evidence has reinforced Devignat's original hypothesis. Lucier and Brubaker explained that the Spel DNA patterns of eight strains of Y. pestis were closely related to their respective biovars (28). Rakin and Heesemann independently confirmed those results (29). Other studies concluded that several new ribotypes of the biovar Orientalis had originated within the past century: the original Y. pestis strain had spread all over the world and also had undergone chromosomal rearrangements, leading to the local emergence of new ribotypes (30). Thus, it appears that distinct ribosomal RNA profiles of Y. pestis may evolve in short periods of time and in specific geographical areas. Isolation of new ribotypes of biovar Orientalis from Madagascar, Vietnam, and India in recent years may be explained by the modification of the original Y. pestis strain that had spread through the entire world during the third pandemic. The questions that remain to be answered are whether these new variants have acquired selective advantages in a new environment and, if so, what the nature of the advantages could be. These challenging questions need to be addressed.
PATHOGENESIS AND CLINICAL PRESENTATION
The pathogenicity of Y. pestis results from its remarkable ability to overcome the defense mechanisms of mammalian hosts and to overwhelm them with massive growth. The rapid multiplication of the organism occurs mainly extracellularly (31). The entrance of the organism into the mammalian host (temperature 37°C) induces the expression of several virulence factors followed by rapid replication of the organism. Y. pestis induces an inflammatory response at the site of its inoculation, which is typically the site of a fleabite. From there, the organisms spread via lymphatics to regional lymph nodes.
Experimental studies identified several virulence factors that are essential to the survival of Y. pestis in the mammalian host. Three plasmids have been identified in Y. pestis. One plasmid encodes for the low calcium response (LCR) genes, which are responsible for the expression of 12 proteins that act specifically at 37°C and in the presence of low amounts of calcium (24). These proteins include a secreted protein (V antigen) and 11 surface and secreted proteins called Yersinia outer (membrane) proteins (Yops). The Yops seem to be especially important for the survival of Y. pestis: Yop H has specific antiphagocytic activity, Yop E is cytotoxic, and Yop M binds human thrombin. Two other plasmids encode for a plasminogen activator (Pla), bacteriocin pesticin (Pst), murine toxin (Ymt), and the structural gene for the fraction 1 (F1) protein capsule. The F1 capsule is also expressed at 37°C and has antiphagocytic activity against neutrophils and monocytes. Although Y. pestis can survive in macrophages, it can be killed by neutrophils. Therefore, the F1 antigen is essential for the survival of the organism in the mammalian host. These and many other antigens enable Y. pestis to survive in the mammalian (human) host by facilitating the use of host nutrients, causing damage to host cells, and escaping phagocytosis and other host defense mechanisms.
The initial local lesion and inflammation are accompanied by rapid spread and multiplication of the bacteria. The earliest response to the infection is probably a profuse protein- and mucopolysaccharide-rich effusion. This initial vascular phase of the inflammatory response is combined with the direct endothelial toxicity of yersinial toxins. In later stages of the infection, necrosis leads to vascular destruction and local hemorrhages. These occur without further bacterial invasion of vascular structures. A prominent neutrophilic infiltrate is present; however, because of F1, Y. pestis escapes phagocytosis and destruction. And even though macrophages actively phagocytose bacilli, they are unable to kill them. Instead, the bacillary toxin destroys the macrophages and other phagocytic cells of the host defense system. The lesions caused by plague result from the local destruction of tissue and from the systemic effects of endotoxins. Some of these toxins cause peripheral vascular collapse and disseminated intravascular coagulation.
The infection with Y. pestis in humans occurs in one of three primary clinical forms: bubonic plague is characterized by regional lymphadenopathy resulting from cutaneous or mucous membrane exposure, primary septicemic plague is an overwhelming plague bacteremia usually following cutaneous exposure, and primary pneumonic plague follows the inhalation of aerosolized droplets containing Y. pestis (3, 20, 25).
In most cases of naturally occurring human plague (the classic form of bubonic plague), the victims are bitten by plague-infected fleas (Figure 6); however, contamination of open skin lesions with plague-infected material has also been described. The bacteria then migrate through cutaneous lymphatics to regional lymph nodes, where they are phagocytosed but resist destruction. The result is inflammation and swelling in those affected lymph nodes (buboes, Figure 7). After the incubation period of 2 to 6 days, patients typically experience a sudden onset of the illness with severe malaise, headache, shaking chills, and fever. Initially lymphadenopathy may not be striking, but with the progression of the disease, buboes are the dominating and prominent feature of the disease. Buboes measure between 1 and 10 cm, and the overlying skin is often erythematous. They are extremely tender, nonfluctuant, and warm and are often associated with surrounding edema. Lymphangitis, however, seldom occurs. With appropriate treatment in uncomplicated cases, fever and general clinical symptoms resolve usually within 3 to 5 days. The buboes, however, may remain enlarged and tender for many weeks following an otherwise satisfactory clinical recovery.
Figure 6.
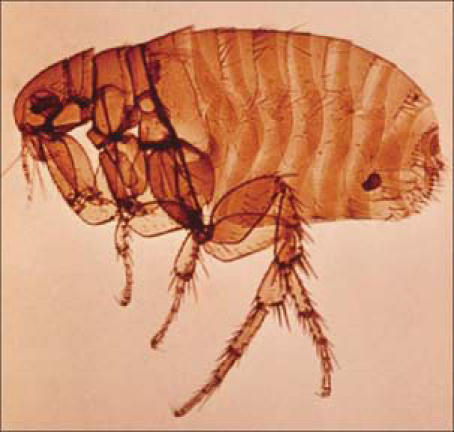
Oriental rat flea. Photo from the World Health Organization; used courtesy of the Public Health Image Library.
Figure 7.
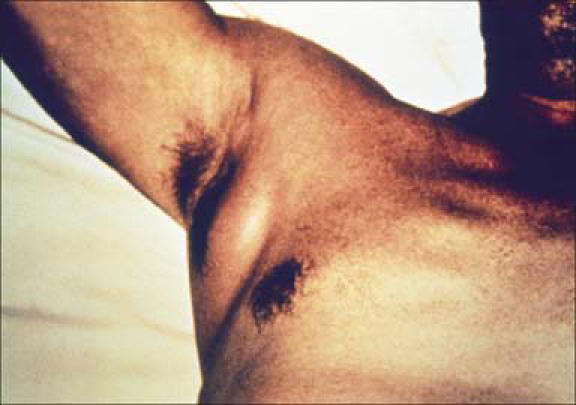
Plague patient with an axillary lymphadenopathy. Courtesy of the Public Health Library of Medicine, CDC.
Primary septicemic plague, which accounts for 10% to 15% of all cases of plague, is an overwhelming and progressive bacteremia in the absence of a primary lymphadenopathy. Septicemia may also arise secondary to bubonic plague. Septicemic plague affects all age groups; however, the elderly appear to be at a greater risk of developing septicemia. The presence of rapidly replicating gram-negative bacilli in the bloodstream leads to a self-perpetuating immunological cascade that is typically linked with host response to severe injury and bacterial endotoxin. The host response includes a wide spectrum of symptoms, including disseminated intravascular coagulopathy, multiple organ failure, and adult respiratory distress syndrome. Disseminated intravascular coagulopathy can lead to arteriolar thrombosis, hemorrhage in skin and serosal surfaces, and gangrenous necrosis of acral regions (Figure 8). Plague septicemia, whether primary or secondary, may also result in metastatic infection of other organs and organ systems. Common complications of septicemia include plague pneumonia, plague meningitis, plague endophthalmitis, hepatic and splenic abscesses, and generalized lymphadenopathy.
Figure 8.
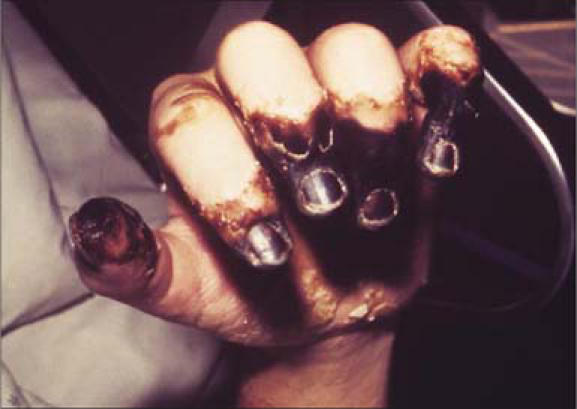
This patient presented with symptoms of plague that included gangrene of the hand causing necrosis of the fingers. Photo from CDC/Dr. Jack Poland; used courtesy of the Public Health Image Library.
Pneumonic plague is the most fulminant form of the disease, resulting in almost 100% mortality. The incubation period is usually 1 to 3 days, with a sudden disease onset characterized by chills, fever, headache, generalized body pains, weakness, and chest discomfort. The initial segmental pneumonitis rapidly progresses to lobar pneumonia and then to bilateral lung involvement (Figures 9 and 10). Typical pulmonary complications include localized areas of necrosis and cavitation, pleurisy with prominent effusion, and adult respiratory distress syndrome. As the disease rapidly progresses, the most prominent clinical features are cough, sputum production, increasing chest pain, dyspnea, hypoxia, and hemoptysis. These symptoms may be further complicated by concomitant septicemia. Death usually ensues if specific antibiotic therapy is not begun within 18 to 24 hours of the disease onset, and even then the mortality rate remains extremely high.
Figure 9.
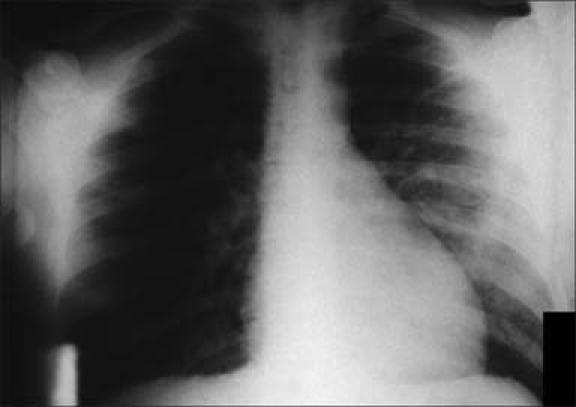
Chest x-ray in a patient with secondary pulmonary plague. Photo from CDC/Dr. Jack Poland; used courtesy of the Public Health Image Library.
Figure 10.
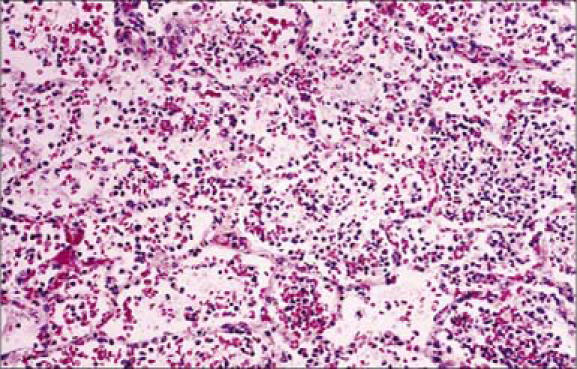
This photomicrograph illustrates the histopathologic changes in lung tissue in a case of fatal human pneumonic plague (hematoxylin and eosin stain, × 160). Note the moderate suppurative pneumonia including the presence of many polymorphonuclear leukocytes, capillary engorgement, and intraalveolar debris, all indicative of an acute infection. Photo from CDC/Dr. Marshal Fox; used courtesy of the Public Health Image Library.
Pneumonic plague occurs in two distinct and epidemiologically significant forms. Secondary pneumonic plague results from hematogenous spread of Y. pestis to the lungs. Patients typically have symptoms of severe bronchopneumonia, chest pain, dyspnea, cough, and hemoptysis (25). The invasive infection initiates an inflammatory response resulting in bacterial multiplication in the pulmonary parenchyma. When this process spills over into the alveolar spaces, it provides a mechanism by which Y. pestis can be expelled during coughing episodes. The spread of Y. pestis to close patient contacts via respiratory droplet transmission can initiate an epidemic of primary pneumonic plague.
Primary pneumonic plague, the second form in which the disease may occur, initially presents as an infectious pneumonitis with onset of symptoms often within 24 to 48 hours of exposure. Sudden disease onset and rapid disease progression in an otherwise healthy patient is the typical clinical presentation of primary pneumonic plague. In contrast, a patient with secondary pneumonic plague has usually been ill for several days prior to lung invasion and development of pulmonary symptoms. Many patients succumb to their infection before they develop a well-advanced pneumonia. Patients with primary pneumonic plague have an almost 100% mortality and succumb to the disease after rapid progression of the infection. However, primary pneumonic plague is rare in the USA (32). A 1997 report by the CDC revealed two recent cases of primary pneumonic plague, contracted after handling cats with pneumonic plague. Both patients had classic symptoms of pneumonic plague in addition to gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea). Since diagnosis and treatment were delayed more than 24 hours after onset of symptoms, both patients succumbed to the disease (33, 34). Pneumonic plague must be considered highly contagious whenever it occurs. Person-to-person transmission appears to be most likely in cold, humid environments coupled with overcrowding. Y. pestis is not considered truly airborne; person-to-person transmission requires face-to-face exposure within 2 m of a coughing and ill patient (20, 35, 36).
When patients present initially, often with vague symptoms of an infection, several differential diagnoses have to be considered. Bubonic plague can be confused with streptococcal or staphylococcal lymphadenitis, infectious mononucleosis, cat-scratch fever, lymphatic filariasis, tickborne typhus, tularemia, and other causes of lymphadenopathy. Involvement of intraabdominal lymph nodes may mimic appendicitis, acute cholecystitis, or enterocolitis.
Septicemic plague constitutes a medical emergency, which, unless the clinician has good reason to suspect the specific etiology, usually has a working diagnosis that is nonspecific (e.g., sepsis syndrome, gram-negative sepsis). Fortunately, some of the empiric antibiotic regimens for gram-negative sepsis are effective against Y. pestis. The most serious point of confusion in the differential diagnosis of plague sepsis may come from the clinical laboratory. For example, an improperly decolorized gram-stain examination of a blood smear or lymph node aspirate may result in the interpretation of Y. pestis bipolarity as a gram-positive diplococcus. In addition, automated bacterial identification devices may not code for Y. pestis and may also result in misidentification (37).
Pneumonic plague can be confused with several other causes of severe and acute community-acquired pneumonia, such as pneumococcal pneumonia, streptococcal pneumonia, and Haemophilus influenza. In addition, confusion with pulmonary anthrax, tularemia, Legionella pneumophila, Hantavirus pulmonary syndrome, and influenza virus pneumonia can occur. For an early diagnosis of plague, a high index of suspicion is required in naturally occurring cases and especially when biowarfare is suspected.
PLAGUE AS A BIOLOGICAL WEAPON
Although plague was widespread in ancient and medieval times, several outbreaks occurred following the deliberate use and propagation of this disease. During the second plague pandemic, which swept through Europe, the Near East, and North Africa in the 14th century, plague was deliberately used as a weapon during military conflicts. During the siege of Caffa, a well-fortified Genoese-controlled seaport (now Feodosia, Ukraine), in 1346, the attacking Tartar force experienced an epidemic of plague (1, 2). The Tartars, however, converted their misfortune into an opportunity by hurling the cadavers of their deceased into the city, thus initiating a plague epidemic in the city. An outbreak of plague followed, forcing a retreat of the Genoese forces. This major incident is described by Gabriel de Mussis, a notary born in Piacenza north of Genoa (38). This technique was repeated with various “success rates” during the next centuries.
Advances in living conditions, public health, and antibiotic treatment made outbreaks less likely in the years after the third pandemic. However, the threat of plague being used as a biological weapon remained. During World War II, the Japanese army, Unit 731, is reported to have experimented on plague and to have dropped plague-infected fleas over populated areas in China and Manchuria (1, 2). In the years following World War II, several countries, including the USA and the former Soviet Union, among many others, performed research on plague as a potential biological weapon. The former Soviet Union focused on the possibility of releasing plague in aerosolized form, thereby eliminating the dependence on the flea vector. In 1970 the World Health Organization (WHO) published a comprehensive report on the outcome of the possible use of biological weapons over populated areas (39). It was reported that, in a worst-case scenario—the deliberate release of 50 kg of Y. pestis in aerosolized form over a city of 5 million—pneu-monic plague could occur in as many as 150,000 persons, 36,000 of whom were expected to die from the disease (39). Furthermore, plague bacilli would remain viable in the area for 1 hour and up to a distance of 10 km. The expert panel was concerned that in such a scenario significant numbers of city inhabitants might attempt to escape, hence further spreading the disease.
While the USA did not succeed in making quantities of plague bacilli sufficient to use as an effective weapon, Soviet scientists were able to produce large quantities of plague organisms suitable for placing into weapons (40). There is little published information indicating actions of autonomous groups or individuals seeking to develop plague as a biological weapon. However, in Ohio in 1995, a microbiologist with doubtful motives was arrested after deceitfully acquiring Y. pestis by mail (41).
After the attacks on the World Trade Center on September 11, 2001, the threat of bioterrorism has reemerged. In October 2001, the aforementioned hypothetical concerns regarding the use of biological warfare as bioterrorism became reality when a Florida man died of pulmonary anthrax. Over the next months, another 10 individuals developed symptoms of inhalational anthrax (42).
Four of these individuals also succumbed to the disease. Eight nonfatal cases of cutaneous anthrax also occurred. In retrospect it became clear that a series of letters containing anthrax spores, sent through the US mail system, were responsible for this outbreak. Since then, new antiterrorism legislation has been introduced, and appropriate steps have been taken to educate and prepare the public and the medical community to ensue a reasoned response to future threats.
The epidemiology of plague in bioterrorism would differ substantially from that in naturally occurring infections. The organism would most likely be released as an aerosol. An outbreak of pneumonic plague would follow, and patients would present with symptoms initially resembling those of other severe respiratory infections. The size of the outbreak would depend on the quantity of biological agent used for the attack, the characteristics of the strain, and the environmental conditions at the time of the release of the organism. Symptoms would most likely occur within 1 to 6 days following exposure, and most people would die quickly after onset of symptoms. The occurrence of cases in areas not known to have enzootic infections together with no known risk factors for infection and an absence of great numbers of dead rodents all indicate the deliberate dissemination of plague.
TREATMENT OPTIONS AND PREVENTION
Streptomycin was approved by the Food and Drug Administration (FDA) for plague; historically, it has been the preferred treatment (25). When administered early in the disease, streptomycin has reduced the overall mortality from plague to the 5%-to-15% range (32). Because US supplies of streptomycin are limited, many experts have suggested gentamicin as an alternative form of treatment, although it is not FDA approved for this indication. Its efficacy was equal to or better than that of streptomycin in some in vitro as well as in vivo studies in mice (43). In addition, gentamicin is widely available, inexpensive, and can be given as a single daily dose. In a contained casualty setting, streptomycin and gentamicin are the preferred choices for treatment of adults and children, and gentamicin is the preferred choice for pregnant women. However, both drugs have to be administered via intramuscular or intravenous injection.
In a mass casualty setting, oral drugs may be needed. The Working Group on Civilian Biodefense (3) has recommended several oral drugs for the treatment and prophylaxis of plague, acknowledging that many are not FDA approved for that indication. These other antibiotics are tetracycline, doxycycline, chloramphenicol, and fluoroquinolones. Within the latter group, preference is given to ciprofloxacin, which has been shown to be at least as efficacious as aminoglycosides and tetracyclines. Chloramphenicol has been recommended for the treatment of plague meningitis because of its ability to cross the blood-brain barrier (25). Beta-lactam antibiotics are not effective in the treatment of plague. Antibiotics that have been shown in animal studies to have poor efficacy against Y. pestis include rifampin, aztreonam, ceftazidime, cefotetan, and cefazolin. These antibiotics should therefore not be used in the treatment of plague. Resistance patterns must be considered when choosing an antibiotic for the treatment of plague, and antibiotic susceptibility testing should be performed at a reference laboratory because of the lack of standardized susceptibility procedures for Y. pestis (44).
Consensus recommendations were made for special groups based on the clinical and evidence-based judgments of the working group (3); again, these recommendations do not necessarily correspond to FDA-approved use, indications, or labeling. In contained and/or mass casualty settings, children should be treated with streptomycin or gentamicin. Chloramphenicol is also considered safe in children aged ≥2 years. In mass casualty settings, children aged ≥8 years may be safely treated with tetracyclines. Given the adverse effects of tetracyclines and fluoroquinolones, the working group agreed that in mass casualty settings children can be safely treated with doxycycline (3, 45). Special recommendations have also been made for pregnant women, in whom aminoglycosides should be avoided (45). However, in cases of severe illness and/or in a contained casualty setting, treatment with gentamicin is recommended for pregnant women (3). Balancing the risks of pneumonic plague infection with those associated with doxycycline and ciprofloxacin use during pregnancy, the working group recommended that in pregnant women doxycycline should be used if gentamicin is not available. No specific recommendation have been made for the treatment of immunocompromised patients due to a lack of studies or animal models of pneumonic plague infection in the immunosuppressed population. At this point, the best recommendation is to proceed with the treatment option given to immunocompetent adults and children (3).
Postexposure prophylaxis for plague should be administered to individuals with close contact (<2 m) with an infectious case and to those who had potential respiratory exposure. The recommended regimen is doxycycline or ciprofloxacin given for 7 days on the same schedule as for treatment. The working group recommends doxycycline as the first-choice antibiotic for post-exposure prophylaxis (3). In addition, all persons developing a temperature of 38.5°C or higher or with symptoms of a new-onset cough should promptly begin antibiotic treatment. For infants in this setting, tachypnea would also qualify as an indication for immediate treatment. In a mass casualty setting, special consideration should be given to surveillance of the targeted population in order to identify individuals and communities at risk requiring postexposure prophylaxis. Many of these individuals may not be aware of the outbreak and therefore require special assistance.
Currently, no preexposure prophylaxis or vaccine is available for plague. Until 1999, a formalin-killed whole-cell vaccine was available in the USA for military personnel and researchers; however, it was discontinued after studies found that the vaccine was protective only for bubonic plague and completely lacked protection for pneumonic plague. A similar vaccine was in use in Canada, the United Kingdom, and Australia. With the reemergence of the bioterrorism threat, new efforts have been made to develop a new, possibly genetic-based, vaccine. The recent efforts have focused on the development of immunity to the F1 capsular protein and the V antigen (46, 47). Research in the pursuit of developing a vaccine that can effectively protect against primary pneumonic plague is ongoing in military research institutions in the USA, Israel, and the United Kingdom. Further information on vaccine development is available from these institutions and will hopefully be available through publication soon.
To date, no evidence exists that plague bacilli pose an environmental threat to the population. In fact, Y. pestis is very sensitive to sunlight and heat and does not survive long outside the host (39). Although some reports suggest that Y. pestis may survive in the soil and decaying animal carcasses, there is no evidence suggesting an environmental risk to humans in this specific setting. In the WHO risk analysis (39), it was estimated that a plague aerosol would remain effective and infectious for as long as 1 hour after its release. In the setting of a clandestine attack with plague, the aerosol would therefore long be dissipated before the first patient of pneumonic plague would come to hospital emergency departments.
Modern experience with person-to-person transmission of pneumonic plague is extremely limited. In large plague epidemics in earlier centuries, wearing masks prevented pneumonic plague transmission. Given the available historical evidence, the working group recommends that patients should remain isolated for the first 48 hours of antibiotic therapy and until clinical improvement occurs. Other standard respiratory droplet precautions such as gown, gloves, and eye protection should be implemented as well. In mass casualty settings, individual isolation of patients may become impossible. In this scenario, patients with (pneumonic) plague may be cohorted in isolation while undergoing antibiotic treatment. Should there be a need to transport patients to other facilities, the patients should wear surgical masks. Bodies of patients who have died from plague should be handled with strict routine precautions. However, aerosol-generation procedures such as bone sawing associated with surgery or postmortem examination is not recommended, since those activities are associated with a high risk of disease transmission. If such procedures are ultimately necessary, high-efficiency particulate air-filtered masks and negative-pressure rooms should be used (48).
In recent years, there is increased concern that a possible bioterrorism attack with plague might employ a natural or bio-engineered drug-resistant strain. Natural resistance of Y. pestis to antibiotics was rare; however, in 1995 a plague isolate from Madagascar contained a multidrug-resistant transferable plasmid (49). The organism produced TEM-1 (β-lactamase, chloramphenicol acetyltransferase, and a streptomycin-modifying enzyme. Later that year, a second strain was identified with a plasmid that encoded for the streptomycin-modifying phosphotransferase gene, which resulted in high-level streptomycin resistance (50). Both organisms were shown to contain plasmids that were easily transferred to other strains of Y. pestis as well as to Escherichia coli. Y. pestis is a member of the Enterobacteriaceae family, and as such it will be able to exchange genetic material with multiple genera within this family of organisms. As there are reports that the bioweapons operations of the former Soviet Union engineered multidrug-resistant and fluoroquinolone-resistant strains of Y. pestis (3, 40), this new evidence of naturally occurring drug resistance in isolates of Y. pestis underlines the importance of continuous reevaluation of guidelines for diagnosis and treatment of plague. More research is needed for effective treatment and vaccine development.
CONCLUSION
In the past centuries, plague has caused social and economic devastation on a scale unmatched by any other infectious agent except for smallpox. Although at the present time the organism is not a major health concern, still approximately 2000 cases annually are reported worldwide. It is evident that plague has not been eradicated and will not be eradicated soon. The WHO recently categorized plague as a reemerging infectious disease. Despite the major advances in the knowledge of the disease, in public health, and in diagnosis and treatment that were made since the discovery of the causative agent Y. pestis, the main reasons for the persistence of the disease are found in its epidemiology: plague is essentially a disease of wild rodents that is transmitted by fleas. The control of this wild animal population is inherently difficult, since the burrows are most often located in inaccessible areas. And even if at some point the infected animal reservoir could be completely destroyed, this would not guarantee the extinction of the disease: Y. pestis can survive in animal carcasses and litter for several years, thus being a source of reinfection of other rodents. With the new evidence of the reemergence of plague in Africa and India, the possibility of a fourth pandemic has to be considered. Furthermore, the recent emergence of variant strains and the possibility of resistance to current treatment regimens should lead to continuous research on Y. pestis and identification of possible new treatment modalities.
In the era of bioterrorism, several other issues have to be addressed. The medical community as well as the public should be educated about the basic infectious disease epidemiology and control measures to increase the possibility of a calm and reasoned response if an outbreak should occur. Furthermore, improved culture methods, biosafety facilities, and methods for susceptibility testing are necessary to allow for a more rapid identification of diseases such as plague. Continuous efforts should be made to seek new treatment modalities. This last concern is of great importance, since concerns about bioengineered organisms have been raised. It is in fact highly feasible to construct extremely virulent organisms resistant to standard antibiotics used for treatment and prophylaxis. A defense plan built on prophylactic antibiotics is highly vulnerable, given the fact that multidrug-resistant plague bacilli have recently occurred naturally. Vaccines have been used in the prevention of diseases for many decades and play a central role in the biodefense against a smallpox attack (51,52). It seems logical that our current national biodefense strategy must include the development of vaccines against multidrug-resistant strains of anthrax and plague to effectively protect the population.
A threat that is less likely but must be taken very seriously is the creation of genetic constructs through recombination technology. Such organisms—called chimeras—would combine the traits of several pathogens to create a highly virulent, transmissible, and multidrug-resistant organism. Alibek and Handelman described work on chimeras being conducted by Soviet military scientists (40). These organisms would challenge our ability to respond effectively to a public health threat with bioweapons. Most recent epidemics like HIV and severe acute respiratory syndrome have taught us that we can indeed respond quickly to global public health emergencies and develop diagnostic methods, therapies, and, hopefully, vaccines. However, proper education of the medical community as well as the public remains an essential cornerstone to ensure an effective safeguard for tragedies such as bioweapons attacks. Let us hope that we will not have to face such a challenge that is caused by the construction of a deadly pathogenic microorganism developed for the sole purpose of killing humans.
References
- 1.Riedel S. Biological warfare and bioterrorism: a historical review. BUMC Proceedings. 2004;17:400–406. doi: 10.1080/08998280.2004.11928002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eitzen EM, Jr, Takafuji ET. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 1997. Historical overview of biological warfare. In Sidell FR, Takafuji ET, Franz DR, eds; pp. 415–423. Available at http://www.bordeninstitute.army.mil/cwbw/default_index.htm; accessed August 31, 2004. [Google Scholar]
- 3.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K, Working Group on Civilian Biodefense Plague as a biological weapon: medical and public health management. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 4.Sticker G. Die Geschichte der Pest. Giessen: A Toepelmann Verlag; 1908. Abhandlungen aus der Seuchengeschichte und Seuchenlehre. Band I. [Google Scholar]
- 5.Haeser H. Geschichte der epidemischen Krankheiten. Jena: G. Fischer Verlag; 1882. Lehrbuch der Geschichte der Medizin und der epidemischen Krankheiten. Band 3. [Google Scholar]
- 6.Hirst LF. The Conquest of Plague: A Study of the Evolution and Epidemiology. Oxford, UK: Clarendon Press; 1953. [Google Scholar]
- 7.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of the plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan IA. Plague: the dreadful visitation occupying the human mind for centuries. Trans R Soc Trop Med Hyg. 2004;98:270–277. doi: 10.1016/S0035-9203(03)00059-2. [DOI] [PubMed] [Google Scholar]
- 9.Slack P. The black death past and present. Trans R Soc Trop Med Hyg. 1998;83:461–463. doi: 10.1016/0035-9203(89)90247-2. [DOI] [PubMed] [Google Scholar]
- 10.Eckert EA. The retreat of plague from Central Europe, 1640–1720: a geomedical approach. Bull Hist Med. 2000;74:1–28. doi: 10.1353/bhm.2000.0015. [DOI] [PubMed] [Google Scholar]
- 11.Leven KH. Jahrhundert. Landsberg/Lech: Ecomed Verlag; 1997. Die Geschichte der Infektionskrankheiten von der Antike bis ins 20. [Google Scholar]
- 12.Fracastoro G. De contagione et contagiosis morbis: German edition: Drei Buecher von den Kontagien, den Kontagioesen Krankheiten und deren Behandlung 1546: Uebers und eingel Von Victor Fossel. Leipzig: Earth Verlag; 1910. [Google Scholar]
- 13.Link VB. A History of Plague in the United States of America [Public Health Service Monograph No. 26] Washington, DC: Government Printing Office; 1955. [Google Scholar]
- 14.Kitasato S. The bacillus of bubonic plague. Lancet. 1894;2:428–430. [Google Scholar]
- 15.Yersin A. La peste bubonique a Hong Kong. Ann Inst Pasteur. 1894;8:662–667. [Google Scholar]
- 16.Bibel DJ, Chen TH. Diagnosis of plague: an analysis of the Yersin-Kitasato controversy. Bacteriol Rev. 1976;40:633–651. doi: 10.1128/br.40.3.633-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross L. How the plague bacillus and its transmission through fleas were discovered: reminiscences from my years at the Pasteur Institute in Paris. Proc Natl Acad Sci USA. 1995;92:7609–7611. doi: 10.1073/pnas.92.17.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simond P-L. La propagation de la peste. Ann Inst Pasteur. 1998;12:625–687. [Google Scholar]
- 19.Zietz BP, Dunkelberg H. The history of the plague and the research on the causative agent Yersinia pestis. Int J Hyg Environ Health. 2004;207:165–178. doi: 10.1078/1438-4639-00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. Plague Manual: Epidemiology, Distribution, Surveillance and Control [Document# WHO/CDS/CSR/EDC/99.2] Geneva: World Health Organization, Communicable Disease Surveillance and Response; 1999. Available at http://www.who.int/emc-documents/plague/whocdscsredc992c.html; accessed January 3, 2005. [Google Scholar]
- 21.Schrag SJ, Wiener P. Emerging infectious diseases: what are the relative roles of ecology and evolution. Trends Evol Ecol. 1995;10:319–324. doi: 10.1016/s0169-5347(00)89118-1. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Plague in Malawi and India. 2002. Available at http://www.who.int/csr/don/archive/disease/plague/en/; accessed January 25, 2005.
- 23.World Health Organization Plague in Algeria. 2003. Available at http://www.who.int/csr/don/2003_06_24a/en/; accessed January 25, 2005.
- 24.Bockemuhl J, Wong JD. Murray PR. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 2003. Yersinia; pp. 672–683. [Google Scholar]
- 25.Boyce JM, Butler T. Yersinia species (including plague). In Mandell GL, Bennett JE, eds. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 2070–2078. [Google Scholar]
- 26.Devignat R. Varietes de 1'espece Pasteurella pestis. Nouvelle hypothese. Bull Org Mond Sante. 1951;4:247–263. [PMC free article] [PubMed] [Google Scholar]
- 27.Guiyoule A, Rasoamanana B, Buchrieser C, Michel P, Chanteau S, Carniel E. Recent emergence of new variants of Yersinia pestis in Madagascar. J Clin Microbiol. 1997;35:2826–2833. doi: 10.1128/jcm.35.11.2826-2833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucier TS, Brubaker RR. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulse-field gel electrophoresis. J Bacteriol. 1992;174:2078–2086. doi: 10.1128/jb.174.7.2078-2086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakin A, Heesemann J. The established Yersinia pestis biovars are characterized by typical patterns of I-CeuI restriction fragment length polymorphism. Mol Gen Mikrobiol Virusol. 1995;3:26–29. [PubMed] [Google Scholar]
- 30.Ramalingaswami V. Plague in India. Nat Med. 1995;1:1237–1239. doi: 10.1038/nm1295-1237. [DOI] [PubMed] [Google Scholar]
- 31.Cornelis GR. Molecular and cell biology aspects of plague. Proc Natl Acad Sci USA. 2000;97:8778–8783. doi: 10.1073/pnas.97.16.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention Fatal human plague—Arizona and Colorado, 1996. JAMA. 1997;278:380–382. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention Pneumonic plague—Arizona. MMWR Morb Mortal Wkly Rep. 1992;41:737–739. [PubMed] [Google Scholar]
- 34.Werner SB, Weidmer CE, Nelson BC, Nygaard GS, Goethals RM, Poland JD. Primary plague pneumonia contracted from a domestic cat in South Lake Tahoe, Calif. JAMA. 1984;251:929–931. [PubMed] [Google Scholar]
- 35.Meyer K. Pneumonic plague. Bacterial Rev. 1961;25:249–261. doi: 10.1128/br.25.3.249-261.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tieh TH, Landauer E, Miyagawa F, Kobayashi G, Okayasu G. Primary pneumonic plague in Mukden, 1946, and report of 39 cases and 3 recoveries. J Infect Dis. 1948;82:52–58. doi: 10.1093/infdis/82.1.52. [DOI] [PubMed] [Google Scholar]
- 37.Wilmoth BA, Chu MC, Quan TJ. Identification of Yersinia pestis by BBL Crystal Enteric/Nonfermenter Identification System. J Clin Microbiol. 1996;34:2829–2830. doi: 10.1128/jcm.34.11.2829-2830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derbes VJ, De Mussis and the great plague of 1348 A forgotten episode of bacteriological warfare. JAMA. 1966;196:59–62. [PubMed] [Google Scholar]
- 39.WHO Group of Consultants . Health Aspects of Chemical and Biological Weapons. Geneva: World Health Organization; 1970. pp. 98–109. [Google Scholar]
- 40.Alibek K, Handelman S. Biohazard. New York: Random House; 1999. [Google Scholar]
- 41.Carus WS. Bioterrorism and Biocrimes: The Illicit Use of Biological Agents in the 20th Century. Washington, DC: Center for Counterproliferation Research, National Defense University; 1998. [Google Scholar]
- 42.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA, Anthrax Bioterrorism Investigation Team Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrne WR, Welkos SL, Pitt ML, Davis KJ, Brueckner RP, Ezzell JW, Nelson GO, Vaccaro Jr, Battersby LC, Friedlander AM. Antibiotic treatment of experimental pneumonic plague in mice. Antimicrob Agents Chemother. 1998;42:675–681. doi: 10.1128/aac.42.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MD, Vinh DX, Nguyen TT, Wain J, Thung D, White NJ. In vitro antimicrobial susceptibilities of strains of Yersinia pestis. Antimicrob Agents Chemother. 1995;39:2153–2154. doi: 10.1128/aac.39.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Hospital Formulary Service . AHFS Drug Information. Bethesda, MD: American Society of Health System Pharmacists; 2000. [Google Scholar]
- 46.Nierengarten MB, Lutwick LI. Vaccine development for plague. Medscape Infectious Diseases. 2002;4(2) [Google Scholar]
- 47.Williamson ED. Plague vaccine research and development. J Appl Microbiol. 2001;91:606–608. doi: 10.1046/j.1365-2672.2001.01497.x. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization . Safety Measures for Use in Outbreaks in Communicable Diseases. Geneva: World Health Organization; 1986. [Google Scholar]
- 49.Galimand M, Guiyoule A, Gerbaud G, Rasoamanana B, Chanteau S, Carniel E, Courvalin P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N Engl J Med. 1997;337:677–680. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 50.Guiyoule A, Gerbaud G, Buchrieser C, Galimand M, Rahalison L, Chanteau S, Courvalin P, Carniel E. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg Infect Dis. 2001;7:43–48. doi: 10.3201/eid0701.010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riedel S. Smallpox and biological warfare: a disease revisited. BUMC Proceedings. 2005;18:13–20. doi: 10.1080/08998280.2005.11928026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breman JG, Henderson DA. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]


