It is widely recognized that malnutrition is the commonest cause of immunodeficiency worldwide (14). In fact, a recent investigation has determined that food intake increases the levels of gamma interferon (IFN-γ) but not interleukin-4 (IL-4) production, whereas starvation enhances the IL-4 response but not IFN-γ levels of T lymphocytes (76). Therefore, the interactions between certain nutrients and immunity exert a crucial role that should be analyzed from a biological and a clinical point of view. In recent years, a large number of studies have been conducted to investigate the relevance of certain fatty acids in the alteration of immune system functions in both humans and animals. At the beginning, the epidemiological findings contributed to demonstrating that certain fatty acids supplied in their diets (particularly long-chain n-3 polyunsaturated fatty acids contained in fish oil) affect the immune responses of Greenland Eskimos, as indicated by the low prevalence of inflammatory disorders in this population (11, 36, 47). Subsequently, on the basis of these epidemiological studies, experimental investigations determined the main processes by which several polyunsaturated or monounsaturated fatty acids are capable of altering the immune system, as well as the mechanisms of action involved in the regulation of immune functions.
At least four modes of action have been proposed to explain the potential action of fatty acids on the modulation of immune system in both animals and humans. Accordingly, immune system modulation by dietary lipids may be attributed to changes in the composition of membrane phospholipids, lipid peroxidation, alteration of gene expression, or eicosanoid production. In addition, recent in vitro and ex vivo studies have demonstrated the involvement of several fatty acids (such as eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA], arachidonic acid [AA], or palmitic acid [PA]) in the induction or inhibition of programmed cell death or apoptosis (21, 22). The participation of different fatty acids in apoptosis opens new approaches for the study of the properties that different fatty acids exert as modulators of immune system functions.
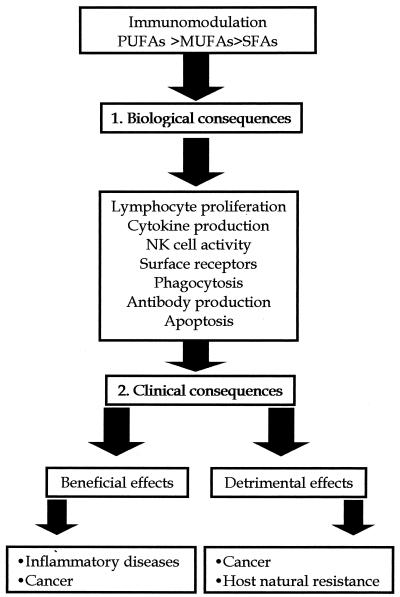
In this review, we summarize the effects of dietary lipids on the immune functions and the mechanisms responsible for these alterations as well as the crucial aspects that relate fatty acids to the triggering of apoptotic mechanisms. Similarly, we analyze the clinical consequences derived from dietary lipid administration, the effects of these substances on tumor promotion, and finally, the adverse effects that several dietary lipids may exhibit as a direct consequence of an immunosuppressive process (Fig. 1).
FIG. 1.
Schematic representation of potential role of dietary lipids and biological and clinical consequences of the administration of several dietary lipids. PUFAs, polyunsaturated fatty acids; MUFAs, monounsaturated fatty acids; SFAs, saturated fatty acids.
TYPES OF FATTY ACIDS THAT ALTER IMMUNE SYSTEM FUNCTIONS
Overall, the integrated action of several polyunsaturated or monounsaturated fatty acids on the immune system includes the alteration of different immune functions in both humans and animals. Therefore, lymphocyte proliferation (30, 53, 81), production of cytokines (30, 79), modification of cell surface molecules (38), changes in phagocytic activity (12, 23), and alteration of natural killer (NK) cell activity (24, 43, 80) are susceptible to be modulated by dietary lipid administration or by culture of cells in the presence of several free fatty acids. However, it is important to note that not all of the fatty acids have the same immunomodulatory properties. Thus, long-chain n-3 polyunsaturated fatty acids, such as EPA (20:5n-3) or DHA (22:6n-3) (which belong to n-3 fatty acid series derived from fish oil) are particularly known to exert a profound influence on the immune system, reducing many of these functions previously described for both humans and animals (30, 53, 81). Other fatty acids belonging to n-6 fatty acid series such as AA (20:4n-6) have also been reported to affect the immune system; although AA has not been shown to produce an immunosuppression as relevant as long-chain n-3 fatty acids, it is also considered an immunosuppressive agent. Nevertheless, both n-6 polyunsaturated fatty acids and saturated fatty acids have been associated to a chemopromotive role in cancer, probably due in part to a suppression of apoptosis (64). However, oleic acid (a monounsaturated fatty acid [18:1n-9] that is the main component of olive oil belonging to n-9 fatty acid series) is also capable of acting as a modulator of immune functions (23, 24, 40, 83). Accordingly, olive oil (a traditional and essential component of the Mediterranean diet) also plays an important role in the modulation of immune functions, and therefore it has been efficiently applied in the alleviation of the symptomatology caused by rheumatoid arthritis (44, 48).
BIOLOGICAL CONSEQUENCES ON IMMUNE FUNCTIONS ATTRIBUTED TO FATTY ACID ADMINISTRATION
Differential properties of fatty acids and their role in the modulation of immune system functions.
The changes attributed to fatty acid administration include the reduction of lymphocyte proliferation, which is modified by polyunsaturated fatty acids (n-3 or n-6) or monounsaturated fatty acids (n-9). Studies carried out in both humans and animals have revealed that the administration of high levels of dietary n-3 polyunsaturated fatty acids or the inclusion in parenteral regimens of lipid emulsions rich in n-3 or n-9 fatty acids are related to the reduction of lymphocyte proliferation during the supplementation (30, 35, 53, 56, 81). Thus, concanavalin-A or lipopolysaccharide-stimulated lymphocytes have reduced the cellular proliferation in assays carried out in vitro or ex vivo in the presence of free fatty acids or in cell cultures from both animals and humans fed dietary lipids, respectively (10, 30). Cytokine production is reduced by the action of n-3 polyunsaturated fatty acids or n-9 monounsaturated fatty acids (79), cytokine receptor expression is also affected (56), natural killer (NK) activity is significantly suppressed (24, 43, 80), phagocytic activity of macrophages is modified (12, 23), and the antigen-presenting function of human monocytes is inhibited (7, 20). Based on these experimental observations, we can affirm that n-3 polyunsaturated fatty acids rather than n-6 polyunsaturated or n-9 monounsaturated fatty acids are directly associated to the alteration of immune and inflammatory response. This modulation depends on different factors, such as the nature of fatty acids added to diets, the concentration of fatty acids, the duration of supplementation with dietary lipids, or differences among animal species fed dietary lipids (5).
Mechanisms of action.
As mentioned previously, for several years numerous studies have attempted to elucidate the mechanisms by which some dietary lipids produce a potential effect on immune system functions (52). Several hypotheses have been suggested as possible mechanisms. Experimental investigations have confirmed that several fatty acids exert changes in the phospholipids of plasma membrane which affect the membrane fluidity (81); they also alter eicosanoid production (34), produce lipid peroxides (1), or regulate the transcription factors (42). Overall, long-chain n-3 polyunsaturated fatty acids, such as EPA, are incorporated into cell membranes, replacing AA (the most important of the eicosanoid precursors). This process reduces the production of a biological mediator as prostaglandin E2 responsible for inhibiting IL-1 and tumor necrosis factor (TNF) production (30). Similarly, EPA inhibits gene transcription because it reduces the translocation of NF-κB (50). In addition to these important considerations, a crucial role of lymphocyte subsets has been suggested to explain the effects of different dietary polyunsaturated fatty acids on the immune system, because they alter the number of lymphocyte subsets as well as the proliferation of these cells (41, 78). Thus, antigen presentation and the proportion of T-cell subsets are modified after dietary lipid manipulation. In fact, recent studies have determined that polyunsaturated fatty acids inhibit the surface expression of major histocompatibility complex (MHC) class II molecules, as well as some adhesion molecules on human monocytes (38, 39). Similarly, consumption of a monounsaturated fatty acid-rich diet by humans is also involved in the reduction of adhesion molecules from peripheral blood mononuclear cells (83). Overall, these actions may explain the different expression of CD4 or CD8 on the T-cell surfaces of peripheral blood mononuclear cells of mice that were fed diets supplemented with DHA (69). The biological consequences of these changes are still unclear. It is probable that the alteration experimented by the lymphocyte population enhances the host susceptibility against opportunistic infections. In other words, the modulation of lymphocyte subsets may lead in part to a modification of cytokine production, NK cell activity, antibody production, etc. Accordingly, Th1-type cytokines such as IL-2 are more susceptible to polyunsaturated fatty acid effects than are Th2-type cytokines, such as IL-4, which indicates that these fatty acids are capable of altering the balance of Th1- and Th2-type cytokines in mice (77). As a direct consequence of a reduction of IL-2 production, Th1 cell activation is suppressed, whereas an increase of Th2 cell activation due to an enhancement of IL-4 production suppresses Th1 cell proliferation in animals fed a diet containing fish oil (3). Therefore, the anti-inflammatory effects derived from long-chain n-3 polyunsaturated fatty acid administration may be the result of these two interconnected mechanisms. Hence, it is obvious that these alterations may affect significantly the interaction between host natural resistance and infectious agents due to the crucial importance of Th1-mediated response in the infectious processes promoted by viruses or bacteria (54).
Although results from epidemiological, experimental, or clinical studies have demonstrated an important association between n-3 polyunsaturated fatty acids and immunosuppressive properties in both humans and animals, their effects are complex and still not clearly known. In fact, some discrepant results have suggested that n-3 polyunsaturated fatty acids have stimulatory effects on TNF and IL-1 production (57).
Evidence for the involvement of fatty acids in apoptosis modulation.
Programmed cell death or apoptosis has been defined as an essential mechanism responsible for the regulation of homeostasis, tissue development, or immune functions. Thus, damaged, aberrant, or unnecessary cells must be eliminated to ensure the correct development of multicellular organisms. Irrespective of its crucial role in normal cell control, apoptosis regulates pathological processes including human clinical disorders such as cancer, autoimmune diseases, viral or bacterial infections, and neurodegenerative disorders. Several biological factors (many viruses, bacteria, or parasites), chemical factors (glucocorticoids, ceramides, etc.), and physical factors (irradiation) are responsible for apoptosis induction (60). Moreover, different studies with both animals and humans, involving in vitro assays or administration of dietary lipids, have recently described an important role of several fatty acids in the induction or inhibition of apoptosis. As a result, different mechanisms of action have been proposed in order to explain the action of fatty acids on apoptosis modulation. Thus, polyunsaturated fatty acids, such as EPA and DHA, saturated fatty acids, such as PA, or fats such as fish oil administered in the diets have been defined as substances capable of inducing cell death via a mitochondrial process (27) or by downregulation of Bcl-2 (67). Different mechanisms have been proposed in order to explain the induction of apoptosis by certain fatty acids. Thus, PA (a saturated fatty acid) added to cellular cultures in in vitro assays induces apoptosis via a direct effect on mitochondria, because it causes a dissipation of the mitochondrial transmembrane potential (ΔΨm), an event that precedes nuclear apoptosis (27). Another possible partial explanation for the modulation of apoptosis by long-chain n-3 polyunsaturated fatty acids is that it is caused by direct action of these substances on the cells and by activation of the caspase cascade through cytochrome c release coupled with a modulation of mitochondrial membrane depolarization (2). Recent studies have also determined the crucial importance of dietary fatty acids in the reduction of Bcl-2 expression as well as an increase of Fas ligand (Fas-L) expression. Therefore, when the concentration of polyunsaturated fatty acids augments, Bcl-2 expression is reduced and cell death occurs (67, 75). In other words, long-chain fatty acids contained in fish oil trigger apoptosis by suppressing Bcl-2 expression and increasing Fas-L expression. On the other hand, it has been recently determined that Ras membrane localization (a protein that plays a critical role in the cell growth and apoptosis) is reduced after administration of fish oil, which indicates that dietary fish oil may play a protective role against colon cancer development (19). Finally, it is important to note that lipid peroxidation is of crucial importance in apoptosis induction. This argument suggests that the effects of polyunsaturated fatty acids can be directly associated to specific alterations on the expression of Bcl-2, Fas, and Ras (21).
CLINICAL CONSEQUENCES OF FATTY ACID ADMINISTRATION
Early studies revealed the importance of n-3 polyunsaturated fatty acids as possible therapeutic substances capable of reducing the incidence of inflammatory disorders in humans (11, 47). Autoimmune diseases such as rheumatoid arthritis, psoriasis, or systemic lupus erythematosus are characterized by the production of an inflammatory response due to a marked increase of proinflammatory cytokines. In recent years, numerous epidemiological studies have described widely the reduction in the incidence of rheumatoid arthritis in populations who consume fish oil or olive oil in their diets, which are related to the prevention of inflammatory disorder incidence, although these events occur after a prolonged ingestion of these fats (38, 44-46, 48, 70). Nevertheless, in spite of the importance of these substances as biological mediators in the inflammation and their application in the resolution of inflammatory diseases, in the present review we will summarize other important features of fatty acids from a clinical point of view, focusing on the potential effect of fatty acids in cancer incidence and the involvement of these substances in the alteration of host natural resistance against infectious agents.
Crucial role of fatty acids in the incidence of cancer.
Many lines of evidence have indicated the essential role of fish oil in the incidence of cancer (13). Numerous reports have suggested that high intakes of long-chain n-3 fatty acids or n-9 fatty acids (the most important fatty acid contained in olive oil) may reduce the risk of breast cancer (51, 72) in both humans and animals. Thus, n-3 polyunsaturated fatty acids may inhibit colon cancer in rats (65) as well as reduce the risk of colorectal cancer development (13). In contrast, n-6 fatty acids or saturated fatty acids may be involved in the increase of mammary or colon tumorigenesis by altering membrane phospholipid turnover. Accordingly, AA is released from plasma membrane that produces an alteration in the synthesis of prostaglandins via cyclooxygenase enzyme (64).
Epidemiological studies have determined the protective role of n-3 polyunsaturated fatty acids against colon cancer in populations of Alaskan and Greenland Eskimos (7, 28). Subsequently, experimental studies demonstrated that a fish oil diet appears to exert a protective effect against experimentally induced colon cancer (66). Similarly, a recent study has determined that olive oil also exerts a protective role against the development of colorectal cancer (71). Although the mechanisms that contribute to this protective effect have not been elucidated, a recent investigation has reported that fish oil diets are able to increase apoptosis and cell differentiation in induced rat colon tumorigenesis (17). Based on current knowledge, an interesting study has recently determined that the Western diet (which consists predominantly of a mixture of saturated, monounsaturated, and polyunsaturated fats) produces dysplastic lesions in the colon, which is indicative of a tumorigenic process. As a result, the administration of a diet containing mixed lipids and high levels of saturated fatty acids (similar to the lipids contained in the Western diet) induces colon carcinogenesis in mice by an unknown mechanism (68). Nevertheless, the most interesting results from this investigation reveal that an alteration of cyclooxygenase activity as well as apoptosis suppression appears to play a crucial role in the colon tumorigenesis induced by this diet in animal models (64). In addition, our experimental observations have indicated that the survival of mice fed a diet containing fish oil and transplanted with a T-lymphoma cell line is reduced in comparison to that of mice fed a diet containing low fat. We speculate that this event may be attributed to the impairment in the function of T cells or NK cells due to polyunsaturated fatty acid administration, which reduces the cytotoxic activity of NK cells (61). Recently, an interesting investigation has shown that n-3 fatty acids alter cell transformation in a mouse epidermal cell line because DHA or EPA inhibited transcription activator protein AP-1 whereas AA did not produce any effect on AP-1 activity. Therefore, this finding confirms previous reports of the chemoprotective role of diets containing n-3 fatty acids (49). In contrast, the most significant relationship between fatty acids and reduction of cancer incidence is found in the Mediterranean diet, which could decrease the development of cancer by up to 10 to 15% in the populations of highly developed Western countries (73).
In spite of the wide information currently available, the potential role of dietary lipids in cancer etiology remains controversial. In fact, although most of the studies have shown that diets containing n-3 polyunsaturated fatty acids might be beneficial during inflammatory conditions, their effects are not elucidated because they promote an impairment of the host defense to infection. Therefore, future studies are needed in order to confirm or refute the different hypotheses and arguments suggested hitherto.
Relevance of dietary lipids in the reduction of host natural resistance and susceptibility to infection.
As mentioned above, nutrient intake is considered a critical determinant of immunocompetence. Many investigations have reported the modulatory functions exerted by fatty acids and the clinical benefits of dietary lipid supplementation with fish oil or olive oil in both humans and animals. As a consequence, this dietary supplementation has been applied in the treatment of patients suffering from inflammatory diseases, because unsaturated fatty acids (mainly n-3 or n-9 fatty acids) reduce the levels of many biological mediators associated to the promotion of the inflammatory events that participate in an inappropriate immune response. However, the reported reduction of immune response caused by the administration in the diet of some fatty acids may exert a detrimental effect, and therefore, they may impair the cellular immunity to pathogenic agents (26).
For obvious reasons, the altered resistance to bacterial infection has been analyzed in animal models in which the administration of diets containing fish oil reduces the clearance of bacteria from liver or spleen and significantly reduces the survival during the course of Listeria monocytogenes infection. As a result, the elimination of pathogenic agents (bacteria, viruses, or parasites) is more difficult. Different reports have described the clinical consequences of dietary supplementation rich in n-3 fatty acids that suppress the immune system functions. Thus, a significant reduction of survival percentage after feeding experimental mice with a diet containing fish oil has been determined (25, 31) whereas an increase of survival percentage was observed after challenge with L. monocytogenes in animals fed diets containing either lard oil, hydrogenated coconut oil, or palm oil (15, 25, 31). These mice were inoculated with a lethal dose of a virulent L. monocytogenes strain, a facultative intracellularly growing bacterium that has been used as a model of infectious and pathogenic processes. After the administration of this diet different results were observed. Bacterial clearance from liver or spleen was increased in these animals (25, 31), bactericidal activity of peritoneal cells was significantly reduced (62), and cytotoxic effects due to bacterial infection were increased (63), whereas the susceptibility of cells to adhesion or invasion by L. monocytogenes infection was substantially modified (63). These observations indicate the loss of capacity of the immune system from animals fed a diet containing fish oil to destroy and eliminate the infectious agents (25, 31, 62, 63). A recent investigation has contributed to explaining in part the reasons for which n-3 polyunsaturated fatty acids reduce host defense against L. monocytogenes: consumption of EPA or DHA (both contained in fish oil) impairs the production of IL-12 and IFN-γ, cytokines that play an essential role in the innate and adaptive responses of host immune system (32). Hence, the reduction of IL-12 levels may explain the impaired bacterial clearance and the reduction of survival to L. monocytogenes infection (33). Another possible explanation for the reduction of host resistance is based on the inhibition of the expression of MHC class II (called Ia in mice) that is reduced in mice fed a fish oil diet and infected with L. monocytogenes (37, 55). Similarly, it is important to note that these effects may be associated with the reported increase of reactive oxygen species, whose numbers were significantly increased in mice after the animals were experimentally infected with this bacterium and fed a diet containing hydrogenated coconut oil (63). In fact, the role of cellular oxidative processes requires considerable attention because they play an important role in the elimination of infectious agents.
However, not all polyunsaturated fatty acids are capable of exerting the same effects, since many studies are not in accordance with the previously described arguments. Thus, a recent investigation has determined that conjugated linoleic acid does not alter the resistance of mice after L. monocytogenes infection (74). Similarly, a previous study indicated the irrelevant effect of dietary lipid manipulation on the survival of mice in two models of peritonitis, one with Pseudomonas aeruginosa and the other with Salmonella enterica serovar Typhimurium (18).
Irrespective of these findings, other experimental studies have demonstrated the adverse effects of dietary lipids containing n-3 or n-6 polyunsaturated fatty acids in the impairment of immune functions in animals experimentally infected with different pathogenic agents such as S. enterica serovar Typhimurium (16, 29), P. aeruginosa (59), Staphylocococcus aureus (20), or Mycobacterium tuberculosis (58). By contrast, it has been reported that the administration of diets containing fish oil does not reduce the survival percentage after experimental infection with Klebsiella pneumoniae (4). In fact, dietary fish oil supplementation increased resistance to infection due to an enhancement of IL-1 and TNF production by peritoneal cells in mice (5, 6). It is probable that the discrepancy in these findings is due to different factors that include the duration of the supplementation period, the concentration of fatty acids used, or the cell population affected by fatty acid administration.
Finally, experimental observations with viruses have also demonstrated that influenza virus infection in animals fed fish oil diet delays the clearance due to an impairment of primary virus-specific T-cell cytotoxicity but has no effect on NK cytotoxicity (8, 9).
CONCLUDING REMARKS
Nutritional status is generally recognized as an essential factor involved in the modulation of immune response, which may be determinant in the development of the clinical effects derived from a malnutrition process (14). Thus, in recent years the effects that different dietary lipids exert upon immune functions have received considerable attention, because different functions of immune system are altered after dietary lipid administration. Hence, lymphocyte proliferation, cytokine production, phagocytic activity, adhesion molecule expression, and NK cell activity are susceptible to modification by the action of certain lipids in both animals and humans. Different mechanisms of action have been proposed to be involved in these processes, such as lipid peroxidation, changes in the plasma membrane, eicosanoid production, or alteration of gene expression. Both epidemiological and experimental studies have applied the beneficial properties associated to a modulation of the immune functions. Thus, different fats such as fish oil or olive oil have been applied in the amelioration of symptoms related to rheumatoid arthritis and other inflammatory disorders due to their anti-inflammatory properties. Similarly, they have also been applied as biological mediators capable of exerting a protective effect against colon or breast cancer, whereas other fatty acids such as n-6 polyunsaturated fatty acids exert a chemopromotive role in tumorigenic processes. In addition, these effects may be related to the ability of several fatty acids to modulate apoptosis by different pathways. Nevertheless, the immunosuppressant role associated with dietary polyunsaturated fatty acids containing fish oil may produce adverse effects because they reduce the host's natural resistance against pathogenic agents. Therefore, in the light of current experimental observations, the benefits of administering these dietary lipids should be weighed before they are applied as immunosuppression factors in order to prevent detrimental or adverse effects caused by an excessive suppression of immune functions.
REFERENCES
- 1.Allard, J. P., R. Kurian, E. Aghdassi, R. Muggli, and D. Royall. 1997. Lipid peroxidation during n-3 fatty acid and vitamin E supplementation in humans. Lipids 32:535-541. [DOI] [PubMed] [Google Scholar]
- 2.Arita, K., H. Kobuchic, T. Utsumia, Y. Takeharab, J. Akiyamad, A. A. Hortone, and K. Utsumib. 2001. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 62:821-828. [DOI] [PubMed] [Google Scholar]
- 3.Arrington, J. L., R. S. Chapkin, K. C. Switzer, J. S. Morris, and D. N. McMurray. 2001. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin. Exp. Immunol. 125:499-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornsson, S., I. Hardardottir, E. Gunnarsson, and A Haraldsson. 1997. Dietary fish oil supplementation increases survival in mice following Klebsiella pneumoniae infection. Scand. J. Infect. Dis. 29:491-493. [DOI] [PubMed] [Google Scholar]
- 5.Blok, W. L., M. B. Katan, and J. W. M. van der Meer. Modulation of inflammation and of cytokine production by dietary (n-3) fatty acids. J. Nutr. 126:1515-1533. [DOI] [PubMed]
- 6.Blok, W. L., M. T. Vogels, J. H. Curfs, W. M. Eling, W. A. Buurman, and J. W. van der Meer. Dietary fish-oil supplementation in experimental gram-negative infection and in cerebral malaria in mice. J. Infect. Dis. 165.:898-903. [DOI] [PubMed]
- 7.Blot, W. J., A. Lanier, J. F. Fraumeni, Jr., and T. R. Bender. 1975. Cancer mortality among Alaskan natives, 1960-69. J. Natl. Cancer Inst. 55:547-554. [DOI] [PubMed] [Google Scholar]
- 8.Byleveld, M., G. T. Pang, R. L. Clancy, and D. C. K. Roberts. 2000. Fish oil feeding enhances lymphocyte proliferation but impairs virus-specific T lymphocyte cytotoxicity in mice following challenge with influenza virus. Clin. Exp. Immunol. 119:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byleveld, P. M., G. T. Pang, R. L. Clancy, and D. C. Roberts. 1999. Fish oil feeding delays influenza virus clearance and impairs production of interferon-gamma and virus-specific immunoglobulin A in the lungs of mice. J. Nutr. 129:328-335. [DOI] [PubMed] [Google Scholar]
- 10.Calder, P. C. 1995. Fatty acids, dietary lipids and lymphocyte functions. Biochem. Soc. Trans. 23:302-309. [DOI] [PubMed] [Google Scholar]
- 11.Calder, P. C. 1998. Fat chance of immunomodulation. Immunol. Today 19:244-247. [DOI] [PubMed] [Google Scholar]
- 12.Calder, P. C., J. A. Bond, D. J. Harvey, S. Gordon, and E. A. Newsholme. 1990. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem. J. 269:807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caygill, C. P., A. Charlett, and M. J. Hill. 1996. Fat, fish, fish oil and cancer. Br. J. Cancer 74:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra, R. K. 1996. Nutrition, immunity and infection: from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc. Natl. Acad. Sci. USA 93:14304-14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra, R. K. 1996. Influence of palm oil on immune responses and susceptibility to infection in a mouse model. Nutr. Res. 16:61-68. [Google Scholar]
- 16.Chang, H. R., A. G. Dulloo, I. R. Vladoianu, P. F. Piguet, D. Arsenijevic, L. Girardier, and J. C. Pechere. 1992. Fish oil decreases natural resistance of mice to infection with Salmonella typhimurium. Metabolism 41:1-2. [DOI] [PubMed] [Google Scholar]
- 17.Chang, W. L., R. S. Chapkin, and J. R. Lupton. 1998. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J. Nutr. 128:491-497. [DOI] [PubMed] [Google Scholar]
- 18.Clouva-Molyvdas, P., M. D. Peck, and J. W. Alexander. 1992. Short-term dietary lipid manipulation does not affect survival in two models of murine sepsis. J. Parenter. Enteral Nutr. 16:343-347. [DOI] [PubMed] [Google Scholar]
- 19.Collett, E. D., L. A. Davidson, Y. Y. Fan, J. R. Lupton, and R. S. Chapkin. 2001. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am. J. Physiol. Cell Physiol. 280:1066-1075. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambola, J. B., E. E. Aeberhard, N. Trang, S. Gaffar, C. T. Barrett, and M. P. Sherman. 1991. Effect of dietary (n-3) and (n-6) fatty acids on in vivo pulmonary bacterial clearance by neonatal rabbits. J. Nutr. 121:1262-1269. [DOI] [PubMed] [Google Scholar]
- 21.Das, U. N. 1999. Essential fatty acids, lipid peroxidation and apoptosis. Prostaglandins Leukot. Essent. Fatty Acids 61:157-163. [DOI] [PubMed] [Google Scholar]
- 22.de Pablo, M. A., and G. Alvarez de Cienfuegos. 2000. Modulatory effects of dietary lipids on immune system functions. Immunol. Cell Biol. 78:31-39. [DOI] [PubMed] [Google Scholar]
- 23.de Pablo, M. A., E. Ortega, A. M. Gallego, C. Alvarez, P. L. Pancorbo, and G. Alvarez de Cienfuegos. 1998. The effect of dietary fatty acid manipulation on phagocytic activity and cytokine production by peritoneal cells from Balb/c mice. J. Nutr. Sci. Vitaminol. 44:57-67. [DOI] [PubMed] [Google Scholar]
- 24.de Pablo, M. A., E. Ortega, A. M. Gallego, C. Alvarez, P. L. Pancorbo, and G. Alvarez de Cienfuegos. 1998. Influence of diets containing olive oil, sunflower oil or hydrogenated coconut oil on the immune response of mice. J. Clin. Biochem. Nutr. 25:11-23. [Google Scholar]
- 25.de Pablo, M. A., M. A. Puertollano, A. Galvez, E. Ortega, J. J. Gaforio, and G. Alvarez de Cienfuegos. 2000. Determination of natural resistance of mice fed dietary lipids to experimental infection induced by Listeria monocytogenes. FEMS Immunol. Med. Microbiol. 27:127-133. [DOI] [PubMed] [Google Scholar]
- 26.de Pablo, M. A., M. A. Puertollano, and G. Alvarez de Cienfuegos. 2000. Immune cell functions, lipids and host natural resistance. FEMS Immunol. Med. Microbiol. 29:323-328. [DOI] [PubMed] [Google Scholar]
- 27.de Pablo, M. A., S. A. Susin, E. Jacotot, N. Larochette, P. Costantini, L. Ravagnan, N. Zanzami, and G. Kroemer. 1999. Palmitate induces apoptosis via a direct effect on mitochondria. Apoptosis 4:81-87. [DOI] [PubMed] [Google Scholar]
- 28.Dyerberg, J., and H. O. Bang. 1979. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet 2:433-435. [DOI] [PubMed] [Google Scholar]
- 29.Eicher, S. D., and D. S. McVey. 1995. Dietary modulation of Kupffer cell and splenocyte function during a Salmonella typhimurium challenge in mice. J. Leukoc. Biol. 58:32-39. [DOI] [PubMed] [Google Scholar]
- 30.Endres, S., R. Ghorbani, V. E. Kelley, K. Georgilis, G. Lonemann, J. M. W. van der Meer, J. G. Cannon, T. S. Rogers, M. S. Klempner, P. C. Weber, E. J. Schaeffer, M. S. Wolff, and C. A. Dinarello. 1989. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 320:265-271. [DOI] [PubMed] [Google Scholar]
- 31.Fritsche, K. L., L. M. Shahbazian, C. Feng, and J. N. Berg. 1997. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin. Sci. 92:95-101. [DOI] [PubMed] [Google Scholar]
- 32.Fritsche, K. L., M. Anderson, and C. Feng. 2000. Consumption of eicosapentaenoic acid and docosahexaenoic acid impair murine interleukin-12 and interferon-gamma production in vivo. J. Infect. Dis. 182:S54-S61. [DOI] [PubMed] [Google Scholar]
- 33.Fritsche, K. L., M. Byrge, and C. Feng. 1999. Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol. Lett. 65:167-173. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin, J. S., and J. Ceuppens. 1983. The regulation of immune responses by prostaglandins. J. Clin. Immunol. 3:295-308. [DOI] [PubMed] [Google Scholar]
- 35.Granato, D., S. Blum, C. Rössle, J. Le Boucher, A. Malnoë, and G. Dutot. 2000. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. J. Parenter. Enteral Nutr. 24:113-118. [DOI] [PubMed] [Google Scholar]
- 36.Horrobin, D. F. 1987. Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of eicosapentaenoic acid (EPA), a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med. Hypotheses 22:421-428. [DOI] [PubMed] [Google Scholar]
- 37.Huang, S. C., M. L. Misfeld, and K. L. Fritsche. 1992. Dietary fat influences Ia expression and immune cell populations in the murine peritoneum and spleen. J. Nutr. 122:1219-1231. [DOI] [PubMed] [Google Scholar]
- 38.Hughes, D. A., A. C. Pinder, Z. Piper, I. T. Johnson, and E. K. Lund. 1996. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 63:267-272. [DOI] [PubMed] [Google Scholar]
- 39.Hughes, D. A., and A. C. Pinder. 2000. N-3 polyunsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am. J. Clin. Nutr. 71:357S-360S. [DOI] [PubMed] [Google Scholar]
- 40.Jeffery, N. M., P. Yaqoob, E. A. Newsholme, and P. C. Calder. 1996. The effects of olive oil upon rat serum lipid levels and lymphocyte functions appear to be due to oleic acid. Ann. Nutr. Metab. 40:71-80. [DOI] [PubMed] [Google Scholar]
- 41.Jenski, L. J., G. M. Bowker, M. A. Johnson, W. D. Ehringer, T. Fetterhoff, and W. Stillwell. 1995. Docosahexaenoic acid-induced alteration of Thy-1 and CD8 expression on murine splenocytes. Biochim. Biophys. Acta 1236:39-50. [DOI] [PubMed] [Google Scholar]
- 42.Jump, D. B., and S. D. Clarke. 1999. Regulation of gene expression by dietary fat. Annu. Rev. Nutr. 19:63-90. [DOI] [PubMed] [Google Scholar]
- 43.Kelley, D. S., P. C. Taylor, G. J. Nelson, and B. E. Mackey. 1998. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids 33:559-566. [DOI] [PubMed] [Google Scholar]
- 44.Kremer, J., D. L. Lawrence, W. Jubiz, R. DiGiacomo, R. Rynes, L. E. Bartholomew, and M. Sherman. 1990. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis: clinical and immunologic effects. Arthritis Rheum. 33:810-820. [DOI] [PubMed] [Google Scholar]
- 45.Kremer, J. M. 2000. n-3 fatty acid supplements in rheumatoid arthritis. Am. J. Clin. Nutr. 71:349S-351S. [DOI] [PubMed] [Google Scholar]
- 46.Kremer, J. M. 1996. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids 31:S243-S247. [DOI] [PubMed] [Google Scholar]
- 47.Kromann, N., and A. Green. 1980. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Med. Scand. 208:401-406. [PubMed] [Google Scholar]
- 48.Linos, A., E. Kaklamanis, A. Kontomerkos, Y. Koumantaki, S. Gazi, G. Vaiopoulos, G. C. Tsokos, and P. Kaklamanis. 1991. The effect of olive oil and fish consumption on rheumatoid arthritis—a case control study. Scand. J. Rheumatol. 20:419-426. [DOI] [PubMed] [Google Scholar]
- 49.Liu, G., D. M. Bibus, A. M. Bode, W. Y. Ma, R. T. Holam, and Z. Dong. 2001. Omega 3 but not omega 6 fatty acids inhibit AP-1 activity and cell transformation in JB6 cells. Proc. Natl. Acad. Sci. USA 98:7510-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo, C. J., K. C. Chiu, M. Fu, R. Lo, and S. Helton. 1999. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering Nfkappa B activity. J. Surg. Res. 82:216-221. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Moreno, J. M., W. C. Willett, and L. Gorgojo. 1994. Dietary fat, olive intake and breast cancer risk. Int. J. Cancer. 58:774-780. [DOI] [PubMed] [Google Scholar]
- 52.McMurray, D. N., C. A. Jolly, and R. S. Chapkin. 2000. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model. J. Infect. Dis. 182:S103-S107. [DOI] [PubMed] [Google Scholar]
- 53.Meydani, S. N., S. Endres, M. M. Woods, B. R. Goldin, C. Soo, A. Morrill-Labrode, C. A. Dinarello, and S. L. Gorbach. 1991. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J. Nutr. 121:547-555. [DOI] [PubMed] [Google Scholar]
- 54.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 55.Mosquera, J., B. Rodriguez-Iturbe, and G. Parra. 1990. Fish oil dietary supplementation reduces Ia expression in rat and mouse peritoneal macrophages. Clin. Immunol. Immunopathol. 56:124-129. [DOI] [PubMed] [Google Scholar]
- 56.Moussa, M., J. Le Boucher, J. Garcia, J. Tkaczuk, J. Ragab, G. Dutot, E. Ohayon, J. Ghisolfi, and J. P. Thouvenot. 2000. In vivo effects of olive oil-based lipid emulsion on lymphocyte activation in rats. Clin. Nutr. 19:49-54. [DOI] [PubMed] [Google Scholar]
- 57.Netea, M. G., B. J. Kullberg, W. L. Blok, and J. W. M. Van der Meer. 1999. Immunomodulation by n-3 polyunsaturated fatty acids. Immunol. Today 20:103.. [DOI] [PubMed] [Google Scholar]
- 58.Paul, K. P., M. Leichsenring, M. Pfisterer, E. Mayatepek, D. Wagner, M. Domann, H. G. Sonntag, and H. J. Bremer. 1997. Influence of n-6 and n-3 polyunsaturated fatty acids on the resistance to experimental tuberculosis. Metabolism 46:619-624. [DOI] [PubMed] [Google Scholar]
- 59.Peck, M. D., J. W. Alexander, C. K. Ogle, and G. F. Babcock. 1990. The effect of dietary fatty acids on response to Pseudomonas infection in burned mice. J. Trauma 30:445-452. [PubMed] [Google Scholar]
- 60.Penninger, J. M., and G. Kroemer. 1998. Molecular and cellular mechanisms of T lymphocyte apoptosis. Adv. Immunol. 68:51-144. [DOI] [PubMed] [Google Scholar]
- 61.Puertollano, M. A., I. Algarra, E. Ortega, M. A. de Pablo, and G. Alvarez de Cienfuegos. 2001. Loss of cell natural killer cell activity after murine tumor transplantation appears as a consequence of dietary lipid administration. Anticancer Res. 21:2697-2702. [PubMed] [Google Scholar]
- 62.Puertollano, M. A., M. A. de Pablo, and G. Alvarez de Cienfuegos. 2001. Immunomodulatory effects of dietary lipids alter host natural resistance of mice to Listeria monocytogenes infection. FEMS Immunol. Med. Microbiol. 32:47-52. [DOI] [PubMed] [Google Scholar]
- 63.Puertollano, M. A., M. A. de Pablo, and G. Alvarez de Cienfuegos. 2002. Relevance of dietary lipids as modulators of immune functions in cells infected with Listeria monocytogenes. Clin. Diagn. Lab. Immunol. 9:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao, C. V., Y. Hirose, C. Indranie, and B. S. Reddy. 2001. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 61:1927-1933. [PubMed] [Google Scholar]
- 65.Reddy, B. S., and H. Maruyama. 1986. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 46:3367-3370. [PubMed] [Google Scholar]
- 66.Reddy, B. S., C. Burill, and J. Rigotty. 1991. Effect of diets high in omega-3 and omega-6 fatty acids on initiation and postinitiation stages of colon carcinogenesis. Cancer Res. 51:487-491. [PubMed] [Google Scholar]
- 67.Reddy Avula, C. P., A. K. Zaman, R. Lawrence, and G. Fernandes. 1999. Induction of apoptotic mediators in Balb/c splenic lymphocytes by dietary n-3 and n-6 fatty acids. Lipids 34:921-927. [DOI] [PubMed] [Google Scholar]
- 68.Risio, M., M. Lipkin, H. Newmark, K. Yang, F. P. Rossini, V. E. Steele, C. W. Boone, and G. J. Kelloff. 1996. Apoptosis, cell replication, and Western-style diet-induced tumorigenesis in mouse colon. Cancer Res. 56:4910-4916. [PubMed] [Google Scholar]
- 69.Sasaki, T., Y. Kanke, M. Nagahashi, M. Toyokawa, M. Matsuda, J. Shimizu, Y. Misawa, and T. Takita. 2000. Dietary docosahexaenoic acid can alter the surface expression of CD4 and CD8 on T cells in peripheral blood. J. Agric. Food Chem. 48:1047-1049. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro, J. A., T. D. Koepsell, L. F. Voigt, C. E. Dugowson, M. Kestin, and J. L. Nelson. 1996. Diet and rheumatoid arthritis in women: a possible protective effect of fish consumption. Epidemiology 7:256-263. [DOI] [PubMed] [Google Scholar]
- 71.Stoneham, M., M. Goldacre, V. Seagroatt, and L. Gill. 2000. Olive oil, diet and colorectal cancer: an ecological study and a hypothesis. J. Epidemiol. Community Health 54:756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trichopoulos, A., K. Katsouyanni, S. Stuver, L. Tzala, C. Gnardellis, E. Rimm, and D. Trichopoulos. 1995. Consumption of olive oil and specific food groups in relation to breast cancer risk in Greece. J. Natl. Cancer Inst. 87:110-116. [DOI] [PubMed] [Google Scholar]
- 73.Trichopoulos, A., P. Lagiou, H. Kuper, and D. Trichopoulos. 2000. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomarkers Prev. 9:869-873. [PubMed] [Google Scholar]
- 74.Turnock, L., M. Cook, H. Steinberg, and C. Czuprynski. 2001. Dietary supplementation with conjugated linoleic acid does not alter the resistance of mice to Listeria monocytogenes infection. Lipids 36:135-138. [DOI] [PubMed] [Google Scholar]
- 75.Tyurina, Y. Y., V. A. Tyurin, G. Carta., P. J. Quinn, N. F. Schor, and V. E. Kagan. 1997. Direct evidence for antioxidant effect of Bcl-2 in PC12 rat pheochromocytoma cells. Arch. Biochem. Biophys. 344:413-423. [DOI] [PubMed] [Google Scholar]
- 76.Van den Brink, G. R., D. E. M. van den Boogaardt, S. J. H. van Deventer, and M. P. Peppelenbosch. 2002. Feed a cold, starve a fever? Clin. Diagn. Lab. Immunol. 9:182-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wallace, F. A., E. A. Miles, C. Evans, T. E. Stock, P. Yaqoob, and P. C. Calder. 2001. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J. Leukoc. Biol. 69:449-457. [PubMed] [Google Scholar]
- 78.Wang, Y. W., C. J. Field, and J. S. Sim. 2000. Dietary polyunsaturated fatty acids alter lymphocyte subset proportion and proliferation, serum immunoglobulin G concentration, and immune tissue development in chicks. Poult. Sci. 79:1741-1748. [DOI] [PubMed] [Google Scholar]
- 79.Yaqoob, P., and P. C. Calder. 1995. The effects of dietary lipid manipulation on the production of murine T-cell-derived cytokines. Cytokine 7:548-553. [DOI] [PubMed] [Google Scholar]
- 80.Yaqoob, P., E. A. Newsholme, and P. C. Calder. 1994. Inhibition of natural killer cell activity by dietary lipids. Immunol. Lett. 41:241-247. [DOI] [PubMed] [Google Scholar]
- 81.Yaqoob, P., E. A. Newsholme, and P. C. Calder. 1994. The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology 82:603-610. [PMC free article] [PubMed] [Google Scholar]
- 82.Yaqoob, P., E. A. Newsholme, and P. C. Calder. 1995. Influence of cell culture conditions on diet-induced changes in lymphocyte fatty acid composition. Biochim. Biophys. Acta 1255:333-340. [DOI] [PubMed] [Google Scholar]
- 83.Yaqoob, P., J. A. Knapper, D. H. Webb, C. M. Williams, E. A. Newsholme, and P. C. Calder. 1998. Effect of olive oil on immune function in middle-aged men. Am. J. Clin. Nutr. 67:129-135. [DOI] [PubMed] [Google Scholar]