Cytokines are important mediators for host defense and inflammation to bacterial infections. Chlamydia pneumoniae, an obligate intracellular bacterium that causes acute respiratory infections in human hosts, has been shown to induce cytokine expression in various human and murine model systems (2, 7). In most in vitro studies of C. pneumoniae, a centrifugation step is employed, and it has been demonstrated that the addition of this centrifugation step can increase the formation of inclusion bodies (6, 9). However, the stress of centrifugation may also affect some cellular activities, such as proliferation and gene expression; certain cytokines, such as interleukin-1β (IL-1β), can also be induced under stress conditions (3, 4, 8). Therefore, the effects of centrifugation of cells should be considered when performing in vitro studies of C. pneumoniae, particularly studies using lung epithelial cells, which serve as a major target for C. pneumoniae infection.
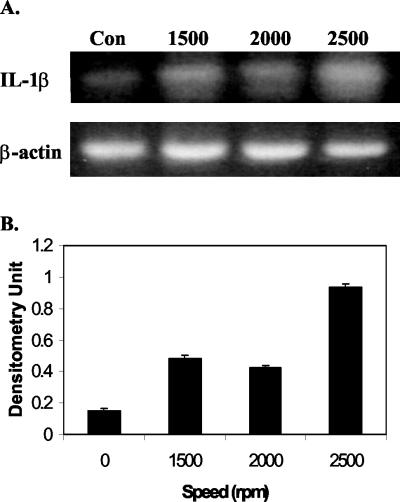
We intended to determine if centrifugation stress can affect the expression of proinflammatory cytokine IL-1β in a human lung epithelial carcinoma A549 cell line. Centrifugation of A549 cells for 1 h at 2,000 rpm (∼760 × g) at room temperature did not affect cell proliferation or cell viability. Furthermore, C. pneumoniae infection with or without centrifugation (direct inoculation) also did not alter cell proliferation or viability. The effect of centrifugation stress on IL-1β expression was then examined by reverse transcription-PCR (RT-PCR). The primers used were 5′-AAA CAG ATG AAG TGC TCC TTC CAG G-3′ and 5′-TGG AGA ACA CCA CTT GTT GCT CCA-3′ (1). β-Actin was used as an amplification control, detected by primer sequences 5′-CGG GAC CTG ACT GAC TAC-3′ and 5′-GAA GGA AGG CTG GAA GAG-3′ (5). As shown in Fig. 1, the stress of centrifugation clearly increased the expression of the IL-1β gene. This increase in expression of IL-1β was present even at 24 h after centrifugation.
FIG. 1.
Centrifugation stress up-regulates IL-1β gene expression. A549 cells were centrifuged at 427 × g, 760 × g, or 1,187 × g for 1 h at room temperature. Four hours later, total RNA was extracted, and cellular levels of IL-1β were measured by RT-PCR. β-Actin was used as internal control (Con). (A) Electrophoretogram of RT-PCR. The RT-PCR products were run on agarose gels, stained with ethidium bromide, and visualized using UV. (B) Densitometric quantification of the electrophoretogram in panel A after normalization to β-actin.
The inclusion bodies of C. pneumoniae in both centrifuged and uncentrifuged A549 cells were also counted, and the inclusion body formation units (IFU) were calculated as (3.58 ± 0.61) × 108 and (1.03 ± 0.49) × 108 IFU/ml, respectively. There was a significant difference between the two values as determined by the Student t test (P < 0.01), suggesting that centrifugation does increase the formation of inclusion bodies. However, even without the centrifugation, C. pneumoniae could still efficiently infect A549 cells.
Although centrifugation has been shown to improve inclusion body formation, it is always better to eliminate as many confounding factors as possible that may interfere with the host-pathogen interactions. In this case, we have shown that the stress of centrifugation alone can up-regulate the expression of the IL-1β gene without affecting cell proliferation and viability. Thus, the IL-1β induction data from some earlier studies where centrifugation was used to infect cells may be compromised. Therefore, reevaluation of earlier data to identify the true effects of C. pneumoniae infection on cytokine expression may be warranted. Although we did not test the effects of centrifugation on cytokines other than IL-1β, they may also be affected by centrifugation. Cellular responses to stress are very complex, and different cell types have differing responses to the same stressor. Thus, it is noted that the results we observed using A549 cells may or may not be applicable to other cell types. Proper controls should always be run to clarify the role that the stress of centrifugation plays in cellular cytokine gene expression.
Acknowledgments
We gratefully thank the colleagues of the Respiratory Diseases Laboratory Section at the Centers for Disease Control and Prevention for their help and advice. J. Yang is an American Society for Microbiology/National Center for Infectious Diseases Postdoctoral Research Associate.
REFERENCES
- 1.Gan, X., and B. Bonavida. 1999. Preferential induction of TNF-α and IL-1β and inhibition of IL-10 secretion by human peripheral blood monocytes by synthetic aza-alkyl lysophospholipids. Cell. Immunol. 193:125-133. [DOI] [PubMed] [Google Scholar]
- 2.Jahn, H.-U., M. Krull, F. N. Wuppermann, A. C. Klucken, S. Rosseau, J. Seybold, J. H. Hegemann, C. A. Jantos, and N. Suttorp. 2000. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J. Infect. Dis. 182:1678-1687. [DOI] [PubMed] [Google Scholar]
- 3.Korneva, E. A., S. N. Shanin, and E. G. Rybakina. 2001. The role of interleukin-1 in stress-induced changes in immune system functions. Neurosci. Behav. Physiol. 31:431-437. [DOI] [PubMed] [Google Scholar]
- 4.Lee, D. H., J. C. Park, and H. Suh. 2001. Effect of centrifugal force on cellular activity of osteoblastic MC3T3-E1 cells in vitro. Yonsei Med. J. 42:405-410. [DOI] [PubMed] [Google Scholar]
- 5.Liechty, K. W., T. M. Cromblehome, D. L. Cass, B. Martin, and N. S. Adzick. 1998. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J. Surg. Res. 77:80-84. [DOI] [PubMed] [Google Scholar]
- 6.Pruckler, J. M., N. Masse, V. A. Stevens, L. Gang, Y. Yang, E. R. Zell, S. F. Dowell, and B. S. Fields. 1999. Optimizing culture of Chlamydia pneumoniae by using multiple centrifugations. J. Clin. Microbiol. 37:3399-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn, T. C., and C. A. Gaydos. 1999. In vitro infection and pathogenesis of Chlamydia pneumoniae in endovascular cells. Am. Heart J. 138:S507-S511. [DOI] [PubMed] [Google Scholar]
- 8.Theilig, C., A. Bernd, G. Leyhausen, R. Kaufmann, and W. Geurtsen. 2001. Effects of mechanical force on primary human fibroblasts derived from the gingiva and the periodontal ligament. J. Dent. Res. 80:1777-1800. [DOI] [PubMed] [Google Scholar]
- 9.Tjhie, J. H. T., R. Roosendaal, D. M. MacLaren, and C. M. Yandenbroucke-Grauls. 1997. Improvement of growth of Chlamydia pneumoniae on HEp-2 cells by pretreatment with polyethylene glycol in combination with additional centrifugation and extension of culture time. J. Clin. Microbiol. 35:1883-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]