Abstract
Anthrax, a potentially fatal infection, is a virulent and highly contagious disease. It is caused by a gram-positive, toxigenic, spore-forming bacillus: Bacillus anthracis. For centuries, anthrax has caused disease in animals and, although uncommonly, in humans throughout the world. Descriptions of this naturally occurring disease begin in antiquity. Anthrax is primarily a disease of herbivores, which are infected by ingestion of spores from the soil. With the advent of modern microbiology, Pasteur developed the first successful anthrax vaccine in 1881. The incidence of the disease has continually decreased since the late 19th century, and animal vaccination programs drastically reduced the animal mortality from the disease. However, anthrax spores continue to be documented in soil samples from throughout the world. Research on anthrax as a biological weapon began more than 80 years ago, and today at least 17 nations are believed to have offensive biological weapons programs that include anthrax. Recent events in the USA have shown how society is affected by both hoax and real threats of anthrax bioweapons. This fourth article in the series on weapons of biowarfare/bioterrorism summarizes the historical background of anthrax as well as clinical and laboratory information useful for bioterrorism preparedness.
Infectious Disease is one of the few genuine adventures left in the world.
—Hans Zinsser (1878–1940)
Of the numerous biological agents that could be used as a biological weapon, anthrax is particularly suitable because it can cause widespread illness and death and eventually cripple a city or region (1, 2). The Centers for Disease Control and Prevention (CDC) has classified Bacillus anthracis as a category A organism. Organisms in this category are easily disseminated and/or transmitted from person to person, resulting in high mortality rates. However, Bacillus anthracis, which can be transmitted easily with the proper technical know-how, does not result in person-to-person transmission. Mortality rates in patients with inhalational anthrax are high despite appropriate antibiotic treatment. The Biological Weapons and Toxins Convention of 1972 prohibited offensive bioweapons research and development and was signed by most countries. However, the former Soviet Union (FSU) and Iraq, both signatories of the convention, have subsequently acknowledged offensive bioweapons research and production. Considering the events that followed the World Trade Center attacks on September 11, 2001, it seems opportune to review the disease of anthrax and its causative organism, B. anthracis.
This article in the series of papers addressing biowarfare and bioterrorism is based on an extensive literature review, which included the Consensus Statements of the Working Group on Civilian Biodefense regarding anthrax (3). This consensus paper served as the basis of the final risk assessment in 1999 and as material for the re-evaluation of the current threat. The final draft published in May 1999 provided a good basis for the development of strategies to counteract the potential threats posed by the use of anthrax as a bioweapon and was helpful in the response to the attacks in 2001. However, because the threat of anthrax as a weapon of bioterrorism continues to exist, these conclusions and recommendations need to be regularly reassessed as new information becomes available. The medical community should continue its education and preparedness efforts to ensue a calm and reasoned response to those threats.
THE HISTORY OF ANTHRAX AND THE CURRENT THREAT
For centuries, anthrax has caused disease in animals and, although uncommonly, in humans throughout the world. Human anthrax in its various forms (inhalational, cutaneous, and gastrointestinal) is historically a disease of those with close contact with animals or animal products contaminated with B. anthracis spores. The disease is very well described in texts of antiquity, and it has been suggested that the famous Plague of Athens (430–427 bc) was an epidemic of inhalational anthrax (4). In fact, the term anthrax is derived from the Greek word anthracites, meaning coal-like, referring to the typical black eschar seen in the cutaneous form of the disease.
Another excellent ancient description is that of the murrain of Noricum (the ancient Roman name for the Danube River delta and the eastern Alps) by the Roman poet Virgil (5). Virgil (70–19 bc), most renowned for his Aeneid, also wrote four Georgics, didactic verse works on agriculture. The third Georgic is devoted to animal husbandry and contains a section on veterinary medicine. It details an epizootic that occurred in the Roman district of Noricum. The disease affected sheep, cattle, and horses, as well as dogs and other domestic and wild animals. The symptoms of anthrax are described in great detail, and although the narrative contains some errors and traces of poetic license, it includes much factual material, showing that Virgil indeed understood the hardiness of the infectious source, as well as the potential for transmission among animals and humans.
Anthrax continued to affect livestock and humans throughout the Middle Ages. During the 18th century, anthrax epidemics destroyed approximately half the sheep population in Europe (6). In Victorian England the disease became known as “woolsorters' disease” because it was frequently observed among mill workers exposed to animal fibers contaminated with B. anthacis spores. However, the name is somewhat a misnomer: the infection was more often a result of contact with goat hair or alpaca than with wool and sheep (7). Other names for the disease included “ragpickers' disease,” charbon, milzbrand, black bain, “tanners' disease,” and Siberian (splenic) fever.
In the 19th century, anthrax was a major point of interest in developing biomedical research. In 1850, Pierre Rayer and Casimir-Joseph Davaine discovered small filiform bodies “about twice the length of a blood corpuscle” in the circulation of sheep with anthrax (8). Initially, this discovery was not given any significance, as the filiform bodies were regarded as disease products. However, Davaine subsequently suggested that the corpuscles described were organisms causing the disease.
In the 1870s, anthrax was extensively studied by several researchers in Europe, including Robert Koch in Berlin and Louis Pasteur in Paris (Figure 1). In 1876, Koch, using suspended drop culture techniques, was able to trace the complete life cycle of the anthrax bacillus for the first time in history. He found that the bacillus formed spores that could remain viable for long periods even in unfavorable environmental conditions (8). Furthermore, he postulated that the anthrax bacillus could be transmitted from one host to another, and in 1877 he grew the organism in vitro and induced the disease in healthy animals by inoculating them with material from these bacterial cultures. Anthrax served as the prototype for Koch's famous postulates regarding the transmission of infectious diseases.
Figure 1.
(a) Robert Koch and (b) Louis Pasteur. Courtesy of the Images from the History of Medicine database of the National Library of Medicine.
However, at the same time, Louis Pasteur felt that Koch's work was inconclusive and announced his goal to provide his own incontrovertible demonstration of infectious disease transmission. As one can imagine, this incited a long and acrimonious dispute between the two men. In May 1881, Louis Pasteur inoculated 25 cattle with his anthrax vaccine at a farm in Pouilly-le-Fort, a small village outside of Paris (8, 9). This first vaccine contained live attenuated organisms. Subsequently, Pasteur inoculated the vaccinated animals as well as other cattle with a virulent strain of B. anthracis. All the vaccinated animals survived; however, the others died. To Pasteur, it was this experiment and not the work of Robert Koch that had proven the germ theory of disease.
From our perspective, it may seem insignificant whose work finally provided the proof for the germ theory of disease. Together, the work of both Koch and Pasteur, who were highly regarded medical authorities, led to a broad acceptance of their theories and opened possibilities for further work in medical microbiology.
In the early 1900s, human cases of anthrax continued to occur occasionally, and human cases of inhalational anthrax were reported in the USA among workers in the textile and tanning industries that processed goat hair, goat skin, and wool (7, 10, 11). With improvements in industrial hygiene practices and restrictions on imported animal products, the number of cases fell dramatically in the latter parts of the 20th century. However, death rates remained high (>85%) when inhalational anthrax occurred. Among animal-processing workers and farmers, the decrease of anthrax was postulated to be due to vaccination of both animals and humans, together with improvements in animal husbandry and processing of animal products (9, 12). In the 1950s, a human anthrax vaccine was developed by the US Army Chemical Corps. After nearly 20 years of use, it was replaced by a new, improved, and licensed vaccine in 1970 (13). In 1997, the US armed forces mandated vaccination for all active and reserve troops. However, this resulted in some well-publicized refusals by military personnel to be vaccinated based on concerns about the vaccine's safety (13, 14).
Although naturally occurring human anthrax has significantly decreased over the past century, it remains fairly common worldwide, particularly in Asia and Africa, with an annual occurrence of 20,000 to 100,000 cases recorded during the first half of the 20th century. In addition, it remains common among herbivores worldwide, and large epidemics of animal anthrax are reported on occasion in Africa, Asia, and South America. During a large outbreak in Iran in 1945, 1 million sheep died (15).
In humans, cutaneous anthrax is now the most common form of anthrax worldwide, with an estimated 2000 cases reported annually (16). In the USA, 224 cases of cutaneous anthrax were reported between 1945 and 1994 (17). The largest reported epidemic occurred in Zimbabwe between 1979 and 1985, when more than 10,000 human cases of anthrax were reported, nearly all of them cutaneous. Gastrointestinal anthrax is a rather rare form of the disease in humans, with only a few cases being reported worldwide (16, 18, 19). However, outbreaks have been reported on occasion in Africa and Asia (20-23). Typically, gastrointestinal anthrax follows the consumption of insufficiently cooked contaminated meat. In 1982, 24 cases of oropharyngeal anthrax were reported from a rural area in northern Thailand, where the outbreak followed consumption of contaminated buffalo meat (20). In 1987, 14 cases of gastrointestinal and oropharyngeal anthrax were reported from northern Thailand (22). As mentioned before, the incidence of inhalational anthrax rapidly declined in the second half of the 20th century.
In 1980, Philip Brachman of the CDC published a review of inhalational anthrax (7). Only 18 cases were reported in the USA between 1900 and 1978, with the majority of those cases occurring in special risk groups, including goat hair mill or goatskin workers and wool and tannery workers. Two of these cases were laboratory-related accidents, and 16 cases were fatal (7). Brachman concluded with what was the common understanding in the western medical community: inhalational anthrax was “now primarily of historical interest.” This opinion was not to be borne out by subsequent events.
The conception of Koch's postulates and the development of modern microbiology during the 19th century made the production of stocks of specific pathogens possible, and several countries worked to develop these agents for biological warfare purposes (1). Substantial evidence suggests the existence of ambitious biological warfare programs in Germany, England, and France during World War I. These programs allegedly involved covert operations featuring agents such as B. anthracis (anthrax) and Pseudomonas pseudomallei (glanders) (1, 24, 25).
During World War II, some of the aforementioned countries, as well as other countries like Russia and Japan, began biological warfare research programs. Various allegations and countercharges clouded the events during and after World War II. Japan conducted biological weapons research in occupied Manchuria from approximately 1932 until the end of World War II (1). Again, B. anthracis was among the organisms most extensively researched and used. Although the German biowarfare program during World War II was small compared with that of other nations, medical researchers infected prisoners with disease-producing organisms like Rickettsia prowazeki, hepatitis A virus, and malaria (2, 26). Despite these efforts, which clearly lagged behind those of other countries, a German offensive biological weapons program never fully materialized.
On the other hand, German officials accused the Allies of using biological weapons. Some of these allegations were believable, since the British were experimenting with at least one organism of biological warfare: B. anthracis Dr. Paul Fildes headed the British effort at Porton Down in the 1940s. By November 1940 he had determined that the most effective way to use a biological warfare agent would be to disseminate an aerosol of particles that could be retained in the lungs. It appeared that the most suitable device for dissemination was a bursting ammunition filled with a liquid suspension of bacteria, so that effective concentrations of bacteria would be inhaled by everyone in the target area (27, 28). The British biological warfare program concentrated on B. anthracis, and bomb experiments of weaponized spores of B. anthracis were conducted on Gruinard Island near the northwest coast of Scotland (29). The so-called N bomb contained 106 special bomblets filled with anthrax spores. These experiments led to heavy contamination of the island with persistence of viable spores. Events toward the end of World War II overtook plans to put these efforts into operation. In 1986, Gruinard Island was finally decontaminated using formaldehyde and seawater, but it remains restricted from public access.
Anthrax would be unlikely to cause severe disruption to military operations, although residual contamination of the ground would occur (19). This has been shown by the experiments on Gruinard Island. Therefore, anthrax is more of a danger to the civilian population. In 1970, a World Health Organization (WHO) expert committee estimated that an aircraft release of 50 kg of anthrax over an urban, developed population of 5 million would result in 250,000 casualties, of whom 95,000 would be expected to die without treatment and an additional 125,000 would be severely incapacitated (30). The strain on medical resources in such a scenario would be tremendous, ultimately leading to hospital bed requirements for 13,000 people, antibiotics for 60 days for 125,000 people, and the disposal of 95,000 dead. This would almost certainly result in a rapid and total breakdown in medical resources and civilian infrastructures. Newer assessments, including those made by the US Congress Office of Technology Assessment in 1993, confirmed the original WHO data (31). The CDC developed an economic model suggesting a cost of $26.2 billion per 100, 000 persons exposed in a bioweapons attack with anthrax (32).
Prior to the bioterrorism-related occurrence of inhalational anthrax in the USA in 2001, only one case had been reported in the country in the 20th century (33). In addition, the only large-scale outbreak of (inhalational) anthrax during the 20th century occurred in the FSU. On April 2, 1979, an epidemic of anthrax occurred among the citizens of Sverdlovsk (now Ekaterinburg), a city of 1.2 million people, 1400 km east of Moscow. The epidemic occurred among those who lived and worked within a narrow downwind zone of a Soviet military microbiology facility known as Compound 19. In addition, a large number of livestock died of anthrax in the same area, out to a distance of 50 km (1, 34).
The first reports of the outbreak emerged in October 1979 by way of a Russian-language newspaper in Frankfurt, West Germany, that was close to the Soviet émigré community. It ran a brief report about a major germ accident in Russia leading to deaths estimated in the thousands (35). At the same time, early European and US intelligence suspected that this facility conducted biological warfare research and attributed the epidemic to an accidental release of anthrax spores. Later, in early February 1980, the widely distributed German newspaper Bild Zeitung carried a story about an accident in a Soviet military settlement in Sverdlovsk in which an anthrax cloud had resulted (36). Afterwards, other major western newspapers and magazines began to take an interest in the anthrax outbreak in Sverdlovsk. In an initial 1980 publication, Soviet officials attributed the disease outbreak in Sverdlovsk to cutaneous and gastrointestinal anthrax caused by the consumption of contaminated meat. Later that year several articles occurred in Soviet medical, veterinary, and legal journals reporting an anthrax outbreak among livestock.
Little further information was published until 1986, when Matthew Meselson (Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA) renewed previously unsuccessful requests to Soviet officials to bring independent scientists to Sverdlovsk to investigate the incident (1, 36). His request finally resulted in the invitation to come to Moscow to discuss the incident with four Soviet physicians who had gone to Sverdlovsk to deal with the outbreak. They concluded that further investigation of the epidemiologic and patho-anatomical data was needed. In 1988, two of these Soviet physicians accepted an invitation to come to the USA for further discussions of the incident with government and private specialists. The Soviet Union maintained that the anthrax outbreak was caused by consumption of contaminated meat that was purchased on the black market, and according to the account of these two Soviet scientists, contaminated animals and meat from an epizootic south of the city caused 96 cases of human anthrax. Of these cases, 79 were said to be gastrointestinal and 17 cutaneous, with 64 deaths among the first group and none among the latter (36).
After the collapse of the Soviet Union, Boris Yeltsin, then the president of Russia, directed his counselor for ecology and health to determine the origin of the epidemic in Sverdlovsk. In 1994, Meselson and his team returned to Russia to aid in these investigations (1, 36). They reviewed the studies of pathologists at a local hospital in Sverdlovsk. One of the lead authors, Dr. Faina Abramova, made available her private records from a series of 42 autopsies, representing the majority of the fatalities from the outbreak (37). Demographic, ecologic, and atmospheric data were also reviewed. The conclusion was that the pattern of these 42 cases of fatal anthrax bacteremia and toxemia was typical of inhalational anthrax as seen in experimentally infected nonhuman primates. In summary, the narrow zone of human and animal anthrax cases extending downwind from Compound 19 indicated that the outbreak resulted from an aerosol that originated there (36, 37). At the time of these investigations, several high-ranking officials in the FSU military and Biopreparat had defected to western countries. In his detailed account of the FSU bioweapons program, Ken Alibek (former chief deputy director of Biopreparat) described in detail the anthrax outbreak in Sverdlovsk, thus confirming the other investigations by US scientists and physicians (38).
After the Sverdlovsk incident, the FSU continued the anthrax research at a remote military facility in Stepnogorsk in Kazakhstan (1, 38, 39). As a result of this intensified research on anthrax, a new and more virulent strain of anthrax was produced in both powdered and liquid form. This strain was resistant to many commonly used antibiotics such as penicillin and streptomycin (38). It is clear from these revelations that the FSU demonstrated the ability to wage biological warfare on a scale matched by no other nation in history. After the final collapse of the FSU and the reorganization of its provinces and states, few of the installations of Biopreparat were subject to international disarmament procedures. The fate of some of the research and know-how remains unknown.
Before October 2001, the last case of inhalational anthrax in the USA occurred in 1976 (33). The identification of inhalational anthrax in a journalist in Florida on October 4, 2001, was the first confirmed case associated with the intentional release of the organism (40-42). The following 10 cases of cutaneous and inhalational anthrax were seen in postal workers who had handled contaminated mail. An additional case of cutaneous anthrax was reported in March 2002, in a laboratory worker processing B. anthracis samples for the CDC investigation of the above cases (9, 10). A thorough analysis of these cases suggested a domestic form of bioterrorism using the Ames strain of B. anthracis. The analysis of the cases also provided a better understanding of the pathogenesis and variable clinical presentation of inhalational anthrax. In contrast to previous studies indicating a death rate of >90%, the cases in 2001 suggested that survival can be markedly improved by early diagnosis, improved intensive care, and combination antimicrobial therapy (10). However, further studies will be needed to better define the antimicrobial regimens and to explore the role of adjunctive treatment modalities, such as immunoglobulin, antitoxin, corticosteroids, and other toxin inhibitors.
MICROBIOLOGY
The Bacillus genus has 70 species, including B. cereus, B. subtilis, B. anthracis, and B. thuringiensis Some species have been lost to other genera by proposals for new Bacillus species. Members of the B. cereus group—B. anthracis, B. cereus, and B. thuringi-ensis-are really pathovars of a single species (43). However, it is convenient to classify B. anthracis within the B. cereus group, which then comprises B. cereus, B. anthracis, B. thuringiensis, and B. mycoides by phenotype (44).
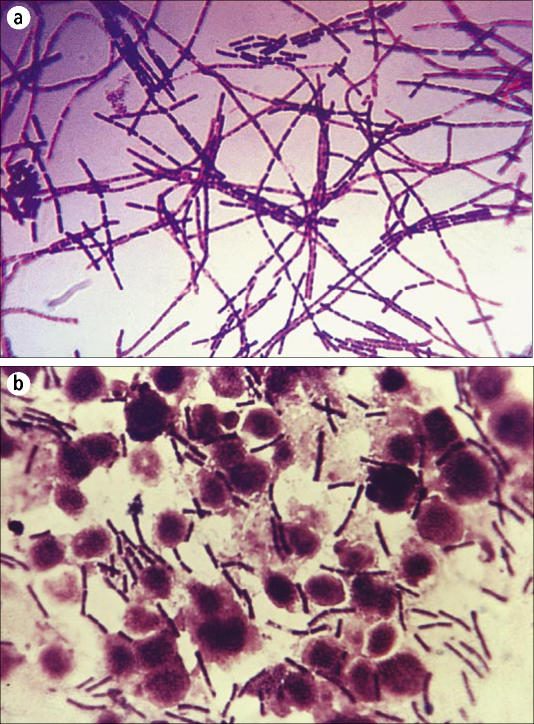
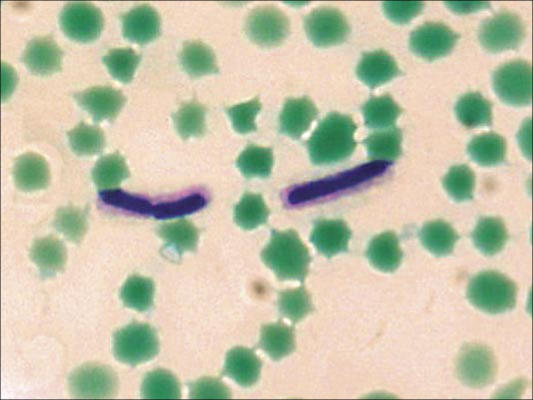
B. anthracis is a large, aerobic, gram-positive, spore-forming, nonmotile bacillus (Figure 2) The nonflagellated vegetative bacillus is 1–1.5 by 3–10 μm in size and is the only obligate pathogen within the genus (43, 45). In infected blood or tissues, the bacilli are frequently present in short chains, surrounded by the polypeptide capsule, which can be visualized under the microscope if stained with polychrome methylene blue (McFadyean stain; Figure 3) or highlighted with India ink. However, in stained smears from anthrax colonies grown on plates, no capsule is identified unless the medium contains 0.7% bicarbonate or 5% serum and the plates are incubated in a 5% to 10% carbon dioxide atmosphere (46).
Figure 2.
Gram stain of Bacillus anthracis: (a) from culture; (b) from infected tissue. Courtesy of CDC/Public Health Image Library.
Figure 3.
McFadyean capsule stain of Bacillus anthracis, grown at 35°C in defibrinated horse blood. Courtesy of CDC/Larry Stauffer/Public Health Image Library.
It is impossible to discriminate between species of the B. cereus group by 16S rRNA sequencing, although B. anthracis can be distinguished by multiple-locus variable-number tandem repeat analysis and amplified fragment-length polymorphism (47, 48). B. anthracis is one of the most molecularly monomorphic bacteria known. However, based on variable numbers of tandem repeats in the region of the VrrA gene, all known B. anthracis strains can be separated into five categories for geographical identification (49).
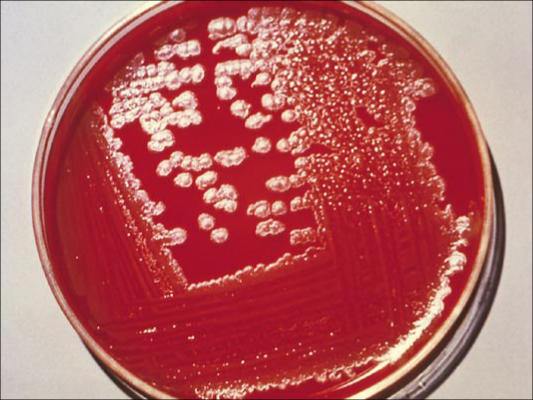
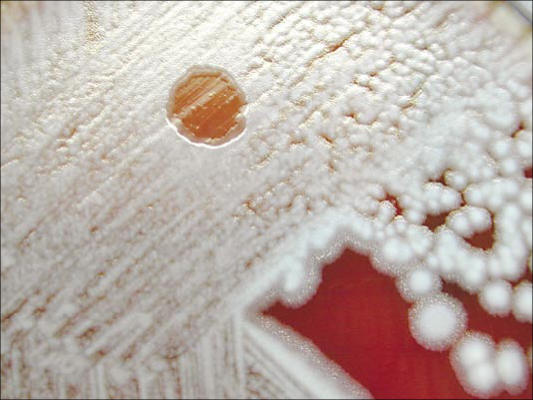
Unlike other members of the B. cereus group, B. anthracis is nonmotile and nonhemolytic on sheep blood agar. It grows readily on a variety of laboratory media at 37°C, forming typical white-gray colonies with an oval, slightly granular appearance. The colonies of B. anthracis are about 2 mm in diameter and somewhat smaller when compared with those of other species in the B. cereus group (Figure 4). It is generally easy to distinguish the virulent B. anthracis from members of the B. cereus group by colony morphology, susceptibility to the diagnostic gamma phage (Figure 5), and the ability to produce the characteristic capsule as visualized by the McFadyean stain.
Figure 4.
Colonies of Bacillus anthracis on sheep blood agar plate. Courtesy of CDC/Public Health Image Library.
Figure 5.
Gamma phage lysis of Bacillus anthracis on sheep blood agar. Courtesy of CDC/Larry Stauffer/Public Health Image Library.
Bacilli will form spores when local nutrients in the environment are exhausted or when anthrax-infected body fluids are exposed to ambient air (47). However, under favorable conditions, anthrax spores germinate and rapidly multiply into vegetative anthrax bacilli. Vegetative bacilli have poor survival outside of an animal or human host; colony counts decline rapidly to undetectable numbers within 24 hours after inoculation with water. This clearly contrasts with the environmentally hardy properties of the B. anthracis spore, which can survive for decades (3, 43, 50).
PATHOGENESIS
Recently, the genome of B. anthracis (Ames strain) was ana-lyzed, and to date the pXOl and pXO2 plasmids responsible for the anthrax toxins have been sequenced (51, 52). These plasmids have also been identified in other non—B. cereus Bacillus species and can be transferred among these species of Bacillus (personal communication, researchers at the Center for Biological Defense College of Public Health at the University of South Florida). The major virulence factors and their role in the pathogenesis of anthrax will briefly be discussed, since current and future research for development of new treatment options and vaccines will focus on these virulence factors.
Full virulence of the bacilli requires the presence of both an antiphagocytic capsule and three plasmid-encoded toxin components. The capsule is composed of a high-molecular-weight polypeptide (poly-D-glutamic acid) and is encoded by the pXO2 plasmid (51). This small plasmid (95.3 kilobase pairs) encodes the three genes capB, capC, and capA The capsule inhibits host phagocytosis of the vegetative form of B. anthracis (47). Plasmid pXOl encodes for three toxins: it is 184.5 kilobase pairs in size and comprises the 83-kd lethal factor, the 89-kd edema factor (calmodulin-dependent adenylate cyclase), and the 85-kd protective antigen (52-54). These proteins are individually nontoxic; however, they act in binary combinations and produce two distinct toxic responses in the host organism: edema and cell death (55). Assembly of the toxic complexes begins when the protective antigen binds to a receptor on the host cells. The membrane-bound protective antigen is then cleaved by a member of the furin class proteases and in part endocytosed. This process also facilitates the entry of the edema factor and lethal factor into the host cell. The endocytosed part of the protective antigen combines with the lethal factor to form the lethal toxin (56). The edema factor inhibits phagocytosis by neutrophils and the oxidative process. The lethal toxin (lethal factor combined with protective antigen) is cytolytic for macrophages and causes the release of tumor necrosis factor and interleukin-1. Both tumor necrosis factor and interleukin-1 in turn cause damage to endothelial cells.
Infection with anthrax occurs after introduction of spores through a break in the skin (cutaneous anthrax) or entry through the mucosa (gastrointestinal anthrax). After spores are ingested by macrophages at the port of entry, germination of the vegetative form occurs and the bacilli rapidly multiply. Rapid extracellular multiplication is accompanied by the production of the lethal toxin and edema factor. In inhalational anthrax, spores (1–2μm in diameter) are inhaled and deposited in the alveolar spaces. From there, they are transported to local lymphatics and the mediastinal lymph nodes, where they germinate and cause hemorrhagic lymphadenitis. Vegetative bacilli then further spread via the bloodstream and lymphatics, causing septicemia. The large amounts of toxin produced by the bacilli, together with the host response (release of tumor necrosis factor and interleukin-1) are responsible for the rapid decline and the overt symptoms of the host organism. If untreated, patients die within a few days. Indeed, the Sverdlovsk accident in 1979 has shown that disease and death occur within 6 weeks of the accidental release of anthrax spores. Studies in rhesus monkeys, which serve as a good model for the study of inhalational anthrax, have shown that germination can occur up to 60 days after exposure and that infected animals die within a week of full symptomatic disease (57). These observations are the basis for recommending antibiotic prophylaxis for 60 days in response to inhalation exposure.
CLINICAL MANIFESTATIONS AND PATHOLOGIC CHARACTERISTICS OF ANTHRAX
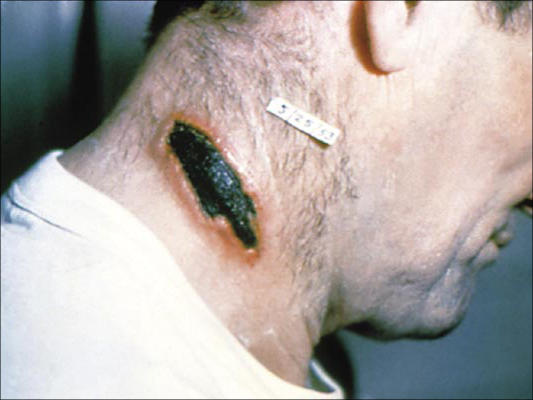
In humans, the most common form of anthrax is cutaneous disease, accounting for 90% or more of all cases worldwide. Areas of exposed skin, such as arms, hands, face, and neck, are more frequently affected. No available data suggest a prolonged latency period for the development of cutaneous anthrax after exposure. In fact, in Sverdlovsk, cases of cutaneous anthrax occurred only as late as 12 days after the incident (36). The literature reports incubation periods as short as 12 hours and as long as 19 days (58). After germination of spores in skin and soft tissues, toxin production results in local edema, and a ring of vesicles develops. These symptoms occur in most cases over 24 hours. Edema and vesicle formation are followed by ulceration of the central papule, which then dries to form the classic black eschar (Figure 6) The eschar dries, loosens, and falls off during the following 1 to 2 weeks, often resulting in complete healing with minimal or no scar formation. Cutaneous lesions often heal irrespective of treatment (59). If the disease becomes systemic, lymphangitis and painful lymphadenopathy usually occur as first symptoms. In untreated cutaneous anthrax, about 20% of patients develop septicemia and die. However, with the use of appropriate antibiotics, the mortality rate is <1% (60).
Figure 6.
The eschar of cutaneous anthrax. Courtesy of CDC/Public Health Image Library.
Gastrointestinal anthrax follows the deposition and subsequent germination of spores in the upper or lower gastrointestinal tract. Commonly, the disease is associated with the consumption of undercooked meat from animals contaminated with anthrax. The oropharyngeal form of the disease is characterized by oral or pharyngeal ulcers, regional lymphadenopathy, edema, and sepsis (20, 22). In the abdominal form of the disease, ulcers and eschars usually form in the wall of the terminal ileum; however, cecum, colon, stomach, and duodenum can also be involved. Presenting symptoms include nausea, vomiting, anorexia, and fever. As the disease progresses, severe abdominal pain, hematemesis, and bloody diarrhea occur, followed by septicemia and death (47). These symptoms result from severe and widespread necrosis of the initial eschar, together with marked edema of the intestines and mesentery. Lymphadenopathy is also prominent. If the diagnosis can be established at an early stage, patients can be treated and cured. However, with the rather nonspecific initial symptoms of gastrointestinal anthrax, early diagnosis is difficult, ultimately resulting in a high mortality rate for this form of the disease.
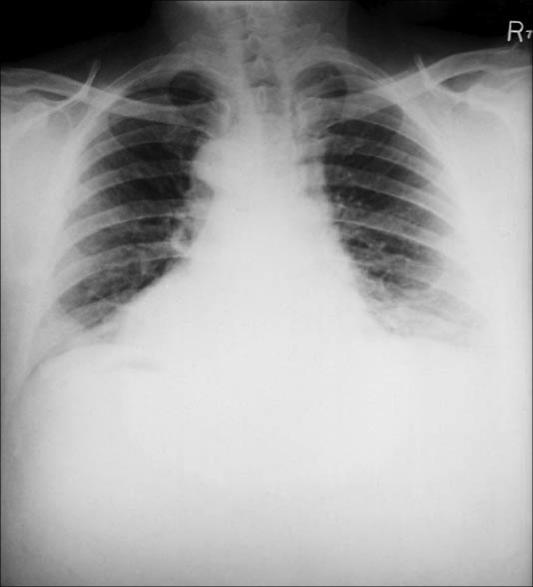
Inhalational anthrax is classically described as a two-stage illness. It begins insidiously with flulike symptoms: mild fever, fatigue, malaise, and a nonproductive cough. These symptoms (prodromal phase) begin approximately 2 to 5 days after the initial exposure and last for about 48 hours. The prodromal phase then ends suddenly with the development of a rather acute illness characterized by severe and acute dyspnea, stridor, fever, and cyanosis. At this stage, massive lymphadenopathy and expansion of the mediastinum are noted. A chest radiograph taken during this stage most often shows the widened mediastinum consistent with lymphadenopathy (Figure 7) (61). On clinical examination, the findings include fever, tachypnea, cyanosis, tachycardia, moist rales, and evidence of pleural effusion. Without treatment, the illness progresses rapidly: terminally, the pulse becomes extremely rapid and faint, and dyspnea and cyanosis worsen. Extreme disorientation is quickly followed by coma and death (62, 63). Up to 50% of patients with inhalational anthrax develop hemorrhagic meningitis with concomitant meningismus, delirium, and obtundation. However, anthrax meningitis can occur as an end-stage process in any of the three forms of the disease. The clinical signs together with the appearance of blood in cerebrospinal fluid are usually followed by loss of consciousness and death (47). The prognosis for patients with inhalational anthrax and anthrax meningitis is extremely poor.
Figure 7.
A posteroanterior chest radiograph taken shortly after onset of inhalational anthrax in a 46-year-old man who contracted the disease from working in a goat hair processing mill. The radiograph reveals bilateral pulmonary effusion and a widened mediastinum, which are hallmarks of the disease process. Courtesy of CDC/Arthur E. Kaye/Public Health Image Library.
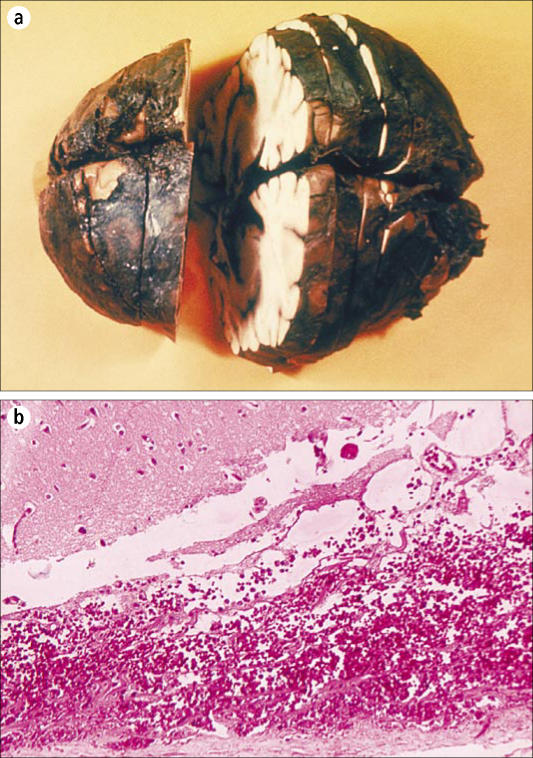
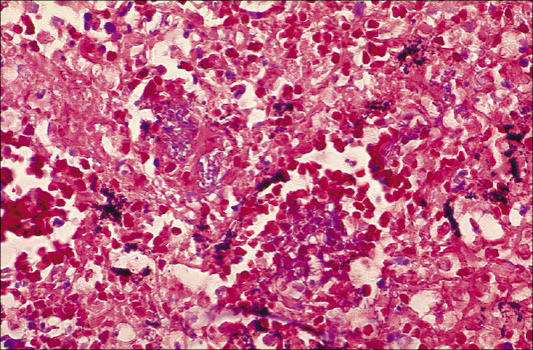
The hallmarks of anthrax are hemorrhage and edema. In cutaneous cases of anthrax, these findings are limited to the tissue immediately adjacent to the site of inoculation. Except for the site of initial entry and the mediastinal edema in patients with inhalational anthrax, the nature and distribution of lesions associated with septicemic spread of anthrax are virtually identical regardless of the route of infection (36, 37). The severe localized reaction is caused by the toxins produced by bacilli at the site of introduction. As they proliferate, the bacteria tend to colonize the lungs and gastrointestinal tract rapidly. Often, preexisting lesions from other causes are colonized. Examples are parasitic nodules, neoplasms, or foci of chronic active inflammation. The local lesion of anthrax then resembles an abscess, and the organisms are disseminated via the bloodstream. Overwhelming septicemia with involvement of spleen, brain, liver, and virtually any other organ will occur within a few hours to days (37). The hemorrhagic meningitis is striking and is often referred to as the “cardinal's cap” (Figure 8) (37). When tissue from patients with disseminated anthrax is evaluated, light microscopy finds hemorrhage, edema, necrosis, fibrin deposition, and a variable degree of inflammatory cell infiltrate, predominantly consisting of neutrophils (Figure 9) Splenic lesions show fibrinopurulent necrosis and depletion of white pulp. The hemorrhagic meningitis is characterized by hemorrhage, fibrinopurulent inflammation, and a myriad of bacilli in and around blood vessels (37). Necrotizing vasculitis and disseminated intravascular coagulation may also occur. Touch preparations from tissue and blood smears also demonstrate a myriad of typically “box car”–shaped, encapsulated gram positive bacilli.
Figure 8.
Hemorrhagic meningitis due to inhalational anthrax. (a) Gross pathology of a formalin-fixed and cut brain. Courtesy of CDC/Public Health Image Library. (b) Photomicrograph of meninges, ×125. Courtesy of CDC/Dr. LaForce/Public Health Image Library.
Figure 9.
Histopathologic study of mediastinal lymph node in fatal human anthrax. Courtesy of CDC/Dr. Marshall Fox/Public Health Image Library.
Until recently, the mortality rate for inhalational anthrax was estimated to be >95%. Most of these cases were occupationally acquired and had occurred before critical care units were in use and in some cases before effective antibiotics were developed (7). At Sverdlovsk, of the reported 79 people who contracted inhalational anthrax, 68 died. However, the reliability of these data and diagnoses remains somewhat questionable (36). It was reported that patients who were diagnosed 30 or more days after the accidental release of the organism had a higher survival rate than those with an earlier diagnosis or onset of disease. Antibiotics, antianthrax globulin, and vaccines were used to treat the residents in the area of Sverdlovsk. However, detailed data from these procedures, hospital records, and data on patients who had received such interventions are not available (36). In many of the fatal cases, reports indicate that the interval between onset of symptoms and death averaged 3 days.
On the contrary, of the 11 known cases to date in the USA, six of the patients survived, providing a mortality rate of 45%. This lower figure may reflect the success of rapid diagnosis and appropriate and immediate antibiotic treatment, together with full intensive care support.
TREATMENT AND PROPHYLAXIS
Penicillin has long been considered the drug of choice for the treatment of (cutaneous) anthrax, and only rarely has penicillin resistance been found in naturally occurring B. anthracis strains. In vitro testing shows B. anthracis to be susceptible to penicillins, fluoroquinolones, tetracycline, chloramphenicol, aminoglycosides, macrolides, imipenem, rifampicin, and vancomycin. The organism is usually resistant to cephalosporins, trimethoprim, and sulfonamides (47).
The initial recommendations for the treatment of (inhalational) anthrax in the setting of a bioterrorist attack were made based on a limited number of studies in experimental animals and the experience from the Sverdlovsk incident (3). These recommendations take into account the understanding of antibiotic resistance patterns and the possible requirement for treatment of large numbers of casualties. Given the rapid development and often fatal course of inhalational anthrax, early antibiotic administration is essential. Furthermore, the experience from the 10 most recent cases of inhalational anthrax in the USA reaffirmed the correctness of this approach. Because the Ames strain causing these recent infections has shown the presence of constitutive and inducible (β-lactamase, the treatment of systemic anthrax with penicillin or amoxicillin alone is no longer recommended. For mild cases of cutaneous anthrax, the use of ciprofloxacin (500 mg twice daily), doxycycline (100 mg twice daily), or amoxicillin (500 mg 3 times daily) is recommended (3). Recommendations were made for contained and mass casualty settings with differences in the mode of administration of these antibiotics.
In experimental animals, the use of antibiotics prevented the development of an adequate immune response (57). Data from these studies suggest that even when appropriate antibiotics are used, a substantial risk for disease recurrence exists for at least 60 days. It has been postulated that germination of dormant spores may occur as late as 60 days after infection. The Working Group on Civilian Biodefense therefore recommended that antibiotic treatment should last at least 60 days (3) (Table 1). In cases of naturally occurring (cutaneous) anthrax, in contrast, treatment is given for 7 to 10 days. Guidelines for postexposure prophylaxis remain the topic of controversy and are not yet fully developed. Such prophylaxis is currently not recommended for asymptomatic people, unless public health or police authorities consider the exposure substantial and a significant risk for development of disease symptoms exists (3, 47). Again, a 60-day period is recommended for prophylaxis based on the possibility of delayed germination of inhaled anthrax spores (Table 2).
Table 1.
Treatment protocol for inhalational anthrax associated with bioterrorist events*
| Category | Initial therapy (intravenous) | Follow-up therapy/duration |
| Adults (including pregnant women† and immunocompromised) | Ciprofloxacin 400 mg BID or doxycycline 100 mg BID and one or two additional antimicrobials | Switch to oral therapy when clinically appropriate: Ciprofloxacin 500 mg BID or doxycycline 100 mg BID Continue for 60 days (intravenous and oral combined) |
| Children (including immunocompromised) | Ciprofloxacin 10–15 mg/kg BID‡ or doxycycline: >8 y and >45 kg: 100 mg BID >8 y and = 45 kg: 2.2 mg/kg BID ≤ 8 y:2.2 mg/kg BID and one or two additional antimicrobials | Switch to oral therapy when clinically appropriate: Ciprofloxacin 10–15 mg/kg BID‡ or doxycycline: >8 y and >45 kg: 100 mg BID >8 y and = 45 kg: 2.2 mg/kg BID ≤ 8 y:2.2 mg/kg BID Continue for 60 days (intravenous and oral combined) |
*Source: MMWR 2001;50:909–919 (reference 41). Patient information sheets available at http://www.bt.cdc.gov.
†High death rate from infection outweighs risk of antimicrobials.
‡Ciprofloxacin not to exceed 1 g daily in children.
BID indicates twice a day.
Table 2.
Recommended postexposure prophylaxis to prevent inhalational anthrax*
| Category | Initial therapy (oral) | Duration |
| Adults (including pregnant women and immunocompromised) | Ciprofloxacin 500 mg BID or doxycycline 100 mg BID | 60 days |
| Children | Ciprofloxacin 10–15 mg/kg BID† or doxycycline: >8 y and >45 kg: 100 mg BID >8 y and ≤ 45 kg: 2.2 mg/kg BID ≤ 8 y: 2.2 mg/kg BID | 60 days; change to amoxicillin if susceptible |
*Patient information sheets available at http://www.bt.cdc.gov.
†Ciprofloxacin not to exceed 1 g daily in children.
BID indicates twice a day.
Naturally, vaccination seems the most effective form of mass protection. Pasteur produced the first anthrax animal vaccine in 1881; however, human vaccines did not emerge until the middle of the 20th century (47). The principal antigen responsible for induction of immunity is the protective antigen (16, 19). The US anthrax vaccine for animals, an inactivated cell-free product (Sterne 34F2), has proved to be extremely safe and effective. A vaccine for use in humans was licensed in 1970 by the Food and Drug Administration. This vaccine was based on cultures in a synthetic medium, which was initially termed “528 medium.” Modifications were made, and the US vaccine used today is an alhydrogel-absorbed, cell-free culture filtrate of the anthrax strain V770-NPI-R (an avirulent, nonencapsulated, nonproteolytic variation of a 1951 bovine isolate from Florida). It was initially licensed in 1972 for administration to people in at-risk occupations. The vaccine is given in a 6-dose series over 18 months and has recently been mandated for all US military active- and reserve-duty personnel (64). Despite some minor side effects, no serious adverse events were reported from the military use of the vaccine (3). In addition, a study in monkeys inoculated with the vaccine showed the vaccine to be completely protective against an aerosol challenge of anthrax spores at 8 and 38 weeks and 88% protective at 100 weeks (65). The FSU and China vaccinate humans with live spores, either by scarification or subcutaneous injection (65). The FSU also uses a strain (ST-1) that is analogous to the US animal vaccine (Sterne 34F2). The use of live attenuated vaccines in the West is considered unsuitable for humans.
Considerable effort has been made in recent years to develop newer and safer anthrax vaccines that would meet current licensing criteria and offer improved efficacy (65, 66). Most of the current research focuses on subunit vaccines that contain whole-length recombinant protective antigen as the active ingredient. Ideally, such new vaccines would be given orally or intranasally and would protect after a single dose.
SUMMARY
Despite its horrifying potential as a biological weapon, anthrax has been a regularly occurring natural disease worldwide. In the early days of microbiology, some 125 years ago, anthrax was important as a disease devastating to domestic animals and the farming economy. The problem was identified and appropriate countermeasures were taken, including the development and use of vaccines. Research and development of anthrax as a biological weapon during the first half of the 20th century focused on easy dissemination of the bioweapon and the development of multidrug-resistant strains. Over time it became clear that anthrax would be unlikely to severely disrupt military operations. However, residual contamination of the ground would occur, and anthrax would pose a great danger to the civilian population. The analysis provided by the WHO in 1970 provided a grim outlook on the estimated casualties should dried anthrax be released in aerosolized form on large cities (1).
With the experience in Sverdlovsk and the discovery of bioweapons research in numerous countries (e.g., FSU and Iraq), it became clear that despite international disarmament agreements, the threat of biological warfare agents continues to exist. Most experts agree that the ability to manufacture and use a lethal anthrax aerosol is beyond the capacity of individuals or groups without access to advanced biotechnology laboratories. However, after the attacks on the World Trade Center on September 11, 2001, and the subsequent 10 cases of inhalational anthrax, concerns have been raised that these attacks are the beginning, rather than the end, of terrorism.
Despite improvements in treatment, anthrax remains a deadly infection. Primary prevention rests on creating a strong global norm that rejects development of such weapons. Secondary prevention implies early detection and prompt treatment of disease. Unfortunately, the tools of primary and secondary prevention are imperfect. As international political efforts are made to assist nations that have been targets of biological weapons, the medical and law enforcement communities must be prepared to face the sequelae should the unthinkable happen.
References
- 1.Riedel S. Biological warfare and bioterrorism: a historical review. BUMC Proceedings. 2004;17:400–406. doi: 10.1080/08998280.2004.11928002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eitzen EM, Jr, Takafuji ET. Historical overview of biological warfare. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 1997. pp. 415–423. Available at http://www.bordeninstitute.army.mil/cwbw/default_index.htm; accessed May 11, 2005. [Google Scholar]
- 3.Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: medical and public health management. JAMA. 1999;281:1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 4.McSherry J, Kilpatrick R. The plague of Athens. J R Soc Med. 1992:85–713. [PMC free article] [PubMed] [Google Scholar]
- 5.Virgil . The Georgics. Chicago: The University of Chicago Press; 1956. [Google Scholar]
- 6.Dirckx JH. Virgil on anthrax. Am J Dermatopatkol. 1981;3:191–195. doi: 10.1097/00000372-198100320-00012. [DOI] [PubMed] [Google Scholar]
- 7.Brachman PS. Inhalation anthrax. Ann N Y Acad Sci. 1980;353:83–93. doi: 10.1111/j.1749-6632.1980.tb18910.x. [DOI] [PubMed] [Google Scholar]
- 8.Carter KG. The Koch-Pasteur dispute on establishing the cause of anthrax. Bull Hist Med. 1988;62:42–57. [PubMed] [Google Scholar]
- 9.Sternbach G. The history of anthrax. J Emerg Med. 2003;24:463–467. doi: 10.1016/s0736-4679(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 10.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA, Anthrax Bioterrorism Investigation Team Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon TC, Meselson M, Guillemin J, Hanna P. Anthrax. N Engl J Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 12.Brachman PS. Bioterrorism: an update with a focus on anthrax. Am J Epidemiol. 2002;155:981–987. doi: 10.1093/aje/155.11.981. [DOI] [PubMed] [Google Scholar]
- 13.Morris K. US military face punishment for refusing anthrax vaccine. Lancet. 1999:353–130. doi: 10.1016/S0140-6736(05)76173-0. [DOI] [PubMed] [Google Scholar]
- 14.Charatan F. Fears over anthrax vaccination driving away US reservists. BMJ. 2000:321–980. [PMC free article] [PubMed] [Google Scholar]
- 15.Kohout E, Sehat A, Ashraf M. Anthrax: a continuous problem in southwest Iran. Am J Med Sci. 1964;247:565–575. [PubMed] [Google Scholar]
- 16.Brachman PS, Friedlander A. Anthrax. In: Plotkin SA, Orenstein WA, editors. Vaccines. 3rd ed. Philadelphia: WB Saunders Co; 1999. pp. 629–637. [Google Scholar]
- 17.Centers for Disease Control and Prevention Summary of notifiable diseases, 1945–1994. MMWR Morb Mortal Wkly Rep. 1994;43:70–78. [Google Scholar]
- 18.Tekin A, Bulut N, Unal T. Acute abdomen due to anthrax. BrJ Surg. 1997;84:813. [PubMed] [Google Scholar]
- 19.Friedlander AM. Anthrax. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 1997. pp. 467–478. Available at http://www.bordeninstitute.army.mil/cwbw/default_index.htm; accessed April 23, 2005. [Google Scholar]
- 20.Sirisanthana T, Nelson KE, Ezzell JW, Abshire TG. Serological studies of patients with cutaneous and oral-oropharyngeal anthrax from northern Thailand. Am J Trop Med Hyg. 1988;39:575–581. doi: 10.4269/ajtmh.1988.39.575. [DOI] [PubMed] [Google Scholar]
- 21.Dutz W, Saidi F, Kohout E. Gastric anthrax with massive ascites. Gut. 1970;11:352–354. doi: 10.1136/gut.11.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunanusont C, Limpakarnjanarat K, Foy HM. Outbreak of anthrax in Thailand. Ann Trop Med Parasitol. 1989;84:507–512. doi: 10.1080/00034983.1990.11812502. [DOI] [PubMed] [Google Scholar]
- 23.Sirisanthana T, Navachareon N, Tharavichitkul P, Sirisanthana V, Brown AE. Outbreak of oral-oropharyngeal anthrax: an unusual manifestation of human infection with Bacillus anthracis. Am J Trop Med Hyg. 1984;33:144–150. doi: 10.4269/ajtmh.1984.33.144. [DOI] [PubMed] [Google Scholar]
- 24.Hugh-Jones M. Wickham Steed and German biological warfare research. Intelligence and National Security. 1992;7:379–302. [Google Scholar]
- 25.Stockholm International Peace Research Institute . The Problem of Chemical and Biological Warfare. Volume 1: The Rise of CB Weapons. New York: Humanities Press; 1971. [Google Scholar]
- 26.Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM., Jr Biological warfare—a historical perspective. JAMA. 1997;278:412–4l7. [PubMed] [Google Scholar]
- 27.Carter GB. Chemical and Biological Defence at Porton Down: 1916–2000. London: HMSO; 2000. [Google Scholar]
- 28.Carter GB. Biological warfare and biological defence in the United Kingdom: 1940–1979. Journal of the Royal United Services Institute for Defence Studies. 1992;137:67–74. [Google Scholar]
- 29.Manchee RJ, Stewart R. The decontamination of Gruinard Island. Chem Br. 1988;4:690–691. [Google Scholar]
- 30.WHO Group of Consultants . Health Aspects of Chemical and Biological Weapons. Geneva: World Health Organization; 1970. [Google Scholar]
- 31.Office of Technology Assessment, US Congress . Proliferation of Weapons of Mass Destruction. Washington, DC: US Govern ment Printing Office; 1993. pp. 53–55. (Pub. No. OTA-ISC-559) [Google Scholar]
- 32.Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg Infect Dis. 1997;3:83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suffin SC, Carnes WH, Kaufmann AF. Inhalational anthrax in a home craftsman. Hum Pathol. 1978;9:594–597. doi: 10.1016/s0046-8177(78)80140-3. [DOI] [PubMed] [Google Scholar]
- 34.US Army Medical Research Institute for Infectious Diseases . Medical Management of Biological Casualties Handbook. 4th ed. Frederick, MD: Fort Detrick; 2001. [Google Scholar]
- 35.Miller J, Engelberg S, Broad W. Germs: Biological Weapons and America's Secret War. New York: Simon & Schuster; 2001. pp. 76–80. 93–94, 134–135, 143–144, 175, 178, 221. [Google Scholar]
- 36.Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, Yampolskaya O. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266:1202–1208. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 37.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci USA. 1993;90:2291–2294. doi: 10.1073/pnas.90.6.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alibek K. Biohazard. New York: Random House; 2000. pp. 70–86. [Google Scholar]
- 39.Caudle LC., III . The biological warfare threat. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 1997. pp. 451–466. Available at http://www.bordeninstitute.army.mil/cwbw/default_index.htm; accessed May 11, 2005. [Google Scholar]
- 40.Centers for Disease Control and Prevention Notice to readers: ongoing investigation of anthrax—Florida, October 2001. MMWR Morb Mortal Wkly Rep. 2001:50–877. [Google Scholar]
- 41.Centers for Disease Control and Prevention Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR Morb Mortal Wkly Rep. 2001;50:909–919. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Update: investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. MMWR Morb Mortal Wkly Rep. 2001;50:941–948. [PubMed] [Google Scholar]
- 43.Logan NA, Turnbull PCB. Bacillus and other aerobic endospore-forming bacteria. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of Clinical Microbiology. 8th ed. Vol. 32. Washington, DC: ASM Press; 2003. pp. 445–460. [Google Scholar]
- 44.Turnbull PCB. Definitive identification of Bacillus anthracis—a review. J Appl Microbiol. 1999;87:237–240. doi: 10.1046/j.1365-2672.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 45.Baillie L, Read TD. Bacillus anthracis, a bug with attitude! Curr Opin Microbiol. 2001;4:78–81. doi: 10.1016/s1369-5274(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 46.Carman JA, Hamblelon P, Melling J. Bacillus anthracis, in isolation and identification of micro-organisms of medical and veterinary importance. In: Collins CH, Grange GM, editors. Society of Applied Bacteriology Technical Series 21. London: Academic Press; 1985. pp. 207–214. [Google Scholar]
- 47.Spencer RC. Bacillus anthracis. J Clin Pathol. 2003;56:182–187. doi: 10.1136/jcp.56.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keim P, Price LB, Klevytska AM, Smith KL, Schupp JM, Okinaka R, Jackson PJ, Hugh-Jones ME. Multiple-locus variable number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J Bacteriol. 2000;182:2928–2936. doi: 10.1128/jb.182.10.2928-2936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson PJ, Hugh-Jones ME, Adair DM, Green G, Hill KK, Kuske CR, Grinberg LM, Abramova FA, Keim P. PCR analysis of tissue samples from the 1979 Sverdlovsk anthrax victims: the presence of multiple Bacillus anthracis strains in different victims. Proc Natl Acad Sci USA. 1998;95:1224–1229. doi: 10.1073/pnas.95.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Titball RW, Turnbull PC, Hutson RA. The monitoring and detection of Bacillus anthracis in the environment. Soc Appl Bacterial Symp Ser. 1991;20:9S–18S. [PubMed] [Google Scholar]
- 51.Uchida I, Sekizaki T, Hashimoto K, Terakado N. Association of the encap sulation of Bacillus anthracis with a 60 megadalton plasmid. J Gen Microbiol. 1985;131:363–367. doi: 10.1099/00221287-131-2-363. [DOI] [PubMed] [Google Scholar]
- 52.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leppla SH. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eukaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- 54.O'Brien J, Friedlander A, Dreier T, Ezzell J, Leppla S. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985;47:306–310. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leppla SH, Ivins BE, Ezzell JW., Jr . Anthrax toxin. In: Lieve L, editor. Microbiology 1985. Vol. 666. Washington, DC: American Society for Microbiology; 1985. [Google Scholar]
- 56.Mogridge J, Cunningham K, Borden Lacy D, Mourez M, Collier RJ. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci USA. 2002;99:7045–7048. doi: 10.1073/pnas.052160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedlander AM, Welkos SL, Pitt ML, Ezzell JW, Worsham PL, Rose KJ, Ivins BE, Lowe Jr, Howe GB, Mikesell P, et al. Postexposure prophylaxis against experimental inhalation anthrax. Infect Dis. 1993;167:1239–1243. doi: 10.1093/infdis/167.5.1239. [DOI] [PubMed] [Google Scholar]
- 58.Abdenour D, Larouze B, Dalichaouche M, Aouati M. Familial occurrence of anthrax in Eastern Algeria. J Infect Dis. 1987;155:1083–1084. doi: 10.1093/infdis/155.5.1083. [DOI] [PubMed] [Google Scholar]
- 59.Turner M. Anthrax in humans in Zimbabwe. Cent Afr J Med. 1980;26:160–161. [PubMed] [Google Scholar]
- 60.Lew D. Bacillus anthracis (anthrax) In: Mandell GL, Bennett JE, editors. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone; 1995. [Google Scholar]
- 61.Vessal K, Yeganehdoust J, Dutz W, Kohout E. Radio logic changes in inhalation anthrax. A report of radiological and pathological correlation in two cases. Clin Radiol. 1975;26:471–474. doi: 10.1016/s0009-9260(75)80100-0. [DOI] [PubMed] [Google Scholar]
- 62.Albrink WS, Brooks SM, Biron RE, Kopel M. Human inhalation anthrax: a report of three fatal cases. Am J Pathol. 1960;36:457–471. [PMC free article] [PubMed] [Google Scholar]
- 63.Plotkin SA, Brachman PS, Utell M, Bumford FH, Atchison MM. An epi demic of inhalation anthrax, the first in the twentieth century. I. Clinical features. Am J Med. 1960;29:992–1001. doi: 10.1016/0002-9343(60)90079-6. [DOI] [PubMed] [Google Scholar]
- 64.Anthrax vaccine, military use in the Persian Gulf region [press release] Washington, DC: US Department of Defense; September 8, 1998. [Google Scholar]
- 65.Turnbull PC. Anthrax vaccines: past, present, and future. Vaccine. 1991;9:533–539. doi: 10.1016/0264-410x(91)90237-z. [DOI] [PubMed] [Google Scholar]
- 66.Baillie L. The development of new vaccines against Bacillus anthracis. J Appl Microbiol. 2001;91:609–613. doi: 10.1046/j.1365-2672.2001.01498.x. [DOI] [PubMed] [Google Scholar]