Abstract
Sexual reproduction in animals and plants is far more prevalent than asexual reproduction, and there is no dearth of hypotheses attempting to explain why. Even bacteria and viruses, which reproduce by cloning, engage in promiscuous horizontal gene exchange (“parasexual reproduction”) on such short time scales that they evolve genotypic diversity even more rapidly than eukaryotes. (We confront this daily in the form of antimicrobial resistance.) The host-parasite and host-pathogen arms race purports to explain the prevalence of sexual reproduction, yet there are over a dozen other hypotheses, including the proposition that sexual reproduction purges the genome of deleterious mutations. An equally daunting challenge is to understand, in terms of evolutionary logic, the jungle of diverse courtship and mating strategies that we find in nature. The phenotypic plasticity of sex determination in animals suggests that the central nervous system and reproductive tract may not reach the same endpoint on the continuum between our stereotypic male and female extremes. Why are there only two kinds of gametes in most eukaryotes? Why are most flowering plants, and few animals, hermaphroditic? Why do male animals compete more for access to females than the other way around in most animals that have been studied?This review presents more questions than answers, but an extraordinary wealth of data has been collected, and new genetic techniques will provide new answers. The possible relevance of these data to human sexuality will be discussed in a future article.
Children learn that most plants and animals reproduce sexually and come in two sexes. They also learn that many plants and a few animals have both sexual organs in the same body (hermaphrodites) yet still reproduce with two distinct kinds of gametes, eggs and sperm. They also learn that sexual reproduction is much more common than asexual.
It is critical to distinguish between natural selection and sexual selection, whenever this is possible. The psychologist Geoffrey Miller stated: “Natural selection is about living long enough to reproduce; sexual selection is about convincing others to mate with you.” This is an excellent comparison in a nutshell, even though it leaves out male-male competition. The Table compares natural and sexual selection.
Table.
Comparison of natural and sexual selection
| Natural selection |
| Genes conferring protection against pathogens and parasites, or against immune systems, and coding for changes that adapt their bearers to changed environmental conditions should achieve better representation in future generations, assuming a variety of genes can become more common in the gene pool of a species at the expense of others. There is a good analogy with human selection in the domestication of animals, plants, and microorganisms, except for the “artifact” of human cognitive choice. |
| Sexual selection |
| Female choice (less commonly male choice): One sex (the one with greater parental investment) is more choosy, and the other sex competes more intensely for access to that sex. |
| Male-male competition (less commonly female-female competition): The more competitive sex may become larger and may evolve more showy ornaments or weaponry. |
Before looking at hypotheses about the ubiquity of sexuality, I'd like to describe some of the extraordinary ways the two sexes differ in form and behavior, especially during courtship and mating. I'll stick to nonhuman animals in this article and venture into the more controversial waters of human behavior in a future article.
SEXUAL SELECTION: EXAMPLES OF SEXUAL DIMORPHISM AND MALE-MALE COMPETITION
When we observe animals in nature or pets in our homes, do we see a difference in form or behavior between the sexes? I am referring to more than just the anatomy of their reproductive organs. Is there a difference in size, color, ornaments, or behavior between the sexes of the same species of animal? More often than not, the answer is a resounding yes. The difference is called “sexual dimorphism.”
Birds provide a striking example of sexual dimorphism. In many species the male is more colorful or ornamented than the female (Figure 1) and is the one who sings, displays, and chases away other males. It is surprising that the huge peacock tail is compatible with survival in the wild (Figures 2 and 3), as the male is encumbered with a monstrous burden of feathers and is surely handicapped if attacked by a predator. Such baggage could hardly evolve by natural selection, as it would be a handicap, not a survival advantage. The color and displays of males also render them more visible to predators. So how could such handicaps evolve? The answer is by female choice, one kind of sexual selection in which one sex prefers to mate with a partner having a particular trait or resource. Sexual selection is not the same as natural selection, even though it is “natural.” It can even be antagonistic to natural selection; if it goes too far, it is countered by natural selection, in some cases because males become vulnerable to predators.
Figure 1.
(a) Male Resplendent Quetzal in nest hole in Costa Rica, his long tail still pointing the way it did when he entered. The long tail and bright colors of many male birds are examples of female choice, a form of sexual selection, distinct from natural selection, even though it is still “natural.” The ornaments and colors of such male birds are actually survival handicaps, and research has shown that choice of such mates by females increases their reproductive success by providing them with robust genes and the likelihood that their male offspring will also be more attractive to females. (b) Female Resplendent Quetzal in Costa Rica, with a much shorter tail than the male and less bright colors.
Figure 2.
Peacock with a highly ornamented tail which, like the male quetzal's tail, evolved by female choice. If some “eyes” are removed from his tail, he becomes less attractive to peahens. It is hard to imagine how such an enormous encumbrance would be compatible with escape from predators, and indeed further enlargement of the tail may have been constrained by natural selection.
Figure 3.

Gary Larson got this one right!
Sexual selection is a little-appreciated but critically important evolutionary mechanism, moving certain alleles preferentially into the future just as natural selection does. Females who choose showy mates have showy male offspring, who will in turn be more attractive to females in the future, so genes that promote the preference are passed on.
Experimental support for the evolutionary advantages of female choice is abundant and well accepted. In one study, the long tails of male widowbirds in Africa were trimmed, and the removed portions were glued onto those of other males, making their tails abnormally long. Those males had the highest mating success, and the males with short tails had the lowest success. Peacocks with eyes trimmed from their tails have the least mating success. Roosters with large bright combs are the most attractive to females. Male swordtail fish with the longest tails or brightest tail coloration are the most attractive to females. The brightest and most ornamented male birds, or those with territory, are the most attractive to females. Tungara frogs with the loudest calls attract the most females. Female crickets prefer males whose songs have the greatest complexity. In most cases the choices made by females are sound in an evolutionary sense, in that the males they choose have the greatest freedom from parasites and greatest fitness. Female choice is usually not arbitrary but based on genetically determined preferences for traits in males that are “badges” of quality genes.
Sexual selection is not just about female choice but also about male-male competition (Figures 4–6), which may result in the evolution of males that are much larger than females and endowed with weaponry. Elephant tusks and deer antlers are larger in males and confer greater competitive ability on their owners; in some cases female choice may also contribute to a male's weaponry. In the case of elk, sea lions, and gorillas, the strongest male gains a harem by male-male competition (Figure 7). Any alleles that contribute to his greater strength and size are passed on to his male offspring. The weaponry may exact a high cost to the bearer; Irish elk from the Siberian Arctic became extinct about 7700 years ago, and it is estimated that the huge antlers of the male contained up to 16 pounds of calcium and 8 pounds of phosphate, a high nutritional price to pay as antlers are regrown every year.
Figure 4.
Young male giraffes in East Africa gently spar for as long as an hour, in preparation for more serious sparring as adults in competition for females. The head and neck are also used as powerful weapons in killing predators.
Figure 6.
Adult male impalas clash in deadly earnest in competition for females and territory, in the Okavango Delta of Botswana, southern Africa.
Figure 7.
Male elk with his harem in Yellowstone National Park. His antlers and larger size result from male-male competition, a form of sexual selection.
Natural selection, in contrast, fine-tunes adaptations of both sexes to the dynamic changes that occur constantly in the biotic and abiotic environment, such as changing virulence of pathogens and parasites, changing relationships between mutualists, changing immune system challenges to pathogens, changing climate, and changing food availability. The latest mass extinction, caused by humans, is occurring at such a breakneck pace that evolutionary change can't keep up, except perhaps in micro-organisms.
A remarkable study of both natural and sexual selection operating simultaneously describes an “antiaphrodisiac” chemical deposited on a female moth by the male who mates with her (1). The chemical, benzyl cyanide, repels other male moths, serving as a kind of chastity belt and increasing the male's certainty of paternity. This is not a conscious activity on his part, of course, but simply a programmed behavior brought about by genes that are favored by sexual selection—in this case, male-male competition. All else being equal, his genes may outcompete those of males whose genes don't promote the behavior. But there is a catch: a tiny parasitic wasp has learned to home in on the smell of the chemical and hitches a ride on the mated female to the place where she will lay her eggs. The female wasp then injects her own eggs into the larger moth eggs, which serve as food for her offspring. The “antiaphrodisiac” chemical, then, repels other male moths but inadvertently dooms his genetic contribution. We are seeing natural selection constraining sexual selection, with a kind of selection arms race going on. If this strategy is widespread in nature, it could constrain the evolution of sexual communication between hosts, as parasites learn to home in on host pheromones.
Lionesses in East Africa mate with many males, including males outside of their pride, before ovulating. In effect, they delay ovulation for a month or longer after inviting males to mate (Figure 8). Infanticide by a coalition of takeover males is common in lion prides. Mating females may be delaying “commitment” of their eggs until they are more confident that their pride males are strong enough to resist invasion and takeover by itinerant males, because replacement means that any newborn cubs are likely to be killed by the takeover males (Figure 9). The females are of course not doing this consciously but with behavioral predispositions imposed by genes that survive preferentially over other genes because they promote greater reproductive success.
Figure 8.
Lions mating in the Masai Mara of Kenya, with the male's grimace and female's reaction at the moment of ejaculation. The female mates with many males before “committing” her eggs to fertilization. When she accepts a male, they often copulate every 15 minutes for 3 days and nights, the male never letting her wander more than a few feet away. Infanticide by new males that take over a pride may help explain her delay in ovulating, as she may be testing the stability of her pride's male coalition.
Figure 9.
A lioness licks her newborn cubs that were just delivered in a protected place away from the pride, probably for the safety of the cubs in the case of takeover by a new male coalition. Females tend to coordinate their pregnancies, and when their cubs are returned to the pride they are suckled by all lactating females. Lions are the only highly “social” cat; other cats around the world are solitary as adults, except for cheetah brothers, which may remain together.
Lions are the only highly “social” cat, forming prides in which females are permanent members and male “coalitions” come and go. All other cats are solitary as adults, except perhaps cheetahs. Cheetah brothers sometimes remain together as adults.
Infanticide by males is a significant source of infant mortality in mammals. In primates it has been found in 35 different species (2). It is clearly an example of male-male competition (one form of sexual selection) with a gain only to the takeover males and a loss to the females and the social group.
Why are chimpanzee testes four times the size of gorilla testes, even though male chimpanzees are one fourth the size of male gorillas? The likely explanation is that male gorillas keep harems, and sperm quantity is not important as a form of male-male competition, whereas female chimpanzees mate promiscuously and the larger the male's sperm volume, the more likely his success in impregnating a female who has mated (or will mate) with other males.
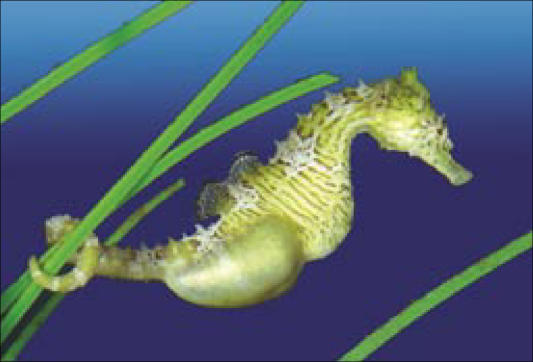
Some male seahorses carry their young in an abdominal pouch into which the female injects her eggs (Figure 10). He fertilizes them there, certain of his paternity, and—like gorillas—enjoys low sperm competition and has a relatively low sperm count. I will say more about seahorses later in this article.
Figure 10.
“Pregnant” male seahorse, carrying his young in an abdominal pouch. His certainty of paternity is absolute, as the female injects her eggs into his pouch, where he fertilizes them. In some male seahorses there is a placenta-like structure for nourishing the young. His sperm count is low, as is predicted by evolutionary theory in the face of absent sperm competition with other males.
Male-male competition may go to extremes. A recent study of the giant Australian cuttlefish (a kind of squid) describes the behavior of small “sneaker males” that swim into the danger zone of other larger males courting females and rapidly mimic the appearance and behavior of females (squids and octopuses are master quick-change artists when it comes to color and pattern change). When his disguise works, he is tolerated long enough for him to inseminate the female and quickly make his escape (3).
Male-male competition has a more sacrificial side. Males of some cannibalistic spiders and insects allow the female to eat them after mating. The male yellow garden spider, Argiope aurantia, has been shown to collaborate in his own instantaneous death: he dies by his own “decision” after inserting the second of his two pedipalps (mating appendages) inside one of the female's genital apertures. He becomes unresponsive, and his heartbeat ceases within minutes of insertion. His body may thus serve as a mating plug, a kind of temporary chastity belt that delays or prevents other males from taking their turn. His genes, which program the behavior, carry the day. Their ephemeral creation, his body, is merely a vehicle (Figure 11) (4).
Figure 11.
A female Argiope garden spider on her web in North Texas, with a zig-zag structure called the stabilimentum, the function of which is unknown. The male is a tiny fraction of her size, and after inserting his mating appendage into her genital aperture he dies spontaneously, of his own accord, remaining in place. One hypothesis is that he thus serves as a mating plug, a kind of chastity belt, temporarily blocking any other male from taking his turn.
THE BEWILDERING VARIETY OF REPRODUCTIVE STRATEGIES IN NATURE
The movie Finding Nemo has made the orange clownfish a lovable and popular fish, living on coral reefs in the protected shelter of its anemone. In each clownfish “family,” the female is largest, the male second largest, and nonbreeders smaller (Figure 12). If the female dies, the male changes sex and becomes the breeding female, and the largest nonbreeder becomes the breeding male (5). In fish that can change sex, both male and female gonads are present but only one is active at any time; the social environment can switch the gender of an individual by turning some genes on and others off, activating one gonad and suppressing the other, with gender-appropriate behavior following the change.
Figure 12.
Orange clownfish share a protective anemone on a Philippine coral reef. The female is largest and the male second largest. If the female dies, the male changes sex and takes her place, and the largest nonbreeder becomes the breeding male. Subordinates in the group queue for breeding positions.
True hermaphroditism, on the other hand, involves both male and female reproductive tracts remaining functional simultaneously in the same individual. Sea hares are hermaphroditic mollusks that look like snails without a shell; they are male in front and female behind. They form daisy chains on the ocean floor, with a dozen or more individuals copulating fore and aft; they sometimes form a closed circle!
In hermaphroditic bass, one individual releases sperm and the other eggs, then they reverse roles and release gametes again. In this way they achieve mutual outcrossing. Is this not more efficient than the assignment of one sex to one individual? Surely it is, but no one knows why it is not more universal.
Teleost fishes (most bony fishes, the largest class of vertebrates) range in sexual phenotypes from species with permanent sexes, such as cichlids, to species that change sex once in their lives and to those that change sex multiple times. “Sequential hermaphrodites” alternate between donating sperm and eggs without permanent commitment to the male or female gender. Others reproduce first as males and then switch permanently to the female sex, and others do it the other way around. Some fish have two distinct male phenotypes: one, usually larger, that guards a female, and a second type, usually smaller, that moves into the other male's space and “sneaks” a quick copulation with the female. Fertilization is external in most fish species.
Parental care shows great diversity in fishes. Some provide none at all, like Atlantic herring, which form huge schools of both sexes and freely shed their eggs and sperm (milt) into the water and then leave. Other fishes build nests and care for both the eggs and newly hatched young. Others carry the fertilized eggs with them, often in their mouths but also in gill cavities or in special pouches on the body (seahorses and pipefishes).
Deep-sea anglerfish have such a bizarre reproductive strategy that it was difficult to discover. At their low population densities in the deep sea, it is hard for them to find mates. In some species, when the much smaller male finds a female, he attaches himself to her and the dermis of his snout and lower jaw becomes completely fused to her body (6). Apparently, a continuity is established between the female blood vascular system and that of the male, although critical proof of this is lacking. Degenerative changes in all organs of the male have been seen except for the relatively large testis, which remains functional. The male thus becomes a degenerate parasite, not much more than a male reproductive organ attached to the female. Now with a guaranteed source of sperm, she supports “him” nutritionally for the rest of his life. He is in effect a xenograft that is not rejected, a challenge for an understanding of how the immune system accommodates this foreign tissue. One could even question whether what remains is a female with a parasitic male or a single hermaphroditic fish. Sperm release into the water is coordinated with her release of eggs. Once fertilized, the eggs are buoyant and float to the surface of the ocean. The hatchlings that survive feed on plankton until they mature and return to deeper waters.
There are 24,500+ species of fishes, 2½ times more species than birds and 5 times more than mammals, and their bewildering variety of reproductive strategies offers limitless opportunities for understanding how two sexes and two gamete types engage in an arms race for future genetic representation, in the same or different bodies. We have just begun to explore the patterns and the evolutionary logic.
The biologist Daniel Janzen commented that plants move twice in their lives: as pollen and seed. Both moves involve reproduction. Plants have an amazing body organization that is often forgotten: there are no gonads for producing sperm and eggs; the body is instead modular with repetitive structures that include totipotent stem cells capable of producing flowers with gametes every flowering season. “Immortal” germ cells arise de novo from somatic cells on branches and even on the trunk of some trees (cauliflory, as in the cacao tree, the source of chocolate). The great longevity of plants (some bristlecone pines are over 4000 years old) is made possible by this ability to maintain unspecialized cells in the adult over long periods of time. Plants can even be propagated from small pieces of tissue, or from single cells, because many somatic cells remain totipotent throughout life. In contrast, the stem cells of adult mammals, such as hematopoietic cells in the bone marrow, can produce only a restricted spectrum of cell types and are “pluripotent,” one step down from totipotent.
Most flowering plants (angiosperms) are hermaphroditic, and every flower is usually bisexual, with both male and female elements in the same flower. Oaks are hermaphroditic but have separate male and female flowers on the same tree, as do most gymnosperms (conifers and cycads). Most hermaphroditic plants have mechanisms that prevent self-fertilization, avoiding inbreeding depression.
In mammals, including humans, true hermaphroditism occurs occasionally as a genetic mistake. The causes are not understood, but a few cases have been attributed to the movement of the Sry gene (which triggers testis development) to a site on the X chromosome (7). Testes, ovaries, and ovotestes can occur in various combinations. For unknown reasons, hermaphroditism in humans is more common in Africa and the Middle East.
The evolutionary biologist Olivia Judson wrote that the battle of the sexes is an eternal war (8). With mating strategies, she added, the only rule is that there are no rules. True monogamy with partner fidelity is “so rare that it is one of the most deviant behaviors in biology.” It is rare because it rarely serves the genetic interest of either party, let alone both.
You may argue, however, that over 90% of bird species are monogamous, but it turns out that the monogamy is more often “social” (i.e., lacking sexual fidelity). Both partners collaborate in rearing the young, but more and more genetic studies show partner infidelity during the mating period, so that males often help raise young that are not theirs. Monogamy may also be “serial,” lasting for only one breeding season.
The Wandering Albatross provides a striking exception to partner infidelity. This albatross is one of the most remarkable animals in the world (Figure 13). These enormous birds, with a wingspan that measures 11 feet, the longest of any bird (one wing is as long as your outstretched arms), mate for life, which is often 6 decades or longer. After leaving its natal nest on a subantarctic island such as South Georgia Island, a newly fledged bird—the size of an adult but a darker color—takes to the air and lives on the wing for the next 5 to 7 years, before coming to land and finding a lifelong mate. It feeds during those years by landing briefly on the water and catching squid, fish, and offal thrown off of fishing boats. Albatrosses are masters of gliding skills, using their narrow, long wings to drift effortlessly on the winds surrounding the bottom of the world.
Figure 13.
This Wandering Albatross fledgling, on its nest on South Georgia Island, is almost ready to start an uninterrupted 5- to 7-year-long flight over the southern ocean surrounding the bottom of the world, returning to land after all those years to mate monogamously for life. Even at only 9 months of life, it has a wingspan of 11 feet, the longest of any bird; we were 20 feet away and it seemed like a giant.
After raising one young, the two partners depart for another year or two of gliding over the southern waters and return to the same place at the same time to breed again. Sadly, long-line fishing boats with nets up to 80 miles long have killed tens of thousands of females, who fly farther north than the males and thus encounter more fishing boats. Males are now returning to land to find their mate missing. They either fail to reproduce that year or must find a younger, more inexperienced female, who may die in a fishing net the following year.
The Rime of the Ancient Mariner, written by Samuel Taylor Coleridge in 1797, chronicled the killing of a Wandering Albatross (a “pious bird of good omen”) as it followed a ship. The Wandering Albatross is my favorite of all birds, and I long to see it again effortlessly following our ship in the stormy waters of the southern ocean.
Birds mate by approximating their cloacae, as the males of most bird species lack a penis. The cloaca is the common outlet of the alimentary canal, bladder, and reproductive tract, in both sexes. A minority of birds, including ducks, geese, flamingos, and ostriches, have a retractable grooved penis fixed to the wall of the cloaca. The ostrich penis may reach 8 inches in length.
Amphibians (frogs, toads, salamanders) lay eggs that lack a shell and the embryonic membranes that allow egg survival on dry land. The moist environments in which frogs lay eggs are endlessly varied (9). Some eggs are deposited in water, some in a foam nest, some in burrows, and some high in trees in temporary water pools in plants such as bromeliads. Most amphibians fertilize their eggs externally, but a few frogs are “viviparous,” the eggs fertilized inside the female reproductive tract and little froglets delivered after hatching. In one frog species the female swallows the fertilized eggs and broods them in her stomach. In another species the male broods the eggs in his mouth. Females of so-called “marsupial” frogs brood the eggs and carry the tadpoles in a pouch on their back. The female dart-poison frog of the Amazon simply carries her tadpoles on her back and feeds them with unfertilized eggs once she carries them to water in a bromeliad.
All frogs, salamanders, and newts studied to date have separate sexes, and gender is determined by sex chromosomes. Exposure to high incubation temperatures, however, can reverse gonadal sex determination in some species.
Dung beetles in East Africa (Figure 14) bury and recycle enormous amounts of dung from elephants and other herbivores. Males compete fiercely for the opportunity to form and roll away a ball of dung, in the process attracting a female who rides passively on the rolling ball. The easiest way for a male to obtain a ball of dung is to steal it from another beetle who has assiduously formed it. Whoever the owner, he rolls the ball across a long stretch of African savanna by standing on his hands and pushing with his feet. At some point he decides to start digging and buries the ball along with himself and the female to a depth of several feet, safe from competition, predation, parasitism, and adverse climatic conditions above ground. The male and female copulate and she lays eggs, which feed on the dung (“coprophagy,” meaning “feeding on feces”). In some species the pair remains together to care for the brood. Among beetles they are rare in the extent of their parental investment. When dung beetles are killed by the antiparasitic drug ivermectin administered to cattle, dung accumulates to a thickness of a foot or more on the African savanna.
Figure 14.
Standing on his hands and rolling a ball of herbivore dung with his hind legs, a male dung beetle in East Africa courts a female in the process, who rides along on the ball. At some point he decides to start digging, and the party disappears slowly down the hole, to a depth of several feet. Once there the female deposits one or more eggs in the dung, now safe from predators. Dung beetles are unsung heroes of nutrient recycling and soil turnover in the tropics. We wear them on our body in the form of scarab jewelry.
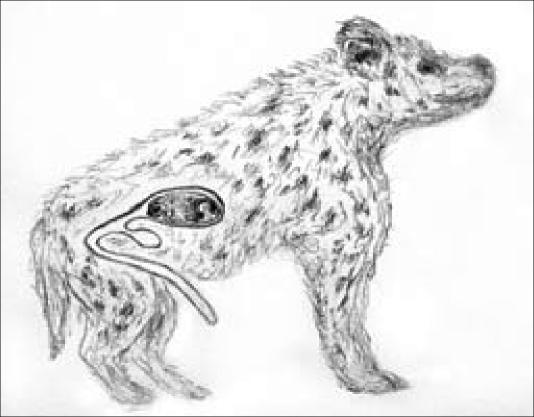
Spotted hyenas (Crocuta crocuta) in East Africa have a unique mating system that has prompted outrageous and comical speculations in the past. Females are larger than males, and the dominant “alpha” female has precedence at kills (along with her offspring). Females have higher circulating androgen levels than males, which may explain their size and tendency to be more aggressive. The clitoris is greatly enlarged and resembles a penis, to such a degree that researchers have trouble distinguishing males from females. The vagina and urogenital canal of the female pass through the clitoris, as in no other known mammal (Figure 15). The clitoris must expand to allow a 4-pound fetus to pass through. Dystocia (obstructed labor) has been observed in captivity and has resulted in the death of many primiparous females, and in those that survive, the fetus often suffocates during the prolonged passage.
Figure 15.
The reproductive tract of the spotted hyena, an African carnivore, is unique in all the world and is outrageous in its risk factors. The fetus (shown) must pass through a tract which turns at almost 180 degrees and continues through the canal of the enlarged clitoris. The clitoris, a homolog of the penis, is enlarged in female spotted hyenas because of high androgen levels, thought to have evolved because they conferred survival benefits upon the female and her young by increasing her body size and dominance at highly competitive feeding opportunities at kills. The clitoris and penis look so much alike that experienced researchers have trouble distinguishing males from females, except by adult size. The vagina and urogenital canal of the female pass through the clitoris, as in no other known mammal. Obstructed labor and suffocation result in the death of a high percentage of neonates.
How could such a dangerous anatomy evolve, seemingly at the edge of survivability? One hypothesis, difficult to test, states that under the conditions of competing for food at a kill, where a clan of 30 or more adults and juveniles compete fiercely at a single carcass on the East African savanna, mutations conferring greater size and strength on a female would provide a survival advantage for her and her offspring, and such genes would spread. Another question remains: Why have high androgen levels not evolved in females of other mammals?
The late Stephen Jay Gould argued that the male-mimicking genitalia of female spotted hyenas are “spandrels,” or coincidental spin-offs, of high testosterone levels. In a famous paper co-authored with Richard C. Lewontin, Gould argued that many traits of animals and plants did not arise as adaptations at all but as by-products of other traits that were adaptive. He used the term “spandrel” as a metaphor, comparing these traits to the curved triangular spaces formed between the arches of the great central dome of St. Mark's Cathedral in Venice. Each spandrel in the cathedral is elaborately decorated but is in essence a secondary by-product or “spin-off” of the vaulted arches supporting the dome.
Gould reminds us that the mammalian penis and clitoris are homologous pairs of organs, as are the scrotal sac and labia majora. In female spotted hyenas the clitoris and labia majora have enlarged to resemble penis and scrotum, but only as “spandrels,” not as adaptive organs used in social interactions, as the earlier hyena researcher Hans Kruuk had proposed. Gould admitted that his interpretation may not be the last word and that ongoing research may eventually clarify the origins of the anatomy and behavior of spotted hyenas (10).
Karen Blixen (Isak Dinesen) quoted a myth about spotted hyenas in Out of Africa (1938):
All hyenas, you will know, are hermaphrodites, and in Africa … on a full-moon night they will meet and join in a ring of copulation wherein each individual takes the double part of male & female… . Do you consider now … that it should be, on account of this fact, harder to a hyena than to other animals to be shut up by itself in a cage ? Would he feel a double want, or is he, because he unites in himself the complementary qualities of creation, satisfied in himself, and in harmony? In other words, since we are all prisoners in life, are we happier, or more miserable, the more talents we possess? (11)
Belief that spotted hyenas are hermaphrodites persists today in parts of Africa.
Another remarkable East African carnivore, the dwarf mongoose, can be seen on any safari to the Serengeti Plains or Masai Mara, living in abandoned termite mounds. Dwarf mongooses are no larger than small squirrels, and their social system is extraordinary, with an alpha male and alpha female forming a lifelong pair-bond. They dominate all other members and are likely to be the parents of all young born in the pack. The alpha male drives off any other male who attempts to mate with his partner. The most striking feature of their social system is the rearing of young by both related and unrelated individuals. Some pack members have emigrated from other packs and are not related to the pack members. Nevertheless, some of the unrelated females serve as “wet nurses,” lactating and feeding the babies of the dominant female!
The naked mole-rat has perhaps the most remarkable social organization of all mammals, living underground in extensive colonies in East Africa. A caste system divides them up into specialized morphotypes that perform specific functions, the closest that a mammal society comes to a social insect colony (see discussion under illustration, Figure 16). Workers excavating tunnels emerge at the surface kicking out dirt, an activity dubbed “volcanoing,” with their uplifted rear ends a tempting target for a snake or a human researcher!
Figure 16.
Naked mole-rats, which live underground in East Africa, are among the most unusual mammals in the world, almost hairless and almost blind, with only one reproductive female and several reproductive males. Other colony members specialize in colony tasks and do not reproduce; the caste system has been compared to eusocial insects, which also have nonproductive workers. This small group of nonreproductive workers was collected by a researcher in East Africa whom we encountered on a dirt road, where he was capturing workers that appeared at the surface while excavating cavity tunnels. They are caught by snakes in the same way, as they emerge at the surface. A colony may comprise 80 individuals, or sometimes up to several hundred, and feed on plant roots and tubers underground. This diet is high in cellulose, which is difficult to digest. Their gut microbiota help with digestion, and coprophagy (ingestion of feces) allows maximal extraction of nutrients.
Male African elephants have a unique male mating strategy called “musth,” in which males come into a sexually active estrus-like state unknown in other mammals. Their serum testosterone levels and aggressive behavior both rise markedly, and two males who are simultaneously in musth may inflict serious injuries on each other. Perhaps for this reason, in one study population the males were found to “straddle” the times of the year when they came into musth. Elephants have a very close and personal family life, which has been severely disrupted by poaching and capture of juveniles. When an elephant family is gunned down and the juveniles captured, those same juveniles often show seriously disturbed behavior as adolescents, resembling posttraumatic stress disorder in humans, with murderous aggression on other animals (12).
Bonobos (close relatives of chimpanzees, the other great ape most closely related to us) engage in “recreational” sex as a daily activity—females with females, males with males, and adults with young. In anthropomorphic psychiatric jargon, this behavior might be called “polymorphous perverse,” even though it is often initiated by the juveniles! It is seen in both wild and captive bonobos; one zookeeper told me she had to explain this behavior daily to visitors, with some embarrassment. Females engage in frontal “genital-genital rubbing” and seem to become excited as they do. Males usually do not ejaculate during recreational sex. In a behavior dubbed “penis-fencing,” two males hang face to face from a branch while rubbing their erect penises together. Bonobo behavior resembles that proposed for early humans in three ways: females are sexually receptive for long periods; sexual life is rich and serves purposes other than reproduction; and bonobos walk bipedally with ease much of the time. Frans de Waal, the primate researcher who in all the world may know as much about chimpanzees and bonobos as Jane Goodall, has studied their behavior in captivity for decades at the Yerkes Primate Research Center in Georgia and has written extensively on their complex social interactions.
The above examples of reproductive strategies in animals and plants are a small sample of the endless varieties, known and unknown. How do we make sense of the jungle of variations?
HYPOTHESES FOR THE PREVALENCE OF SEXUAL REPRODUCTION
Why is sexual reproduction almost universal in eukaryotes (animals, plants, fungi, protists)? Reproduction per se doesn't require sex, and sex is expensive: only one half of an individual's genes are passed on to each offspring, and courtship and mating are risky and energetically costly.
Over a dozen hypotheses attempt to explain the overwhelming prevalence of sexuality over asexuality. The two that are supported by the most evidence are 1) the host-parasite (pathogen) arms race (Red Queen hypothesis) and 2) the purging of the genome of deleterious mutations.
The Red Queen hypothesis is described in more detail in my paper in BUMC Proceedings (13) and in great detail in Matt Ridley's book, The Red Queen (14). This hypothesis states that in a world of dynamically changing biotic and abiotic environments, different lottery tickets (different genotypes created by sexual reproduction) provide a hedge against loss of all of one's offspring to pathogens, parasites, predators, or harsh environmental conditions. Support for this hypothesis comes from the fact that pathogens and parasites are best adapted to defeat the most common host genotypes (uncommon genotypes have an advantage) and from the extraordinary diversity of major histocompatibility complex (MHC) genes, which code for proteins involved in recognition of pathogens and transport of protein fragments to the cell surface for recognition by T cells of the immune system. Some MHC genes have over 100 alleles in the population of a species, maintained by selection; this number of alleles is unheard of for other genes.
To understand the second hypothesis for the ubiquity of sex, which is called “Muller's ratchet,” consider photocopying a document, then copying the copy, and repeating this process again and again. Each copy becomes more degraded than the last. This is what happens in asexual reproduction, with an organism cloning itself again and again, each time accumulating more mutations, ratcheting down the quality. DNA repair mechanisms correct some of those mutations, but not all. In sexual reproduction, the crossing-over of chromosome segments in meiosis allows for greater error correction, as there is a template on the other chromosome. In addition, with the reshuffling of genes in sexual reproduction, you can imagine a bell curve of individuals in the population, with one end of the curve harboring members with the most mutations and the other end having those with the fewest mutations. Those at the end with more mutations will have lower reproductive success than those at the other, thereby purging the gene pool of many harmful mutations. The advantage of this distribution is summed up by George Bernard Shaw's reply to the actress who suggested that they have a child together, which would have her beauty and his brains: “Yes, madam, but what if it had my beauty and your brains?” (the wrong end of the bell curve).
The privilege of purging mutations by means of a bell curve (Muller's ratchet) is denied an asexual organism. It may be a valid explanation for sexuality, but it does not rule out the Red Queen hypothesis. Both may contribute to the benefits of sexual reproduction.
The bottom line is that we do not know why sexuality is the winning strategy, by a wide margin. Can we learn from the examples when it is not?
ASEXUALITY
Some invertebrates reproduce sexually when the environment turns nasty, as with abrupt parasite infestation or the drying up of their habitat. This gives them the advantage of a diversity of genotypes in their progeny, some of which may survive the environmental change. During favorable environmental conditions, these invertebrates choose asexual reproduction, cloning the genotype that worked well under those conditions. They therefore have the best of both worlds, sexual and asexual.
Other rare invertebrates are exclusively asexual. Bdelloid rotifers, tiny aquatic invertebrates that have lived and thrived for 40 million years without sex, are “something of an evolutionary scandal,” according to the late John Maynard Smith, who studied the evolution of sexuality for most of his life. These rotifers have flagrantly falsified the hypothesis that asexual organisms should become extinct in a relatively short time because of accumulation of harmful mutations in nonrecombining genomes. Males are unknown in these little animals, which are found in fresh water and moist habitats worldwide. A study of their DNA suggests that their genome “froze” millions of years ago, when meiosis ceased and they became asexual.
One such rotifer is named Philodina roseola. The biologist Olivia Judson, in Dr. Tatiana's Sex Advice to All Creation, quoted Miss Philodina, who explained the “scandalous” longevity of her kind:
We bdelloids travel in both space and time. Of course, we can't travel backward in time: nobody can do that. But we can go forward. We have a trick called anhydrobiosis. It's a state of suspended animation. Essentially, we dry up and blow away… . It's risky. Anhydrobiosis is difficult… . Many bdelloids never recover. But if you do survive, you come back to life in a new place and time, healthier and happier than before (8).
Another case of long-lasting asexuality is that of mycorrhizal fungi, which collaborate with flowering plants in an ancient and widespread mutualism. The fungal filaments invade plant roots and provide the plants with minerals from the soil, in turn receiving carbohydrates from the plant (products of photosynthesis, unknown in fungi). Like bdelloid rotifers, these fungi have been asexual for millions of years. But there is a catch: their cells contain hundreds of nuclei, and a recent study found that each nucleus contains many different sequences of the same genes for ribosomal DNA. This may be a unique kind of sexuality that protects this ancient lineage from accumulating harmful mutations over time.
A small number of asexual, parthenogenetic salamanders in North America have persisted for millions of years (15). These lineages are all-female but require sperm from males of a different, sexual species to initiate egg division and embryogenesis. Fertilization does not occur, so the male's genes are wasted. Why should males of a sexual species waste their time and sperm in such a manner? Why should genes promoting that behavior persist? The answer, at least in one case, seems to be that the males become more attractive to females of their own species if they are observed consorting with females of the other species. Females of both species look alike, and those of the sexual species may mistakenly identify the other females as some of their own. Female choice may thus maintain the behavior (16), as long as the parthenogenetic females mimic the appearance of the sexual females.
Only lizards, among vertebrates, have fully parthenogenetic species, with no need for stimulation of egg development by sperm from a male of another species. These lizards have fully parthenogenetic populations able to reproduce without males. Some tropical lizards have both sexual and parthenogenetic populations; with habitat disturbance, only one parthenogenetic female need find her way into a favorable habitat to found a new colony, which can outproduce the bisexuals in a short time. Her offspring are identical copies of herself and so can reproduce without mating, twice as fast as a sexual colony. Parthenogenesis may survive as a viable strategy because of this sporadic advantage.
A study published in 2005 found that the favorite laboratory plant Arabidopsis can repair damaged genes and restore normal floral morphology without the help of the template of a second normal copy of the damaged gene (17). This is an unprecedented finding with no known explanation, and if it is found to be more widespread it may help explain the occasional evolutionary longevity of asexual species such as bdelloid rotifers. One suggestion is that there may be an RNA “backup copy” of the entire genome that has been entirely undetected and can act as a template. Computer metaphors in biology are multiplying like rabbits!
BACTERIALLY INDUCED “ASEXUALITY” IN ARTHROPOD HOSTS
Sexuality may be ubiquitous because of infectious and parasitic diseases, but there are specialized bacteria that may literally eliminate sexuality in their host. The tiny bacterium Wolbachia spends its entire life within the ovaries and testes of many insects and is transmitted from female to offspring through the egg's cytoplasm (18). Because sperm are almost empty of cytoplasm, males are usually unable to pass on the bacterium. That fact provides a clue to the bacterium's strategy: eliminate males, or at least reduce them to insignificance, because the bacterium's genes are spread only by females. The result is a skewed sex ratio with few males, and in some cases loss of all males, with resulting parthenogenesis. We once thought this was an evolved trait of the host species, but now it emerges as a takeover by Wolbachia.
Some wasp species that are hosts of Wolbachia become parthenogenetic for as many generations as the bacterium is there. Asexuality can be “cured” by treating the wasps with antibiotics or subjecting them to heat in the laboratory, either of which kills the bacteria. Males reappear among the offspring. If the antibiotic is continued over several generations, the population becomes permanently sexual and does not revert after cessation of treatment, unless it becomes reinfected in the wild.
How common is Wolbachia infection? It occurs in members of over 90% of arthropod species, including 5 orders of insects, an isopod, and a spider mite. In most cases a skewed sex ratio, not asexual reproduction, results. Wolbachia is only one of many male-killing bacteria that are common in insects (19).
Wolbachia may soon become more newsworthy, as they are essential symbionts of the major pathogenic filarial nematode parasites of humans, including Onchocerca volvulus, which causes river blindness. Antimicrobials that kill the Wolbachia symbionts also kill the nematode hosts and may become useful in treating the debilitating diseases caused by the nematodes (20, 21).
ARE BACTERIA AND VIRUSES ASEXUAL?
Although bacteria and archaebacteria replicate asexually, and viruses are cloned asexually by their host cell, rampant horizontal gene transfer (from one cell to another, not from parent to offspring) also shapes their genomes. In bacteria we see plasmid-mediated transfer of genes that confer antibiotic resistance and virulence, and in the influenza A virus we see mixing of genes and gene segments when both an avian and a mammalian strain infect an intermediate mammal host simultaneously, such as pigs in southeast Asia, where ducks, swine, and humans have regular and intimate contact. Such mixing of genes in bacteria and viruses, called “parasexuality,” is as effective as any form of sexual reproduction in generating genotypic diversity and can occur instantaneously, not having to wait for a new generation of progeny. Microbes enjoy much more rapid evolution and adaptation than their larger hosts.
We once thought the “tree of life” could be traced back to an U rorganism that would represent the earliest life form. The biologist Carl Woese laid this idea to rest by proposing that the earliest life forms engaged in a promiscuous horizontal exchange of genes, so that the trunk of the tree of life was actually a soup of genes traded freely by primitive microbes.
CONFLICT BETWEEN THE SEXES: IMPRINTING
We should always ask: How do alleles get themselves better represented in future generations, and do they do this by different strategies in the two sexes?
There is no teleology here; natural and sexual selection act by the logic of what works best. The use of words like “strategy” is only a convenient anthropomorphic way to describe what works best. We could avoid such words, but the alternative is a cumbersome description such as, “Behavioral predispositions have evolved in the competitive setting of specific variables.” Nevertheless, there is an ongoing debate about whether to use such anthropocentric descriptions. I favor their use for the sake of simplicity and economy of expression, as long as we understand what we are doing.
Strategies of courtship and mating should, by evolutionary logic, be different for the sexes, which sometimes seem to be from different planets. They obviously need each other, but they do not have the same priorities. Anthropologist Meredith F. Small has elegantly captured the essence of male-female reproductive conflict:
Compelled by the urge to pass on genetic material to the next generation, the sexes must often cooperate in mating and parenting, but each sex cooperates only under duress because females and males operate under different reproductive rules set down in opposing directions eons ago. Like an open wound that never heals, the conflict between males and females will never be resolved because the evolutionary interests of the two sexes are forever locked in opposing position. There is no right or wrong here, no sex better than the other, just two types of individuals trying to win in the game of reproductive success. The ground rules of the battle include cooperation, conflict, and exploitation, and both sexes use these tactics equally (22).
Conflict between the sexes, at the most fundamental level, takes the form of imprinting of DNA in egg and sperm. Imprinted genes are epigenetically marked during gametogenesis so that they are exclusively expressed in either egg or sperm genomes with no change in their DNA sequence. A child's genes are therefore not all equal: in some cases, the copy from one parent is turned off, and this affects the child's ability to acquire resources in the uterus and after birth. Imprinting may even last through life, the alleles retaining a “memory” of their parental origin. There is growing evidence that imprinting may contribute to disease susceptibility, possibly by altering resource allocation to organs over the course of a lifetime.
The asymmetry of interests of maternally and paternally inherited genes may take the form of differences in the placental tissues interposed between mother and fetus. Gene knockout experiments have shown that eliminating genes expressed from sperm reduces the surface area for nutrient exchange, whereas knocking out genes expressed in the egg increases the area for exchange. The offshoot is that a shift toward greater nutrient provisioning to the fetus is promoted by the father's genes and suppressed by those of the mother, both being compatible with fetal life support but the one enhancing fetal survival and the other preserving maternal resources for future reproductive opportunities (23). The conflict of the sexes is no more dramatically revealed.
Parthenogenesis does not occur in mammals, probably because there is only one parental genome (the maternal genome) and some of the maternal gene copies are silenced by imprinting, with no unimprinted partner alleles to provide the critically important gene products. Biparental reproduction is necessary because of epigenetic modifications (such as imprinting) that occur during gametogenesis. This results in unequal expression of genes from parental chromosomes. In 2004 the first parthenogenetic mouse was artificially created in the laboratory and developed to adulthood with the ability to reproduce normally by mating with a male and delivering normal offspring (but was only one of many that did not develop normally) (24). With a stretch of the imagination and laboratory intervention, we can finally propose that male mammals are unnecessary!
HOW MANY SEXES?
If you were a single-celled alga in a pond, you wouldn't see the world as splitting into males and females, argues a prominent sex researcher, Laurence Hurst of the University of Bath in the United Kingdom. In some species of algae every member is sexually equivalent. One slime mold has 13 gamete types, which compete in a hierarchy, and somehow (for the time being), the arrangement seems to be working. The biological diversity of sexual reproduction has produced a different take on what sex is. There is even an ant species believed to have a three-sex system—a female and two types of males (25).
The bottom line is that we have adopted a stereotyped, anthropomorphic view of sex. Some algae, fungi, and protists dispense with a hard-and-fast division of sexes, and we can view them as having multiple “sexes” or just mixing genes in any of a myriad of ways, as bacteria and viruses do.
On the other hand, only two sexes—and two kinds of gametes—have evolved in the vast majority of eukaryotic organisms. How would you answer your child's question, “Why are there two sexes?”
The simplest answer might start with the explanation that gametes could be identical and still provide genetic diversity in offspring. Gene reshuffling could occur even if sperm and egg were identical (isogametes). So why are they different, with eggs bloated with food resources and mitochondria, and sperm consisting of anemic cytoplasm, a full complement of nuclear genes, and a tail powered by a few mitochondria that aren't allowed into the egg?
According to one model, one sex abandons its cytoplasm and avoids conflict with mitochondria of the other sex. (Conflict inevitably results when two entities interact in reproduction, with natural selection weaning out the one or the other because of small differences.) Sperm and egg diverged, so that each performed very different functions. The one provided resources in the form of cytoplasmic food and mitochondria (and chloroplasts, in plants); the other provided another set of genes, without mitochondria or chloroplasts to compete with those in the other gamete. “Runaway” selection pushed each to its own extreme.
Although this is only one model, it is a popular one and probably a good one for a tentative answer. Having two sexes and two gamete types may be the best way to avoid conflict and get along. The result is a form of genomic conflict management, important for organisms that reproduce by fusing two cells, bringing together both nuclear and cytoplasmic DNA. (The genome is no longer the cooperative “republic of genes” it was once thought to be.) The verdict: if you're going to fuse, there should be different sexes, and if there are different sexes, the number should usually be two.
SEX DETERMINATION
Sex determination in animals is highly complex and poorly understood. In mammals, males are the “heterogametic” sex (XY) and females are “homogametic” (XX), but in birds and lepidopterans (butterflies and moths) it is just the opposite, females being heterogametic and males homogametic.
In crocodilians (crocodiles, alligators, caimans, and gavials), many turtles, and several lizards, sex assignment is by means of temperature during embryogenesis. In the leopard gecko, an egg incubation temperature of 26°C produces only females, 30°C produces a female-biased sex ratio, 32.5°C results in a male-biased ratio, and 34°C again produces almost all females (26). David Crews, who has studied temperature-based sex determination for decades at the University of Texas at Austin, believes that incubation temperature has direct organizational effects on the development of brain nuclei, notably in limbic structures such as the hypothalamus. Phenotypic plasticity is abundantly in evidence in these reptiles. Sexual behavior, and not just gender, is determined by temperature (27).
In a number of litter-bearing mammals such as mice, gerbils, and rats, females sandwiched between two males in utero are less attractive to males, have a masculinized anatomy, urine-mark more often, and are more aggressive than females sandwiched between two other females or between one female and one male. The surge in testosterone levels in male fetuses diffuses through the amniotic fluid, with higher concentrations reaching adjacent fetuses. The opposite, feminizing effect is seen in males flanked by females. Crews reminds us that sexuality is different from sex. sexuality “goes beyond the components of sex and represents the continuously variable suite of traits that emerge during the organism's lifetime, making each individual unique” (27).
Our stereotypical division of animals into male and female is thus challenged. If we think of a continuum between male and female, it appears that the reproductive tract and the central nervous system may end up at different points on the continuum.
The latest research—in its early stages—suggests that male and female brains sometimes start down different developmental paths from the outset, before hormones enter the picture; both body and brain of a zebra finch were male on one side and female on the other, hinting that more than sex hormones guided their development (28).
“Homosexuality” in nonhuman animals has been disputed endlessly with no meaningful conclusion, probably because the term itself is anthropomorphic. In bonobos, as mentioned previously, brief “homosexual” pairings between either sex are common, but they occur along with heterosexual and adult-juvenile pairings and are not long-term exclusive choices. Brief sexual pairings between males of many animals are seen in nature and in zoos; penguin males often engage in copulatory behavior together, and male dolphins have paired with other males and with turtles sharks and eels! (One male Amazon River dolphin was even observed to penetrate another's blowhole!) Young male Masai giraffes engage in long “necking” bouts, which seem to be a kind of practice for later contests between adult males over access to females (Figure 4). Young male sea lions also practice sparring behavior (Figure 5). Males of almost any species of land vertebrate mount other males, as juveniles and adults.
Figure 5.
Young male Australian sea lions confront each other in practice bouts of sparring, in preparation for competition for females as adults. Male-male competition is one form of sexual selection and often results in males being larger than females and having more formidable weaponry.
X AND Y CHROMOSOMES
Female mammals inherit an X chromosome from each parent, whereas males inherit a single maternal copy. The mammalian X and Y chromosomes are believed to have evolved from one pair of autosomes (i.e., all the other chromosomes) within the last 300 million years, when one member of the pair acquired a male-determining locus. Male-advantage alleles accumulated on the proto Y, and the combination was preserved by cessation of recombination between the X and Y during meiosis in males, except for a small “pseudoautosomal” region on the tip of the X that recombines with equivalent segments on the Y. This opened the door to a progressive degeneration of the Y as it accumulated mutations, deletions, and repetitive elements that could not be eliminated by DNA repair during meiosis. An X chromosome spends two thirds of its time in a woman, where it can recombine with another X, dodging the Muller's ratchet that has so eroded the Y. Some genes on the Y have been found to recombine with palindromic copies in a hairpin-like fold, and this may serve to repair some copying errors. But the decay of the Y chromosome is continuing; two groups of rodents have already lost their Y (29). Jokes about males have taken full advantage of this degeneracy (modified from Maureen Dowd, The New York Times): Why, oh Y, are men so insecure? Not only are men dwindling away as their Y chromosomes disintegrate, but now their Y chromosomes have been found to have sex with themselves. Narcissistic to the core. Better to be an X chromosome than an ex-chromosome.
In female mammals, the nucleus of somatic cells developed a way to silence most of the genes on one or the other X chromosome in each cell, the choice being random from cell to cell (the calico cat demonstrates this random pattern in its fur colors). This silencing prevents a double dose of gene products in females.
Males pass their X chromosome to their daughters (XX) but never to their sons (XY), whereas females pass their X chromosomes to daughters and sons with equal frequency. Male mammals are vulnerable to genetic diseases that are much more rare in females, because males have only one X chromosome. If a mutated gene occurs on that X, there is no equivalent allele on the Y to provide the proper code for the trait. Like autosomal single-gene disorders, X-linked diseases can be either recessive or dominant. X-linked recessive diseases include hemophilia and some forms of muscular dystrophy. These diseases are much more common in males than females because two copies of the mutant allele are required for the disease to occur in females, while only one copy is needed in males.
In 2005 the human X chromosome was sequenced (30) and revealed a big surprise: some 15% of genes on the X are not silenced, thereby providing a double dose of those gene products to females. Another 10% are sometimes silenced and sometimes not. All together, human females have 15% to 25% more active X genes than males, giving them more of those gene products and in some cases different alleles. Human females may therefore have greater genetic diversity than males, and the X chromosome is the home of many genes for brain structures.
The sexes not only have different priorities but also are differently endowed genetically.
THREE “GREAT DIVIDES”
Sexual vs asexual reproduction is one “great divide” in reproductive biology. There are two others to consider: internal vs external fertilization and parental investment (male vs female).
Eggs are fertilized internally in mammals and birds, and these males are rarely absolutely certain of their paternity. “Sneaky copulations” are implicated by DNA analysis in more and more bird species that were thought to be monogamous. Sperm competition occurs in vertebrates and invertebrates across taxa, because females are now known to be highly “promiscuous” (another anthropomorphic term) in a majority of animal species. Sperm wars are universal among males, except in rare truly monogamous species and in seahorses and pipefishes, where the male fertilizes the eggs in his pouch.
External fertilization usually enables males to be more certain of their paternity than internal fertilization. When a female fish deposits her eggs on the sea floor, a lucky male deposits his sperm onto the egg mass and stays there to guard it from insemination by other males and from predation. Genes promoting male parental care are likely to be adaptive and selected for, as they promote survival of his offspring. Male frogs ride on the backs of females in order to deposit their sperm on the eggs the moment she lays them (Figure 17). External fertilization changes the dynamic of male parental care; the male is now more likely to spread his genes if he provides parental care. The female may now be free to desert the eggs after laying them.
Figure 17.

Golden toads, this pair in amplexus in the cloud forest of Monteverde, Costa Rica, have never been seen since 1989. The small and isolated populations, which occupied only a few square mi les of cloud forest, are now apparently extinct, and their social behavior has never been studied in detail. Many biologists have searched for them in vain. At large pools of rainwater in the rainy season, as many as several hundred males were once seen at one time; for the lucky few who saw them, the sight was unforgettable.
Sperm competition occurs with internal or external fertilization. Female fish that release their eggs into the water invite sperm competition from males who rush in to fertilize them. Male red jungle fowl release more sperm in their ejaculate when they mate with a new female (after repeated matings with the same female) and also if they perceive a high level of competition with other nearby males (31).
Parental investment, the third “great divide,” sets the stage for which sex competes the most for access to the other. It is considered by many to be the single most important difference between the sexes. The sex that invests the most in the next generation is the “limiting resource.” Consider mammals: the female becomes pregnant (with tremendous costs and risks), delivers the young (with still more risks), nurses the young (expensive in terms of effort and nutritional demands), and usually raises the young by herself. She thus provides much greater parental investment than the male, who has only to desert her after mating and seek another mating opportunity. Female mammals are thus the limiting resource; that is, each female is competed for by more males than each male is competed for by females. Females can thus be more choosy than males, and there is less variance in female reproductive success than in male reproductive success: females enjoy mating success with relatively little differences, but there is great variation in male success (some males are highly successful and others are total losers). Female choice is a powerful form of sexual selection in animals.
Parental investment is usually greater in females than in males in animals with internal fertilization. This is why in mammals and birds we usually see greater competition among males for access to females, rather than the other way around. Females are usually the limiting resource. The rare exceptions are instructive: consider phalaropes (shorebirds) and seahorses.
The three species of phalaropes in North America show “reversed sexual dimorphism,” the females being larger and more brightly colored than the males. The female competes aggressively with other females for mates. She abandons her eggs after laying them, and the male incubates them and raises the young by himself. This is a rare case of polyandry (“many males,” in which females compete for mating with males), and begs an explanation. One hypothesis: males can be counted on to rear the young and females can increase their mating opportunities by deserting; paternal investment is relatively large. But the real question is, How did this strategy arise in phalaropes when it is so rare elsewhere? And why is it rare?
The second instructive example of sex role reversal is that of seahorses and pipefishes, in the family Syngnathidae (collectively called syngnathids). As I mentioned, males of these fishes have a specialized egg-brooding structure on the abdomen or tail into (or onto) which females deposit their eggs. This arrangement assures the male of his paternity, as he inseminates the eggs in his own pouch or on his own tail. As with phalaropes, paternal investment is high. The most complex pouches of seahorses have placenta-like tissues that provide nourishment and oxygen to the young. The chemical composition of the pouch fluid changes from that of body fluids to that of seawater as “pregnancy” progresses. Males protect, nourish, and oxygenate the developing embryos for several weeks and then release them into the water as independent young. “Labor” may last hours or days.
Many seahorse species with complex brood pouches are monogamous, and females of these species are busy guarding their mate from other females. In other seahorse and pipefish species, both males and females are polygamous, and both have multiple mating partners. In these polygamous species, it is often the female who seeks more than one partner (polyandry); some of these latter females are vividly colored, unlike the males, and compete with other females for access to males.
Sex role reversal may thus occur as in phalaropes, but mostly in nonmonogamous pairs. Mating habits may thus influence sex roles just as parental investment does. Some seahorse males may compete strongly with other males, and in these cases sex role reversal is absent, except for carrying the young. Sexual selection in syngnathids needs more study, as it is diverse and may provide a wealth of insight into how mating strategies evolve (32, 33).
SUMMARY
This review of sexuality has several important messages:
Sexual reproduction is far more common than asexual reproduction in plants and animals.
Hypotheses for the prevalence of sexuality include the hostparasite and host-pathogen arms race and the purging of the genome of deleterious mutations.
Sexual selection is distinct from natural selection and is a key player in evolution.
Courtship and mating strategies are almost infinitely variable.
Conflict between the sexes occurs at all levels, from mating strategies to imprinting of genes.
Parental investment may be the most important difference between the sexes.
In most animals with internal fertilization, females provide more parental investment and are the “limiting resource.”
When females invest more, they are the choosier sex and there is more overt male-male competition for access to mates, resulting in variance in reproductive success (with a more even distribution of success in females).
Alleles move into the future according to evolutionary logic, which is usually different for the two sexes.
Some findings in this paper may change overnight, but the lesson remains that evolutionary logic is important in the understanding of reproductive biology, at the level of genes, individuals, and populations.
RECOMMENDED READING
Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. New York: Pantheon Books, 1999. Sarah Blaffer Hrdy, anthropologist at the University of California at Davis, provides the rare perspective of a scientist who integrates anthropology with biology. Her book is hard to put down, as she interweaves stories about mothers across cultures, mother-father conflict, oxytocin, lactation, menopause, infanticide, and the relevance of evolutionary theory to parenting. Her own research has been primate social systems and reproductive biology.
Judson O. Dr. Tatiana's Sex Advice to All Creation. New York: Metropolitan Books, 2002. This is an original and highly entertaining exposition of animal sexuality by an Oxford biologist. Judson discusses a wide variety of sexual strategies in the format of letters written to an imaginary Dr. Tatiana, in the fashion of Dear Abby columns. Her coverage of the biology and behavior is accurate.
Ridley M. The Red Queen: Sex and the Evolution of Human Nature. New York: Macmillan Publishing Company, 1993. Although Ridley has written two book since this one, there is no treatise as good or as stimulating as this one on the Red Queen Hypothesis, which proposes that the host-parasite (-pathogen) arms race provides the stage for the reproductive advantage of genotypic diversity in offspring. This is covered in the first half of the book, through chapter 5. The last half of the book moves into speculations on human nature, and I recommend that you read this last part with skepticism or skip it all together.
Moss C. Portraits in the Wild: Animal Behavior in East Africa, 2nd ed. Chicago: University of Chicago Press, 1982. This little paperback and Richard Estes' Behavior Guide (below) may be the two best books ever written on the behavior, sexual and otherwise, of African mammals, and they are both a must to travelers to eastern and southern Africa.
Moss C. Elephant Memories: Thirteen Years in the Life of an Elephant Family. New York: William Morrow & Co, Inc, 1988. This is undoubtedly the most scholarly coverage of the social life of the African elephant and a pleasure to read.
Estes RD. The Behavior Guide to African Mammals. Berkeley, CA: University of California Press, 1991. Richard Estes has produced the definitive and authoritative reference guide to the behavior of African mammals, in the form of a paperback field guide. We have spent time in East Africa with Dick, and his understanding of the mammals is boundless. His life research has been on the reproductive behavior and biology of wildebeest.
de Waal F. Bonobo: The Forgotten Ape. Berkeley, CA: University of California Press, 1997. Frans de Waal, who probably knows bonobos better than anyone else alive, has written a definitive description of their social life, replete with the recreational sexuality which is unique to these apes, our closest living relatives along with chimpanzees.
References
- 1.Fatouros NE, Huigens ME, van Loon JJA, Dicke M, Hilker M. Chemical communication: butterfly anti-aphrodisiac lures parasitic wasps. Nature. 2005:433–704. doi: 10.1038/433704a. [DOI] [PubMed] [Google Scholar]
- 2.Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. New York: Pantheon Books; 1999. [DOI] [PubMed] [Google Scholar]
- 3.Hanlon RT, Naud MJ, Shaw PW, Havenhand JN. Behavioural ecology: transient sexual mimicry leads to fertilization. Nature. 2005:433–212. doi: 10.1038/433212a. [DOI] [PubMed] [Google Scholar]
- 4.Foellmer MW, Fairbairn DJ. Spontaneous male death during copulation in an orb-weaving spider. Proc R Soc Land B Biol Sci. 2003;270(Suppl 2):S183–S185. doi: 10.1098/rsbl.2003.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buston P. Social hierarchies: size and growth modification in clownfish. Nature. 2003;424:145–146. doi: 10.1038/424145a. [DOI] [PubMed] [Google Scholar]
- 6.Munk O. Histology of the fusion area between the parasitic male and the female in the deep-sea anglerfish Neoceratias spinifer Pappenheim, 1914 (Teleostei, Ceratioidei) Acta Zool. 2000;81:315–324. [Google Scholar]
- 7.MacLaughlin DT, Donahoe PK. Mechanisms of disease: sex determination and differentiation. N Engl J Med. 2004;350:367–378. doi: 10.1056/NEJMra022784. [DOI] [PubMed] [Google Scholar]
- 8.Judson O. Tatiana's Sex Advice to All Creation. New York: Metropolitan Books; 2002. Dr. [Google Scholar]
- 9.Duellman WE. Reproductive strategies of frogs. Sci Am. 1992;267:80–87. doi: 10.1038/scientificamerican0792-80. [DOI] [PubMed] [Google Scholar]
- 10.Gould SJ. The Structure of Evolutionary Theory. Cambridge, MA: Belknap/Harvard University Press; 2002. [Google Scholar]
- 11.Dinesen I, Blixen K. Out of Africa. New York: Random House, Vintage Books; 1972. original first edition by Random House, 1938. [Google Scholar]
- 12.Bradshaw GA, Schore AN, Brown JL, Poole JH, Moss CJ. Elephant breakdown. Nature. 2005:433–807. doi: 10.1038/433807a. [DOI] [PubMed] [Google Scholar]
- 13.Dimijian GG. Pathogens and parasites: insights from evolutionary biology. BUMC Proceedings. 1999;12:175–187. Available at http://www.BaylorHealth.com/proceedings/12_3/12_3_dimijian.html; accessed April 7, 2005. [Google Scholar]
- 14.Ridley M. The Red Queen: Sex and the Evolution of Human Nature. New York: Macmillan Publishing Company; 1993. [Google Scholar]
- 15.Spolsky CM, Phillips CA, Uzzell T. Antiquity of clonal salamander lineages revealed by mitochondrial DNA. Nature. 1992;356:706–708. doi: 10.1038/356706a0. [DOI] [PubMed] [Google Scholar]
- 16.Schlupp I, Marler C, Ryan MJ. Benefit to male sailfin mollies of mating with heterospecific females. Science. 1994;263:373–374. doi: 10.1126/science.8278809. [DOI] [PubMed] [Google Scholar]
- 17.Lolle SJ, Victor JL, Young JM, Pruitt RE. Genome-wide non-mendelian inheritance of extra-genomic information in Arabidopsis. Nature. 2005;434:505–509. doi: 10.1038/nature03380. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer C. Wolbachia: a tale of sex and survival. Science. 2001;292:1093–1095. doi: 10.1126/science.292.5519.1093. [DOI] [PubMed] [Google Scholar]
- 19.Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GDD. A functional dosage compensation complex required for male killing in Drosophila. Science. 2005;307:1461–1463. doi: 10.1126/science.1107182. [DOI] [PubMed] [Google Scholar]
- 20.Pennisi E. New culprit emerges in river blindness. Science. 2002;295:1809–1811. doi: 10.1126/science.295.5561.1809. [DOI] [PubMed] [Google Scholar]
- 21.Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, Büttner DW. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 22.Small M. Female Choices: Sexual Behavior of Female Primates. New York: Cornell University Press; 1993. pp. 10–11. [Google Scholar]
- 23.Constância M, Kelsey G, Reik W. Resourceful imprinting. Nature. 2004;432:53–57. doi: 10.1038/432053a. [DOI] [PubMed] [Google Scholar]
- 24.Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo J, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–864. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield J. Everything you always wanted to know about sexes. PLoS Biol. 2004;2:el83. doi: 10.1371/journal.pbio.0020183. Available at http://biology.plosjournals.org/perlserv/?request-getrdocument&doi=10.1371/journal.pbio.0020183; accessed April 4, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhen T, Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation.) J. Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 27.Crews D. Sex determination: where environment and genetics meet. Evol Dev. 2003;5:50–55. doi: 10.1046/j.1525-142x.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- 28.Dennis C. Brain development: the most important sexual organ. Nature. 2004;427:390–392. doi: 10.1038/427390a. [DOI] [PubMed] [Google Scholar]
- 29.Graves JAM. Recycling the Y chromosome. Science. 2005;307:50–51. doi: 10.1126/science.1107295. [DOI] [PubMed] [Google Scholar]
- 30.Ross MT, Grafham DV, Coffey AJ, Scherer S, Mclay K, Muzny D, Platzer M, Howell GR, Burrows C, Bird CP, et al. The DN A sequence of the human X chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizzari T, Cornwallis CK, Lovlie H, Jakobsson S, Birkhead TR. Sophisticated sperm allocation in male fowl. Nature. 2003;426:70–74. doi: 10.1038/nature02004. [DOI] [PubMed] [Google Scholar]
- 32.Pagel M. Evolutionary biology: polygamy and parenting. Nature. 2003;424:23–24. doi: 10.1038/424023a. [DOI] [PubMed] [Google Scholar]
- 33.Vincent A. A seahorse father makes a good mother. Natural History. 1990;99:34–43. [Google Scholar]