Abstract
The overall incidence of tumor lysis syndrome in adults is not well defined, and its occurrence can be unpredictable. Several strategies are available for the prevention and treatment of tumor lysis syndrome, with rasburicase being the most recent. Rasburicase is a recombinant urate oxidase enzyme approved for use by the Food and Drug Administration in patients who are at risk of developing tumor lysis syndrome or for the management of elevated uric acid levels. Clinical trials have demonstrated rasburicase to be effective in both the pediatric and adult populations, although the drug is currently indicated only for use in the pediatric population. Adverse effects associated with rasburicase can be significant, ranging from anaphylactic reactions to methemoglobinemia. To ensure accuracy of uric acid test results, special laboratory handling procedures must be followed while patients receive rasburicase. Compared with allopurinol and intravenous sodium bicarbonate, rasburicase is costly, and therefore judicious use of the medication is warranted.
Tumor lysis syndrome occurs in malignancies that are highly proliferative and have high tumor burdens, such as lymphomas and leukemias. Metabolic abnormalities—including hyperphosphatemia, hyperkalemia, hyperuricemia and/or hypocalcemia, and renal dysfunction—usually accompany tumor lysis syndrome. Often, hyperuricemia (generally a uric acid level ≥8 mg/dL) is a hallmark finding of tumor lysis syndrome. The current strategies to prevent tumor lysis syndrome are the recognition of risk factors and preventive treatment. In the USA, the standard therapy for preventing tumor lysis syndrome is hydration, alkalinization of the urine with sodium bicarbonate, and allopurinol (1–3). Allopurinol, however, only prevents the formation of uric acid and does not affect the uric acid developed prior to treatment.
Since 1975, a nonrecombinant form of urate oxidase (Uricozyme) has been available in Europe and found to be effective in reducing uric acid levels (3). Within the past few years, a more purified form of urate oxidase has been developed. On July 12, 2002, the Food and Drug Administration (FDA) approved rasburicase, a recombinant urate oxidase enzyme, for elevated plasma uric acid.
INDICATION
Rasburicase is indicated for use in the pediatric population for the management of elevated uric acid in patients who are receiving chemotherapy for leukemia, lymphoma, or solid tumors and are anticipated to develop tumor lysis and elevated uric acid levels (4).
PHARMACOLOGY
Rasburicase is derived from a cDNA code from a modified Aspergillus flavus strain and expresssed in a modified yeast strain of Saccharomyces cerevisiae (4). A recombinant urate oxidase enzyme, rasburicase converts existing uric acid to allantoin, which is 5 to 10 times more soluble in urine than uric acid. Rasburicase differs from allopurinol since it can affect existing plasma uric acid; allopurinol affects only the future production of uric acid by inhibiting xanthine oxidase (3). The oxidation of uric acid to allantoin by rasburicase produces hydrogen peroxide and carbon dioxide (5).
PHARMACOKINETICS
The elimination half-life of rasburicase is approximately 16 and 21 hours with the 0.15 mg/kg and 0.2 mg/kg dosing regimens, respectively. In general, the overall elimination half-life of rasburicase is 18 hours. There was no observed accumulation of rasburicase on days 1 and 5 of dosing when the area under the curve was evaluated (4, 6). Clearance of rasburicase is independent of renal and hepatic function because it is degraded via peptide hydrolysis (2). Because of limited pharmacokinetic evaluation in the adult and geriatric populations, no definitive data can be presented for these groups (4).
CLINICAL TRIALS
Five clinical trials were evaluated to assess the effectiveness of rasburicase in pediatric and adult patients. Most patients included in the trials, with the exception of study #5, were pediatric patients.
Study #1
Pui et al (6) evaluated the effectiveness of rasburicase in 131 children, adolescents, and young adults <20 years old, with the median age being 7. Patients included in the study were recently diagnosed with B-cell acute lymphoblastic leukemia (ALL); had ALL with an initial leukocyte count of at least 50 × 109/L or a lymphomatous presentation and a large tumor burden; had stage III or IV small noncleaved cell or lymphoblastic non-Hodgkin's lymphoma (NHL) with a large tumor burden; or had any leukemia or NHL with a plasma uric acid level ≥8 mg/dL and either a serum creatinine or lactate dehydrogenase level >2 times the upper limit of normal. Rasburicase was administered for 5 to 7 days, with chemotherapy scheduled to start as soon as 4 hours after the first dose of rasburicase.
A dose-validation phase was incorporated into the study to determine the most effective dosing regimen. Initially, 0.15 mg/kg was administered; however, hyperuricemia was not corrected in 14 consecutive patients and, per the study design, the dose was increased to 0.2 mg/kg, which was determined to be effective in correcting or preventing hyperuricemia. Rasburicase could be administered every 12 hours during the first 48 hours of the study. Half of the patients included in the study had hyperuricemia (i.e., uric acid concentrations >6.5 mg/dL in patients younger than 13 or >7.5 mg/dL in patients older than 13). The average uric acid level of all patients at study entry was 5.7 mg/dL. After rasburicase administration, the average uric acid concentration remained at or near 0.5 mg/dL throughout treatment. Decreases in uric acid levels occurred within 4 hours after administration of rasburicase.
Even though rasburicase was found to be efficacious, rasburicase was not compared with allopurinol and urine alkalinization, and therefore no conclusions could be drawn about whether rasburicase is more clinically beneficial than standard treatment. The duration of rasburicase therapy was not clearly defined, nor was it stated how many patients required rasburicase every 12 hours within the first 48 hours of therapy. The authors did not discuss whether the duration of treatment or permitting 12-hour dosing during the initial treatment period affected clinical outcomes.
Study #2
Goldman et al (7) compared rasburicase with oral allopurinol in 52 pediatric patients (average age, 7 years) with lymphoma or leukemia at high risk of tumor lysis syndrome in this open-label, randomized, comparative, multicenter trial. Patients received either rasburicase at 0.2 mg/kg daily or oral allopurinol dosed at the investigators' discretion for 5 to 7 days. The median dose of allopurinol administered was 300 mg/day. Urine alkalinization was allowed at the investigators' discretion, and all patients received hydration. The average uric acid level of the rasburicase and allopurinol groups was 7.1 mg/dL and 7.8 mg/dL, respectively. Patients were considered hyperuricemic if their uric acid level was >8 mg/dL. The study found that the rasburicase group had a more rapid decline in uric acid levels 4 hours after drug administration than the allopurinol group and determined that the exposure to uric acid during the first 4 days of chemotherapy was 2.6-fold less than in the allopurinol group. In the hyperuricemic subgroup analysis, which included 10 patients in the rasburicase group and 9 in the allopurinol group, uric acid levels fell below 8 mg/dL in all patients within 4 hours after rasburicase administration vs 24 hours in the allopurinol group.
Because of the small number of patients, the study could not conclusively determine whether a more rapid decline in uric acid levels translated to a decrease in metabolic complications and morbidity associated with tumor lysis as well as a decrease in utilization of supportive care measures. Also, the investigators did not address whether allopurinol was as effective as rasburicase in the prophylactic setting. The duration of treatment and the number of patients who received urine alkalinization were not clearly stated; therefore, it is unknown whether treatment duration and urine alkalinization contributed to differences in outcomes.
Study #3
Bosly et al (8) performed an international compassionate-use trial evaluating the efficacy of rasburicase in 219 children and adults. Forty percent of study patients were adults. The median ages were 6 and 54 in the pediatric and adult populations, respectively. Any cancer patient at high risk of developing hyperuricemia was included in the study. The predominant disease states were leukemia and lymphoma. Rasburicase was administered at 0.2 mg/kg daily for 1 to 7 days; however, if necessary, rasburicase could be administered twice daily within the first 3 days of treatment. Allopurinol was not permitted. Hyperuricemia was defined as plasma uric acid >7.5 mg/dL except in children <13 years of age, where it was defined as a uric acid level >6.5 mg/dL. Fifty-nine patients were excluded from the analysis due to the unavailability of uric acid results. Of the remaining 160 patients, rasburicase decreased the plasma uric acid levels in all patients. In the hyperuricemic subgroup, the mean uric acid level prior to rasburicase administration was 13.1 mg/dL, which decreased to 0.3 mg/dL after drug administration. Average treatment duration was 5 days in the children and nonhyperuricemic adult groups versus 6 days in the hyperuricemic adult group.
Though the study showed that rasburicase was effective in the adult population, it did not provide definitive guidelines for selecting which patients would benefit most from rasburicase. In addition, no recommendations were provided on how long adults should be treated with rasburicase once they were no longer hyperuricemic.
Study #4
Pui et al (9) evaluated the effectiveness of rasburicase in treating and preventing hyperuricemia in 173 children and 72 adults in a compassionate-use trial performed in the USA. The median ages of the pediatric treatment and prophylaxis groups were 8 and 4 years, respectively. For the adults, the median ages were 52 and 54 years in the treatment and prophylactic groups, respectively. Most patients had leukemia or lymphoma. Rasburicase was administered at 0.2 mg/kg daily for 1 to 7 days; however, patients could be administered rasburicase twice daily in the first 3 days if they were receiving chemotherapy. Concomitant use of additional hypouricemic agents was not allowed. Uric acid levels greater than 6.4, 5.9, 6.4, 7.2, or 6.4 mg/dL in patients aged 0–2, 2–12, 12–14, >14 (males) or >14 (females), respectively, were considered to be hyperuricemic. All patients responded to rasburicase with the exception of two patients in the pediatric treatment group, who received inadequate therapy. In the hyperuricemic pediatric subgroup, the median uric acid level declined from 9.7 mg/dL before treatment to 0.6 mg/dL after treatment. The median uric acid level declined from 11.9 mg/dL to 0.7 mg/dL in the adult hyperuricemic subgroup. The median number of doses administered was three in the pediatric and adult groups.
Although the study determined that rasburicase was efficacious in adults, the investigators did not address whether rasburicase was warranted for prophylaxis considering its costliness. In addition, rasburicase was not compared with the current treatment strategies for hyperuricemia, so it is difficult to determine whether, especially in the prophylactic setting, the clinical outcomes would have been the same or different without rasburicase.
Study #5
Coiffier et al (10) evaluated the efficacy of rasburicase in patients ≥18 and <80 years of age who were diagnosed with NHL in this phase II, single-arm, open-label study performed in Europe. All of the patients were considered to be at risk of developing hyperuricemia, which was defined as uric acid levels >7.56 mg/dL. The dose of rasburicase was 0.2 mg/kg daily for 3 to 7 days. Patients were hydrated with dextrose 5% every 8 hours, and diuretics could be administered to maintain adequate urine outflow. Allopurinol was permitted only if hyperuricemic treatment was warranted beyond 7 days.
Ninety-five patients were evaluated for efficacy, and all the patients had a decrease in uric acid levels within 4 hours after rasburicase administration. Decreased uric acid levels were maintained throughout the treatment period. Eighty-one patients received rasburicase for 3 days, and the remaining 14 patients were treated for 4 to 6 days. No patients received allopurinol. Results of the study indicated that rasburicase is effective in reducing uric acid levels in adult patients at risk of tumor lysis syndrome who are receiving chemotherapy. The duration of therapy for rasburicase was not clearly defined; however, it could be inferred that 3 days of therapy is effective.
Differences in practice philosophies between Europe and the USA could be gathered from the study. An example is that the investigators did not design the study to compare rasburicase with allopurinol because they felt that urate oxidase was the standard of care for hyperuricemia, which is different from current practice standards for adults in the USA. Although the investigators considered rasburicase part of the standard of care for hyperuricemia, it could not be clearly determined from the study whether rasburicase should always be utilized for prevention and/or treatment of hyperuricemia because a direct comparison with allopurinol was not performed.
Of note, a prospective clinical trial, the BENEFIT trial, is being conducted in the USA comparing rasburicase with allopurinol to determine whether controlling uric acid levels confers renal protection in adults diagnosed with acute leukemia or NHL and at high risk of tumor lysis syndrome.
ADVERSE EFFECTS
Information regarding adverse effects for rasburicase was collected from various clinical trials, which were conducted under varying protocol designs. Therefore, the adverse reactions observed in rasburicase clinical trials may not reflect clinical practice. Table 1 compares adverse effects associated with rasburicase and intravenous allopurinol.
Table 1.
Comparison of adverse effects reported with rasburicase and intravenous allopurinol*
| Type of reaction | Incidence with rasburicase (n = 347) | Incidence with allopurinol (reported from clinical trials) |
| Vomiting | 50% | 1.2% |
| Fever | 46% | <1% |
| Nausea | 27% | 1.3% |
| Headache | 26% | <1% |
| Abdominal pain | 20% | None reported |
| Constipation | 20% | <1% |
| Diarrhea | 20% | <1% |
| Mucositis | 15% | None reported |
| Rash | 13% | 1.5% (most common) |
An additional 356 patients were assessed for adverse events with rasburicase. Of the total 703 patients (including the 347 patients represented in Table 1), the most serious adverse reactions attributed to rasburicase were anaphylaxis, rash, hemolysis, and methemoglobinemia, which all occurred in < 1 % of patients. Other serious adverse reactions were fever, neutropenia with fever, respiratory distress, sepsis, neutropenia, and mucositis. Antibody development can occur with rasburicase. Time to detection of antibodies can range from 1 to 6 weeks after administration (4).
CONTRAINDICATIONS
Rasburicase is contraindicated in patients who are glucose-6-phosphate dehydrogenase deficient because these patients cannot break down hydrogen peroxide, a byproduct of rasburicase, which can lead to hemolysis (12). Screening for glucose-6-phosphate dehydrogenase deficiency is recommended in patient populations who are at greater risk for this deficiency (e.g., those of African or Mediterranean descent).
Additional contraindications are a history of anaphylactic or hypersensitivity reactions, methemoglobinemia due to rasburicase, or previous hemolytic reactions. The risk of developing methemoglobinemia or hemolytic anemia in patients deficient in cytochrome-b5 reductase or enzymes with antioxidant activity is unknown (4).
DOSING AND ADMINISTRATION
The FDA-recommended dosing guidelines for pediatric patients are 0.15 mg/kg or 0.2 mg/kg administered once daily for a maximum of 5 days. Treatment beyond 5 days or for more than one course of therapy is not recommended. The first dose of rasburicase should be administered 4 to 24 hours before starting chemotherapy. Rasburicase is given as an intravenous infusion over 30 minutes and should not be given as a bolus infusion (4). Currently, rasburicase does not have FDA approval for use in the adult population; however, the dose often utilized in practice is 0.2 mg/kg.
LABORATORY TESTING
Rasburicase can degrade uric acid in blood samples at room temperature, leading to lower uric acid results than are actually present, so special handling of samples is necessary. Blood needs to be collected in prechilled tubes containing heparin, either in the heparin sodium or heparin lithium forms. After collection, the sample must immediately be placed in an ice water bath and tested within 4 hours of collection (4). These laboratory testing procedures for uric acid must be followed for 4 days after the last dose of rasburicase is administered (personal communication, medical information department at Sanofi-Synthelabo, October 6, 2004).
PREGNANCY CATEGORY
Rasburicase has not been evaluated in animal reproduction studies, and therefore it is not known whether it can cause fetal harm. In addition, it is unknown whether rasburicase can affect reproduction abilities. Rasburicase is considered to be pregnancy category C (4).
DRUG INTERACTIONS
No drug interaction studies have been conducted in humans; however, rasburicase has been shown to not have a metabolic-based interaction with allopurinol, cytarabine, methylprednisolone, methotrexate, 6-mercaptopurine, thioguanine, etoposide, daunorubicin, cyclophosphamide, and vincristine in vitro. Rasburicase has not been shown to inhibit or induce CYP1A, CYP2A, CYP2B, CYP2C, CYP2E, and CYP3A isoenzymes in preclinical in vivo studies (4).
DOSAGE FORM
Rasburicase is supplied as three 1.5-mg single-use vials per box, with 3 ampules of 1 -mL diluents included (4).
ECONOMIC ISSUES
Rasburicase is a costly medication compared with current standard therapies for the prevention of tumor lysis syndrome (Table 2). There is a significant difference in the 5-day cost of oral allopurinol/ sodium bicarbonate and rasburicase.
Table 2.
Comparison of cost for rasburicase and allopurinol/sodium bicarbonate
| Drug | Average dose per day | Maximum duration | Cost per day* | Cost of 5 days of therapy |
| Allopurinol sodium (intravenous) | 300 mg | NA | $473.89 | $2369.45 |
| Oral allopurinol | 600 mg | NA | $0.28 | $1.40 |
| Sodium bicarbonate intravenous (90 mEq)‡ | 4000 ml (rate, 150–200 mL/h) | NA | $0.74 | $29.60 |
| Rasburicase | 13.5 mg‡ (based on 0.2 mg/kg for 70-kg patient) | 5 days | $2724.84 | $13, 624.20 |
NA indicates not applicable.
*Cost is based on Baylor University Medical Center's pharmacy acquisition cost.
†Mixed with 1000-mL dextrose 5% in water (D5W). The cost figures given exclude the D5W bag.
‡Dose is rounded to the nearest vial size.
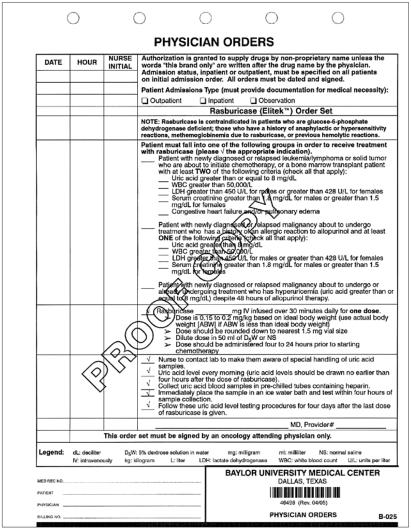
Because of the expense, judicious use of the medication is necessary to prevent unwarranted utilization and unnecessary cost to the patient and institution. Although rasburicase has been shown to be effective for prophylaxis in patients who are not hyperuricemic, it should not be utilized in this population at Baylor University Medical Center (BUMC) since current strategies with hydration, urine alkalinization, and allopurinol are effective. Also, clinical studies have not clearly addressed whether rasburicase used prophylactically is more beneficial than other prophylactic modalities. However, for patients who are at a high risk of developing tumor lysis syndrome, rasburicase is an option. BUMC's policy is to restrict the medication to the oncology and bone marrow transplant service lines. Orders must be written by an attending physician from one of these service lines before rasburicase can be dispensed from the pharmacy (Figure).
Figure.
Draft physician orders for rasburicase.
SUMMARY
The overall incidence of tumor lysis syndrome in adults is not clearly defined, and its occurrence can be unpredictable. Complications from tumor lysis syndrome are serious (e.g., acute renal failure) and therefore preventing tumor lysis syndrome is necessary, especially in patients who have high tumor burdens and highly proliferative malignancies (10). Clinical trials have demonstrated that rasburicase is effective in lowering plasma uric acid levels in pediatric and adult patients in both prophylactic and treatment settings (6–10). Of note, rasburicase is not FDA approved for use in the adult and geriatric populations (4). Rasburicase affects already developed uric acid by converting uric acid to allantoin, whereas allopurinol prevents the formation of new uric acid from purine catabolism (3). Because rasburicase has a different mechanism than other available treatment strategies for hyperuricemia, it provides oncologists with another pharmacologic option in preventing and treating tumor lysis syndrome.
Acknowledgment
Special thanks to Valerie Sheehan, PharmD, for her contributions to this article.
References
- 1.Warrell RP. Metabolic emergencies. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2633–2644. [Google Scholar]
- 2.Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, Goldman SC, Jeha SC, Shanholtz CB, Leonard JP, McCubrey JA. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a meeting report, Dallas, Texas, January 2002. Leukemia. 2003;17:499–514. doi: 10.1038/sj.leu.2402847. [DOI] [PubMed] [Google Scholar]
- 3.Cairo MS. Prevention and treatment of hyperuricemia in hematological malignancies. Clin Lymphoma. 2002;3(Suppl 1):26–31. doi: 10.3816/clm.2002.s.012. [DOI] [PubMed] [Google Scholar]
- 4.Sanofi-Synthelabo . Elitek(™) (Rasburicase) Prescribing Information [package insert] New York: Sanofi-Synthelabo; 2002. Available at http://www.sanofi-synthelabous.com/products/pi_elitek/pi_elitek.html; accessed April 12, 2005. [Google Scholar]
- 5.Ribeiro RC, Pui CH. Recombinant urate oxidase for prevention of hyperuricemia and tumor lysis syndrome in lymphoid malignancies. Clin Lymphoma. 2003;3:225–232. doi: 10.3816/clm.2003.n.003. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, Hastings C, Blaney SM, Relling MV, Reaman GH. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma. J Clin Oncol. 2001;19:697–704. doi: 10.1200/JCO.2001.19.3.697. [DOI] [PubMed] [Google Scholar]
- 7.Goldman SC, Holcenberg JS, Finkleslein JZ, Hutchinson R, Kreissman S, Johnson FL, Tou C, Harvey E, Morris E, Cairo MS. A randomized comparison between rasburicase and allopurinol in children wilh lymphoma or leukemia al high risk for lumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]
- 8.Bosly A, Sonet A, Pinkerton CR, McCowage G, Bron D, Sanz MA, Van den Berg H. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients wilh cancer: report of an international compassionate use study. Cancer. 2003;98:1048–1054. doi: 10.1002/cncr.11612. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Jeha S, Irwin D, Camitta B. Recombinant urate oxidase (rasburicase) in the prevention and treatment of malignancy-associated hyperuricemia in pediatric and adult patients: results of a compassionate-use trial. Leukemia. 2001;15:1505–1509. doi: 10.1038/sj.leu.2402235. [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B, Mounier N, Bologna S, Ferme C, Tilly H, Sonet A, Christian B, Casasnovas O, Jourdan E, Belhadj K, Herbrecht R, Groupe d'Etude des Lymphomes de l'Adulte Trial on Rasburicase Activity in Adult Lymphoma Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin's lymphoma: results of the GRAAL1 (Groupe d'Etude des Lymphomes de l'Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol. 2003;21:4402–4406. doi: 10.1200/JCO.2003.04.115. [DOI] [PubMed] [Google Scholar]
- 11.Bedford Laboratories . Allopurinol Sodium for Injection [package insert] Bedford, OH: Bedford Laboratories; June 2004. Available at http://www.bedfordlabs.com/pdf/allopurinol_pi.pdf; accessed June 1, 2005. [Google Scholar]
- 12.Yim BT, Sims-McCallum RP, Chong PH. Rasburicase for ihe treatment and prevention of hyperuricemia. Ann Pharmacother. 2003;37:1047–1054. doi: 10.1345/aph.1C336. [DOI] [PubMed] [Google Scholar]