Abstract
Purpose of Review
Induced pluripotent stem cells (iPSCs) have become widely adopted tools in cardiovascular biology due to their ability to differentiate into patient-specific cell types. Here, we describe the current protocols, important discoveries, and experimental limitations from the iPSC-derived cell types of the human heart: cardiomyocytes, cardiac fibroblasts, vascular smooth muscle cells, endothelial cells, and pericytes. In addition, we also examine the progress of 3D-based cell culture systems.
Recent Findings
There has been rapid advancement in methods to generate cardiac iPSC-derived cell types. These advancements have led to improved cardiovascular disease modeling, elucidation of interactions among different cell types, and the creation of 3D-based cell culture systems able to provide more physiologically relevant insights into cardiovascular diseases.
Summary
iPSCs have become an instrumental model system in the toolbox of cardiovascular biologists. Ongoing research continues to advance the use of iPSCs in (1) disease modeling, (2) drug screening, and (3) clinical trials in a dish.
Keywords: Induced pluripotent stem cells, Cardiovascular disease modeling, Precision medicine, Cardiac regeneration, 3D cell culture systems
Introduction
Since the first human pluripotent stem cells (hPSCs) were derived from human embryos [1], these cells were expected to have broad clinical and translational applications. Over the past several years, it has become clear that the time has arrived. Prior to the derivation of hPSCs and hPSC-derived cell types, it was difficult to obtain certain human primary cells, such as cardiomyocytes. Even when acquired, many primary cell types show changes in cellular morphology and cell loss during extended cell culture. In contrast, hPSCs, by definition, can propagate indefinitely and can differentiate into all cell types from tissues in the body. By leveraging these fundamental biological features, hPSCs represent a paradigmatic advancement in our ability to interrogate cell-specific molecular mechanisms of the human heart. In recent years, they have also provided unparalleled opportunities to evaluate patient-specific disease phenotypes “in a dish” for precision medicine.
Human embryonic stem cells (hESCs), the first hPSCs, had limitations that prevented full usage for their original hypothesized applications in disease modeling and precision medicine. For example, the creation of a single hESC line requires the destruction of the embryonic blastocyst, which in part led to ethical, scientific, and legal reconsiderations and the adoption of laws and policies to restrict hESC use and research funding, particularly in the USA [2]. Moreover, the disease phenotype is often undeterminable, because most donors of embryonic blastocysts or gametes are from fertility clinics and healthy donors without a predilection for particular diseases. For these reasons, the use of hESCs became largely impracticable for investigating the genetic and molecular mechanisms of human diseases.
After the development of hESCs, two other methods were established to derive hPSCs: [1] reprogramming somatic cells into hESCs by somatic cell nuclear transfer (SCNT) and [2] the derivation of induced pluripotent stem cells (iPSCs). SCNT involves the transplantation of patient-specific somatic cell nuclei into mature, metaphase II-arrested oocytes [3]. Despite presciently theorized [3] and demonstrated [4] uses in disease modeling and personalized therapies, there were continued ethical and policy dilemmas regarding the use of SCNT. In addition, technical challenges such as low efficiencies have prevented the widespread adoption of SCNT as a technique to tackle challenging questions in human diseases.
The iPSC is the most widely used hPSC, particularly in cardiovascular biology. The seminal derivation of iPSCs was first demonstrated in 2006 by Nobel Laureate Dr. Shinya Yamanaka and colleagues, who reprogrammed somatic cells into pluripotent stem cells in mice [5] and in human cells [6] by the introduction of four factors, OCT3/4, SOX2, c-MYC, and KLF4. At first, there were questions as to whether the reprogrammed iPSCs retained epigenetic signatures of their original somatic cell types and whether iPSCs were truly similar to hESCs [7]. Nonetheless, these initial concerns were addressed by subsequent studies indicating that iPSCs and hESCs are molecularly and functionally equivalent, and are genetically, epigenetically, or transcriptionally indistinguishable [8]. It is now possible to take easily accessible patient-specific somatic cell types and dedifferentiate them into iPSCs (i.e., turn back the clock). Thus, iPSCs have become widely adopted in medical research and have made significant contributions to understanding the pathological mechanisms underlying genetic and acquired diseases, including heart diseases [9].
Heart muscle cells (cardiomyocytes) account for roughly 70% of the heart by mass and 25–35% by the total number of cells. The remainder is comprised of other cell types, including fibroblasts, vascular smooth muscle cells, endothelial cells, and pericytes [10••]. Consequently, in this review, we begin with a brief introduction to iPSCs. We then summarize current protocols, uses, important discoveries, and limitations of the cardiac iPSC-derived cell types: cardiomyocytes, cardiac fibroblasts, coronary vascular smooth muscle cells, coronary endothelial cells, and cardiac pericytes (Fig. 1). We conclude by describing current methods and new advances in 3D-based cell culture systems.
Fig. 1.
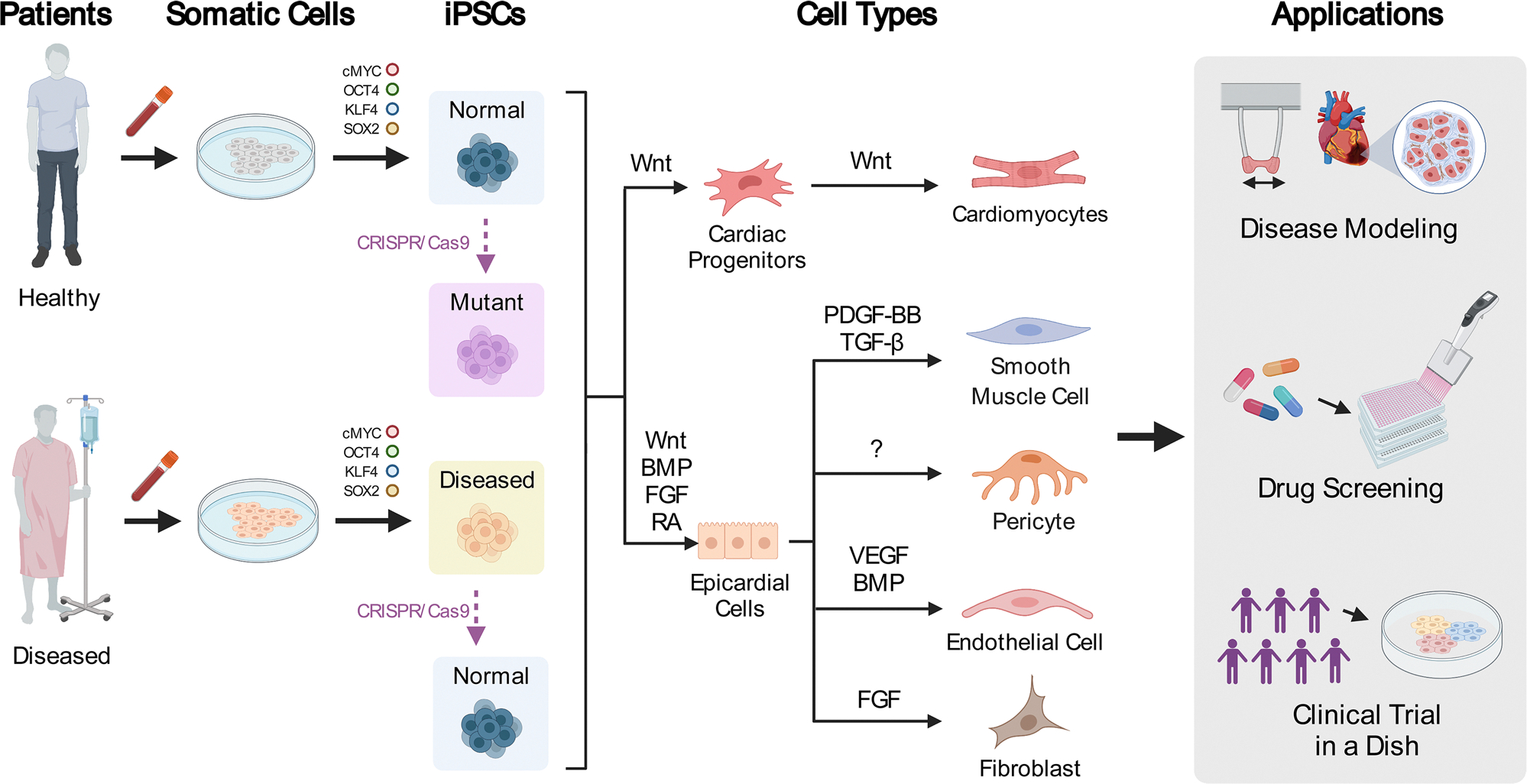
Schematic diagram summarizing the general process of induced pluripotent stem cell (iPSC) derivation, differentiation into cardiac cell types, and their applications. Initially, patient-derived somatic cells (often T cells from routine blood draws, as indicated in the figure) may be obtained from either healthy or diseased patients. These cells can then be reprogrammed to iPSCs by introducing the Yamanaka factors (c-MYC, OCT4, KLF4, and SOX2) with the use of the Sendai virus, episomal vectors, or synthetically modified messenger RNA. Often, genetic editing techniques such as CRISPR/Cas9 are used to generate isogenic controls for experimentation. These iPSCs can be differentiated into the various cell types of the heart through the modulation of several pathways. For example, iPSC-derived cardiomyocytes can be generated by modulation of WNT/β-catenin signaling. iPSC-derived cardiac fibroblasts, coronary endothelial cells, coronary vascular smooth muscle cells, and cardiac pericytes can be generated by first specifying iPSCs to an epicardial lineage by modulating some combination of WNT/β-catenin, bone morphogenic peptide (BMP), fibroblast growth factor (FGF), and retinoic acid (RA) signaling pathways. From epicardial cells, iPSC-derived coronary vascular smooth muscle cells can be generated by treatment of epicardial cells with platelet-derived growth factor (PDGF-BB) and transforming growth factor (TGF-β). iPSC-derived coronary endothelial cells can be generated by treatment with vascular endothelial growth factor (VEGF) in a cocktail of other factors that increase the yield of mesodermal cells (e.g., BMP4). iPSC-derived cardiac fibroblasts can be generated by treatment with basic fibroblast growth factor (FGF2). There are currently no published protocols for the generation of iPSC-derived cardiac pericytes. These iPSC-derived cell types can be used for disease modeling (e.g., modeling the disease phenotype with engineered heart tissues), drug screening (e.g., with high-throughput screening with reporter gene assays), and clinical trials in a dish (e.g., using patient-derived iPSCs to evaluate the efficacy and safety of drugs)
iPSCs
There are two main decisions in the reprogramming of patient-derived somatic cells into iPSCs [9]. The first decision is the choice of somatic cell type for pluripotent reprogramming. Typically, choices include using dermal fibroblasts from skin punch biopsies [11], T cells from routine blood draw [12], renal tubular cells from urine [13], and keratinocytes from harvested hair follicles [14]. Skin, blood, urine, and hair are all easily accessible patient biomaterials. The second decision is choosing the molecular delivery vehicle to introduce pluripotency factors (i.e., OCT3/4, SOX2, c-MYC, and KLF4) into the chosen somatic cell type. Common vehicles include integrating viruses (e.g., lentiviruses or retroviruses) to transfect the genes, but choosing integrating viruses requires subsequent excision of highly undesirable integrated transgenes [15]. Non-integrating methods include the Sendai virus [16], episomal vectors [17], minicircle DNA vectors [18], and synthetically modified messenger RNA [19]. While there are differences in the reprogramming efficiencies, success rates, and workloads of the three major non-integrating reprogramming methods, namely the Sendai virus, episomal vectors, and synthetically modified messenger RNA, they also have distinct advantages and disadvantages [20]. The choice of which reprogramming method to use depends on the particular requirements and capabilities of each laboratory. Subsequent to reprogramming, each iPSC line should be evaluated for its capacity to form teratomas, genomic integrity, clearance of reprogramming vectors, and ability to yield the desired differentiated cell type [9].
iPSC-Derived Cardiomyocytes
The fundamental contractile unit of the heart is the cardiomyocyte. In the past decade, several methods to direct the differentiation of iPSCs to cardiomyocytes (iPSC-CMs) have emerged. Current protocols generally rely on the modulation of WNT/β-catenin-signaling to achieve cardiogenesis [21]. First, iPSCs are induced to a mesendoderm lineage by activation of the WNT/β-catenin-signaling pathway by small molecule inhibitors of GSK3β (a negative regulator of canonical WNT signaling) such as CHIR99021. A few days after mesendoderm induction occurs, cells are differentiated to cardiac progenitors through inhibition of WNT/β-catenin by small molecule inhibitors of WNT such as IWR compounds. An important characteristic of iPSC-CMs is their ability to survive in glucose-free media, thereby allowing the generation of highly purified iPSC-CM cultures through glucose starvation in the weeks after IWR administration [22]. Details on widely used protocols for a step-by-step process for iPSC-CM differentiation and purification can be found elsewhere [22, 23•].
Disease modeling of inherited cardiovascular diseases, such as channelopathies, cardiomyopathies, and cardiometabolic diseases, through iPSC-CMs, constitutes the largest body of cardiovascular research from iPSCs to date. These disease models have allowed investigators to study the genetic contributions of each disease and glean granular molecular insights into their disease mechanisms. For instance, one of the first channelopathies studied by using iPSC-CMs was long QT syndrome type 1 in patients with an R190Q missense mutation in the KCNQ1 gene. Studies indicated that there was a negative trafficking defect leading to altered channel activation and deactivation properties. These abnormalities could be attenuated by beta-blockers [24]. Several other mutations in long QT syndrome, including mutations in KCNH2 [25•] and SCNA5 [26, 27], have also been probed with iPSC-CMs. These studies have delineated arrhythmogenic characteristics, channel properties, and therapeutic strategies to mitigate the effects of gene mutations, showing proof-of-concept of the capacity of iPSC-CMs for patient-specific disease modeling. Other cardiac rhythm disorders that have been investigated with iPSC-CMs include Brugada syndrome [28–30], a disease characterized by precordial ST-segment elevation, and catecholaminergic polymorphic ventricular tachycardia [31], a disorder characterized by ventricular arrhythmias during exercise or strong emotion. Recently, familial forms of atrial fibrillation [32], including familial atrial fibrillation with multiple mutated genes [33•], have also been modeled with iPSC-CMs.
Cardiomyopathies have also been extensively characterized by iPSC-CM studies. Cardiomyopathies are organized into five major types: (1) dilated cardiomyopathy (DCM), (2) hypertrophic cardiomyopathy (HCM), (3) restrictive cardiomyopathy (RCM), (4) non-compaction cardiomyopathy (NCCM), and (5) arrhythmogenic cardiomyopathy (ACM). As these cardiomyopathies are among the most well-characterized of inherited cardiovascular disorders, there has been significant interest in understanding how the spectrum of affected mutations and genes lead to their respective disease phenotypes. An important example is the sarcomeric protein titin, the most common genetic cause of dilated cardiomyopathy (DCM). Using iPSC-CMs derived from DCM patients, investigators found exactly how titin mutations lead to deficiencies in functional sarcomeres, particularly in response to mechanical and adrenergic stressors [34]. Other important genes in DCM that have been evaluated with iPSC-CMs include cardiac troponin T (TNNT2) [35, 36], lamin A/C (LMNA) [37], phospholamban (PLN) [38], and desmin (DES) [39]. These evaluations show that iPSC-CMs not only recapitulate the disease phenotype of DCM, but they also advance our understanding of disease mechanisms. Hypertrophic cardiomyopathy (HCM) is another example. In HCM, the patient’s left ventricular heart wall thickens. Using iPSCs derived from patients with mutations in myosin heavy chain beta (MYH7), research showed activation of HCM-associated genes, arrhythmia, and cellular hypertrophy [40]. In addition, iPSC-CMs derived from HCM patients were more susceptible to endothelin-1-mediated hypertrophy [41]. A long-term benefit of these studies is that once these diseases can be modeled, it may then be possible to determine potential drug effects for disease management.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a disease where myocardial tissue is replaced by fibrofatty tissue. The most common mutation in patients with ARVC is in plakophilin-2 (PKP2). ARVC patients’ derived iPSC-CMs are characterized by increased lipid accumulation, apoptosis, and abnormal calcium handling after treatment with adipogenic factors [42, 43]. These lipid-laden cardiovascular phenotypes could be reversed with compounds discovered in a high-throughput screen, including cell-permeable inhibitors of glycogen synthase kinase 3 [44]. These studies in HCM and ARVC indicate that to recapitulate disease phenotypes in iPSC-CMs, there may be a need for stimulatory cofactors. However, these additional stimuli may not be physiologically relevant, and the iPSC-CM phenotype provoked by these stimuli needs to be compared with cardiomyocytes in patient heart tissues to ensure physiological significance. In contrast with DCM, HCM, and ARVC, the molecular mechanisms underlying NCCM and RCM are not as well characterized. Kodo et al. used NCCM patient-specific iPSCs to show that mutations in TBX20, a gene important in congenital heart development, is linked with transforming growth factor (TGF-β) signaling activation, leading to reduced cardiomyocyte proliferation [45]. Moreover, inhibition of TGF-β could reverse the NCCM disease phenotype. Regarding RCM, to the best of our knowledge, there have been no published papers on patient-specific iPSC disease modeling of RCM, although a case report exists that introduced a likely pathogenic RCM mutation into iPSCs [46].
Diseases in metabolism have also been investigated using iPSC-CMs. These have primarily been inborn errors of metabolism such as glycogen storage disorders (e.g., Pompe disease [47] and Danon disease [48]), mitochondrial diseases (e.g., Barth syndrome [49]), and lysosomal storage disorders (e.g., Fabry disease [50]). The cardinal feature of each disease could be modeled using iPSC-CMs [51]. The pathogenic mechanisms of each disease, once elucidated, may lead to novel therapies (e.g., L-carnitine for dysfunctional iPSC-CMs in Pompe disease) [47]. A few reports have gone beyond inherited errors in metabolic disease, including studies that modeled diabetes-induced cardiomyopathy. In diabetes-induced cardiomyopathy, there are initially increased levels of circulating fatty acids, leading to increased fatty acid uptake and oxidation in cardiomyocytes, increased lipid accumulation, and cardiac dysfunction. One report observed that a diabetogenic extracellular environment leads to cardiomyocyte hypertrophy and lipid accumulation [52]. Furthermore, a high-throughput screen identified small molecules that can prevent the in vitro development of diabetes-induced cardiomyopathy [52].
Despite advances in our understanding of cardiovascular disease through iPSC-CMs, there are several limitations of this technology. First, iPSC-CMs may represent an immature analog of adult cardiomyocytes. For example, iPSC-CMs appear rounder and shorter than adult cardiomyocytes [53]. Moreover, they lack characteristic sarcolemma invaginations (and thus, depend more upon L-type calcium channels for contraction and less on calcium-induced calcium release), have fewer mitochondria [54], have less negative resting membrane potentials, are mononucleated, and have gene expression profiles more like fetal cardiomyocytes [9]. The heterogeneity of differentiated cardiomyocytes is another important limitation, as some differentiated cells may resemble atrial, ventricular, or even nodal cells [55, 56]. Cell sorting is often necessary to isolate specific cardiomyocyte subtypes. These attributes may limit iPSC-CMs from precisely modeling some human cardiovascular diseases, such as atrial fibrillation. Consequently, strategies to minimize these limitations are ongoing and active areas of research. For instance, prolonged culture time [57], changes in energy substrate [58], changes in the extracellular matrix [59], and physical stimulation (e.g., electrical stimulation or mechanical stress) [60] have yielded more mature iPSC-CM phenotypes in morphology, structure, and physiology. There has also been recent progress toward differentiating specific cardiomyocyte subtypes, including atrial [56, 61•] and nodal cells [62]. Much more research, however, is needed to refine iPSC-CM differentiation protocols to increase the efficiency and efficacy of modeling cardiovascular disease.
iPSC-Derived Cardiac Fibroblasts
Cardiac fibroblasts play important roles in cardiac development and physiological and pathological remodeling through the production of extracellular matrix, cytokines, and growth factors. Several protocols have emerged in the last 2 years to differentiate iPSCs to cardiac fibroblasts (iPSC-CFs). One method, in a similar first step as iPSC-CM differentiation, inhibits GSK3β with CHIR99021 to stimulate the formation of mesoderm and cardiac progenitors [63••]. After 2 days, these cardiac mesodermal progenitors were continuously treated with basic fibroblast growth factor (FGF2), generating iPSC-CFs similar to primary cardiac fibroblasts. Primary cardiac fibroblasts can be ascertained by the expression of cardiac-specific markers such as GATA4. Giacomelli et al. and Zhang et al. use another protocol to generate iPSC-CFs [64, 65]. They first differentiated iPSCs to cardiac mesoderm progenitors with CHIR99021, followed by epicardial progenitor differentiation with retinoic acid. They then made use of TGF-β inhibition with SB431542 to expand the epicardial cells. Finally, epicardial cells were treated with FGF2 to generate cardiac fibroblasts.
Although the differentiation and characterization of iPSC-CFs is a recent development, iPSC-CFs are likely to play important roles not only in disease models but also in exploring putative interactions among the various heart cell types. This is because of the importance of cardiac fibroblasts in heart failure and arrhythmias through chronic beta-adrenergic stimulation, pathological fibrosis, and secretion and degradation of the extracellular matrix. For example, 3D microtissues of iPSC-CMs have been grown together with iPSC-CFs to allow researchers to refine the effects of iPSC-CFs on the electrophysiology of iPSC-CMs [64] and uncover the pathogenic contributions of cardiac fibroblasts in cardiovascular disease [63••]. Ultimately, these iPSC-CF protocols may serve as a cellular source for drug discovery and therapy.
iPSC-Derived Vascular Smooth Muscle Cells
Vascular smooth muscle cells contract and relax in the coronary vasculature to control cardiac blood flow [10••]. For example, coronary smooth muscle cells, in response to metabolic stimuli, are coupled with myocardial oxygen demand to regulate blood flow distribution to the coronary arteries. Recently, there has been the development of lineage-specific iPSC-derived vascular smooth muscle cell (iPSC-VSMC) differentiation protocols [66] that can generate coronary vascular smooth muscle cells and aortic vascular smooth muscle cells. Coronary vascular smooth muscle cells normally develop from lateral plate mesoderm to become proepicardial cells and move inward during embryonic development [67]. Consequently, iPSC-VSMC protocols mimic this pattern. First, we see the derivation of early mesoderm cells from iPSCs treated with bone morphogenic peptide (BMP4), FGF2, and a phosphoinositide 3-kinase inhibitor (LY294002), followed by differentiation into lateral plate mesoderm with continued treatment with BMP4 and FGF2 [66]. Activation of BMP, WNT (through WNT3A), and retinoic acid signaling then generate epicardial cells [68]. Other more refined protocols show it is possible to directly generate epicardial cells from iPSCs by modulation of WNT signaling with the 8-day treatment of CHIR99021 alone [69]. Regardless of the method to generate epicardial cells, these early mesoderm/epicardial cells can then be specified to lateral plate mesoderm-derived iPSC-VSMCs by treatment with platelet-derived growth factor (PDGF-BB) and TGF-β [66, 70]. In contrast, vascular smooth muscle cells from the ascending aorta are differentiated from the neuroectoderm. Therefore, the differentiation process differs: the first differentiation step treats iPSCs with FGF2 and SB431542, an inhibitor of the TGF-β/activin/nodal pathway, to generate an intermediate neuroectodermal population [66]. This is followed by PDGF-BB and TGF-β treatment to differentiate this neuroectodermal population into neuroectoderm-derived iPSC-VSMCs. For an in-depth review on the in vitro differentiation of iPSC-VSMCs from distinct progenitors, we refer to this article by Shen et al. [71].
For modeling vascular diseases, one study showed that iPSC-VSMCs from patients with hypertension contracted more in response to endothelin-1 and were more responsive to inflammatory signals compared to control cells [72]. Other studies with iPSC-VSMCs have elucidated disease mechanisms from congenital aortic defects, including supravalvular aortic stenosis [73] and bicuspid aortic valves [74], as well as systemic diseases such as Marfan syndrome [75] and Hutchinson–Gilford Progeria syndrome [76]. Finally, iPSC-VSMCs have also been used for tissue-engineered vascular grafts [77].
iPSC-Derived Endothelial Cells
Coronary endothelial cells make up the inner wall of coronary vessels to regulate vascular tone, coagulation, inflammation, and passage of proteins from blood into the tissue. Coronary endothelial cells, as with cardiac fibroblasts and coronary vascular smooth muscle cells, are derived from the epicardium. Several investigators have used CHIR99021 to commit iPSCs to a cardiac progenitor and epicardial lineage [78], similar to the generation of iPSC-CFs and iPSC-VSMCs. Once generated, they then be differentiated to iPSC-ECs by adding vascular endothelial growth factor (VEGF) [79] in a cocktail of several other factors that increase the yield of mesodermal cells (e.g., BMP4) [70]. These cells are then purified with fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS) to obtain iPSC-ECs. Research is ongoing to improve the yield and homogeneity of iPSC-ECs through alterations in cell culture conditions.
Patient-specific iPSC-ECs have been used as cardiovascular disease models and for drug screening. One study used iPSC-ECs to show how mutations that cause aortic valve calcification may regulate the epigenetic landscape [80]. In addition, several studies used iPSC-ECs to model familial forms of pulmonary arterial hypertension [81]. Another study used iPSC-ECs in coculture with iPSC-CMs from patients with LMNA-related DCM to show how crosstalk between ECs and CMs can contribute to the pathogenesis of DCM [82•]. However, given that many diseases in vascular research are multifactorial with flow-induced shear stress as a disease modifier (e.g., atherosclerosis), it is likely that methodological advances and increased use of 3D-based cell culture systems (see section “From 2D to 3D Cell-based Culture Systems”) will be needed to model the full spectrum of vascular diseases.
iPSC-Derived Pericytes
Cardiac pericytes are found along the walls surrounding endothelial cells in the cardiac microvasculature where they regulate various functions in vessel development and vascular homeostasis. Moreover, cardiac pericytes may play a part in diverse beneficial and pathological mechanisms in the heart, including angiogenesis after myocardial infarction, cardiac fibrosis, and coronary microvascular stability. The developmental origins of cardiac pericytes are thought to stem from the epicardium [83]. Therefore, although there are currently no protocols to derive cardiac pericytes from iPSCs, one may reasonably infer from the derivation of brain pericytes [84, 85•] that differentiating iPSCs to epicardial cells by modulating WNT/β-catenin signaling followed by treatment with important pericyte factors, such as PDGF-BB with TGF-β inhibition to prevent mural cell commitment toward a smooth muscle cell lineage, should generate iPSC-derived cardiac pericytes (iPSC-CPs). Given the relatively unknown contribution of cardiac pericytes to heart function [83], future studies on iPSC-CPs may be able to provide both protocols to generate cardiac-specific pericyte populations and functional studies needed to elucidate the role of cardiac pericytes in heart disease.
From 2D to 3D Cell-based Culture Systems
The above sections have reviewed primarily 2D cell-based culture systems for iPSC-derived cell types. We next discuss 3D cell-based culture systems.
Human diseases exist in the context of multicellular systems. Recent research has shifted focus to the engineering of iPSCs within 3D cell-based systems. These systems include the use of novel biomaterials (e.g., scaffolds that can mimic natural extracellular matrices) to form 3D-engineered heart tissues [86], microfluidic organ-on-a-chip that simulate physiological functions of the heart or vasculature in a controlled manner [87], and cardiac organoids composed of multicellular aggregates that are able to differentiate and self-organize to recapitulate basic heart architecture and function (often also within a Matrigel scaffold) [88•].
Each of these platforms has yielded important discoveries to answer many different physiological and biological questions. For example, 3D-engineered heart tissues have recently been used to show that iPSC-CMs can form remarkably organized ultrastructures, which possess increased maturity if cultured into fibrin hydrogen stretched between two flexible pillars [89••]. These findings indicate that the use of 3D-engineered heart tissues may provide a more physiologically relevant reference for disease modeling than 2D cell-based culture systems [90].
Microfluidics is a recent technology that involves processing minute volumes of fluids in etched or molded microchannels tens of micrometers (~ 10 μm) across to form a so-called “organ-on-a-chip.” By altering the architecture, flow conditions, and even surface properties, microfluidics in combination with the embedding of various cell types can mimic more complex interactions between organs and organ systems [91]. Indeed, by controlling the chip microenvironment to mimic disease conditions, microfluidic technology is rapidly becoming a useful tool in the expanding toolbox of cardiovascular researchers. Such a tool may be particularly relevant for many vascular diseases (e.g., evaluating thrombosis pathogenesis or atherosclerosis).
Finally, cardiac organoids by virtue of the self-assembling nature of hPSCs to form heart-like structures [92•] are suitable to probe cardiac development [88•] and the organotypic features of certain diseases, such as heart remodeling after myocardial infarction [93•]. Despite the potential of these 3D cell-based culture systems, they have limitations such as increased cost, complexity, and time.
Conclusions
There has been rapid advancement in iPSC technologies, leading to significant discoveries in the derivation of iPSC-CMs, iPSC-CFs, iPSC-VSMCs, iPSC-ECs, and iPSC-CPs. New 2D and 3D cell-based culture systems and protocols have provided researchers better models for understanding the pathophysiology of both genetic and acquired cardiovascular diseases. These research developments continue to advance the use of iPSCs as a platform for patient- and disease-specific clinical trials in a dish for precision medicine.
Acknowledgements
This work was supported by a Stanford Medical Scholars Research Fellowship (BY) and by a National Institutes of Health (NIH) grant R01 HL123968, R01 HL141851, R01 HL145676, and R01 HL150693 (JCW).
Footnotes
Compliance with Ethical Standards
Competing Interests JCW is a cofounder of Greenstone Biosciences but has no competing interests. The other authors declare that they have no known competing financial interests or personal relationships that may have influenced this paper.
Human and Animal Rights This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145. 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Io Medicine, Council NR. Guidelines for human embryonic stem cell research. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 3.Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182(4627):64–5. 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- 4.Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510(7506):533–6. 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–90. 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J, Lee S, Mallard W, Clement K, Tagliazucchi GM, Lim H, et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat Biotechnol. 2015;33(11):1173–81. 10.1038/nbt.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musunuru K, Sheikh F, Gupta RM, Houser SR, Maher KO, Milan DJ, et al. Induced pluripotent stem cells for cardiovascular disease modeling and precision medicine: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018;11(1):e000043. 10.1161/HCG.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. Cells of the adult human heart. Nature. 2020;588(7838):466–72. 10.1038/s41586-020-2797-4. •• Important paper using single-cell and single-nucleus RNA sequencing to assess spatial and temporal heterogeneity of the complete human heart. This has helped characterized the different cell types that constitute the adult human heart.
- 11.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–9. 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7(1):15–9. 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou T, Benda C, Duzinger S, Huang Y, Li X, Li Y, et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22(7):1221–8. 10.1681/asn.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2011;108(21):8797. 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommer CA, Sommer AG, Longmire TA, Christodoulou C, Thomas DD, Gostissa M, et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells. 2010;28(1):64–74. 10.1002/stem.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62. 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, et al. A non-viral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–9. 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–30. 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33(1):58–63. 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109(27):E1848–57. 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Li G, Rajarajan K, Hamaguchi R, Burridge PW, Wu SM. Derivation of highly purified cardiomyocytes from human induced pluripotent stem cells using small molecule-modulated differentiation and subsequent glucose starvation. J Vis Exp. 2015;(97). 10.3791/52628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma A, McKeithan WL, Serrano R, Kitani T, Burridge PW, Del Álamo JC, et al. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc. 2018;13(12):3018–41. 10.1038/s41596-018-0076-8. • Detailed protocol for the generation of iPSC-CMs.
- 24.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–409. 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 25. Garg P, Oikonomopoulos A, Chen H, Li Y, Lam CK, Sallam K, et al. Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J Am Coll Cardiol. 2018;72(1):62–75. 10.1016/j.jacc.2018.04.041. • Paper using iPSC-CMs to show that genome editing of patient-specific iPSC-CMs can be used to evaluate the pathogenicity of variants of uncertain significance in cardiac channelopathies.
- 26.Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB, et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol. 2013;168(6):5277–86. 10.1016/j.ijcard.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, et al. Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol. 2013;141(1):61–72. 10.1085/jgp.201210899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Roche J, Angsutararux P, Kempf H, Janan M, Bolesani E, Thiemann S, et al. Comparing human iPSC-cardiomyocytes versus HEK293T cells unveils disease-causing effects of Brugada mutation A735V of Nav1.5 sodium channels. Sci Rep. 2019;9(1):11173. 10.1038/s41598-019-47632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Stauske M, Luo X, Wagner S, Vollrath M, Mehnert CS, et al. Disease phenotypes and mechanisms of iPSC-derived cardiomyocytes from Brugada syndrome patients with a loss-of-function SCN5A mutation. Front Cell Dev Biol. 2020;8:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, et al. Patient-specific and genome-edited induced pluripotent stem cell–derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol. 2016;68(19):2086–96. 10.1016/j.jacc.2016.07.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatima A, Xu G, Shao K, Papadopoulos S, Lehmann M, Arnáiz-Cot JJ, et al. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell Physiol Biochem. 2011;28(4):579–92. 10.1159/000335753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong L, Zhang M, Ly OT, Chen H, Sridhar A, Lambers E, et al. Human induced pluripotent stem cell-derived atrial cardiomyocytes carrying an SCN5A mutation identify nitric oxide signaling as a mediator of atrial fibrillation. Stem Cell Rep. 2021;16(6):1542–54. 10.1016/j.stemcr.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benzoni P, Campostrini G, Landi S, Bertini V, Marchina E, Iascone M, et al. Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICal in patient-derived cardiomyocytes. Cardiovasc Res. 2020;116(6):1147–60. 10.1093/cvr/cvz217. • Paper on the use of iPSC-CMs to model a complex familial form of atrial fibrillation.
- 34.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, et al. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349(6251):982–6. 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4(130):130ra47–ra47. 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Lee J, Vincent LG, Wang Q, Gu M, Lan F, et al. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell. 2015;17(1):89–100. 10.1016/j.stem.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Termglinchan V, Diecke S, Itzhaki I, Lam CK, Garg P, et al. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature. 2019;572(7769):335–40. 10.1038/s41586-019-1406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karakikes I, Stillitano F, Nonnenmacher M, Tzimas C, Sanoudou D, Termglinchan V, et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun. 2015;6(1):6955. 10.1038/ncomms7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tse HF, Ho JC, Choi SW, Lee YK, Butler AW, Ng KM, et al. Patient-specific induced-pluripotent stem cells-derived cardiomyocytes recapitulate the pathogenic phenotypes of dilated cardiomyopathy due to a novel des mutation identified by whole exome sequencing. Hum Mol Genet. 2013;22(7):1395–403. 10.1093/hmg/dds556. [DOI] [PubMed] [Google Scholar]
- 40.Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12(1):101–13. 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka A, Yuasa S, Mearini G, Egashira T, Seki T, Kodaira M, et al. Endothelin-1 induces myofibrillar disarray and contractile vector variability in hypertrophic cardiomyopathy-induced pluripotent stem cell-derived cardiomyocytes. J Am Heart Assoc. 2014;3(6): e001263. 10.1161/jaha.114.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494(7435):105–10. 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi O, Huber I, Gepstein A, Arbel G, Maizels L, Boulos M, et al. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ Cardiovasc Genet. 2013;6(6):557–68. 10.1161/circgenetics.113.000188. [DOI] [PubMed] [Google Scholar]
- 44.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6(240):240ra74. 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kodo K, Ong S-G, Jahanbani F, Termglinchan V, Hirono K, InanlooRahatloo K, et al. iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol. 2016;18(10):1031–42. 10.1038/ncb3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brodehl A, Pour Hakimi SA, Stanasiuk C, Ratnavadivel S, Hendig D, Gaertner A, et al. Restrictive cardiomyopathy is caused by a novel homozygous desmin (DES) mutation p.Y122H leading to a severe filament assembly defect. Genes. 2019;10(11):918. 10.3390/genes10110918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang HP, Chen PH, Hwu WL, Chuang CY, Chien YH, Stone L, et al. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum Mol Genet. 2011;20(24):4851–64. 10.1093/hmg/ddr424. [DOI] [PubMed] [Google Scholar]
- 48.Hashem SI, Perry CN, Bauer M, Han S, Clegg SD, Ouyang K, et al. Brief report: oxidative stress mediates cardiomyocyte apoptosis in a human model of Danon disease and heart failure. Stem Cells. 2015;33(7):2343–50. 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudek J, Cheng IF, Balleininger M, Vaz FM, Streckfuss-Bömeke K, Hübscher D, et al. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 2013;11(2):806–19. 10.1016/j.scr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Itier JM, Ret G, Viale S, Sweet L, Bangari D, Caron A, et al. Effective clearance of GL-3 in a human iPSC-derived cardiomyocyte model of Fabry disease. J Inherit Metab Dis. 2014;37(6):1013–22. 10.1007/s10545-014-9724-5. [DOI] [PubMed] [Google Scholar]
- 51.Chanana AM, Rhee J-W, Wu JC. Human-induced pluripotent stem cell approaches to model inborn and acquired metabolic heart diseases. Curr Opin Cardiol. 2016;31(3):266–74. 10.1097/HCO.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drawnel Faye M, Boccardo S, Prummer M, Delobel F, Graff A, Weber M, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–20. 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67(18):2161–76. 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai D-F, Danoviz ME, Wiczer B, Laflamme MA, Tian R. Mitochondrial maturation in human pluripotent stem cell derived cardiomyocytes. Stem Cells Int. 2017;2017:5153625. 10.1155/2017/5153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301(5):H2006–17. 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21(4):579–87. 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebert A, Joshi AU, Andorf S, Dai Y, Sampathkumar S, Chen H, et al. Proteasome-dependent regulation of distinct metabolic states during long-term culture of human iPSC-derived cardiomyocytes. Circ Res. 2019;125(1):90–103. 10.1161/circresaha.118.313973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correia C, Koshkin A, Duarte P, Hu D, Teixeira A, Domian I, et al. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci Rep. 2017;7(1):8590. 10.1038/s41598-017-08713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee JB, et al. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2015;67:52–64. 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroll K, Chabria M, Wang K, Häusermann F, Schuler F, Polonchuk L. Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog Biophys Mol Biol. 2017;130(Pt B):212–22. 10.1016/j.pbiomolbio.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 61. Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3(12): e99941. 10.1172/jci.insight.99941. • Paper providing methods to differentiate specific subtypes of iPSC-CMs (e.g., atrial vs. ventricular cardiomyocytes).
- 62.Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2017;35(1):56–68. 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 63. Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat Commun. 2019;10(1):2238. 10.1038/s41467-019-09831-5. •• Paper providing a method for the generation of iPSC-CFs.
- 64.Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 2020;26(6):862–79.e11. 10.1016/j.stem.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Tian L, Shen M, Tu C, Wu H, Gu M, et al. Generation of quiescent cardiac fibroblasts from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis. Circ Res. 2019;125(5):552–66. 10.1161/CIRCRESAHA.119.315491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30(2):165–73. 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, et al. Pericytes are progenitors for coronary artery smooth muscle. eLife. 2015;4:e10036. 10.7554/eLife.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iyer D, Gambardella L, Bernard WG, Serrano F, Mascetti VL, Pedersen RA, et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development. 2015;142(8):1528–41. 10.1242/dev.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bao X, Lian X, Hacker TA, Schmuck EG, Qian T, Bhute VJ, et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat Biomed Eng. 2016;1(1):0003. 10.1038/s41551-016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17(8):994–1003. 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen M, Quertermous T, Fischbein MP, Wu JC. Generation of vascular smooth muscle cells from induced pluripotent stem cells: methods, applications, and considerations. Circ Res. 2021;128(5):670–86. 10.1161/circresaha.120.318049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biel NM, Santostefano KE, DiVita BB, El Rouby N, Carrasquilla SD, Simmons C, et al. Vascular smooth muscle cells from hypertensive patient-derived induced pluripotent stem cells to advance hypertension pharmacogenomics. Stem Cells Trans Med. 2015;4(12):1380–90. 10.5966/sctm.2015-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge X, Ren Y, Bartulos O, Lee MY, Yue Z, Kim KY, et al. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation. 2012;126(14):1695–704. 10.1161/circulationaha.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao J, Xiong W, Wang L, Yang J, Qiu P, Hirai H, et al. Differentiation defect in neural crest-derived smooth muscle cells in patients with aortopathy associated with bicuspid aortic valves. EBioMedicine. 2016;10:282–90. 10.1016/j.ebiom.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Granata A, Serrano F, Bernard WG, McNamara M, Low L, Sastry P, et al. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat Genet. 2017;49(1):97–109. 10.1038/ng.3723. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8(1):31–45. 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Luo J, Qin L, Zhao L, Gui L, Ellis MW, Huang Y, et al. Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. Cell Stem Cell. 2020;26(2):251–61.e8. 10.1016/j.stem.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X, Qi J, Xu X, Zeisberg M, Guan K, Zeisberg EM. Differentiation of functional endothelial cells from human induced pluripotent stem cells: a novel, highly efficient and cost effective method. Differentiation. 2016;92(4):225–36. 10.1016/j.diff.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Paik DT, Tian L, Lee J, Sayed N, Chen IY, Rhee S, et al. Large-scale single-cell RNA-seq reveals molecular signatures of heterogeneous populations of human induced pluripotent stem cell-derived endothelial cells. Circ Res. 2018;123(4):443–50. 10.1161/CIRCRESAHA.118.312913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theodoris CV, Li M, White MP, Liu L, He D, Pollard KS, et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160(6):1072–86. 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.West JD, Austin ED, Gaskill C, Marriott S, Baskir R, Bilousova G, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307(5):C415–30. 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sayed N, Liu C, Ameen M, Himmati F, Zhang JZ, Khanamiri S, et al. Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci Transl Med. 2020;12(554):eaax9276. 10.1126/scitranslmed.aax9276. • Paper showing “clinical trial in a dish” of the effects of statins on LMNA iPSC-ECs.
- 83.Murray IR, Baily JE, Chen WCW, Dar A, Gonzalez ZN, Jensen AR, et al. Skeletal and cardiac muscle pericytes: functions and therapeutic potential. Pharmacol Ther. 2017;171:65–74. 10.1016/j.pharmthera.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stebbins MJ, Gastfriend BD, Canfield SG, Lee M-S, Richards D, Faubion MG et al. Human pluripotent stem cell–derived brain pericyte–like cells induce blood-brain barrier properties. Sci Adv. 2019;5(3):eaau7375. 10.1126/sciadv.aau7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Faal T, Phan DTT, Davtyan H, Scarfone VM, Varady E, Blurton-Jones M, et al. Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Rep. 2019;12(3):451–60. 10.1016/j.stemcr.2019.01.005. • Protocol on brain pericyte formation that may be useful for the generation of cardiac pericytes.
- 86.Lemoine MD, Mannhardt I, Breckwoldt K, Prondzynski M, Flenner F, Ulmer B, et al. Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci Rep. 2017;7(1):5464. 10.1038/s41598-017-05600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16(3):599–610. 10.1039/C5LC01356A. [DOI] [PubMed] [Google Scholar]
- 88. Drakhlis L, Biswanath S, Farr C-M, Lupanow V, Teske J, Ritzenhoff K, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 2021;39(6):737–46. 10.1038/s41587-021-00815-9. • Paper showing that the generation of highly structured cardiac organoids can be used to probe questions in cardiac development.
- 89. Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556(7700):239–43. 10.1038/s41586-018-0016-3. •• Paper showing organized microstructures and improved maturity of engineered heart tissues compared with naïve iPSC-CMs.
- 90.Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 2018;145(5). 10.1242/dev.156166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wnorowski A, Yang H, Wu JC. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv Drug Deliv Rev. 2019;140:3–11. 10.1016/j.addr.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lewis-Israeli YR, Wasserman AH, Gabalski MA, Volmert BD, Ming Y, Ball KA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12(1):5142. 10.1038/s41467-021-25329-5. • Paper showing cardiac organoids exihibit several basic structural and functional characteristics of the heart and can mimic complex metabolic disorders associated with congenital heart disease.
- 93. Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020;4(4):446–62. 10.1038/s41551-020-0539-4. • Paper indicating cardiac organoids may be especially well-suited to model entire organ features of certain heart diseases, such as myocardial infarction.


