Abstract
Objective
Dermatologists regularly encounter patients having expanded fields with numerous actinic keratosis (AK) lesions on the face and scalp. Field-directed red light photodynamic therapy (PDT) is a well-established treatment, yet published data on the safety of PDT on large areas is scarce. We aimed to evaluate the safety and tolerability of red light PDT in treating expanded AK fields on the face and scalp.
Methods
This was a non-randomized, open-label, multicenter study. After lesion preparation, 6g of 10% aminolevulinic acid (ALA) gel were applied to the treatment field (60 cm2) and incubated for three hours under a light-blocking, occlusive dressing before 10-minute illumination with a red light lamp (~635nm, 37 J/cm2). Safety and tolerability were assessed throughout the study.
Results
All participants (n=100) had adverse reactions. No previously unknown effects, serious adverse events, or deaths were reported. The most frequent application site reactions were pain/burning (96.0%), exfoliation (87.0%), and erythema (86.0%). Most treatment-emergent adverse events were of mild to moderate severity and lasted slightly longer compared to those experienced after treatment of smaller areas. The mean maximum pain during PDT was 7.4±2.1 on an 11-point numeric rating scale. A transient increase in blood pressure on the day that PDT was performed was not clinically significant.
Limitations
Although the allowed use of pain-reducing measures might have influenced evaluation of pain, it reflects how the procedure is managed in current practice.
Concklusion
PDT with 10% ALA gel and red light illumination on an expanded treatment field was generally well tolerated.
Keywords: Photodynamic therapy, 10% ALA gel, BF-200 ALA, red light, actinic keratosis, actinic keratoses, RhodoLED, clinical research, safety, aminolevulinic acid
Actinic keratoses (AKs) result from atypical proliferating keratinocytes in the epidermis of the skin and are considered a consequence of cumulative ultraviolet (UV) damage. With the prevalence of the disease rising,1 the burden on the healthcare system is increasing.2,3
AKs are an early precursor form of invasive cutaneous squamous cell carcinoma (SCC).4,5 Multiple AK lesions6,7 and time since initial diagnosis8 increase the risk of progression. In addition to clinically distinctive lesions which appear as rough, reddish or scaly patches, subclinical precancerous changes can occur in the field around those lesions. As AKs of all severity grades have a risk of progression to SCC,9 international guidelines strongly recommend early and field-directed treatment of AKs.4,5,10–17 Photodynamic therapy (PDT) can be applied in a lesion- and field-directed manner4,5,10,18 and has advantages compared to other medications or procedures.4,5,10,19,20 PDT with 10% aminolevulinic acid (ALA) gel, has demonstrated excellent efficacy outcomes20–23 including comparably favorable long-term efficacy.24–26 A meta-analysis by Vegter et al27 concluded that PDT using 10% ALA gel and narrow-spectrum red light was the most efficacious treatment option.
Depending on the protocol used, one common adverse reaction of PDT is pain, especially in larger treatment areas.28 Nevertheless, most side effects of PDT resolve within a few days, and cosmetic results are favorable.20
Dermatologists often face treatment of expanded fields with numerous AKs;29 however, separate PDT sessions might further compromise treatment compliance and increase costs. Several sessions can be avoided if more clinical data is published on red light PDT of large AK areas29–33 to confirm safety.34,35
This study was performed to assess the safety and tolerability of red light PDT with 10% ALA gel in an expanded field-directed treatment of mild to severe AK on the face and scalp.
METHODS
Trial design. This was a non-randomized, open-label, multicenter Phase I study to evaluate the safety and tolerability of PDT with 10% ALA gel and a narrow-spectrum red light lamp in the treatment of extended AK fields located on the face and scalp (NCT05060237). It was designed for a submission to the United States (US) Food and Drug Administration (FDA), targeting a label extension for the 10% ALA gel. The study was approved by the Institutional Review Board (IRB) and competent authority prior to start of the study and was performed according to national drug laws, Good Clinical Practice Guidelines, and the Declaration of Helsinki. Informed consent was obtained by all participants prior to any study procedure. Data was collected between December 2021 and April 2023 at nine sites in the US. The study consisted of five study visits: A screening visit within 14 days before treatment, a baseline/treatment visit, two interim visits for safety assessment approximately 7 and 14 days after PDT; and the final visit approximately 28 days after PDT.
Participants. Eligible participants were aged 18 years or older, with at least eight mild to moderate clinically confirmed AK lesions of ≥4mm in diameter (according to Olsen36) on the face and/or scalp. Presence of severe AK lesions was allowed, however diagnosis of each severe lesion had to be confirmed histopathologically (2mm punch biopsy at screening; assessment performed by a central laboratory). Lesions classified as malignant or benign tumors inside or in close proximity (<10cm distance) to the treatment field led to exclusion of the participants from the study. The treatment field (continuous or in several patches) had to be located within one effective illumination area of the lamp (see specifics in the treatment section). Participants with a history of hypersensitivity to ALA or any other ingredient of 10% ALA gel, presence of porphyria, hypersensitivity to porphyrins, or a clinically significant medical condition rendering implementation of the protocol or interpretation of the study results difficult were excluded. The identities and personal data of all participants were safeguarded according to data protection regulations.
Treatment. 10% ALA gel (BF-200 ALA, Ameluz®; Biofrontera Pharma GmbH, Leverkusen, Germany) was manufactured and released according to Good Manufacturing Practice and relevant regulations and delivered in aluminum tubes containing 2g of gel. Treatment field was prepared by degreasing, removal of scabs and crusts, and debridement consisting of roughening of the surface, if applicable. Subsequently, three tubes (6g) of 10% ALA gel (total dose of 600mg ALA-HCl) were applied to the treatment field of 60cm2 in an ~1mm thick layer (excluding eyes, nostrils, ears, and mouth). After three-hour incubation period under a light-blocking, occlusive dressing, the dressing and remaining gel were removed.
The treatment field was illuminated for 10 minutes using the RhodoLED® XL (~635nm red light, 37 J/cm2; Biofrontera Pharma GmbH, Leverkusen, Germany) at a treatment distance between 11 to 14cm. The maximum effective illumination area (using all 5 panels, curved configuration) is 667cm²; however, a minimum of three adjacent panels had to be used.
Assessments. At screening, medical history was documented. In addition, treatment fields were selected and target lesions (size, number, location, clinical severity)36 were assessed. Throughout the study, safety and tolerability assessments were performed. The frequency of (serious) adverse events (AEs), treatment-emergent AEs, (TEAEs, all AEs with onset or worsening after application of 10% ALA gel) and adverse drug reactions (ADRs, TEAEs which were considered at least possibly related to treatment), including their duration and severity (mild, moderate, severe), were evaluated. AEs, TEAEs and ADRs were coded according to MedDRA Version 26.0. Application site skin reactions (assessed by the investigator) and application site discomfort (reported by the participants) were assessed during and after PDT. Immediately following PDT, the participants retrospectively rated the maximum pain during illumination on an 11-point numeric rating scale (NRS-11) ranging from 0 (no pain) to 10 (worst imaginable pain). Further, vital signs (blood pressure, heart rate, body temperature) were measured at all clinical visits. At screening and the final visit, hematology and clinical chemistry parameters were assessed, and participants were physically examined. Neurological assessments were performed at screening and repeatedly on the day that PDT was performed.
Statistical methods. Continuous data was summarized by means of descriptive statistics, ie number of participants, mean, standard deviation (SD), median, quartiles and range (minimum and maximum). Categorical variables were summarized by absolute and relative frequencies (percentages) of participants by category. Missing values were not included.
RESULTS
Demographics. One hundred and twelve participants were assessed for eligibility, of which eight participants were excluded as screening failures and four participants withdrew their consent prior to treatment. In total, 100 participants received treatment and were analyzed for the outcomes. No participant discontinued the study. Participant and lesion characteristics at screening are summarized in Table 1.
TABLE 1.
Participant demographics and baseline disease characteristics
| PARTICIPANT DEMOGRAPHICS | N=100 |
|---|---|
| Sex, n (%) | |
| Female | 12 (12.0) |
| Male | 88 (88.0) |
| Age at inclusion [years] | |
| Mean±SD | 67.9±7.4 |
| Median (range) | 69.0 (37 – 83) |
| Race, n (%) | |
| White | 99 (99.0) |
| Other | 1 (1.0) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 99 (99.0) |
| Hispanic or Latino | 1 (1.0) |
| Fitzpatrick skin type, n (%) | |
| I – III | 99 (99.0) |
| IV – VI | 1 (1.0) |
| Disease characteristics (per participant) | N=100 |
| Total number of AK target lesions | |
| Mean±SD | 16.7±11.1 |
| Total area of AK target lesions ≥ 4mm diameter [mm²] | |
| Mean±SD | 575.9±579.3 |
| AK lesion characteristicsa | N=1,299 |
| Location, n (%) | |
| Face | 692 (53.3) |
| Scalp | 607 (46.7) |
| Individual lesion area [mm²] | |
| Mean±SD | 44.3±78.4 |
| Median (range) | 25.0 (16 – 2,304) |
| Clinical severityb, n (%) | |
| Mild/grade 1 | 685 (52.7) |
| Moderate/grade 2 | 599 (46.1) |
| Severe/grade 3 | 15 (1.2) |
Lesions with a diameter <4mm are not considered. The lesion area of each lesion is calculated by multiplying largest diameter and perpendicular diameter.
aThe location, diameters and lesion area are displayed for all lesions with a diameter ≥4mm.
bAccording to Olsen et al36 AK: actinic keratosis, N: Total number of participants or lesions; n: Number of participants or lesions; SD: Standard deviation.
Safety and tolerability. Frequency of AEs. All participants (n=100, 100%) experienced at least one AE. In total, 888 AEs were reported, of which 871 were TEAEs and 811 were ADRs. No serious AEs and no deaths were reported.
All participants experienced at least one ADR. Of 811 events in total, 749 were general disorders or administration site conditions. The most frequently reported ADRs at the application site (percent of participants) reflected the mode of action of PDT: pain/burning (96.0%), exfoliation (87.0%), erythema (86.0%) and pruritus (65.0%). Table 2 provides a comparison of frequently reported ADRs at the application site for treatment fields of 60cm2 compared to already known ADRs for treatment fields of 20cm2.37 Regarding ADRs which occurred not at the application site, headache (17.0%) was the most frequently reported.
TABLE 2.
Most frequently reported adverse drug reactions at the application site in ALA-AK-CT018 compared to 10% ALA gel US PIa
| MEDDRA PREFERRED TERM | ALA-AK-CT018 (THREE TUBES) N=100 | US PIA (ONE TUBE) N=212 | |
|---|---|---|---|
| NAE | N (%) | N (%) | |
| Pain/burning | 193 | 96 (96%) | 195 (92%) |
| Exfoliation | 117 | 87 (87%) | 41 (19%) |
| Erythema | 97 | 86 (86%) | 195 (92%) |
| Pruritus | 79 | 65 (65%) | 72 (34%) |
| Scab | 58 | 49 (49%) | 41 (19%) |
| Edema | 40 | 35 (35%) | 75 (35%) |
| Induration | 18 | 18 (18%) | 26 (12%) |
| Warmth | 20 | 17 (17%) | – |
| Erosion | 18 | 16 (16%) | 6 (3%) |
| Paraesthesia | 17 | 15 (15%) | 18 (9%) |
| Vesicles | 16 | 14 (14%) | 25 (12%) |
| Discomfort | 13 | 11 (11%) | 7 (3%) |
| Hyperaesthesia | 10 | 10 (10%) | 13 (6%) |
| Bleeding/hemorrhage | 9 | 9 (9%) | 3 (1%) |
| Swelling | 8 | 6 (6%) | – |
| Discoloration | 6 | 6 (6%) | – |
| Dryness | 4 | 4 (4%) | – |
| Discharge | 4 | 4 (4%) | 4 (2%) |
| Pustules | 3 | 3 (3%) | 3 (1%) |
Adverse drug reactions (treatment-emergent adverse events, at least possibly related to treatment) at the application site experienced by two or more participants are presented.
a10% ALA gel US PI37 includes lesion-directed and field-directed treatments of max. 20 cm2.
N: number of participants; n: number of participants with event; nAE: number of events; PI: Prescribing information; US: United States
Duration of ADRs. Further, the mean duration of ADRs was analyzed by severity category (mild, moderate, severe), whereas only ADRs which occurred in at least two participants and with complete start/stop dates were analyzed. Most ADRs were of mild to moderate severity and resolved by the end of the study. Overall, the mean duration of the majority of ADRs was seven days or fewer and was rarely longer than 14 days (Figure 1). In the total study population, 29.6 percent of analyzed ADRs lasted on average four days or less, 62.9 percent lasted between 5 and 14 days, and 7.4 percent lasted over 15 days. Nausea (1.00±0.00 days), chills, (1.60±0.89 days) and application site warmth (2.13±1.75 days) were the ADRs with the shortest mean duration. Application site exfoliation (13.62±9.81 days), application site induration (15.71±29.67 days), and application site erythema (19.04±12.78 days) lasted the longest. The period of events classified as severe was longest for application site pain (1.87±3.47 days) and application site erythema (1.00±4.8 days).
FIGURE 1.
Duration and severity of adverse drug reactions after expanded field-directed photodynamic therapy of actinic keratosis. Mean duration of adverse drug reactions after photodynamic therapy in days is presented, including proportions of mild, moderate and severe severity categories. Only events which occurred in at least two participants and with complete start and stop dates are displayed.
Application site reactions. Application site skin reactions and application site discomfort were analyzed separately. Application site skin reactions were reported for 96.0 percent of participants (402 events). Exfoliation (117 events in 87.0% of participants), erythema (97 events in 86% of participants), and scab (58 events in 49.0% of participants) were the most frequently reported application site skin reactions. Application site discomfort was reported by 97.0 percent of participants (334 events). Pain (193 events in 96.0% of participants), pruritus (79 events in 65.0% of participants), and warmth (20 events in 17.0% of participants) were the most frequently reported application site discomfort categories.
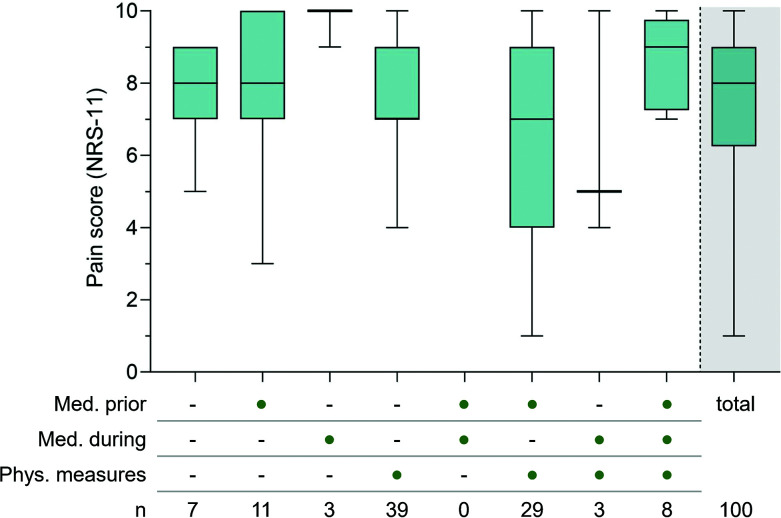
Pain. All participants experienced pain during PDT. Overall, the mean maximum pain intensity reported on NRS-11 during PDT was 7.4±2.1 (median 8.0). The highest mean pain intensity was reported by participants treated on the scalp (n=39; 7.8±2.3) compared to face (n=40; 7.2±2.2) and face and scalp (n=21; 7.3±1.7). As pain during PDT is related to the size of the treatment area, higher pain was anticipated.Thus, different measures or medications to relieve pain were allowed. At study start, medication during illumination (eg, applied or injected fast-acting anesthetic) and/or physical measures (eg, interruption of illumination and/or cooling with an air stream and/or nebulized water) were allowed. After some participants prematurely terminated the illumination due to severe pain, and several interruptions of the illumination were documented, the study protocol was revised to include prophylactic analgesic treatment (eg, oral non-steroidal anti-inflammatory drugs, applied or injected fast-acting anesthetic). However, as prophylactic analgesic treatment might impact data integrity for pain evaluation, all pain-relieving measures were documented accordingly. Participants receiving no measure, all measures, and any of the combinations were analyzed separately (Figure 3). No relevant effect of the measures was noticeable.
FIGURE 3.
Pain intensity during expanded field-directed PDT of actinic keratosis with and without pain relieving measures and/or medications. The maximum pain during illumination was rated retrospectively directly after illumination by the participants on a scale of 0 (no pain) to 10 (worst imaginable pain). Participants might have received different measures or medications to relieve pain, including medication prior to illumination (e.g. oral non-steroidal antirheumatic drugs, applied or injected fast-acting anesthetic), medication during illumination (e.g. applied or injected fast-acting anesthetic) and/or physical measures (e.g. interruption of illumination and/or cooling with an air stream and/or nebulized water), as indicated by dots in the adjacent table below. Each box represents participants to whom the same type of pain-relieving measures had been applied to. The box in the grey highlighted area shows data of all participants combined, irrespective of the pain-relieving measure used.
Box displays 1st and 3rd quartile, median value is indicated by horizontal line, whiskers are drawn from the box to the most extreme points.
N: Number of participants, NRS-11: Numeric rating scale, PDT: Photodynamic therapy.
Vital signs. In general, the PDT procedure had no effect on the body temperature or heart rate (Table 3). Mean blood pressure was slightly elevated prior and shortly after illumination but were normal at all following visits (Table 3, Figure 2). The highest mean values were measured within 10 minutes after illumination: The mean change from baseline was 17.3±16.5mmHg for systolic blood pressure and 7.1±9.3mmHg for diastolic blood pressure.
TABLE 3.
Vital sign measurements, change from baseline
| VITAL SIGNS MEASUREMENTS | BASELINE | CHANGE FROM BASELINE | |||||
|---|---|---|---|---|---|---|---|
| ARRIVAL | DAY OF PDT | 7 DAYS AFTER PDT | 14 DAYS AFTER PDT | 28 days after PDT | |||
| WITHIN 10 MIN BEFORE ILLUMINATION | WITHIN 10 MIN AFTER ILLUMINATION | 60 MIN AFTER ILLUMINATION | |||||
| Blood pressure [mmHg] | |||||||
| Systolic Mean±SD | 131.6±16.4 | 5.7±12.8 | 17.3±16.5 | 10.8±15.0 | 1.1±12.5 | -1.1±13.4 | -0.9±14.5 |
| Systolic Median (min, max) | 128.5 (100, 175) | 5.0 (-21, 47) | 16.0 (-24, 72) | 8.0 (-30, 56) | 1.0 (-30, 39) | -1.5 (-35, 25) | -1.0 (-51, 35) |
| Diastolic Mean±SD | 79.7±8.7 | 2.3±6.6 | 7.1±9.3 | 4.4±7.9 | 1.0±8.8 | -0.6±8.3 | 0.8±8.5 |
| Diastolic Median (min, max) | 80.0 (57, 102) | 1.0 (-12, 18) | 6.5 (-10, 31) | 4.0 (-17, 23) | 1.0 (-25, 30) | -0.5 (-19, 25) | 1.0 (-30, 25) |
| Heart rate [beats/min] | |||||||
| Mean±SD | 100±11.8 | -2.1±10.0 | -1.2±10.2 | -2.8±9.5 | 0.7±9.8 | 0.7± 0.0 | 1.0±9.2 |
| Median (min, max) | 70.5 (43, 101) | -2.0 (-30, 36) | -2.0 (-28, 20) | -3.0 (-27, 24) | 0.0 (-26, 33) | -1.0 (-19, 43) | 0.0 (-26, 32) |
| Body temperature [°C] | |||||||
| Mean±SD | 36.69 (0.33) | -0.11±0.41 | -0.17±0.60 | -0.08±0.43 | 0.04±0.40 | -0.05±0.39 | -0.03±0.39 |
| Median (min, max) | 36.70 (35.7, 37.7) | 0.00 (-2.0, 1.0) | -0.05 (-2.4, 1.3) | -0.10 (-1.1, 1.5) | 0.00 (-1.0, 1.3) | 0.00 (-1.4, 0.9) | 0.00 (-1.0, 1.1) |
Vital signs data from blood pressure (systolic and diastolic), heart rate and body temperature at different time points at day of PDT and up to 28 days after PDT is presented as change from baseline. The first measurement after the participants arrived at the site on the day of PDT served as baseline value and is displayed in the first column.
Max: maximum, min: minimum, PDT: photodynamic therapy, SD: standard deviation.
FIGURE 2.
Blood pressure before and after expanded field-directed photodynamic therapy of actinic keratosis. Systolic blood pressure and diastolic blood pressure were measured at different time points on the day of PDT and up to 28 days after PDT. Box displays 1st and 3rd quartile, median value is indicated by horizontal line, whiskers are drawn from the box to the most extreme points. PDT: Photodynamic therapy.
Other safety assessments. Whereas no clinically significant urinalysis findings were observed, two participants (2%) had clinically significant hematology (elevated liver enzymes) and clinical chemistry (elevated blood glucose) findings 22 and 29 days after PDT, which were of mild severity and considered unrelated to the treatment. Results of physical examinations and neurological assessments were normal.
DISCUSSION
This study showed the safety and tolerability of 10% ALA gel in the expanded field-directed treatment of AK on the face and scalp with red light PDT. Moreover, its results were decisive for a recent FDA-approved label extension concerning the treatment of expanded AK fields up to 60cm2 using red light PDT with up to three tubes of 10% ALA gel.
Field-directed treatments are recommended in current guidelines.10,14,15 Several studies indicated good efficacy and tolerability for ALA-PDT on extended fields with numerous AK,29–34 including daylight PDT.38–41 In particular, one retrospective study assessed the tolerability of PDT with 10% ALA gel in areas ranging from 75cm2 to 300cm2 in 203 participants with AK. The results indicated that PDT treatment of areas larger than 20cm2 is safe.34 Furthermore, two maximal use studies assessed pharmacokinetics using 2g 10% ALA gel on 20cm2 skin and 6g on 60cm2 skin. The authors showed that a similar, minor fraction of topical ALA was systemically absorbed under both dosing regimens,35 which was well below the daily rate of ALA synthesis (200–354mg).42
In our study, all participants had at least one AE, which is to be expected as the therapeutic principle of PDT is based on phototoxic effects. Most ADRs were consistent with those already known to occur on treatment fields up to 20cm2.21–23,37
Although most of the ADRs and TEAEs were transient, the mean duration of ADRs was slightly prolonged compared to those experienced after treatment of smaller areas.37 This can be explained by the recovery time of the large area which was treated. The duration of the recovery depends on the different stages of wound healing. These involve the removal of dead or devitalized atypical keratinocytes and epithelialization of the area.43 Larger skin areas thus need longer to recover.
One of the most common side effects of PDT is pain. In general, pain starts within seconds after start of illumination, peaks usually within the first minute, and decreases gradually afterwards.44 This should be considered when interpreting maximum pain intensities reported during PDT. Furthermore, PDT is more painful when performed on well-innervated areas of the skin, such as the face.28,45 When compared to pain scores in areas <20cm2, maximum pain during illumination was slightly higher in our study.21–23 This might be expected, as pain during PDT presumably correlates with the size of the treatment field and the extent of the disease.28 Nevertheless, the pain score in our study was lower than in another recent study with 10% ALA gel on larger areas.35 In general, it might be useful to offer physical pain-relieving measures to patients: although the mean pain was not measurably influenced by the different mitigation methods, the participants were more compliant with the whole illumination duration. A psychological component, eg, feeling more secure or being taken care of, might explain the effect.
Males have been previously described as more susceptible to pain.28 However, the higher rate of male participants in our study did not distort the mean maximum pain scores. Males have consistently higher rates of AKs compared to females.46,47 In addition, the risk of developing AKs have been shown to increase significantly with age, particularly in patients with fair skin,17,48 and a strong risk factor for the development of more than 10 AKs was baldness in males.49 Thus, the ratio of male and female participants in our study reflect the prevalence of the disease in the studied population.
Interestingly, previous studies reported that pain and blood pressure during PDT correlate. They showed that PDT was associated with a significant increase in blood pressure both during and shortly after illumination.50–52 The slight transient increase in blood pressure in the current study might be due to the pain experienced during illumination. Furthermore, the slight increase in blood pressure within 10 minutes prior to illumination might be due to agitation and nervousness before illumination. Aiyer et al53 defined a significant increase in blood pressure as an increase in systolic blood pressure of greater than 20mmHg and an increase in diastolic blood pressure of greater than 10mmHg. Thus, according to these criteria, the average increase in systolic and diastolic blood pressure observed in the current study would not be considered clinically significant.
CONCLUSION
From the results of this study, it can be concluded that PDT with three tubes of 10% ALA gel and red light illumination on an expanded treatment field of 60cm2 was generally well tolerated. No previously unknown side effects, serious AEs, deaths, or other clinically relevant AEs were reported during the study and no subject discontinued the study due to an AE.
AcknowledgementS
We thank all clinical research staff and participants involved in the study, and Prof. Dr. Hermann Lübbert, Dr. Jon Lyons, Dr. Nicole Pospiech, and Ruth Schäning for intellectual input.
REFERENCES
- Yeung H, Baranowski ML, Swerlick RA et al. Use and cost of actinic keratosis destruction in the Medicare part b fee-for-service population, 2007 to 2015. JAMA Dermatol. 2018;154(11):1281–1285. doi: 10.1001/jamadermatol.2018.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warino L, Tusa M, Camacho F et al. Frequency and cost of actinic keratosis treatment. Dermatol Surg. 2006;32(8):1045–1049. doi: 10.1111/j.1524-4725.2006.32228.x. [DOI] [PubMed] [Google Scholar]
- Lim HW, Collins SAB, Resneck JS, Jr et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–972.e2. doi: 10.1016/j.jaad.2016.12.043. [DOI] [PubMed] [Google Scholar]
- Eisen DB, Asgari MM, Bennett DD et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209–e233. doi: 10.1016/j.jaad.2021.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppt MV, Leiter U, Steeb T et al. S3 guideline "actinic keratosis and cutaneous squamous cell carcinoma"- update 2023, part 1: Treatment of actinic keratosis, actinic cheilitis, cutaneous squamous cell carcinoma in situ (Bowen's disease), occupational disease and structures of care. J Dtsch Dermatol Ges. 2023;21(10):1249–1262. doi: 10.1111/ddg.15231. [DOI] [PubMed] [Google Scholar]
- Heppt MV, Leiter U, Steeb T et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma - short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J Dtsch Dermatol Ges. 2020;18(3):275–294. doi: 10.1111/ddg.14048. [DOI] [PubMed] [Google Scholar]
- Costa C, Scalvenzi M, Ayala F et al. How to treat actinic keratosis? An update. J Dermatol Case Rep. 2015;9(2):29–35. doi: 10.3315/jdcr.2015.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA, Naylor MF et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- Fernández-Figueras MT, Carrato C, Sáenz X et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(5):991–997. doi: 10.1111/jdv.12848. [DOI] [PubMed] [Google Scholar]
- Werner RN, Stockfleth E, Connolly SM et al. Evidence- and consensus-based (S3) Guidelines for the treatment of actinic keratosis - International League of Dermatological Societies in cooperation with the European Dermatology Forum - short version. J Eur Acad Dermatol Venereol. 2015;29(11):2069–2079. doi: 10.1111/jdv.13180. [DOI] [PubMed] [Google Scholar]
- Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):8–11. doi: 10.1111/jdv.14092. [DOI] [PubMed] [Google Scholar]
- Berman B, Bienstock L, Kuritzky L et al. Actinic keratoses: sequelae and treatments. Recommendations from a consensus panel. J Fam Pract. 2006;55(5):1–8. Suppl. [PubMed] [Google Scholar]
- Calzavara-Pinton P, Hædersdal M, Barber K et al. Structured expert consensus on actinic keratosis: treatment algorithm focusing on daylight PDT. J Cutan Med Surg. 2017;21(1_Suppl):3S–16S. doi: 10.1177/1203475417702994. [DOI] [PubMed] [Google Scholar]
- Jetter N, Chandan N, Wang S et al. Field cancerization therapies for management of actinic keratosis: a narrative review. Am J Clin Dermatol. 2018;19(4):543–557. doi: 10.1007/s40257-018-0348-7. [DOI] [PubMed] [Google Scholar]
- de Berker D, McGregor JM, Mohd Mustapa MF et al. British Association of Dermatologists' guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- Cornejo CM, Jambusaria-Pahlajani A, Willenbrink TJ et al. Field cancerization: treatment. J Am Acad Dermatol. 2020;83(3):719–730. doi: 10.1016/j.jaad.2020.03.127. [DOI] [PubMed] [Google Scholar]
- Willenbrink TJ, Ruiz ES, Cornejo CM et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83(3):709–717. doi: 10.1016/j.jaad.2020.03.126. [DOI] [PubMed] [Google Scholar]
- Braathen LR, Szeimies RM, Basset-Seguin N et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. J Am Acad Dermatol. 2007;56(1):125–143. doi: 10.1016/j.jaad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Han H, Berman B. Clinical management of actinic keratosis: review and update. Skin (Milwood). 2022;6(4):274–285. [Google Scholar]
- Ozog DM, Rkein AM, Fabi SG et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827. doi: 10.1097/DSS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- Dirschka T, Radny P, Dominicus R et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166(1):137–146. doi: 10.1111/j.1365-2133.2011.10613.x. [DOI] [PubMed] [Google Scholar]
- Reinhold U, Dirschka T, Ostendorf R et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz®) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED® lamp. Br J Dermatol. 2016;175(4):696–705. doi: 10.1111/bjd.14498. [DOI] [PubMed] [Google Scholar]
- Szeimies RM, Radny P, Sebastian M et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol. 2010;163(2):386–394. doi: 10.1111/j.1365-2133.2010.09873.x. [DOI] [PubMed] [Google Scholar]
- Steeb T, Wessely A, Petzold A et al. Evaluation of long-term clearance rates of interventions for actinic keratosis: a systematic review and network meta-analysis. JAMA Dermatol. 2021;157(9):1066–1077. doi: 10.1001/jamadermatol.2021.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirschka T, Radny P, Dominicus R et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168(4):825–836. doi: 10.1111/bjd.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov. Safety and efficacy study for the field-directed treatment of actinic keratosis (AK) with photodynamic therapy (PDT). Updated 28 Jul 2023. Accessed 12 Feb 2025. https://clinicaltrials.gov/study/NCT01966120.
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9(6):e96829. doi: 10.1371/journal.pone.0096829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CB, Karai LJ, Vidimos A et al. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol. 2009;61(6):1033–1043. doi: 10.1016/j.jaad.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Garelli V, Pranteda G et al. Dermoscopy and methyl aminolevulinate: a study for detection and evaluation of field cancerization. J Photochem Photobiol B. 2016;162:72–76. doi: 10.1016/j.jphotobiol.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Schmieder GJ, Huang EY, Jarratt M. A multicenter, randomized, vehicle-controlled phase 2 study of blue light photodynamic therapy with aminolevulinic acid HCl 20% topical solution for the treatment of actinic keratoses on the upper extremities: the effect of occlusion during the drug incubation period. J Drugs Dermatol. 2012;11(12):1483–1489. [PubMed] [Google Scholar]
- Touma D, Yaar M, Whitehead S et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140(1):33–40. doi: 10.1001/archderm.140.1.33. [DOI] [PubMed] [Google Scholar]
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: A pilot study. Dermatol Surg. 2014;40(10):1094–1102. doi: 10.1097/01.DSS.0000452662.69539.57. [DOI] [PubMed] [Google Scholar]
- Taub AF, Garretson CB. A randomized, blinded, bilateral intraindividual, vehicle-controlled trial of the use of photodynamic therapy with 5-aminolevulinic acid and blue light for the treatment of actinic keratoses of the upper extremities. J Drugs Dermatol. 2011;10(9):1049–1056. [PubMed] [Google Scholar]
- Moore AY, Moore S. Tolerability of photodynamic therapy using 10% 5-aminolevulinic acid hydrochloride gel for treating actinic keratoses on surface areas larger than 75cm2. J Clin Aesthet Dermatol. 2020;13(9):45–48. [PMC free article] [PubMed] [Google Scholar]
- Novak B, Dubois J, Chahrour O et al. Clinical pharmacokinetics and safety of a 10% aminolevulinic acid hydrochloride nanoemulsion gel (bf-200 ala) in photodynamic therapy of patients extensively affected with actinic keratosis: Results of 2 maximal usage pharmacokinetic trials. Clin Pharmacol Drug Dev. 2021;11(4):535–550. doi: 10.1002/cpdd.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen EA, Abernethy ML, Kulp-Shorten C et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24(5 Pt 1):738–743. doi: 10.1016/0190-9622(91)70113-g. [DOI] [PubMed] [Google Scholar]
- Full Prescribing Information, Ameluz® (aminolevulinic acid hydrochloride) gel 10%; US: Biofrontera Bioscience GmbH. October 2024. https://us.ameluz.com/fileadmin/AmeluzUSImages/05-pdf/Ameluz-Full-Prescribing-Information.pdf
- von Dobbeler C, Schmitz L, Dicke K et al. PDT with PPIX absorption peaks adjusted wavelengths: safety and efficacy of a new irradiation procedure for actinic keratoses on the head. Photodiagnosis Photodyn Ther. 2019;27:198–202. doi: 10.1016/j.pdpdt.2019.05.015. [DOI] [PubMed] [Google Scholar]
- Falkenberg C, Schmitz L, Dicke K et al. Pretreatment with ablative fractional carbon dioxide laser improves treatment efficacy in a synergistic PDT protocol for actinic keratoses on the head. Photodiagnosis Photodyn Ther. 2021;34:102249. doi: 10.1016/j.pdpdt.2021.102249. [DOI] [PubMed] [Google Scholar]
- Salido-Vallejo R, Jiménez-Nájar F, Garnacho-Sucedo G et al. Combined daylight and conventional photodynamic therapy with 5-aminolaevulinic acid nanoemulsion (BF-200 ALA) for actinic keratosis of the face and scalp: a new and efficient approach. Arch Dermatol Res. 2020;312(9):675–680. doi: 10.1007/s00403-019-02028-2. [DOI] [PubMed] [Google Scholar]
- Sáenz‐Guirado S, Cuenca‐Barrales C, Vega‐Castillo J et al. Combined versus conventional photodynamic therapy with 5‐aminolaevulinic acid nanoemulsion (BF‐200 ALA) for actinic keratosis: a randomized, single‐blind, prospective study. Photodermatol Photoimmunol Photomed. 2022;38(4):334–342. doi: 10.1111/phpp.12753. [DOI] [PubMed] [Google Scholar]
- Hamblin MR. Covalent photosensitizer conjugates, part 2: Peptides, polymers, and small molecules for targeted photodynamic therapy. In: Hamblin MR, Mroz P, eds. Advances in Photodynamic Therapy: Basic, Translational, and Clinical. Artech House. 2008:217–233. [Google Scholar]
- Tottoli EM, Dorati R, Genta I et al. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735. doi: 10.3390/pharmaceutics12080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shi L, Zhang YF et al. Gain with no pain? Pain management in dermatological photodynamic therapy. Br J Dermatol. 2017;177(3):656–665. doi: 10.1111/bjd.15344. [DOI] [PubMed] [Google Scholar]
- Gholam P, Denk K, Sehr T et al. Factors influencing pain intensity during topical photodynamic therapy of complete cosmetic units for actinic keratoses. J Am Acad Dermatol. 2010;63(2):213–218. doi: 10.1016/j.jaad.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Yaldiz M. Prevalence of actinic keratosis in patients attending the dermatology outpatient clinic. Medicine (Baltimore). 2019;98(28):e16465. doi: 10.1097/MD.0000000000016465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer I, Augustin M, Spehr C et al. Prevalence and risk factors of actinic keratoses in Germany - analysis of multisource data. J Eur Acad Dermatol Venereol. 2014;28(3):309–313. doi: 10.1111/jdv.12102. [DOI] [PubMed] [Google Scholar]
- Landis ET, Davis SA, Taheri A et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20(4):22368. [PubMed] [Google Scholar]
- Flohil SC, van der Leest RJ, Dowlatshahi EA et al. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol. 2013;133(8):1971–1978. doi: 10.1038/jid.2013.134. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Keller A, Enk A et al. Hemodynamic changes during conventional and daylight photodynamic therapy of actinic keratoses–a randomized controlled trial. J Dermatolog Treat. 2022;33(7):3022–3027. doi: 10.1080/09546634.2022.2097160. [DOI] [PubMed] [Google Scholar]
- Keller A, Hartmann J, Enk A et al. Pulse rate and blood pressure changes during low-irradiance PDT compared to conventional PDT in the treatment of facial actinic keratoses: a retrospective study. Photodermatol Photoimmunol Photomed. 2022;38(5):435–441. doi: 10.1111/phpp.12764. [DOI] [PubMed] [Google Scholar]
- Borroni RG, Carugno A, Rivetti N et al. Risk of acute postoperative hypertension after topical photodynamic therapy for non-melanoma skin cancer. Photodermatol Photoimmunol Photomed. 2013;29(2):73–77. doi: 10.1111/phpp.12019. [DOI] [PubMed] [Google Scholar]
- Aiyer AN, Kip KE, Mulukutla SR et al. Predictors of significant short-term increases in blood pressure in a community-based population. Am J Med. 2007;120(11):960–967. doi: 10.1016/j.amjmed.2007.06.021. [DOI] [PubMed] [Google Scholar]