Abstract
Analysis of the virologic and immunomodulatory effects of an association of efavirenz (EFV), nelfinavir (NFV), and stavudine (d4T) was performed in 18 human immunodeficiency virus (HIV)-infected and highly active antiretroviral therapy (HAART)-experienced patients who failed multiple therapeutic protocols. Patients (<500 CD4+ cells/μl; >10,000 HIV copies/ml) were nonnucleoside reverse transcriptase inhibitor (NNRTI)-naive and were treated for 10 months with EFV (600 mg/day) in association with NFV (750 mg three times daily) and d4T (30 or 40 mg twice daily). Measurement of HIV peptide- and mitogen-stimulated production of interleukin-2 (IL-2), gamma interferon (IFN-γ), IL-4, and IL-10 as well as quantitation of mRNA for the same cytokines in unstimulated peripheral blood mononuclear cells were performed at baseline and 2 weeks (t1), 2 months (t2), and 10 months (t3) into therapy. The results showed that HIV-specific (but not mitogen-stimulated) IL-2 and IFN-γ production was augmented and IL-10 production was reduced in patients who received EFV, NFV, and d4T. Therapy was also associated with a reduction in HIV RNA in plasma and an increase in CD4+ cell count. These changes occurred in the first year of therapy (t2 and t3) and were confirmed by quantitation of cytokine-specific mRNA. Therapy with EFV, NFV, and d4T increases HIV-specific type 1 cytokine production as well as CD4 counts and reduces plasma viremia. This therapeutic regimen may be considered for use in cases of advanced HIV infection.
The progression of human immunodeficiency virus (HIV) infection to AIDS is associated with increasing HIV viral load (20) and qualitative and quantitative defects affecting CD4 T lymphocytes and cell-mediated immunity (11, 31, 32, 34, 35). Drugs designed as therapeutic tools against HIV infection should thus ideally be capable of stimulating cell-mediated immunity and reducing HIV viremia. Nevertheless, whereas highly active antiretroviral therapy (HAART) can achieve suppression of HIV viremia in HIV-infected individuals, the efficacy of these antiretroviral therapies in reconstituting immune function has been less dramatic (2, 3, 10, 17, 18, 22, 23, 27, 33).
Nonnucleoside reverse transcriptase inhibitors (NNRTI), nucleoside reverse transcriptase inhibitors (NRTI), and protease inhibitors (PI) are currently used in the treatment of HIV-1 infection (reviewed in references 6, 12, 15, and 30). Efavirenz (EFV) is an NNRTI which in combination with other antiretroviral drugs induces viral suppression as well as increases in CD4 counts. Treatment with EFV may be provided on a once-a-day, no-food-interaction schedule that makes its compliance higher than that of other drugs. Furthermore, although cross-resistance within the NNRTI class is extensive and the presence of point mutations (K103N, Y181C) may exclude the efficacy of the entire class, there is some evidence that EFV may retain full activity against more than 25% of Y181C-mutated viruses (7, 37), even though an NNRTI sequential treatment is still not recommended by international guidelines (28). Because of these interesting pharmacokinetic properties we decided to evaluate the potential usefulness of this compound in advanced HIV infection. Therefore, we investigated immune and virologic parameters in patients undergoing therapy with an EFV-containing regimen. In particular, because progression to AIDS and loss of CD4+ T cells is associated with the impairment of type 1 cytokine production, we verified that EFV-containing antiviral therapies stimulated cell-mediated immunity and a type 1 cytokine profile.
MATERIALS AND METHODS
Patients.
Eighteen HIV-infected patients were randomly selected among those enrolled in a study to evaluate the efficacy of an EFV-, nelfinavir (NFV)-, and stavudine (d4T)-based rescue therapy (32). The only selection bias was the patient's willingness to be included in the study. These patients had failed previous combination therapies (including multiple nucleoside analogues and at least one PI) and they (i) were failing the current PI-containing therapy, (ii) were naive for NNRTIs, (iii) had never received NFV, (iv) had no evidence of active HIV-related infections or neoplasms; and (v) had never been treated with immunomodulants (e.g., interleukin-2 [IL-2]). EFV was administered at a dose of 600 mg daily at bedtime, NFV was given at 750 mg every 8 h, and d4T was given at 30 or 40 mg, depending on body weight, twice daily.
Blood sample collection.
Whole blood was collected by venipuncture immediately before treatment (t0) and 2 weeks (t1) as well as 2 months (t2) and 10 months (t3) after the start of therapy in EDTA Vacutainer tubes (Becton Dickinson & Co., Rutherford, N.J.). Peripheral blood mononuclear cells (PBMC) were separated on lymphocyte separation medium (Organon Teknika Corp., Durham, N.C.) and washed twice in phosphate-buffered saline, and the number of viable leukocytes was determined by trypan blue exclusion. PBMC were resuspended in RPMI 1640 (Sigma, St. Louis, Mo.) plus 2% AB serum (Sigma). All experiments were performed using cryopreserved PBMC.
Antigen- and mitogen-stimulated cytokine production.
Gamma interferon (IFN-γ), IL-2, IL-4, and IL-10 production by PBMC was determined by culturing 3 × 106 PBMC per well in 24-well LINBRO plates (Flow Laboratories, Inc.) (37°C; 7% CO2). PBMC were unstimulated or stimulated with phytohemagglutinin (PHA) (M form; Sigma, St. Louis, Mo.) (final dilution, 1:100) for 48 h or a pool of five synthetic peptides from the gp160 envelope (Env) of HIV type 1 (HIV-1) (8) (2.5 mM final concentration) for 5 days. The following peptides were utilized: T1 (KQIINMWQEVGKAMYA), HIV-1/IIIb Env amino acid residues 428 to 443; T2 (HEDIISLWDQSLK), HIV-1/IIIb Env amino acid residues 112 to 124; TH4.1 (DRVIEVVQGAYRAIR), HIV-1/IIIb Env amino acid residues 834 to 848; p18-IIIb (RIQRGPGRAFVTIGK), HIV-1/III IIIB Env amino acid residues 315 to 329; and p18-MN (RIHIGPGRAFYTTKN), HIV-1 (MN) Env region homologous to p18-IIIB.
Cytokine production was evaluated in the supernatants with commercially available enzyme-linked immunosorbent assays (Endogen, Woburn, Mass.).
RNA extraction.
RNA was extracted from unstimulated lymphocytes with the acid guanidium thiocyanate-phenol-chloroform method and dissolved in RNase-free water; purity was determined by spectrophotometry. Genomic DNA contamination was removed by RNase-free DNase (RQ1 DNase; Promega, Madison, Wis.).
Reverse transcription (RT).
One microgram of RNA was reverse transcribed into first-strand cDNA in a 20-μl final volume containing 1 mM random hexanucleotide primers, 1 mM oligo(dT), and 200 U of Moloney murine leukemia virus reverse transcriptase (Clontech, Palo Alto, Calif.); cytokine mRNA expression was normalized for β-actin cDNA content by semiquantitative PCR (Clontech).
Quantification of cytokine cDNA by PCR.
PCR was performed in 50 μl of reaction mixture containing 10 μl of RT reaction mixture, 1× PCR buffer (20 mM Tris-HCl, 100 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.5% Tween 20, 0.5% Nonidet P-40, 50% glycerol), 200 mM dNTPs, 1.25 U of Taq polymerase (Takara, Otsu, Japan), 0.4 mM β-actin primers, and 0.4 mM (each) cytokines. The following primers were used: IL-4 5′ primer, 5′CGGCAATTTGACCACGGACACCCGTGCATA3′; IL-4 3′ primer, 5′ACGTACTCTGGTTGGCTTCCTTCACAGGACAG3′; IL-10 5′ primer, 5′AAGCTGAGAACCAAGACCCAGACATCAAGGCG3′; IL-10 3′ primer, 5′AGCTAT CCCAGAGCCCCAGATCCGATTTTGG3′; IFN-γ 5′ primer, 5′GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC3′; IFN-γ 3′ primer, 5′CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG3′; tumor necrosis factor alpha (TNF-α) 5′ primer, 5′GAGTGACAAGCCTGTAGCCCATGTTGTAGCA3′; TNF-α 3′ primer, 5′GCAATGATCCCAAAGTAGACCTGCCCAGACT3′; TNF-β 5′ primer, 5′ATGACACCACCTGAACGTCTCTTC3′; TNF-β 3′ primer, 5′GAAGGCTCCAAAGAAGACAGTACT3′. Thermal cycling was performed in a Touchdown Hybaid apparatus (Celbio, Milan, Italy) as follows: initial denaturation at 95°C for 10 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s; and a final amplification step at 72°C for 10 min. PCR products were electrophoresed in a 10% acrylamide gel and stained with 0.5 mg of ethidium bromide per ml; the size of cDNA products was determined by comparison to a DNA size marker, pbR322 (Sigma). The bands on the gels were scanned by transmission densitometry to quantify relative levels of gene expression, and the areas of the peaks were calculated in arbitrary units. To evaluate the relative levels of expression of the target genes in RT-PCR, the value of the internal standard (β-actin) was used as the baseline gene expression of that sample, and the relative value was calculated for the target genes amplified in that reaction.
Statistical analysis.
Statistical analysis was based on a nonparametric Jonckheere-Terpstra test for trends. Data were also analyzed by a nonparametric Kruskal-Wallis test; P values were two sided.
RESULTS
Demographic profile of the study cohort.
The patients enrolled in the protocol (7 females and 11 males) had a mean age of 33.8 years. Four had a diagnosis of AIDS in their past medical history and eight had a CD4+ cell count below 200 cells/μl at the baseline. None of the patients had been immunized with candidate HIV vaccines; patients had previously received a mean of 3.6 NRTI and 2 PI. All of the patients were tested at the time points shown in the text.
CD4+ cell counts progressively increased in all individuals after the initiation of therapy. Thus, mean (range) CD4 cell counts were as follows: t0, 212 (115-328) CD4 cells/μl; t1, 227 (131- 350) CD4 cells/μl; t2, 291 (174-444) CD4 cells/μl; t3, 399 (312-497) CD4 cells/μl. The change was significant (P = 0.011) when the baseline value was compared with the value observed after 10 months of therapy (t3). HIV genomic RNA was detectable in all patients at the baseline (mean, 109,217; range, 62,488-198,714 copies/μl) and decreased during therapy in all cases (t1 mean, 104,100; range, 58,227-196,515 copies/μl; t2 mean, 39,744; range, 21,057-74,480 copies/μl; t3 mean, 11,518; range, 499 to 15,511 copies/μl). HIV RNA at t3 was undetectable for 12 of 18 subjects. A trend toward a greater increase in CD4 counts was seen in the 12 patients who achieved suppression of plasma viremia below the limit of detection.
Modulation of immune functions.
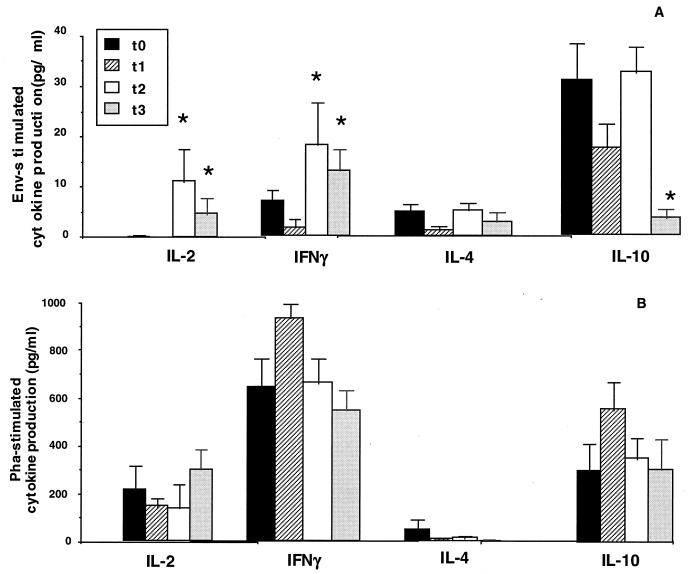
Env-stimulated production of IL-2 and IFN-γ was augmented whereas IL-10 production was reduced in the patients. These results (means ± standard errors) are shown in Fig. 1. Thus, Env-stimulated IL-2 and IFN-γ production was significantly increased 2 months after initiation of therapy (t2); the increase was still observed at t3 (P < 0.01 in all cases). IL-10 production was significantly diminished at the last time point (t3) (P < 0.01) (Fig. 1A). Mitogen-stimulated IL-2 production was marginally improved at t3; the production of both IFN-γ and IL-10 upon mitogen stimulation was augmented, albeit not significantly, at t1 and t2 and returned to baseline by the end of the study period (Fig. 1B).
FIG. 1.
(A) Env-stimulated cytokine production (IL-2, IFN-γ, IL-4, and IL-10) by PBMC of HIV-infected individuals at different time points (before treatment [t0] and 2 weeks [t1], 2 months [t2], and 10 months [t3] into therapy). The results are shown as means ± standard errors. (B) PHA-stimulated production of the same cytokines. *, values are significantly different from those at t0.
Modulation of cytokine mRNA.
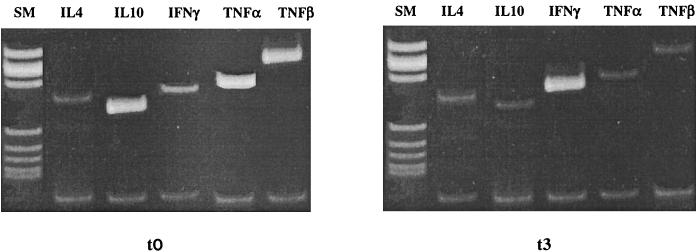
Cytokine-specific mRNA was quantified in resting PBMC of all patients at the beginning and after 10 months of therapy (cytokine mRNA could not be examined at t1 and t2 because of limitations in the number of available PBMC). Because TNF-α exerts a direct effect on viral replication and TNF-β is suggested to be a mediator of apoptotic cell death in HIV infection, the expression of these two cytokines was measured as well. Results showed that after 10 months of therapy, (i) the expression of IFN-γ was higher and (ii) the expression of IL-10, IL-4, TNF-α, and TNF-β was lower. IL-2 was not detectable in resting PBMC (Fig. 2 and Table 1).
FIG. 2.
Quantification of mRNA for IL-4, IL-10, IFN-γ, TNF-α, and TNF-β in resting PBMC of one representative HIV-infected individual before (t0) and after 10 months of (t3) therapy. The upper bands show cDNA retrotranscribed from cytokine mRNA extracted from patient PBMC; the lower bands show cDNA retrotranscribed from β-actin mRNA extracted from patient PBMC and used to normalize the PCRs.
TABLE 1.
Cytokine-specific mRNA in HIV-infected patients undergoing a rescue therapy
| Time point | Cytokine-specific mRNA (cytokine/β-actin ratio) for:a
|
||||
|---|---|---|---|---|---|
| IL-4 | IL-10 | IFN-γ | TNF-α | TNF-β | |
| t0 | 2.71 (1.5-3.4) | 3.08 (1.3-3.4) | 2.77 (1.2-4.2) | 15.15 (5.1-19.5) | 2.83 (0.7-3.3) |
| t3 | 0.2 (0.1-1.2)∗ | 0.3 (0.2-1.1)∗ | 4.61 (1.7-12.8) | 5.2 (3.2-12.2)∗ | 0.55 (0.3-1.3) |
Mean values (ranges) are shown at baseline and after 10 months of therapy. ∗, values are significanly different from those at t0 (P < 0.05).
DISCUSSION
The activities of an EFV-, NFV-, and d4T-based therapy on immunologic and virologic parameters were evaluated by analyzing the effects of this association in 18 randomly selected, HIV-infected, heavily pretreated individuals who failed previous therapeutic regimens (32). The combination was well tolerated, resulted in increased CD4 cell counts and reduced HIV plasma viremia, and at the same time induced a positive trend in HIV-specific type 1 cytokine production as well as a significant reduction in the generation of IL-10. To verify if the variations in cytokine production were secondary to modifications in their patterns of expression, the amount of cytokine-specific mRNA was analyzed in resting PBMC. The results showed that IFN-γ mRNA was more abundant while IL-10 and IL-4 mRNAs were expressed less after 10 months of therapy. Because the generation of type 1 and type 2 cytokines is cross-regulatory, it is not surprising that during follow-up the decreased expression and production of IL-10 coincided with an augmented generation of IL-2 and IFN-γ. Env-stimulated IL-2 and IFN-γ production increased before down modulation of IL-10 production could be observed. A speculative hypothesis could be that the drug regimen studied here preferentially stimulates type 1 cytokine production and that the increase of these cytokines results in the subsequent cross-regulation of IL-10 production. TNF-α expression was reduced in this study. Because TNF-α directly stimulates viral replication (13), the modulation of this cytokine could contribute to the reduction of HIV viral load observed in our patients. CD4 cell counts progressively improved concomitantly with a reduction of TNF-β expression in the study presented here. Because TNF-β is known to be a mediator of apoptotic cell death in HIV infection (9, 19) it is tempting to speculate that increases in CD4 counts are at least partially secondary to a reduced susceptibility of CD4 cells to apoptosis.
The analysis of the efficacy data relative to the entire cohort of patients showed that plasma viral load was reduced by 1.7 log at 12 months with a sustained increase in CD4+-T-cell count (21). HIV plasma viremia was undetectable in 12 of the 18 patients after 1 year of therapy. In light of recent data suggesting that HIV-specific T-cell function would not be recovered in patients with fully suppressed viral replication (reviewed in reference 4), we compared Env-specific cytokine production in patients in whom viremia was or was not still detectable after therapy. We did not observe any correlation between residual viremia and improvement in immune function. This result can probably be explained considering the small number of individuals studied and the fact that our patients had advanced illness and had failed previous combination therapies. It is interesting to observe that modulation of HIV-specific immune responses was detected in our patients within a few months. These results contrast with recent data showing that improvement in HIV-specific immunity may not be seen in the first year of antiviral therapy (1, 4). The discrepancy could be secondary to the fact that, whereas we measured Env-specific responses, Angel et al. (1) and Blankson et al. (5) stimulated PBMC with Gag or Pol. Numerous studies showed that HAART positively influences quantitative and qualitative immune parameters in both acute and chronic HIV infection, but the immune reconstitution that ensues nevertheless is never complete (2, 3, 10, 17, 22, 23, 27, 33). Thus, triple therapy is associated with improvement in CD4 counts and functions that are more easily seen in early- than in late-stage patients (3, 5, 16, 17, 20, 21, 22, 24, 25, 26, 29, 36; M. Albrecht, D. Katzenstein, R. Bosch, and S. Liou, Abstr. 7th Conf. Retrovir. Opportunistic Infect., abstr. 531, 2000). Our data show that partial modulation of immune functions in chronic HIV infection can be seen even in patients who had previously failed multiple regimens.
NNRTIs, in addition to NRTIs and PIs, have gained a definite place in the treatment of HIV-1 infection (reviewed in references 6, 12, 15, and 30). EFV is an NNRTI which is used in combination with other antiviral compounds in the treatment of HIV infection and is shown to knock out virus replication and to delay virus resistance from arising (37; Albrecht et al., 7th Conf. Retrovir. Opportunistic Infect.). Treatment guidelines were recently modified to include NNRTI as an acceptable substitute for a PI in primary therapy (5). Results stemming from a number of protocols in which this therapeutic approach was used in HIV-infected individuals confirmed that NNRTI-including regimens are capable of achieving a very consistent suppression of HIV plasma viremia. For the 006 study participants were randomized to receive EFV in association either with nucleoside analogues or with PIs. The results showed that suppression of plasma HIV-1 RNA to undetectable levels was achieved in a higher percentage of patients in the NNRTI plus nucleoside analogues group than in the PI plus nucleoside analogues group (36). In the ACTG 302 study NNRTI- and PI-naive patients who had previously failed an NRTI therapy were randomized to receive EFV, NFV, or both drugs in combination to 1 or 2 NRTIs. By week 40 the percentage of individuals with a viral load below the detection limit was significantly higher in the EFV- than in the NFV-based arms (Albrecht et al., 7th Conf. Retrovir. Opportunistic Infect.). Finally, recent data comparing the virologic effect of NNRTI- and PI-based antiretroviral combinations suggest that the use of NNRTI is associated with a faster and more frequent initial virologic response (14, 23).
In conclusion, our results suggest that NNRTI-employing antiviral therapies could be useful as a rescue regimen in advanced cases of HIV infection. Because these data are based on a small group of patients and because the design of this preliminary study did not involve a control arm, further studies will be necessary to clarify the best way to utilize this strategy in advanced HIV disease.
Acknowledgments
This paper was supported by grants from Istituto Superiore di Sanità “III Programma Nazionale di Ricerca sull' AIDS 1999.”
REFERENCES
- 1.Angel, J. A. B., K. G. Parato, A. Kumar, S. Kravcik, A. D. Badley, C. Fex, D. Ashby, E. Sun, and D. W. Cameron. 2001. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J. Infect. Dis. 185:546-554. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J. A. B., A. Kumar, A. Parato, L. G. Filion, F. Diaz-Mitoma, P. Daftarian, B. Pham, E. Sun, J. M. Leonard, and D. W. Cameron. 1998. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J. Infect. Dis. 177:898-904. [DOI] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 4.Autran, B., and G. Carcelain. 2000. Boosting the immunity to HIV. Can the virus help? Science 290:946-949. [DOI] [PubMed] [Google Scholar]
- 5.Blankson, J. N., J. E. Gallant, and R. F. Siliciano. 2001. Proliferative responses to human immunodeficiency virus type 1 antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657-661. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter, C. C., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzardn, S. M. Hammern, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA Panel. JAMA 283:381-390. [DOI] [PubMed] [Google Scholar]
- 7.Casado, J. L., K. Hertogs, L. Ruiz, F. Dronda, A. Van Cauwenberge, A. Arno, I. Garcia-Arata, S. Bloor, A. Bonjoch, J. Blazquez Clotet, and B. Larder. 2000. Non-nucleoside reverse transcriptase inhibitor resistance among patients failing a nevirapine plus protease inhibitor-containing regimen. AIDS 14:F1-F7. [DOI] [PubMed] [Google Scholar]
- 8.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. C. Bernstein, D. L. Mann, G. M. Shearer, and J. A. Berzofsky. 1989. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature 339:383-385. [DOI] [PubMed] [Google Scholar]
- 9.Clerici, M., A. Sarin, J. A. Berzofsky, A. L. Landay, H. A. Kessler, F. Hashemi, C. W. Hendrix, S. P. Blatt, J. Rusnak, M. J. Dolan, R. L. Coffman, P. A. Henkart, and G. M. Shearer. 1996. Antigen-stimulated CD4+ T cell death in HIV infection is selective for CD4+ T cells, regulated by cytokines and effected by lymphotoxin. AIDS 10:603-611. [DOI] [PubMed] [Google Scholar]
- 10.Clerici, M., E. Seminari, F. Suter, F. Castelli, A. Pan, M. Biasin, F. Colombo, D. Trabattoni, F. Maggiolo, G. Carosi, and R. Maserati. 2000. Different immunologic profiles characterize HIV infection in highly active antiretroviral therapy-treated and antiretroviral-naive patients with undetectable viraemia. AIDS 14:109-116. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, O. J., A. Kinter, and A. S. Fauci. 1997. Host factors in the pathogenesis of HIV disease. Immunol. Rev. 159:31-48. [DOI] [PubMed] [Google Scholar]
- 12.de Jong, M. D., C. A. Boucher, S. A. Danner, B. Gazzard, P. D. Griffiths, C. Katlama, J. M. Lange, D. D. Richman, and S. Vella. 1998. Summary of the international consensus symposium on management of HIV, CMV and hepatitis virus infections. Antiviral Res. 37:1-16. [DOI] [PubMed] [Google Scholar]
- 13.Emilie, D., M. C. Maillot, J. F. Nicolas, R. Fior, and P. Galanaud. 1992. Antagonistic effect of IFN gamma on tat-induced transactivation of HIV by long terminal repeat. J. Biol. Chem. 267:20565-20570. [PubMed] [Google Scholar]
- 14.Fiedl, A. C., B. Ledergerber, M. Flepp, B. Hirschel, A. Telenti, H. Furrer, H. C. Bucher, E. Bernasconi, and R. Webb for the Swiss HIV Cohort Study. 2001. Response to first protease inhibitor- and efavirenz-containing antiretroviral combination therapy. AIDS 15:1793-1800. [DOI] [PubMed] [Google Scholar]
- 15.Flexner, C. 1998. HIV-protease inhibitors. N. Eng. J. Med. 338:1281-1292. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, G. R., M. Bloch, J. J. Zaunders, D. Smith, and D. A. Cooper. 2000. Long-term immunological response in HIV-1 infected subjects receiving potent antiretroviral therapy. AIDS 14:959-969. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher, A. D., A. Carr, J. Zaunders, and D. A. Cooper. 1996. Alterations of the immune response of HIV-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J. Infect. Dis. 173:321-329. [DOI] [PubMed] [Google Scholar]
- 18.Kelleher, A. D., L. Al-Harti, and A. L. Landay. 1997. Immunological effects of antiretroviral and immune therapies for HIV. AIDS 11:S149-S155. [PubMed] [Google Scholar]
- 19.Lazdins, J. K., M. Grell, M. R. Walker, K. Woodscook, P. Scheurich, and K. Pfizenmaier. 1997. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFr60 and TNFr80 favors induction of cell death rather than virus production in HIV-infected T cells. J. Exp. Med. 185:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lederman, M. M., E. Connick, A. Landay, D. R. Kuritzkes, J. Spritzler, M. St. Clair, B. L. Kotzin, L. Fox, M. H. Chiozzi, J. M. Leonard, F. Rousseau, M. Wade, J. D. Roe, A. Martinez, and H. Kessler. 1998. Immunologic responses associated with 12 weeks of combination therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS clinical trial group protocol 315. J. Infect. Dis. 178:70-79. [DOI] [PubMed] [Google Scholar]
- 21.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T cell function and viral load reduction after HAART in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 22.Martinon, F., C. Michelet, M. Peguillet, Y. Taoufik, P. Lefebvre, C. Goujard, J. Guillet, J. Delfraissy, and O. Lantz. 1999. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after 1 year of highly active antiretroviral therapy. AIDS 13:185-194. [DOI] [PubMed] [Google Scholar]
- 23.Matthews, G. V., C. A. Sabin, S. Mandalaia, F. Lampe, A. N. Phillips, M. R. Nelson, M. Bower, M. A. Johnson, and B. G. Gazzard. 2002. Virological suppression at 6 months is related to choice of initial regimen in antiretroviral naive patients: a cohort study. AIDS 16:53-61. [DOI] [PubMed] [Google Scholar]
- 24.Pakker, N. G., M. T. L. Roos, and R. van Leeuwen. 1997. Patterns of T cell repopulation, viral load reduction, and restoration of T cell function in HIV-infected persons during therapy with different antiretroviral agents. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:318-326. [DOI] [PubMed] [Google Scholar]
- 25.Pialoux, G., F. Raffi, F. Brun-Vezinet, V. Meiffredy, P. Flandre, J. A. Gastaut, P. Dellamonica, P. Yeni, J. F. Delfraissy, and J. P. Aboulker. 1998. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. N. Engl. J. Med. 339:1269-1276. [DOI] [PubMed] [Google Scholar]
- 26.Plana, M., F. Garcia, T. Gallart, C. Tortajada, A. Soriano, E. Palou, M. J. Maleno, J. J. Barcelo, C. Vidal, A. Cruceta, J. M. Miro, and J. M. Gatell. 2000. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS 14:1921-1933. [DOI] [PubMed] [Google Scholar]
- 27.Pontesilli, O., S. Grade-Kerkhof, F. W. Noterman, N. A. Foudraine, M. T. L. Roos, M. R. Klein, S. A. Danner, J. M. A. Lange, and F. Miedema. 1999. Functional T cell reconstitution and HIV-1-specific cell-mediated immunity during highly active antiretroviral therapy. J. Infect. Dis. 180:76-86. [DOI] [PubMed] [Google Scholar]
- 28.Pozniak, A. L. 2000. Why switch from protease inhibitors (PI) to non-nucleoside reverse transcriptase inhibitors (NNRTI)? HIV Med. 1:7-10. [DOI] [PubMed] [Google Scholar]
- 29.Reijers, M. H., G. J. Weverling, S. Jurriaans, M. T. Roos, F. W. Wit, H. M. Weigel, R. W. Ten Kate, J. W. Mulder, C. Richter, H. J. Ter Hofstede, H. Sprenger, R. M. Hoetelmans, H. Schuitemaker, and J. M. Lange. 1998. Maintenance therapy after quadruple induction therapy in HIV-1 infected individuals. Amsterdam Duration of Antiretroviral Medication (ADAM) study. Lancet 352:185-190. [DOI] [PubMed] [Google Scholar]
- 30.Richman, D. D. 1996. HIV therapeutics. Science 272:1886-1888. [DOI] [PubMed] [Google Scholar]
- 31.Saag, M. S., M. Holodniy, D. R. Kuritzkes, W. A. O'Brien, R. Coombs, M. E. Poscher, D. M. Jacobsen, G. M. Shaw, D. D. Richman, and P. A. Volberding. 1996. HIV viral load markers in clinical practice. Nat. Med. 2:625-629. [DOI] [PubMed] [Google Scholar]
- 32.Seminari, E., F. Maggiolo, P. Villani, F. Suter, and R. Maserati. 1999. Efavirenz, nelfinavir and stavudine rescue combination therapy in HIV-1 positive patients heavily pretreated with nucleoside analogues and protease inhibitors. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 22:453-460. [DOI] [PubMed] [Google Scholar]
- 33.Seth, A., J. Markee, A. Hoering, A. Sevin, D. E. Sabath, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, M. S. Hirsch, A. C. Collier, N. L. Letvin, and M. J. McElrath. 2001. Alterations in T cell phenotype and HIV-1 specific cytotoxicity after potent antiretroviral therapy. J. Infect. Dis. 183:722-729. [DOI] [PubMed] [Google Scholar]
- 34.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Immunity 9:587-593. [DOI] [PubMed] [Google Scholar]
- 35.Shearer, G. M., and M. Clerici. 1998. Cytokine profiles in HIV type 1 disease and protection. AIDS Res. Hum. Retrovir. 14:S49-S52. [PubMed] [Google Scholar]
- 36.Staszewski, S., J. Morales-Ramirez, K. T. Tashima, A. Rachlis, D. Skiest, J. Stanford, R. Stryker, P. Johnson, D. F. Labriola, D. Farina, D. J. Manion, N. M. Ruiz, et al. 1999. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N. Engl. J. Med. 341:1865-1873. [DOI] [PubMed] [Google Scholar]
- 37.Winslow, D. L., S. Garber, C. Reid, H. Scarnati, D. Baker, M. M. Rayner, and E. D. Anton. 1996. Selection conditions affect the evolution of specific mutations in reverse transcriptase gene associated with resistance to DMP-266. AIDS 10:1205-1209. [DOI] [PubMed] [Google Scholar]