As the use of imaging studies in medicine increases, so does the discovery of unexpected pathologic findings. One of the more common unexpected findings revealed by computed tomography (CT), magnetic resonance imaging (MRI), or ultrasonography is an adrenal mass, called an incidental adrenal mass, or incidentaloma (Figure 1).
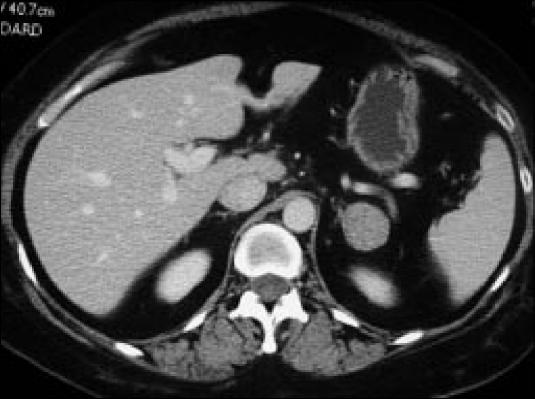
Figure 1.
A characteristic incidentaloma. This is an encapsulated solid lesion in the left adrenal gland. Final diagnosis cannot be made without further workup.
The incidence of adrenal masses found on abdominal CT scans is between 0.6% and 1.3%. The incidence of these masses on all CT scans, including thoracic, abdominal, and pelvic, is between 0.4% and 4.4%. The indication for the CT scan varies (Table). In contrast, the incidence of adrenal masses at autopsy in patients who had no evidence of adrenal disease is between 1.4% and 9%.
Table.
Indications for computed tomography scans in which an incidentaloma was found*
| Indication | Series 1 (%) | Series 2 (%) | Series 3 (%) |
| Abdominal pain | 60 | 17 | 6 |
| Urologic complaints | 20 | 3 | 4 |
| Suspicion of abscess | 10 | — | 15 |
| Splenomegaly | 5 | — | — |
| Nonspecific symptoms | 5 | 30 | — |
| Increased blood pressure | — | 41 | — |
| Back pain | — | 3 | — |
| Pancreatitis | — | — | 9 |
| Trauma | — | — | 24 |
| Vascular (aneurysm) | — | — | 6 |
| Oncologic evaluation | — | — | 31 |
| Other | 6 | 5 |
It is difficult to answer a patient's questions when a mass is found. This article attempts to simplify the workup and direct the evaluation of the mass. It begins by discussing the differential diagnosis and imaging studies that can be used and then comments on several hyperfunctioning and malignant adrenal masses.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of the adrenal mass comprises a long list: adenoma, myelolipoma, cyst, lipoma, pheochromocytoma, adrenal cancer, metastatic cancer, hyperplasia, and tuberculosis (3). More than half of incidentalomas are benign or hyperfunctioning adenomas that need evaluation. Myelolipomas, cysts, and lipomas are characteristic lesions on CT scans. Pheochromocytomas are often evident from the clinical picture and discussion with the patient. The most difficult single adrenal masses to diagnose are adrenal cancer and metastatic cancer because they can often mimic adenomas at smaller sizes.
IMAGING STUDIES
Evaluation of the mass usually begins with further imaging studies, although fineneedle aspiration (FNA) biopsy and cytology are also available. In addition, the differences in discrete masses of the adrenal gland can be biochemically evaluated. Adrenal vein sampling is also available but is not often used.
The imaging studies that are most commonly and most effectively used to identify and discriminate the different types of adrenal masses are noncontrast CT, MRI, and adrenal scintigraphy.
Noncontrast CT
Cysts, myelolipomas, and adrenal hemorrhage, which can be found incidentally on CT scans, have diagnostic features on a noncontrast CT scan (Figure 2). They look nothing like an adenoma or carcinoma, so these diagnoses can be excluded. Non-surgical management is usually indicated unless they are large myelolipomas or painful, symptomatic cysts. Benign adenomas are usually homogeneous lesions with smooth, regular, encapsulated borders; they are discrete masses. Adrenal carcinomas typically look different from adenomas, but until they are large, it is hard to differentiate between the two. Adrenal carcinomas are typically inhomogeneous lesions with irregular borders; they may have evidence of local invasion or associated lymphadenopathy.
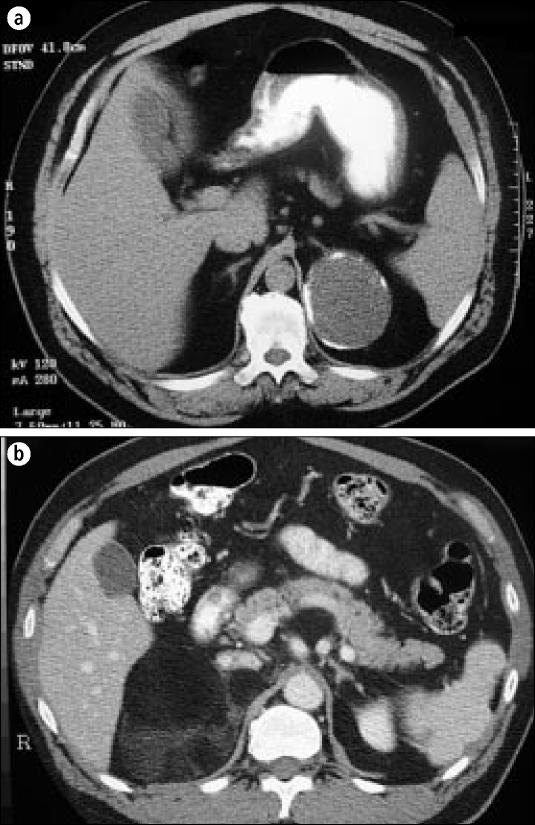
Figure 2.
(a) A left adrenal cyst with a fluid-filled cavity and calcified rim. (b) A right myelolipoma with predominantly lipid density.
Radiologists are constantly seeking ways to differentiate adenomas from carcinomas on CT scan. The most recent method uses Hounsfield units. The density of the lesion or mass found on the CT scan is compared against the density of water, which is assigned the value of 0 Hounsfield units. Adenomas typically have low attenuation values of ≤10 units; some studies report values up to 14 units, but <10 units almost certainly indicates an adenoma (3). CT scans, however, cannot determine if a mass is hyperfunctioning vs nonfunctioning or benign vs malignant.
Carcinomas are typically >18 units, although 15% of benign masses evaluated by measuring Hounsfield units on nonenhanced CT scans will be misinterpreted as malignant. A small percentage (<1%) of malignant lesions will be misinterpreted as benign.
MRI
MRI is also a good method for evaluating adrenal masses. It avoids exposing the patient to ionizing radiation, but it is more expensive than CT. Adenomas usually have a low signal on T2-weighted images, which is the major sequence used for adrenal pathology. Pheochromocytomas have a brighter signal and are easier to see on these T2-weighted sequences (Figure 3).
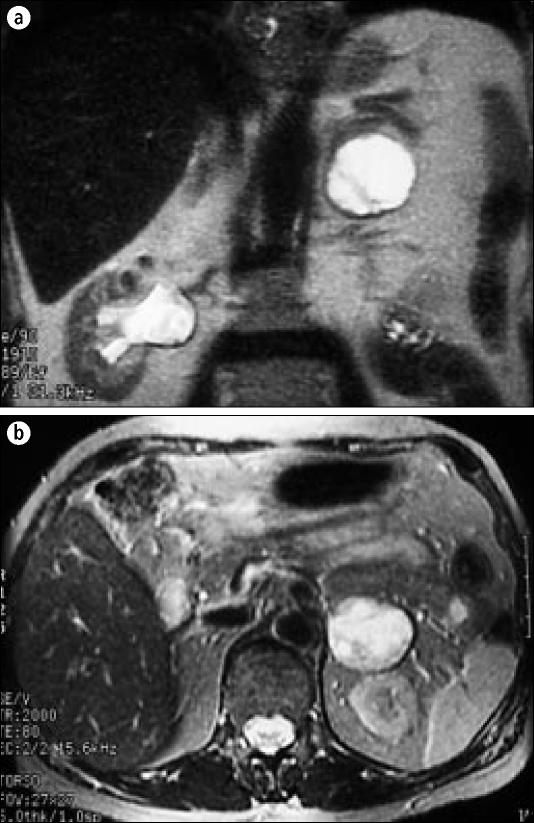
Figure 3.
A characteristic (a) coronal and (b) axial MRI T2-weighted sequence of a pheochromocytoma. The left adrenal mass is very bright and easy to identify.
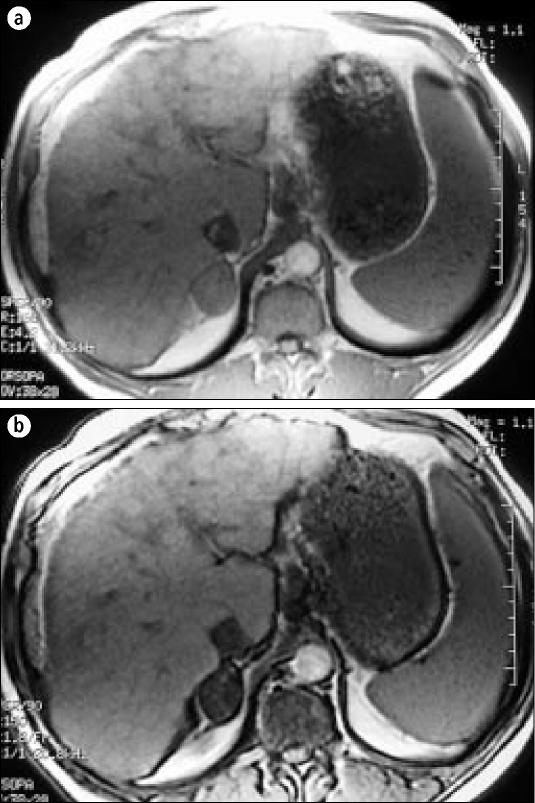
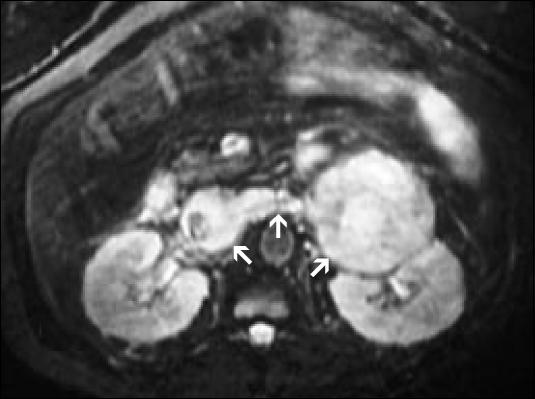
Recently, in-phase/opposed-phase, chemical-shift MRIs have been used to discriminate between benign and malignant lesions. Benign lesions, which have a high lipid content, typically show a loss of signal intensity, meaning they darken on the chemical-shift, opposed-phase sequences. In contrast, malignant lesions show no loss of signal intensity (Figure 4). In one study, the finding of signal loss on opposed-phase images was 100% specific for an adenoma (4).
Figure 4.
In-phase and (b) opposed-phase chemical-shift MRI. On the opposed-phase chemical shift, the right adenoma darkens—a finding 100% specific for a benign adenoma.
Adrenal scintigraphy
Adrenal scintigraphy is another way of discerning benign and malignant lesions. There are 2 types, metaiodobenzylguanidine (MIBG) scintigraphy and 1-6-beta-iodomethyl-norcholesterol (NP-59) scintigraphy.
MIBG scans are used specifically for pheochromocytomas or any tissues that take up MIBG in the adrenergic vesicles in the adrenal medulla. The normal adrenal medulla has too few vesicles and therefore not enough uptake to produce an image. An early amine precursor uptake and decarboxylation tumor, however, has extra vesicles and will take up enough MIBG to produce an image on scintigraphy. MIBG scintigraphy is useful for localizing recurrent tumors or multiple extraadrenal pheochromocytomas in different places if they are suspected.
Unlike other imaging, such as MRI, MIBG scintigraphy detects extraadrenal disease and indicates whether the tumor is functioning. The disadvantages of MIBG scans are radiation exposure, higher cost, and limited availability. The sensitivity ranges from 77% to 89%, and specificity ranges from 88% to 100% for diagnosing a pheochromocytoma.
The NP-59 study uses radiolabeled iodine attached to a norcholesterol, which is taken up by the adenomatous or lipid-containing lesions and not by nonadenomatous or malignant lesions. In a series at the University of Michigan, all lesions that took up NP-59 were adenomas (5). However, NP-59 scintigraphy cannot determine the difference between a hyperfunctioning and benign mass.
A patient given NP-59 must wait 5 to 7 days before the mass can be imaged. As with MIBG, NP-59 is a radiolabeled iodine, so potassium iodide must be given orally for 1 week before and after imaging to block thyroid uptake of the agent. The waiting period and extra medications are inconvenient for the patient. Other limitations are that an institutional review board permit is needed and that NP-59 scintigraphy is available only from the University of Michigan.
FNA biopsy, adrenal vein sampling, and biochemical testing
FNA biopsy is another useful tool in distinguishing adrenal masses. However, it is difficult to distinguish among normal adrenal tissue, adenoma, and well-differentiated carcinoma. Before performing an FNA biopsy, it is important to exclude pheochromocytoma to prevent a hypertensive crisis or worse. FNA biopsy is usually reserved for patients with known extraadrenal malignancy when tissue diagnosis of the adrenal metastasis is necessary to guide therapy. In this setting, it is almost 100% accurate.
Adrenal vein sampling is not done very often. It has a high complication rate of pneumothorax, pancreatitis, and hemorrhage and is indicated only when the physician is trying to rule out idiopathic hyperaldosteronism, which is treated by removing both glands. In these cases, adrenal vein sampling can distinguish separate bilateral from unilateral secretion of aldosterone.
A variety of biochemical tests can be performed after an adrenal mass is found, whether the mass is biochemically active or not. Tests can determine levels of dexamethasone suppression; 24-hour urinary free cortisol; urinary 17-hydroxycortisol and 17-ketosteroid; plasma androgens, testosterone, androstenedione, renin, and aldosterone; urinary vanillylmandelic acid; plasma catecholamines; and plasma metanephrine and normetanephrine.
ALDOSTERONOMA
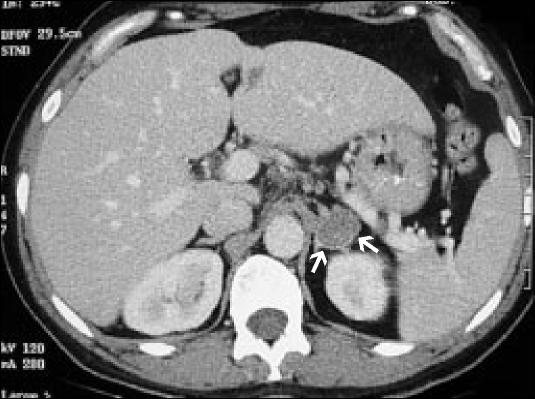
The first type of hyperfunctioning adrenal adenoma is an aldosterone-producing tumor, or aldosteronoma (Figure 5), which accounts for up to 2% of all incidentally discovered adrenal masses. Aldosteronoma is the primary cause of hyperaldosteronism (70%–80% of cases), which is a syndrome of hypertension and hypokalemia. Hypokalemia may be masked when the patient is on a low-sodium diet, so an aldosteronoma should be considered in a hypertensive patient who needs an evaluation for an adrenal mass.
Figure 5.
A typical well-encapsulated aldosteronoma of the left adrenal gland. It is benign but is producing aldosterone.
A characteristic aldosteronoma has a speckled appearance, is well encapsulated, and looks benign. Most aldosteronomas are small (average, 1–2 cm); therefore, they are usually determined first clinically and then are located by CT. To evaluate a patient who has an adrenal mass and hypertension and/or hyperkalemia, the physician should measure plasma aldosterone concentration and plasma renin activity (after the patient has been upright for at least 2 hours). A ratio of plasma aldosterone to plasma renin activity >20 mg/mL/hr is highly suggestive of primary hyperaldosteronism. Patients with an elevated plasma aldosterone-to-renin ratio should undergo testing of 24-hour urinary aldosterone secretion after saline loading or while on a high-sodium diet. Before testing, the patient should be off spironolactone for 6 weeks, diuretics for 4 weeks, and sympathetic inhibitors for 2 weeks.
CORTISOL-PRODUCING ADENOMA
The second type of hyperfunctioning adrenal tumor is a cortisol-producing adenoma. Between 2% and 15% of incidentalomas produce cortisol. All patients who have adenomas should be biochemically screened for hypercortisolism before being treated.
Cushing syndrome is produced by hypercortisolism and includes hypertension, weight gain, easy bruisability, diabetes, and centripetal obesity. Up to 20% of cases of Cushing syndrome are caused by hypersecretory adenomas; this is in contrast to bilateral adrenal hyperplasia and pituitary-based hypercortisolism, which are the other causes of Cushing syndrome.
In the patient with an adrenal mass, a low-dose dexamethasone suppression test is a good screening tool. The patient is given 1 mg of dexamethasone at 11:00 pm, and the cortisol level is checked the next morning at 8:00. If the level is >3 (μg/dL, the patient has hypercortisolism and needs further evaluation. It is important to analyze diurnal rhythm; some people have a constant secretion of cortisol and can have subclinical Cushing syndrome. Measurements of 24-hour free cortisol and plasma adrenocorticotropic hormone levels will indicate whether the mass is pituitary or adrenal based. If the patient has a positive dexamethasone suppression test and a low adrenocorticotropic hormone level, the cause of cortisol suppression is adrenal based and not pituitary based.
Small adrenal adenomas can be associated with a subclinical Cushing syndrome secondary to either mildly supraphysiologic cortisol production or a constant secretion of cortisol. It is important to identify these patients for 2 reasons. First, if the adenoma is undetected, the patient can develop serious osteoporosis and the resulting complication of secondary pathological fractures. Second, a patient can develop sudden severe adrenal insufficiency after a cortisol-producing mass is removed.
PHEOCHROMOCYTOMA
Pheochromocytomas fit into the functioning adenoma category of adrenal masses. Up to 11 % of all incidentally found adrenal masses are pheochromocytomas. Characteristic symptoms are episodic hypertension associated with spells of headache, sweating, palpitations, anxiety, pallor, and tremors. Syndromes that can suggest a pheochromocytoma are von Recklinghausen (neurofibromatosis and café au lait spots), von Hippel-Lindau disease (retinal angiomas), and multiple endocrine neoplasm II (medullary thyroid carcinoma). If a patient has these syndromes, urine catecholamines and metanephrines in a 24-hour collection should be measured, and then CT or MRI scans should be checked for a pheochromocytoma. As shown earlier, the pheochromocytoma shows bright heterogeneous signal intensity on T2-weighted MRI and is easy to detect.
Abnormal levels of plasma catecholamines (>2000 pg/mL) are diagnostic for a pheochromocytoma, as are urinary metanephrines >1.6 mg/24 hr. Often catecholamines are between 1000 and 2000 pg/mL. In this case, a clonidine suppression test can be used. The patient is given 15 mg of clonidine; if the cat-echolamine level is still elevated, even in the gray zone, the test is positive, and the patient most likely has a pheochromocytoma.
ADRENOCORTICAL CARCINOMA
Adrenocortical carcinomas are rare; the incidence is 1 in 1.7 million. When an incidental adrenal mass is found, however, the incidence of carcinoma is between 0% and 25%. A recent metaanalysis of 6 published series reported an incidence of 4% (6), and a Mayo Clinic series of 342 adrenal masses had an incidence of 1.2% for adrenocortical carcinoma (7).
Characteristic adrenocortical carcinomas are large with irregular borders on CT (Figure 6). Size is important in diagnosing these tumors. By the time they are found, they are usually 12 cm in diameter. Over 90% of adrenocortical carcinomas are >6 cm at presentation. However, in one series, 16% of the carcinomas were <5 cm in diameter (8), so there may be a risk of missing the smaller carcinomas if only masses >6 cm are removed.
Figure 6.
A left adrenocortical carcinoma with renal vein and inferior vena cava invasion on a T2-weighted MRI.
If the CT scan does not indicate that an adrenal mass is an adrenocortical carcinoma, a dehydroepiandrosterone sulfate level should be obtained. This test can indicate, but not diagnose, a carcinoma. Inhomogeneous masses with irregular margins that enhance after administration of intravenous contrast on CT and have increased signal intensity with T2-weighted MRI strongly indicate an adrenocortical carcinoma. If uncertainty remains, an FNA biopsy, if taken from a good sample, will look different than normal adrenal tissue on cytology.
ADRENAL METASTASIS
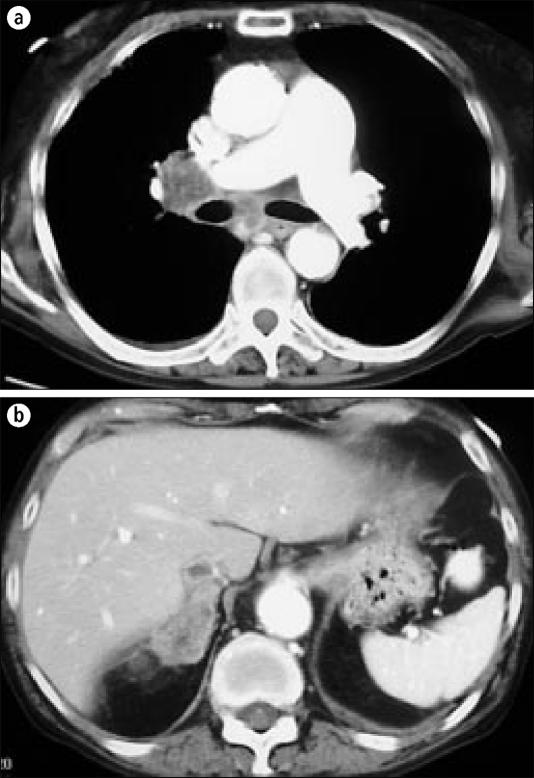
The final adrenal mass that can be confused with a benign mass is adrenal metastasis. Metastases are the second most common cause (up to 21%) of adrenal incidentalomas. At autopsy, 8% to 38% of patients with extraadrenal malignancies will have metastasis to the adrenal gland (9). The primary tumors that most often metastasize to the adrenal gland are breast, lung (Figure 7), and renal cell carcinomas, as well as multiple myeloma and lym-phoma.
Figure 7.
(a) Chest CT scan with right hilar and mediastinal lymphadenopathy in a patient with squamous cell lung cancer, (b) Images through the abdomen demonstrate a right adrenal mass, proven to be a squamous cell cancer metastasis.
The finding of a unilateral adrenal metastasis in an otherwise disease-free patient should prompt consideration for surgical resection. Radiologic examination should be performed to exclude other metastatic disease, and FNA biopsy should be performed to confirm that the mass is a metastasis. However, in the absence of clinically apparent endocrine disease, adrenal masses identified in the setting of obvious metastatic disease in other sites require no further workup.
Any patient with both a hyperfunctioning adrenal mass of any size and proof of hormone hypersecretion should have the mass removed. Opinion varies, however, when the mass is non-functioning and has not been proven to be cancerous. The standard is that any mass >6 cm should be removed. Copeland was first to estimate a threshold diameter of 6 cm; at this size, approximately 60 adrenalectomies would need to be performed to remove 1 adrenocortical carcinoma (10). In Herrera's series from 1991, removal of all lesions >4 cm resulted in a benign/malignant ratio of 8:1 (7). Recently, Thompson recommended that healthy patients who were <60 years old with lesions >3 cm should have their masses removed (11).
SUMMARY
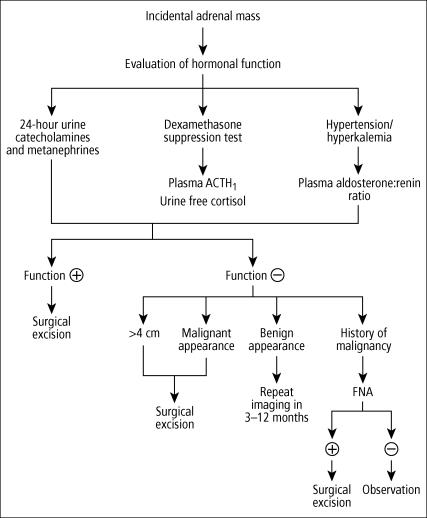
Incidentally discovered adrenal masses are becoming more common with the increased number of imaging studies that are performed. Each of these masses deserves attention, and appropriate radiographic and biochemical evaluation is essential and often directed by clinical findings (Figure 8).
Figure 8.
Algorithm for managing adrenal incidentalomas. ACTH indicates adreno-corticotropic hormone; FNA, fine-needle aspiration.
A patient with a nonfunctioning mass <3 cm should be followed up with appropriate CT scans (or MRI if a CT scan was the original test). If the mass has not grown at the 3-month or 1-year follow-up, no further testing is recommended. If the mass does grow within 1 year, adrenalectomy is recommended. Immediate adrenalectomy is recommended for a hyperfunctioning mass of any size and for nonfunctioning masses >4 cm.
References
- 1.Virkkala A, Valimaki M, Pelkonen R, Huikuri K, Kahri A, Kiuisaari L, Korhonen T, Salmi J, Seppala P. Endocrine abnormalities in patients with adrenal tumours incidentally discovered on computed tomography. Acta Endrocrinol (Copenh) 1989;121:67–72. doi: 10.1530/acta.0.1210067. [DOI] [PubMed] [Google Scholar]
- 2.Saruta T. Adrenal incidentaloma Presented at the Ninth International Congress of Endocrinology, Nice, France, August 30, 1992.
- 3.Cook DM. Adrenal mass. Endocrinol Metab Clin North Am. 1997;26:829–852. doi: 10.1016/s0889-8529(05)70284-x. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell DG, Crovello M, Matteucci CT, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology. 1992;185:345–351. doi: 10.1148/radiology.185.2.1410337. [DOI] [PubMed] [Google Scholar]
- 5.Korobkin M, Francis IR. Imaging of adrenal masses. Urol Clin North Am. 1997;24:603–622. doi: 10.1016/s0094-0143(05)70404-3. [DOI] [PubMed] [Google Scholar]
- 6.Siren JE, Haapiainen RK, Huikuri KT, Sivula AH. Incidentalomas of the adrenal gland: 36 operated patients and review of literature. World J Surg. 1993;17:634–639. doi: 10.1007/BF01659129. [DOI] [PubMed] [Google Scholar]
- 7.Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Inciden tally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110:1014–1021. [PubMed] [Google Scholar]
- 8.Fishman EK, Deutch BM, Hartman DS, Goldman SM, Zerhouni EA, Siegelman SS. Primary adrenocortical carcinoma: CT evaluation with clinical correlation. AJR Am J Roentgenol. 987;148:531–535. doi: 10.2214/ajr.148.3.531. [DOI] [PubMed] [Google Scholar]
- 9.Brunt LM, Moley JF. Adrenal incidentaloma. Worm J Surg. 2001;25:905–913. doi: 10.1007/s00268-001-0029-0. [DOI] [PubMed] [Google Scholar]
- 10.Copeland PM. The incidentally discovered adrenal mass. Ann Surg. 1984;199:116–122. doi: 10.1097/00000658-198401000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson GB, Grant CS, van Heerden JA, Schlinkert RT, Young WF, Jr, Farley DR, Ilstrup DM. Laparoscopic versus open posterior adrenalectomy: a case-control study of 100 patients. Surgery. 1997;122:1132–1136. doi: 10.1016/s0039-6060(97)90218-x. [DOI] [PubMed] [Google Scholar]