Abstract
Quinine is widely used for the common symptom of leg cramps. Quinine tablets require a prescription, but quinine and the product from which it is derived, cinchona, are also available without prescription. They are components of over-the-counter remedies for many common symptoms, of nutrition products, and of beverages such as tonic water and bitter lemon. Although quinine has been used for centuries, initially as an extract from the bark of the cinchona tree, allergic reactions to quinine can be severe and can affect multiple organs. These allergic reactions can cause thrombocytopenia, neutropenia, anemia, disseminated intravascular coagulation, acute renal failure, liver toxicity, and neurological abnormalities. Because quinine use is often intermittent, defining quinine as a cause of an acute disorder may be difficult. Moreover, since quinine use is often self-regulated, patients may not mention it in response to direct questions about medication use, adding to diagnostic difficulty. The diversity and severity of quinine-associated disorders and the difficulties of diagnosis are illustrated by the presentation of 4 case histories. Awareness of the variety of potential quinine-associated reactions is important for accurate diagnosis and critical for prevention of recurrent illness.
Quinine has been a commonly used medication for over 350 years. It was first listed in the London Pharmacopoeia in 1677 (1, 2). Quinine is derived from the bark of the cinchona tree, native to the Andes Mountain region of South America. Production of synthetic quinine, which began in 1944, was a wartime priority because of its importance in treating malaria (1). Synthetic quinine and natural quinine in the form of powdered cinchona bark have been used for many indications, such as pain, fever, diarrhea, and exhaustion, as well as to induce abortion (1, 3). For the past 60 years, quinine has been the traditional remedy for nocturnal leg cramps (4), a very common symptom among older people (5). Because of increasing reports of severe reactions to quinine and the absence of evidence for its efficacy in the treatment of leg cramps, over-the-counter marketing of quinine was banned by the US Food and Drug Administration (FDA) in 1994 (6). The frequency of quinine-induced thrombocytopenia has decreased since the regulatory action of the FDA, but reports continue (7). Quinine continues to be provided by prescription, is easily available as a component of remedies and supplements from Internet sources and stores such as Wal-Mart and General Nutrition Center (Table 1), and is a component of common beverages such as tonic water and bitter lemon.
Table 1.
Availability of quinine (or cinchona bark) for online purchase at selected Internet sites
| Manufacturer | Active ingredient | Manufacturer suggested use | Internet site |
| Hyland | Cinchona officinalis 3X (quinine), viscum album 3X, gnaphalium polycephalum 3X, rhus toxicodendron 6X, aconitum napellus 6X, ledum palustre 6X, magnesia phosphorica 6X, in a base of lactose | Leg cramps | http://www.gnc.com [through http://www.drugstore.com] http://shopping.yahoo.com/[14 products from various sites available] |
| Boiron | Cinchona officinalis 30C (cinchona bark) | Loss of vital fluids, exhaustion from excessive sweating, diarrhea, distended abdomen, gas | http://www.VitaminUSA.com |
| Various single and multiingredient products | Cinchona officinalis | Various | http://shopping.yahoo.com/ [11 stores with 29 products available] |
| Boericke and Tafel | Cinchona officinalis 6X | Loss of vital fluids, exhaustion from excessive sweating, diarrhea, distended abdomen, gas | http://www.VitaminUSA.com |
The first report of quinine-induced thrombocytopenia was published in the Lancet in 1865 (8); 3 patients were described who had purpura and extensive mucocutaneous bleeding associated with quinine ingestion. One woman had recurrent purpura with repeated use of quinine for chest pain (8). The first description of multiorgan failure caused by quinine was reported in 1979 (9). A 41-year-old woman who was apparently sensitized by quinine in the beverage bitter lemon developed acute abdominal pain with vomiting and diarrhea, disseminated intravascular coagulation (DIG) with thrombocytopenia, microangiopathic red cell changes, and acute renal failure immediately after taking a quinine tablet prescribed for leg cramps (9). Not only is the small amount of quinine present in beverages (3–8 mg/100 mL) sufficient to cause sensitization, it can also induce severe thrombocytopenia and multiorgan failure in allergic subjects (10–16).
Physicians must be alert for quinine allergic reactions because 1) quinine can cause many different manifestations of multiorgan failure (Table 2), 2) quinine allergic reactions can be fatal, either from thrombocytopenic bleeding (17) or multiorgan failure (18), 3) quinine is widely used—often as a component of an over-the-counter remedy or herbal supplement, which may not be reported by patients as part of a medication history, and 4) even with prescribed quinine tablets, dosing is typically self-regulated and underreported (3).
Table 2.
Clinical manifestations of quinine toxicity*
| Clinical manifestations | References |
| Hematologic | |
| Thrombocytopenia | 27 |
| Neutropenia | 35–37 |
| Lymphocytopenia | 38 |
| Autoimmune hemolytic anemia | 35, 36 |
| Microangiopathic hemolytic anemia | 18, 23 |
| Disseminated intravascular coagulation | 9, 28, 33 |
| Renal manifestations | |
| Anuric acute renal failure | 18, 23 |
| Gastrointestinal manifestations | |
| Nausea, vomiting, diarrhea | 18 |
| Hepatic manifestations | |
| Acute liver failure simulating hepatitis | 38, 39 |
| Neurological manifestations | |
| Headache | 18 |
| Confusion | 18 |
| Coma | 18 |
| Blindness | 40 |
| Psychosis | 41 |
| Hypoglycemia | 42 |
| Systemic manifestations | |
| Fever, chills simulating sepsis | 24, 43 |
*These manifestations may occur alone but often occur together. For example, thrombocytopenia, microangiopathic hemolytic anemia, coma, fever, and acute renal failure fulfill all diagnostic criteria for thrombotic thrombocytopenic purpura–hemolytic uremic syndrome (TTP-HUS). Patients with manifestations of TTP-HUS may also have leukopenia, liver failure, and disseminated intravascular coagulation, abnormalities not associated with the typical TTP-HUS syndrome. Selected references are provided.
We became aware of the diversity of quinine-induced systemic reactions as part of our studies on thrombotic thrombocytopenic purpura—hemolytic uremic syndrome (TTP-HUS) (19, 20). We previously reported 17 patients with quinine-associated TTP-HUS (18). In addition to exhibiting the diagnostic features of TTP-HUS, these patients manifested many other systemic abnormalities. The 4 patients described here demonstrate the diversity of the clinical presentations of adverse reactions to quinine, including fever, chills, nausea, vomiting, diarrhea, neutropenia, DIG, acute renal failure, and acute liver injury. These patients also demonstrate the difficulty of documenting quinine use and the risks of recurrent illness with repeated quinine ingestion.
METHODS
The patients described here are from the Oklahoma TTP-HUS registry (20) and were included in a previous report describing quinine-associated TTP-HUS (18). The case definition (18) was the presence of diagnostic criteria for TTP-HUS in the absence of another apparent etiology and the regular ingestion of quinine. In some patients, as in the first patient described here, the association of acute illness with quinine was clear, due to repeated quinine ingestion with immediate onset of symptoms. In other patients, as in the second patient described here, the documentation of quinine use was difficult, requiring persistent investigation. In the third and fourth patients described here, the only history was of regular but intermittent quinine use, without clear proximity to the onset of symptoms. In these patients, the association of symptoms with quinine must be suspected but cannot be established with certainty.
Analysis of quinine-dependent antiplatelet and antineutrophil antibodies in patient 1 was performed by Janice McFarland, MD, at the Blood Center of Southeastern Wisconsin (21).
CASE REPORTS
Patient 1 is now a 55-year-old woman (patient 2 in our previous report [18]) who had several days of fatigue and fever after Christmas 1994. At 3:00 one morning, she awoke with severe muscle cramps. She had previously had leg cramps approximately once every 4 to 10 months, for which she had taken quinine tablets. On this occasion, approximately an hour after she took a quinine tablet, she developed chills, fever, and continuous vomiting and diarrhea. She rapidly became confused and obtunded. On admission to the hospital she was anuric and her hematocrit was 33%; her white blood cell count was 2600/μL with 63% neutrophils; platelet count, 47,000/μL; creatinine level, 6.5 mg/dL; and lactate dehydrogenase (LDH) level, 964 U/L. The increased LDH level was considered to be due to both hemolysis and muscle ischemia, as both typically occur in TTP-HUS (22). Her serum bilirubin level was markedly increased at 8.2 mg/dL. Although the direct- and indirect-reacting bilirubin fractions were not measured, the increased total bilirubin probably reflected hemolysis rather than a liver function abnormality since the alkaline phosphatase level was normal (74 U/L), the aspartate aminotransferase (AST) level was only slightly increased (131 U/L), and serum haptoglobin was undetectable. Prothrombin time (PT, 12.5 seconds) and activated partial thromboplastin time (aPTT, 28.5 seconds) were normal. TTP-HUS was diagnosed; plasma exchange and hemodialysis were begun on day 2. Her hematocrit and platelet count decreased further to 23% and 24,000/μL by day 3 and then gradually recovered. She required 10 hemodialysis and 15 plasma exchange treatments. Her platelet count was normal on day 9; her creatinine level was normal 1 month after admission.
In June, the patient had a recurrence of severe muscle cramps. She took a quinine tablet and within 2 hours had the same reaction of fever and chills with continual vomiting and diarrhea. On admission to the hospital, her hematocrit was 32%; white blood cell count, 1500/μL with 50% neutrophils; platelet count, 58,000/μL; creatinine level, 1.0 mg/dL; and LDH level, 1015 U/L. Because of the fever and chills with neutropenia, the initial impression was sepsis. However, when the previous history of TTP-HUS was recognized, plasma exchange was begun. Blood cultures were negative. With this episode there was no impairment of renal function. Her white blood cell count returned to normal in 1 day, and her platelet count was normal in 9 days.
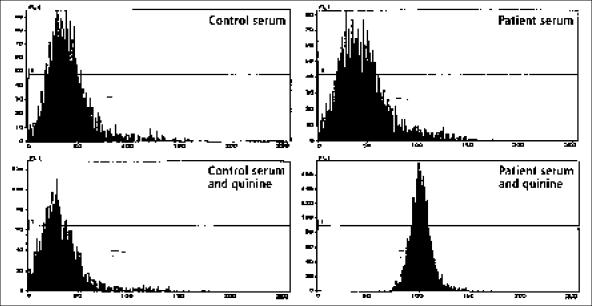
In 1995, quinine-associated TTP-HUS had been recently reported (16, 23), and it was during this episode that the association of her episodes with quinine was recognized. She had taken no quinine tablets between January and June. The patient was cautioned about the risks of quinine but perhaps not strongly enough, since she considered taking quinine again when muscle cramps recurred in late June. Because of the warning that quinine may have caused her previous episodes of TTP-HUS, she hesitated. She told her husband, “This [quinine tablet] is going to tell the story.” Her husband replied, “Don't take it.” She told him, “I've got to know.” She took the tablet and told her husband, “OK, buddy, it's 8:45. Let's see what happens.” She didn't have to wait long. Twenty minutes later nausea began, followed in an hour by fever and chills with continuous vomiting and diarrhea. She went immediately to the hospital. Her temperature was 102.3°F; her white blood cell count was only 700/μL with 53% neutrophils; her hematocrit was 34%, decreasing rapidly to 24%; her platelet count was 186,000/μL, decreasing rapidly to 87,000/μL. Her LDH level was 352 U/L, and her creatinine level was normal at 1.0 mg/dL and remained normal. Plasma exchange was begun immediately, and her neutropenia recovered the following day. Her platelet count was normal on day 6. A serum sample from day 1 demonstrated quinine-dependent antiplatelet and antineutrophil antibodies (Figure). Since 1995, this patient has not taken quinine and has had no further episodes of acute illness.
Figure.
Demonstration of quinine-dependent antiplatelet antibodies. The 4 panels demonstrate patterns of flow cytometry analysis, with the number of platelets recorded on the vertical axis and the intensity of brightness of the fluorescence on the horizontal axis. Normal platelets were incubated with normal serum or patient serum, with or without quinine. After the incubation, fluorescein-labeled antihuman immunoglobulin G antibodies were added and flow cytometry performed. In the presence of quinine, antiplatelet antibodies from the patient's serum bound to platelets and were detected by the fluorescein-labeled anti-human immunoglobulin G, as shown in the bottom right panel by the shift of the platelet fluorescence to the right. Antibodies from the patient's serum did not react with platelets in the absence of quinine (top right panel). Quinine did not cause any reaction between normal control serum and platelets (bottom left panel). Quinine-dependent antineutrophil antibodies were also detected in the patient's serum by the same methods, only normal neutrophils were substituted for normal platelets (data not shown).
Comment: This patient's story illustrates several points. First, quinine ingestion in a sensitive patient may cause the immediate onset of severe systemic symptoms simulating sepsis (24). Second, the rate of serum creatinine increase in patients with quinine-induced acute renal failure may far exceed the commonly accepted maximum values for daily increases in serum creatinine level (0.5–1.0 mg/dL/day) derived from observations on anephric patients. This presumably occurs because of severe systemic muscle ischemia caused by systemic microvascular thrombosis (22), the principal pathogenic feature of TTP-HUS. The extremely high serum LDH values document the severe muscle damage (22). In this patient, the presence of an initial serum creatinine value of 6.5 mg/dL with only a few hours' history of systemic symptoms led to the inappropriate impression of preexisting chronic renal failure, since it was assumed that the creatinine value could not increase from normal to this level in less than a week. Third, although these syndromes have been previously referred to as quinine-associated HUS, rather than TTP, since acute renal failure was the prominent abnormality, this patient demonstrates that acute renal failure is not always present, since her renal function remained normal throughout her second and third documented episodes. Fourth, the documentation of both quinine-dependent antiplatelet and anti-neutrophil antibodies demonstrates the variety of cell targets for quinine-dependent antibodies, which are responsible for the diverse clinical manifestations. Quinine-dependent antibodies reacting with red cells, lymphocytes, and endothelial cells have also been reported.
Patient 2 was 64 years old (patient 5 in our previous report [18]) when she was admitted to the hospital in November 1995 with a 1-day history of severe lower abdominal pain, vomiting, and diarrhea. She also had fever with several hard, shaking chills, and she had been anuric since the onset of the symptoms. On examination, she was alert and oriented. Laboratory data indicated multiorgan failure: her hematocrit was 40% but fell to 20%; white blood cell count was 4000/μL, falling to 1500/μL with 5% neutrophils; platelet count was 58,000/μL, falling to 17,000/μL; creatinine level was 3.3 mg/dL, rising to 6.7 mg/dL in 2 days; LDH level was 4358 U/L; AST level was 992 U/L; and alanine aminotransferase (ALT) level was 331 U/L. Coagulation studies were normal: PT, 10.4 seconds; aPTT, 21.1 seconds; fibrino-gen, 214 mg/dL. Because of the acute hematologic and renal abnormalities, TTP-HUS was suspected, and plasma exchange and hemodialysis were begun the day after admission. Blood culture results were negative. The etiology of the liver function abnormalities was unknown. Fever and abdominal symptoms resolved within 3 days. Her hospitalization was complicated by 2 further episodes of fever and chills with recurrent severe neu-tropenia and thrombocytopenia, attributed to sepsis from the central venous catheter, but repeated blood cultures remained negative. Results of a bone marrow biopsy, done to investigate the recurrent neutropenia, were normal. Although urine output began to recover during her first week in the hospital, she became anuric again after her recurrent acute symptoms and required continued hemodialysis. Plasma exchange treatments were discontinued, and she was discharged 1 month after admission.
Throughout January 1996, the patient required hemodialysis 3 times each week. She remained asymptomatic, and her blood counts remained normal. Then in February she had another episode of fever with severe, shaking chills associated with severe muscle pain. Examination demonstrated no neurological or mental status abnormalities. Laboratory data demonstrated a hematocrit of 22%; white blood cell count of only 100/μL with 28% neutrophils; platelet count of 33,000/(μL; creatinine level of 7.6 mg/dL; and LDH level of 908 U/L. A diagnosis of recurrent TTP-HUS was made, and plasma exchange was resumed.
During this time, we were developing an assay for quinine-dependent antiplatelet antibodies, based on the method described in the Figure. The patient's serum from her second episode was tested as part of the assay development and, unexpectedly, it was strongly positive. She had said at the time of her first hospitalization in November 1995 that she had not taken quinine for more than 6 months, although she described having nausea and vomiting when she had previously taken quinine for leg cramps. At the time of her second hospitalization, in February 1996, she said again that she had not taken quinine for more than 6 months. Her daughter was asked to search her home, but she could not find any quinine tablets. Her primary care physician was called, but he said that he had not prescribed any quinine for her. Then her home health nurse was called, and she said, “Check her husband's medicines. They take each other's medicines all the time.” During her first hospitalization, her husband had slept on a cot in her hospital room. When the patient was asked if she had had leg cramps during her first hospitalization, she said, “Yes, sometimes they would keep me awake all night.” When asked what she did for them, she said, “My husband gave me some of his pills, and they helped my cramps.” How often had she done this? “I did this several times while I was in the hospital.” She also said she had taken some of her husband's pills when she had severe muscle cramps just before this second hospitalization. The home health nurse had a record that her husband had received prescriptions for quinine in October 1995 and January 1996. At this time, she was instructed to take only her own medicines and to strictly avoid quinine. She never again had thrombocytopenia or neutropenia.
The patient's last plasma exchange for the second episode of TTP-HUS was in March; she was discharged 3 days later. She had end-stage renal disease and required permanent hemodialysis. Over the next 4 years she had progressive heart failure, and she died in May 2000.
Comment: This patient, like patient 1, had recurrent episodes of fever and chills with thrombocytopenia and severe neutropenia. These symptoms and signs were interpreted as sepsis (24) related to recurrent infectious complications of the central venous catheter, a common and critical complication of plasma exchange treatment (25). The occult source of the quinine emphasizes the extent of investigation required to exclude quinine as a potential etiology for systemic illness. The continued use of quinine after the onset of acute renal failure probably caused the end-stage renal disease, which ultimately contributed to this patient's death.
Patient 3 was a 59-year-old woman (patient 9 in our previous report [18]) when she was admitted to the hospital in April 1997 with epigastric pain and vomiting. On examination, she was lethargic though oriented, jaundiced, and oliguric. Laboratory data demonstrated a hematocrit of 39%, falling to 21%; white blood cell count, 16,700/μL; platelet count, 70,000/μL, falling to 51,000/μL; creatinine level, 4.6 mg/dL, rising to 9.4 mg/ dL in 3 days; LDH level, 3756 U/L; and bilirubin level, 16.1 mg/ dL (13.4 mg/dL direct-reacting), falling to 0.8 mg/dL in 6 days. Results of other liver function tests were also abnormal: AST, 741 U/L; ALT, 231 U/L; alkaline phosphatase, 154 U/L. Coagulation studies were normal: PT, 11.5 seconds; aPTT, 24.0 seconds; fibrinogen, 440 mg/dL. Hepatitis serologies were negative.
TTP-HUS was suspected, and plasma exchange and hemodialysis were begun. The etiology of the liver function abnormalities was unknown. Her response was prompt, with recovery to a platelet count of 203,000/(μL within 3 days. Hemodialysis was required for 8 weeks.
The patient had used quinine regularly though intermittently for leg cramps. She could not recall the time of her most recent quinine tablet prior to the onset of her illness. However, quinine was suspected not only for this acute episode, but also for multiple previous episodes of acute, transient jaundice, for which she had had liver biopsies on 2 occasions. After April 1997, she never took quinine again, and she never had a recurrence of jaundice, hematologic abnormalities, or acute renal failure. However, chronic renal failure, with serum creatinine levels of 1.4 to 2.7 mg/dL, and hypertension persisted. She died of heart failure in October 2001.
Comment: In addition to the features highlighted by the stories of the previous patients, this patient also had acute liver toxicity. The coexistence of liver disease with the signs of TTP-HUS, the absence of another apparent etiology, and the prompt resolution are consistent with quinine as the etiology for her multiple organ failure. Quinine has been reported to cause acute liver toxicity (Table 2); liver toxicity was also apparent in patient 2. Since the patient had taken quinine regularly, it was assumed that her previous episodes of jaundice were also caused by quinine-induced liver toxicity. No tests for quinine-dependent antibodies were performed.
Patient 4 was a 70-year-old woman (patient 10 in our previous report [18]) when she was admitted to the hospital in August 1997 with a 3-day history of fever, abdominal pain, and vomiting. On examination, she was lethargic but responsive. Laboratory data demonstrated a hematocrit of 41%; white blood cell count of 13,000/μL; platelet count of 58,000/μL; creatinine level of 1.2 mg/dL; LDH level of 1539 U/L; bilirubin level of 4.3 mg/dL; ALT level of 3460 U/L; AST level of 3735 U/L; and alkaline phosphatase level of 170 U/L. There was also evidence for DIC: PT, 37.9 seconds; aPTT, 31 seconds; fibrinogen, 68 mg/ dL; D-dimer, 4.0 (ig/mL. The initial diagnosis was acute hepatic failure with sepsis, but blood culture results were negative. On the second hospital day, the diagnosis of TTP-HUS was considered because no alternative explanation was available for her acute systemic illness, and plasma exchange was begun. On the following day, acute renal failure developed with a creatinine level of 2.6 mg/dL, and she became less responsive, with cyanosis of her hands and feet. She died 3 days after admission.
Comment: Only in retrospect was quinine suspected as a possible cause for this patient's acute and fatal illness. She had a history of regular use of quinine tablets for leg cramps, and there was no clear alternative explanation for her multiorgan failure. No tests for quinine-dependent antibodies were performed. In addition to the systemic features suggesting sepsis that were similar to those of patients 1 and 2, this patient also had severe DIC. The presence of DIC initially diverted the physicians from the diagnosis of TTP-HUS, since DIC is believed by many to be evidence against a diagnosis of TTP-HUS. However, quinine can also cause DIC (Table 2). This patient also had evidence of acute liver injury, like patients 2 and 3.
DISCUSSION
These case histories document the diversity and severity of quinine allergic reactions. They also document the difficulty of obtaining an accurate history of quinine exposure and the uncertainty that quinine was the cause of the acute multisystem disorder in some patients. These stories are a warning to physicians about the risks of recurrent severe illness, with many different manifestations, that may occur with quinine allergy.
Although FDA regulations require a prescription for quinine tablets, quinine is readily available in lower concentrations in a variety of remedies and supplements. Because of its long history of use for many common symptoms, and its established role (though without evidence of efficacy [6]) in the treatment of muscle cramps (4), quinine remains a familiar remedy to many people. Participants in Internet chat rooms and discussion groups for people suffering from leg cramps commonly discuss how to obtain quinine without a prescription and how much of the different preparations are required to be equivalent to a prescription quinine tablet. Use of popular Internet search engines (http://www.google.com and http://www.yahoo.com) to search for “quinine and leg cramps” yields several herbal sites that sell products as well as other sites dedicated to the subject. Note in Table 1 the wide variety of common symptoms for which manufacturers recommend their preparations. This continued use is not surprising for a drug that has been used for centuries. The current popularity of herbal remedies (26) may increase the use of quinine in its natural form as an extract of cinchona bark. The many preparations of quinine and cinchona available must all be assumed to be dangerous for people who are allergic to quinine.
If the efficacy of quinine is uncertain, its potential toxicities are clear, in spite of the common notion that natural remedies are safe. Quinine was the first drug to be associated with acute thrombocytopenia (8), and it remains the second most commonly reported cause of drug-induced thrombocytopenia (27). Only qui-nidine, an isomer of quinine, has been reported more often as a cause of isolated thrombocytopenia (27), but reports of quinidine-induced thrombocytopenia are now uncommon, as it is used much less often for treatment of cardiac arrhythmias. Quinine was the first drug reported to cause TTP-HUS (9, 28); it remains the most common cause of drug-induced TTP-HUS (18) and the only drug for which there is definite evidence of a causal role in TTP-HUS (29). There are no reports of quinidine-induced TTP-HUS. Further, quinine is the only drug, other than heparin (30), that has been reported to cause acute DIC (Table 2), but DIC is an extremely rare complication of heparin and may occur only as a result of tissue necrosis. Beyond these toxicities, quinine can cause a variety of other severe, potentially fatal allergic reactions.
The mechanism for the diverse allergic reactions caused by quinine is the development of quinine-dependent antibodies to antigens on different types of cells. Quinine can bind to surface molecules, altering their structure and exposing new parts of the cell membrane molecule to plasma; these revealed sequences are then recognized by the immune system as foreign antigens (31). The frequency and diversity of quinine-induced disorders must be related to the ability of quinine to bind to surface molecules on many different cell types and perturb their structure to reveal neoepitopes. Diversity may also occur in a single patient at different times, as in patient 1 in this report. Another report describes a patient who first had quinine-induced thrombocytopenia and then, with repeat exposure, had TTP-HUS with severe hemolytic anemia and acute renal failure in addition to thrombocytopenia (32).
Drug-dependent antibodies can be detected by flow cytometry (Figure) (21), but these studies are performed only in a few specialized reference laboratories that have established ability to discriminate significant abnormalities from the wide range of values that occur in healthy subjects. However, even sensitive flow cytometry assays can fail to detect drug-dependent antibodies. The titer of quinine-dependent antibodies may not be detectable until later in the course of disease (16, 33). Furthermore, the immune reaction causing disease may be due to a metabolite of the drug, not the actual drug used in the assay (34).
The patients in this report were managed by plasma exchange because of their critical illness and their criteria for the diagnosis of TTP-HUS, which is considered to have a high mortality without plasma exchange (19). Whether withdrawal of quinine and supportive care could have been equally effective is not known.
The important conclusions from these observations are that quinine is a commonly used medication, that quinine allergy can cause severe multiorgan failure, and that recognition of quinine-induced disorders is critical for prevention of recurrent disease.
References
- 1.Quinine bark, Available at http://www.rain-treecom/quinine.htm. Ac*cessed July 23, 2002.
- 2.Kreig M. In Green Medicine: The Search for Plants That Heal. Chicago: Rand-McNally; 1964. The incredible history of quinine; pp. 165–206. [Google Scholar]
- 3.Kojouri K, Perdue JJ, Medina PJ, George JN. Occult quinine-induced thrombocytopenia. J Okla State Med Assoc. 2000;93:519–521. [PubMed] [Google Scholar]
- 4.Moss HK, Herrmann LG. The use of quinine for the relief of “night cramps” in the extremities. JAMA. 1940;115:1358–1359. [Google Scholar]
- 5.Oboler SK, Prochazka AV, Meyer TJ. Leg symptoms in outpatient veterans. West J Med. 1991;155:256–259. [PMC free article] [PubMed] [Google Scholar]
- 6.Drug products for the treatment and/or prevention of nocturnal leg muscle cramps for over-the-counter human use Federal Register. 1994;59:43234–43252. [Google Scholar]
- 7.Brinker AD, Beitz J. Spontaneous reports of thrombocytopenia in associa tion with quinine: clinical attributes and timing related to regulatory ac tion. Am J Hematol. 2002;70:313–317. doi: 10.1002/ajh.10148. [DOI] [PubMed] [Google Scholar]
- 8.Vipan WH. Quinine as a cause of purpura. Lancet. 1865;2:37. [Google Scholar]
- 9.Elliott HL, Trash DB. Intravascular coagulation induced by quinine. Un expected hazard of “bitter lemon.”. Scott Med J. 1979;24:244–245. doi: 10.1177/003693307902400314. [DOI] [PubMed] [Google Scholar]
- 10.Belkin GA. Cocktail purpura. An unusual case of quinine sensitivity. Ann Intern Med. 1967;66:583–586. doi: 10.7326/0003-4819-66-3-583. [DOI] [PubMed] [Google Scholar]
- 11.Korbitz BC, Eisner E. Cocktail purpura. Quinine-dependent thrombocy topenia. Rocky Mt Med J. 1973;70:38–41. [PubMed] [Google Scholar]
- 12.Barrett AP, Tversky J, Griffiths CJ. Thrombocytopenia induced by quinine. Oral Surg Oral Med Oral Pathol. 1983;55:351–354. doi: 10.1016/0030-4220(83)90188-3. [DOI] [PubMed] [Google Scholar]
- 13.Wagner GH, Diffey BL, Ive FA. “I'll have mine with a twist of lemon.” Quinine photosensitivity from excessive intake of tonic water. Br J Dermatol. 1994;131:734–735. doi: 10.1111/j.1365-2133.1994.tb04995.x. [DOI] [PubMed] [Google Scholar]
- 14.Brasic JR. Quinine-induced thrombocytopenia in a 64-year-old man who consumed tonic water to relieve nocturnal leg cramps. Mayo clin Proc. 2001;76:863–864. doi: 10.1016/s0025-6196(11)63235-7. [DOI] [PubMed] [Google Scholar]
- 15.Barr E, Douglas JF, Hill CM. Recurrent acute hypersensitivity to quinine. BMJ. 1990;301:323. doi: 10.1136/bmj.301.6747.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschall JL, Elliot W, Lianos E, McFarland JG, Wolfmeyer K, Aster RH. Quinine-induced immune thrombocytopenia associated with hemolytic uremic syndrome: a new clinical entity. Blood. 1991;77:306–310. [PubMed] [Google Scholar]
- 17.Freiman JP. Fatal quinine-induced thrombocytopenia. Ann Intern Med. 1990;112:308–309. doi: 10.7326/0003-4819-112-4-308. [DOI] [PubMed] [Google Scholar]
- 18.Kojouri K, Vesely SK, George JN. Quinine-associated thrombotic throm-bocytopenic purpura—hemolytic uremic syndrome: frequency, clinical fea tures, and long-term outcomes. Ann Intern Med. 2001;135:1047–1051. doi: 10.7326/0003-4819-135-12-200112180-00008. [DOI] [PubMed] [Google Scholar]
- 19.George JN. How I treat patients with thrombotic thrombocytopenic pur pura-hemolytic uremic syndrome. Blood. 2000;96:1223–1229. [PubMed] [Google Scholar]
- 20.George JN, Vesely SK. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: diagnosis and treatment. Cleve Clin J Med. 2001;68:857–860. doi: 10.3949/ccjm.68.10.857. [DOI] [PubMed] [Google Scholar]
- 21.Visentin GP, Wolfmeyer K, Newman PJ, Aster RH. Detection of drug- dependent, platelet-reactive antibodies by antigen-capture ELISA and flow cytometry. Transfusion. 1990;30:694–700. doi: 10.1046/j.1537-2995.1990.30891020326.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JA, Brecher ME, Bandarenko N. Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Citn Apheresis. 1998;13:16–19. doi: 10.1002/(sici)1098-1101(1998)13:1<16::aid-jca3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Gottschall JL, Neahring B, McFarland JG, Wu GO, Weitekamp LA, Aster RH. Quinine-induced immune thrombocytopenia with hemolytic uremic syndrome: clinical and serological findings in nine patients and review of literature. Am J Hematol. 1994;47:283–289. doi: 10.1002/ajh.2830470407. [DOI] [PubMed] [Google Scholar]
- 24.Schattner A. Quinine hypersensitivity simulating sepsis. Am J Med. 1998;104:488–490. doi: 10.1016/s0002-9343(98)00082-5. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi MA, Vesely SK, George JN, Chandler L, Duvall D, Smith JW, Gilcher RO. Complications of plasma exchange in 71 consecutive patients treated for clinically suspected thrombotic thrombocytopenic purpura—hemolyticuremic syndrome. Transfusion. 2000;40:896–901. doi: 10.1046/j.1537-2995.2000.40080896.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaptchuk TJ, Eisenberg DM. Varieties of healing. 1: medical pluralism in the United States. Ann Intern Med. 2001;135:189–195. doi: 10.7326/0003-4819-135-3-200108070-00011. [DOI] [PubMed] [Google Scholar]
- 27.George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Vondracek T. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129:886–890. doi: 10.7326/0003-4819-129-11_part_1-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 28.Webb RF, Ramirez AM, Hocken AG, Pettit JE. Acute intravascular haemolysis due to quinine. N Z Med J. 1980;91:14–16. [PubMed] [Google Scholar]
- 29.Medina PJ, Sipols JM, George JN. Drug-associated thrombotic thrombocy topenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2001;8:286–293. doi: 10.1097/00062752-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Bell WR, Tomasulo PA, Alving BM, Duffy TP. Thrombocytopenia occur ring during the administration of heparin. A prospective study in 52 patients. Ann Intern Med. 1976;85:155–160. doi: 10.7326/0003-4819-85-2-155. [DOI] [PubMed] [Google Scholar]
- 31.Burgess JK, Lopez JA, Berndt MC, Dawes I, Chesterman CN, Chong BH. Quinine-dependent antibodies bind a restricted set of epitopes on the glycoprotein Ib-IX complex: characterization of the epitopes. Blood. 1998;92:2366–2373. [PubMed] [Google Scholar]
- 32.Glynne P, Salama A, Chaudhry A, Swirsky D, Lightstone L. Quinineinduced immune thrombocytopenic purpura followed by hemolytic uremic syndrome. Am J Kidney Dis. 1999;33:133–137. doi: 10.1016/s0272-6386(99)70269-6. [DOI] [PubMed] [Google Scholar]
- 33.Spearing RL, Hickton CM, Sizeland P, Hannah A, Bailey RR. Quinineinduced disseminated intravascular coagulation. Lancet. 1990;336:1535–1537. doi: 10.1016/0140-6736(90)93309-d. [DOI] [PubMed] [Google Scholar]
- 34.Curtis BR, McFarland JG, Wu GO, Visentin GP, Aster RH. Antibodies in sulfonamide-induced immune thrombocytopenia recognize calcium-dependent epitopes on the glycoprotein Ilb/IIIa complex. Blood. 1994;84:176–183. [PubMed] [Google Scholar]
- 35.Stroncek DF, Vercellotti GM, Hammerschmidt DE, Christie DJ, Shankar RA, Jacob HS. Characterization of multiple quinine-dependent antibodies in a patient with episodic hemolytic uremic syndrome and immune agranulocytosis. Blood. 1992;80:241–248. [PubMed] [Google Scholar]
- 36.Maguire RB, Stroncek DF, Campbell AC. Recurrent pancytopenia, coagulopathy, and renal failure associated with multiple quinine-dependent an tibodies. Ann Intern Med. 1993;119:215–217. doi: 10.7326/0003-4819-119-3-199308010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland R, Vincent PC, Raik E, Burgess K. Quinine-induced agranulocytosis: toxic effect of quinine bisulphate on bone marrow cultures in vitro. Br Med J. 1977;1:605–607. doi: 10.1136/bmj.1.6061.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou M, Homey E, Stockelberg D, Jacobsson S, Kutti J, Wadenvik H. Multiple quinine-dependent antibodies in a patient with episodic throm bocytopenia, neutropenia, lymphocytopenia, and granulomatous hepatitis. Blood. 1997;90:4806–4811. [PubMed] [Google Scholar]
- 39.Farver DK, Lavin MN. Quinine-induced hepatotoxicity. Ann Pharmacother. 1999;33:32–34. doi: 10.1345/aph.18172. [DOI] [PubMed] [Google Scholar]
- 40.Waddell K. Blindness from quinine as an antimalarial. Trans R Soc Trop Med Hyg. 1996;90:331–332. doi: 10.1016/s0035-9203(96)90277-1. [DOI] [PubMed] [Google Scholar]
- 41.Verghese C. Quinine psychosis. Br J Psychiatry. 1988;153:575–576. doi: 10.1192/bjp.153.4.575. [DOI] [PubMed] [Google Scholar]
- 42.Limburg PJ, Katz H, Grant CS, Service FJ. Quinine-induced hypoglycemia. Ann Intern Med. 1993;119:218–219. doi: 10.7326/0003-4819-119-3-199308010-00007. [DOI] [PubMed] [Google Scholar]
- 43.Schlutz M, Zinneman HH, Hall WH. Drug fever caused by quinine and quinidine. Minn Med. 1973;56:668–670. [PubMed] [Google Scholar]