The Charles A. Sammons Cancer Center at Baylor University Medical Center (BUMC) in Dallas, Texas, opened in 1976. Unlike freestanding cancer centers, Sammons is an integral part of a large tertiary care hospital whose medical staff is composed of physicians in private practice. Thus, it is “a center within a center.” Multidisciplinary interaction among physicians from different specialties has been the pivotal concept underlying the organization and development of the cancer center. Ongoing cooperative interaction with the hospital and with physicians in various communities is a key objective. The principal goals are to provide patients with personalized, high-quality care and to conduct educational and research programs that advance knowledge in the field.
The term cancer refers to more than 100 separate diseases that share the common biologic characteristic of abnormal growth. These malignant cells can, if untreated, spread to other parts of the body and ultimately cause death of the patient. Five percent to 10% of cancers are hereditary; individuals carrying an abnormal gene transmitted in the germline are at very high risk of developing certain malignancies. The vast majority of cancers are not hereditary but develop from mutations in various genes (DNA) due to internal or external agents.
Cancer remains a major public health problem in the USA and the most feared diagnosis. In the year 2002, the American Cancer Society estimated that 1,285,000 new cases and 555,500 deaths occurred from these malignant diseases (1). In Texas, 79,700 new cases and 34,500 deaths were anticipated. In other words, 1 in every 4 deaths in the USA is related to cancer; this translates to more than 1500 people dying each day. Nearly one third of cancer deaths are caused by tobacco, especially cigarette smoking. Men have a 1 in 2 lifetime risk of developing cancer, and for women the risk is 1 in 3. The 3 most common cancers in men (prostate, lung, and colon) and women (breast, lung, and colon) account for about 50% of new cases and 50% of cancer deaths. Nearly 80% of all new cancer diagnoses are made in persons aged 55 and older; this figure will increase as our population ages. The overall annual costs for cancer in the USA during 2001 were estimated to be $156.7 billion, $56.4 billion of which was due to direct medical costs.
On the brighter side, over 9 million Americans are alive today who have a history of cancer. Cancer survival was rare in the early part of the 20th century. By the 1990s, more than 40% of cancer patients survived. The mortality rate from cancer in the USA began to decline for the first time during the 1990s and is continuing to fall (2). The 5-year relative survival rate for all cancers is now approximately 62% (1). Better outcomes are due to advances in research and education. Future progress will require ongoing advances in cancer prevention, detection, and treatment.
A SHORT HISTORY OF CANCER
The philosophies of one age have become the absurdities of the next, and the foolishness of yesterday has become the wisdom of tomorrow.
The greater the ignorance the greater the dogmatism.
—William Osier, 1902 (3)
Early history and scientific beginnings
Cancer is older than humans (4). Tumors have been identified in dinosaur bones from the Jurassic period, more than 150 million years ago. All 5 classes of vertebrate animals and some invertebrates develop some form of cancer (5). A few cases of bone tumors in mummified Egyptians from up to 5000 years ago have been described. Medical texts from India and folklore from China refer to cancers of various types 2000 years ago. Egyptian papyri written between 1500 and 3000 bc refer to tumors of the breast (4, 6, 7) (Figure 1). The Ebers papyrus describes large tumors of the leg. Skull lesions suggestive of metastatic cancer have been found in skeletal remains from the Bronze age, 1900 to 1600 bc.
Figure 1.

The Ebers papyrus, one of the earliest known descriptions of cancer believed to have been written in Egypt about 1600 bc. Reprinted courtesy of the National Library of Medicine.
Hippocrates (ca. 460–370 bc) or a Hippocratic writer compared the long, distended veins radiating from a breast tumor to the limbs of a crab; the Greek word was karkinoma, while its later Latin equivalent was cancer (4, 7, 8) (Figure 2). Neoplasm (new formation) and oncology (the study of masses) are other words derived from Greek. The term cancer was applied to both ulcerating tumors and to inflammatory conditions and cysts. Hippocrates described cancers of the breast, nasopharynx, stomach, skin, cervix, and rectum. Accessible cancers, such as those of the breast, were removed surgically. The wounds and superficial tumors were treated by the application of coal tar and herbal poisons, including hemlock, belladonna, and arsenic. For internal cancers, Hippocrates stated, “It is better not to apply any treatment in cases of a cancer; for the ones who are treated die sooner, while those who are not treated survive a longer time.”
Figure 2.

Cancer the crab in the 15th-century Italian Book of Hours. Reprinted with permission from the Morgan Library, New York.
Galen (129–201), a second-century Greek physician, is often regarded as the founder of clinical medicine and the first oncologist. He wrote about cancers of multiple different organs, including the female reproductive tract, the intestines, and the breast. Hippocrates and Galen thought cancer was due to an imbalance in the 4 humors (blood, phlegm, yellow bile, and black bile)—in this case, an excess of black bile (4, 8, 9). Such thinking dominated Western medicine for over 1500 years. The humoral theories of disease were prevalent in ancient Greece and Rome and led to the grim metaphorical references to evil, insidious behavior as cancerous.
Life expectancy for humans has changed only in the past 250 years. Prior to the mid 18th century, persons had less than a 50% chance of surviving long enough to produce children (10). The Renaissance ushered in the rediscovery of creativity and rebellion against dogma. Paracelsus (1493–1542), a controversial reformer, thought that cancer was a product of excess or deficiency of certain fluids rather than an imbalance in the body's humors, and he burned Galen's works (8, 9). Paracelsus refused to accept medical teaching not based on experience. He pioneered a natural philosophy founded on chemical principles and used laudanum, sulfur, lead, and mercury therapeutically.
The 17th century saw the beginnings of modern science—questions became “how” rather than “why.” Newton's laws of gravitation and instruments such as the microscope and Galileo's telescope led to a new understanding of the universe. William Harvey's demonstration of the circulation of the blood was the most significant advance in medicine. The humoral theory of cancer and other diseases finally was discarded, and scientists began to look elsewhere for explanations.
In 1665, Robert Hooke examined a slice of cork under a microscope and described small compartments that he termed “cells” (7, 8). Marcello Malpighi (1628–1694), one of the first microscopists and the founder of histology, described capillaries, glomerular tufts of the kidneys, and the Malpighian bodies of the spleen. Antony van Leeuwenhoek (1632–1723), a Dutch textile merchant from Delft, produced his own microscopes and identified spermatozoa, protozoa, bacteria, and human red blood cells (7, 8, 11, 12) (Figure 3). The great clinician and teacher Herman Boerhaave (1668–1738) thought that blood was the essence of life and if stasis occurred in the circulation, the resulting inflammation would lead to a scirrus or tumor capable of developing into cancer (9).
Figure 3.

Leeuwenhoek's microscope, one of many made by the Dutch textile merchant in the 17th century. Only a few still exist. Reprinted with permission from the National Museum of Health and Medicine of the Armed Forces Institute of Pathology at Walter Reed Army Medical Center, Washington, DC.
After the lymphatic system was discovered in the 17th century, attention began to be focused on lymph and lymph nodes as possible sources of cancer. William Hewson's (1739–1774) studies on the function of the lymphatic system as well as his description of leukocytes and blood coagulation were major contributions (12). John Hunter (1728–1793) (Figure 4), the leading surgeon and medical scientist of the 18th century, thought cancer was the most unfavorable outcome of inflammation. Hunter felt that inflammation often was a healthy reaction to injury. In his view, cancer was related to “coagulable lymph,” a component described by Hewson that we now call plasma (8, 12). This relationship between cancer and inflammation, which dated to the Greeks, would be revisited later by Virchow and again recently (12–15). Hunter thought if a tumor were movable, it could be surgically removed. If enlarged glands were present, he advised against surgery.
Figure 4.

John Hunter (1728–1793). Reprinted from the frontispiece in Paget S. John Hunter: Man of Science and Surgeon. London: T. Fisher Unwin, 1897.
Xavier Bichat's (1771–1802) concept of tissues, developed without the use of a microscope at the end of the 18th century, laid the groundwork for structural and pathologic anatomy (8, 9, 12, 13). Bichat stated that each system of tissues had its own characteristic lesions. Cancer was thought to be cellular tissue. Bichat's pupil, René Laennec (1781–1826), better known as the inventor of the stethoscope than as a pathologist, made a distinction between inflammation, such as gangrene, and cancer, which was an accidental tissue. He separated inflammatory from true tumors and pointed out that disease processes were both local and general. Thus, Laennec took Bichat's tissues of the body and made them into a classification of disease.
The development of pathologic anatomy was aided by the removal of bans against dissection and autopsy. In 1761, Giovanni Morgagni (1682–1771) for the first time used postmortem findings in 700 cases to correlate anatomic findings with the symptoms experienced during life. Matthew Baillie (1761–1823) produced the first systemic illustrated pathology textbook based on organs (1793). Cancers of breast, stomach, rectum, testes, bladder, pancreas, and esophagus were detailed in Morgagni's and Baillie's works. The contributions of these 2 great pioneering pathologists were milestones in the development of morbid anatomy (7, 8, 13).
Joseph J. Lister (1786–1869), the surgeon's father, devised improved achromatic lenses for the microscope that provided higher resolution and led to a scientific revolution in histology after 1830 (7, 8, 12, 13). Cells were identified as the units of structure and function in animal tissues and in tumor tissue. The pathologic anatomy of cancer remained at the gross level until the 1830s and the application by Johannes Muller (1801–1858) of the microscope and Schwann's cell theory to the study of tumors (13). Theodor Schwann (1810–1882), a student of Müler's, in 1837 published his view that the cell was the unit of structure and that its nucleus was the reproductive organ. By the 1850s, Schwann's theory gave way to a belief in cell continuity.
Rudolph Virchow (1821–1902) (Figure 5), the dominant figure in German medical research for half a century, published his landmark scientific treatise Cellular Pathology in 1858 and applied the cell theory to pathology, proclaiming his doctrine of “omnis cellula e cellula” (every cell arises from another cell) (16, 17). Thus, cells could not develop by spontaneous generation but only through the growth and division of other cells. This focus on the cell rather than tissues or organs became a fundamental tenet of modern biology (8, 13, 18). Virchow, another former student of Müller, believed that tumors develop from immature cells scattered through the connective tissue. In 1863, Virchow noted the association between inflammation and cancer and suggested that the 2 processes were related (the irritation hypothesis) (6, 12–14). In addition to his monumental contributions in pathology, Virchow was a vigorous proponent of public health measures and a supporter of the new field of anthropology (17). He was also appointed to civic offices in Berlin and elected to the Prussian parliament.
Figure 5.

Rudolph Virchow (1821–1902). For photo, see print version.
Wilhelm Waldeyer (1836–1921) laid the foundation for current views about cancer by suggesting it arose from transformation of individual normal cells into malignant cells by external factors (8, 9, 13). The mechanism of local spread involved the active or passive movement of cancer cells into adjacent tissues, whereas the mechanism of metastatic spread involved the transport of cancer cells to distant sites via blood or lymph.
Leukemias, lymphomas, and myeloma joined the list of malignant neoplastic diseases during the 19th century (12, 19–24). Leukemia was described in 1845 by John Hughes Bennett (1812–1875) and Virchow and was named by Virchow. Lymphomas—the term was a general name given to any neoplastic disease derived from a cellular component of the immune system—originated with the description of malignant disease of the lymph glands in 1832 by Thomas Hodgkin (1798–1866) and was named “Hodgkin's disease” in 1856 by Samuel Wilks (1824–1911) (Figure 6). For Wilks, the disease appeared to be somewhat between a cancer and a tubercle. The controversy as to whether leukemias and lymphomas represented true neoplasms continued well into the 20th century. Non-Hodgkin's lymphomas were not clearly recognized as entities separate from Hodgkin's disease and leukemia until 1925, though Virchow had suggested the concept in 1863 by using the term “aleukemic leukemia.” Multiple myeloma was first described in 1844. One year later, Henry Bence Jones (1813–1873) found the unusual urinary protein that became widely utilized for the diagnosis of myeloma (Figure 7).
Figure 6.

Thomas Hodgkin (1798–1866). Reprinted with permission from the Gordon Museum, Guy's Hospital Medical School, London.
Figure 7.

Henry Bence Jones (1813–1873), “the best chemical doctor in London.” Reprinted by kind permission of the Royal Society of Medicine, London.
Microscopic histopathology emerged as the basis for diagnosis and typing of malignant neoplasms (25, 26). William Osier's first clinical paper in 1871 described the microscopic findings in a patient with breast cancer. By the latter part of the 19th century, much of the framework for oncology was in place (8, 9,12, 13). True neoplasms were distinguished from inflammatory lesions and many other swellings that had been grouped together for over 2000 years. Pathologists treated tumors as having a cellular nature, originating in normal cells and tissues of corresponding types, and retaining many of the features of the originating structures. They were composed of tumor cells that multiplied by mitotic division. In this view, tumors were supported in most instances by blood vessels and connective tissues and were nourished by the blood of the host organism. They could be either malignant or benign. Malignant neoplasms were characterized by invasiveness into surrounding tissues and colonization of distant body sites after being transported in blood or lymph. Benign tumors were local circumscribed growths that were derived from epithelial or connective tissue and failed either to invade or metastasize. Both malignant and benign tumors were classified according to their derivation from the 3 embryonic germ layers (ectoderm, mesoderm, and endoderm) or from epithelial and nonepithelial cells. The malignant epithelial neoplasms were termed carcinomas and their nonepithelial analogues, sarcomas. Benign neoplasms were given names such as lipoma, chondromas, and myomas, according to their histological derivation—from fat, cartilage, and muscle, respectively.
Other major events in the 19th century led to scientific advances in medicine (6, 7). Darwin published his theory of evolution, Pasteur invented bacteriology, and Claude Bernard began the study of experimental medicine. Anesthesia and antisepsis allowed surgery to develop into an effective clinical discipline. Conrad Rontgen's accidental discovery of x-rays in 1895 and the Curies' discovery of radium shortly thereafter had an immediate impact on diagnosis and on establishing the new specialties of radiology and radiotherapy (8, 27–30). In the 1890s, Dr. William Coley attracted attention with his anecdotal reports of injections of bacterial extracts from organisms causing erysipelas (streptococci) that resulted in regression of advanced cancers (8, 18, 31, 32). These extracts, known as “Coley's toxins,” generated much controversy. Coley's work was one of the earliest attempts at immunotherapy of cancer by stimulating the host's immune system. Nearly a century later, interest in this type of approach was revived with the discovery of tumor necrosis factor and other immune-stimulating cytokines.
Carcinogens
The history of carcinogens is usually traced back to the identification by London surgeon Percival Pott (1714–1788) of scrotal cancer among chimney sweeps (4, 7, 8, 33). He attributed this association to the chronic irritating effect of soot and thus identified the first occupational cancer. Lung cancer among Black Forest miners was reported in 1879, and urinary bladder cancer among dye workers was reported in 1895. Research began for irritants that might cause cancer. In the 1930s, a London research group identified active chemicals as polycyclic hydrocarbons. Many others have been added to the list since then. The work of Bruce Ames (b. 1928) showed that carcinogenicity correlated with the ability to induce mutations.
The carcinogenic action of radiation had been known since the early 20th century (27, 28, 30). Though critics maintained that safety precautions were underemphasized, that situation changed dramatically after 1945 with the atomic explosion at Hiroshima, which raised new fears of cancer. Study of survival showed that exposure to ionizing radiation produced myelocytic leukemia and increases in thyroid and other cancers.
Tobacco had been cited as a possible carcinogen from the 19th century on, but the medical profession generally showed little concern about it. By the end of the Second World War, the fear of rising mortality from lung cancer began to intensify. Epidemiologic evidence in Britain and America linked this rise with cigarette smoking, which had been growing in popularity over the period of the 20th century, especially in the 1940s. By 1962 in England and 1964 in the USA, the link between smoking and cancer was officially endorsed (4, 7, 10, 33). The rising tide of evidence about cigarette smoking finally led to major changes in the law and to large financial awards in the court that sought to limit access to cigarettes. By the late 1980s and 1990s, the number of individuals in the USA who were cigarette smokers had dropped from 40% to 20%, and the rise in lung cancer, which had been steep in the early and mid parts of the century, began to decline. Nevertheless, in 2001, over 170,000 cancer deaths in the USA were caused by tobacco (1). This figure amounts to one third of the total.
Through the 1960s and 1970s, environmental issues began to gain momentum in other areas as well. Asbestos leading to mesothelioma and vinyl chloride leading to angiosarcoma of the liver were well publicized. Aniline dyes were linked to bladder cancer, and aflatoxin (peanut mold), to liver cancer. Sun exposure increased the risk of skin cancers, including melanoma. Reduction of exposure to environmental carcinogens and the resources required for such reduction remain controversial topics (34, 35).
Viruses have been implicated as a cause of cancer for nearly a century (4, 6, 7, 14, 36, 37). The virogene-oncogene theory of Huebner and Todaro and the concept of proto-oncogenes are discussed in the next section. Cancers also are caused by common viruses; it has been estimated that as many as 15% to 20% of all cancers worldwide are due to persistent infection with common viruses or other microbial organisms (4, 14). Examples include the association of liver cancer (hepatocellular carcinoma) with hepatitis B and C, nasopharyngeal carcinoma and African Burkitt's lymphomas with Epstein-Barr virus, Kaposi's sarcoma with a recently discovered herpes virus (HHV8), and an unusual type of adult leukemia in Southern Japan and the Caribbean area with an RNA or retrovirus named HTLV-1. Cervical cancer is related to human papillomavirus, especially the unusual strains 16 and 18. Lymphomas associated with Epstein-Barr virus occur in immunosuppressed patients such as those with AIDS and recipients of solid organ transplants. Rather than acting as complete carcinogens, viruses associated with human cancer appear to drive the infected cell toward malignancy in the pathway of multistep tumor formation (14, 18). These and other viruses may have an oncogenic role in the etiology of additional human tumors (14).
Other microbial organisms associated with certain types of cancer include the liver fluke Clonorchis sinensis, with bile duct cancer; schistosomiasis, with bladder cancer; and the common bacterium Helicobacter pylori, with gastric carcinoma and lymphoma. Persistent infection, age at which infection occurs, and underlying status of the immune system are important factors in determining the eventual outcome of the clash between a microbe and its human host.
Tumor biology
Research on the etiology of cancer generally shifted between 2 kinds of mechanisms in the 20th century: explanations favoring actions of external factors such as viruses, environmental chemicals, or physical agents such as radiation; and those favoring endogenous factors such as genetic mutation (4,6,9,18,33,37). In 1911, American researcher Peyton Rous (1879–1970) reported the transmission of a chicken sarcoma into healthy chickens by a submicroscopic, filterable agent, i.e., virus, but subsequently the search for an infectious agent fell into disrepute. New data reawakened interest in the viral etiology of cancer, and a half century later, Rous was awarded the Nobel Prize (1966). The cyclic fashions of cancer research as exemplified by the work of Coley and Rous underline the wisdom of William Osier's aphorisms printed at the beginning of this section.
In 1914, the somatic mutation theory of Theodore Boveri (1862–1915) stated that cancer was caused by chromosome abnormalities in single cells or by agents that produced them. This view was reinforced in 1927 by H. J. Muller (1890–1967), who demonstrated the mutagenic properties of x-rays (9, 18). By explaining how exogenous factors affected the genetic behavior of cells, the mutation theory challenged theories that explained cancer production in terms of chemical reactivity. Later work showed that carcinogenic chemicals could produce mutations in bacterial or animal systems.
The most important discovery in biology during the 20th century was elucidation of the double helical structure of DNA by James Watson (b. 1928) and Francis Crick (b. 1916) in 1953 (38) (Figure 8). By the middle of the next decade, the genetic code had been unraveled and found to be essentially the same in all organisms, i.e., universal. Viral theories began to become attractive again, especially with the advances in basic science by fundamental research on the polio virus (9). Both DNA and RNA viruses were shown to cause a number of animal neoplasms, especially leukemias and lymphomas in chickens, cats, and cattle. By 1960, the discovery of tumor-specific transplantation antigens revived interest in tumor immunity by suggesting that stimulating an immune response might lead to tumor regression. An effective prophylactic vaccine was developed for Marek's disease, a form of chicken lymphoma caused by a herpes virus (4). Nevertheless, critics of tumor immunity pointed out that immunotherapy of cancer had a long and unsuccessful history (39).
Figure 8.
James Watson, Francis Crick, and the DNA double helix. Reprinted from Watson JD. The double helix: a personal account of the discovery of the structure of DNA. In Stent GS, ed. The Double Helix: Text, Commentary, Reviews, Original Papers. New York: WW Norton & Co, 1980.
In 1960, the first chromosome abnormality in cancer, the Philadelphia (Phi) chromosome, was identified in chronic myelogenous leukemia (40). The field of cytogenetics has grown in importance in the leukemias ever since. It was later shown that the Phi chromosome arises from a translocation involving chromosomes 9 and 22. This translocation results in the formation of a hybrid gene (bcr/abl), which, in turn, codes for a hybrid protein that predisposes cells to become leukemic (18). It is now known that most cancer cells show karyotypic changes with a variety of chromosomal abnormalities.
In 1970, Howard Temin (b. 1934) and David Baltimore (b. 1938) independently reported the discovery of an enzyme, reverse transcriptase (7, 9, 18). This enzyme was found in a class of viruses responsible for many types of animal tumors and some rare forms of human leukemia and lymphoma. The genetic core of the retrovirus consisted of RNA rather than DNA. Temin and Baltimore found that once a retrovirus had infected a cell, it employed its reverse transcriptase enzyme to turn its RNA core into a strand of DNA, thus explaining the mechanism by which RNA viruses convert their genetic information into DNA. This discovery changed cancer research and biology in general because it refuted the central dogma of molecular genetics—that DNA made RNA but not the reverse.
The virogene-oncogene theory of Robert Huebner (b. 1914) and George Todaro (b. 1937) became the modern parallel to Boveri's early theory of chromosome changes as the cause of cancer. The Huebner-Todaro theory postulated that in the course of evolution, portions of RNA virus became incorporated into the genome and existed there as a silent infection prior to birth. These fragmented viral genes would normally be suppressed but might be activated by many carcinogens. Such cancer-causing viral gene fragments were termed oncogenes (7, 18). Normal genes with latent carcinogenic potential were called proto-oncogenes. Thus, normal cells carried genes with the potential of becoming oncogenic. The relationship between a retroviral oncogene, v-src, and a closely related proto-oncogene, c-src, identified by J. Michael Bishop (b. 1936) and Harold Varmus (b. 1939), closed a 65-year loop. The virus they used for their Nobel Prize—winning work was the long-neglected Rous sarcoma agent. Many other retroviral oncogenes have been identified since. Proto-oncogenes can be activated not only by retroviruses, usually in animals, but also by somatic mutations involving base substitution, gene amplification, or chromosomal translocation (40).
Normal cells also carry genes that can limit the growth of malignant cells. When these tumor suppressor genes are inactivated, tumor growth can occur (18, 40, 41). Tumor suppressor genes such as the retinoblastoma gene and p53 have been widely investigated. Alfred Knudson (b. 1922) put forth a “2-hit” hypothesis after studying children with the hereditary and sporadic forms of retinoblastoma (18, 40, 41). Both genes must be mutated in order for malignancy to develop. The p53 gene is the most frequently mutated gene in human tumors; its mutations are more subtle than those occurring in the rb gene. The tumor suppressor gene p53 is frequently mutated in colon, lung, breast, esophageal, liver, and brain tumors as well as leukemia-about 50% of all human tumors. Each suppressor gene codes for a signal-transducing protein that relays growth-inhibiting messages from one part of the cell to another. If the suppressor gene is eliminated or inactivated, the normal growth-inhibiting signals are no longer present, and the “brake” to uncontrolled cell growth is removed. Thus, tumor suppressor genes work in a manner opposite to that of oncogenes: they prevent cancer rather than allowing it to develop.
These 2 general classes of genes control the life cycles of cells: 1) proto-oncogenes control growth and differentiation of cells under their control, and 2) tumor suppressor genes code for enzymes that control DNA transcription, DNA repair, and other functions. Damage to these genes, whether by a chemical carcinogen, virus, or ionizing radiation, can lead to mutations and malignancy (18, 40, 41). The discovery of oncogenes and tumor suppressor genes during the past 30 years is a milestone in tumor biology.
Other mechanisms of genetic alteration of cell growth involve gene amplification. Many additional factors influence cell growth, a basic biological phenomenon. Angiogenesis is clearly a critical step in tumor progression: new blood vessels are necessary if tumors are to grow beyond 2 to 3 mm in size (18, 42, 43). Expression of the telomerase enzyme allows cells to persistently grow and may be a prerequisite for development of malignancy (4, 44). This enzyme is active in up to 90% of human cancers. Other mechanisms influencing cell proliferation include transcription factors, cytokines and other growth factors, repair enzymes, adhesion molecules, and programmed cell death (apoptosis). This myriad of complicated factors and pathways offers opportunities to limit tumor cell growth by blocking crucial points in proliferation and regulation with specific antibodies or small molecule inhibitors.
Most cancers result from gene alteration in somatic cells, i.e., mutations that affect a given cell and its progeny rather than every cell in the host and its descendants. Accumulation of mutations in DNA often occurs over many years and has led to the concept of multistep carcinogenesis, whereby gradual progression from a precancerous to an overtly malignant process evolves (18). Perhaps the best example is the sequence of genetic events leading to colon cancer as delineated by B. Vogelstein (b. 1949) et al (40, 41). Other examples include cancers of the breast, uterine cervix, and prostate. The progression to overt malignancy is accompanied by the tumor cells taking on autonomous characteristics. This acquired independence is the hallmark of cancer; it signifies a growth state determined by the tumor cells rather than by external growth-controlling factors (18).
Almost all malignant tumors are monoclonal, i.e., a single normal cell undergoes transformation, often through multiple mutations, into a cancer cell (4, 18). Its descendants proliferate over many years, producing a large population and the signs and symptoms of cancer. It is difficult to detect <1 billion (109 or 1 g) of tumor cells, which result from 30 cell divisions (45, 46). Most patients have 1010 tumor cells or more by the time they develop symptoms causing them to seek medical attention. After 40 cell divisions, 1012 (1 trillion) cells or 1 kg of tumor is present, and unless it is reduced, death occurs. These kinetic data indicate that most cancers are in the late stage of their natural history before they can even be found by a blood test, x-ray, or scan. Because early detection enhances the possibility of cure for many cancers (e.g., breast and colon), much more sensitive and accurate tests are sorely needed. Molecular techniques hold promise if they can be applied cost effectively.
The development of hybridoma technology by G. Köhler (1946–1995) and C. Milstein (1927–2002) revolutionized immunology after 1975. These investigators demonstrated that antibody-producing cells of virtually any desired specificity could be fused with a myeloma cell line, the result being unlimited amounts of homogeneous (monoclonal) antibodies carrying that specificity (47, 48). The impact of these “designer” monoclonal antibody reagents on diagnostic pathology has been immense. Together with other recently developed analytic methods such as flow cytometry, the polymerase chain reaction, fluorescence in situ hybridization, DNA microarrays, and gene rearrangement techniques, the immunological, molecular, and genetic characterization of tumor cells can be accomplished with astonishing accuracy. These methodological innovations have contributed substantially to the understanding of tumor biology as well as providing new dimensions in clinicopathological diagnosis.
Therapy: surgery and radiation
Hippocrates and Galen cautioned against treatment of hidden cancers, arguing that treatment more often than not hastened death. Galen recommended the use of purging, bleeding, and proper diet and the limited local use of poisons and caustics in breast cancer. Until the 19th century, treatment was a combination of crude surgery, cauterization, and the use of topical preparations containing scar-forming corrosive agents such as arsenic. Purging, herbal substances, and magical preparations also were utilized (9). After the introduction of anesthesia in the 1840s, Joseph Lister (1827–1912) developed the concept of antisepsis using carbolic acid. Lister's rationale was based on Pasteur's theory that bacteria caused infection. Anesthesia and antisepsis (later asepsis) allowed surgery to be done more safely on an elective basis.
Cancer surgery became widely applied in the late 19th and early 20th century (49). William S. Halsted (1852–1922), the first professor of surgery at Johns Hopkins, introduced Lister's methods for antisepsis to America and emphasized the importance of meticulous handling of tissues during surgery. He also defined the principles of en bloc resection, applying it to radical mastectomy in 1890. It is noteworthy that Halsted worked closely and cooperatively with his colleague, William Osier, first professor of medicine at Johns Hopkins. Osier often recommended surgery, particularly for abdominal tumors. The Halsted-Osler interaction might be considered one of the earliest examples of multidisciplinary cancer management, an approach that was to become a dominant theme after 1970.
Other cancer surgeons made important contributions. Theodore Billroth (1829–1894) performed the first gastrectomy, esophagectomy, and laryngectomy. Prostatectomy, radical hysterectomy, and abdominoperineal resection were first performed by Hugh Young, Ernst Wertheim, and W Ernest Miles, respectively, during the period from 1900 to 1910. By the 1920s, Harvey Gushing was able to remove brain tumors. The justification for many of these operations was that the total removal of the affected part would reduce the likelihood of recurrence. Many patients with solid tumors (e.g., colon, breast) were cured with surgery alone. General improvements in surgery during the 20th century included better techniques and tools, more effective control of shock, blood transfusions, and antibiotics, which permitted more extensive surgical procedures for cancer (7). In the 1940s and 1950s, new radical procedures were performed; examples included the supraradical mastectomy, hemicorporectomy, and hemipelvectomy. Survival figures showed at best marginal improvements and, not surprisingly, severe reductions in quality of life (9). Consequently, surgeons and patients turned away from supraradical operations in the 1960s and 1970s. Often, however, patients were not offered the choice between radical and conservative surgery. This was particularly true in England and the USA with regard to surgical treatment of breast cancer. After 1980, breast conservation became much more widely utilized as combined modality therapy and patient advocacy assumed greater importance.
Surgeons are key members of the multidisciplinary cancer team. They often provide the entry point for patients, establish the diagnosis, and carry out staging. Surgeons thus obtain the various consultants and coordinate effective treatment planning.
As noted, almost immediately after the discovery of x-rays in 1895 and radium in 1898, radiation became a valuable cancer treatment modality alone and as an adjunct to surgery (Figure 9). The growing enthusiasm for x-rays and radium as possible alternatives or supplements to surgery assumed major importance. By 1914, virtually every European capital had a radium institute, with the first being proposed in Paris around 1906 (27, 28). The importance of x-rays in diagnosis and treatment of cancer led to the establishment of the new specialties of diagnostic radiology and radiation therapy. It was soon apparent that x-ray exposure sometimes produced severe side effects, including burns and even cancer itself (27, 28, 30). The recognition of such dangers led to the development of more powerful and safer machines that delivered radiation by external beam. The other major alternative to surgery was radium implantation, particularly useful in treatment of carcinoma of the cervix.
Figure 9.
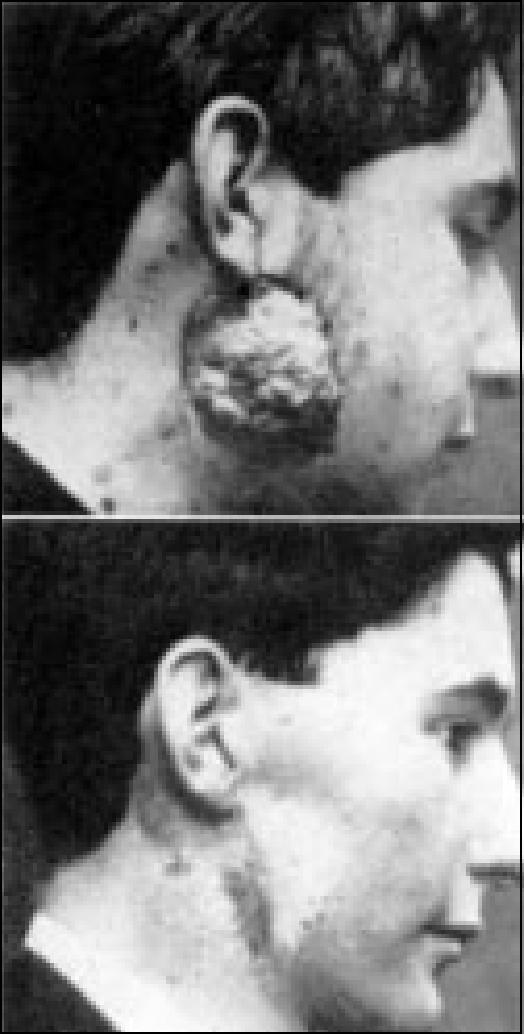
X-ray therapy of recurrent sarcoma (1901). Before therapy and 11 months after beginning treatment. Reprinted with permission from Pfahler GE. The early history of roentgenology in Philadelphia. XUfi 1956;75:14–22.
By the 1950s, the modern era of external beam therapy began as cobalt replaced radium and orthovoltage equipment. Linear accelerators were developed in the 1950s and 1960s. Modern equipment and advances in dosimetry improved the effectiveness and safety of radiotherapy. Radioisotopes such as iridium 192 and iodine 125 are now in wide use for implantation. Diagnostic radiology and the development of remarkably accurate imaging techniques such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography scanning during the latter part of the 20th century were to become especially important in oncology (27, 29). Radiation therapy is an important treatment modality in oncology, especially for patients with lymphomas, seminomas, neuroblastomas, small cell cancers, and retinoblastomas. It is also useful in treatment of breast, head and neck, prostate, gynecologic, rectal, and lung cancers—especially as a component of multimodality therapy. During the 1940s and 1950s, surgery and radiotherapy were joined by a new modality in anticancer treatment, chemotherapy with drugs.
Chemotherapy
Paul Ehrlich (1854–1915) was the father of chemotherapy (50), describing the first alkylating agent in 1898. Following his discovery of Salversan for syphilis in 1910, interest increased in developing drugs that could be administered systemically to treat infectious diseases and cancer. During the 1940s, cancer chemotherapy was shown to be effective initially in lymphomas treated with nitrogen mustard (12, 21, 22, 51) and in childhood acute leukemia treated with folate antagonists (12, 20, 52). By 1956, the first cure of a disseminated tumor, choriocarcinoma, was reported by Li et al (4, 53). This advance heralded the age of modern chemotherapy. The use of combination chemotherapy using agents having differing mechanisms of action and nonoverlapping toxicities led to unrivaled success in the treatment of leukemias and Hodgkin's disease in the 1960s and 1970s. Heterogeneity of the tumor cell population was identified as a major obstacle to the effectiveness of chemotherapy, and the combination drug approach partially overcame this barrier. Multimodal treatment employing surgery, chemotherapy, and radiotherapy proved to be beneficial in the adjuvant treatment of patients with breast cancer.
The use of multimodal treatment necessitated multidisciplinary interaction among clinicians from different specialties and resulted in major advances in oncology, including the designation of medical oncology as a recognized subspecialty by the American Board of Internal Medicine in 1973. The advances brought about by multidisciplinary interaction also led to the formation of cancer centers in the 1970s following implementation of the National Cancer Act.
Early trials of cytotoxic agents were focused especially on childhood leukemias and lymphomas. The search for chemotherapy drugs became actively supported and consumed almost half of the budget of the National Cancer Institute (NCI). By 1970, some 400,000 drugs had been tested (7). As with radiotherapy, some short- and long-term side effects limited the effectiveness of chemotherapy; it was difficult to target cancer cells without damaging normal cells. Both modalities were sometimes associated with serious side effects, including even cancer itself. Early critics contended that chemotherapy was of little to no use against many of the common cancers, especially lung and colon cancer. However, combination chemotherapy has proven to be of significant benefit in treatment of non-Hodgkin's lymphomas, disseminated testicular cancer, breast cancer, and other solid tumors (40, 45, 54). Agents such as doxorubicin, cisplatin, and the taxanes became important in the treatment of multiple types of malignancies. More recently, nucleoside analogues have been shown to be useful in certain leukemias and lymphomas. Targeted therapy directed specifically at the cancer cell has been developed recently, the best example being imatinib (Gleevec), an oral tyrosine kinase inhibitor of the bcr/abl oncogene in chronic myelogenous leukemia (55). Imatinib also inhibits the growth of gastrointestinal stromal tumors. Initial results with this highly active new agent have been exciting and illustrate the potential for targeted anticancer therapy in the future.
In the early 1940s, Charles B. Huggins, a urologist at the University of Chicago, showed experimentally that growth of the prostatic epithelium was stimulated by testosterone and inhibited by estrogen. These findings led to the use of surgical castration and “chemical” castration (via estrogen administration) in patients with metastatic prostate cancer. Remissions for short periods (occasionally, several years) were seen in some patients. This work led to an entire field focusing on the role of hormonal therapy of cancer. Huggins was awarded the Nobel Prize in physiology and medicine in 1966 for his pioneering work. A variety of hormonal agents are now widely employed in the treatment of breast, prostate, and other endocrine-responsive malignancies (54).
Immunotherapy
Treatment of cancer by immunological methods has a long and unimpressive history dating from Coley's toxins in the 1890s. Although the British immunologist Almroth Wright predicted in 1909 that “the physician of the future will be an immunisator,” the following 60 years saw little progress (39). The use of interferon and interleukin-2 roused interest in the 1980s for diseases like hairy cell leukemia, chronic myelogenous leukemia, renal cell carcinoma, and melanoma. Attempts at vaccine treatment of established cancer (e.g., renal cell and melanoma) have not yielded reproducible successes. However, recent studies utilizing antigen-loaded dendritic cells, those elements that direct the immune response, are promising (56). Prophylactic vaccines as developed for Marek's disease in chickens have been sought. Hepatitis B vaccine to prevent hepatoma and papillomavirus vaccine to prevent cervical cancer appear effective. Such immunologic approaches have major public health implications.
As noted previously, monoclonal antibodies revolutionized diagnostic immunology after the hybridoma technique for making them was discovered in 1975 (47, 48). With respect to their therapeutic application, directing monoclonal antibodies against tumors has received special attention. Because of their high specificity, such reagents have been termed “guided missiles” or “magic bullets.” Monoclonal antibodies are not magic, but they are bullets and clearly represent a quantum jump in targeted anticancer therapy. Though initial progress was slow in coming, monoclonal antibodies have demonstrated clear-cut efficacy in some patients with lymphoma and breast cancer. It is likely that this approach will prove useful for patients with other malignancies. Radioisotopes, toxins, and drugs can be linked to monoclonal antibodies to provide greater antitumor effect. For optimal results, it seems clear that each reagent needs to be studied methodically, alone and in combination with other modalities, in each clinical circumstance.
Psychosocial aspects
The mythology surrounding cancer dates back hundreds of years (57). During the 20th century, cancer became the dominant disease metaphor, replacing tuberculosis (58, 59). Popular and medical opinion in Western society suggested that industrial and urban growth exacerbated the dangers of cancer (4, 7, 8, 10, 33). At the very time that interest in cancer emerged in the 19th century, however, the disease tended to disappear from view. The New York Cancer Hospital was renamed Memorial Hospital in 1899 because the word cancer was unacceptable to its patients. The disease was rarely mentioned in obituary notices (60). Moreover, popular medical fears that cancer was contagious inhibited public discussion, as did the suggestion that cancer ran in families.
Many prominent people developed cancer in the 1950s and 1960s and were never told their diagnosis (61). A survey in 1961 showed that most physicians preferred not to tell their patients of the diagnosis. The silence around the disease began to break down in America about that time. Changes in society and advances in medical diagnosis and treatment have reversed this trend. Additional factors responsible include growth in recognition of the importance of informed consent and patient autonomy during the past 40 years. Patient advocacy has emerged as a potent force, especially in breast cancer (62). Accompanying the progress in cancer research, diagnosis, and treatment, the mortality due to these diseases in the USA began to decline for the first time in the 1990s (2).
Increased attention has been focused on palliative care and on care of dying patients, some of whom have cancer. Cicely Saunders (b. 1918) developed the hospice movement in England in the early 1960s; it has spread to other parts of the world, including the USA (63). Much more effort has been directed toward educating physicians about the importance of end-of-life care, e.g., the Education for Physicians on End-of-Life Care courses offered by the American Medical Association (64).
Much of the fear about cancer has been due to ignorance (58, 59) (Figure 10). As noted, until recently many patients were never told their diagnosis, and those who were had few sources of factual information. These circumstances favored the emergence of unproven methods of treatment touted by well-known individuals in some cases and frank quackery in others (4, 8, 49, 58, 59). Controversies raged about agents such as Laetrile and krebiozen, and, later, vitamin C. Each of these agents had enthusiastic support from well-known scientists such as Andrew Ivy for krebiozen and Linus Pauling for vitamin C.
Figure 10.
The American Society for the Control of Cancer, later the American Cancer Society, emphasized public education as in this illustration from 1939. Reprinted with permission from the American Cancer Society.
Harry M. Hoxsey, one of the longest-running unorthodox practitioners of cancer therapy, perpetuated a scandalous hoax on cancer patients from 1936 to 1960 (4, 59, 65). Hoxsey, a naturopath, worked out of his clinic on Gaston Avenue almost in the shadow of Baylor Hospital (Figure 11). The author of a book entitled You Don't Have to Die, Hoxsey alleged a number of cures supported largely by testimonial evidence. He administered an herbal tonic discovered in 1840 by his great-grandfather, whose horse recovered from cancer of the leg after grazing in a field of mixed weeds. The tonic consisted of prickly ash bark, red clover blossoms, barberry root, liquorice root, pokeweed, alfalfa, buckthorn bark, and burdock root—all dissolved in cascara. In 1956, it was estimated that Hoxsey treated 8000 patients and grossed $1.5 million. The Food and Drug Administration (FDA) finally stopped Hoxsey in 1960, but not until cancer patients had been bilked out of an estimated $50 million.
Figure 11.
Harry Hoxsey's cancer clinic on Gaston Avenue in Dallas treated thousands of patients between 1936 and 1960. Courtesy American Medical Association Archives.
Fraud and quackery aside, the growth of alternative and complementary medicine in cancer patients has been vast (66, 67). The dramatic increase in patients' desire for information, the Internet, and lack of effective treatment for some types of malignancies are 3 factors influencing this striking growth. Amid this array of confusing information (sometimes misinformation) and the understandable anxiety about making crucial decisions with incomplete scientific data, cancer patients and their families need reliable sources and effective communication with their doctors. Both patients and physicians have written helpful, informative, and uplifting monographs (68–70).
Financial support of cancer research
After the Second World War, the emphasis on cancer research shifted to the USA. Through the enthusiasm and support of philanthropists such as Mary Lasker, increased attention was focused on better diagnosis and treatment of cancer and especially on cancer research. The American Society for the Control of Cancer was renamed the American Cancer Society and raised money for research as well as public education. The NCI was founded in 1937 (7, 71). Its budget jumped from $1.75 million in 1946 to $14 million in 1947 and to $110 million in 1961. The vigorous advocacy of Lasker and her associates eventually resulted in President Nixon signing the National Cancer Act in 1971. The NCI appropriation for 1973 jumped to $400 million, rising to $1 billion by 1976, $2.25 billion by 1996, and $3.3 billion by 2000 (8, 9, 59).
The military metaphors of a cancer crusade and a war to be waged against disease encountered difficulty. Susan Sontag warned about warlike imagery, saying that such images distracted from the real scientific nature of the disease (4, 58). The huge increase in financial commitment drew attention to the small payoff in cures and prevention, and some suggested it was a waste of tax dollars. Enthusiasm for the National Cancer Act had been fueled in part by the revival of viral theories of cancer and the hopeful prospect of developing a vaccine. The success of the space program led some proponents to believe that a similar triumph could be achieved against cancer. Critics likened the National Cancer Act to “moonshot” medicine, stating that unlike with the space program, the fundamental information necessary for success against cancer (i.e., basic understanding of the biology of normal and malignant growth) was not available. However, much of the progress described above in molecular biology, genetics, and immunology has indeed provided crucial information about the biology of normal and malignant growth during the past 30 years (72). These basic science insights have resulted in significant improvement for patients with certain types of cancer and will continue to benefit others in the future. Some milestones in the history of cancer are shown in Table 1.
Table 1.
Some milestones in the history of cancer
| 1600 BC | Egyptian papyri describe tumors of breast and leg |
| 400 BC | Hippocrates, the father of medicine, likens cancer to a crab; disease is due to imbalance among the 4 humors |
| ad 200 | Galen, a prolific writer whose opinions were unchallenged for almost 1500 years, believes cancer is due to an excess of black bile and is best left alone |
| 1500 | Paracelsus rebels against dogma, defies Galen, and introduces chemicals into Western medical therapeutics |
| 1680 | A microscope makes possible the discovery of spermatozoa, protozoa, bacteria, and red blood cells (Leeuwenhoek) |
| 1700 | Inflammation can result in a tumor which may become malignant (Boerhaave) |
| 1766 | Clinicopathological correlation (Morgagni) |
| 1775 | Scrotal cancer is identified in chimney sweeps (Pott) |
| 1780 | Inflammation and cancer (Hunter) |
| 1793 | Pathological anatomy (Baillie) |
| 1800 | Concept of tissues (Bichat) |
| 19th century | |
| 1830 | Improved achromatic lenses for the microscope (Lister) |
| 1832 | Lymphoma (Hodgkin) |
| 1838 | Cell theory (Schwann) |
| 1840 | Microscopic appearance of tumors (Müller) |
| 1845 | Leukemia (Bennett, Virchow) |
| Myeloma urinary protein (Bence Jones) | |
| 1858 | Cellular pathology (Virchow) |
| 1880–1905 | Cancer surgery (Billroth, Halsted, Wertheim, others) |
| 1893 | Coley's toxins |
| 1895 | X-rays (Röntgen) |
| 1898 | Radium (Curies) |
| 20th century | |
| 1900 onward | Radiation therapy for cancer |
| 1900–1910 | Chemotherapy (Ehrlich) |
| 1911 | Viral etiology of cancer (Rous) |
| 1914 | Chromosomes and cancer (Boveri) |
| 1927 | X-rays cause mutations (Muller) |
| 1937 | National Cancer Institute founded |
| 1940s | Chemotherapy for cancer—nitrogen mustard/folate antagonists |
| 1953 | DNA structure (Watson and Crick) |
| 1960s | Link between cigarette smoking and lung cancer |
| Hospice care (Saunders) | |
| Combination cancer chemotherapy | |
| 1966 | Genetic code |
| 1970 | Reverse transcriptase (Temin and Baltimore) |
| 1971 | National Cancer Act |
| “2-hit” hypothesis—retinoblastoma (Knudson) | |
| 1973 | Medical oncology established as a subspecialty of internal medicine |
| 1970s | Carcinogens are mutagenic (Ames) |
| Monoclonal antibodies (Köhler and Milstein) | |
| Proto-oncogenes (Varmus and Bishop) | |
| 1970s–1990s | Tumor suppressor genes (rb, p53) |
| Human multistep carcinogenesis—colon cancer (Vogelstein and others) | |
| Angiogenesis (Folkman) | |
| Telomerase (Shay) |
FORMATION OF THE CANCER CENTER
It is within this context that Baylor created the Charles A. Sammons Cancer Center in 1976 (Table 2). Why was the cancer center created? First, an increasing number of cancer patients were being seen at Baylor as it grew under the leadership of Boone Powell, Sr. (Figure 12) and the talented medical staff. Hemato-pathologist Dr. Joseph Hill organized the founding meetings of the International Society of Hematology (1946) and the American Association of Blood Banks (1947) at Baylor. Thoracic surgeons Dr. Robert Shaw and Dr. Donald Paulson with radio- therapists Dr. John Mallams and Dr. Richard Collier employed preoperative radiation followed by extended resection for selected patients with bronchogenic carcinoma in the superior pulmonary sulcus (Pancoast tumors) beginning in 1956. Dr. Billie Aronoff was a pioneer in the development of laser surgery in the early 1970s. Second, the National Cancer Act signed by President Nixon in 1971 gave major impetus to the cancer center concept. Third, medical oncology became established as a new subspecialty of internal medicine in 1973. Recognition of this new field was the result of a number of recent advances in cancer care in the USA. Fourth, the effectiveness of multidisciplinary interaction and combined modality therapy for certain types of cancer had been demonstrated.
Table 2.
Baylor Sammons Cancer Center time line
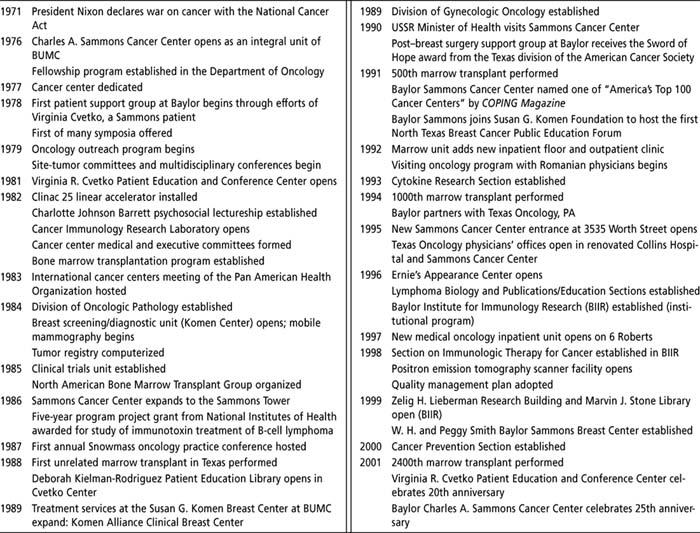
Figure 12.

Boone Powell, Sr.
These developments led Baylor's administration and medical staff to design a new component at the medical center. This effort was spearheaded by Boone Powell, Sr., who engaged the consulting firm of Booz, Allen and Hamilton. Their report outlined possible organizational schemes and desirable qualifications of key personnel. Part of the difficulty in designing Baylor's cancer center was that no such attempt had been made previously on this scale at a private hospital. Charles A. Sammons (Figure 13), a longtime Baylor benefactor, graciously donated $1 million. Mr. Sammons had previously provided funds for the virology laboratory and for the purchase of the first cobalt radiation unit at Baylor. Because of Mr. Sammons' ongoing generosity to Baylor, Boone Powell, Sr., named the cancer center for him.
Figure 13.

Charles A. Sammons.
The Charles A. Sammons Cancer Center opened on May 1, 1976, as an integral unit of BUMC. Its objective was to coordinate and facilitate patient care, education, and research in oncology at Baylor. Although the Sammons Cancer Center building was the most visible evidence of the institution's expanded commitment to caring for patients with malignant diseases, the cancer center was organized as a “center without walls,” encompassing oncology activities throughout the medical center. This key concept, although a simple one, proved challenging to implement. The Department of Oncology was established through the medical staff structure; it was the first and remains the only multidisciplinary department at Baylor. Approximately 140 members of the medical staff are members of the Department of Oncology with primary appointments in the departments of surgery (and surgical specialties), internal medicine, radiology, obstetrics-gynecology, and pathology. Criteria for initial appointment were established in 1976; those for reappointment were developed later after experience had been obtained with the existing structure and the Baylor credentials committee, chaired initially by Dr. Marvin Stone in 1992, was established.
Many individuals made key contributions to the new Sammons Cancer Center at Baylor. In addition to Boone Powell, Sr., and Mr. Sammons, a number of members of the medical staff provided major input and assistance (73). These included Drs. Billie Aronoff in surgery, George Race in pathology, Merrick (Mike) Reese in medical oncology, and Richard Collier in radiation oncology. A search committee chaired by Dr. Reuben Adams considered various candidates for the positions of director and chief of oncology. Dr. Marvin Stone was selected. Dr. Stone had been a member of the Division of Hematology-On-cology at the University of Texas Southwestern Medical School (UT Southwestern), where he was associate professor of internal medicine. He previously trained at the University of Chicago, Barnes Hospital, and the National Institutes of Health (NIH), where he distinguished himself in the fields of hematology, medical oncology, and immunology (73). Dr. Stone joined the Baylor medical staff in May 1976 when the Sammons Cancer Center opened and appointed Drs. Aronoff, Reese, and Collier as division directors of surgical oncology, medical oncology-hematol-ogy, and radiation oncology, respectively.
Initial efforts dealt mainly with establishing a solid patient base at the cancer center. Since Baylor had little previous experience with outpatients, the cancer center offered new challenges; administrative staff had extensive knowledge about inpatient care but less knowledge about the details of running an office. When the cancer center opened, radiation oncology was located on the first floor, medical oncology-hematology was based on the second floor, and surgical oncology was on the third floor. The top 2 floors of the Sammons building were shelled in. The sixth and seventh floors of the cancer center were added in the mid 1980s when the adjacent Sammons Tower was constructed.
SITE-TUMOR COMMITTEES
Site-tumor committees were formed shortly after cancer center activities began in 1976. It was felt that the old concept of a “tumor board,” where a small group of physicians would hear about patients with a variety of different types of cancer, was outmoded. With the new advances in multidisciplinary and combined modality approaches, separate committees were established for each of the major sites: bone and soft tissue, skin, head and neck, chest, breast, gastrointestinal tract, female reproductive tract, urinary tract, circulatory system, lymphatic system, and nervous system. Each committee was organized with multidisciplinary representation (from medicine, surgery, and radiation oncology plus specialists from other departments) and was responsible for conducting a conference at regular intervals and coordinating educational and research activities related to that site. The committees reported initially to the cancer center director and subsequently to the cancer center medical committee.
The site-tumor committees and their conferences have played a central role in the growth and development of the cancer center. Early on, category I continuing medical education credit was given to those attending the site-tumor conferences through Baylor's A. Webb Roberts Center for Continuing Education. The goal was to have patients with interesting or difficult diagnostic and management problems presented for educational purposes and to make access equally available for any staff member who wished to have his or her patient discussed. The evolution of these conferences resulted in certain members of the medical staff becoming recognized experts by acquiring significant breadth and depth of experience in areas related to their interests.
All the regularly held site-tumor conferences remain multidisciplinary and educational in scope. Continuing medical education credit is now necessary both for licensure in Texas and for credentialing for reappointment in the BUMC Department of Oncology. The various site-tumor conferences are attended by members of the medical staff as well as fellows, residents, medical students, nurses, and other allied health personnel. Over 200 of these site-tumor conferences are held annually, with a total attendance exceeding 4000 participants.
MEDICAL AND EXECUTIVE COMMITTEES
Initially, the cancer center was positioned on the Baylor organizational chart at the same level as the housekeeping department. By the early 1980s, it was evident that a revised structure was necessary if the cancer center was to function in a more effective and multidisciplinary fashion. Dr. Z. H. (Zeck) Lieberman, who had been active in cancer center activities and conferences since their inception, and Dr. Stone designed a 2-tiered committee system: a medical committee to oversee site-tumor committees, quality of care, and outreach activities and serve in an advisory capacity to the director (Table 3); and an executive committee responsible for overall policy and integration of cancer center programs into BUMC (Table 4).
Table 3.
Sammons Cancer Center Medical Committee, 2002
| R. Pickett Scruggs, MD, Chair | Douglas W. Orr, MD |
| James D. Bates, DDS/MD | Paul G. Pin, MD |
| John S. Bradfield, MD | John Pippen, MD |
| Claude A. Denham, MD | John T. Preskitt, Sr., MD |
| Peter A. Dysert II, MD | Charles Richardson, MD |
| Joshua K. Fine, MD | Daniel A. Savino, MD |
| John N. Harrington, MD | Weldon L. Smith, MD |
| Ronald C. Jones, MD | Wynne M. Snoots, MD |
| Stephen E. Jones, MD | Marvin J. Stone, MD |
| Patricia Krakos, MD | Dana Choate, RHIA, Cancer Registry |
| Joseph A. Kuhn, MD | Janet Kirklen, RN, Cvetko Center |
| Z. H. Lieberman, MD | Diane Cook, RN, Program Manager |
| Carolyn M. Matthews, MD | Charles Cooper, Foundation |
| Todd McCarty, MD | Tim Parris, EVP/COO, BUMC |
| Robert G. Mennel, MD | Janet Reynolds, Sammons Administration |
| John C. O'Brien, Jr., MD | Maureen Sweeny, Vice President |
Table 4.
Sammons Cancer Center Executive Committee, 2002
| Marvin J. Stone, MD, Chair | Göran Klintmalm, MD |
| Edward D. Agura, MD | Z. H. Lieberman, MD |
| Joel Allison | Robert G. Mennel, MD |
| Joanne L. Blum, MD | R. Steven Paulson, MD |
| J. Harold Cheek, MD | Tim Parris |
| Charles Cooper | John T. Preskitt, MD |
| Chuck Dowling | R. Pickett Scruggs, MD |
| Peter A. Dysert II, MD | Michael Smerud, MD |
| Michael Emmett, MD | C. Allen Stringer, Jr., MD |
| Perry Gross, MD | Maureen Sweeny, Administration |
| J. B. Howell, MD | R. Gilbert Triplett, DDS, PhD |
| Ronald C. Jones, MD |
This organizational structure was inaugurated in 1982 and remains operative. Both are standing committees of the medical staff and thus report to the medical board. Both have high-level administrative representatives as members in addition to physicians. Dr. Lieberman was the first chairman of the medical committee and served until 1992, at which time the chair was assumed by Dr. R. Pickett (Pick) Scruggs. Both of these physicians subsequently became president of the Baylor medical staff. Dr. Stone has served as chairman of the cancer center executive committee since its inception. This 2-tiered committee system has proven valuable in providing broad-based input from the medical staff, which is both necessary and desirable for the multidisciplinary organization of the cancer center. It also has served to provide integrated implementation of cancer center activities into the medical center as a whole.
The Sammons Cancer Center celebrated its 25th anniversary in 2001. Dr. Stone continues as director and chief of oncology at Baylor, positions he has held since 1976. The cancer center's strengths and accomplishments have been due to Baylor's talented and dedicated medical staff and administration. Boone Powell, Sr., and Boone Powell, Jr., were always enthusiastic and very supportive. Joel Allison has continued this pattern of involvement, actively participating in cancer center activities and further reinforcing the mission and goals of the institution. William Carter and Tim Parris have made important and continuing contributions to the growth and development of the cancer center. Paula Holder, Sylvia Coats, and Maureen Sweeny have provided inestimable assistance with cancer center administrative activities (Figure 14). Diane Cook, Margaret Albright, and many other members of Baylor's excellent nursing staff have been vital in the ongoing effort to provide consistently high-quality care for oncology patients.
Figure 14.

Upper panel: William Carter, Paula Holder. Lower panel: Maureen Sweeny, Sylvia Coats.
The Baylor Sammons Cancer Center maintains a tumor registry similar to those at other leading cancer centers across the nation. The registry has been in continuous operation since January 1960. In a fairly typical year (1999), the BUMC cancer registry abstracted 2574 analytical cases (viz, cases in which the patient was diagnosed or initially treated at BUMC). Texas Oncology, PA, with offices at the cancer center, reported an additional 1929 new analytic cases. Hence, the total number of new cancer patients seen on the BUMC campus was over 4500 in a single year. The 5 most frequent cancer sites were breast, lung, colon and rectum, prostate, and corpus uteri. For new cases, women made up 57.4% of the total and men, 42.6% compared with national figures of 48.9% and 51.1%, respectively. The higher percentage of women reflects the large number of breast cancer cases seen at Baylor (74).
The BUMC tumor registry is essential to effective patient care, education, and research at Baylor. Its reports also are made available to the Commission on Cancer of the American College of Surgeons, which accredits hospital cancer programs. The Sammons Cancer Center has earned such accreditation since its founding in 1976.
The Department of Oncology is composed of 5 divisions: radiation oncology, medical oncology-hematology, surgical oncology, oncologic pathology, and gynecologic oncology (Figure 15).
Figure 15.
Division directors: Drs. Pick Scruggs, John Preskitt, Dan Savino, Robert Mennel, and Allen Stringer.
DIVISION OF RADIATION ONCOLOGY
In the early 1960s, Baylor radiologists included Drs. Jerry Miller, A. D. Sears, and Richard E. Collier. Only Dr. Collier was doing full-time radiation therapy then. Dr. John Mallams joined the group to practice radiation therapy with Dr. Collier. In 1967, Dr. Sears became chief of radiology, and Dr. Collier was named director of radiation therapy. Several other physicians had completed their general radiology training and worked with Dr. Collier and Dr. Mallams from 1968 through 1973. These included Drs. Jesse Tomme, Herb Steinbach, and Felix Vendrell.
From 1968 through 1976, the department was located in the Truett-Veal area and was well equipped for that era. A cobalt 60 unit, a cesium 137 unit, a 100-kV x-ray machine, and a 250-kV orthovoltage machine were utilized. The most sophisticated and state-of-the-art equipment for the time was a General Electric 2-mV resonance transformer, which was dedicated with great fanfare by Ronald Reagan, then a spokesman for the company (Figure 16). During some of those years, radiation therapy was not available at Parkland Hospital and, consequently, patients from Parkland were treated over the noon hour at Baylor.
Figure 16.

Ronald Reagan, spokesperson for General Electric, visited Baylor's supervoltage unit in 1959.
Diagnostic radiology and radiation therapy had advanced as specialties, and the American College of Radiology (ACR) developed and recognized separate board certification. Drs. Mallams and Tomme left Baylor for positions elsewhere. In 1973, Dr. John S. Bradfield joined Dr. Collier and Dr. Vendrell to be the third full-time staff member in radiation therapy at BUMC. Dr. Bradfield had trained at Mallinkrodt Institute in St. Louis and was the first physician at Baylor to have been solely trained in radiation therapy instead of general radiology. In 1974, Dr. Herb Steinbach began working full-time in nuclear medicine at Baylor.
Dr. R. Pickett Scruggs joined the radiation therapy staff in 1976 shortly before the department moved to the first floor of the new Sammons Cancer Center. Boone Powell, Sr., felt it was important for radiation therapy to be above ground rather than in the basement, where facilities at many other hospitals were located. He personally supervised the selection of attractive and scenic wall coverings for each of the treatment rooms (Figure 17). The department was designated in honor of Charles and Elizabeth Prothro of Wichita Falls. For the first time, the department had a simulator (a rarity at that time) and 2 new linear accelerators (a 4-mV and a 10-mV with 5-electron beam energies) as well as a cobalt 60 unit. Shortly thereafter, a 25-mV linear accelerator was installed; this machine, the first of its kind, was jointly developed by Varian Corporation with Baylor radiation oncologists and administrators.
Figure 17.
Radiotherapy treatment room in the Sammons Cancer Center.
The physicians were part of the radiology group at Baylor called Radiology Associates of Dallas. Dr. Sears was president of the group, and Dr. Collier was director of radiation therapy. Dr. Neil Senzer joined the group in 1984 shortly before Dr. Vendrell retired. In 1989, the oncologists formed a separate group called Dallas Radiation Oncology Associates (DROA) and were instrumental in developing and staffing departments in Midland, Plano, and Sherman and recruiting radiation oncologists for those centers. In 1994, Dr. Collier retired, and the members of DROA joined Texas Oncology. Dr. Barry Wilcox joined Drs. Bradfield, Scruggs, and Senzer at Baylor in 1999.
Through the years, the radiation oncology department has had outstanding physicists. Valuable support has been provided by Herb Barnes, Chris James, and Thaddeus Sokolosky. A training program for radiation technologists (therapists) was established at BUMC in 1979. Lana Andrews directed the school from 1986 through 1996. Dr. Bradfield was medical director, and the physicians participated in the clinical lectures. During this era, the school was the largest in North Texas, had more than 65 graduates with a 100% pass rate on board certification exams, and received an outstanding accreditation review.
The department participated in the early application of hyper-thermia treatments using equipment from the 2 major manufacturers seeking FDA approval. Conformal 3-dimensional treatment planning has been utilized in the department since 1999.
Dr. Senzer serves as research director of radiation oncology for Texas Oncology and US Oncology. He is particularly involved in combined modality therapy using chemotherapy agents to sensitize tumor cells to radiation (75, 76). All physicians in the department work closely with those from other disciplines in the multimodal treatment of cancer.
DIVISION OF MEDICAL ONCOLOGY-HEMATOLOGY
The new medical oncology-hematology unit in 1976 was staffed by Drs. Reese, J. Richard Williams, John C. Bagwell, and Stone. Dr. Reese had been at Baylor since 1967 and was its first hospital-based medical oncologist. He also helped Department of Internal Medicine Chief Ralph Tompsett recruit new house-staff. Dr. Williams had joined Dr. Reese in the early 1970s. Dr. Bagwell had recently started in practice at Baylor after completing his fellowship at UT Southwestern under Drs. Eugene Frenkel and Stone.
Outreach activities began in 1979 and were based on the expressed needs of the outlying communities. Local physicians most often requested medical oncology consultation. Drs. Reese, Williams, Bagwell, Stone, Lewis Duncan, Lloyd Kitchens, Leon Dragon, Bob Mennel, and Barry Cooper provided coverage, originally to Odessa, Texas, and later to other cities. The efforts were well received and led to the concept of multicity group practice and the subsequent development of Texas Oncology. After beginning once a month, it soon was necessary to send 2 physicians per week to the outreach communities. Shortly thereafter, Dr. Charles Rietz and other members of the Baylor pathology department were included as well. Programs were soon established in Paris, Midland, Corsicana, and other Texas cities. In all these sites, the principal objective of the cancer center outreach program was, whenever possible, to provide care for patients in their local communities. Initially, this led to a reduction in referrals to Dallas. However, the more complex cases were referred to Baylor, and referrals to other members of the Baylor medical staff increased as a result of the close relationships that developed between Sammons oncologists and physicians in the outlying cities.
The visionary leadership of Dr. Mike Reese was responsible for Texas Oncology's growth into one of the largest oncology practice groups in the country and played a major role in developing the cancer center at Baylor. The long-standing relationship between Baylor's medical oncologists and the institution has been productive and mutually supportive. In 1994, Baylor and Texas Oncology became more closely affiliated through the Sammons Cancer Center, thus further augmenting the strengths of both organizations. Now Sammons Cancer Center is a joint arrangement between Baylor and Texas Oncology, with its management company, US Oncology (Figure 18).
Figure 18.
Sammons Cancer Center, Worth Street entrance.
Dr. Robert Mennel replaced Dr. Reese as division chief of medical oncology-hematology in 1996. Drs. Stone and Michael Emmett appointed Dr. Mennel professor of oncology and internal medicine in 2000. He has maintained an active teaching role with internal medicine medical students, residents, and medical oncology fellows throughout his 23-year career at Baylor. Dr. Barry Cooper also has had a key role in teaching students, residents, and fellows since he joined the Baylor staff in 1979. Dr. Cooper has been the principal physician caring for patients with hematological malignancies, especially leukemia. Drs. Mennel and Stone are codirectors of the Division of Medical Oncology in the Department of Internal Medicine. Drs. Cooper and Stone serve as codirectors of the Division of Hematology in the Department of Internal Medicine. Drs. Mennel, Cooper, and Stone all have won teaching awards from the internal medicine housestaff. Dr. Stone also directs the internal medicine clerkship for the third-year UT Southwestern medical students who come to Baylor for 6-week rotations.
Dr. R. Steven Paulson, a leader and longtime member of the Baylor Sammons staff, became president of Texas Oncology in 2001. Other members of the Division of Medical Oncology -Hematology include Drs. Joanne Blum, Claude Denham, Houston Holmes, Vinay Jain, Stephen Jones, David McCollum, Douglas Orr, Joyce O'Shaughnessy, John Pippen, and Mark Walberg. Drs. Blum, Jones, O'Shaughnessy, and Pippen comprise the Breast Medical Oncology Section. Drs. Edward Agura, Brian Berryman, Joseph Fay, Luis Pineiro, and Estil Vance are members of the Blood and Marrow Stem Cell Program. Drs. John Nemunaitis and Casey Cunningham direct the Mary Crowley Research Clinic. Many physicians in the medical oncology- hematology division have participated in a wide range of research activities; these are described in later sections. The Leukemia Association of North Central Texas has made a number of grants for patient care and research to members of the medical oncology-hematology division of the Sammons Cancer Center.
DIVISION OF SURGICAL ONCOLOGY
The surgical oncology program has been developed to foster integrated management by effectively coordinating both surgical and medical physicians committed to oncology patients. These efforts have received major support from representatives of the administration, the tumor registry, the Baylor Foundation, and the marketing department.
Surgical members of the various site-specific tumor committees were selected by each department chief and Dr. Stone so that they would represent their areas of expertise and serve as liaison between the cancer center and the primary department. All BUMC surgeons were offered the opportunity of becoming members of the surgical oncology division if they maintained adequate training, participated in the conferences, allowed their patients' management to be reviewed, and assisted in the development of protocols. This concept was adopted because the hospital is a community and tertiary referral hospital rather than a freestanding cancer center. Similar requirements for reappointment were implemented for members of the other divisions of the cancer center on the recommendation of a committee chaired by Dr. John Preskitt. These criteria for initial appointment and reappointment were formally adopted by the Department of Oncology and subsequently by the Baylor credentials committee. Dr. Preskitt became the first chair of the cancer center quality committee when it was formed in 1998. In 2001, Dr. Preskitt was appointed division chief of surgical oncology, replacing Dr. Lieberman.
In addition to Drs. Aronoff, Lieberman, and Preskitt, members of the Department of Surgery who have been especially active in surgical oncology at BUMC have included Drs. Ron Jones, Harold Cheek, Miller Bell, Howard Derrick, Michael Grant, Sally Knox, Joseph Kuhn, Todd McCarty, Thomas Newsome, John O'Brien, Bruce Smith, and Jeffrey Stephens, as well as Drs. Göran Klintmalm and Robert Goldstein from the Division of Transplant Services. Members of other surgical departments also have made significant contributions to cancer center programs. Dr. Wynne Snoots has chaired the bone and soft tissue site committee and conference since their inception. Drs. Rick Hebeler, Tom Meyers, and Richard Wood (thoracic surgery), Warren Lichliter and Rick Dignan (colon and rectal surgery), and Fritz Barton, Byron Brown, Paul Pin, and William Carpenter (plastic and reconstructive surgery) have been valuable participants in cancer center activities.
DIVISION OF ONCOLOGIC PATHOLOGY
As noted previously, Dr. George Race, chief of the Department of Pathology for 27 years, was one of the medical staff leaders instrumental in supporting the concept and planning for a cancer center at Baylor. He also served as dean of the A. Webb Roberts Center for Continuing Education from 1972 until 1994 and was founding editor of BUMC Proceedings. When Dr. Stone joined Baylor as chief of oncology and director of the Sammons Cancer Center, he also became director of immunology in the Department of Pathology. Dr. Joseph Newman has worked with Dr. Stone in the immunology laboratory since 1976. Drs. Weldon Tillery and Peter Dysert succeeded Dr. Race as chief of pathology, and both have played important roles in the growth and development of the cancer center. The Division of Oncologic Pathology was established by Drs. Stone and Race in 1984; Dr. William Kingsley, chief of surgical pathology, was appointed division director. Upon Dr. Kingsley's retirement, Dr. Daniel Savino became chief of surgical pathology and director of oncologic pathology. Dr. Savino has served as moderator for the popular biweekly breast conference for several years. Many other members of the pathology department participate in cancer center activities, especially Drs. Charles Rietz, George Netto, David Watkins, Richard Meyer, and William Herlihy.
It is not possible to practice modern oncology without an excellent pathology department. Through the leadership of Drs. Race, Tillery, and Dysert, Baylor clinicians and patients have benefited immeasurably from the expertise in pathology at this institution. The involvement of Drs. Savino, Rietz, and Netto has been key to the development and maintenance of high-quality cancer center educational and research activities and to patient care. Members of the department of pathology, including residents, actively participate in all site-tumor conferences. Their contributions continue to be crucial to the care of oncology patients at Baylor.
DIVISION OF GYNECOLOGIC ONCOLOGY
Gynecologic oncology formally began at the Sammons Cancer Center in 1989 with the arrival of Dr. C. Allen Stringer, Jr. Dr. Stringer, having finished his fellowship at M. D. Anderson Cancer Center in 1984, had remained on the faculty there. Drs. Joe Jacobs and Carolyn Matthews joined Dr. Stringer upon completion of their fellowships at M. D. Anderson.
Busy outreach clinics were developed in Tyler, Longview, Paris, Midland, and Odessa, and later in Corsicana and Abilene. In 1992, Dr. Jacobs moved to Missouri to be closer to family. He was replaced by Dr. Alan Gordon, who trained a year ahead of Dr. Stringer in Houston.
Dr. Gordon rapidly displayed his leadership as head of gynecology research, joining the nationwide cooperative gynecologic oncology group as affiliate members of Ohio State University. In addition, he became principal investigator for several Baylor studies, presenting regularly at American Society of Clinical Oncology conferences and many international meetings. He has been senior author on a number of peer-reviewed articles dealing with ovarian cancer (77–79). Dr. Gordon was invited to join the editorial board of the journal of the Society of Gynecologic Oncologists in 2001.
In addition to practicing gynecologic oncology, Dr. Stringer became chief of the obstetrics and gynecology department at BUMC in 1993. He also serves as head of gynecologic oncology for Texas Oncology and US Oncology. Dr. Matthews became the residency program director in 1993 and remained in that position until 1999. In 2002, Dr. Stringer assumed the additional position of medical director of the Virginia R. Cvetko Patient Education and Conference Center at the Sammons Cancer Center. Drs. Stringer, Matthews, and Gordon remain heavily invested in the teaching program, and all have won teaching awards from residents and medical students.
Dr. Mark Doherty joined the group in 2000, having been in private practice in San Antonio for 7 years preceded by 7 years of teaching at the University of Texas Medical Branch at Galveston. He, too, was trained at M. D. Anderson, finishing there in 1986. Dr. Doherty was charged with expanding gynecologic oncology services to Methodist Hospital in addition to working at Sammons Cancer Center.
PROGRAMS
Breast cancer
Development and organization. The care of patients with breast cancer has had a long history of growth and development at Baylor. Dr. J. Harold Cheek provided the initial impetus by becoming one of the first surgeons in the Southwest to limit his practice to breast disease and by establishing the Breast Cancer Education and Research Fund in the Department of Surgery. Subsequently, the Seeger Endowed Fellowship in Surgical Oncology of the Breast, one of the first of its kind in the USA, was established at Baylor. Dr. A. D. Sears, former chief of radiology, established with the support of Dr. Cheek the first mammography unit at Baylor. The Susan G. Komen Breast Center and the Komen Alliance Clinical Breast Center were developed in 1984 and 1989, respectively (see breast imaging section). The W. H. and Peggy Smith Baylor Sammons Breast Center was established in 1999 (Figure 19). Over 600 patients with newly diagnosed breast cancer were seen at Baylor in the year 2000 (80).
Figure 19.
Drs. Pat Krakos, Joyce O'Shaughnessy, John Pippen, and Joanne Blum in the W. H. and Peggy Smith Breast Center.
The breast center offers free breast cancer risk assessments using a computerized model, breast health information through community programs, and breast cancer prevention and treatment research trials. A breast cancer risk evaluation program directed by Dr. Joanne L. Blum offers genetic counseling and testing (81). Dr. Blum is also active in breast cancer therapy research (82, 83). Her article on the use of the chemotherapy agent capecitabine in patients with metastatic disease was named as a classic article by the Journal of Clinical Oncology.
The mammography unit, formerly known as the Komen Breast Center, became the Baylor Sammons Breast Imaging Center in 2002 and is directed by Dr. Patricia Krakos. The breast imaging center is one of the largest of its kind.
Dr. Joyce A. O'Shaughnessy was named director of cancer prevention research in 2000 and directs a breast cancer risk assessment project in the W. H. and Peggy Smith Breast Cancer Center. She is also an active investigator in research, including a project on ductal lavage that is aimed at developing a test to give individual women more information about premalignant changes that may be occurring in their breasts (84–86). Ductal lavage has become available for high-risk women as part of standard risk assessment. Dr. O'Shaughnessy also directs an intervention clinical trial using selective estrogen receptor modulator agents.
The breast site-tumor committee has been one of the most active at the Sammons Cancer Center. This committee was headed by Dr. W. Phil Evans through 2001, at which time Dr. John E. Pippen became chairman. The breast cancer site conference, moderated by Dr. Dan Savino, is held at 2-week intervals and regularly has a standing-room-only crowd from multiple medical and allied health disciplines.
Breast cancer symposia were held in 1978, 1983, 1988, 1991, 1993, 1995, and 1997. These have generally been 2-day meetings in which 10 to 12 internationally known experts in various aspects of multidisciplinary breast cancer care participated. Several of these special breast conferences were cosponsored by the Department of Surgery with the active collaboration and support of Drs. Robert S. Sparkman, Jesse Thompson, and Ron Jones. The 1997 symposium, sponsored by Sammons Cancer Center and the Department of Surgery, included 14 international authorities providing a multidisciplinary update on various aspects of diagnosis and treatment. Boone Powell, Jr., and Drs. Stone and Jones named that symposium in honor of Dr. Cheek. At the dedication of the W. H. and Peggy Smith Baylor Sammons Breast Center in 1999, Dr. Cheek received the Wings of Eagles Award from Boone Powell, Jr. (Figure 20).
Figure 20.
Boone Powell, Jr., presenting the Wings of Eagles Award to J. Harold Cheek, MD.
Psychosocial support activities in the Cvetko Center have been expanded to include distinct breast cancer support groups. The medical staff and administration remain strongly committed to further growth and development of breast cancer care, research, and education.
Clinical trials. The clinical trials program at the Sammons Cancer Center was established in 1985 when Dr. Stephen Jones joined the staff. He had previously been professor of medicine and chief of the Division of Hematology/Oncology at the University of Arizona. Dr. Jones came to Baylor to establish clinical investigative studies, primarily in breast cancer. A number of important trials were designed and completed through his efforts (87–91). Among the most significant has been one of the earliest trials for adjuvant therapy of patients with node-negative breast cancer. In addition, Dr. Jones' studies have played an important role in gaining FDA approval for new drugs used in breast cancer.
The Sammons Cancer Center also has participated in trials sponsored by the National Surgical Adjuvant Breast and Bowel Program (NSABP), administered initially by Dr. George Peters and, since 1995, by Dr. Michael Grant. Baylor Sammons and satellites at Presbyterian and Parkland Hospitals participated in the NSABP Breast Cancer Prevention Trial, which closed to accrual in 1997. Sammons Cancer Center was one of the leading participants in this randomized study in which women considered to be at high risk of developing breast cancer were given either the antiestrogen agent tamoxifen or placebo (92). Over 13,000 women participated in the study nationwide; results indicated a 50% reduction in breast cancer in the tamoxifen-treated group. Dr. Grant is directing Sammons Cancer Center's participation in the second NSABP prevention trial, which will compare tamoxifen with raloxifene for prevention of breast cancer in high-risk postmenopausal women.
Breast imaging. Breast imaging began at Baylor in 1968 when Dr. A. D. Sears attended a radiology equipment meeting and saw the first-ever dedicated x-ray unit designed solely for breast imaging. He obtained the mammography equipment and soon was demonstrating to the medical staff that breast cancer could be found before it could be felt. This was a difficult concept for some surgeons to accept. Others, such as Dr. Cheek, embraced the concept and strongly supported the notion that women could be screened for breast cancer (93).
A new problem was soon recognized. Breast cancer manifested by calcifications was being found by mammography that not only was not palpable but could not be seen grossly in tissue specimens. Dr. Sears would x-ray breast surgical specimens and direct the pathologists to the very small cancers. With the able cooperation of Dr. William Kingsley of the pathology department, the method of “specimen radiography” was developed. A tabletop x-ray unit was employed to examine breast surgical specimens in the pathology department immediately after surgery to document that the cancers had been found and removed.
By the early 1970s, a new process called xeromammography had become available and provided improved breast imaging with blue and white images printed on paper rather than black and white images on film. With this technique and the realization that breast cancer could be found mammographically several years before it became palpable, the utilization of mammography began to grow.
Prior to the mid 1970s, few women ever publicly discussed their diagnosis of breast cancer. That paradigm changed when Betty Ford, the wife of President Gerald Ford, and Happy Rockefeller, the wife of Governor Nelson Rockefeller of New York, announced to the world that they had breast cancer and openly discussed their treatment plans (62). Public reaction was surprisingly swift and supportive, and a veritable flood of frightened women sought mammography.
Then in 1976, a scientific article was published that shocked the mammographic world (94). Dr. John Bailar raised concern that radiation doses required for mammographic imaging in the 1960s could induce as many cancers as might be cured from early mammographic detection. This pessimistic evaluation resulted in a reassessment of potential radiation risk and reviews of the available data. Simultaneously, research was stimulated and resulted in the development of mammography systems that produced markedly improved images at doses an order of magnitude lower than those used previously. But public confidence in mammography was shaken; fewer women requested mammography, and fewer physicians recommended it. Because of these concerns about breast radiation, other methods to detect breast cancer were suggested. Heat-sensitive brassieres and thermography (photography of breast heat patterns) were utilized briefly but were found to be inaccurate.
Another modality with no ionizing radiation that showed potential in imaging breast cancer was ultrasound. In 1980, the first dedicated breast ultrasound screening unit was evaluated at Baylor. It soon became clear that breast ultrasound was not ready for screening but had great potential as a diagnostic tool, differentiating cystic from solid breast masses and demonstrating the internal matrices of tumors. Studies done at Baylor and scientific presentations made throughout the country by Baylor physicians began to convince physicians of the value of breast ultrasound.
In response to the need for a specialized breast imaging center, the Baylor Breast Center opened in the Barnett Tower in 1984. The unit had 2 unique aspects. First, self-referred patients (women who did not have a physician or women seeking consultation about a breast problem) were accepted. Referrals to surgeons were made in rotation from a referral list approved by Dr. Jesse Thompson, chief of the Department of Surgery. Second, patients were given the results of their exams at the completion of the visit. This “results-before-you-leave” policy was designed to minimize the anxiety associated with waiting several days or weeks for results. The rapid reporting of results led to tremendous customer satisfaction and prompted changes by other facilities in Dallas and the nation.
Other breast cancer activities in Dallas also were beginning. Nancy Brinker, a Dallas resident originally from Peoria, Illinois, had recently lost her younger sister, Susan G. Komen, to breast cancer. Nancy promised her sister that she would do everything she could to find a cure for the disease, and she founded the Susan G. Komen Breast Cancer Foundation in 1982 (Figure 21). The Sammons Cancer Center organized educational programs for the public in conjunction with the Komen Foundation. Because of the emphasis Baylor was placing on breast cancer, the Foundation presented Baylor with a $100,000 gift in 1984. In response to this generous gift, Boone Powell, Jr., changed the name of the breast imaging unit to the Susan G. Komen Breast Center at BUMC.
Figure 21.

Nancy Brinker.
Another larger Komen Breast Center location opened in the spring of 1986 on the third floor of the Sammons Cancer Center. The proforma called for 2500 patients to be seen the first year, but over 8000 came. In addition, a mobile unit began service so that women could obtain mammograms at various locations throughout the metroplex (95).
In 1987 and 1988, the breast center participated in the American Cancer Society's Texas Breast Cancer Screening Project. During these 2 years, over 100,000 Texas women participated in the program. The Susan G. Komen Breast Centers at Baylor had the highest participation rate of any facility in the state (96). Such projects in Texas and other states led to the realization that the quality of mammography was not the same in all facilities. The ACR began a voluntary program for mammography accreditation in 1988. This program had 2 parts: an evaluation of the mammography units by physicists and review of the clinical images by expert mammographers. The Susan G. Komen Breast Center was one of the first in the nation to receive ACR accreditation in mammography.
In 1988, breast cancer was diagnosed by imaging-guided needle biopsy for the first time at the Komen Center. Later that year, the findings of 50 cases were presented at the Radiologic Society of North America annual meeting (97). This experience and that of others led to the development of stereotactic and ultrasound-guided core-needle biopsy.
In 1989, Nancy Brinker and other Komen Foundation volunteers met with Boone Powell, Jr., of Baylor and Kern Wildenthal of UT Southwestern to explore the idea of a joint venture between the 3 institutions that would result in Dallas becoming a widely recognized center for breast cancer research and treatment. Baylor was to be responsible for the clinical program; UT Southwestern, the research program; and the Komen Foundation, the fundraising effort. Baylor physicians Drs. Marvin Stone and Z. H. Lieberman joined Powell, Brinker, and Wilden-thal in the creation of the Komen Alliance.
The Komen Alliance Clinical Breast Center opened in 1989 following expansion of the Komen Breast Center at Sammons. The new center occupied over 8000 square feet and provided full service breast imaging, multidisciplinary consultation, breast cancer risk assessment, access to clinical trials, a program for underinsured women, and a breast cancer information hotline. The Komen Foundation also generously provided a $30,000 endowment for economically disadvantaged or uninsured women needing a screening mammogram. The funds for this endowment continue to the present and have provided hundreds of women with a mammogram they otherwise could not afford. Breast cancers have been detected in an early stage that would not have been found for several years.
In 1990, as a result of a grant from Mabel Caruth and the Hillcrest Foundation, the Komen Center obtained a dedicated prone table for performing stereotactic mammographically guided core-needle breast biopsy. Later in 1991, the ultrasound-guided breast biopsy program began. These new procedures provided accurate diagnosis of breast cancer using needle biopsy at approximately one third the cost of surgical biopsy and with less morbidity. Baylor physicians including J. Harold Cheek, Ron Jones, Steve Jones, George Peters, and Dan Savino were very supportive of these programs and contributed greatly to their success (98).
The breast imaging fellowship began in 1992 as a 6-month program providing board-certified diagnostic radiologists with specialized training in screening, diagnostic, and interventional breast imaging procedures and research. The curriculum was later changed to 1 year with 6 months of clinical breast imaging and 6 months of research. Expansion to 2 fellows peryear began in 1999.
Also in 1992, Dr. Steve Harms, a diagnostic radiologist at Baylor with extensive experience in MRI and a special interest in breast cancer, developed a technique called rotating delivery of excitation off resonance (RODEO). The subsequent breast MR images provided some of the clearest depictions of breast cancer ever seen in vivo. Physicians at the Komen Center worked closely with Dr. Harms in clinical research comparing mammography with MRI (99–101).
In 1993, the Komen Center participated in the largest multi-institutional study of stereotactic biopsy up to that time. Over 6000 patients were enrolled, with the Komen Center recruiting the second largest number of cases. This study and others demonstrated that many institutions could achieve the same success with imaging-guided needle biopsy that was found at Baylor (102–104).
Physicians at the Komen Center helped develop the ACR stereotactic biopsy accreditation program in 1996 and the ACR ultrasound-guided biopsy accreditation program in 1997. The center was one of the first in the country to achieve accreditation in both areas. Komen Center physicians serve as clinical reviewers for the ACR mammography, stereotactic breast biopsy, and breast ultrasound accreditation programs.
By 1996, there was another major controversy with mammographic screening. Many physicians questioned the benefit of screening women between the ages of 40 and 49 for breast cancer. The Komen Center had a large database of women with a diagnosis of breast cancer. Physicians reviewed the data and found that the relative incidence of ductal carcinoma in situ (DCIS) compared with that of invasive ductal carcinoma (IDC) was greater in women <50 years vs women ≥50 years. This study supported the theory that DCIS was a precursor to IDC and that finding DCIS in women under 50 should reduce the incidence of IDC in later years. The data were presented at a Radiological Society of North America meeting, received national attention, and were welcomed by proponents of screening women <50 years of age (105). The role of screening mammography in the 40- to 49-year age group continues to be vigorously debated.
In 1998, the ACR produced a video describing how to perform both stereotactic and ultrasound-guided breast biopsy and how to apply for accreditation. The procedures were performed by the physicians and technologists of the Komen Center and narrated by Dr. David Dershaw of Memorial Sloan-Kettering Cancer Center in New York and Dr. Evans of the Komen Center at Baylor. The video serves as a teaching tool for the Armed Forces Institute of Pathology, the ACR National Conferences on Breast Cancer, and many residency programs and is available to radiologists and facilities on request.
By the year 2000, most medical imaging was performed in a digital format. However, because of the complexity of the mam-mographic image, film images continued to be the standard in breast imaging. The Komen Center participated with General Electric in a study to determine the feasibility of using computer-assisted detection with digital imaging. The center was the first in the country to evaluate digital mammography with computer-assisted detection. At the end of 2000, another milestone was achieved. The Komen Center and its mobile mammography vans performed over 50,000 exams that year.
In 2002, the mammography unit was renamed the Baylor Sammons Breast Imaging Center and was integrated more fully into the W. H. and Peggy Smith Baylor Sammons Breast Center. Dr. Patricia Krakos became medical director following the departure of Dr. Evans. Drs. Michele R. Miles, Joseph Spigel, and William deLeon, all dedicated mammographers, staff the imaging center with Dr. Krakos and other Baylor radiologists. The breast imaging unit at the Sammons Cancer Center has become one of the most widely recognized services on the Baylor campus.
Marrow and blood stem cell transplantation
During Baylor's long-term planning process in the late 1970s, the cancer center task force recommended development of a bone marrow transplantation program. This was its top priority and was based on recent major advances in the field as well as the medical staff's interest in hematologic malignancies such as leukemia, lymphoma, and multiple myeloma. Dr. Joseph W. Fay, an experienced marrow transplant physician from Duke University and the NIH, was recruited to run this new program. The Baylor Sammons program began in 1982. To our knowledge, no full-range allege -neic and autologous marrow transplant program had been independently established at a private hospital at that time. Such an undertaking required a large commitment from the Baylor administration, including construction of new laboratories and a specialized inpatient unit in the Collins wing.
The Marrow Transplant Section was the first transplant program established at Baylor and became a major component of the clinical and research effort at the cancer center. The first unrelated marrow transplant in Texas was accomplished at Baylor in 1988 and, by 1994, 1000 transplants had been performed. The program focused on design and implementation of novel pre-transplant conditioning regimens for hematological malignancies, marrow aplasia, and selected solid tumors. Several of these regimens have been published and are still used at Baylor and at other transplant centers. Investigator-initiated research was launched in the areas of hematopoietic cytokines, prevention and treatment of infectious complications in transplant patients, and new methods for prevention and treatment of graft-vs-host disease after al-logeneic hematopoietic stem cell transplantation (106, 107).
The program at Baylor was among the first in the USA to establish clinical transplantation using unrelated hematopoietic stem cell donors. In addition to being a transplant center, the Baylor center was one of few in the nation that recruited donors and procured hematopoietic stem cells for the National Blood and Marrow Donor Program. Survival rates for Baylor patients with leukemia, myeloma, and aplastic anemia have been among the highest after an unrelated transplant compared with rates from other transplant centers, as recently published by the National Blood and Marrow Donor Program. Baylor was among the founding transplant centers for the Society of Blood and Marrow Transplantation. Physicians of the Baylor transplant program helped the society establish a journal for peer-reviewed manuscripts in clinical and laboratory transplantation science. In addition, Baylor's transplant program helped to create an accreditation body, the Foundation for the Accreditation of Hematopoietic Cell Therapy. Accreditation by the foundation is important for transplant centers in the USA, as this agency mandates excellence in patient care, teaching, and research. Members of the Baylor Blood and Marrow Transplant Program have significantly contributed to the success of the International Research Registry of Blood and Marrow Transplantation and the American Registry for Autologous Blood and Marrow Transplantation.
Recently, BUMC established the Baylor Institute for Immunology Research (BUR) on campus. Dr. Fay is actively involved with the design and implementation of clinical research studies involving dendritic cell vaccination for patients with cancer (56, 108). These studies have been funding by the NIH. Other investigative activities with BUR scientists include studies of the pathogenesis of graft-vs-host disease, posttransplant induction of immune tolerance, and detection of cancer cells in the blood after dendritic cell immunotherapy.
Drs. Robert Collins, Luis Pineiro, and Ed Agura joined Dr. Fay during the early 1990s. Dr. Collins organized a registry that collected data on the effect of donor leukocyte infusions in patients with relapsed malignancy after allogeneic bone marrow transplantation (109). Dr. Collins left to establish the bone marrow transplant program at UT Southwestern in 1998 and was replaced by Dr. Estil Vance. Dr. Agura became interim director of the Blood and Marrow Transplant Program when Dr. Fay assumed the position of director of immunological research for cancer in 1998. Dr. Brian Berryman joined the transplant team in 2001 (Figure 22). Dr. Pineiro serves as medical director of the marrow/stem cell processing laboratory and the apheresis unit. Both stem cell autografts as well as allogeneic transplants continue to be performed at Baylor. More than 200 such procedures were accomplished in 1999 and, by 2001, the 2400th blood/ marrow stem cell transplant had been performed. An annual reunion is held each spring for previous transplant recipients and their families (Figure 23).
Figure 22.
Marrow/stem cell transplant physicians: Drs. Luis Pineiro, Ed Agura, Brian Berryman, Estil Vance, and Joe Fay.
Figure 23.
Bone Marrow Transplant Reunion, April 2000.
Virginia R. Cvetko Patient Education and Conference Center
Patient education and psychosocial activities have been an important part of the Sammons Cancer Center since its inception. The initial efforts were made by Virginia Cvetko, a patient of Dr. Reese, who was dedicated to the concept of having a support group for cancer outpatients at Baylor. It soon became clear that support groups provided a valuable missing ingredient for many patients. Virginia's patient support group was the first at Baylor; now there are over 30. When she died in 1980, her family and friends established the Virginia R. Cvetko Center in the Sammons Cancer Center, and it opened the following year. The center celebrated its 20th anniversary in 2001 (Figure 24).
Figure 24.
Cvetko Center 20th anniversary. Back: Dr. Pick Scruggs, Will Rodriguez, Bill Barrett, Ed Cvetko. Front: Tim Parris, Travis Maxwell.
The center serves as the fulcrum of educational and psychosocial support activities for cancer patients at Baylor. The original Cvetko group became so popular that its members refused to disband after the program was completed, so an alumni group was formed. Among the support groups now offered by the Cvetko Center are a caregiver program, colorectal cancer support group, inpatient support programs, melanoma education and support group, New Beginnings, North Texas myeloma support group, oral and head and neck cancer support program, ovarian cancer support group, post-breast surgery support program, prostate cancer education and support group, self-help group, young women's breast cancer group, and the BUMC employee and caregiver support group. In collaboration with the American Cancer Society, the Cvetko Center also offers the CanSurmount, Dialogue, and Look Good … Feel Better programs. These educational and psychosocial support activities reach over 2800 patients and family members annually. All are provided free of charge due to support from BUMC and Texas Oncology.
One of Virginia Cvetko's close friends, Charlotte Barrett, helped in the activities of the initial outpatient group and took the lead in continuing the group after Virginia's death. Virginia and Charlotte had been longtime friends before either became a cancer patient (Figure 25). When Charlotte died, her family and friends established the Charlotte Johnson Barrett Lectureship in Psychosocial Medicine, which annually brings to Baylor a recognized authority in one of the disciplines related to patient education and psychosocial support. The Barrett lecturers are listed in Table 5. They meet with members of the staff and with patient groups during their visit to the Sammons Cancer Center.
Figure 25.
Virginia Cvetko and Charlotte Barrett.
Table 5.
Charlotte Johnson Barrett lecturers
| Jimmie Holland, MD |
| Eric Cassell, MD |
| Barrie Cassileth, PhD |
| Sr. Rosemary Therese Moynihan, ACSW |
| David K. Wellisch, PhD |
| Deborah Welch-McCaffrey, RN, MSN, OCN |
| Fitzhugh Mullan, MD |
| David Spiegel, MD |
| Jimmie Holland, MD |
| J. William Worden, PhD |
| Wendy S. Harpham, MD |
| Fawzy I. Fawzy, MD |
| Kathy LaTour, BA, MFA |
| Harold Vanderpool, PhD, ThM |
| Leslie R. Schover, PhD |
| Joanna Bull, MA, MFCC |
| Christina Puchalski, MD, MS |
| Alastair Cunningham, PhD |
In 1988, Dr. and Mrs. Marvin Stone established the Deborah Kielman-Rodriguez Library in the Cvetko Center in memory of an inspirational young woman who died of Hodgkin's disease. The library provides informative books, tapes, and other educational materials for patients and families. In 1996, the Cvetko Center launched the Healing Environment program to complement and enhance patient treatment through the use of music, humor, art, and relaxation techniques. This popular program has now been extended to other Baylor hospital departments and has been instrumental in championing the Interfaith Garden of Prayer, which will be located directly in front of the cancer center and dedicated during Baylor's centennial in October 2003.
The Cvetko staff is multidisciplinary and includes nurses, social workers, chaplains, and many patient volunteers. Dr. Lloyd W. Kitchens, Jr., served as the medical director of the Cvetko Center from its founding in 1981 until his death in 2001 (Figure 26). His contributions to the growth and development of Cvetko Center activities were beyond measure. Dr. C. Allen Stringer, Jr., was appointed medical director for the Cvetko Center in 2002. Kathy Thomas-Welch, LMSW, and Travis Maxwell, MDiv, have been longtime social worker and chaplain, respectively, on the Cvetko staff. Jann Aldredge-Clanton, PhD, MDiv, Pam Reinke-Walter, LMSW, ACP, and Phyllis Yount, LMSW, ACP, also staff the Cvetko Center with Janet Kirklen, RN, MHCA, the oncology nurse educator and program manager.
Figure 26.

Lloyd W. Kitchens, Jr., MD.
Ernie's Appearance Center, a specialty boutique that assists patients with their cosmetic needs during and after cancer therapy, opened in 1996. Proceeds from Ernie's are designated for Sammons Cancer Center research projects.
RESEARCH
Progress in medicine comes about through carefully conducted research and the discovery and application of new knowledge. Advances in basic sciences, especially molecular biology, immunology, and genetics, have brought us to the threshold of a new era in oncology at the beginning of the 21st century. Research has been a key activity in Sammons Cancer Center since its founding in 1976. Research is also part of the mission of BUMC and the Baylor Health Care System. Some of the cancer center research activities are briefly described below. Research projects related to breast cancer and marrow/blood stem cell transplantation were described in those sections.
US Oncology
In the year 2000, the 850 physicians in US Oncology saw over 500,000 patients (1 in 7 US cancer patients) at 496 sites in 27 states. More than 3500 patients were placed on clinical trials, more than through any other US medical enterprise (110). These trials have had a significant impact in the FDA approval of 8 new oncology drugs during the previous 5 years. The physicians at Sammons Cancer Center have played a major role in clinical trials research (111). Fifty different clinical trials were open at Sammons Cancer Center at the end of 2000. The number of patients put on clinical studies at Sammons was nearly 3-fold higher than the US Oncology national average. Many Sammons physicians serve on US Oncology research committees. Dr. Joyce O'Shaughnessy chaired the research steering committee, and Drs. Casey Cunningham, Alan Gordon, Steve Jones, John Nemunaitis, and Neil Senzer were members. Drs. Jones, Nemunaitis, O'Shaughnessy, and Senzer also served on the US Oncology clinical research advisory board. One or more Sammons physicians served on 12 other site committees, which develop, review, and manage new and ongoing clinical trials within the US Oncology network. Drs. Cunningham, Gordon, Holmes, Jain, Jones, Nemunaitis, O'Shaughnessy, Senzer, and Stringer served as principal investigators for various clinical trials in 2000. Thus, Sammons physicians played a leading role in US Oncology research and were authors on 61 publications in medical journals and 52 abstracts for presentation at medical meetings during 2000. Dr. Nemunaitis has authored many articles in peer-reviewed journals on gene therapy (112–114). The extensive involvement of Sammons physicians in clinical research provides patients with the latest therapies.
Cancer immunology
The Cancer Immunology Research Laboratory has been directed by Dr. Stone and Dr. Alex Tong since it was established in 1982. This laboratory was made possible by a generous gift from Max Thomas, a patient of Dr. Bagwell. Dr. Tong's work has focused on the generation and characterization of monoclonal antibodies for diagnosis and treatment of cancer patients, the mechanisms of drug resistance in myeloma and hepatoma cells, and factors influencing the differentiation and proliferation of myeloma and breast cancer cells (115–118). Dr. Tong also has conducted studies with Dr. Nemunaitis dealing with gene therapy and the use of ribozymes to inhibit lung cancer growth in experimental animals.
Collaborative research
BIIR. Dr. Jacques Banchereau, an expert in cellular and molecular immunology, directs the BIIR in the Z. H. Lieberman Building, which opened in 1999. Named for the outstanding Baylor clinician and key contributor to cancer center activities, the Lieberman Building houses modern laboratories, the John S. Fordtran Conference Room, and the Marvin J. Stone Library. The laboratory designated for transplantation research is named for Dr. Thomas Starzl. The goal of BIIR is to bring together talented basic scientists and clinicians to increase understanding of how the immune system works. The institute is devoted to translating basic discoveries about the immune system into more effective treatments for patients. A key objective of BIIR is to establish and define the potential therapeutic use of dendritic cells as vaccines in various diseases, with initial emphasis on cancer. Dendritic cells are made in the bone marrow and act as “directors” or “commanders” of the immune system. Drs. Banchereau, Karolina Palucka, and Joe Fay have initiated an exciting series of NIH-funded studies in collaboration with investigators at the Rockefeller University using dendritic cells loaded with tumor peptides to treat patients with malignant melanoma. Initial results have been promising and suggest a correlation between immune status and clinical condition (56). Further development of this innovative biological treatment modality for melanoma and other cancer patients is eagerly awaited.
Liver transplantation studies. Collaborative studies with Dr. Göran Klintmalm and the liver transplant team have principally dealt with 3 areas: 1) the use of neoadjuvant chemotherapy in transplant patients with hepatocellular carcinoma (hepatoma), 2) prevention of recurrent thrombosis in patients transplanted for the Budd-Chiari syndrome (BCS), and 3) identification and investigation of graft-vs-host disease in liver transplant recipients.
The hepatoma study examined the use of pre-, intra-, and postoperative chemotherapy with doxorubicin to delay tumor recurrence after transplantation. Hepatoma patients transplanted without chemotherapy usually develop tumor recurrence within 2 years. Initial results at Baylor were promising in that 50% of the chemotherapy-treated patients were tumor-free at 4 years, though late recurrences followed (119, 120). The chemotherapy appeared to have “shifted the curve to the right.” Because the waiting time for donor livers lengthened considerably after the first study was completed, further exploration of this approach has been delayed. Hopefully, this project can be resumed at a later date when donor waiting times become shorter and it is again feasible to employ neoadjuvant chemotherapy for patients with primary hepatic malignancies. This combined-modality approach seems worthy of further study since there is no clearly effective treatment for patients with hepatocellular carcinoma. Moreover, the incidence of this malignancy likely will increase in the USA in the future due to its association with hepatitis C.
BCS results from hepatic vein obstruction, usually thrombosis, and often leads to liver failure. The BCS study was carried out over a 14-year period and involved all patients transplanted at Baylor with this disorder through the year 2000 (121). Approximately 70% of patients with BCS in the USA have evidence of underlying myeloproliferative disorders, which are acquired clonal bone marrow stem cell diseases. Treatment of this subgroup of BCS patients with antiplatelet therapy has proven effective and safer than long-term anticoagulation, the standard treatment for such patients in the past.
Graft-vs-host disease is a rare complication of liver allografting, but it has been identified in 12 recipients since 1990 (122, 123). Although it is usually fatal, some patients survive with variable degrees of immunologic reconstitution. The Baylor studies have focused on recognition and treatment for this unusual but serious complication of liver transplantation.
UT Southwestern. In 1985, Drs. Jonathan Uhr and Ellen Vitetta, investigators at UT Southwestern, initiated a collaborative research project with Dr. Stone involving the use of immunotoxin molecules for treatment of patients with B-cell non-Hodgkin's lymphoma. Drs. Uhr and Vitetta were well-known immunologists who had become recognized experts in preclinical studies utilizing monoclonal antibodies linked to a portion of a toxin. The toxin used, ricin, became well known in the 1970s when the KGB used a drop of it on an umbrella to assassinate a Bulgarian diplomat. The clinical trials were performed at Baylor under Dr. Stone's direction with Drs. Fay, Collins, Jain, and Joe Newman using the immunotoxins made at UT Southwestern (124–126). The Baylor program was among the earliest clinical trials in the USA utilizing monoclonal antibodies tagged with cellular toxins for treatment of lymphoma patients. This research was funded by the NIH as a program project grant, initially for 5 years and renewed for 4 more years. Patients were treated at Baylor and subsequently at a limited number of institutions throughout the world, including the Royal Free Hospital in London and the NIH in Bethesda. Over $1.5 million in NIH funds came to Baylor in support of these clinical studies. This work preceded later advances in monoclonal antibody therapy of patients with non-Hodgkin's lymphoma, now a widely used biologically targeted modality. The project was valuable for providing Baylor patients with new types of immunotherapy as well as establishing a long-term and productive collaboration with colleagues at UT Southwestern. The immunotoxin studies also provided cutting-edge experience for Baylor physicians and staff prior to FDA approval of the first monoclonal antibody for B-cell lymphoma in 1997.
EDUCATIONAL ACTIVITIES
Since the opening of the Baylor Sammons Cancer Center in 1976, education has been an important activity. In addition to the previously mentioned ongoing site-tumor conferences, a number of Sammons Cancer Center symposia have been held, including 7 meetings related to breast cancer. Oncology educational activities for medical students and residents (in medicine, surgery, and gynecology) have been a regular part of multiple training programs. In addition, medical oncology fellows have been trained at Baylor since 1976. The medical oncology fellowship program director is Dr. Stone, and the director for the Seeger Surgical Breast Oncology Fellowship is Dr. Ron Jones. A list of the fellows in medical oncology and breast surgery is shown in Table 6.
Table 6.
Fellows in medical oncology and surgical oncology of the breast at Baylor University Medical Center
Dr. Stone observed in 2000, “Our medical staff actively participates in the education of medical students and residents, but we devote the most time and effort to our fellows. When they finish their training, they will be doing what we do—practicing medical oncology-hematology. We're proud of the men and women who've graduated from our fellowship program. They teach us as much as we teach them. We appreciate the ongoing support of our colleagues in Texas Oncology and US Oncology for funding our medical oncology fellowship.”
The Baylor Sammons Cancer Center medical oncology fellowship program is designed to train internal medicine physicians to become highly skilled clinicians for patients with neoplastic diseases. Medical oncology fellows gain not only the ability to provide excellent patient care and teaching but also the capacity to conduct sound clinical studies and to interpret and expand knowledge within the field of oncology. Teaching in the fellowship program is patient oriented. Oncology rotations are designed so that each trainee spends 1 or 2 months with one attending oncologist and participates fully in inpatient, outpatient, and consultative care. In addition, Dr. Stone meets with fellows and residents at weekly “microscope rounds,” where they evaluate histologic material as unknowns and then discuss the cases. Through the years, fellows have consistently rated these microscope conferences as one of the most valuable parts of their training.
“The focus on patient care is one of the reasons the Baylor Sammons program is rare, if not unique,” said Dr. Robert Mennel, associate director of the fellowship program. “Unlike some purely academic oncology programs, Baylor's program clearly gives fellows role models and some idea of how to actually practice oncology.” Physicians at Sammons Cancer Center treat patients referred by Baylor internists, as well as those with unusual oncology problems referred from outside the Dallas–Fort Worth metroplex. In addition, Sammons oncologists treat many patients with immunosuppression-related cancers, since Baylor has one of the nation's largest adult solid organ transplant centers.
In addition to the medical oncology and surgical breast oncology fellowships, advanced training is offered to fellows in breast imaging through the Department of Radiology.
The Sammons Cancer Center has cosponsored numerous conferences related to various aspects of medical and surgical oncology. These included the Annual Perspectives in Oncology conferences organized by Dr. Steve Jones and held at Snowmass, Colorado, between 1987 and 1995. An oncology nursing conference has been held each year since 1993. The previously mentioned Barrett psychosocial lecture is held annually, and the cancer center has cosponsored other educational meetings with the departments of urology and surgery. The cancer center also regularly participates in patient education and screening activities, particularly for melanoma and prostate cancer. Dr. James B. Howell has led the melanoma screening program since its inception in 1985 and also has established an annual melanoma lectureship in the Department of Surgery. Drs. Ben Schnitzer, Mike Goldstein, and Myron Fine have spearheaded patient education and screening activities in prostate cancer since 1989.
Dr. Vinay Jain, who came to Baylor from the NIH and is an authority on malignant lymphomas, has organized national conferences since 1997. These meetings have dealt with solid tumor malignancies, hematologic malignancies, lung cancer, breast cancer, and supportive care. Numerous internationally recognized speakers have participated in each of the 3- to 4-day annual conferences. A national meeting of medical oncology fellows was inaugurated in 2000, and the First International Congress on Monoclonal Antibody Therapy of Cancer was held in 2001. Most of these meetings are sponsored by the Sammons Cancer Center.
PUBLICATIONS
Many physicians in the department of oncology have made important contributions to the field by making presentations at national meetings and publishing their findings in peer-reviewed medical journals. In addition, a Cancer Center Publications Section was established in 1996 and is headed by Dr. Vinay Jain. He is also president of Physicians' Education Resource, a private company. The Cancer Center Publications Section annually provides over 180 newsletters and conference summaries and other publications to practicing oncologists. Such information is based on material presented at recent national meetings and is written by the investigators who performed the work. The rapid availability of such new data narrows the window between the report of new advances and their translation to patient care. Five peer-reviewed oncology journals for clinicians on lymphoma, lung cancer, breast cancer, colon cancer, and prostate cancer were established in 1999. Each of these journals has an editorial board composed of internationally recognized experts in the field. Clinical Lymphoma was accepted for inclusion in Index Medicus in 2001 and Clinical Breast Cancer in 2002. In 2002, the premier issue of CURE (Cancer Updates, Research, and Education) was sent to over 425,000 laypersons. This new magazine received an enthusiastic reception from the public and the press.
FUTURE DIRECTIONS
At the outset of this article, it was stated that one factor leading to the formation of the cancer center was the impetus given by the National Cancer Act of 1971. Enhanced federal support provided major emphasis on recognition of oncology as a specialty and focus on care of cancer patients through improved diagnosis and treatment. Moreover, important insights in basic science that were lacking in 1971 have been achieved during the past 30 years, particularly in molecular biology, immunology, and genetics. These advances were perhaps most dramatically underlined by the mapping of the human genome announced in June 2000. Genomics and proteomics are active areas of basic research certain to yield valuable information for cancer investigators, clinicians, and patients. In addition, major attention has focused on end-of-life care, a long-neglected area (60, 63, 64, 70, 127–129).
We look toward the 21st century with expectations that oncology will be one of the most exciting areas in medicine and that the significant advances made during the past 30 years will be multiplied (130). The multidisciplinary approach that has proved so valuable during the latter part of the 20th century will continue to be the linchpin of cancer care. Further advances in molecular genetics and immunology will provide new dimensions of prevention, earlier detection, more accurate diagnosis, and improved outcomes for cancer patients (40, 131, 132).
In his presidential address to the American Society of Clinical Oncology in 2002, Dr. Larry Norton referred to imatinib (Gleevec) as “our penicillin” and commented about the transforming power of targeted therapy. He predicted that in a few years, it is likely that cancer will be diagnosed by the affected oncogene rather than by cell type-analogous to classifying pneumonia according to the causative organism. It will be important to integrate these advances in a cost-effective manner. Dr. Al Jonsen has emphasized this point by stating that “the major task of medicine is to distinguish what is scientifically available from what is clinically useful and to apply this distinction appropriately in patient care.” This will be a major challenge as technological advances generally increase cost. Sir David Weatherall has warned, “There is a genuine danger that, in our haste to improve the lot of our patients and to curb the costs of medical care, we may fail to appreciate the importance of the medical sciences for our future well-being. This would be particularly unfortunate, because all the signs are that we are entering the most exciting and productive phase of their long history” (131). Optimal care for patients will become even more challenging, as it has been estimated that the number of cancer patients in the USA will double during the next 50 years due to population growth and aging (2).
The Baylor Charles A. Sammons Cancer Center celebrated its 25th anniversary in 2001. In September, the founding physicians, Drs. Aronoff, Collier, Reese, Race, Lieberman, and Stone, were recognized (Figure 27), and Dr. Stone received the Wings of Eagles Award from Joel Allison (Figure 28). Shortly thereafter, a 25th anniversary community symposium entitled “The New Era in Cancer Treatment and Research” was held. The keynote speakers were Dr. James Holland, distinguished professor of neo-plastic diseases, Mount Sinai School of Medicine in New York City, and Dr. Jimmie Holland, chair, Department of Psychiatry and Behavioral Sciences at the Memorial Sloan-Kettering Cancer Center in New York City. Dr. James Holland spoke at the dedication of the Sammons Cancer Center in 1977, and Dr. Jimmie Holland gave the first Charlotte Johnson Barrett Lecture in 1982 and the 10th anniversary lecture in 1992. Therefore, we were particularly honored to have these 2 internationally acclaimed leaders in oncology return to Baylor on this auspicious occasion.
Figure 27.
Sammons Cancer Center 25th anniversary. Back: Tim Parris, Dr. George Race, Dr. Zeck Lieberman, Dr. Marvin Stone, Joel Allison. Front: Dr. Mike Reese, Dr. Billie Aronoff.
Figure 28.
Joel Allison presenting the Wings of Eagles Award to Marvin Stone, MD.
At the 25th anniversary symposium, Dr. Stone made the following remarks:
When the Sammons Cancer Center was dedicated 25 years ago, the theme was “Mission Impossible.” Our guest was Peter Graves, the lead actor in that popular television show. During the past quarter century, the principal objective of the Sammons Cancer Center has been to facilitate multidisciplinary interaction among specialists from different fields in order to provide the most effective care for patients. Major advances have been made in diagnosis and treatment of many patients with cancer. When we opened in 1976, medical oncology was a new specialty, and we didn't have CT or MRI scanners, growth factors, antinausea drugs, monoclonal antibodies, and many of the other valuable diagnostic and therapeutic tools that we employ every day now. Cancer is 100 separate diseases, so it is not surprising that advances have come in some of them faster than in others. It's an asymmetrical kind of progress, but it has been significant and, for patients with some types of cancer, the progress has been spectacular.
Advances in medicine and in oncology come about through carefully conducted research and high-quality educational programs. We've been involved in research and education since our inception. Research and education continue to be crucially important components of the cancer center's, and indeed Baylor's, charge.
What about “Mission Impossible”? I know of no more dramatic example of what can and has been achieved than Lance Armstrong's story. Five years ago, he had disseminated testicular cancer with metastases to multiple organs, including lungs and brain. After undergoing surgery and combination chemotherapy successfully at the Indiana University Medical Center, he has won the Tour de France, perhaps the most grueling sporting event of all, for the past 3 years. It seems especially meaningful that this cancer survivor's remarkable feats have bridged 2 centuries, as he triumphed in the Tour in 1999, 2000, and 2001 [and 2002]. He is a shining beacon to cancer patients, physicians, researchers, and the public because Lance Armstrong is living proof that our mission is not impossible, though for him it certainly would have been so only a few years before.
Lance Armstrong's inspiring book, It's Not About the Bike, tells of his experience as a cancer patient (133). What clearly comes through is the enormous scientific progress that allowed Lance Armstrong to regain his health and ride a bike better than anyone else in the world, and also the courageous and positive way he dealt with his illness. Armstrong's acronym for cancer is shown in Table 7.
Oncology has emerged as one of the most exciting fields in biology and medicine. Progress in basic science research, especially in genetics and immunology, has brought scientific medicine to the dawn of a new age as we begin the new millennium. Most of what we know and can do for sick patients has been learned during the past 70 years. We will be able to do much more for patients with cancer and other diseases during the next generation. By the year 2025, people may look back at 1990s medicine in the same way we recall conditions in the preantibiotic era. We remain firmly committed to making additional progress that results in better care and outcomes for our patients. William Osier's words still ring true:
“To wrest from nature the secrets which have perplexed philosophers in all ages, to track to their sources the causes of disease, to correlate the vast stores of knowledge, that they may be quickly available for the prevention and cure of disease—these are our ambitions” (3).
Table 7.
Lance Armstrong's acronym for cancer*
| Courage |
| Attitude |
| Never give up |
| Curability |
| Enlightenment |
| Remembrance of fellow patients |
*From reference 133.
Acknowledgment
This article is dedicated to all Baylor Sammons Cancer Center patients and their families. They are the reasons why Baylor physicians, nurses, and administrators strive for excellence. Ms. Linda Miller provided expert assistance with manuscript preparation. I thank my colleagues for their thoughtful and informative contributions. However, any errors in judgment or content are mine (MJS).
Footnotes
Historical articles published in Proceedings will be reprinted in the centennial history of Baylor University Medical Center. Readers who have any additional information, artifacts, photographs, or documents related to the historical articles are asked to forward such information to the Proceedings' editorial office for possible inclusion in the book version.
References
- 1.American Cancer Society. Cancer Facts and Figures, (2002), Available at http://www.cancer.Org/downloads/STT/CancerFacts&Figures2002TM.pdf.
- 2.Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the sta tus of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 3.Osier W. In Aequanimitas and Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. New York: Blakiston; 1932. Chauvinism in medicine; pp. 265–289. [Google Scholar]
- 4.Greaves M. Cancer: The Evolutionary Legacy. Oxford: Oxford University Press; 2000. [Google Scholar]
- 5.Huxley J. Biological Aspects of Cancer. London: Allen and Unwin; 1958. [Google Scholar]
- 6.Oberling C. The Riddle of Cancer. Woglom WH, New Haven: Yale University Press; 1952. [Google Scholar]
- 7.National Cancer Institute . Closing In on Cancer: Solving a 5,000-Year-Did Mystery (NIH publication 87–2955) Bethesda, Md: National Institutes of Health; 1987. Available at http://rex.nci.nih.gov/behindthenews/cioc/ciochome.htm. [Google Scholar]
- 8.Porter R. The Greatest Benefit to Mankind. New York: Norton; 1997. pp. 574–580. [Google Scholar]
- 9.Cantor D. Cancer. In: Bynum WE, Porter C, editors. Companion Encyclopedia of the History of Medicine. Vol. 1. London: Routledge; 1993. pp. 537–561. [Google Scholar]
- 10.Cairns J. Matters of Life and Death: Perspectives on Public Health, Molecular Biology, Cancer, and the Prospects for the Human Race. Princeton: Princeton University Press; 1997. [Google Scholar]
- 11.Dobell C. Antony van Leeuwenhoek and His “Little Animals.”. New York: Harcourt, Brace and Co; 1932. [Google Scholar]
- 12.Wintrobe MM. Blood, Pure and Eloquent. New York: McGraw-Hill; 1980. [Google Scholar]
- 13.Rather LJ. The Genesis of Cancer: A Study in the History of Ideas. Baltimore: Johns Hopkins University Press; 1978. [Google Scholar]
- 14.Parsonnett J. Microbes and Malignancy: Infection as a Cause of Human Cancers. New York: Oxford; 1999. [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Virchow R. Cellular Pathology as Based Upon Physiological and Pathological Histology. Chance F, London: John Churchill; 1860. [DOI] [PubMed] [Google Scholar]
- 17.Ackerknecht EH. Doctor, Statesman, Anthropologist. Madi son: University of Wisconsin Press; 1953. RudolfVirchow. [Google Scholar]
- 18.Varmus H, Weinberg RA. Genes and the Biology of Cancer. New York: Sci entific American Library; 1993. [Google Scholar]
- 19.Gunz FW. Leukemia in the past. In: Henderson ES, Lister TA, editors. Leuke mia. 5th ed. Philadelphia: WB Saunders; 1990. [Google Scholar]
- 20.Freireich EJ, Lemak NA. Milestones in Leukemia Research and Therapy. Baltimore: Johns Hopkins University Press; 1991. [Google Scholar]
- 21.Kaplan HJ. Hodgkin's Disease. 2nd ed. Cambridge: Harvard University Press; 1980. [Google Scholar]
- 22.Aisenberg AC. Historical overview of malignant lymphoma. In: Wiernik PH, Camellos GP, Dutcher JP, Kyle RA, editors. Neopiastic Diseases of the Blood. 3rd ed. Vol. 36. New York: Churchill Livingstone; 1996. [Google Scholar]
- 23.Kyle RA. History of multiple myeloma. In: Wiernik PH, Camellos GP, Dutcher JP, Kyle RA, editors. Neoplastic Diseases of the Blood. 3rd ed. Vol. 22. New York: Churchill Livingstone; 1996. [Google Scholar]
- 24.Stone MJ. Henry Bence Jones and his protein. J Med Biogr. 1998;6:53–57. doi: 10.1177/096777209800600112. [DOI] [PubMed] [Google Scholar]
- 25.Arnott H. Cancer, Its Varieties, Their Histologies and Diagnoses. London: JH Churchill; 1872. [Google Scholar]
- 26.Sutton JB. Their Clinical Features and Appropriate Treatment. London: Cassel and Co Ltd; 1893. Tumours, Innocent and Malignant. [Google Scholar]
- 27.Eisenberg RL. An Illustrated History. St. Louis: Mosby; 1992. Radiology. [Google Scholar]
- 28.Hayter CRR. The clinic as laboratory: the case of radiation therapy, 1896–1920. Bull Hist Med. 1998;72:663–688. doi: 10.1353/bhm.1998.0213. [DOI] [PubMed] [Google Scholar]
- 29.Kevles BH. Medical Imaging in the Twentieth Century. Reading, Mass: Addison-Wesley; 1997. Naked to the Bone. [Google Scholar]
- 30.Mullner R. The Radium Dial Worker Tragedy. Washington, DC: American Public Health Association; 1999. Deadly Glow. [Google Scholar]
- 31.Israel L. Conquering Cancer. New York: Random House; 1978. [Google Scholar]
- 32.Hall SS. A Commotion in the Blood. New York: Holt; 1997. [Google Scholar]
- 33.Cairns J. Cancer: Science and Society. San Francisco: WH Freeman; 1978. [Google Scholar]
- 34.Epstein SS. The Politics of Cancer Revisited. The Fremont Center, New York: East Ridge Press; 1998. [Google Scholar]
- 35.Rodu B, Cole P. The fifty-year decline of cancer in America. J Clin Oncol. 2001;19:239–241. doi: 10.1200/JCO.2001.19.1.239. [DOI] [PubMed] [Google Scholar]
- 36.Gye WE, Purdy WJ. The Cause of Cancer. London: Cassell and Co Ltd; 1931. [Google Scholar]
- 37.Berenblum I. The Story of Cancer Research. Baltimore: John Hopkins University Press; 1952. Man Against Cancer: [Google Scholar]
- 38.Judson HE. The Eighth Day of Creation. New York: Simon and Schuster; 1979. [Google Scholar]
- 39.Currie GA. Eighty years of immunotherapy: a review of immunological methods used for the treatment of human cancer. Br J Cancer. 1972;26:141–153. doi: 10.1038/bjc.1972.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendelsohn J, Howley PM, Israel MA, Liotta LA. The Molecular Ba sis of Cancer. 2nd ed. Philadelphia: WB Saunders; 2001. eds. [Google Scholar]
- 41.Vogelstein B, Kinzler KW. The Genetic Basis of Human Cancer. New York: McGraw-Hill; 1998. eds. [Google Scholar]
- 42.Folkman J. Tumor angiogenesis. In: Bast RC, Kufe DW, Pollock RE, Weich-selbaum RR, Holland JF, Frei E, editors. Cancer Medicine. 5th ed. Hamilton: BC Decker; 2000. pp. 132–152. [Google Scholar]
- 43.Cooke R. Dr. Foikman's War. New York: Random House; 2001. [Google Scholar]
- 44.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 45.Stone MJ. Recent advances in oncology. Dallas Medicol Journal. 1978;64:406–414. [Google Scholar]
- 46.Fidler IJ. Cancer biology: invasion and metastasis. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical Oncology. 2nd ed. New York: Churchill Livingstone; 2000. pp. 29–53. [Google Scholar]
- 47.Cambrosio A, Keating P. The Monoclonal Antibody Revolution. New York: Oxford; 1995. Exquisite Specificity. [Google Scholar]
- 48.Stone MJ. Monoclonal antibodies in the prehybridoma era: a brief histori cal perspective and personal reminiscence. Clin Lymphoma. 2001;2:148–154. doi: 10.3816/clm.2001.n.020. [DOI] [PubMed] [Google Scholar]
- 49.Bainbridge WS. The Cancer Problem. New York: Macmillan; 1914. [Google Scholar]
- 50.Mann J. The Search for the Perfect Drug. Oxford: Oxford University Press; 1999. The Elusive Magic Bullet. [Google Scholar]
- 51.Gilman A, Phillips FS. The biological actions and therapeutic applica tions of b-chloroethyl amines and sulfides. Science. 1946;103:409–415. [PubMed] [Google Scholar]
- 52.Lazlo J. Into the Age of Miracles. New Brunswick, NJ: Rutgers University Press; 1995. The Cure of Childhood Leukemia. [Google Scholar]
- 53.Li MC, Hertz R, Spence DB. Effect of methotrexate therapy upon cho- riocarcinoma and chorioadenoma. Proc Soc Exp Biol Med. 1956;93:361. doi: 10.3181/00379727-93-22757. [DOI] [PubMed] [Google Scholar]
- 54.Perry MC, Anderson CM, Donebower RC. Chemotherapy. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinicaloncology. 2nd ed. New York: Churchill Livingstone; 2000. pp. 378–422. [Google Scholar]
- 55.Savage DG, Antman KH. Imatinib mesylate—a new oral targeted therapy. N Engl J Med. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 56.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with meta- static melanoma to CD34+ progenitor-derived dendritic cell vaccine. Can* cer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 57.Demaitre L. Medieval notions of cancer: malignancy and metaphor. Bull Hist Med. 1998;72:609–637. doi: 10.1353/bhm.1998.0212. [DOI] [PubMed] [Google Scholar]
- 58.Sontag S. Illness as Metaphor. New York: Farrar, Straus and Giroux; 1977. [Google Scholar]
- 59.Patterson JT. The Dread Disease: Cancer in Modem American Culture. Cam bridge: Harvard University Press; 1987. [Google Scholar]
- 60.Holland JC. Psycho-oncology. New York: Oxford University Press; 1998. pp. 3–11. [Google Scholar]
- 61.Lerner BH. The illness and death of Eva Peron: cancer, politics, and se crecy. Lancet. 2000;355:1988–1991. doi: 10.1016/S0140-6736(00)02337-0. [DOI] [PubMed] [Google Scholar]
- 62.Lerner BH. The Breast Cancer Wars. New York: Oxford University Press; 2001. [Google Scholar]
- 63.Saunders CM. The Management of Terminal Disease. London: Edward Arnold; 1978. [Google Scholar]
- 64.Emanuel L. EPEC Trainer's Guide. Chicago: American Medical Associa tion; 1999. [Google Scholar]
- 65.Hoxsey HM. You Don't Have to Die: The Amazing Story of the Hoxsey Treatment. New York: Milestone Books; 1956. [Google Scholar]
- 66.Cassileth BR. The Alternative Medicine Handbook. New York: Norton; 1998. [Google Scholar]
- 67.American Cancer Society's Guide to Complementary and Alternative Cancer Methods. Atlanta: American Cancer Society; 2000. [Google Scholar]
- 68.LaTour K. The Breast Cancer Companion. New York: William Morrow; 1993. [Google Scholar]
- 69.Harpham WS. Diagnosis: Cancer, Your Guide Through the First Few Months. New York: Norton; 1997. [Google Scholar]
- 70.Holland JC, Lewis S. The Human Side of Cancer. New York: Harper Collins (Quill); 2000. [Google Scholar]
- 71.40th anniversary issue J Ntl Cancer Inst. 1977;59(2 Supp) [Google Scholar]
- 72.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 73.Henderson L. Baylor University Medical Center: Yesterday, Today and To- morrow. Waco: Baylor University Press; 1978. pp. 240–246. [Google Scholar]
- 74.Baylor-Charles A. Sammons Cancer Center Annual Report 2000. Dallas: Baylor-Sammons Cancer Center; [Google Scholar]
- 75.Senzer NN. Docetaxel and radiation therapy for management of locally advanced prostate cancer. Advances in Prostate Cancer. 2001;2001(3):10–13. [Google Scholar]
- 76.Senzer NN. Prostate cancer: multimodality approaches with docetaxel. Semin Oncol. 2001;28(4 Suppl 15):77–85. doi: 10.1016/s0093-7754(01)90160-5. [DOI] [PubMed] [Google Scholar]
- 77.Gordon AN, Hancock KG, Matthews CM, Stringer CA, Boston J, Nemunaitis J. A phase I/I I dose escalation study of carboplatin in the treat ment of newly diagnosed patients with advanced ovarian cancer receiv ing paclitaxel. Am J Clin Oncol. 1999;22:601–605. doi: 10.1097/00000421-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, Resales R, Sharpington T. Phase II study of liposomal doxorubicin in plati num- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 79.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 80.Baylor-Cfiarles A. Sammons Cancer Center Annual Report 2001. Dallas: Baylor-Sammons Cancer Center; [Google Scholar]
- 81.Wistuba II, Tomlinson GE, Behrens C, Virmani A, Geradts J, Blum JL, Minna JD, Gazdar AF. Two identical triplet sisters carrying a germline BRCA1 gene mutation acquire very similar breast cancer somatic mutations at multiple other sites throughout the genome. Genes Chromosomes Cancer. 2000;28:359–369. doi: 10.1002/1098-2264(200008)28:4<359::aid-gcc1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 82.Blum JL, Jones SE, Fay JW, Senzer N, Mennel RG. Guidelines for systemic therapy of early stage breast cancer. Breast Cancer Res Treat. 1997;43:259–276. doi: 10.1023/a:1005705300012. [DOI] [PubMed] [Google Scholar]
- 83.Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. also Classic Papers and Current Comments: Breast Cancer 2001;5:790–799. [DOI] [PubMed] [Google Scholar]
- 84.O'Shaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B, Burger HU, Laws S. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 85.O'Shaughnessy JA, Ljung BM, Dooley WC, Chang J, Kuerer HM, Hung DT, Grant MD, Khan SA, Phillips RF, Duvall K, Euhus DM, King BL, Anderson BO, Troyan SL, Kim J, Veronesi U, Cazzaniga M. Ductal lavage and the clinical management of women at high risk for breast carcinoma: a commentary. Cancer. 2002;94:292–298. doi: 10.1002/cncr.10238. [DOI] [PubMed] [Google Scholar]
- 86.O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, Sigman CC, Bertagnolli MM, Stratton SP, Lam S, Nelson WG, Meyskens FL, Alberts DS, Pollen M, Rustgi AK, Papadimitrakopoulou V, Scardino PT, Gazdar AF, Wattenberg LW, Sporn MB, Sakr WA, Lippman SM, Von Hoff DD. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 87.Jones S, Winer E, Vogel C, Laufman L, Hutchins L, O'Rourke M, Lembersky B, Budman D, Bigley J, Hohneker J. Randomized comparison of vinorelbine and melphalan in anthracycline-refractory advanced breast cancer. J Clin Oncol. 1995;13:2567–2574. doi: 10.1200/JCO.1995.13.10.2567. [DOI] [PubMed] [Google Scholar]
- 88.Jones SE, Schottstaedt MW, Duncan LA, Kirby RL, Good RH, Mennel RG, George TK, Snyder DA, Watkins DL, Denham CA, Hoyes FA, Rubin AS. Randomized double-blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate-dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. J Clin Oncol. 1996;14:2976–2983. doi: 10.1200/JCO.1996.14.11.2976. [DOI] [PubMed] [Google Scholar]
- 89.Jones S, Vogel C, Arkhipov A, Fehrenbacher L, Eisenberg P, Cooper B, Honig S, Polli A, Whaley F, di Salle E, Tiffany J, Consonni A, Miller L. Multicenter, phase II trial of exemestane as third-line hormonal therapy of postmenopausal women with metastatic breast cancer. Aromasin Study Group. J Clin Oncol. 1999;17:3418–3425. doi: 10.1200/JCO.1999.17.11.3418. [DOI] [PubMed] [Google Scholar]
- 90.Jones S, Clark G, Koleszar S, Ethington G, Mennel R, Paulson S, Brooks B, Kerr R, Denham C, Savin M, White C, Blum J, Kirby R, Stone M, Pippen J, Kitchens L, George T, Cooper B, Peters G, Knox S, Grant M, Cheek H, Jones R, Kuhn J, Lieberman Z, Savino D, Rietz C. Low proliferative rate of invasive node-negative breast cancer predicts for a favorable outcome: a prospective evaluation of 669 patients. Clin Breast Cancer. 2001;1:310–314. doi: 10.3816/CBC.2001.n.005. [DOI] [PubMed] [Google Scholar]
- 91.Jones SE, Clark G, Koleszar S, Ethington G, Mennel R, Paulson S, Brooks B, Kerr R, Denham C, Savin M, Blum J, Kirby R, Stone M, Pippen J, George T, Orr D, Knox S, Grant M, Peters G, Savino D, Rietz C. Adjuvant chemotherapy with doxorubicin and cyclophosphamide in women with rapidly proliferating node-negative breast cancer. Clin Breast Cancer. 2002;3:147–152. doi: 10.3816/CBC.2002.n.019. [DOI] [PubMed] [Google Scholar]
- 92.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-l Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 93.Cheek JH, Sears AD. Results of breast biopsies for mammographic findings. Am J Surg. 1978;136:726–729. doi: 10.1016/0002-9610(78)90345-8. [DOI] [PubMed] [Google Scholar]
- 94.Bailar JC., III Mammography: a contrary view. Ann Intern Med. 1976;84:77–84. doi: 10.7326/0003-4819-84-1-77. [DOI] [PubMed] [Google Scholar]
- 95.Mootz AR, Glazer-Waldman H, Evans WP, Peters ON, Kirk LM. Mammography in a mobile setting: remaining barriers. Radiology. 1991;180:161–165. doi: 10.1148/radiology.180.1.2052686. [DOI] [PubMed] [Google Scholar]
- 96.Peters ON, Vogel VG, Evans WP, Bondy M, Halabi S, Lord J, Laville EA. The Texas Breast Screening Project: Part I. Mammographic and clinical results. South Med J. 1993;86:385–390. doi: 10.1097/00007611-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Evans WP, Cade SH. Needle localization and fine-needle aspiration biopsy of nonpalpable breast lesions with use of standard and stereotactic equipment. Radiology. 1989;173:53–56. doi: 10.1148/radiology.173.1.2675189. [DOI] [PubMed] [Google Scholar]
- 98.Cross MJ, Evans WP, Peters ON, Cheek JH, Jones RC, Krakos P. Stereotactic breast biopsy as an alternative to open excisional biopsy. Ann Surg Oncol. 1995;2:195–200. doi: 10.1007/BF02307023. [DOI] [PubMed] [Google Scholar]
- 99.Pierce WB, Harms SE, Flamig DP, Griffey RH, Evans WP, Hagans JE. Three-dimensional gadolinium-enhanced MR imaging of the breast: pulse sequence with fat suppression and magnetization transfer contrast. Work in progress. Radiology. 1991;181:757–763. doi: 10.1148/radiology.181.3.1947093. [DOI] [PubMed] [Google Scholar]
- 100.Harms SE, Flamig DP, Evans WP, Harries SA, Brown S. MR imaging of the breast: current status and future potential. AJR Am J Roentgenol. 1994;163:1039–1047. doi: 10.2214/ajr.163.5.7976873. [DOI] [PubMed] [Google Scholar]
- 101.Soderstrom CE, Harms SE, Copit DS, Evans WP, Savino DA, Krakos PA, Farrell RS, Jr, Flamig DP. Three-dimensional RODEO breast MR imaging of lesions containing ductal carcinoma in situ. Radiology. 1996;201:427–432. doi: 10.1148/radiology.201.2.8888235. [DOI] [PubMed] [Google Scholar]
- 102.Evans WP. Stereotactic breast biopsy. In: Harris JR, Lippman ME, Morrow M, Hellman S, editors. Diseases of the Breast. Philadelphia: JB Lippincott Co; 1996. pp. 144–158. [Google Scholar]
- 103.Bassett L, Winchester DP, Caplan RB, Dershaw DD, Dowlatshahi K, Evans WP, III, Fajardo LL, Fitzgibbons PL, Henson DE, Mutter RV, Morrow M, Paquelet JR, Singletary SE, Curry J, Wilcox-Buchalla P, Zinninger M. Ste reotactic core-needle biopsy of the breast: a report of the Joint Task Force of the American College of Radiology, American College of Surgeons, and College of American Pathologists. CA Cancer J Clin. 1997;47:171–190. doi: 10.3322/canjclin.47.3.171. [DOI] [PubMed] [Google Scholar]
- 104.Brenner RJ, Bassett LW, Fajardo LL, Dershaw DD, Evans WP, III, Hunt R, Lee C, Tocino I, Fisher P, McCombs M, Jackson VP, Feig SA, Mendelson EB, Margolin FR, Bird R, Sayre J. Stereotactic core-needle breast biopsy: a multi-institutional prospective trial. Radiology. 2001;218:866–872. doi: 10.1148/radiology.218.3.r01mr44866. [DOI] [PubMed] [Google Scholar]
- 105.Evans WP, III, Starr AL, Bennos ES. Comparison of the relative incidence of impalpable invasive breast carcinoma and ductal carcinoma in situ in cancers detected in patients older and younger than 50 years of age. Radiology. 1997;204:489–491. doi: 10.1148/radiology.204.2.9240541. [DOI] [PubMed] [Google Scholar]
- 106.Fay JW, Lazarus H, Herzig R, Saez R, Stevens DA, Collins RH, Jr, Pineiro LA, Cooper BW, DiCesare J, Campion M, Fetzer JM, Herzig G, Bernstein GH. Sequential administration of recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for malignant lymphoma: a phase I/II multicenter study. Blood. 1994;84:2151–2157. [PubMed] [Google Scholar]
- 107.Fay JW, Wingard JR, Antin JH, Collins RH, Pineiro LA, Blazar BR, Saral R, Bierer BE, Przepiorka D, Fitzsimmons WE, Maher RM, Weisdorf DJ. FK506 (tacrolimus) monotherapy for prevention of graft-versus-host disease after histocompatible sibling allogenic bone marrow transplantation. Blood. 1996;87:3514–3519. [PubMed] [Google Scholar]
- 108.Fay JW. Hematopoietic growth factors, dendritic cell biology, and vaccine therapy of cancer. Curr Opin Hematol. 2002;9:202–206. doi: 10.1097/00062752-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, List A, Dalton W, Ognoskie N, Chetrit A, Antin JH, Nemunaitis J. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 110.US Oncology Annual Report. Houston: US Oncology; 2000. [Google Scholar]
- 111.Research . US Oncology Annual Report 2000. Houston: US Oncology; [Google Scholar]
- 112.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 113.Nemunaitis J, Eager R, Twaddell T, Corey A, Sekar K, Tkaczuk K, Thompson J, Hoff PM, Pazdur R. Phase I assessment of the pharmacokinetics, metabolism, and safety of emitefur in patients with refractory solid tumors. J Clin Oncol. 2000;18:3423–3434. doi: 10.1200/JCO.2000.18.19.3423. [DOI] [PubMed] [Google Scholar]
- 114.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 115.Tong AW, Lee J, Stone MJ. Characterization of two human small cell lung carcinoma-reactive monoclonal antibodies generated by a novel immunization approach. Cancer Res. 1984;44:4987–4992. [PubMed] [Google Scholar]
- 116.Tong AW, Lee JC, Stone MJ. Characterization of a monoclonal antibody having selective reactivity with normal and neoplastic plasma cells. Blood. 1987;69:238–245. [PubMed] [Google Scholar]
- 117.Tong AW, Zhang BQ, Mues G, Solano M, Hanson T, Stone MJ. Anti-CD40 antibody binding modulates human multiple myeloma clonogenicity in vitro. Blood. 1994;84:3026–3033. [PubMed] [Google Scholar]
- 118.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, Stone MJ. Growth-inhibitory effects of CD40 ligand (CD154) and its en dogenous expression in human breast cancer. Clin Cancer Res. 2001;7:691–703. [PubMed] [Google Scholar]
- 119.Stone MJ, Klintmalm GB, Polter D, Husberg BS, Mennel RG, Ramsay MA, Flemens ER, Goldstein RM. Neoadjuvant chemotherapy and liver transplantation for hepatocellular carcinoma: a pilot study in 20 patients. Gastroenteroiogy. 1993;104:196–202. doi: 10.1016/0016-5085(93)90852-4. [DOI] [PubMed] [Google Scholar]
- 120.Stone MJ. Transplantation for primary hepatic malignancy. In: Busuttil RW, Klintmalm GB, editors. Transplantation of the Liver. Philadelphia: WB Saunders; 1996. pp. 120–129. [Google Scholar]
- 121.Melear JM, Goldstein RM, Levy MF, Molmenti EP, Cooper B, Netto GJ, Klintmalm GB, Stone MJ. Hematologic aspects of liver transplantation for Budd-Chiari syndrome with special reference to myeloproliferative dis orders. Transpiantation. 2002;74:1090–1095. doi: 10.1097/00007890-200210270-00006. [DOI] [PubMed] [Google Scholar]
- 122.Collins RH, Jr, Anastasi J, Terstappen LW, Nikaein A, Feng J, Fay JW, Klintmalm G, Stone MJ. Brief report: donor-derived long-term multilineage hematopoiesis in a liver-transplant recipient. N Engl J Med. 1993;328:762–765. doi: 10.1056/NEJM199303183281104. [DOI] [PubMed] [Google Scholar]
- 123.Collins RH, Jr, Sackler M, Pitcher CJ, Waldrop SL, Klintmalm GB, Jenkins R, Picker LJ. Immune reconstitution with donor-derived memory/effector T cells after orthotopic liver transplantation. Exp Hematol. 1997;25:147–159. [PubMed] [Google Scholar]
- 124.Vitetta ES, Stone M, Amlot P, Fay J, May R, Till M, Newman J, Clark P, Collins R, Cunningham D, Ghetie V, Uhr JW, Thorpe PE. Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res. 1991;51:4052–4058. [PubMed] [Google Scholar]
- 125.Amlot PL, Stone MJ, Cunningham D, Fay J, Newman J, Collins R, May R, McCarthy M, Richardson J, Ghetie V, Ramilo O, Thorpe PE, Uhr JWS, Vitetta ES. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conven tional therapy. Blood. 1993;82:2624–2633. [PubMed] [Google Scholar]
- 126.Stone MJ, Sausville EA, Fay JW, Headlee D, Collins RH, Figg WD, Stetler-Stevenson M, Jain V, Jaffe ES, Solomon D, Lush RM, Senderowicz A, Ghetie V, Schindler J, Uhr JW, Vitetta ES. A phase I study of bolus ver sus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–1197. [PubMed] [Google Scholar]
- 127.Abrahm JL. Guide to Pain and Symptom Management in Cancer Patients. Baltimore: Johns Hopkins University Press; 2000. A Physician's. [Google Scholar]
- 128.Stone MJ. Goals of care at the end of life. BUMC Proceedings. 2001;14:134–137. doi: 10.1080/08998280.2001.11927748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snyder L, Quill TE. Physician's Guide to End-of-Life Care. Philadelphia: American College of Physicians; 2001. [Google Scholar]
- 130.Stone MJ, Roberts WC. Marvin Jules Stone, MD, MACP: a conversation with the editor. BUMC Proceedings. 2001;14:422–438. doi: 10.1080/08998280.2001.11927795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weatherall D. Science and the Quiet Art: The Role of Medical Research in Health Care. New York: Norton; 1995. [Google Scholar]
- 132.Ramaswamy S, Golub TR. DNA microarrays in clinical oncology. J Clin Oncol. 2002;20:1932–1941. doi: 10.1200/JCO.2002.20.7.1932. [DOI] [PubMed] [Google Scholar]
- 133.Armstrong L, Jenkins S. It's Not About the Bike. Vol. 274. New York: Putnam; 2000. [Google Scholar]
















