Abstract
Background
Vascular calcification (VC) always has poor cardiovascular outcomes, but it is still difficult to control. Exosomes secreted from activated macrophages can affect VC through microRNAs (miRNAs). Research has suggested that miRNA-204 inhibits VC. We previously demonstrated that angiotensin II type 2 receptor (AT2R) plays an important role in VC; however, its underlying mechanisms are not yet clear.
Methods and results
Rat aortic smooth muscle cells (RASMCs) and rat alveolar macrophages were cocultured with or without the phosphate and/or AT2R agonist compound 21 (C21). Calcium deposition was assessed by alizarin red staining. Protein expression was assessed by immunofluorescence staining and immunoblot analysis. The level of microRNA-204 was detected via qPCR, and its target mRNA was tested via a luciferase activity assay. C21 treatment improved the additional calcification of RASMCs cocultured with macrophages more than it did those cultured alone. The expression of miRNA-204-5p in exosomes secreted from macrophages markedly increased after C21 treatment. The decrease in the degree of calcification of RASMCs cocultured with macrophages and the expression of BMP-2, OCN, Wnt3a, β-catenin and RUNX2 induced by C21 treatment were significantly weakened after transfection with the miRNA-204-5p inhibitor. RUNX2 mRNA was the target of miRNA-204-5p in RASMCs cocultured with macrophages after C21 treatment.
Conclusions
Our results suggested that miRNA-204-5p in exosomes secreted from macrophages was at least partly involved in the AT2 receptor-mediated improvement in VC induced by phosphate through targeting RUNX2 mRNA, inhibiting the Wnt/β-catenin signalling pathway and decreasing the expression of calcification-related proteins.
Keywords: Vascular calcification, AT2 receptor, VSMC, Macrophage, Exosome, MiRNA-204
Introduction
Vascular calcification (VC) is a complex pathological process that is characterized by the deposition of minerals in the vasculature [1]]. VC is an independent predictor of cardiovascular morbidity and mortality, which remain important public health issues worldwide [2, 3]. Even though some studies have been published in recent years, VC is still difficult to control and has poor outcomes [4]. In the process of VC, vascular smooth muscle cells (VSMCs) in the media are a major source of calcifying vascular cells [5]. The key to VSMC calcification is the transformation from a contractile phenotype to an osteoblast-like phenotype [6]. Many researchers have recently recognized that VC is caused not only by a single cell but also by cell-to-cell interactions through a variety of biomolecules and signalling pathways [7]. However, the mechanisms of VSMC calcification are still unclear, which may be why no therapeutic strategy is effective in preventing and reversing the progression of VC.
Macrophages play an important regulatory role in intimal calcification because they mediate the inflammatory response [8]. They can undergo two distinct polarization states: the proinflammatory M1 phenotype and the anti-inflammatory M2 phenotype [9]. M1 macrophages can release proinflammatory factors (e.g., TNF-α and IL-1β), whereas M2 macrophages can release anti-inflammatory factors (e.g., IL-10 and TGF-β) [10]. However, few studies have investigated the role of macrophages in medial artery calcification. Recent research revealed that macrophages can infiltrate the media of calcified arteries, suggesting the potential regulatory role of macrophages in VSMC calcification [11]. Emerging evidence has demonstrated that coculture of VSMCs with macrophages profoundly affects the degree of VSMC calcification [8]. Therefore, exploring the crosstalk between VSMCs and macrophages in VSMC calcification is meaningful. Exosomes secreted from activated macrophages are involved in cell‒cell interactions with VSMCs and affect VC through microRNAs (miRNAs) or other cargo [12]. Studies have suggested that miRNA-204 can downregulate RUNX2 expression, inhibit the formation of VSMC calcification in vitro and in vivo [13, 14] and improve the calcification of human aortic valve interstitial cells [15, 16]. However, it is unknown whether exosomal miRNA-204 can be secreted from activated macrophages and exert any effects on the crosstalk between VSMCs and macrophages during VSMC calcification.
Angiotensin II is the effector peptide of the renin angiotensin system, which is known to be a major pathogenic factor in VC [17, 18]. Angiotensin II binds two receptor subtypes, type 1 receptor (AT1R) and type 2 receptor (AT2R). Our previous research revealed that AT2R overexpression ameliorated phosphate-induced VC, which was partially due to PPARγ activation [19]. Interestingly, AT2R prevented VC induced by β-glycerophosphate, at least in part, through the inhibition of bone morphogenetic protein-2 (BMP-2) and osteocalcin (OCN) expression. These results fully demonstrated that AT2R plays an important role in the improvement of VC. In addition, AT2R activation modulates macrophage polarization [20, 21]. Therefore, this study aims to elucidate the effects of macrophages and exosomal miRNA-204 on AT2R-mediated improvement in VC and to investigate the underlying mechanisms, which will provide novel insight into new therapeutic strategies for VC.
Methods
Cell culture
Rat aortic smooth muscle cells (RASMCs, RAT-iCell-c004) and rat alveolar macrophages (NR8383, iCell-r021) were purchased from iCell. RASMCs were cultured alone or cocultured with macrophages in Dulbecco’s modified Eagle’s medium (DMEM, iCell, PriMed-iCELL-004) supplemented with 10% fetal bovine serum (FBS, Gibco, 10100147 C), 1% GlutaMAX (Invitrogen, iCell-0900), and 1% penicillin and streptomycin (Gibco, 15070063) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The influence of macrophages on RASMCs was assessed via transwell plates (Corning, 3413). A 6.5 mm transwell with a 0.4 µm pore polycarbonate membrane insert was placed in a culture plate, with macrophages seeded into the upper chamber and equal numbers of RASMCs seeded into the lower surface. Cells at passages 3–6 were used for the experiments. Some cells were cultured with or without 10 mmol/L β-glycerophosphate (Sigma, G9422), 0.25 mmol/L ascorbic acid (Sigma, A7506) and/or 10 µmol/L C21 (MedChemExpress, HY-100113) treatment. In this study, the groups are were divided into control group (CON), C21 treatment group (C21), β-glycerophosphate and ascorbic acid treatment group (Pi), and β-glycerophosphate and ascorbic acid plus C21 treatment group (Pi+C21). The miRNA-204-5p inhibitor (100 nmol/L) was transferred into some RASMCs via Lipofectamine 3000 Transfection Reagent (Invitrogen, L3000015). The medium was changed every 2 days with new medium containing freshly prepared reagents.
Alizarin red staining
Calcium deposition was detected by staining with 2% Alizarin red (Cyagen, ALIR-10001). Briefly, RASMCs were cultured for 14 days, fixed with 4% paraformaldehyde for 30 min and washed twice with distilled water. After the addition of Alizarin red solution, the cells were stained for 20 minutes at room temperature. Excess stain was added, and the samples were washed five times with distilled water. The cells were photographed at ×100 magnification via microscopy (Olympus, CKX53). Alizarin red staining was quantified via ImageJ, and the results are presented as the OD values.
Immunofluorescence staining
The expression of BMP-2 and OCN in RASMCs and the expression of CD68, CD11c, CD206, IL-1β, TNF-α, IL-10 and TGF-β in macrophages were evaluated via immunofluorescence staining. The cells were cultured for 14 days, fixed with 4% paraformaldehyde for 10 minutes and incubated with an anti-BMP-2 antibody (Abcam, ab214821), anti-OCN antibody (Proteintech, 23418-1-AP), anti-CD68 antibody (Abcam, ab303565), anti-CD11c antibody (Proteintech, 81853-1-RR), anti-CD206 antibody (Abcam, ab300621), anti-IL-1β antibody (Abcam, ab315084), anti-TNF-α antibody (Abcam, ab307164), anti-IL-10 antibody (Abcam, ab9969) or anti-TGF-β antibody (Abcam, ab315254) in phosphate-buffered saline overnight at 4 °C. The next day, the cells were incubated with the corresponding fluorescently labelled secondary antibodies (Cy3-conjugated AffiniPure goat anti-rabbit IgG (H+L), Proteintech, SA00009-2; CoraLite488-conjugated goat anti-rabbit IgG (H+L), Proteintech, SA00013-2; HRP-conjugated AffiniPure goat anti-rabbit IgG (H+L), Proteintech, SA00001-2) for 1 hour before the nuclei were stained with DAPI. The cells were photographed at ×100 magnification via microscopy (Olympus, CKX53). Immunofluorescence staining was quantified via ImageJ and is presented as the relative fluorescence intensity. The relative fluorescence intensity was calculated as the ratio of the fluorescence intensity to that of the CON group.
Immunoblot analysis
RASMCs were cultured and exposed to different experimental conditions for 14 days. After being washed with ice-cold phosphate-buffered saline containing 1 mmol/L sodium orthovanadate, the cells were lysed in RIPA lysis and extraction buffer (Thermo, 89900) supplemented with Halt™ protease inhibitor cocktail (Thermo, 87786) and Halt™ phosphatase inhibitor cocktail (Thermo, 78420) for 15 minutes on ice. The insoluble material was removed by centrifugation at 14,000 × g for 20 minutes at 4 °C. The protein concentration in the cleared supernatant was measured via a BCA protein quantitative analysis kit (Thermo, 23227). Proteins were subjected to SDS‒PAGE and immunoblotted with Wnt3a antibody (Abcam, ab219412), β-catenin antibody (Abcam, ab305261), RUNX2 antibody (Abcam, ab236639) or β-actin antibody (Abcam 8226). The protein bands were visualized with an enhanced chemiluminescence (ECL) system (Amersham Biosciences). Densitometric analysis was performed via NIH image software. The data were normalized to β-actin and calculated as a ratio to the CON group.
Exosome isolation and identification
RAMCs and macrophages were cocultured on the plate with transwells in normal medium until they reached 80% confluence. The medium was then replaced with exosome-depleted medium (DMEM containing 10% exosome-depleted FBS; Gibco, A2720-801). After 72 hours, 30 ml of the conditioned medium was collected from each dish and subjected to polymerization precipitation, ultrafiltration, and ultracentrifugation to harvest the exosomes. Briefly, the conditioned medium was successively centrifuged at 300× g for 10 minutes, 3,000× g for 10 minutes, and 10,000× g for 30 minutes at 4 °C to remove cell fragments, cell debris, and large extracellular vesicles. Then, 3 ml of the supernatant in an ultrafiltration tube (Merck Millipore) was centrifuged at 100000 × g for 120 minutes at 4 °C to separate the exosome fraction from the soluble exosome fraction. Exosomes were then purified according to the experimental protocol of Total Exosome Isolation Reagent (Invitrogen, 4478359).
The amount and concentration of exosomes were analysed by nanoparticle tracking analysis (NTA) via a NanoSight LM10 system (Malvern Instruments). The expression of the exosome markers ALIX (Abcam, ab275377), TSG101 (Abcam, ab125011) and CD9 (Abcam, ab82390) was detected via immunoblot analysis after the protein concentration of the exosomes was measured via a BCA protein assay kit (Thermo Fisher Scientific, 23225). In addition, the morphology of the exosomes was observed via transmission electron microscopy (Thermo Scientific).
Quantitative PCR
Total RNA was extracted from the exosomes via TRIzol Reagent (Invitrogen, 15596026) according to the manufacturer’s instructions. miRNA was reverse transcribed into cDNA via a miRNA First Strand cDNA Synthesis Kit (Tailing Reaction) (Sangon Biotech, B532451). We then utilized the 7300Plus Real-Time PCR System (Thermo Fisher Scientific) to carry out quantitative PCR (qPCR). The data were collected and normalized to U6 levels. The primers were synthesized by RiboBio. The sequences of primers used were as follows: miRNA-204-5p forward: 5-GTCGTATCCAGTGCAGGGTCGAGGTTCGCACTGGATACAGAGAGGCATA-3′; miRNA-204-5p reverse: 5′-GCAGTTCCCTTTGTCATCCT-3′; U6 forward: 5′-CTCGTCGGCAGCAA-3′; and U6 reverse: 5′-AACGCTTCACGAATTTTGCGT-3′.
Luciferase activity assay
To investigate the interaction between miRNA-204-5p and the 3ʹ untranslated region (UTR) of the RUNX2 gene, plasmid vectors containing the wild-type (WT) and mutant versions (MUT) of the RUNX2 3ʹ UTR with predicted miRNA-204-5p binding sites were constructed. These constructs were transfected into HEK293 T cells. Additionally, a Renilla luciferase vector was cotransfected in all the transfections to monitor the efficiency of the transfection. Relative luciferase activity was quantified as relative light units, with the average activity of the Photinus pyralis firefly luciferase normalized to the average activity of the Renilla luciferase vector according to the manufacturer’s protocol (Promega, #E1910).
Statistical analysis
All values are expressed as the means ± SEMs in the text and figures. Statistical analyses were performed with Statcel-3 (OMS, Saitama). The data were evaluated via analysis of variance (ANOVA). The variation in the data among all the groups was similar. If a statistically significant effect was found, we performed a Tukey‒Kramer post hoc test for multiple comparisons. A difference of p < 0.05 was considered statistically significant.
Results
Effects of macrophages on the AT2R-mediated improvement of vascular calcification
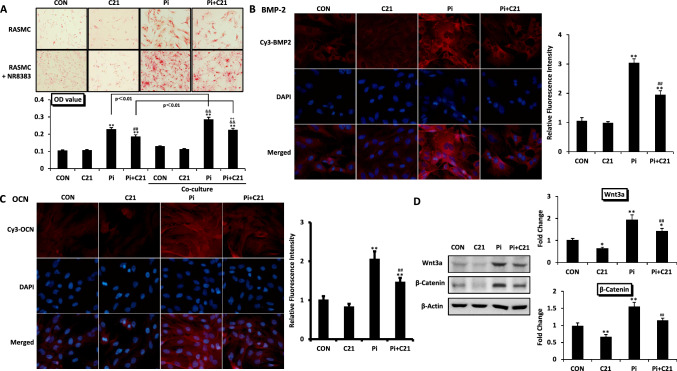
Alizarin red staining revealed that in both the RASMCs cultured alone and the RASMCs cocultured with macrophages, the number of calcified nodules and the OD value representing calcium deposition were significantly greater in the Pi group than in the CON group and were significantly lower in the Pi group than in the CON group. When RASMCs were cocultured with macrophages, the number of calcified nodules and the OD value further increased in the Pi group compared with those in the control group, indicating that coculture of RASMCs with macrophages significantly enhanced the formation of RASMC calcification induced by phosphate. In RASMCs cultured alone, C21 improved RASMC calcification by approximately 18%. However, C21 improved the calcification of additional RASMCs cocultured with macrophages by approximately 31%. These findings indicate that macrophages have important effects on AT2R-mediated improvements in RASMC calcification (Fig. 1A).
Fig. 1.
Effects of macrophages on the AT2R-mediated improvement of vascular calcification. A Effects of macrophages on RASMC calcification after β-glycerophosphate (10 mmol/L) and/or C21 (10 µmol/L) treatment for 14 days. Representative images of calcified nodules evaluated by Alizarin red staining (original magnification ×100). The results of the quantitative analysis of Alizarin red staining are shown as the OD value. B, C The expression of calcification-related proteins associated with the calcification of RASMCs cocultured with macrophages after C21 treatment for 14 days. Photos of BMP-2 and OCN expression determined by immunofluorescence staining (original magnification ×100). The results of the quantitative analysis of the immunofluorescence staining are shown as the relative fluorescence intensity. D The expression of calcification-related signalling pathway proteins in RASMCs cocultured with macrophages after C21 treatment for 14 days. The expression of Wnt3a and β-catenin was determined by Western blotting. The values are the means ± S.E.M.s n = 3 for each group. *p < 0.05, **p < 0.01 vs. CON. ##p < 0.01 vs. Pi. &&p < 0.01 vs. CON (coculture). ++p < 0.01 vs. Pi (coculture). Co-culture RASMCs cocultured with macrophages
When RASMCs were cocultured with macrophages, the fluorescence intensities of BMP-2 and OCN and the protein expression levels of Wnt3a and β-catenin were significantly greater in the Pi group than in the CON group and were significantly lower after C21 treatment (Fig. 1B-D). These findings suggested that in RASMCs cocultured with macrophages, C21 improved RASMC calcification by inhibiting the Wnt/β-catenin signalling pathway and reducing the expression of calcification-related proteins.
Polarization and inflammation of macrophages in AT2R-mediated improvement of vascular calcification
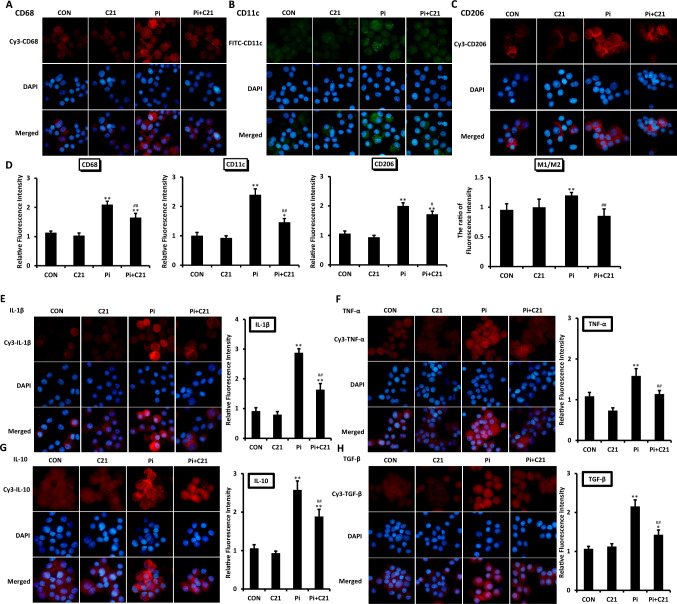
The total number of macrophages evaluated by CD68 staining, the number of M1-polarized macrophages (M1) evaluated by CD11c staining and the number of M2-polarized macrophages (M2) evaluated by CD206 staining were significantly greater in the Pi group than in the CON group and were significantly lower after C21 treatment (Fig. 2A-D). To compare the polarization of macrophages, we calculated the ratio of M1 to M2 macrophages. Compared with that in the CON group, the ratio of M1 to M2 significantly increased in the Pi group, which could be significantly reduced by C21 treatment (Fig. 2D). This evidence suggested that there was a greater ratio of the M1 fraction and a lower ratio of the M2 fraction induced by phosphate, while C21 treatment reversed the above proportion.
Fig. 2.
Polarization and inflammation of macrophages in AT2R-mediated improvement of vascular calcification. A–C Polarization of macrophages after β-glycerophosphate and/or C21 treatment for 14 days. Representative images of CD68, CD11c and CD206 expression determined by immunofluorescence staining (original magnification ×100). D Results from the quantitative analysis of immunofluorescence staining are shown as the relative fluorescence intensity. The polarization of macrophages was evaluated by the ratio of the relative fluorescence intensity of M1 to that of M2. E–H The expression of proinflammatory and anti-inflammatory markers released by macrophages after β-glycerophosphate and/or C21 treatment for 14 days. Representative images of IL-1β, TNF-α, IL-10 and TGF-β expression, as determined by immunofluorescence staining (original magnification × 100). The results of the quantitative analysis of the immunofluorescence staining are shown as the relative fluorescence intensity. The values are the means ± S.E.M.s n = 3 for each group. *p < 0.05, **p < 0.01 vs. CON. #p < 0.05, ##p < 0.01 vs. Pi
The fluorescence intensity of IL-1β, TNF-α, IL-10 and TGF-β in macrophages was significantly greater in the Pi group than in the CON group and was significantly reduced by C21 treatment (Fig. 2E-H). These findings suggested that there were increased levels of both proinflammatory factors released by M1 macrophages and anti-inflammatory factors released by M2 macrophages induced by phosphate, which may be related to the increase in the numbers of both M1 and M2 macrophages.
Exosomes secreted by macrophages in AT2R-mediated improvement of vascular calcification
To identify the exosomes extracted from macrophages, we tested three aspects: size, surface markers, and morphology. The NTA curve revealed that the particle size of the exosomes was approximately 30–200 nm (Fig. 3A). The expression of exosome surface proteins was qualitatively analysed (Fig. 3B). Exosomes exhibited a tea tray-shaped structure with clear membrane boundaries under electron microscopy (Fig. 3C). These results demonstrated that exosomes could be successfully extracted from macrophages.
Fig. 3.
Exosomes secreted by macrophages in AT2R-mediated improvement of vascular calcification. A–C Identification of macrophage-secreted exosomes after β-glycerophosphate and/or C21 treatment for 72 hours. Size‒concentration curve of macrophage-secreted exosomes drawn after nanoparticle tracking analysis (NTA). The expression of surface markers of macrophage-secreted exosomes (ALIX, TSG101 and CD9) was determined by Western blotting. Representative images of macrophage-secreted exosomes taken via electron microscopy (original magnification × 30000, × 49000). D, E The relative expression of macrophage-secreted exosomal miRNA-204-5p determined by qPCR after β-glycerophosphate and/or C21 treatment with or without miRNA-204-5p inhibitor stimulation for 72 hours. The values are the means ± S.E.M.s n = 3 for each group. *p < 0.05, **p < 0.01 vs. CON. ##p < 0.01 vs. Pi. &&p < 0.01 vs. C21. Inhibitor miRNA-204-5p inhibitor
Compared with that in the CON group, the expression of miRNA-204-5p in exosomes secreted by macrophages markedly decreased in the Pi group and significantly increased after C21 treatment. Moreover, the expression of miRNA-204-5p in the Pi+C21 group was significantly greater than that in the CON and Pi groups and significantly lower than that in the C21 group (Fig. 3D). These findings suggest that miRNA-204-5p in exosomes secreted by macrophages might play a key role in the effects of macrophages on AT2R-mediated improvements in RASMC calcification. Compared with that before transfection, the expression of miRNA-204-5p was mostly suppressed after transfection with the miRNA-204-5p inhibitor (Fig. 3E).
Effects of miRNA-204-5p on AT2R-mediated improvement of vascular calcification
Alizarin red staining revealed that when RASMCs were cocultured with macrophages, after transfection with the miRNA-204-5p inhibitor, the number of calcified nodules and the OD value were also significantly greater in the Pi group than in the CON group and could also be significantly reduced by C21 treatment. Moreover, the number of calcified nodules and the OD value were greater in the Pi+C21 group than in the control group after transfection with the miRNA-204-5p inhibitor (Fig. 4A). These findings suggest that miRNA-204-5p protects against RASMC calcification when cocultured with macrophages after C21 treatment. Therefore, AT2R-mediated improvement in vascular calcification was partially achieved by increasing the level of miRNA-204-5p.
Fig. 4.
Effects of exosomal miRNA-204-5p on AT2R-mediated improvements in RASMC calcification. A Effects of exosomal miRNA-204-5p on RASMC calcification after β-glycerophosphate and/or C21 treatment for 14 days. Representative images of calcified nodules evaluated by Alizarin red staining (original magnification ×100). The results of the quantitative analysis of Alizarin Red S staining are shown as the OD value. B Effects of exosomal miRNA-204-5p on the expression of calcification-related signalling pathway proteins during RASMC calcification after β-glycerophosphate and/or C21 treatment for 14 days. The expression of Wnt3a and β-catenin was determined by Western blotting. C, D Effects of exosomal miRNA-204-5p on the expression of calcification-related proteins involved in RASMC calcification after β-glycerophosphate and/or C21 treatment for 14 days. Photos of BMP-2 and OCN expression determined by immunofluorescence staining (original magnification ×100). The results of the quantitative analysis of the immunofluorescence staining are shown as the relative fluorescence intensity. E Effects of exosomal miRNA-204-5p on the expression of RUNX2 during RASMC calcification after β-glycerophosphate and/or C21 treatment for 14 days. The expression of RUNX2 was determined by Western blotting. F The interaction between miRNA-204-5p and RUNX-2 mRNA was determined via a luciferase activity assay. The results of the quantitative analysis of the luciferase activity assay are shown as the relative luciferase activity. The values are the means ± S.E.M.s n = 3 for each group. *p < 0.05, **p < 0.01 vs. CON. ## p < 0.01 vs. Pi. &p < 0.05, &&p < 0.01 vs. CON (Inhibitor). ++p < 0.01 vs. Pi (Inhibitor). Inhibitor miRNA-204-5p inhibitor, NC negative control of miRNA-204-5p, WT the wild-type of RUNX2 mRNA, MUT the mutant versions of RUNX2 mRNA
When RASMCs were cocultured with macrophages, after transfection with the miRNA-204-5p inhibitor, the protein expression of Wnt3a, β-catenin and RUNX2 and the fluorescence intensities of BMP-2 and OCN were significantly greater in the Pi group than in the CON group and were significantly reduced by C21 treatment. Moreover, they were significantly greater in the Pi+C21 group after transfection with the miRNA-204-5p inhibitor than before transfection (Fig. 4B-E). To verify whether miRNA-204-5p targeted RUNX2 mRNA to exert its effects, we determined the interaction between miRNA-204-5p and RUNX-2 mRNA via a luciferase activity assay. The results revealed that the relative luciferase activity after the transfection of agomiRNA-204-5p was significantly lower than before, which indicated that RUNX2 mRNA was the target of miRNA-204-5p in RASMCs cocultured with macrophages after C21 treatment (Fig. 4F).
Discussion
In this study, the calcification of RASMCs cocultured with macrophages was greater than that of RASMCs cultured alone and was significantly reduced by C21 treatment. C21 treatment improved the additional calcification of RASMCs cocultured with macrophages more than that of those cultured alone. Moreover, C21 treatment significantly attenuated the increase in the expression of BMP-2, OCN, Wnt3a and β-catenin induced by phosphate when RASMCs were cocultured with macrophages. The polarization of M1 to M2 macrophages induced by phosphate could be significantly reversed by C21 treatment. On the other hand, the expression of miRNA-204-5p in the exosomes secreted from macrophages markedly increased after C21 treatment. The decrease in the degree of calcification of RASMCs cocultured with macrophages and the expression of BMP-2, OCN, Wnt3a, β-catenin and RUNX2 induced by C21 treatment were significantly weakened after transfection with the miRNA-204-5p inhibitor. RUNX2 mRNA was the target of miRNA-204-5p in RASMCs cocultured with macrophages after C21 treatment.
VC is one of the major complications of cardiovascular disease and is associated with increased risk of morbidity and mortality [22]. VC can be classified into 3 types according to the location of calcium accumulation: VC in the intima, VC in the media, and cardiac valve calcification [23]. Because the medial layer of the artery wall is composed of VSMCs, the osteogenic differentiation of VSMCs is now considered one of the main mechanisms underlying VC in media [18]. However, relatively little research has been conducted on the mechanisms of VC formation in media. Angiotensin II, the main active compound of the renin angiotensin system, was found to play a key role in promoting the osteogenic differentiation of VSMCs, which could contribute to VC formation in media [24]. Angiotensin II binds with high affinity to two distinct receptors: AT1R and AT2R. AT2R is abundantly and widely expressed in fetal tissues, but its expression decreases rapidly after birth. Interestingly, AT2R is re-expressed in certain pathological conditions, such as VC [25]. Our results first demonstrated that AT2R overexpression ameliorated phosphate-induced VSMC calcification, which was partially due to PPAR-γ activation [19]. Another study suggested that AT2R activation prevents VSMC calcification induced by phosphate, at least in part through decreasing BMP-2 and OCN expression (26). Therefore, our findings provide novel insight into the possibility that AT2R might be a new therapeutic target for VSMC calcification. However, the underlying mechanisms of AT2R-mediated improvements in VSMC calcification are not yet clear.
Macrophages are the major participants in innate immunity and exist in all human tissues, including the artery wall [27]. The role of macrophages in intimal calcification has been intensively studied [8]. Previous studies have demonstrated that macrophages infiltrate the media of calcified arteries [11], suggesting the potential regulatory effects of macrophages on VC in media. However, little is known about the corresponding mechanisms of infiltrating macrophages in VSMC calcification. In recent years, studies have shown that macrophages promote phosphate-induced VSMC calcification via the let-7b-5p/TGFBR1 axis [12] and arterial calcification in mice with type 2 diabetes through the inhibition of VSMC autophagy [28]. Our results are consistent with those of recently published articles, further confirming that coculture of RASMCs with macrophages significantly enhances the degree of RASMC calcification. Moreover, this study revealed that C21 treatment improved the additional calcification of RASMCs cocultured with macrophages more than the calcification of RASMCs cultured alone did (Fig. 1A). Therefore, macrophages are at least partly involved in AT2R-mediated improvements in RASMC calcification.
Macrophages are the main inflammatory cells, and their dynamic changes in response to different phenotypes play important roles in the inflammatory response. M1-type macrophages can enhance the formation of VC by releasing inflammatory mediators and promoting the osteogenic differentiation of VSMCs [9]. Recent findings have shown that AT2R stimulation modulates macrophage polarization from the proinflammatory M1 phenotype to the anti-inflammatory M2 phenotype in a rat model of collagen-induced arthritis [20] and in mechanical ventilation-induced lung injury [29]. It has also been reported that the M1-to-M2 ratio in adipose tissue is lower in AT2R-interacting protein 1 transgenic mice than in wild-type mice [21]. Our results suggest that the polarization of M1 to M2 macrophages induced by phosphate could be significantly reversed by C21 treatment. Studies provided compelling evidence that VC is associated with inflammatory status and is enhanced by proinflammatory cytokines such as IL-1β and TNF-α, which play an activating role in the initiation of VC by promoting the expression of osteogenic growth factors [30]. On the contrary, anti-inflammatory cytokines such as TGF-β inhibit VSMC osteoblastic differentiation and VC formation. [31]. However, in this study, the expression of both proinflammatory and anti-inflammatory factors was significantly increased by phosphate (Fig. 2E-H), possibly because of the increase in the number of both M1 and M2 macrophages. Therefore, there may be other mechanisms contributing to the effects of macrophages on AT2R-mediated improvement in RASMC calcification.
Crosstalk between macrophages and VSMCs is essential for regulating VSMC calcification. One such mechanism involves the secretion of exosomes from macrophages, which contributes considerably to intercellular communication and subsequent functional reprogramming of VSMCs [32]. Macrophage-derived exosomes affect VSMC transdifferentiation in atherosclerosis and vascular injury through miRNAs or other cargo [33]. Serum miRNA-204 levels are associated with long-term cardiovascular disease risk on the basis of the Framingham risk score in patients with type 2 diabetes [34]. Previously, one study demonstrated that downregulation of miRNA-204 contributes to phosphate-induced VSMC calcification by regulating RUNX2 in vitro and in vivo [14]. Our results suggest that miRNA-204-5p has important effects in protecting against RASMC calcification when cocultured with macrophages after C21 treatment. Therefore, AT2R-mediated improvement in vascular calcification was partially achieved by increasing the level of miRNA-204-5p. Moreover, the canonical Wnt/β-catenin pathway, a key mediator of osteogenic differentiation, has been shown to be activated during the formation of VC in vivo and in vitro [35]. RUNX2 is a key transcription factor in the regulation of VSMC calcification [36]. Our findings suggests that when RASMCs were cocultured with macrophages, C21 improved RASMC calcification by increasing the level of miRNA-204-5p, targeting RUNX2 mRNA, inhibiting the Wnt/β-catenin signalling pathway and reducing BMP-2 and OCN expression.
However, this study still has some limitations. Firstly, the work focuses on rat cell-lines alone, and lacks in other models such as human vascular tissues/cell lines or in vivo models to further validate the findings obtained and strengthen the conclusions made. Secondly, the study did not carry more unbiased approaches (i.e. transcriptomics) to expand further cellular cross-talk. These all need further improvement and research in the future. C21 recently granted orphan drug status for the treatment of idiopathic pulmonary fibrosis, and has also shown a potent potential in heart failure, myocardial infarction, chronic inflammatory diseases, and neurological diseases [37]. Future works focusing on the expansion of researches of C21 treatment and the promotion of its potential clinical applications, will be very anticipated.
Conclusions
Our results suggested that miRNA-204-5p in the exosomes secreted from macrophages was at least partly involved in the AT2 receptor-mediated improvement of VC induced by phosphate through targeting RUNX2 mRNA, inhibiting the Wnt/β-catenin signalling pathway and decreasing the expression of calcification-related proteins. Our data also provide more evidence that AT2R stimulation may be a novel therapeutic strategy for preventing and even reversing VC.
Acknowledgements
None.
Abbreviations
- VC
Vascular calcification
- VSMC
Vascular smooth muscle cell
- M1
M1-polarized macrophage
- M2
M2-polarized macrophage
- miRNA
MicroRNA
- AT1R
Angiotensin II type 1 receptor
- AT2R
Angiotensin II type 2 receptor
- BMP-2
Bone morphogenetic protein-2
- OCN
Osteocalcin
- RASMC
Rat aortic smooth muscle cell
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- C21
Compound 21
- UTR
Untranslated region
- NTA
Nanoparticle tracking analysis
Author contributions
HL and BSS conceived and designed the project. HYB and XRL conducted the cell experiments. HBG worked on data acquisition and interpretation and was responsible for the statistical analysis. HYB and XRL wrote the manuscript and prepared the figures. HL and BSS helped write and revise the manuscript and approved the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by the Science and Technology Development Plan Project of Suzhou: grant number SKJY2021099; the General Project of Basic Science Research of Jiangsu Province in Higher Education Institutions: grant number 22 KJD320001; the National Natural Science Foundation of China for Young Scientists: grant number 82200458; and the Gusu Health Talent Plan Talent Research Project of Suzhou: grant number GSWS2023053.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participation
RASMCs (RAT-iCell-c004) and rat alveolar macrophages (NR8383, iCell-r021) were purchased from iCell. All ethical issues were considered appropriately.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui-Yu Bai and Xiao-Rui Lv have contributed equally to this work.
Contributor Information
Hui Li, Email: 9911263@163.com.
Bao-Shuai Shan, Email: shanbssuzhou@outlook.com.
References
- 1.Ge Q, Ruan CC, Ma Y, Tang XF, Wu QH, Wang JG et al (2017) Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci Rep 7:40253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Chen X, Chen Z, Zhang M (2022) Arterial calcification and its association with stroke: implication of risk, prognosis, treatment response, and prevention. Front Cell Neurosci 16:845215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng W, Teng Y, Zhong Q, Zhang Y, Zhang J, Zhao P et al (2023) Biomimetic grapefruit-derived extracellular vesicles for safe and targeted delivery of sodium thiosulfate against vascular calcification. ACS Nano 17(24):24773–24789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ et al (2020) Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol 76(22):2635–2646 [DOI] [PubMed] [Google Scholar]
- 5.Leopold JA (2015) Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med 25(4):267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao W, Yang R, Yang Y, Jin S, Li Y, Yuan F et al (2018) Stellate ganglion block ameliorates vascular calcification by inhibiting endoplasmic reticulum stress. Life Sci 193:1–8 [DOI] [PubMed] [Google Scholar]
- 7.Bardeesi ASA, Gao J, Zhang K, Yu S, Wei M, Liu P et al (2017) A novel role of cellular interactions in vascular calcification. J Transl Med 15(1):95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waring OJ, Skenteris NT, Biessen EAL, Donners M (2022) Two-faced Janus: the dual role of macrophages in atherosclerotic calcification. Cardiovasc Res 118(13):2768–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song X, Song Y, Ma Q, Fang K, Chang X (2023) M1-type macrophages secrete TNF-α to stimulate vascular calcification by upregulating CA1 and CA2 expression in VSMCs. J Inflamm Res 16:3019–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peled M, Fisher EA (2014) Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol 5:579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Qu L, Matz AJ, Murphy PA, Liu Y, Manichaikul AW et al (2022) AtheroSpectrum reveals novel macrophage foam cell gene signatures associated with atherosclerotic cardiovascular disease risk. Circulation 145(3):206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Zhang C, Shi J, Yang Y, Xing X, Wang Y et al (2022) High-phosphate-stimulated macrophage-derived exosomes promote vascular calcification via let-7b-5p/TGFBR1 axis in chronic kidney disease. Cells 12(1):161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuo L, Song H, Jiang D, Bai X, Song G (2021) Mesenchymal stem cells transfected with anti-miRNA-204-3p inhibit acute rejection after heart transplantation by targeting C-X-C motif chemokine receptor 4 (CXCR4) in vitro. J Thorac Dis 13(8):5077–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu X et al (2012) MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res 96(2):320–329 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chen S, Deng C, Li F, Wang Y, Hu X et al (2015) MicroRNA-204 targets Runx2 to attenuate BMP-2-induced osteoblast differentiation of human aortic valve interstitial cells. J Cardiovasc Pharmacol 66(1):63–71 [DOI] [PubMed] [Google Scholar]
- 16.Song R, Zhai Y, Ao L, Fullerton DA, Meng X (2019) MicroRNA-204 deficiency in human aortic valves elevates valvular osteogenic activity. Int J Mol Sci 21(1):76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaori K, Iwatani H, Yamato M, Ito T (2021) User of angiotensin-converting-enzyme inhibitor and/or angiotensin II receptor blocker might be associated with vascular calcification in predialysis chronic kidney disease patients: a retrospective single-center observational study : ACEI/ARB and vascular calcification. BMC Nephrol 22(1):7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Hong SW, Kim MJ, Moon SJ, Kwon H, Park SE et al (2024) Glucagon-like peptide receptor agonist inhibits angiotensin II-induced proliferation and migration in vascular smooth muscle cells and ameliorates phosphate-induced vascular smooth muscle cells calcification. Diabetes Metab J 48(1):83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kukida M, Mogi M, Kan-No H, Tsukuda K, Bai HY, Shan BS et al (2019) AT2 receptor stimulation inhibits phosphate-induced vascular calcification. Kidney Int 95(1):138–148 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Tu J, Jiang J, Zhang Q, Liu Q, Körner H et al (2020) Angiotensin II type 2 receptor modulates synovial macrophage polarization by inhibiting GRK2 membrane translocation in a rat model of collagen-induced arthritis. J Immunol (Baltimore, Md : 1950) 205(11):3141–3153 [DOI] [PubMed] [Google Scholar]
- 21.Jing F, Mogi M, Min LJ, Ohshima K, Nakaoka H, Tsukuda K et al (2013) Effect of angiotensin II type 2 receptor-interacting protein on adipose tissue function via modulation of macrophage polarization. PloS one 8(4):e60067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G, Stormont RM, Gangahar DM, Agrawal DK (2012) Role of matrix Gla protein in angiotensin II-induced exacerbation of vascular calcification. Am J Physiol Heart Circ Physiol. 303(5):H523-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M (1996) Medial artery calcification. a neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16(8):978–983 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Ott KM, Kagiyama S, Phillips MI (2000) The multiple actions of angiotensin II in atherosclerosis. Regul Pept 93(1–3):65–77 [DOI] [PubMed] [Google Scholar]
- 25.Matsubara H (1998) Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res 83(12):1182–1191 [DOI] [PubMed] [Google Scholar]
- 26.Bai HY, Shan BS, Jiang YN (2021) The protective effects of renin-angiotensin system componts on vascular calcification. J Hum Hypertens 35(5):410–418 [DOI] [PubMed] [Google Scholar]
- 27.Ravi S, Martin LC, Krishnan M, Kumaresan M, Manikandan B, Ramar M (2024) Interactions between macrophage membrane and lipid mediators during cardiovascular diseases with the implications of scavenger receptors. Chem Phys Lipids 258:105362 [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Chen C, Chen Q, Gao Y, Zhao Z, Yuan Q et al (2022) Extracellular vesicle miR-32 derived from macrophage promotes arterial calcification in mice with type 2 diabetes by inhibiting VSMC autophagy. J Transl Med 20(1):307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Xu Z, Xu L, Wang L, Qin S, Ying L et al (2024) Angiotensin II Type 2 receptor inhibits M1 polarization and apoptosis of alveolar macrophage and protects against mechanical ventilation-induced lung injury. Inflammation 48(1):165–180 [DOI] [PubMed] [Google Scholar]
- 30.Bessueille L, Magne D (2015) Inflammation: a culprit for vascular calcification in atherosclerosis and diabetes. Cell Mol Life Sci 72(13):2475–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Xi Z, Yu Z, Yang C, Tan C (2022) LincRNA-EPS increases TGF-β expression to inhibit the Wnt/β-catenin pathway, VSMC osteoblastic differentiation and vascular calcification in diabetic mice. Exp Ther Med 23(6):425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu C, Wang X, Zhao M, Cai T, Liu P, Li J et al (2016) Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc 5(10):e004099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L et al (2019) Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 9(23):6901–6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Ding YD, Gao W, Pei YQ, Yang JX, Zhao YX et al (2020) Serum microRNA-204 levels are associated with long-term cardiovascular disease risk based on the Framingham risk score in patients with type 2 diabetes: results from an observational study. J Geriatr Cardiol : JGC 17(6):330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herencia C, Rodríguez-Ortiz ME, Muñoz-Castañeda JR, Martinez-Moreno JM, Canalejo R, Montes de Oca A et al (2015) Angiotensin II prevents calcification in vascular smooth muscle cells by enhancing magnesium influx. Eur J Clin Invest 45(11):1129–1144 [DOI] [PubMed] [Google Scholar]
- 36.Zheng G, Zhao Y, Li Z, Hua Y, Zhang J, Miao Y et al (2023) GLSP and GLSP-derived triterpenes attenuate atherosclerosis and aortic calcification by stimulating ABCA1/G1-mediated macrophage cholesterol efflux and inactivating RUNX2-mediated VSMC osteogenesis. Theranostics 13(4):1325–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey A, Gaikwad AB (2017) AT2 receptor agonist compound 21: a silver lining for diabetic nephropathy. Eur J Pharmacol 15(815):251–257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.