Abstract
Background
Dark chocolate, rich in flavanols, may support vascular health by reducing arterial stiffness and blood pressure across menstrual phases. This study examined the effects of 85% dark chocolate on nitric oxide (NO) levels and vascular function during high-intensity resistance exercise in healthy women across the early follicular and mid-luteal phases.
Methods
Thirty-one healthy women (aged 20–30 years) with regular menstrual cycles completed a randomized, crossover study (conducted at National Chung Cheng University, Sep–Dec 2023). Participants consumed either 85% dark chocolate or milk chocolate (1 g/kg body weight) before high-intensity resistance exercise during the early follicular (days 2–5) and mid-luteal (days 18–24) phases of two menstrual cycles. Finger-toe pulse wave velocity (ftPWV), arterial stiffness, blood pressure, and plasma NO levels were measured at rest, 2 h after chocolate consumption (baseline), immediately post-exercise (T0), and at 60 (T60) and 120 (T120) minutes post-exercise.
Results
Dark chocolate supplementation significantly increased NO levels and reduced systolic blood pressure (SBP), ftPWV, and arterial pressure volume index (API) (p < 0.05) compared to milk chocolate across both menstrual phases. During the early follicular phase, dark chocolate also attenuated exercise-induced increases in arterial stiffness and blood pressure (p < 0.05).
Conclusion
85% dark chocolate supplementation may reduce the negative vascular effects of high-intensity resistance exercise, particularly by lowering blood pressure, arterial stiffness, and API, especially in the early follicular phase. These findings suggest that dark chocolate could be a practical, non-pharmacological intervention for improving cardiovascular health in women.
Trial Registration
ClinicalTrials.gov, NCT06908941. Registered 19 March 2025 — Retrospectively registered, https://clinicaltrials.gov/study/NCT06908941.
Keywords: Isotonic Resistance Exercise, Cocoa Flavanols, Catechins, Epicatechins
Key Points
1. Dark chocolate supplementation significantly improved vascular function by reducing arterial stiffness (ftPWV, API) and SBP compared to milk chocolate, irrespective of menstrual phase.
2. The vascular benefits of dark chocolate were more pronounced post-exercise during the early follicular phase, when estrogen levels were lower.
3. This study underscores the potential of dark chocolate in alleviating vascular stress induced by high-intensity resistance exercise in young women.
Background
According to the 2020 report, the World Health Organization (WHO) indicated that cardiovascular disease (CVD) was ranked as the leading cause of death globally among the top ten causes, highlighting the significant issue of mortality due to CVD [1]. In addition to its health impact, CVD imposed a substantial economic burden due to the costs associated with medical treatment and long-term care [2]. While CVD is often perceived as a condition affecting older populations, recent studies emphasized that the risk for CVD began much earlier, with evidence suggested that cardiovascular risk factors, such as obesity, hypertension, and pregnancy-related complications, often accumulated during adolescence or early adulthood [3]. Furthermore, the incidence of acute myocardial infarction and ischemic stroke among young women was reported to be rising, driven by the cumulative effects of lifestyle-related risk factors such as physical inactivity, poor diet, and smoking [4]. Hormonal factors, such as estrogen, have also been linked to cardiovascular health. The menopausal transition, characterized by a decline in estrogen levels, was associated with impaired endothelial function, which could have begun during perimenopause and worsened with prolonged estrogen deficiency, thereby increasing cardiovascular disease risk [5]. Given these risks, research attention has been directed toward exploring non-pharmacological interventions, such as different exercise patterns, to prevent CVD and improve arterial function in healthy adults [6]. Therefore, initiating exercise training and maintaining exercise habits during the stage of healthy young women was critical for preventing the onset of cardiovascular diseases and maintaining vascular health. Both estrogen and exercise-induced changes have been suggested to play important roles in promoting vascular health and preventing cardiovascular disease in young women, highlighting the potential benefits of early interventions.
The protective effects of estrogen on vascular health were mediated through multiple mechanisms, including the enhancement of nitric oxide (NO) production, the inhibition of endothelin-1 (ET-1) activity, and the suppression of sympathetic nervous system (SNS) activity. First, estrogen enhanced NO bioavailability by activating endothelial nitric oxide synthase (eNOS), leading to improved endothelial function and vasodilation [7]. Second, estrogen reduced ET-1-mediated vasoconstriction by modulating receptor responses, diminishing ETA-mediated vasoconstriction while enhancing ETB-mediated vasodilation [8].Third, estrogen attenuated SNS activity, which contributed to reduced vascular resistance and improved hemodynamic regulation in studies involving estrogen replacement therapy [9]. Collectively, these mechanisms underscored the multifaceted role of estrogen in maintaining vascular health and mitigating cardiovascular risks. Prior research involving healthy, young, and eumenorrheic women found that higher estrogen levels were associated with reductions in the aortic reflected pressure wave and aortic augmentation index (AIx) as well as enhanced endothelial function [10]. In addition, a study focusing on women with regular menstrual cycles revealed that estrogen levels were higher during the mid luteal phase compared to the early follicular phase. In the early follicular phase, when estrogen levels were lower, pulse wave velocity (PWV) tended to be higher [11]. These findings suggested that higher estrogen levels were associated with reduced arterial stiffness. Previous studies have examined baseline (resting) blood pressure values across different menstrual phases, but the findings have been inconsistent. Some studies involving healthy adult women with regular menstrual cycles did not observe significant differences in either systolic blood pressure (SBP) or diastolic blood pressure (DBP) at rest between the follicular and luteal phases [11–13]. However, another study reported significantly higher SBP at rest in the early follicular phase compared to the late follicular and early luteal phases, along with elevated DBP at rest in the early follicular phase compared to the late follicular and early luteal phases [10]. These discrepancies highlight the need for further investigation with larger sample sizes and more comprehensive measurements. In terms of exercise and menstrual cycle effects, a study involving women with regular menstrual cycles found that high-intensity resistance exercise performed during the early follicular phase resulted in increased brachial-ankle pulse wave velocity (baPWV) compared to the mid luteal phase, where no significant changes in arterial stiffness were observed 30 and 60 min post-exercise [12]. However, blood pressure measurements taken at 30 and 60 min post-exercise showed no significant differences between the early follicular and mid luteal phases, indicating that blood pressure responses to exercise were consistent across menstrual phases [12]. These findings highlight the phase-dependent effects of estrogen on vascular responses, with higher estrogen levels in the mid luteal phase mitigating arterial stiffness following high-intensity resistance exercise. In contrast, blood pressure responses remained consistent between menstrual phases.
In past studies, researchers have observed a negative correlation between supplementing with dark chocolate and the risk of cardiovascular disease [14]. Dark chocolate is primarily derived from cocoa, which is rich in flavanols, including monomeric compounds such as catechins and epicatechins [15]. Previous research has explained the mechanisms by which flavanols improve vascular function following ingestion: (I) by stimulating the activity of nitric oxide synthase (NOS), thereby increasing the content of NO within blood vessels; (II) by inhibiting the increase in blood pressure induced by L-nitroarginine methyl ester (L-NAME), an NOS inhibitor; and (III) by suppressing the production of ET-1 [16]. In healthy adult women, a single supplementation of 85% dark chocolate or milk chocolate led to a significant reduction in blood pressure only in the dark chocolate supplementation group [17]. Similarly, acute studies conducted in healthy adults have demonstrated that dark chocolate improved vascular function by significantly reducing the AIx, indicative of decreased wave reflection, and enhancing endothelial function through increased flow-mediated dilation (FMD) [18]. Prior research investigated the acute effects of (-)-epicatechin, a primary flavanol found in cocoa, on healthy adults and reported no significant improvements in brachial artery vasodilation or exercise performance following high-intensity CrossFit® resistance exercise [19]. These findings highlighted the potential vascular benefits of dark chocolate in improving endothelial function and reducing cardiovascular risk, while the lack of significant effects with isolated (-)-epicatechin supplementation following high-intensity CrossFit® resistance exercise suggested that exercise modality, the complexity of cocoa polyphenols, and supplementation protocols might have influenced vascular responses. Further research is warranted to explore the combined effects of traditional resistance exercise and dark chocolate on vascular function and cardiovascular health.
Resistance training was one of the most popular forms of physical activity among young adults (aged 18–34) [20]. Long-term resistance training had been shown to improve muscular strength and endurance, enhance body composition, increase bone mineral density, and reduce cardiovascular disease risk factors in women [21]. However, acute of resistance exercise elevated arterial stiffness and blood pressure, potentially imposing additional stress on the vascular system [22]. To mitigate these acute vascular responses, it was essential to explore strategies that reduced the vascular stress induced by resistance exercise. While previous studies have separately demonstrated the vascular benefits of dark chocolate supplementation and estrogen [7–9, 14, 16, 17], the combined effects of these factors on mitigating resistance exercise-induced vascular stress remain unexplored. Furthermore, the hormonal fluctuations across the menstrual cycle provided a unique model to explore the potential protective interaction between estrogen and dark chocolate factors in response to exercise-induced stress. Therefore, this study aimed to evaluate the effects of pre-exercise supplementation with 85% dark chocolate on vascular elasticity and blood pressure responses during the early follicular and mid-luteal phases following high-intensity resistance exercise.
Method
Participants
This study utilized the G Power software (version 3.1.9.7, Heinrich Heine University, Düsseldorf, Germany) to calculate the required sample size. We used repeated measures analysis (ANOVA, within factors), setting a moderate effect size = 0.25, α = 0.05, power = 0.8, with 4 trials, each measured 5 times. The analysis results indicated that a minimum of 24 participants is required. Anticipating potential dropouts during the experiment, recruitment was increased to 40 participants.
This study recruited 40 healthy adult women, aged 20–30 years, from a local university campus who had a regular menstrual cycles (28 ± 7 days) in the past three months. According to Augustine et al. [13], Regecova et al. [17] and Moran et al. [23], the exclusion criteria for studies on the menstrual cycle and chocolate supplementation were adopted in this study. Participants were excluded based on the following conditions: (1) irregular menstrual cycles (menstrual cycle length < 21 or > 35 days in the past three months), (2) pregnancy or lactation in the past year, (3) history of cardiovascular or uterine surgery in the past six months, (4) use of contraceptives or other female hormone medications, (5) smoking or alcohol consumption habits, (6) hypertension (blood pressure > 140/90 mmHg), chronic diseases, heart disease, or other cardiovascular conditions, (7) allergy to cocoa products, nuts, or fruits. Before the experiment, each participant completed a self-reported questionnaire designed to assess health status, physical activity levels, and menstrual cycle regularity. The questionnaire included detailed inquiries on weekly exercise frequency and exercise duration to evaluate habitual activity levels. Participants were also asked to report whether they were members of collegiate sports teams or professional athletes, as those with high-intensity training loads were excluded to minimize confounding effects. Additionally, the questionnaire assessed medical history to confirm the absence of metabolic or cardiovascular diseases and to verify regular menstrual menstrual cycle length (28 ± 7 days) over the previous three months. In addition, participants had to read the participant information sheet carefully to understand the purpose and process of the study. This study initially recruited 40 women participants, of whom 9 were excluded due to non-adherence to protocols or ineligibility. Ultimately, 31 participants who completed the study and were included in the final analysis. National Chung Cheng University Human Research Ethics Center approved this study (ID: CCUREC111122101) and all participants provided sign the informed consent form if they voluntarily agreed to participate in the experiment. The study was conducted at National Chung Cheng University, Sep–Dec 2023.
Table 1 presents the physical and demographic characteristics of the participants, including their average age, height, weight, BMI, weekly activity levels, and one-repetition maximum (1RM) for the deadlift, bench press, and squat exercises.
Table 1.
Participant characteristics (N = 31)
| Variable | Means ± SD | Range (Min-Max) |
|---|---|---|
| Age (year) | 22.10 ± 1.85 | 20.00–27.00 |
| Height (cm) | 161.26 ± 6.97 | 149.00-182.00 |
| Weight (kg) | 55.47 ± 6.06 | 42.50–70.10 |
| Chocolate Intake (g) | 55.47 ± 6.06 | 42.50–70.10 |
| BMI (kg/m2) | 21.34 ± 1.99 | 16.81–25.22 |
| Activity (min/week) | 115.00 ± 108.74 | 10.00-480.00 |
| 1-RM Deadlift (kg) | 72.19 ± 12.52 | 44.00-100.00 |
| 1-RM Bench Press (kg) | 27.74 ± 6.86 | 17.00–47.00 |
| 1-RM Squat (kg) | 54.84 ± 9.48 | 39.00–77.00 |
| Menstrual Cycle (days) | 31.10 ± 2.33 | 27.00–35.00 |
Note BMI: body mass index; 1RM: 1repetition maximum
Study Design
Before the formal experiment, participants underwent four training sessions to learn correct exercise techniques (squat, bench press, and deadlift) and a 1RM test for resistance exercise to set the training intensity. One week after the training session, participants performed a 1RM test for the resistance exercises in the study, and the 1RM formula was applied to determine the 1RM values for the formal experiment [24].
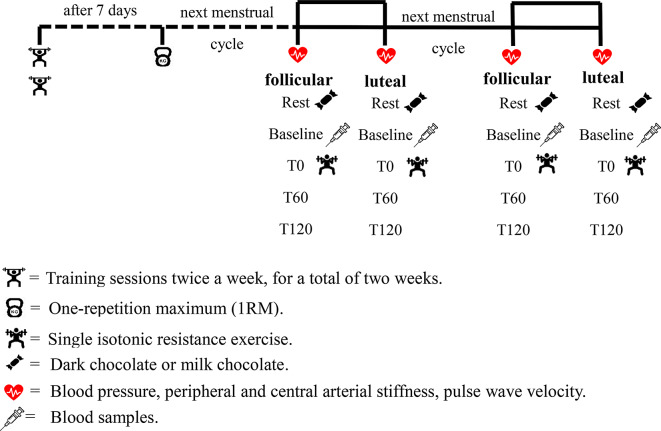
Participants came to the laboratory four times for the formal experiment, conducting the experiment during the early follicular and mid luteal phases in two consecutive months. During the early follicular and mid luteal phases of the two menstrual cycles, participants underwent resistance exercise combined with dark chocolate or milk chocolate, randomly allocated. The same type of chocolate supplementation was not repeated within the same phase. Milk chocolate, which lacks the bioactive flavonoids present in dark chocolate, was selected as the control condition to the effects of flavonoid-rich dark chocolate while ensuring comparable sensory properties and caloric content [25]. Based on previous studies, significant differences in estrogen and progesterone levels during the menstrual cycle’s early follicular and mid luteal phases have been noted [11, 13]. Therefore, this study set the early follicular phase (days 2–5) and mid-luteal phase (days 18–24) for exercise in the same menstrual cycle [11]. The early follicular phase (days 2–5) was fixed for all participants, regardless of menstrual cycle length. For the mid-luteal phase, testing days were adjusted based on participants’ previous menstrual cycle lengths, which were calculated by the researchers. For example, for a 28-day menstrual cycle, the mid-luteal phase was defined as days 18–24; for a 29-day cycle, as days 19–25; and for a 27-day cycle, as days 17–23, and so forth. This approach ensured consistency across participants while limiting inclusion to regular menstrual cycles (28 ± 7 days). The first formal experiment was then conducted in the early follicular and mid luteal phases of the participants’ next menstrual cycle. On the day of the experiment, participants arrived at the laboratory between 8:00 and 10:00 a.m. Upon arrival, participants’ height and weight were measured using a calibrated stadiometer and weighing scale (Ken Zhong Weighing Instruments KC-21 A, Taiwan). Subsequently, blood pressure, peripheral and central arterial stiffness, and pulse wave velocity were measured. The experiments were conducted in a controlled environment with the laboratory temperature maintained at 22–24 °C using air conditioning. The laboratory was an independent space with minimal noise, and only the participant and research personnel were present to ensure focus and eliminate external distractions. After collecting rest values (Rest), a standard breakfast was provided consisting of russian bread (456 kcal; 10.5 g protein, 60.9 g carbohydrates) and unsweetened soy milk (40.7 kcal; 3.4 g protein, 3.5 g carbohydrates), along with supplementation of dark chocolate or milk chocolate. Two hours after supplementation, measurements of the above indicators were taken again (baseline), and blood samples were collected only at baseline. Following warm-up exercises for resistance training, isotonic resistance exercises were performed, and physiological and biochemical indexs were measured immediately after exercise (T0), at 1 h (T60), and at 120 min (T120) post-exercise, for a total of 5 measurements. The same experiment was conducted in the early follicular and mid luteal phases of the following month, following the same procedures. (Fig. 1)
Fig. 1.
Experimental study design
Maximum Repetition (1RM)
To avoid affecting the experimental results, the 1RM test was conducted at least 10 days apart from the formal exercise. This study referenced the method used by Hackett et al. [26] for the maximum repetition test. Before the formal test, participants underwent at least 4 training sessions. Upon arrival at the laboratory, participants began with a standard warm-up process, including dynamic training movements or static stretching. After warming up, the maximum repetition test began. The weight and number of repetitions were recorded when the participant reached muscular failure within 10 repetitions. The successful weight and number of repetitions were then used in the formula to estimate 1RM, which is 「successful weight × (1+ (0.033× successful repetitions)) 」 [27]. If the repetitions exceed 10, the load weight needs to be increased by 5–10%, and the participant should rest for 5 min before attempting again.
Resistance Exercise
In this study, the method of isotonic resistance exercise (squat, bench press, and deadlift) was employed. These movements were selected for their effectiveness in engaging multiple muscle groups and their broad application in resistance training research [28, 29]. All three movements were conducted with participants under high safety conditions, with a certified fitness coach (Health & Exercise Association R.O.C., Taiwan) present throughout the training session. Participant responses were continuously monitored during the exercise, and if any discomfort was reported, the training was immediately halted. For the squat, during the eccentric contraction phase, the knee joint is flexed to approximately less than 90 degrees, and during the concentric contraction phase, force is applied to stand up without locking the knee joint (approximately 180 degrees), completing one repetition. For the bench press, during the eccentric contraction phase, the elbow joint is flexed to approximately less than 90 degrees, and during the concentric contraction phase, the barbell is pushed up until the arms are straight, without locking the elbow joint (approximately 180 degrees), completing one repetition. For the deadlift, the initial setup with knee joint angle of approximately 100 degrees and hip joint angle of approximately 70 degrees, stabilizing the core to keep the back straight, during the concentric contraction phase, the weight is pulled from the ground to a standing position at the hip and knee joints, without locking the joints (knee joint approximately 180 degrees, hip joint approximately 108 degrees), followed by the eccentric contraction placing the weight back on the ground, completing one repetition. The exercise training in this study began with squats, with each warm-up set starting from an empty barbell to half of the target intensity, performed for 3 sets of 5 repetitions [29], with a 3-minute rest between sets. This was followed by the formal exercise training, with the intensity set at 75% 1RM for all resistance exercises in this study, performed for 5 sets of 6 repetitions, with a 3-minute rest between sets, and a 5-minute rest between each movements. Subsequently, bench press and deadlifts were performed in the same pattern as squats. The exercise protocol was identical across all phases of the menstrual cycle to ensure consistency in training volume and intensity.
Chocolate Supplementation
The dosage of dark chocolate supplementation was based on Regecova et al. [17], with a dosage of 1 g/kg of body weight. Participants were instructed to consume either 85% dark chocolate (DiRaja Chocolate, Pingtung, Taiwan) or milk chocolate (Hunya Foods, New Taipei City, Taiwan) (cocoa content < 25%). Both types of chocolate had similar total calorie, fat, and carbohydrate content (Table 2 for full nutritional composition). The dark and milk chocolate samples were analyzed for catechin, epicatechin, and total cocoa flavanol content at the Organic and Health Food Inspection Laboratory (OHC Lab), Chia Nan University of Pharmacy & Science, which is certified by the Taiwan of the Ministry of Health and Welfare. All chocolate was consumed alongside a standardized breakfast during each of the four formal experiments.
Table 2.
Nutritional composition per 100 g of milk and dark chocolate
| Nutritional component | Milk chocolate | Dark chocolate |
|---|---|---|
| Energy (kJ) | 2269.8 | 2618.7 |
| Protein (g) | 8.3 | 12.2 |
| Total fat (g) | 32.8 | 49.1 |
| Saturated fat (g) | 32.0 | 27.7 |
| Trans fat (g) | 0.0 | 0.0 |
| Carbohydrates (g) | 53.8 | 33.8 |
| Sugars (g) | 49.8 | 14.5 |
| Sodium (mg) | 87.5 | 0.0 |
| Total cocoa flavanol (mg) | 773.0 | 4370.0 |
| catechin (mg) | 58.3 | 659.5 |
| epicatechin (mg) | 75.9 | 376.7 |
Finger-Toe Pulse Wave Velocity (ftPWV)
This study used non-invasive ftPWV as an indicator of arterial stiffness. In addition, previous research has demonstrated a significant correlation between ftPWV and the gold-standard cfPWV, supporting the validity of ftPWV as a reliable measure of arterial stiffness [30]. For measurements at the Rest and Baseline time points, participants must lying flat quietly and rest for 15 min upon arrival at the laboratory to stabilize their physiological signs before beginning the ftPWV measurements. Measurements at other time points are taken while participants are resting in the laboratory. Firstly, sensors for photoplethysmography (PPG) were placed on the right index finger and right big toe of the participants. These sensors were marked with an oily pen to ensure consistent placement throughout the experiment. The signals were then amplified using a Biopec MP150 (MP150, Biopac Systems Inc., Goleta, CA, USA), with each physiological signal collected for at least 150 s. The signals were analyzed using MATLAB to calculate the time difference between the two pulse waves. The length of the participant’s body segments ( sternum to the right index finger, and sternum to the big toe of the right foot) was measured, and this value was divided by the average difference between 10 pulse waves (in the stable interval of 150 s) to calculate the ftPWV value [31, 32]. The coefficient of variation (CV) for ftPWV measurements was below 5%.
Blood Pressure and Artery Stiffness
After completing ftPWV measurements, participants were measured for blood pressure and arterial stiffness measurements at each time point. The measured indicators include SBP, DBP, heart rate, arterial pressure volume index (API), and arterial velocity pulse index (AVI), all of which are measured using the PASESA AVE-2000 (PASESA, Tokyo, Japan) with a wrapped around the right upper arm of seated participants. MAP and PP were derived from the SBP and DBP measurements. MAP and PP were subsequently derived from the SBP and DBP measurements. MAP was calculated using the formula: MAP = DBP + [(SBP - DBP) / 3], and PP was calculated as the difference between SBP and DBP (PP = SBP - DBP). The API reflected the stiffness of peripheral arteries by assessing the pressure-volume relationship of the artery, while the AVI primarily reflected the stiffness of central arteries by analyzing the ratio of forward and reflected pressure waves [33, 34]. Each measurement was taken three times, and the average of the three readings was recorded. The CV was below 5% for SBP and DBP measurements, and below 10% for API and AVI measurement.
Blood Analysis
To quantify the concentrations of NO and estradiol, blood samples were drawn from the antecubital vein using standard venipuncture techniques. Blood was collected into EDTA tubes, immediately centrifuged at 3,000 rpm for 10 min to separate plasma, and the plasma samples were stored at -20 °C until analysis.
Plasma concentrations of NO and estradiol were quantified using enzyme-linked immunosorbent assay (ELISA) kits. NO was assessed using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), and estradiol was measured using the Estradiol ELISA Kit (DRG Instruments, Marburg, Germany). All assays were performed following the manufacturers’ instructions, and each sample was analyzed in duplicate. The CV for both assays was maintained below 5%.
Statistical Analysis
The recorded data were analyzed using the statistical software SPSS (version 23.0, IBM Inc., Armonk, NY, USA). Descriptive statistics were used to calculate the mean and standard deviation of the participants’ basic information. Normality of the data was assessed using the Shapiro-Wilk test. A two-way repeated measures analysis of variance (ANOVA) was conducted to compare the effects of different trials on the dependent variables. The trials included early follicular milk chocolate (EF-MC), early follicular dark chocolate (EF-DC), mid luteal milk chocolate (ML-MC), and mid luteal dark chocolate (ML-DC). Measurements were taken at five time points: pre-chocolate and pre-exercise (Rest), 2 h after consuming chocolate (Baseline-C), immediately after exercise (T0), 60 min after exercise (T60), and 120 min after exercise (T120). The analysis examined the trial × time interaction to determine how these factors influenced the outcomes. If a significant interaction was found, Bonferroni post hoc tests were conducted to examine the specific effects of each factor on the results. Additionally, one-way repeated measures ANOVA was performed to compare changes in blood samples, including NO and estrogen levels, across the four trials (EF-MC, EF-DC, ML-MC, ML-DC). The significance level was set at p < 0.05.
Results
Plasma NOx and Estrogen Responses
This study assessed the plasma levels of NOx to evaluate the vascular differences following a 2-hour supplementation with dark or milk chocolate. Additionally, estradiol levels were measured to confirm menstrual cycle phases, as presented in Table 3. In the Estradiol indicator, the ML-MC and ML-DC trials were significantly higher levels than the EF-MC and EF-DC trials (p < 0.05). Additionally, in the NOx indicator, the EF-DC and ML-DC trials showed significantly higher levels than the EF-MC and ML-MC trials (p < 0.05).
Table 3.
The effects of dark or milk chocolate supplementation with a standard breakfast on NOx (nitrate/nitrite) and estradiol levels 2 h post-ingestion during different menstrual cycles
| EF-MC | EF-DC | ML-MC | ML-DC | |
|---|---|---|---|---|
| Estradiol (pg/ml) | 66.53 ± 21.24 | 62.19 ± 24.06 | 128.79 ± 58.54a | 127.11 ± 64.26 a |
|
Nitrite/Nitrate (µmol/l) |
24.26 ± 13.84 | 32.14 ± 14.16b | 25.89 ± 11.67 | 30.31 ± 12.64b |
Note The value are mean ± SD; EF-MC = early follicular-milk chocolate; EF-DC = early follicular-dark chocolate; ML-MC = Mid Luteal-milk chocolate; ML-DC = Mid Luteal-dark chocolate
a = Significant difference compared with EF-MC and EF-DC trials (p < 0.05)
b = Significant difference compared with EF-MC and ML-MC trials (p < 0.05)
FtPWV Responses
Figure 2 shows changes in ftPWV at rest, baseline, 0, 60, and 120 min after resistance exercise, supplemented with either dark chocolate or milk chocolate, across each menstrual cycle. The two-way repeated measures ANOVA revealed a significant main effect of trials on ftPWV (F = 15.546, p < 0.001, 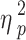 = 0.349) and a significant main effect of time (F = 56.023, p < 0.001,
= 0.349) and a significant main effect of time (F = 56.023, p < 0.001, 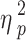 = 0.659). Additionally, a significant trial × time interaction effect was observed (F = 2.970, p = 0.001,
= 0.659). Additionally, a significant trial × time interaction effect was observed (F = 2.970, p = 0.001, 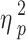 = 0.093). Post hoc Bonferroni comparisons revealed that ftPWV was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The ftPWV was significantly higher in the EF-MC trial at Baseline, T0 and T60 compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). In addition, significant increases in ftPWV were observed from rest to baseline in the EF-MC trial (p < 0.05). The ftPWV in the EF-DC and ML-DC trials showed significant decrease at baseline when compared to the rest condition (p < 0.05). All trials exhibited significant increases in ftPWV at T0 after resistance exercise (p < 0.05).
= 0.093). Post hoc Bonferroni comparisons revealed that ftPWV was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The ftPWV was significantly higher in the EF-MC trial at Baseline, T0 and T60 compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). In addition, significant increases in ftPWV were observed from rest to baseline in the EF-MC trial (p < 0.05). The ftPWV in the EF-DC and ML-DC trials showed significant decrease at baseline when compared to the rest condition (p < 0.05). All trials exhibited significant increases in ftPWV at T0 after resistance exercise (p < 0.05).
Fig. 2.
The changes in ftPWV at each time point in the EF-MC, EF-DC, ML-MC, and ML-DC trials. Note. The value are mean ± SD; EF-MC: early follicular-milk chocolate; EF-DC: early follicular-dark chocolate; ML-MC: Mid Luteal-milk chocolate; ML-DC: Mid Luteal-dark chocolate; ftPWV: finger-toe pulse wave velocity; Rest: pre-chocolate and pre-exercise; Baseline: 2 h after consuming chocolate; T0: 0 min after exercise; T60: 60 min after exercise; T120: 120 min after exercise. a = The EF-MC and EF-DC trials showed a significant difference compared to both the ML-MC and ML-DC trials (p < 0.05). b = The EF-MC trial showed a significant difference compared to the EF-DC, ML-MC and ML-DC trials (p < 0.05). c = Significant difference against rest in the EF-MC, EF-DC and ML-DC trials (p < 0.05). d = Significant difference against rest in the all trials (p < 0.05)
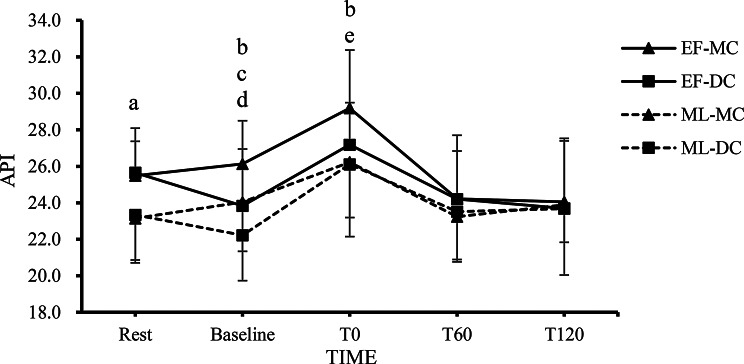
API Responses
Figure 3 shows changes in API at rest, baseline, 0, 60, and 120 min after resistance exercise, supplemented with either dark chocolate or milk chocolate, across each menstrual cycle. The two-way repeated measures ANOVA revealed a significant main effect of trials on API (F = 6.389, p = 0.001, 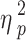 = 0.203) and a significant main effect of time (F = 34.164, p < 0.001,
= 0.203) and a significant main effect of time (F = 34.164, p < 0.001, 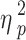 = 0.611). Additionally, a significant trial × time interaction effect was observed (F = 3.443, p < 0.001,
= 0.611). Additionally, a significant trial × time interaction effect was observed (F = 3.443, p < 0.001, 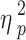 = 0.112). Post hoc Bonferroni comparisons revealed that API was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The API was significantly higher in the EF-MC trial at Baseline and T0 compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). The API was significantly higher in the ML-MC trial at Baseline compared with the ML-DC trials (P < 0.05). The API in the EF-DC and ML-DC trials showed significant decrease at baseline when compared to the rest condition (p < 0.05). All trials exhibited significant increases in API at T0 after resistance exercise (p < 0.05).
= 0.112). Post hoc Bonferroni comparisons revealed that API was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The API was significantly higher in the EF-MC trial at Baseline and T0 compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). The API was significantly higher in the ML-MC trial at Baseline compared with the ML-DC trials (P < 0.05). The API in the EF-DC and ML-DC trials showed significant decrease at baseline when compared to the rest condition (p < 0.05). All trials exhibited significant increases in API at T0 after resistance exercise (p < 0.05).
Fig. 3.
The changes in API at each time point in the EF-MC, EF-DC, ML-MC, and ML-DC trials. Note. The value are mean ± SD; EF-MC: early follicular-milk chocolate; EF-DC: early follicular-dark chocolate; ML-MC: Mid Luteal-milk chocolate; ML-DC: Mid Luteal-dark chocolate; API: arterial pressure volume index; Rest: pre-chocolate and pre-exercise; Baseline: 2 h after consuming chocolate; T0: 0 min after exercise; T60: 60 min after exercise; T120: 120 min after exercise. a = The EF-MC and EF-DC trials showed a significant difference compared to both the ML-MC and ML-DC trials (p < 0.05). b = The EF-MC trial showed a significant difference compared to the EF-DC, ML-MC and ML-DC trials (p < 0.05). c = The ML-MC trial showed a significant difference compared to the ML-DC trial (p < 0.05). d = Significant difference against rest in the EF-DC and ML-DC trials (p < 0.05). e = Significant difference against rest in the all trials (p < 0.05)
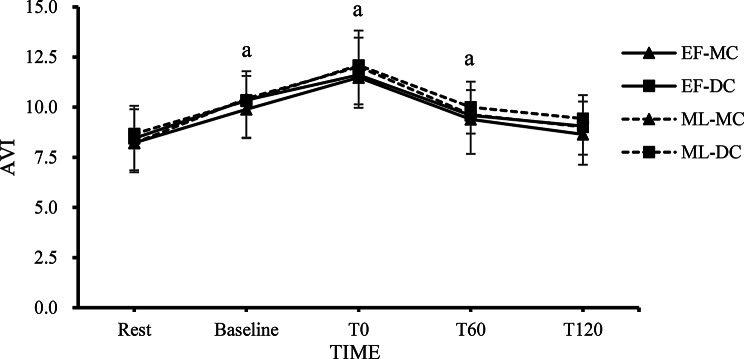
AVI Responses
Figure 4 shows changes in AVI at rest, baseline, 0, 60, and 120 min after resistance exercise, supplemented with either dark chocolate or milk chocolate, across each menstrual cycle. The two-way repeated measures ANOVA revealed no significant main effect of trials on AVI (F = 1.773, p = 0.159, 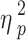 = 0.064) and a significant main effect of time (F = 65.110, p < 0.001,
= 0.064) and a significant main effect of time (F = 65.110, p < 0.001, 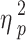 = 0.715). Additionally, the trial × time interaction effect was not significant (F = 0.293, p = 0.990,
= 0.715). Additionally, the trial × time interaction effect was not significant (F = 0.293, p = 0.990, 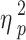 = 0.011). Post hoc Bonferroni comparisons revealed that all trials exhibited significant increases in AVI at baseline, T0 and T60 after resistance exercise (p < 0.05).
= 0.011). Post hoc Bonferroni comparisons revealed that all trials exhibited significant increases in AVI at baseline, T0 and T60 after resistance exercise (p < 0.05).
Fig. 4.
The changes in AVI at each time point in the EF-MC, EF-DC, ML-MC, and ML-DC trials. Note. The value are mean ± SD; EF-MC: early follicular-milk chocolate; EF-DC: early follicular-dark chocolate; ML-MC: Mid Luteal-milk chocolate; ML-DC: Mid Luteal-dark chocolate; AVI: arterial velocity pulse index; Rest: pre-chocolate and pre-exercise; Baseline: 2 h after consuming chocolate; T0: 0 min after exercise; T60: 60 min after exercise; T120: 120 min after exercise. a = Significant difference against rest in the all trials (p < 0.05)
SBP Responses
Figure 5 shows changes in SBP at rest, baseline, 0, 60, and 120 min after resistance exercise, supplemented with either dark chocolate or milk chocolate, across each menstrual cycle. The two-way repeated measures ANOVA revealed a significant main effect of trials on SBP (F = 9.957, p < 0.001, 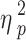 = 0.323) and a significant main effect of time (F = 82.613, p < 0.001,
= 0.323) and a significant main effect of time (F = 82.613, p < 0.001, 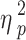 = 0.796). Additionally, a significant trial × time interaction effect was observed (F = 6.235, p = 0.018,
= 0.796). Additionally, a significant trial × time interaction effect was observed (F = 6.235, p = 0.018, 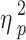 = 0.081). Post hoc Bonferroni comparisons revealed that SBP was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The SBP was significantly higher in the EF-MC trial at Baseline compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). The SBP was significantly higher in the ML-DC trial at Baseline compared with the EF-MC, EF-DC and ML-MC trials (p < 0.05). The SBP was significantly higher in the ML-MC trial at T0 compared with the ML-DC trial (p < 0.05). The SBP in the EF-DC and ML-DC trials significantly decreased from rest to baseline (p < 0.05). All trials exhibited significant increases in SBP at T0 after resistance exercise (p < 0.05). The SBP in the EF-MC and EF-DC trials showed significant decrease at T60 and T120 when compared to the rest condition (p < 0.05).
= 0.081). Post hoc Bonferroni comparisons revealed that SBP was significantly higher in the EF-MC and EF-DC trials at rest compared with the ML-MC and ML-DC trials (p < 0.05). The SBP was significantly higher in the EF-MC trial at Baseline compared with the EF-DC, ML-MC and ML-DC trials (p < 0.05). The SBP was significantly higher in the ML-DC trial at Baseline compared with the EF-MC, EF-DC and ML-MC trials (p < 0.05). The SBP was significantly higher in the ML-MC trial at T0 compared with the ML-DC trial (p < 0.05). The SBP in the EF-DC and ML-DC trials significantly decreased from rest to baseline (p < 0.05). All trials exhibited significant increases in SBP at T0 after resistance exercise (p < 0.05). The SBP in the EF-MC and EF-DC trials showed significant decrease at T60 and T120 when compared to the rest condition (p < 0.05).
Fig. 5.
The changes in SBP at each time point in the EF-MC, EF-DC, ML-MC, and ML-DC trials. Note. The value are mean ± SD; EF-MC: early follicular-milk chocolate; EF-DC: early follicular-dark chocolate; ML-MC: Mid Luteal-milk chocolate; ML-DC: Mid Luteal-dark chocolate; SBP: systolic blood pressure; Rest: pre-chocolate and pre-exercise; Baseline: 2 h after consuming chocolate; T0: 0 min after exercise; T60: 60 min after exercise; T120: 120 min after exercise. a = The EF-MC and EF-DC trials showed a significant difference compared to both the ML-MC and ML-DC trials (p < 0.05). b = The EF-MC trial showed a significant difference compared to the EF-DC, ML-MC and ML-DC trials (p < 0.05). c = The ML-DC trial showed a significant difference compared to the EF-MC, EF-DC and ML-MC trials (p < 0.05). d = The EF-MC trial showed a significant difference compared to the ML-DC trial (p < 0.05). e = Significant difference against rest in the EF-DC and ML-DC trials (p < 0.05). f = Significant difference against rest in the all trials (p < 0.05). g = Significant difference against rest in the EF-MC and EF-DC trials (p < 0.05)
Blood Pressure and Heart Rate Responses
Table 4 shows changes in blood pressure and heart rate at rest, baseline, 0, 60, and 120 min after resistance exercise, supplemented with either dark chocolate or milk chocolate, across each menstrual cycle. The two-way repeated measures ANOVA revealed no significant main effect of trials for DBP (F = 1.221, p = 0.307, 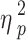 = 0.039) and HR (F = 2.002, p = 0.119,
= 0.039) and HR (F = 2.002, p = 0.119, 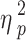 = 0.063), while significant trial effects were found for MAP (F = 4.384, p = 0.006,
= 0.063), while significant trial effects were found for MAP (F = 4.384, p = 0.006, 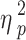 = 0.128) and PP (F = 11.629, p < 0.001,
= 0.128) and PP (F = 11.629, p < 0.001, 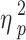 = 0.279). Significant main effects of time were observed for DBP (F = 60.756, p < 0.001,
= 0.279). Significant main effects of time were observed for DBP (F = 60.756, p < 0.001,  = 0.669), MAP (F = 121.057, p < 0.001,
= 0.669), MAP (F = 121.057, p < 0.001, 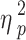 = 0.801), PP (F = 25.377, p < 0.001,
= 0.801), PP (F = 25.377, p < 0.001, 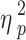 = 0.458), and HR (F = 166.981, p < 0.001,
= 0.458), and HR (F = 166.981, p < 0.001, 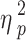 = 0.848). No significant trial × time interaction effects were found for DBP (F = 1.079, p = 0.376,
= 0.848). No significant trial × time interaction effects were found for DBP (F = 1.079, p = 0.376, 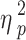 = 0.035), MAP (F = 1.316, p = 0.207,
= 0.035), MAP (F = 1.316, p = 0.207, 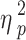 = 0.042), and HR (F = 0.658, p = 0.792,
= 0.042), and HR (F = 0.658, p = 0.792, 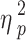 = 0.021), while a significant trial × time interaction effect was found for PP (F = 2.092, p = 0.017,
= 0.021), while a significant trial × time interaction effect was found for PP (F = 2.092, p = 0.017, 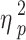 = 0.065). Post hoc Bonferroni comparisons revealed that PP was significantly lower in the EF-DC trial compared to EF-MC trial, and ML-DC trial compared to ML-MC trial at Baseline (p < 0.05). The PP was significantly higher in the EF-MC and EF-DC trials at Rest compared with the ML-MC and ML-DC trials (p < 0.05). The MAP and PP were significantly higher in the EF-MC at T0 compared with the ML-DC (p < 0.05). For DBP and MAP, significant increases was observed from Rest to T0 in all trials (p < 0.05). For PP, significant decreases was observed from Rest to Baseline in EF-DC and ML-DC trials (p < 0.05), significant increases were observed from Rest to T0 in EF-MC and EF-DC trials (p < 0.05), significant decreases were observed from Rest to T60 and T120 in EF-MC and EF-DC trials (p < 0.05). For HR, significant increases were observed from Rest to Baseline, T0 and T60 in all trials (p < 0.05).
= 0.065). Post hoc Bonferroni comparisons revealed that PP was significantly lower in the EF-DC trial compared to EF-MC trial, and ML-DC trial compared to ML-MC trial at Baseline (p < 0.05). The PP was significantly higher in the EF-MC and EF-DC trials at Rest compared with the ML-MC and ML-DC trials (p < 0.05). The MAP and PP were significantly higher in the EF-MC at T0 compared with the ML-DC (p < 0.05). For DBP and MAP, significant increases was observed from Rest to T0 in all trials (p < 0.05). For PP, significant decreases was observed from Rest to Baseline in EF-DC and ML-DC trials (p < 0.05), significant increases were observed from Rest to T0 in EF-MC and EF-DC trials (p < 0.05), significant decreases were observed from Rest to T60 and T120 in EF-MC and EF-DC trials (p < 0.05). For HR, significant increases were observed from Rest to Baseline, T0 and T60 in all trials (p < 0.05).
Table 4.
The changes in blood pressure and heart rate at each time point in the EF-MC, EF-DC, ML-MC, and ML-DC trials
| Rest | Baseline | T0 | T60 | T120 | Trial; Time; Trial×Time |
||
|---|---|---|---|---|---|---|---|
| DBP (mmHg) | EF-MC | 67.48 ± 6.69 | 67.42 ± 8.00 | 76.26 ± 7.94d | 67.52 ± 5.33 | 68.02 ± 6.65 |
p = 0.307; p < 0.001; p = 0.376 |
| EF-DC | 66.85 ± 7.51 | 68.92 ± 7.27 | 76.85 ± 8.81d | 70.10 ± 6.29 | 68.95 ± 8.29 | ||
| ML-MC | 67.94 ± 8.44 | 67.58 ± 7.28 | 75.84 ± 8.97d | 66.21 ± 5.87 | 66.89 ± 7.30 | ||
| ML-DC | 67.15 ± 8.99 | 68.98 ± 7.29 | 75.66 ± 8.61d | 68.89 ± 7.38 | 68.42 ± 8.51 | ||
| MAP (mmHg) | EF-MC | 82.01 ± 6.04 | 81.90 ± 6.96 | 92.97 ± 7.03cd | 80.69 ± 5.29 | 80.92 ± 5.82 |
p = 0.006; p < 0.001; p = 0.207 |
| EF-DC | 81.59 ± 6.92 | 81.66 ± 6.63 | 91.70 ± 7.56d | 82.41 ± 5.91 | 81.30 ± 7.31 | ||
| ML-MC | 80.61 ± 7.60 | 80.59 ± 6.68 | 90.72 ± 8.31d | 78.81 ± 5.59 | 79.50 ± 6.47 | ||
| ML-DC | 79.97 ± 7.98 | 79.90 ± 6.36 | 89.36 ± 7.16d | 81.00 ± 7.43 | 80.36 ± 7.83 | ||
| PP (mmHg) | EF-MC | 43.56 ± 8.28b | 43.45 ± 7.05 | 50.30 ± 12.37 cd | 39.53 ± 7.37d | 38.71 ± 8.46d |
p < 0.001; p < 0.001; p = 0.017 |
| EF-DC | 44.21 ± 9.67b | 38.21 ± 8.49ad | 44.53 ± 9.79 | 36.94 ± 7.84d | 37.05 ± 8.01d | ||
| ML-MC | 38.02 ± 9.27 | 39.03 ± 10.01 | 44.63 ± 11.23d | 37.81 ± 9.76 | 37.84 ± 8.81 | ||
| ML-DC | 38.48 ± 8.57 | 33.08 ± 6.67ad | 41.10 ± 12.67 | 36.34 ± 6.70 | 35.82 ± 9.39 | ||
| HR (bpm) | EF-MC | 72.65 ± 9.03 | 79.21 ± 8.77d | 101.48 ± 11.68d | 80.11 ± 8.08d | 75.13 ± 9.28 |
p = 0.119; p < 0.001; p = 0.792 |
| EF-DC | 72.87 ± 8.38 | 79.37 ± 6.99d | 101.68 ± 14.32d | 82.48 ± 8.35d | 77.19 ± 8.59 | ||
| ML-MC | 75.40 ± 9.24 | 81.55 ± 6.82d | 101.84 ± 16.51d | 82.24 ± 8.49d | 77.52 ± 7.71 | ||
| ML-DC | 74.79 ± 8.25 | 80.06 ± 6.53d | 102.84 ± 13.56d | 83.24 ± 10.73d | 79.21 ± 8.95 |
Note The value are mean ± SD; EF-MC = early follicular-milk chocolate; EF-DC = early follicular-dark chocolate; ML-MC = Mid Luteal-milk chocolate; ML-DC = Mid Luteal-dark chocolate; DBP: diastolic blood pressure; PP: pulse pressure; MAP: mean arterial pressure; HR: heart rate; Rest: pre-chocolate and pre-exercise; Baseline: 2 h after consuming chocolate; T0: 0 min after exercise; T60: 60 min after exercise; T120: 120 min after exercise
a = Significant difference were evaluated within the same menstrual phase (EF-MC vs. EF-DC; ML-MC vs. ML-DC) (p < 0.05)
b = Significant difference were evaluated between menstrual phases (EF-MC and EF-DC vs. ML-MC and ML-DC) (p < 0.05)
c = The EF-MC trial showed a significant difference compared to the ML-DC trial (p < 0.05)
d = Significant difference against rest within the same trial (p < 0.05)
Discussion
Our study demonstrated that dark chocolate supplementation, regardless of the menstrual phase, resulted in significantly lower ftPWV, API, SBP and PP compared to milk chocolate consumption. Additionally, NO levels were markedly higher following dark chocolate consumption, which may play a key role in preventing increases in arterial stiffness and blood pressure following high-intensity resistance exercise. During the rest period, mid luteal phase trials exhibited significantly lower ftPWV, API, SBP and PP than early follicular phase trials, suggesting a phase-specific effect on vascular health. This observation is consistent with the well-documented vascular benefits associated with higher estrogen levels during the mid luteal phase, further supporting the phase-specific impact of hormonal fluctuations on vascular health.
Our study found that two hours after consuming dark chocolate, NO levels were significantly higher in the dark chocolate trial compared to the milk chocolate trial, while arterial stiffness and blood pressure were significantly decreased. Cocoa flavanols and epicatechins in dark chocolate were key contributors to these cardiovascular benefits, prior systematic reviews demonstrated that effective doses of cocoa flavanols (≥ 900 mg) and epicatechin (≥ 100 mg) lowered blood pressure by approximately 1–5 mmHg in normotensive individuals [35]. These effects were attributed to the stimulation of NO production, which enhanced vasodilation and endothelial function, ultimately leading to blood pressure reductions [36]. In our study, participants consumed an average of approximately 2424 mg of cocoa flavanols and 208 mg of epicatechin. These amounts substantially exceeded the thresholds reported in systematic reviews. Consistent with these findings, our study found that ftPWV, API, SBP, and PP significantly decreased two hours after dark chocolate consumption in both menstrual cycle phases. Additionally, the EF-MC trial exhibited significantly higher ftPWV, API, and SBP compared to EF-DC, ML-MC, and ML-DC trials, indicating that both estrogen and cocoa flavanols had protective effects. Notably, in the luteal phase, API and SBP in the ML-DC trial were significantly lower than in the ML-MC trial, further supporting the potential synergistic effect between estrogen and cocoa flavanols.
Furthermore, prior research indicates that consuming high- or low-cocoa-content beverages before moderate-intensity aerobic exercise can enhance flow-mediated dilation (FMD) and mitigate exercise-induced increases in blood pressure [37]. Previous research has also shown that 85% dark chocolate can attenuate blood pressure increases induced by acute stress in healthy adult women [17]. Cocoa flavanols also mitigated exercise-induced acute increases in blood pressure, likely through their ability to enhance NO production and improve endothelial function [38]. While these effects were primarily documented in the context of aerobic exercise, research on the effects of Cocoa flavanols supplementation following high-intensity resistance exercise remained limited. Consistent with these findings, we observed that dark chocolate consumption reduced vascular pressure following resistance exercise, particularly during the early follicular phase, where post-exercise ftPWV and API were significantly lower in the dark chocolate trial compared to the milk chocolate trial. However, no significant differences in arterial stiffness were observed immediately post-exercise (T0) during the mid-luteal phase. Notably, despite the synergistic effect between estrogen and cocoa flavanols observed at rest, this effect was not evident at T0 post-exercise. A possible explanation is the activation of the exercise pressor reflex (EPR), a mechanism that regulates cardiovascular responses during exercise by increasing SNS activity to maintain blood flow and perfusion pressure [39]. The heightened SNS activation induced by EPR elevates vascular resistance and may counteract the vasodilatory effects of estrogen or cocoa flavanols, thereby attenuating their synergistic impact on arterial stiffness post-exercise.
Our study revealed significant variations in arterial stiffness and blood pressure responses to high-intensity resistance exercise across menstrual phases. Specifically, the mid luteal phase trials exhibited a smaller increase in arterial stiffness and blood pressure post-exercise compared to the early follicular phase trials. Estrogen has been shown to enhance NO secretion and inhibit SNS activity, thereby improving endothelial function and mitigating arterial stiffness. These effects were particularly pronounced during the mid luteal phase when estrogen levels were at their peak [7, 9, 40]. Previous studies have demonstrated that high-intensity resistance exercise in women leads to significant increases in SBP and PWV within five minutes post-exercise [41]. This rise in arterial stiffness was attributed to the acute hypertensive response observed during high-intensity resistance exercise, which was associated with a significant reduction in arterial compliance [41]. Additionally, high-intensity resistance training also stimulates SNS activity, further promoting vasoconstriction and increasing arterial stiffness [42, 43]. Studies accounting for the menstrual cycle have reported significant increases in PWV at 30 and 60 min post-exercise during the follicular phase, with no such changes observed during the luteal phase [12]. PWV at 30 and 60 min post-exercise was significantly lower during the luteal phase compared to the follicular phase, indicating that women in the luteal phase are better able to mitigate exercise-induced increases in arterial stiffness [12]. These findings were consistent with our results, which showed that in the trials not consuming dark chocolate, SBP, ftPWV, and API significantly increased immediately post-exercise (T0). However, at the same time point, ftPWV and API were significantly lower during the mid luteal phase compared to the early follicular phase. This trend continued at T60, where ftPWV was again significantly lower in the mid luteal phase. These observations underscored the protective role of estrogen during the mid luteal phase in mitigating exercise-induced increases in arterial stiffness and blood pressure.
In our study, a significant increase in ftPWV, AVI and HR were observed in the EF-MC trial from rest to baseline. This phenomenon may be attributed to physiological changes associated with morning variations. Previous research has demonstrated that baPWV increases significantly from morning to midday, reflecting a circadian pattern in arterial stiffness [44]. Similarly, heart rate is influenced by diurnal rhythms, with a gradual increase during the day and a decrease at night [45]. These natural physiological fluctuations might have contributed to arterial stiffness and heart rate increased significantly from rest to baseline in the EF-MC trial, particularly in the absence of protective effects from estrogen or dark chocolate supplementation. Previous research has also demonstrated that central PWV significantly increases at 1, 10, 20, and 30 min post-exercise during both menstrual phases in healthy adult women [13]. These results align with our findings, where central arterial stiffness, represented by AVI, significantly increased at T0 and T60 across all trials.
Additionally, post-exercise hypotension (PEH) is a well-documented phenomenon, occurring as a result of increased heart rate and cardiac output during exercise, which enhances circulation efficiency and induces vasodilation, reducing vascular resistance [46]. Meta-analyses have shown that various exercise modalities, including aerobic, isotonic resistance, isometric resistance, and combined aerobic-resistance exercise, reduce blood pressure, with greater effects observed in hypertensive or prehypertensive individuals [6]. Our study observed a significant reduction in SBP at T60 and T120 post-exercise in the early follicular phase trials. Specifically, in the EF-MC trial, SBP decreased by 4 mmHg from REST to T60 and by 4.32 mmHg from REST to T120, while in the EF-DC trial, SBP decreased by 4.03 mmHg and 5.06 mmHg, respectively. These reductions were likely due to the higher resting SBP observed during this phase, which amplified the post-exercise hypotensive response. Furthermore, at T0, despite the absence of a synergistic effect on arterial stiffness, a significant reduction in SBP was observed in the ML-DC trial compared to the EF-MC trial, demonstrating a synergistic effect between estrogen and cocoa flavanols. This indicated that the regulatory mechanisms controlling arterial stiffness and blood pressure might have differed immediately post-exercise. Given the complexity of the EPR, which is a multifaceted mechanism regulating cardiovascular responses during exercise [39], further research is needed to elucidate its role in modulating post-exercise hemodynamic responses.
Furthermore, our findings highlighted significant differences in estrogen levels and their impact on cardiovascular markers, including NO, between the early follicular and mid-luteal phases. Higher estrogen levels in the mid-luteal phase were associated with lower resting values of ftPWV, API, and SBP. Prior research reported estrogen levels around 40 pg/mL in the early follicular phase and 120 pg/mL in the mid-luteal phase [47], which aligned with our findings of 66.53 ± 21.24 pg/mL (EF-MC) and 62.19 ± 24.06 pg/mL (EF-DC) in the early follicular phase, and 128.79 ± 58.54 pg/mL (ML-MC) and 127.11 ± 64.26 pg/mL (ML-DC) in the mid-luteal phase. This alignment supported the validity of the selected phases for assessing cardiovascular responses. Prior research has also suggested that higher estrogen levels stimulate NO production, leading to vasodilation, increased vascular elasticity, suppression of SNS activity, and reduced ET-1 production [7–9]. However, in the trials without dark chocolate supplementation, we did not observe significant differences in NO levels between the early follicular and mid-luteal phases. This suggests that estrogen may not substantially influence NO levels under the conditions of our study. Instead, the observed differences in arterial stiffness and blood pressure between menstrual phases may be attributed to other estrogen-mediated mechanisms, such as suppression of SNS activity and reduced ET-1 production, rather than enhanced NO production. Regarding PWV, previous studies have produced inconsistent results when examining PWV across different menstrual phases. Some studies found no differences in rest PWV values among different phases [10, 13]. However, other research has shown that baseline PWV values during the luteal phase (18–24 days) are significantly lower than those in the follicular phase (2–5 days) [10]. These discrepancies may arise from differences in how menstrual phases are defined. For instance, Augustine et al. [12] defined menstrual cycle phases as early follicular (1–7 days) and early luteal (14–19 days), while Adkisson et al. [9] used early follicular (2–4 days), late follicular (12–14 days), early luteal (17–19 days), and late luteal (25–28 days), none of which found significant differences in PWV. It is also important to note that estrogen levels not only increase before ovulation but also rise during the mid-luteal phase [48]. As a result, arterial stiffness measurements taken on different days of the same menstrual cycle may yield varying results, underscoring the importance of considering the duration and timing of the menstrual cycle in cardiovascular research.
Limitations
This study did not confirm ovulation through direct methods, such as urinary luteinizing hormone (LH), progesterone testing or basal body temperature (BBT) monitoring. While phase identification relied on menstrual cycle length calculations, the absence of direct ovulation confirmation may have introduced variability in hormonal status. Additionally, blood samples were collected after the consumption of both the standard breakfast and chocolate supplementation, without measuring NO levels at complete rest. Although this design allowed for the assessment of changes in NO levels by comparing the milk chocolate and dark chocolate trials, it limited the ability to establish absolute baseline values. Moreover, the duration of eccentric and concentric phases during resistance exercises was not explicitly controlled. Participants were instructed to perform the movements in a controlled and deliberate manner under the supervision of a certified instructor to ensure safety and proper technique. Blood pressure was measured in a seated position, while ftPWV was assessed in a supine position. The transition between positions may have introduced minor variability, but efforts were made to minimize this impact. Furthermore, this study exclusively measured NO levels as the primary biomarker of vascular function. While NO plays a key role in endothelial function, the assessment of additional biomarkers such as ET-1, noradrenaline, and oxidative stress markers would have provided a more comprehensive evaluation of the interaction between estrogen and cocoa flavanols. Given the complexity of vascular regulation, future studies should incorporate multiple physiological markers and hemodynamic measurements at each time point to better elucidate the potential synergistic effects of estrogen and cocoa flavanols on vascular function and autonomic regulation.
Conclusions
This study demonstrated that 85% dark chocolate supplementation improved vascular function in healthy adult women, with effects varying by menstrual phase and exercise conditions. At baseline, dark chocolate significantly reduced SBP, PP, ftPWV and API in both the early follicular and mid-luteal phases, likely due to its impact on NO production and enhanced endothelial function. Without dark chocolate supplementation, no significant differences in NO levels or vascular parameters were observed between the early follicular and mid-luteal phases. Post-exercise, dark chocolate attenuated immediate post-exercise increases in ftPWV and API during the early follicular phase, where estrogen levels were lower and hormonal support for vascular function was reduced. However, its effects were less pronounced during the mid-luteal phase, likely because higher estrogen levels already supported vascular function. These findings suggested that the benefits of cocoa polyphenols could have contributed to greater vascular benefits under conditions of reduced hormonal influence, warranting further research into their long-term effects and broader applications in cardiovascular health.
Acknowledgements
The authors would like to thank DiRaja Chocolate, Pingtung, Taiwan, for generously sponsoring the dark chocolate used in this study. Their support was invaluable to the completion of this research. The authors also acknowledge the participants who volunteered for this study.
Abbreviations
- WHO
World Health Organization
- CVD
Cardiovascular disease
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- ET-1
Endothelin-1
- SNS
Sympathetic nervous system
- L-NAME
L-nitroarginine methyl ester
- 1RM
Maximum repetition
- ftPWV
Finger-toe pulse wave velocity
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- API
Arterial pressure volume index
- AVI
Arterial velocity pulse index
- PEH
Post-exercise hypotension
- EF-MC
Early follicular milk chocolate trial
- EF-DC
Early follicular dark chocolate trial
- ML-MC
Mid luteal milk chocolate trial
- ML-DC
Mid luteal dark chocolate trial
Author Contributions
CWW contributed to manuscript writing, data analysis, and supervision of experimental conduct. SHF, TAY, LYC, CKW, and SCW were responsible for experimental conduct and data collection. CSH contributed to funding acquisition and manuscript review. All authors read and approved the final version.
Funding
This study was funded by the Taiwan Ministry of Science and Technology, grant number NSTC112-2410-H-194-076-. The funding body had no role in the design, data collection, analysis, interpretation of data, or writing of the manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval and Consent to Participate
National Chung Cheng University Human Research Ethics Center approved this study (ID: CCUREC111122101). The study was conducted in accordance with the standards of ethics outlined in the Declaration of Helsinki. All participants provided written informed consent to participate in the study, and consented to the use of their data in the publication of this manuscript.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no conflicts of interest relevant to the content of this article. All authors have reviewed this statement and confirm that there are no financial, professional, or personal relationships that could be perceived as influencing the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. (2020). The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke Statistics–2016 update: A report from the American heart association. Circulation. 2016;133(4):447–54. 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Gooding HC, Gidding SS, Moran AE, et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National heart, lung, and blood Institute working group. J Am Heart Association. 2020;9(19):e016115. 10.1161/JAHA.120.016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel B, Acevedo M, Appelman Y, et al. The lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–438. 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 5.Moreau KL, Hildreth KL, Meditz AL, et al. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metabolism. 2012;97(12):4692–700. 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Association. 2013;2(1):e004473. 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lekontseva O, Chakrabarti S, Jiang Y, et al. Role of neuronal nitric-oxide synthase in estrogen-induced relaxation in rat resistance arteries. J Pharmacol Exp Ther. 2011;339(2):367–75. 10.1124/jpet.111.183798. [DOI] [PubMed] [Google Scholar]
- 8.Sebzda KN, Kuczmarski AV, Pohlig RT, et al. Ovarian hormones modulate endothelin-1 receptor responses in young women. Microcirculation. 2018;25(7):e12490. 10.1111/micc.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vongpatanasin W, Tuncel M, Mansour Y, et al. Transdermal Estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103(24):2903–8. 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- 10.Adkisson EJ, Casey DP, Beck DT, et al. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Experimental Biology Med. 2010;235(1):111–8. 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.10 Aminuddin A, Hakim LA, Chan SY, et al. The changes of aortic stiffness during normal menstrual cycle. Med Health-Kuala Lumpur. 2018;13(1):117–29. [Google Scholar]
- 12.Okamoto T, Kobayashi R, Sakamaki-Sunaga M. Effect of resistance exercise on arterial stiffness during the follicular and luteal phases of the menstrual cycle. Int J Sports Med. 2017;38(5):347–52. 10.1055/s-0043-101377. [DOI] [PubMed] [Google Scholar]
- 13.Augustine JA, Nunemacher KN, Heffernan KS. Menstrual phase and the vascular response to acute resistance exercise. Eur J Appl Physiol. 2018;118(5):937–46. 10.1007/s00421-018-3815-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Xu G, Liu X. Chocolate intake reduces risk of cardiovascular disease: evidence from 10 observational studies. Int J Cardiol. 2013;168(6):5448–50. 10.1016/j.ijcard.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Grassi D, Desideri G, Ferri C. Blood pressure and cardiovascular risk: what about cocoa and chocolate? Arch Biochem Biophys. 2010;501(1):112–5. 10.1016/j.abb.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Ludovici V, Barthelmes J, Nägele MP, et al. Cocoa, blood pressure, and vascular function. Front Nutr. 2017;4:36. 10.3389/fnut.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regecova V, Jurkovicova J, Babjakova J, et al. The effect of a single dose of dark chocolate on cardiovascular parameters and their reactivity to mental stress. J Am Coll Nutr. 2020;39(5):414–21. 10.1080/07315724.2019.1662341. [DOI] [PubMed] [Google Scholar]
- 18.Vlachopoulos C, Aznaouridis K, Alexopoulos N, et al. Effect of dark chocolate on arterial function in healthy individuals. Am J Hypertens. 2005;18(6):785–91. 10.1016/j.amjhyper.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz NA, Theodore AP, Funderburg BR, et al. Acute (-)-epicatechin consumption: effects on local vasodilation following resistance exercise and high-intensity exercise performance. Sports. 2020;8(2):22. 10.3390/sports8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries B, Duncan MJ, Mummery WK. Prevalence and correlates of resistance training in a regional Australian population. Br J Sports Med. 2010;44(9):653–6. 10.1136/bjsm.2008.048975. [DOI] [PubMed] [Google Scholar]
- 21.Paluch AE, Boyer WR, Franklin BA, et al. Resistance exercise training in individuals with and without cardiovascular disease: 2023 update: a scientific statement from the American heart association. Circulation. 2024;149(3):e217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiebaud RS, Fahs CA, Rossow LM, et al. Effects of age on arterial stiffness and central blood pressure after an acute bout of resistance exercise. Eur J Appl Physiol. 2016;116(1):39–48. 10.1007/s00421-015-3242-5. [DOI] [PubMed] [Google Scholar]
- 23.Moran VH, Leathard HL, Coley J. Cardiovascular functioning during the menstrual cycle. Clin Physiol. 2000;20(6):496–504. 10.1046/j.1365-2281.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 24.Haff GG, Triplett NT. Essentials of strength training and conditioning (4th ed.). Human Kinetics. 2015.
- 25.Lamport DJ, Christodoulou E, Achilleos C. Beneficial effects of dark chocolate for episodic memory in healthy young adults: A parallel-groups acute intervention with a white chocolate control. Nutrients. 2020;12(2):483. 10.3390/nu12020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackett DA, Cobley SP, Davies TB, et al. Accuracy in estimating repetitions to failure during resistance exercise. J Strength Conditioning Res. 2017;31(8):2162–8. 10.1519/JSC.0000000000001683. [DOI] [PubMed] [Google Scholar]
- 27.Baechle TR, Earle RW. Essentials of strength training and conditioning (2nd ed.). Human Kinetics. 2000.
- 28.Gaynor-Metzinger SHA, Triplett NT, Meucci M, et al. Central arterial stiffness, wave reflection, and heart rate variability following 4-week high-intensity resistance training intervention in young active women. Eur J Appl Physiol. 2022;122(10):2283–93. 10.1007/s00421-022-05003-4. [DOI] [PubMed] [Google Scholar]
- 29.Focht BC, Koltyn KF, Bouchard LJ. State anxiety and blood pressure responses following different resistance exercise sessions. Int J Sport Psycol. 2000;31(3):376–90. [Google Scholar]
- 30.Alivon M, Vo-Duc Phuong T, Vignon V, et al. A novel device for measuring arterial stiffness using finger-toe pulse wave velocity: validation study of the pOpmètre®. Arch Cardiovasc Dis. 2015;108(4):227–34. 10.1016/j.acvd.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Tsai WC, Chen JY, Wang MC, et al. Association of risk factors with increased pulse wave velocity detected by a novel method using dual-channel photoplethysmography. Am J Hypertens. 2005;18(8):1118–22. 10.1016/j.amjhyper.2005.03.739. [DOI] [PubMed] [Google Scholar]
- 32.Chen LY, Wang CW, Chen LA, et al. Low vitamin D status relates to the poor response of peripheral pulse wave velocity following acute maximal exercise in healthy young men. Nutrients. 2022;14(15):3074. 10.3390/nu14153074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komine H, Asai Y, Yokoi T, et al. Non-invasive assessment of arterial stiffness using oscillometric blood pressure measurement. Biomed Eng Online. 2012;11:6. 10.1186/1475-925X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita T, Kitamura K. Seasonal variation of novel arterial stiffness indexes in Japanese hypertensive patients. Clin Exp Hypertens. 2019;41(7):670–4. 10.1080/10641963.2018.1539092. [DOI] [PubMed] [Google Scholar]
- 35.Amoah I, Lim JJ, Osei EO, et al. Effect of cocoa beverage and dark chocolate consumption on blood pressure in those with normal and elevated blood pressure: A systematic review and Meta-Analysis. Foods. 2022;11(13):1962. 10.3390/foods11131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri C, Desideri G, Ferri L, et al. Cocoa, blood pressure, and cardiovascular health. J Agric Food Chem. 2015;63(45):9901–9. [DOI] [PubMed] [Google Scholar]
- 37.Berry NM, Davison K, Coates AM, et al. Impact of cocoa Flavanol consumption on blood pressure responsiveness to exercise. Br J Nutr. 2010;103(10):1480–4. 10.1017/S0007114509993382. [DOI] [PubMed] [Google Scholar]
- 38.Decroix L, Soares DD, Meeusen R, et al. Cocoa Flavanol supplementation and exercise: a systematic review. Sports Med. 2018;48(4):867–92. 10.1007/s40279-017-0849-1. [DOI] [PubMed] [Google Scholar]
- 39.Grotle AK, Macefield VG, Farquhar WB, et al. Recent advances in exercise pressor reflex function in health and disease. Auton Neurosci. 2020;228:102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minson CT, Halliwill JR, Young TM, et al. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102(13):1473–6. 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 41.Grigoriadis G, Rosenberg AJ, Lefferts WK, Wee SO, Schroeder EC, Baynard T. Similar effects of acute resistance exercise on carotid stiffness in males and females. Int J Sports Med. 2020;41(2):82–8. 10.1055/a-1044-2321. [DOI] [PubMed] [Google Scholar]
- 42.Parker BA, Smithmyer SL, Jarvis SS, et al. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol. 2007;292(2):H1148–56. 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- 43.Rosenmeier JB, Dinenno FA, Fritzlar SJ, et al. Rapid report: α1-and α2‐adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547(3):971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada K, Iino S, Isobe S, et al. Relation of plasma catecholamine levels with pulse wave velocity in hypertensive patients compared with normotensive subjects. Heart Vessels. 2012;27(5):493–8. 10.1007/s00380-011-0174-9. [DOI] [PubMed] [Google Scholar]
- 45.Morris CJ, Yang JN, Scheer FAJL. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58. 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29(2):65–70. 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Schmalenberger KM, Tauseef HA, Barone JC, et al. How to study the menstrual cycle: practical tools and recommendations. Psychoneuroendocrinology. 2021;123:104895. 10.1016/j.psyneuen.2020.104895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reschechtko S, Nguyen TN, Tsang M, et al. Postural sway is not affected by Estrogen fluctuations during the menstrual cycle. Physiological Rep. 2023;11(10):e15693. 10.14814/phy2.15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.