Abstract
Wearable medical-grade devices are transforming the standard of care for prevalent chronic conditions like diabetes. Yet, adoption and long-term use remain a challenge for many people. In this study, we investigate patterns of consistent versus disrupted use of continuous glucose monitors (CGMs) through analysis of more than 118,000 days of data, with over 22 million blood glucose samples, from 108 young adults with type 1 diabetes (average: 3 years of CGM data per person). In this population, we found more consistent CGM use at the start and end of the year (e.g., January, December), and more disrupted CGM use in the middle of the year/warmer months (i.e., May to July). We also found more consistent CGM use on weekdays (Monday to Thursday) and during waking hours (6AM - 6PM), but more disrupted CGM use on weekends (Friday to Sunday) and during evening/night hours (7PM - 5AM). Only 52.7% of participants (57 out of 108) had consistent and sustained CGM use over the years (i.e., over 70% daily wear time for more than 70% of their data duration). From semi-structured interviews, we unpack factors contributing to sustained CGM use (e.g., easier and better blood glucose management) and factors contributing to disrupted CGM use (e.g., changes in insurance coverage, issues with sensor adhesiveness/lifespan, and college/life transitions). We leverage insights from this study to elicit implications for next-generation technology and interventions that can circumvent seasonal and other factors that disrupt sustained use of wearable medical devices for the goal of improving health outcomes.
Keywords: Continuous glucose monitors, Type 1 diabetes, Seasonal variations
Subject terms: Biomedical engineering, Type 1 diabetes
Introduction
Wearable medical devices are a key part of the digital health era, especially for ambulatory health monitoring and chronic disease management1–7. Ample evidence shows that adherence to therapy, including sustained and consistent use of medical devices is critical to realize improved health outcomes3,8,9. Yet, adoption and long-term use of wearable medical devices remains a challenge for some people with diabetes (PwD)10. Prior research has identified some factors that affect sustained use of wearable and health-monitoring devices such as users’ motivation, age, education-level, income, risk-benefit perception, device type, context, and more11–24. Yet, a critical gap still exists in understanding patterns of long-term use for wearable medical devices, including seasonal, weekly, and daily patterns. Bridging this knowledge gap is particularly important given established findings on seasonal variations in health-relevant behaviors and outcomes, including blood glucose management, physical activity, mental health, viral infections, mood, behavior, and vital signs25–38. Understanding use patterns associated with medical devices prescribed for disease management is fundamental to design smarter versions of such technology and to develop interventions that can support more consistent use to improve health outcomes.
The diabetes domain is ahead of the curve when it comes to clinical-grade wearable medical devices for personalized care and disease management. Prescription devices like continuous glucose monitors (CGMs) are transforming the standard of care and enabling significant improvements in blood glucose management39–43. Thus, clinical recommendations support the use of CGMs and uptake of this technology is increasing44–47. However, sustained use in the long-term remains a challenge for some people with diabetes, despite the established benefits of using CGMs for diabetes management23,48. Some barriers identified in prior literature include high costs associated with diabetes technologies, lack of insurance coverage, patients’ dislike of wearing a medical device, stigma associated with using a medical device, disruptive alarms, information overload from the devices, adhesive allergies and skin irritation when wearing the devices, as well as inadequate education and technical support for healthcare providers and device users48–54. Albeit these findings, unanswered questions remain around seasonal and individual variations in long-term use of CGMs for diabetes management. Prior work primarily leverages qualitative data collection and analyses, meanwhile quantitative and longitudinal analysis of CGM use patterns is limited. Additionally, evidence shows that CGM and patient-generated data generated from digital health technologies is significantly underutilized in spite of the wealth of knowledge that can be gleaned from this rich data source25,55–59.
In this paper, we focus on the understudied and at-risk population of young adults with type 1 diabetes, many of whom are navigating numerous life transitions amidst increased independence with managing their health condition60–64. Evidence shows that many young adults with type 1 diabetes already experience poor prognosis, including high incidences of both acute and long-term complications, such as above-target blood glucose levels, diabetes ketoacidosis, retinopathy, and renal complications44,65. Given this, there is no better time for transformational research and intervention to change the status quo. Building on the knowledge that the benefit of medical devices is often proportional to frequency of use9,39, our research seeks to understand patterns of long-term use of prescription medical devices, particularly CGMs for diabetes management. More specifically, our research seeks to uncover patterns of consistent and disrupted use of wearable CGMs using over 118,000 days of device data interspersed with 32,000 days with no CGM data from 108 young adults with type 1 diabetes (mean = 3 years of data per participant). We investigate seasonal, weekly, daily, and individual patterns of long-term CGM use at the population- and individual-level within our cohort of young adults with type 1 diabetes. We also investigate associations between individual characteristics (e.g. age, gender and hemoglobin A1 C) and sustained/consistent CGM use over the years. Finally, we conduct semi-structured interviews with 11 young adults with type 1 diabetes to gain a deeper perspective on factors that contribute to sustained versus disrupted CGM use, including variations in seasonal, weekly, and individual use patterns over the years. We hope that insights from this work can inform the design of future technology and targeted interventions that can improve consistent use of prescribed medical devices, and in so doing improve health outcomes.
Method
Study overview
This study includes quantitative analysis of long-term CGM data from 108 young adults with type 1 diabetes followed by semi-structured interviews with some participants to further understand the reasons for variations in CGM use patterns. The quantitative phase of this study involves secondary analysis of CGM data collected during the initial screening phase (i.e., before the intervention phase) of a randomized controlled trial (ClinicalTrials.gov ID: NCT04646473)60. Study participants were recruited remotely through social media and online platforms if they self-reported being in the target age range of 19 to 30 years old, having been diagnosed with type 1 diabetes for more than 18 months, and self-reported having a hemoglobin A1C of greater than 7.5% at a doctor’s visit within the prior 6 months. To confirm eligibility, prospective participants were further screened via a phone/video call, provided a hemoglobin A1C test (AccuBase A1c test kit by DTI Laboratories66) via postal mail, and requested to share their diabetes device data (including CGM data) through Glooko67. This study was approved by the Committee for Protection of Human Subjects at Dartmouth College (STUDY00023559 and STUDY00032988) and all methods were performed in accordance with the relevant guidelines and regulations. Additionally, all participants provided informed consent for being a part of this study.
Clinically-accepted metrics used for assessing blood glucose management include, time in range (TIR), time above range (TAR), and time below range (TBR)68,69. TIR refers to the percentage of time the blood glucose is within the target range of 70 - 180 mg/dL, TAR refers to percentage of time the blood glucose is above the target range, while TBR refers to the percentage of time the blood glucose is below the target range. For an adult with type 1 diabetes, the clinical recommendation is to maintain blood glucose levels within the target range of 70 - 180 mg/dL for at least 70% of each day and aim to achieve a target hemoglobin A1C of less than 7%68,69. Given our research focus on assessing CGM use patterns, we leveraged consensus recommendations68,70 in research and practice to categorize CGM use into high daily CGM use ( wear time), partial daily CGM use (>0 to <70% wear time), and no daily CGM use (0% wear time). Across our cohort of participant’s CGM data, there were days with high CGM use, days with partial CGM use, and days with no CGM use - see Supplementary Figure S1.
Assessing seasonal variations in long-term CGM use
Our approach to assessing seasonal variations in long-term CGM use was guided by prior work investigating seasonal variations in mood, body temperature, weight gain, and glycemic trends25,36,37,71. First, we calculated the ratio of high CGM use for each day of the year for each participant’s data collection period []; where i represents participants 1, 2,..., n, and j represents days between January 1 and December 31. Since our study dataset spanned multiple years of CGM data for each participant, was used to quantify how many times a given participant had high CGM use on each unique day of the year. For example, let us assume that participant 10 has 5 years of CGM data, and thus five occurrences of June 1 in their dataset. If they had high CGM use (i.e., >70% wear time) on June 1 st for 3 years, and partial or no CGM use (i.e., <70% wear time) on June 1 st for 2 years, then will equal 0.6. Next, we calculated the average CGM use () across all participants for each day of the year (January 1 to December 31). Following this, we calculated the change in daily CGM use () by normalizing across the mean CGM use for the year () - see equation 1. This change plot was then smoothed with a 7-day running average. Finally, we quantified monthly variations in CGM use by calculating () the average CGM use () for each month of the year (i.e., January to December).
| 1 |
Assessing weekly and daily variations in long-term CGM use
Similar to the above analysis and guided by prior work25,36,37, we sought to assess weekly and daily variations in long-term CGM use. To achieve this, we calculated for each participant, the ratio of high CGM use on each day of the week throughout their individual’s duration of CGM data (); where i represents participants , and w represents days of the week Monday through Sunday. Since our study dataset spanned multiple months and weeks of CGM data for each participant, was used to quantify how many times a given participant had high CGM use on each unique day of the week (e.g., Monday). For example, let us assume participant 18 has 3 years of CGM data, and thus 156 full weeks in their dataset. If this participant had high CGM use (i.e., >70% wear time) on 100 Mondays and partial/low CGM use (i.e., <70% wear time) on the remaining 56 Mondays in their dataset, then will equal 0.64 (i.e., 100 divided by 156). Next, for each participant, we calculated the change in CGM use for each day of the week () by normalizing across the average (across the week) CGM use for that participant (). Following this, we calculated the average (across all participants) change in CGM use for each day of the week () and normalized across the average (across the week) change in CGM use for all participants () - see equation 2.
| 2 |
Similar to the analysis for assessing weekly variations in CGM use across days of the week, we also calculated the daily variations in CGM use across hours in the day. To achieve this, we calculated for each participant, the ratio of CGM data present for each hour time block on the days that had blood glucose data compared to the expected number of CGM data per hour (i.e., 12 samples per hour). Following this, we calculated for each participant the change in CGM use for each hour of the day and then the average (across all participants) change in CGM use for each hour of the day.
Statistical analysis
We used a paired t-test72 to evaluate the difference in CGM use between weekdays versus weekends (i.e., Monday - Thursday vs. Friday - Sunday) and daytime versus evening/nighttime (i.e., 6 AM - 6PM vs. 7PM - 5 AM). Significance levels of p < 0.05, 0.01, and 0.001 was used to assess the strength of evidence against the null hypothesis.
Following this, we performed multivariate analysis to investigate associations between distinct individual characteristics and long-term CGM use (i.e., > 70% daily wear time on at least 70% of days; CGM duration/participant: 9 months to 6.5 years). Toward this goal, we defined long-term CGM use as a binary variable set to 1 if a participant has high CGM use (i.e., > 70% wear time) across more than 70% of days in their individual data duration, and 0 otherwise. Informed by prior work12,23,24,73, we identified potential predictors of long-term CGM use, including age, gender, years since T1D diagnosis, hemoglobin A1C, insurance type, and high CGM use in the first 30 days of the data collection period. Following this, we selected a reference category for each predictor and calculated the odds ratio (i.e., the strength of association between long-term CGM use and each predictor variable relative to the reference category) and the 95% confidence interval. This analysis was conducted with Python using the scipy.stats library74 and the p-values were calculated based on the Fisher’s exact test.
Semi-structured interviews
Following quantitative analysis, we recruited 11 participants (about half of whom overlapped with the larger study) for semi-structured interviews and visual analysis of their own long-term CGM data. Our interview participants included 9 females and 2 males between the ages of 20 to 30 years old, and the time since type 1 diabetes diagnosis was between 5 to 29 years (mean ± std: 15.4 ± 8.1). All interviews were conducted via Zoom and each session lasted approximately 30 minutes. During the interviews, participants were asked open-ended questions about their journey with diabetes, their experience with using a CGM for managing their glucose levels, variations in their CGM use patterns observed through visual analysis of their device data across different time granualarities (i.e., months of the year, days of the week, and hours in the day) - see Supplementary Fig. S2, and factors affecting sustained or disrupted CGM use. More details on the interview questions can be found in the Supplementary materials. All interviews were recorded to enable transcription and thematic analysis. Upon completion of the interview, each participant received a $25 gift card as compensation for their time.
Results
This section presents our data description and findings on seasonal, weekly, daily, and individual variations in long-term CGM use from analysis of over 118,000 days between year 2015 and 2023. Next, we present results from multivariate analysis of associations between individual characteristics and long-term CGM use. Finally, we present insights from semi-structured interviews to elicit factors contributing to sustained and disrupted CGM use.
Data description
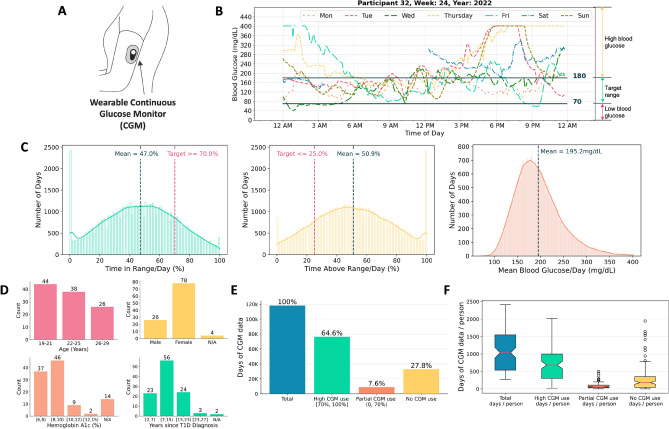
Table 1 and Figure 1provide an overview of our study participants and the dataset leveraged for quantitative analysis of CGM use patterns. In total, this study includes 118,325 days of CGM data with 22.9 million blood glucose samples from 108 young adults with T1D (age: 19 - 29 years, time since T1D diagnosis: 2 - 27 years). Study participants resided in various geographical locations (i.e. 33 distinct states) in the U.S. - see Supplementary Fig. S3. The duration of CGM data for each participant spanned between 9 months to 6.5 years, with an average of 3 years of CGM data per person (mean ± SD: 1096 ± 580 days with an average of 212,831 blood glucose samples per participant). The majority of study participants used Dexcom CGMs75 in combination with insulin pumps (n=88) or multiple daily injection insulin therapy (n=20) for diabetes management. In total, our dataset includes 76,435 days with high CGM use (64.6%), 8,959 days with partial CGM use (7.6%), and 32,931 days with no CGM use (27.8%) - see Table 1.
Table 1.
Overview of the study dataset showing more than 118,000 days of CGM data with over 2.2 million blood glucose samples from 108 young adults with type 1 diabetes. Hemoglobin A1 C values collected via the mail-in AccuBase A1c test kit.
| Attributes | Mean ± SD | Range | Not Available | |
|---|---|---|---|---|
| Demographics | Total no. of participants | 108 | ||
| Age (yrs.) | 23 ± 3 | 19 - 29 | ||
| Male/Female | 26/78 | 4 | ||
| White/Black/Others | 77/12/7 | 12 | ||
| Time since T1D diagnosis (yrs.) | 11.5 ± 5.3 | 2.4 - 27.0 | 2 | |
| Hemoglobin A1C (%) | 8.4 ± 1.3 | 6.3 - 14.4 | 14 | |
| Insulin pump/Multiple daily injections | 88/20 | |||
| Private/Public insurance | 78/18 | 12 | ||
| CGM use | Device (Dexcom/Medtronic) | 103/1 | 4 | |
| Total duration of data (days) | 118325 | |||
| Duration of data/participant (days) | 1096 ± 580 | 272 - 2412 | ||
| Total days with no CGM use | 32931 | |||
| Total days with high CGM use | 76435 | |||
| Blood glucose data | Total BG samples | 22,985,747 | ||
| Number of BG samples/participant | 212,831 ± 139,929 | 7,721 - 601,407 | ||
| Mean blood glucose/day (mg/dL) | 195.2 ± 49.2 | 70.4 - 401.0 | ||
| Mean time in range/day (%) | 47.0 ± 24.1 | 0 - 100 |
Fig. 1.
Data Overview. (A) Wearable CGM used for diabetes management. (B) 1 week of blood glucose recordings from a CGM. (C) Distribution plots of time in range, time above range, and mean blood glucose per day across study participants (n=108). (D) Demographics of study participants. (E & F) CGM use patterns across over 118,000 days of data.
Fig. 1A shows an illustration of a wearable CGM, while Fig. 1B shows a representative example of one week of CGM data from one of our study participants (i.e., participant 32). Fig. 1C shows the distribution of blood glucose metrics across our study participants, with a mean daily time with blood glucose in the target range (i.e., TIR/day) of 47.0% (clinical target: >70%); mean daily time with blood glucose above the target range (i.e., TAR/day) of 50.9% (clinical target: <25%); and mean blood glucose per day (i.e. BG/day) of 195.2 mg/dL. From this analysis, it is evident that the majority of participants in this study do not meet the clinical target for blood glucose management68,69. However, this is expected since participants were initially recruited for an intervention study seeking to improve blood glucose management.
Seasonal variations in long-term CGM use
Fig. 2 shows seasonal trends observed in long-term CGM use across the year. From this figure, we observe higher CGM use at the beginning of the year (i.e., January and February) and toward the end of the year (i.e. September to December) with the exception of November - see Fig. 2A. Across the full cohort, the highest average daily CGM use of 73.5% was observed on December 25th, meanwhile the lowest average daily CGM use of 56.2% was observed on July 16th. Fig. 2B further summarizes the change in CGM use across the year by showing the average CGM use per month for our population of young adults with type 1 diabetes. From this figure, we observe the highest CGM use of 68.8% in the month of January and a steady decline in CGM use until the month of May. Conversely, the lowest CGM use of 61.5% is observed in the month of July after which CGM use begins to increase again albeit a dip in the month of November. The average monthly CGM use was lowest mid-year between the months of May to July (range: 61.5% to 63.2%).
Fig. 2.
Seasonal variations in long-term CGM use observed from analysis of 118,325 days of data between year 2015 and 2023 (n=108). (A) The change in CGM use across all days of the year compared to the baseline (i.e., average CGM use across all days of the year). (B) Average CGM use for each month of the year.
Weekly and daily variations in long-term CGM use
Fig. 3 shows weekly and daily variations in CGM use patterns. More specifically, Fig 3A presents the cumulative change in CGM use for each day of the week across our study population compared to the baseline across all days of the week. From this figure, we observe higher CGM use on weekdays Monday through Thursday as evident from the positive changes above the baseline and lower CGM use on weekends Friday through Sunday as evident from the negative changes below the baseline. In particular, we observe the highest CGM use of 0.63% above the baseline on Tuesdays and the lowest CGM use of 0.85% below the baseline on Sundays. To further understand these differences, we grouped the full week into two categories that best align with the observed direction of change (Monday to Thursday and Friday to Sunday). Fig. 3B shows the cumulative change for each category. In particular, CGM use increased above the baseline by 0.43% between Monday to Thursday and decreased below the baseline by 0.57% on Friday to Sunday. Using a paired t-test to compare the normalized difference in CGM use between weekdays (Monday to Thursday, mean=0.43%, SD=2.30%) and weekends (Friday to Sunday, mean=0.57%, SD=3.07%), we found that the difference was not statistically significant; t(107)=1.92, p=0.057.
Fig. 3.
Weekly and daily variations in CGM use observed from analysis of 118,325 days of data between year 2015 and 2023 (n=108). (A) Variations in CGM use across days of the weeks compared to the baseline (i.e., average CGM use across all days of the week). (B) Variations in CGM use on weekdays (Monday - Thursday) compared to weekends (Friday - Sunday). (C) Variations in CGM use across hours of the day compared to the baseline (i.e., average CGM use across all hours of the day). (D) Variations in CGM use during the daytime (6 AM - 6PM) compared to evening/nighttime (7PM - 5 AM).
Similarly, we evaluated variations in CGM use across the 24 hours of each day as shown in Fig. 3C. From this figure, we observe higher than baseline CGM use during the waking hours of 6 AM - 6PM, and lower than baseline CGM use in the evenings and nighttime from 7PM to 5 AM. The hour of day with the highest CGM use of 0.7% above the baseline was at 9 AM, meanwhile the hour of day with the lowest CGM use of 1.1% below the baseline was at 2 AM. To further quantify variations in daily CGM use patterns, we grouped all hours of the day into two categories that best align with the observed direction of change (i.e., 6 AM to 6PM and 7PM to 5 AM). Fig. 3D shows the cumulative change for each category. We observed that CGM use increased above the baseline by 0.49% between 6 AM - 6PM and decreased by 0.58% between 7PM - 5 AM. Using a paired t-test to compare the normalized difference in CGM use between daytime (6 AM - 6PM, mean=0.49%, SD=8.23%) and evening/nighttime (7PM - 5 AM, mean=−0.58%, SD=9.30%), we found that the difference was statistically significant; t(107)=3.64, p=0.00042.
Individual variations in long-term CGM use
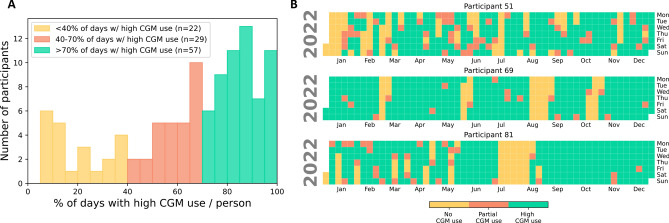
To understand CGM use patterns at the individual-level, we quantified the percent of days with high daily CGM use for all participants in this study - see Fig. 4A. From this figure, we observed that only 52.7% of participants (n=57) had sustained and consistent CGM use (i.e., high daily CGM use on at least 70% of days in their data duration). Conversely, 26.9% of participants (n=29) had average CGM use patterns (i.e., high daily CGM use on 40% - 70% of days in their data duration), while 20.4% of participants (n=22) had low CGM use patterns (i.e., high daily CGM use on less than 40% of days in their data duration). To further understand how CGM use patterns varied across individuals, we visualized each participant’s CGM use patterns with a calendar plot. Figure 4B and Supplementary Fig. S4 show examples of individual CGM use patterns for 6 sample participants in the year 2022. From these figures, we observe high inter- and intra-individual variability in long-term CGM use. From Figure 4, we see that participant 51 had many days with no CGM use in the month of January and more intermittent use patterns across all days on the year. Conversely, participant 69 had high daily CGM use on many consecutive days in the year with only a few block of days with no CGM use in the months of March, June, August to September and October. Meanwhile, participant 81 had more intermittent CGM use patterns in the first half of the year, a period of no CGM use in the months of July to August, and more consistent CGM use in the later months of the year (i.e., September to December). On the other hand, Supplementary Fig. S4 shows the calendar plot for 3 more example participants with notably different CGM use patterns including low CGM use for participant 52, moderate CGM use for participant 74, and high and sustained CGM use for participant 78. These highly varied use patterns present an opportunity for personalized algorithms that can leverage each individual’s own rich health data to understand and possibly predict their unique use patterns.
Fig. 4.
Overview of individual variations in long-term CGM use observed from analysis of 118,325 days of day between year 2015 and 2023 (n=108). (A) Distribution of days with high CGM use (>70% wear time). (B) Calendar plot for 3 example participants showing high inter-person variability in long-term CGM use patterns.
Associations between individual characteristics and long-term CGM use
Table 2 presents the results from multivariate analysis aiming to assess associations between distinct individual characteristics and sustained/consistent CGM use in the long-term (i.e., > 70% wear time on at least 70% of days). From this table, we observe that participants who had low CGM use in the first 30 days of the data collection period (i.e., < 21 days with at least 70% of CGM data) were 10 times less likely to have high CGM use in the long-term compared to participants who had high CGM use in the first 30 days (Odds Ratio [OR]: 0.12, 95% CI: 0.04 - 0.31, p=<0.0001). Additionally, participants who had higher levels of hemoglobin A1C (> 7.5%) were 2 to 2.5 times less likely to have high CGM use in the long-term than participants who had lower levels of hemoglobin A1C between 6% to 7.5% (OR: 0.46, 95% CI: 0.46 - 4.02, p=0.14 and OR: 0.39, 95% CI: 0.10 - 1.52, p=0.14, respectively). While the results of our analysis shows that long-term CGM use is associated with lower hemoglobin A1C (i.e., better diabetes management), the difference was not statistically significant - see Table 2. Finally, our results show that females were 1.85 times more likely to have long-term CGM use compared to males, however this difference was also not statistically significant (OR: 1.85, 95% CI: 0.69 - 5.08, p=0.18). Other factors evaluated including age (range: 19 - 29 years), and years since T1D diagnosis (range: 2 - 27 years) and insurance type were not notably associated with long-term CGM use.
Table 2.
Associations between individual characteristics and long-term CGM use. Results of multivariate analysis. ‘ref’ denotes the reference category used for categorical predictors. Bold indicates .
| Predictor | # Participants | Odds Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Age | 19 - 21 | 44 | ref | ref |
| 22 - 25 | 38 | 1.23 (0.47 - 3.23) | 0.66 | |
| 26 - 29 | 26 | 1.16 (0.40 - 3.45) | 0.81 | |
| Gender | Male | 26 | ref | ref |
| Female | 78 | 1.85 (0.69 - 5.08) | 0.18 | |
| Years since T1D diagnosis | [2 - 7] | 23 | ref | ref |
| (7 - 15] | 57 | 1.39 (0.47 - 4.14) | 0.62 | |
| > 15 | 26 | 0.94 (0.26 - 3.32) | 1.0 | |
| Hemoglobin A1 C | [6 - 7.5] | 25 | ref | ref |
| (7.5 - 9] | 49 | 0.46 (0.14 - 1.37) | 0.14 | |
| > 9 | 20 | 0.39 (0.10 - 1.52) | 0.14 | |
| Insurance Type | Private | 78 | ref | ref |
| Public | 18 | 1.56 (0.49 - 5.29) | 0.44 | |
| High daily CGM use in first 30 days | Y | 70 | ref | ref |
| N | 38 | 0.12 (0.04 - 0.31) | <0.0001 |
Insights from semi-structured interviews on CGM use patterns
To further understand individual perspectives on long-term use of CGMs and factors contributing to underlying variations in CGM use patterns, we thematically organized our interview data into two broad categories, namely factors contributing to sustained and consistent CGM use and factors contributing to disrupted CGM use. Insights from each category are below.
Factors contributing to sustained and consistent CGM use
All interview participants spoke positively about their experience with using a CGM for diabetes management. The most common factors shared that contribute to sustained and consistent CGM use include the ability to achieve easier and better blood glucose management (including lower A1C) with a CGM, the convenience of having the CGM connect to other devices including smartphones and insulin pumps, the ability to have family and peer support with a CGM, and the ability to live independently. On the note of easier management, interviewee P10 said “I have worked shift work in a hospital and that was made much easier by the CGM because I could just glance down at my phone or my watch to see what my blood sugar was while working, not having to stop and go to the break room to check my blood sugar.” Likewise interviewee P02 said, “now I really like having the overnight data, [it] is kind of the biggest thing where I don’t have to worry about if I go high in the middle of the night... I get woken up and I can just fix it right then.” With regard to the convenience of having the CGM connect with other devices, interviewee P07 said “and it also, like, connects to my iPhone so I can just automatically see what’s going on. I don’t have to look at my [insulin] pump if I don’t want to, which makes it really, really convenient.” Similarly, interviewee P06 said, “it’s just easier to look at [the] phone and you’re done... I started on the Tandem t:slim, and now I [use] OmniPod, but both of them have [Dexcom] G6 integration where it does have its own loop built in.”
On the note of achieving better blood glucose management and lower hemoglobin A1C, interviewee P01 said, “I think it’s [diabetes management is] way better... 6.7 is one of the lowest A1C I’ve had in my life... I am coming up on 16 years of having type 1 diabetes, so it [the CGM] definitely helped me lower my overall glucose levels and my A1C.” Likewise interviewee P08 said, “my A1C came down from about twice what it is now.” On the note of enabling family and peer support, interviewee P07 said “as much as I’m fully an adult... I was diagnosed [with type 1 diabetes] at 3 [years old], it is a constant part of my family’s journey and so it [the CGM] really gives them peace of mind to just be able to see what my numbers [blood glucose readings] are.” Likewise, interviewee P11 said “my friends have it [my CGM readings] on the phones, and it makes being a college student just easier. Like, I can go out and drink with my friends at a bar knowing that they know what to do. Interviewee P11 said, “I started dating my boyfriend. In his fraternity, there was a girl who was diabetic... so that’s kind of where I started wearing it [the CGM] more and we became friends”. Finally, on the note of enabling independent living, interviewee P10 said, “I was going to college like eight hours away from home and so it kind of became a non-negotiable if I needed to have something [the CGM] that would wake me up in case I went low [had low blood glucose] in the middle of the night.”
Factors contributing to disrupted CGM use
Albeit the positive sentiment toward using a CGM for diabetes management, varying factors contributed to disrupted CGM use for some participants throughout the year and years including, changes in insurance coverage, high costs and supply issues, sensor malfunction and connectivity issues, college and life transitions, mental health and burnout, as well as issues around the sensor lifespan and adhesiveness of the sensors/patches. Majority of our interview participants (> 60%) highlighted issues with changes in insurance coverage, high costs, and supply issues. For example, when reviewing their visual plots of CGM use patterns, interviewee P06 said “it was 2020, my dad had lost his job meaning I lost my health insurance... the main thing [issue] is just the price without insurance,” similarly interviewee P07 said “I was on Alabama medicaid which didn’t cover CGMs and so I wasn’t able to get one [a CGM] until I went to college.” Likewise, interviewee P04 said “the only times I would not be using the CGM is due to the Dexcom G6 sensor coming off my skin... I can only get a certain amount of sensors per month... so the only time I don’t use the CGM is when there is some error, when I don’t have enough of the equipment [sensors].” Majority of our interview participants (> 60%) also highlighted sensor malfunction and connectivity issues when using the CGM. For example, interviewee P07 said “sometimes, connectivity can be an issue. Sometimes, if it’s [the CGM is] like on the wrong side of my body and the pump is on the other side of my body, it’ll tell me that there are no readings.” Interviewee P10 said, “I know I had a bad batch of sensors at one point where I was constantly changing sensors... and it was constantly disconnecting.” Likewise interviewee P05 said, “I do remember sometimes the app [companion CGM app] just wasn’t working... sometimes it would just like, have a bad connection to my phone, but I think most of those [periods of disrupted CGM use] are just, it’s [the CGM] stopping while I’m in my sleep or coming off in my sleep.”
The next most prevalent factors affecting sustained CGM use relate to college/life transitions, mental health and burnout. While reviewing visual plots of their CGM use patterns, interviewee P11 said “September 2020 until May 2021, that was my first year at college, that played a big factor in it [disrupted CGM use]. I had the mentality that I was unstoppable, and I could do anything... like first taste of independence and honestly, I just kept making myself sick.” Likewise interviewee P09 said “I was in college 2019 through the end of 2021 so I was in school and working. I think it [disrupted CGM use] was kind of a combination of life being crazy... also maybe just depresssion and stuff, as well as just normal mental health stuff, on top of having a chronic illness that can kind of add on to that.” Interviewee P06 said “something of ADHD, and that’s just mental stuff. It’s just like I can get in a habit and then I forget” while interviewee P11 said, “October of 2021, my mom got diagnosed with breast cancer. I hit a very bad depression. I basically said, forget diabetes.” Finally, interviewees spoke about issues with the sensor lifespan and adhesiveness of the sensors/patches. On this note, interviewee P05 said “most times, the sites [CGM sensors] won’t even last the full 10 days,” interviewee P08 said “it’s every 10 days, which is annoying.” Meanwhile, interviewee P04 said, “it looks like May to August, September, it looks like lower [CGM] adherence... I’m going to assume that’s because it’s warmer weather during those months. It’s the summer so it’s [the disrupted CGM use is] because of sweating and the sensor coming off more.” Likewise interviewee P11 said, “I know my hyderhidrosis does not help [with consistent CGM use/adhesiveness of the CGM sensor]. It’s basically a condition where I perspirate too much and I produce more sweat than a normal person.”
Specific factors gleaned from the interviews that contribute to seasonal variations in CGM use for some participants include school/college calendar and adhesiveness of the CGM sensor in warmer weather, meanwhile sensor lifespan was the most prominent factor that contributes to weekly and daily variations in CGM use. With regards to seasonal variations in CGM use, some participants shared that they wear their CGM less when they were home/off-school and thus have a less busy schedule (e.g., interviewee P02), meanwhile some participants experienced more issues with the adhesiveness of the CGM sensor during the summer season when the weather is generally warmer (e.g., interviewee P04 and P05). With regards to weekly and daily variations in CGM use, many participants shared that situations around the sensor lifespan. For example, some participants shared that when their CGM sensor is reaching the end of it’s 10- or 14-day lifespan, they may not replace the sensor until the next day or couple of days (e.g., interviewee P03, P06, and P08) while some participants try to extend the life of the CGM sensor and thus use it for a few more days before replacing it with a new sensor (e.g., interviewee P06).
Discussion
As we move toward a future in which wearable devices are becoming the standard of care for managing increasingly prevalent chronic conditions1,2,76,77, it is critical to understand heterogeneity in long-term use patterns. While this effort aligns with ample research on adoption and adherence to consumer-grade mobile/wearable devices in non-clinical populations15–17,24,78, the non-elective nature of chronic health conditions support a need for targeted research in clinical populations. Unlike many other chronic conditions, the diabetes domain is ahead of the curve when it comes to the availability and prevalence of clinical-grade wearable medical devices with proven efficacy to improve health outcomes40,42,43. Hence the diabetes domain is ideal for studying long-term use patterns of wearable medical devices amongst a clinical population. In this work, we focus on the understudied and at-risk population of young adults with type 1 diabetes64,65, many of whom experience poor prognosis as they navigate various life transitions while dealing with the daily task of managing their condition. We leverage rich longitudinal data (> 118,000 days from 108 young adults with type 1 diabetes) to study population- and individual-level patterns of CGM use across the cohort. To our knowledge, this is the first work that shows longitudinal and seasonal patterns of use associated with prescription wearable medical devices (i.e., CGMs) and our dataset is notably larger than related efforts10,79–81.
One key finding from this work relates to the seasonal, weekly, and daily patterns of CGM use observed from our population of young adults with type 1 diabetes. Our analysis showed that CGM use is generally higher in the early months of the year (i.e., January and February) and on the weekdays (Monday through Thursday). Some of these patterns may be influenced by “the fresh start effect” which supports the notion that people are likely to take on more positive behaviors and/or work toward attaining a goal immediately following salient temporal landmarks (e.g., beginning of a new year, month, or week)82. Additionally, we observed a steady decline in CGM use between the months of January to May, lower CGM use in the months of June and July (warmer months), followed by an increase in CGM use from August to December (albeit a dip in the month of November). We also observed lower than baseline CGM use during the weekends (Friday through Sunday).
Previous research conducted in the context of both type 1 and type 2 diabetes has found that although individual differences exist, contextual and social factors influence the use of mobile medical devices and CGMs in particular49,52,83. For example, some people do not care about others noticing their use of a medical device in a social or public context and/or that they have diabetes, while others tend to be more private and prefer to conceal or not use their device in settings that may attract undue attention. This finding from prior work could provide some additional rationale for the CGM use patterns found in this study such that there is less CGM use in the summer or warmer months when people often have more social events and wear lighter clothes that may not conceal their medical device (e.g., sleeveless outfits, swimming suits, and more). Related to this point, interviewee P11 said “at least with the [Dexcom] G7, it’s kind of inconvenient that we can only wear [it] on our arms. I would love to be able to go outside in a strapless dress and look normal, but it [the CGM sensor] always has to be on my arm.” Likewise, interviewee P03 said “I think just having something on my body... is still something to keep getting used to.” As identified in prior literature54, these pain points and challenges are important to inform the design of next-generation wearable medical devices that are more discreet and can be used interchangeably on different parts of the body.
Another key finding from this work relates to high variability in long-term CGM use. We observed that although population-level patterns exist, there also exists high inter- and intra-individual variability in CGM use patterns. Findings from this study show that higher levels of hemoglobin A1C (i.e., less than ideal blood glucose management) may be associated with lower consistency in CGM use. Although not statistically significant in this study, this insight corroborates with results from prior work which shows that digital health devices may be used more by people who are “healthier” or achieving better disease management outcomes, and less used by people who may need it most11,12,73. Additionally, in this work (and prior work by Faust et al.24), low CGM use in the first 30 days of device use was a strong predictor for discontinued use in the long-term. Given this, early use patterns of wearable medical devices can be assessed by healthcare providers or caregivers to identify pain points and facilitate the provision of support when needed. Additionally, the high degree of variability in long-term CGM use patterns observed from this study presents an opportunity for smarter wearable medical devices that can anticipate disrupted or discontinued use based on a person’s own historical data to guide the timing of feedback, notifications, or other forms of support. Prior work78has shown that long-term wearable data can be used to provide users with insights about their wearing patterns to overcome challenges with missing data and support users with achieving their health goals. Related to this point, interviewee P02 said “I know I had taken a while off [using my CGM], but I had no idea it was that long.” These findings show added potential for insightful data visualization and data interpretation tools that can further support sensemaking and reflection on health-related behaviors84,85.
Summary, limitations, and future work
This study is unique in its use of large scale longitudinal data from clinical-grade wearable devices used by young adults with type 1 diabetes to uncover variations in long-term CGM use. Some key strengths of this work include remote recruitment which enables participant representation from 33 states in the U.S. and use of routinely collected data from wearable medical device at the time of recruitment which allows for studying natural behaviors that are not impacted by participation in a research study. However, while research from this study led to many insights, there are several limitations that should be acknowledged including an imbalance in representation across genders (i.e., over 70% female (n=78)), almost all participants use Dexcom CGMs (i.e., 103 out of 104 in the quantitative study), and recruitment through social media and online platforms which can contribute to bias in the study population. In addition, there are some limitations associated with the qualitative part of this work (i.e., semi-structured interviews). For example, all interview participants shared their CGM data from the Dexcom server via Tidepool, hence when participants used non-Dexcom CGMs or other non-connected blood glucose monitoring tools, these showed up as periods with no CGM use in their long-term data visualizations. Secondly, some of our interview participants took part in a randomized controlled trial with an intervention aiming to improve their diabetes-relevant behaviors/outcomes (ClinicalTrials.gov ID: NCT04646473)60.
Building on the contributions of this work, future research is needed to address modifiable factors that negatively affect CGM use. Insights from these work and prior work48,54identified barriers including high costs of CGMs, stigma associated with wearing a medical devices, issues with adhesiveness when wearing the devices especially in the warmer months when the CGM sensor is more prone to fall off, limited sensor lifespan that leads to medical waste and increased costs for users, and a need for improved sensemaking and data interpretation solutions that facilitate health-behavior change when needed. Future research is also needed to understand the generalizability of insights from our study population to other age groups, including pediatric populations and older adults, as well as generalizability to other health domains with promising wearable-enabled monitoring (e.g., cardiovascular care76,86and mental health87).
Supplementary Information
Acknowledgements
This research was partly funded by grants from the National Institutes of Health (P30 DA029926, R01 DK124428).
Author contributions
Y.C. and T.P. conceived the study. C.S. led data collection. Y.C and T.P. conducted study interviews, quantitative and qualitative data analysis, data interpretation, and wrote the manuscript. All authors have access to the study data, critically reviewed the manuscript, and approved the final version.
Data availability
All data needed to evaluate the results in this manuscript are present in the paper and/or the Supplementary Materials.
Code availability
Code that supports the findings from this study is available on GitHub: https://github.com/Augmented-Health-Lab/CGM-Adherence/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanjun Cui and Temiloluwa Prioleau contributed equally.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98276-6.
References
- 1.Keshet, A., Reicher, L., Bar, N. & Segal, E. Wearable and digital devices to monitor and treat metabolic diseases. Nature Metabolism5, 563–571 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Lu, L. et al. Wearable health devices in health care: narrative systematic review. JMIR mHealth and uHealth8, e18907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson, K. B. et al. Precision medicine, ai, and the future of personalized health care. Clinical and translational science14, 86–93 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim, J., Campbell, A. S., de Ávila, B.E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nature biotechnology37, 389–406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn, J., Runge, R. & Snyder, M. Wearables and the medical revolution. Personalized medicine15, 429–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang, H. S. & Exworthy, M. Wearing the future-wearables to empower users to take greater responsibility for their health and care: Scoping review. JMIR mHealth and uHealth10, 10.2196/35684 (2022). [DOI] [PMC free article] [PubMed]
- 7.Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Science translational medicine14, eabn6036 (2022). [DOI] [PMC free article] [PubMed]
- 8.Group & J. D. R. F. C. G. M. S,. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes care32, 1947–1953 (2009). [DOI] [PMC free article] [PubMed]
- 9.Mäkelä, M. J., Backer, V., Hedegaard, M. & Larsson, K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and copd. Respiratory medicine107, 1481–1490 (2013). [DOI] [PubMed] [Google Scholar]
- 10.de Bock, M. et al. Continuous glucose monitoring adherence: lessons from a clinical trial to predict outpatient behavior. Journal of Diabetes Science and Technology10, 627–632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiners, F., Sturm, J., Bouw, L. J. & Wouters, E. J. Sociodemographic factors influencing the use of ehealth in people with chronic diseases. International journal of environmental research and public health16, 645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekaran, R., Katthula, V. & Moustakas, E. Patterns of use and key predictors for the use of wearable health care devices by us adults: insights from a national survey. Journal of medical Internet research22, e22443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, S. L. et al. Determinants of longitudinal adherence in smartphone-based self-tracking for chronic health conditions: evidence from axial spondyloarthritis. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies5, 1–24 (2021). [Google Scholar]
- 14.Li, H., Wu, J., Gao, Y. & Shi, Y. Examining individuals’ adoption of healthcare wearable devices: An empirical study from privacy calculus perspective. International journal of medical informatics88, 8–17 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Lazar, A., Koehler, C., Tanenbaum, T. J. & Nguyen, D. H. Why we use and abandon smart devices. In Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing, 635–646 (2015).
- 16.Shin, G. et al. Wearable activity trackers, accuracy, adoption, acceptance and health impact: A systematic literature review. Journal of biomedical informatics93, 103153 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Attig, C. & Franke, T. Abandonment of personal quantification: A review and empirical study investigating reasons for wearable activity tracking attrition. Computers in Human Behavior102, 223–237 (2020). [Google Scholar]
- 18.Fendrich, S. J., Balachandran, M. & Patel, M. S. Association between behavioral phenotypes and sustained use of smartphones and wearable devices to remotely monitor physical activity. Scientific reports11, 21501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clawson, J., Pater, J. A., Miller, A. D., Mynatt, E. D. & Mamykina, L. No longer wearing: investigating the abandonment of personal health-tracking technologies on craigslist. In Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing, 647–658 (2015).
- 20.Meyer, J., Wasmann, M., Heuten, W., El Ali, A. & Boll, S. C. Identification and classification of usage patterns in long-term activity tracking. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems, 667–678 (2017).
- 21.Jeong, H., Kim, H., Kim, R., Lee, U. & Jeong, Y. Smartwatch wearing behavior analysis: a longitudinal study. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies1, 1–31 (2017). [Google Scholar]
- 22.Li, L., Peng, W., Kononova, A., Bowen, M. & Cotten, S. R. Factors associated with older adults’ long-term use of wearable activity trackers. Telemedicine and e-Health26, 769–775 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Vhaduri, S. & Prioleau, T. Adherence to personal health devices: A case study in diabetes management. In Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare, 62–72 (2020).
- 24.Faust, L. et al. What 30 days tells us about 3 years: identifying early signs of user abandonment and non-adherence. In Proceedings of the 13th EAI International Conference on Pervasive Computing Technologies for Healthcare, 216–224 (2019).
- 25.Belsare, P., Bartolome, A., Stanger, C. & Prioleau, T. Understanding temporal changes and seasonal variations in glycemic trends using wearable data. Science Advances9, eadg2132 (2023). [DOI] [PMC free article] [PubMed]
- 26.Belsare, P., Lu, B., Bartolome, A. & Prioleau, T. Investigating temporal patterns of glycemic control around holidays. In 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 1074–1077 (IEEE, 2022). [DOI] [PubMed]
- 27.Chiefari, E. et al. Impact of seasonality on gestational diabetes mellitus. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders)17, 246–252 (2017). [DOI] [PubMed]
- 28.Sakamoto, M. et al. Seasonal variations in the achievement of guideline targets for hba1c, blood pressure, and cholesterol among patients with type 2 diabetes: a nationwide population-based study (abc study: Jddm49). Diabetes Care42, 816–823 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Shephard, R. J. & Aoyagi, Y. Seasonal variations in physical activity and implications for human health. European journal of applied physiology107, 251–271 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Ayers, J. W., Althouse, B. M., Allem, J.-P., Rosenquist, J. N. & Ford, D. E. Seasonality in seeking mental health information on google. American journal of preventive medicine44, 520–525 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Wirz-Justice, A. Seasonality in affective disorders. General and comparative endocrinology258, 244–249 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Golder, S. A. & Macy, M. W. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science333, 1878–1881 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Liu, X. et al. The role of seasonality in the spread of covid-19 pandemic. Environmental research195, 110874 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriyama, M., Hugentobler, W. J. & Iwasaki, A. Seasonality of respiratory viral infections. Annual review of virology7, 83–101 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Marti-Soler, H. et al. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart100, 1517–1523 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Pierson, E., Althoff, T., Thomas, D., Hillard, P. & Leskovec, J. Daily, weekly, seasonal and menstrual cycles in women’s mood, behaviour and vital signs. Nature Human Behaviour5, 716–725 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Harding, C. et al. The daily, weekly, and seasonal cycles of body temperature analyzed at large scale. Chronobiology International36, 1646–1657 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Stewart, S., Keates, A. K., Redfern, A. & McMurray, J. J. Seasonal variations in cardiovascular disease. Nature Reviews Cardiology14, 654–664 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Rodbard, D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes technology & therapeutics18, S2-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappon, G., Acciaroli, G., Vettoretti, M., Facchinetti, A. & Sparacino, G. Wearable continuous glucose monitoring sensors: a revolution in diabetes treatment. Electronics6, 65 (2017). [Google Scholar]
- 41.Beck, R. W., Bergenstal, R. M., Laffel, L. M. & Pickup, J. C. Advances in technology for management of type 1 diabetes. The Lancet394, 1265–1273 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Laffel, L. M. et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. Jama323, 2388–2396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galindo, R. J. & Aleppo, G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes research and clinical practice170, 108502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster, N. C. et al. State of type 1 diabetes management and outcomes from the t1d exchange in 2016–2018. Diabetes technology & therapeutics21, 66–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson, A. L., Mullen, D. M. & Bergenstal, R. M. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes technology & therapeutics19, S–4 (2017). [DOI] [PMC free article] [PubMed]
- 46.Taylor, P. J., Thompson, C. H. & Brinkworth, G. D. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. Journal of diabetes investigation9, 713–725 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin, R., Brown, F., James, S., Jones, J. & Ekinci, E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabetic Medicine38, e14528 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Tanenbaum, M. L. et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. Journal of diabetes science and technology11, 484–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanenbaum, M. L. et al. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes care40, 181–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messer, L. H., Berget, C., Beatson, C., Polsky, S. & Forlenza, G. P. Preserving skin integrity with chronic device use in diabetes. Diabetes technology & therapeutics20, S2-54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanenbaum, M. L. & Commissariat, P. V. Barriers and facilitators to diabetes device adoption for people with type 1 diabetes. Current diabetes reports22, 291–299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyyety, V. et al. Barriers and facilitators to uptake of continuous glucose monitoring for management of type 2 diabetes mellitus in youth. The Science of Diabetes Self-Management and Care49, 426–437 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messer, L. H. et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes technology & therapeutics22, 760–767 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Barnard-Kelly, K. D., Martínez-Brocca, M. A., Glatzer, T. & Oliver, N. Identifying the deficiencies of currently available cgm to improve uptake and benefit. Diabetic Medicine e15338 (2024). [DOI] [PubMed]
- 55.Bartolome, A., Shah, S. & Prioleau, T. Glucomine: A case for improving the use of wearable device data in diabetes management. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies5, 1–24 (2021). [Google Scholar]
- 56.Prioleau, T., Bartolome, A., Comi, R. & Stanger, C. Diatrend: A dataset from advanced diabetes technology to enable development of novel analytic solutions. Scientific Data10 (2023). [DOI] [PMC free article] [PubMed]
- 57.Bartolome, A. & Prioleau, T. A computational framework for discovering digital biomarkers of glycemic control. NPJ Digital Medicine5, 111 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morton, S., Li, R., Dibbo, S. & Prioleau, T. Data-driven insights on behavioral factors that affect diabetes management. In 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 5557–5562 (IEEE, 2020). [DOI] [PMC free article] [PubMed]
- 59.Lu, B. et al. Mealtime prediction using wearable insulin pump data to support diabetes management. Scientific Reports14, 21013 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanger, C. et al. A digital health intervention (sweetgoals) for young adults with type 1 diabetes: Protocol for a factorial randomized trial. JMIR Research Protocols10, e27109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monaghan, M., Helgeson, V. & Wiebe, D. Type 1 diabetes in young adulthood. Current diabetes reviews11, 239–250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James, S., Armstrong, M., Abdallah, Z. & O’Kane, A. A. Chronic care in a life transition: challenges and opportunities for artificial intelligence to support young adults with type 1 diabetes moving to university. In Proceedings of the 2023 CHI Conference on Human Factors in Computing Systems, 1–16 (2023).
- 63.Mathias, P. et al. Young adults with type 1 diabetes. Endocrinology and Metabolism Clinics53, 39–52 (2024). [DOI] [PubMed] [Google Scholar]
- 64.Ramchandani, N., Way, N., Melkus, G. D. & Sullivan-Bolyai, S. Challenges to diabetes self-management in emerging adults with type 1 diabetes. The diabetes educator45, 484–497 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Zoffmann, V., Vistisen, D. & Due-Christensen, M. A cross-sectional study of glycaemic control, complications and psychosocial functioning among 18-to 35-year-old adults with type 1 diabetes. Diabetic Medicine31, 493–499 (2014). [DOI] [PubMed] [Google Scholar]
- 66.DTILaboratories, I. Accubase a1c test kit. https://dtilaboratories.com/. Accessed: June 2024.
- 67.Glooko. https://glooko.com/. Accessed: June 2024.
- 68.Danne, T. et al. International consensus on use of continuous glucose monitoring. Diabetes care40, 1631–1640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes care42, 1593–1603 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Battelino, T. et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. The lancet Diabetes & endocrinology11, 42–57 (2023). [DOI] [PubMed] [Google Scholar]
- 71.Helander, E. E., Wansink, B. & Chieh, A. Weight gain over the holidays in three countries. New England Journal of Medicine375, 1200–1202 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Mishra, P., Singh, U., Pandey, C. M., Mishra, P. & Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Annals of cardiac anaesthesia22, 407–411 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pathiravasan, C. H. et al. Factors associated with long-term use of digital devices in the electronic framingham heart study. NPJ Digital Medicine5, 195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fundamental Algorithms for Scientific Computing in Python. Virtanen, P. et al. SciPy 1.0. Nature Methods17, 261–272. 10.1038/s41592-019-0686-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dexcom. Dexcom continuous glucose monitoring. https://www.dexcom.com/ (2024).
- 76.Bayoumy, K. et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nature Reviews Cardiology18, 581–599 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo, Y. et al. A review of wearable and unobtrusive sensing technologies for chronic disease management. Computers in Biology and Medicine129, 104163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang, L. M. & Kay, J. Harnessing long term physical activity data-how long-term trackers use data and how an adherence-based interface supports new insights. Proceedings of the ACM on interactive, mobile, wearable and ubiquitous technologies1, 1–28 (2017). [Google Scholar]
- 79.Giani, E., Snelgrove, R., Volkening, L. K. & Laffel, L. M. Continuous glucose monitoring (cgm) adherence in youth with type 1 diabetes: associations with biomedical and psychosocial variables. Journal of diabetes science and technology11, 476–483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patton, S. R. Adherence to glycemic monitoring in diabetes. Journal of diabetes science and technology9, 668–675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murata, T. et al. Predictive factors of the adherence to real-time continuous glucose monitoring sensors: a prospective observational study (parcs study). Journal of diabetes science and technology15, 1084–1092 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai, H., Milkman, K. L. & Riis, J. The fresh start effect: Temporal landmarks motivate aspirational behavior. Management Science60, 2563–2582 (2014). [Google Scholar]
- 83.O’Kane, A. A., Rogers, Y. & Blandford, A. E. Concealing or revealing mobile medical devices? designing for onstage and offstage presentation. In Proceedings of the 33rd annual ACM conference on human factors in computing systems, 1689–1698 (2015).
- 84.Prioleau, T., Sabharwal, A. & Vasudevan, M. M. Understanding reflection needs for personal health data in diabetes. In Proceedings of the 14th EAI International Conference on Pervasive Computing Technologies for Healthcare, 263–273 (2020).
- 85.Coşkun, A. & Karahanoğlu, A. Data sensemaking in self-tracking: Towards a new generation of self-tracking tools. International Journal of Human-Computer Interaction39, 2339–2360 (2023). [Google Scholar]
- 86.Hughes, A., Shandhi, M. M. H., Master, H., Dunn, J. & Brittain, E. Wearable devices in cardiovascular medicine. Circulation Research132, 652–670 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fedor, S. et al. Wearable technology in clinical practice for depressive disorder. New England Journal of Medicine389, 2457–2466 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the results in this manuscript are present in the paper and/or the Supplementary Materials.
Code that supports the findings from this study is available on GitHub: https://github.com/Augmented-Health-Lab/CGM-Adherence/.