Abstract
This study aimed to investigate the ratios of precore stop mutant (codon 28; TGG to TAG) to total viremia in 53 HBeAg-positive patients with chronic hepatitis B by amplification-created restriction site assays along the course of HBeAg-to-anti-HBe seroconversion. At baseline, 11% had exclusive wild-type hepatitis B virus (HBV), 15% had exclusively precore mutant, and 74% had mixed viral strains. Precore mutant ratios correlated little with age, sex, or HBV DNA levels (all P > 0.1), but correlated modestly with alanine aminotransferase (ALT) levels (P = 0.05). The intervals from presentation to anti-HBe seroconversion correlated significantly with ALT and precore mutant ratios in univariate analysis but with only precore mutant ratios in multivariate analysis (P = 0.003). Precore mutant ratios at baseline were significantly higher (P < 0.001) in six patients with persistent high viremia and ALT elevation after anti-HBe seroconversion (group 1) than in 47 with remission (group 2). All group 1 patients had exclusive precore mutant after anti-HBe seroconversion, as did only 14 (30%) of the group 2 patients (P = 0.003). Among group 2 patients, precore mutant ratios at baseline or after anti-HBe seroconversion showed no significant difference between 34 patients with sustained remission and 13 with relapse. Cirrhosis developed in 50% (5 of 10) of patients with precore mutant ratios >50% at baseline but only in 12% (5 of 43) of those with precore mutant ratios of <50% at baseline (P < 0.05). In conclusion, precore mutant of variable ratios was frequently detected in HBeAg-positive patients with chronic hepatitis B. Precore mutant ratios tended to correlate with ALT levels and anti-HBe seroconversion, but high precore mutant ratios were associated with persistent hepatitis after anti-HBe seroconversion and increased risk of cirrhosis.
In the natural history of chronic hepatitis B virus (HBV) infection, seroconversion from HBVe antigen (HBeAg) to its antibody (anti-HBe) is usually accompanied by a decrease in HBV replication and remission of liver disease (18, 19, 31). However, in areas with high or intermediate HBV endemicity, viral replication and liver damage persist in about 10% of anti-HBe-positive carriers (2, 10, 15, 23). HBV mutants unable to secrete HBeAg because of translational defects in the precore region have been isolated in these patients (1, 4, 7, 14, 36). In the great majority of them (> 90%), an unusual HBV strain was characterized with a G-to-A mutation at nucleotide 1896, which generates a stop codon in the precore sequence (28, 32, 36). Notably, many recent studies have shown that precore mutant also can be detected in HBeAg-positive patients with chronic HBV infection (5, 17, 25, 27, 29). The prevalence of precore mutant and its relative ratio to total HBV in HBeAg-positive patients with chronic HBV infection has varied considerably in the published reports: precore mutant is detected in 0 to 80% of HBeAg-positive patients, and the majority have had coexisting predominant wild-type HBV, but a few even have had exclusively precore mutant (5, 11, 17, 25, 27, 29, 38). The significance of precore mutations in the HBeAg-positive phase of chronic HBV infection, however, remains unclear. Many studies have focused on its relationship to interferon therapy, but the results were very controversial (5, 13, 25, 38). It has been well recognized that several factors such as serum alanine aminotransferase (ALT) and HBV DNA levels are closely related to responsiveness to interferon therapy (3, 30). Unfortunately, these factors were largely not addressed in these studies. Further studies are needed to investigate the relationship between precore mutation and clinical and laboratory data, and also to correlate precore mutation and other relevant factors with HBeAg-to-anti-HBe seroconversion in HBeAg-positive patients with chronic type B hepatitis.
Moreover, although the majority of patients come to have sustained remission of liver disease after seroconversion from HBeAg to anti-HBe, some might have reactivation of HBV with relapse of hepatitis, and a few even remain in a persistent state of high viremia and ALT elevation (12, 20). It is also interesting to know whether determination of precore mutation may help in predicting the different outcomes along the course of HBeAg-to-anti-HBe seroconversion.
Recently, an amplification-created restriction site method has been applied in detection of a G-to-A mutation in the precore region of the HBV genome of nucleotide 1896 (codon 28; TGG to TAG) (11, 17, 22, 39). Compared with sequencing and methods based on hybridization, this method is faster and less elaborate. Moreover, it has the potential of quantitating in detail the ratio of wild-type to mutant HBV (17, 22, 39). In this report, the percentage of precore stop mutant relative to total HBV was studied in sequential sera from 53 patients with chronic hepatitis B using amplification-created restriction site assays, and the results were correlated with the clinical and laboratory characteristics as well as the course of HBeAg-to-anti-HBe seroconversion.
MATERIALS AND METHODS
Patients.
Fifty-three randomly selected HBeAg-positive adult Chinese patients with clinico-pathologically verified chronic type B hepatitis who fulfilled the following criteria were studied: (i) no previous antiviral or immunomodulatory therapy before entry of the study; (ii) no concomitant HCV or HDV superinfection or alcoholism; (iii) documented HBeAg-to-anti-HBe seroconversion during the follow-up; and (iv) a minimal follow-up period of 1 year after anti-HBe seroconversion.
There were 41 males and 12 females, and their mean age was 33 years (range, 18 to 52 years). All were documented to have chronic HBV infection, as they were positive for HBsAg for more than 6 months. No patients were homosexuals or intravenous drug abusers. All were HBV DNA positive by hybridization assays (mean, 1,718 pg/ml; range, 20 to 8,000 pg/ml) and had elevated serum ALT levels (mean, 227 IU/liter; range, 57 to 863 IU/liter; normal value, <36 IU/liter) at presentation.
After chronic hepatitis B was diagnosed, 23 patients received interferon treatment, and the remaining 30 did not receive any specific therapy. Liver biochemical tests were regularly done at 1- to 6-month intervals or more often when clinically indicated. Serial sera collected at initial presentation, 6-month intervals, and during HBeAg-to-anti-HBe seroconversion were stored at −70°C and used for analysis for the presence of precore mutant.
The precore mutant ratios of 15 HBsAg-positive carriers who were HBeAg and HBV DNA positive and had normal ALT at regular examinations every 3 to 6 months for at least 2 years were also studied as controls. Other controls, including 15 HBeAg-positive patients with elevated ALT who did not experience HBeAg-to-anti-HBe seroconversion during the follow-up periods of 1 to 3 years, were also studied.
Serological studies.
Serum was tested for HBsAg, HBeAg, anti-HBe, and total antibody against HDV by radioimmunoassay (Abbott Diagnostics, Chicago, Ill.). Antibodies against HCV were tested by a third-generation enzyme immunoassay (Abbott Diagnostics). HBV DNA was quantitated by sandwich molecular hybridization assays using a Digene Hybrid Capture System (Digene Diagnostics, Inc., Beltsville, Md.). The detection sensitivity was 0.5 pg/ml.
Detection of precore mutant by amplification-created restriction site method.
Detection of the precore stop mutant (codon 28; TGG to TGA) was performed by PCR in combination with a restriction fragment length polymorphism assay, as described in detail before (11, 39). This method uses a mismatched oligonucleotide as one of the PCR primers to generate an artificial restriction enzyme site. The primers used were 5′-GTATGGTGAGGTGAACAATG-3′ (nucleotides 2058 to 2039; antisense) and 5′-GCCTCCAAGCTGTGCCTTCCATGGCTTT-3′ (nucleotides 1868 to 1895; sense). Mismatched nucleotides are underlined. PCR using this pair of primers successfully created a BstXI site (CCAN6TGG) when wild-type, but not mutant-type, sequence was encountered. After amplification, the PCR products were digested with 10 IU of BstXI for 2 h at 45°C. The digested products were later separated by electrophoresis with 8% polyacrylamide gel. The separated bands of HBV were visualized with ethidium bromide staining. The density of each band corresponding to precore stop mutant and wild-type HBV was quantified by a computer-assisted densitometer (imaging densitometer, model GS-670; Bio-Rad, Hercules, Calif.). To obtain the standard curves for calculation of the proportion, amplified wild-type and mutant sequences were first cloned into pT7Blue T-Vector (Novagen, Madison, Wis.) and verified by dideoxy sequencing (Sequenase [version 2.0] DNA sequencing kit; United States Biochemical, Cleveland, Ohio). These two plasmids were used as standards to generate a standard curve for calculation of the percentage. Two serum samples containing only mutant or wild-type HBV precore sequences, which were verified by direct sequencing (SequiTherm Cycle Sequencing Kits; Epicentre Technologies, Madison, Wis.), were selected and used to confirm the standard curve obtained by the plasmids described above. Finally, the ratios of precore mutant in the tested samples were calculated according to these standard curves. See reference 39 for detail. This method can quantitatively assess the ratios of precore stop mutant to total viremia in the range of 5 to 95%, while those with precore stop mutant ratios of <5% and >95% will be read as 0% (exclusively wild-type HBV) and 100% (exclusively precore stop mutant), respectively. All tests were done in duplicate, with a variability of less than 5%.
Statistical analysis.
The data were expressed as mean ± standard error of mean, unless otherwise indicated. The significance of the difference between two means was assayed using Student’s t test, and that between two proportions was assayed using the χ2 test with Yates’ correction. Correlation coefficients (r) were derived by linear regression analysis or multiple regression analysis.
RESULTS
Prevalence of precore mutant in HBeAg-positive patients at baseline.
Of the 53 HBeAg-positive patients with chronic type B hepatitis, 6 (11%) had exclusively wild-type HBV, 8 (15%) had exclusively precore mutant, and the remaining 39 (74%) had mixed viral strains. Of the latter, the ratios of the precore mutant to total HBV ranged from 11 to 20% in 5 patients, 21 to 30% in 25 patients, 31 to 40% in 7 patients, 51 to 60% in 1 patient, and 61 to 70% in 1 patient.
Of the 15 HBeAg-positive carriers with normal ALT levels, 12 had exclusively wild-type HBV, and the remaining 3 had mixed viral strains, with precore mutant ratios in the range of 11 to 20%. Of the 15 HBeAg-positive patients with elevated ALT levels who did not experience HBeAg-to-anti-HBe seroconversion, 3 had exclusively wild-type HBV and 12 had mixed viral strains, with precore mutant ratios being 11 to 20% in 3 patients, 21 to 30% in 8 patients, and 31 to 40% in 1 patient.
Correlation between precore mutant ratios and clinical features in HBeAg-positive patients with chronic type B hepatitis at baseline.
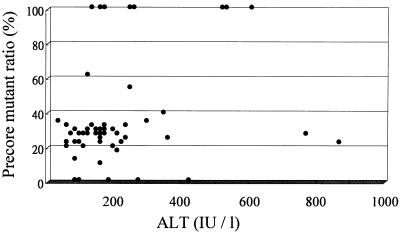
The precore mutant ratios did not correlate with age (r = −0.117; P = 0.4), sex (male = 0, female = 1; r = −0.07; P = 0.62), and levels of HBV DNA in serum (r = 0.193; P = 0.16), but there was a modest degree of positive correlation between precore mutant ratios and ALT levels (r = 0.271; P = 0.05), as depicted in Fig. 1.
FIG. 1.
Correlation between serum ALT levels and precore mutant ratios in 53 HBeAg-positive patients with chronic hepatitis B (r = 0.271; P = 0.05).
Correlation between intervals from presentation to HBeAg-to-anti-HBe seroconversion and clinical and laboratory features at baseline.
Of the 53 HBeAg-positive patients with chronic type B hepatitis, HBeAg-to-anti-HBe seroconversion occurred 2 to 55 months (mean, 18.5 months) after the initial presentation. To identify the possible determinants of HBeAg-to-anti-HBe seroconversion, the intervals from presentation to HBeAg-to-anti-HBe seroconversion were correlated with age, sex, ALT, levels of HBV DNA in serum, and precore mutant ratios at baseline. As shown in Table 1, the intervals from presentation to HBeAg-to-anti-HBe seroconversion correlated significantly with ALT levels and precore mutant ratios by linear regression analysis. The intervals from presentation to HBeAg-to-anti-HBe seroconversion also were not significantly related to interferon treatment. Multiple regression analysis revealed that only precore mutant ratios were independently related to the intervals from presentation to HBeAg-to-anti-HBe seroconversion (partial correlation coefficient = −0.384; P = 0.003), whereas ALT levels were not (partial correlation coefficient = −0.194; P = 0.162).
TABLE 1.
Correlation between clinical and laboratory variables (independent variable: x) at baseline and intervals (months) from presentation to anti-HBe seroconversion (dependent variable: y) in 53 HBeAg-positive patients with chronic hepatitis B
| Independent variable | Regression equation | r | P |
|---|---|---|---|
| Age (yr) | y = 18.3 + 8.09x | 0.004 | 0.976 |
| Sex (male = 0, female = 1) | y = 18.6 − 0.06x | −0.002 | 0.990 |
| ALT (IU/liter) | y = 24.0 − 0.02x | −0.280 | 0.047 |
| HBV DNA (pg/ml) | y = 21.2 − 1.53x | −0.238 | 0.086 |
| Precore mutant ratio (%) | y = 26.1 − 0.21x | −0.428 | 0.002 |
| Interferon treatment (no = 0, yes = 1) | y = 15.9 + 6.18x | 0.214 | 0.124 |
Correlation between clinical and laboratory features at baseline and subsequent course after HBeAg-to-anti-HBe seroconversion.
Forty-seven patients experienced remission of hepatitis (normalization of ALT and undetectable HBV DNA by hybridization assays) within 3 months after HBeAg-to-anti-HBe seroconversion (group 1), while the other six patients remained in a persistent state of high viremia and ALT elevation for more than 6 months after HBeAg-to-anti-HBe seroconversion (group 2). Among patients of group 1, 34 (group 1a) had sustained remission during the follow-up periods of at least 12 months (mean, 48 months; range, 12 to 84 months), while the other 13 (group 1b) had relapse of hepatitis (elevation of ALT and reappearance of HBV DNA by hybridization assays) 7 to 60 months (mean, 25 months) after HBeAg-to-anti-HBe seroconversion. Relapse of hepatitis was accompanied by reversion of HBeAg in serum in 4 of 13 patients. Among patients of group 2, five experienced remission of hepatitis 15, 27, 60, 60, and 63 months, respectively, after HBeAg/anti-HBe seroconversion, and one had persistent high viremia and hepatitis activity during the follow-up period of at least 48 months.
The clinical and laboratory features at baseline showed no significant difference between these three groups of patients, except that patients of group 2 had significantly higher precore mutant ratios than those of group 1a and 1b (Table 2). Six (60%; 95% confidence interval, 30 to 90%) of 10 patients with precore mutant ratios of >50% at baseline remained in a persistent state of high viremia and ALT elevation for at least 6 months after anti-HBe seroconversion, as did none of the 43 with precore mutant ratios of <50% at baseline (P < 0.001). The proportions of patients who received interferon treatment showed no significant difference between these three groups of patients, but the intervals from presentation to HBeAg-to-anti-HBe seroconversion were significantly shorter in patients of group 2 than in those of groups 1a and 1b (Table 2).
TABLE 2.
Clinical and laboratory characteristics of HBeAg-positive patients with chronic hepatitis B at baseline: correlation with the course after HBeAg/anti-HBe seroconversiona
| Characteristic | Group 1a (n = 34) | Group 1b (n = 13) | Group 2 (n = 6) |
|---|---|---|---|
| Age (yr) | 31.8 ± 1.4 | 34.7 ± 2.0 | 32.5 ± 3.1 |
| Sex (no. male/no. female) | 25:9 | 11:2 | 5:1 |
| AST (IU/liter) | 112 ± 12 | 142 ± 53 | 171 ± 61 |
| ALT (IU/liter) | 212 ± 22 | 240 ± 74 | 263 ± 65 |
| HBV-DNA (pg/ml) | 1,714 ± 354 | 1,712 ± 743 | 1,753 ± 1,398 |
| Precore mutant ratio (%) | 31 ± 5 | 27 ± 4 | 86 ± 10* |
| No.(%) of patients with precore mutant ratio of >50% | 4 (11.8) | 0 (0) | 6 (100)# |
| No. (%) of patients with interferon treatment | 15 (44.1) | 6 (46.1) | 2 (33.3) |
| Interval from presentation to anti-HBe seroconversion (mo) | 20.3 ± 2.5 | 21.4 ± 4.5 | 7.8 ± 1.9= |
Unless otherwise noted, results are means ± standard errors of the means. Groups: 1a, patients who experienced sustained remission of hepatitis during follow-up of at least 12 months after anti-HBe seroconversion; 1b, patients who experienced remission of hepatitis after anti-HBe seroconversion but had subsequent relapse; 2, patients who had persistent high viremia and ALT elevation for more than 6 months after anti-HBe seroconversion. Statistical significance: *, P < 0.001 for comparison with both group 1a and 1b; #, P < 0.001 for comparison with both group 1a and b; =, P < 0.001 for comparison with group 1a, P < 0.05 for comparison with group 1b. For other comparisons, P > 0.05.
Changes of precore mutant ratios along the course of HBeAg-to-anti-HBe seroconversion.
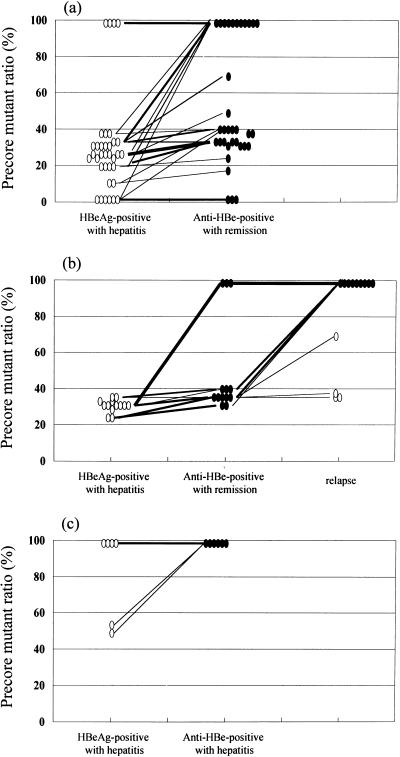
Precore mutant ratios in 15 HBeAg-positive patients with elevated ALT and persistence of HBeAg changed little or none during follow-up periods of 1 to 3 years. Among 47 patients of group 1, the precore mutant ratios also changed little or none during the HBeAg-positive phase, but increased immediately after HBeAg-to-anti-HBe seroconversion (52% ± 5% at the time of 1 to 3 months after HBeAg-to-anti-HBe seroconversion versus 31% ± 5% at baseline; P < 0.05). After anti-HBe seroconversion with remission of liver disease, 3 patients had exclusively wild-type HBV, 14 had exclusively precore mutant, and the remaining 30 had mixed viral strains (Fig. 2a and b). The precore mutant ratios after anti-HBe seroconversion showed no significant difference between patients who had sustained remission (53% ± 6%) and those who had subsequent relapse (47% ± 9%). Of the 34 patients with sustained remission, precore mutant ratios remained stationary during the follow-up periods of 1 to 7 years: 3 had exclusively wild-type HBV, 11 had exclusively precore mutant, and the other 20 had mixed viral strains with precore mutant ratios being 11 to 20% in 1 patient, 21 to 30% in 2 patients, 31 to 40% in 15 patients, 51 to 60% in 1 patient, and 71 to 80% in 1 patient. Of the 13 patients with subsequent relapse, 4 had mixed viral strains and reversion of serum HBeAg and 9 had exclusively precore mutant without reversion of serum HBeAg during reactivation of HBV (Fig. 2b).
FIG. 2.
Sequential changes of precore mutant ratios in 53 HBeAg-positive patients with chronic hepatitis B. (a) Patients who experienced sustained remission of hepatitis during follow-up of at least 12 months after anti-HBe seroconversion (n = 34); (b) Patients who experienced remission of hepatitis after anti-HBe seroconversion but had subsequent relapse (n = 13); (c) patients who had persistent high viremia and ALT elevation for more than 6 months after anti-HBe seroconversion (n = 6). Open circles, HBeAg-positive phase; closed circles, anti-HBe-positive phase.
Among six patients of group 2, all had exclusively precore mutant after HBeAg-to-anti-HBe seroconversion and remained so during the follow-up periods of 3 to 8 years (Fig. 2c).
Relationship between precore mutant ratios at baseline and final outcome.
Histological or clinical evidence of cirrhosis was identified in 10 (19%) of 53 patients at the end of the follow-up. The incidence of cirrhosis was 67% (4 of 6) in group 2, 31% (4 of 13) in group 1b, and 6% (2 of 34) in group 1a (group 2 versus group 1a, P < 0.01). Five (50%; 95% confidence interval, 19 to 81%) of 10 patients with precore mutant ratios of >50% at baseline developed cirrhosis, as did only 5 (12%; 95% confidence interval, 2 to 21%) of 43 with precore mutant ratios of <50% at baseline (P < 0.05). No patients developed hepatocellular carcinoma during the follow-up periods.
DISCUSSION
The onset and duration of HBV infection in patients of the present series are uncertain, but it has been suggested that the vast majority of chronic HBV-infected patients in Taiwan are due to neonatal or childhood infection (33). The natural course of chronic HBV infection has three chronological phases: the first is known as the immune tolerance phase, the second is known as the immune clearance phase, and the third is known as the low-replicative, residual phase (10). In the early immune tolerance phase, the patients are HBeAg positive and have high levels of HBV DNA in serum but are asymptomatic and have normal ALT levels and minimal histological activity (10). This phase usually can last for decades, during which HBeAg is produced and secreted in excess, and, as indicated in the present findings, the precore sequence of infecting HBV belongs predominantly or exclusively to the wild-type strain.
Most patients in the present series presented clinically overt hepatitis between 20 to 35 years of age with elevated serum ALT levels and increased necroinflammatory activity in the liver, representing attempts by the immune system to eliminate HBV-infected hepatocytes. The present results revealed that nearly 90% of these patients had detectable precore mutant, suggesting the emergence of precore mutant during the periods of immune elimination of HBV. The ratios of precore mutant to total HBV, however, showed considerable individual variation. There is no significant correlation between levels of HBV DNA in serum and precore mutant ratios, in keeping with the suggestion that precore mutant had a replicative activity as well as wild-type HBV (8, 9). Perhaps the more important finding of the present study is that there is a modest degree of positive association between the precore mutant ratios and ALT levels (see Fig. 1). These findings might suggest that the levels of emergence of precore mutant in the immune clearance phase might reflect the degrees of immune-mediated lysis of infected hepatocytes.
Previous studies have shown that the average rate of spontaneous HBeAg seroconversion in patients with chronic type B hepatitis is about 10% per year (19, 24). The probability of HBeAg-to-anti-HBe seroconversion is closely related to the necroinflammatory activity in liver (21). The present results revealed that the intervals from presentation to HBeAg-to-anti-HBe seroconversion correlated significantly with ALT levels and precore mutant ratios in univariate analysis. However, in multivariate analysis only precore mutant ratios can significantly predict HBeAg-to-anti-HBe seroconversion. These findings also seem in agreement with the suggestion that the levels of emergence of precore mutant in the immune clearance phase might reflect the degrees of immune-mediated lysis of infected hepatocytes.
After seroconversion from HBeAg to anti-HBe, serum HBV DNA became undetectable by hybridization assays and ALT levels returned to normal ranges in the majority of patients. However, about 10% of patients in the present series had persistent high viremia and ALT elevation in spite of HBeAg-to-anti-HBe seroconversion. The clinical and laboratory features at baseline showed no significant difference between patients who had clinical remission and those who did not, except that precore mutant ratios were significantly higher in the latter than in the former (Table 2). The present results thus for the first time indicate that analysis of precore mutant ratios in HBeAg-positive patients with chronic type B hepatitis can predict remission or persistence of liver disease after anti-HBe seroconversion. All of our patients with predominant wild-type HBV (precore mutant ratios, <50%) at baseline cleared both wild-type HBV and precore mutant from serum to levels undetectable by hybridization assays and had remission of hepatitis after anti-HBe seroclonversion. On the contrary, 60% of patients with predominant precore mutant (precore mutant ratios, >50%) at baseline cleared wild-type HBV only and remained in a persistent state of high viremia with exclusively precore mutant after anti-HBe seroconversion. These findings highly suggest that hepatocytes infected with wild-type HBV appear to be more vulnerable to immune-mediated lysis than hepatocytes expressing precore mutant strain. However, the number of patients with persistent high viremia and ALT elevation after anti-HBe seroconversion in this series was small. Further studies of a larger series of such cases are needed to confirm this postulation.
After anti-HBe seroconversion, all of our patients who remained in a persistent state of high viremia and ALT elevation had exclusively precore mutant (Fig. 2c), in keeping with the previous observations (1, 4, 7, 14, 26, 32, 36, 37). On the contrary, of the patients who had remission of liver disease, only about 30% had exclusively precore mutant, while many had mixed viral strains and a few even had exclusively wild-type HBV (Fig. 2a and b). The prevalence of precore mutant in anti-HBe-positive asymptomatic HBsAg carriers varied considerably in the published reports. For example, all cases in Okamoto’s series from Japan (28) or in Tur-Kaspa’s series from Israel (37) had exclusively precore mutant, while the majority of cases in Carman’s series from Greece (7) and in Naoumov’s series from Bulgaria (35) had mixed viral strains. Whether this discrepancy is related to the difference in the ethnic or geographical origins remains unclear.
Thirteen of 47 patients in this series had reactivation of HBV with relapse of hepatitis during the follow-up. The clinical and laboratory characteristics at presentation showed no significant difference between patients who had sustained remission and those who had subsequent relapse of hepatitis (Table 2). The precore mutant ratios after anti-HBe seroconversion also showed no significant difference between them. As shown in Fig. 2a and b, 3(21%) of 14 patients with exclusively precore mutant after anti-HBe seroconversion underwent relapse of hepatitis, as did 10 (33%) of 30 patients with mixed viral strains. Our findings thus seem in contrast to the previous observations by others. Takeda et al. (34) analyzed six patients who seroconverted to anti-HBe and hypothesized that the presence of precore mutant might predict sustained remission. On the contrary, Brunetto et al. (6) found a significantly higher incidence of relapse in patients with exclusively precore mutant than in those with mixed viral strains. The relationship between precore mutant and relapse of hepatitis thus remains inconclusive. It should be pointed out that the classification of patients into those experiencing sustained remission and those experiencing relapse is artificial, as some patients who are classified as experiencing sustained remission might experience relapse of hepatitis later with prolonged follow-up. The period of observation is usually only several years at most in our series as well as in most of the reported series. Therefore, the present results and the published data on the relationship between precore mutant and relapse of hepatitis are likely subject to change as the duration of follow-up is prolonged.
Of the 13 patients experiencing relapse of hepatitis in our series, only 4 had reversion of serum HBeAg, and the other 9 were persistently anti-HBe positive. These data were similar to those in our previous report, in which reversion of serum HBeAg was found in 8 of 35 anti-HBe-positive patients with reactivation of HBV (20). The sequential changes of precore mutant ratios in these patients as demonstrated here enable us to better understand the relationship between reactivation of HBV and reversion of serum HBeAg (Fig. 2b). As expected, all three patients with exclusive precore mutant after anti-HBe seroconversion had reactivation of precore mutant without reversion of serum HBeAg. Notably, of the 10 patients with mixed viral strains after anti-HBe seroconversion, only 4 had reactivation of both wild-type HBV and precore mutant with reversion of serum HBeAg, while the other 6 had reactivation of precore mutant only without reversion of serum HBeAg. These findings might suggest that the existence of functioning immune response to HBeAg in the hosts after anti-HBe seroconversion (16, 35) will prevent the reactivation of wild-type HBV more efficiently than that of precore mutant.
With regard to the relationship between precore mutant ratios at baseline and the final outcome, 19% of patients in this series were identified as having cirrhosis. Perhaps another important finding of the present study is that patients with predominantly precore mutant (precore mutant ratios, >50%) at baseline were more significantly associated with increased risk of cirrhosis than those with predominantly wild-type HBV (precore mutant ratios, <50%). The reason for this increased risk of cirrhosis in patients with high precore mutant ratios obviously is closely related to the presence of high ALT levels at baseline and the persistence of high viremia and ALT elevation after anti-HBe seroconversion in the majority of these patients.
In conclusion, about 90% of HBeAg-positive patients with chronic type B hepatitis have precore stop mutant detectable in serum. Of these, the majority (80%) of patients have coexisting predominant wild-type HBV, but 20% have predominant or exclusive precore mutant. Precore mutant ratios tend to correlate with ALT and HBeAg-to-anti-HBe seroconversion. High ratios (>50%) of precore mutant in HBeAg-positive patients correlate significantly with persistent high viremia and ALT elevation after anti-HBe seroconversion and thus are associated with an increased risk of cirrhosis. For patients who experience remission of liver disease after anti-HBe seroconversion, precore mutant ratios at baseline or after anti-HBe seroconversion do not help in predicting relapse of hepatitis.
Acknowledgments
This study was supported by grant NSC88-2315-B-182-005 from the National Science of Council of Republic of China.
We thank W. C. Shyu and L. H. Lu for excellent technical assistance and S. C. Chen for preparing the manuscript.
REFERENCES
- 1.Akahane, Y., T. Yamanaka, H. Suzuki, Y. Sugri, F. Tsuda, S. Yotsumoto, S. Omi, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1990. Chronic active hepatitis with hepatitis B virus DNA and antibody against e antigen in the serum. Gastroenterology 90:1113–1119. [DOI] [PubMed] [Google Scholar]
- 2.Bonino, F., F. Rosina, M. Rizzetto, R. Rizzi, E. Chiaberge, R. Tardanico, F. Callea, and G. Verme. 1986. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology 90:1268–1273. [DOI] [PubMed] [Google Scholar]
- 3.Brook, M. G., P. Karayiannis, and H. C. Thomas. 1989. Which patients with chronic hepatitis B virus infection will respond to alpha-interferon therapy? A statistical analysis of predictive factors. Hepatology 10:761–763. [DOI] [PubMed] [Google Scholar]
- 4.Brunetto, M. R., M. Stemler, F. Schodel, H. Will, A. Ottobrelli, M. Rizzetto, G. Verme, and F. Bonino. 1989. Identification of HBV variants which cannot produce precore-derived HBeAg and may be responsible for severe hepatitis. Ital. J. Gastroenterol. 21:151–154. [Google Scholar]
- 5.Brunetto, M. R., M. Giarin, G. Saracco, F. Oliver, P. Calvo, G. Capra, A. Randone, M. L. Abate, P. Manzini, and M. Capalbo. 1993. Hepatitis B virus unable to secrete e antigen and response to interferon in chronic hepatitis B. Gastroenterology 105:846–850. [DOI] [PubMed] [Google Scholar]
- 6.Brunetto, M. R., M. M. Giarin, F. Oliveri, E. Chiaberge, M. Baldi, A. Alfarano, A. Serra, G. Saracco, G. Verme, H. Will, and F. Bonino. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl. Acad. Sci. USA 88:4186–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. McGarvey, M. J. Makris, and H. C. Thomas. 1989. Mutation preventing formation of e antigen in patients with chronic HBV infection. Lancet ii:588–591. [DOI] [PubMed] [Google Scholar]
- 8.Chang, C., G. Enders, R. Sprengel, N. Peters, H. E. Varmas, and D. Ganem. 1987. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J. Virol. 61:3322–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, H. S., M. C. Kew, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Parcell, and R. H. Miller. 1992. The precore gene of the woodchuck hepatitis B virus genome is not essential for viral replication in the natural host. J. Virol. 66:5682–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu, C. M., P. Karayiannis, M. J. F. Fowler, J. Monjardino, Y. F. Liaw, and H. C. Thomas. 1985. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum. Hepatology 5:431–434. [DOI] [PubMed] [Google Scholar]
- 11.Chu, C. M., C. T. Yeh, C. T. Chiu, I. S. Sheen, and Y. F. Liaw. 1996. Precore mutant of hepatitis B virus prevails in acute and chronic infections in an area in which hepatitis B is endemic. J. Clin. Microbiol. 34:1815–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, G. L., J. H. Hoofnagle, and J. G. Waggoner. 1984. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology 86:230–235. [PubMed] [Google Scholar]
- 13.Fattovich, G., G. McIntyre, M. Thursz, K. Colman, G. Giuliano, A. Alberti, H. C. Thomas, and W. F. Carman. Hepatitis B virus precore/core variation and interferon therapy. Hepatology 22:1355–1362. [PubMed]
- 14.Fiordalisi, G., E. Cariani, G. Mantero, A. Zanetti, E. Tanzi, M. Chiaramonte, and D. Primi. 1990. High genomic variability in the pre-C region of hepatitis B virus in anti-HBe, HBV DNA-positive chronic hepatitis. J. Med. Virol. 31:297–300. [DOI] [PubMed] [Google Scholar]
- 15.Hadziyannis, S. J., H. M. Lieberman, G. G. Karvountzis, and D. A. Shafrits. 1983. Analysis of nuclear HBcAg, viral replication and hepatitis B virus DNA in liver and serum of HBeAg vs. anti-HBe positive carriers of hepatitis B virus. Hepatology 3:656–662. [DOI] [PubMed] [Google Scholar]
- 16.Hadziyannis, S. J. 1995. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognization to pathogenesis and treatment. Viral Hepatitis Rev. 1:7–36. [Google Scholar]
- 17.Hamasaki, K., K. Nakata, Y. Nagayama, A. Ohtsurau, M. Daikoku, K. Taniguchi, T. Tsutsumi, Y. Sato, Y. Kato, and S. Nagataki. 1994. Changes in the prevalence of HBeAg-negative mutant hepatitis B virus during the course of chronic hepatitis B. Hepatology 20:8–14. [DOI] [PubMed] [Google Scholar]
- 18.Hoofnagle, J. H., G. M. Dusheiko, L. B. Seeff, A. Jones, J. G. Waggoner, and Z. B. Bales. 1981. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann. Intern. Med. 94:744–748. [DOI] [PubMed] [Google Scholar]
- 19.Liaw, Y. F., C. M. Chu, I. J. Su, M. J. Huang, D. Y. Lin, and C. S. Chang-Chien. 1983. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology 84:216–219. [PubMed] [Google Scholar]
- 20.Liaw, Y. F., D. I. Tai, C. M. Chu, C. C. Pao, and T. J. Chen. 1987. Acute exacerbation in chronic type B hepatitis: comparison between HBeAg and antibody-positive patients. Hepatology 7:20–23. [DOI] [PubMed] [Google Scholar]
- 21.Liaw, Y. F. 1997. Current therapeutic trends in therapy for chronic viral hepatitis. J. Gastroenterol. Hepatol. 12:S346–S353. [DOI] [PubMed] [Google Scholar]
- 22.Lindh, M., Y. Furuta, K. K. Ljunggren, G. Norkans, and P. Horal. 1995. Detection of hepatitis B virus precore TAG mutant by an amplification-created restriction site method. J. Infect. Dis. 171:194–197. [DOI] [PubMed] [Google Scholar]
- 23.Lok, A. S. F., S. J. Hadziyannis, I. V. D. Weller, and H. C. Thomas. 1984. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut 25:1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lok, A. S. F., C. L. Lai, P. C. Wu, E. K. Leung, and T. S. Lam. 1987. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology 92:1839–1843. [DOI] [PubMed] [Google Scholar]
- 25.Lok, A. S. F., U. S. Akarla, and S. Greene. 1995. Predictive value of precore hepatitis B virus mutations in spontaneous and interferon-induced hepatitis B e antigen clearance. Hepatology 21:19–24. [PubMed] [Google Scholar]
- 26.Naoumov, N. V., R. Schneider, T. Grotziner, M. C. Jung, S. Miska, G. R. Pape, and H. Will. 1992. Precore mutant hepatitis B virus infection and liver disease. Gastroenterology 102:538–543. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, X. T., R. Fukuda, and S. Fukumoto. 1994. Precore region mutation in hepatitis B virus genome in early stage of infection: a study in hepatitis B e antigen-positive young carriers. J. Gastroenterol. 29:469–473. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto, H., S. Yotsumoto, Y. Akahane, T. Yamanaka, Y. Miyazaki, Y. Sugai, F. Tsuda, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1990. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to antibody against e antigen. J. Virol. 64:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuse, C., M. Takatori, and S. Iwabuchi. 1997. Quantitative analysis of serum HBV pre-core mutant in HBV carriers. Acta Hepatol. Jpn. 38:60–66. [Google Scholar]
- 30.Perillo, R. P., E. R. Schiff, and G. L. Davis. 1990. A randomized controlled trial of interferon alfa-2b alone and after prednisolone withdrawal for the treatment of chronic hepatitis B. N. Engl. J. Med. 323:295–301. [DOI] [PubMed] [Google Scholar]
- 31.Realdi, G., A. Alberti, F. Rugge, F. Bortolotti, A. M. Rigoli, F. Tremolada, and A. Ruol. 1980. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B infection. Gastroenterology 79:195–199. [PubMed] [Google Scholar]
- 32.Santantonio, T., M. C. Jung, S. Miska, G. Pastore, G. R. Pape, and H. Will. 1991. High prevalence and heterogeneity of HBV preC mutants in anti-HBe-positive carriers with chronic liver disease in Southern Italy. J. Hepatol. 13:S78–S81. [DOI] [PubMed] [Google Scholar]
- 33.Sung, J. L., D. S. Chen, M. Y. Lai, J. Y. Yu, T. H. Wang, C. Y. Wang, C. Y. Lee, S. H. Chen, and T. M. Ko. 1984. Epidemiological study on hepatitis B virus infection in Taiwan. Chin. J. Gastroenterol. 1:1–9. [Google Scholar]
- 34.Takeda, K., Y. Akahane, H. Suzuki, H. Okamoto, F. Tsuda, Y. Miyakawa, and M. Mayumi. 1990. Defects in the precore region of the HBV genome in patients with chronic hepatitis B after sustained seroconversion from HBeAg to anti-HBe induced spontaneously or with interferon therapy. Hepatology 12:1284–1289. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, H. C. 1995. The emergence of envelope and precore/core variants of hepatitis B virus: the potential role of antibody selection. J. Hepatol. 22(Suppl. 1):1–8. [PubMed] [Google Scholar]
- 36.Tong, S., J. Li, L. Vitvitski, and C. Trepo. 1990. Active hepatitis B virus replication in the presence of anti-HBe is associated with viral variants containing an inactive pre-C region. Virology 176:596–603. [DOI] [PubMed] [Google Scholar]
- 37.Tur-Kaspa, R., A. Klein, and S. Aharonson. 1992. Hepatitis B virus precore mutants are identical in carriers from various ethnic origins and are associated with a range of liver disease severity. Hepatology 16:1338–1342. [DOI] [PubMed] [Google Scholar]
- 38.Xu, J., D. Brown, T. Harrison, Y. Lin, and G. Dusheiko. 1992. Absence of hepatitis B virus precore mutants in patients with chronic hepatitis B responding to interferon-alpha. J. Hepatol. 15:1002–1006. [DOI] [PubMed] [Google Scholar]
- 39.Yeh, C. T., C. M. Chu, and Y. F. Liaw. 1998. Progression of the proportion of hepatitis B virus precore stop mutant following acute superinfection of hepatitis C. J. Gastroenterol. Hepatol. 13:19–24. [DOI] [PubMed] [Google Scholar]