Abstract
The glycoprotein B (gB) is highly conserved among distinct human herpesvirus 7 (HHV-7) strains. Similarly to other herpesvirus glycoproteins, gB has been assumed to induce a specific human immune response. However, it did not appear as an immunodominant protein in conventional immunoblot assays. Recombinant gB, obtained from either Escherichia coli or baculovirus expression systems, did react specifically with HHV-7-seropositive sera, and the main corresponding epitopes were located in its N-terminal part. A 24-amino-acid peptide, corresponding to a predicted hydrophilicity peak and presenting no extensive homology with other betaherpesvirus glycoproteins, was selected in this region at positions 129 to 152 of the gB sequence. When tested by enzyme-linked immunosorbent assay (ELISA), this peptide specifically reacted with HHV-7-seropositive sera. This reactivity was significantly inhibited by the preincubation of sera with the peptide itself, lysates of gB-expressing cells, or lysates of HHV-7-infected cells. The reactivity was not significantly modified when sera were preincubated with lysates of either human cytomegalovirus (HCMV)- or HHV-6-infected cells. In cross-sectional studies including both children and adults, 49 out of 61 serum samples (80%) were found to be positive by HHV-7 ELISA, independent of their reactivity to HCMV. A longitudinal serological study of 17 children during the first 4 years of life showed that the level of ELISA-detected antibodies significantly decreased within a few weeks after birth and then increased in the following months, likely reflecting, respectively, the loss of maternal antibodies and the occurrence of seroconversion. These results demonstrate that gB peptide ELISA might be a useful tool for the serological study of HHV-7 infection.
Human herpesvirus 7 (HHV-7) is a recently recognized herpesvirus, isolated initially from the peripheral blood lymphocytes of healthy individuals (18). HHV-7 prevalently infects individuals at young ages (1, 24, 28, 47, 48) and persistently infects CD4+ T lymphocytes (2, 19, 25) as well as salivary glands (31). The virus is excreted in saliva (21, 46), which is the most likely route of transmission. The natural history of HHV-7 infection in the human host is not well known. Primary infection has not yet been consistently associated with a given disease, and the potential association of HHV-7 reactivation with diseases in adults remains to be confirmed (11, 22, 32, 33, 40, 43, 45).
These pending questions point out the need for relevant diagnostic tools targeted to HHV-7 infection. So far, the serological diagnosis of HHV-7 infection has raised concerns about its specificity (3, 14, 38) because HHV-7 has marked homologies with the two other human betaherpesviruses, HHV-6 and, to a lesser extent, human cytomegalovirus (HCMV). The cross-reactive human antibodies have to be removed by preadsorption with heterologous virus antigens, which strongly affects the feasibility, reproducibility, and sensitivity of serological HHV-7 assays. Several attempts have recently been made to identify specific HHV-7 proteins with the aim of developing recombinant diagnostic reagents. As examples, the HHV-7 glycoprotein gp65 homologous to the HHV-6 glycoprotein gp82-105 and the proteins pp85 and p86, encoded by the HHV-7 U14 and U11 genes, respectively, have been investigated (15, 36, 37, 38). In particular, the phosphoprotein pp85 was assumed to be a good candidate because it is an immunodominant protein in immunoblot analyses, which, albeit cumbersome, remain the most specific approach of HHV-7 serology. An HHV-7-specific epitope was mapped to the C-terminal region of pp85, but the development of a readily accessible enzyme-linked immunosorbent assay (ELISA) with peptides carrying this epitope has not yet been successful (38).
All human herpesvirus genomes encode a subset of glycoproteins exposed on the envelope of the virus and the surface of infected cells. These proteins mediate entry of the virus into the cells and the cell-to-cell spread of the infection, which in turn influences both tissue tropism and host range. Moreover, herpesvirus glycoproteins are important elicitors of a protective immune response, as demonstrated for the glycoprotein B (gB) of the herpes simplex virus, varicella-zoster virus, and HCMV (6, 30, 42). Accordingly, glycoproteins are considered to be the best candidates for subunit vaccines and seem appropriate for the development of specific reagents dedicated to the study of HHV-7-specific immune response. The knowledge of biochemical and immunological properties of HHV-7-encoded glycoproteins remains preliminary, mainly because of difficulties in virus culture and scarcity of specific antibodies. However, the genomes of two HHV-7 strains (JI and RK) have been entirely sequenced (26, 27), and the gene encoding gB has been identified (20). In addition, we have previously shown that this sequence is highly conserved among distinct HHV-7 strains (16, 17). HHV-7 gB is a protein consisting of 822 amino acids and shows features characteristic of type I transmembrane proteins such as the gB of other betaherpesviruses (26, 35). These proteins are cleaved into two proteolytic digestion products of 64 and 58 kDa, respectively, in the case of HHV-6 and 58 and 116 kDa in the case of HCMV (5, 12). They have been found to carry species- or subtype-specific epitopes: an HHV-6A-specific epitope was recognized by a monoclonal antibody in a complement-independent neutralizing assay (9, 39); neutralizing antibodies binding to epitopes of HCMV gB have been reported (6, 29, 30, 42, 44).
All these results led us to hypothesize that HHV-7 gB elicited a detectable immune response in HHV-7-infected subjects and was a candidate for the development of HHV-7-specific serological assays. The results we present here indicate that HHV-7-seropositive sera react with recombinant gB and that a gB-derived peptide, located in the N-terminal part of the protein, appears to be a suitable antigenic reagent for HHV-7-specific ELISA.
MATERIALS AND METHODS
Cells and viruses.
SupT1 cells were grown in RPMI 1640 medium (Gibco-BRL, Paisley, United Kingdom) supplemented with 10% fetal calf serum (Gibco-BRL) according to published procedures (8, 34). SupT1 cells were infected with the HHV-7 SB strain as described previously (4). At different times postinfection, HHV-7 replication was monitored by the quantitation of HHV-7 DNA using endpoint dilution PCR with the primer pair HV7-HV8 (2). For this purpose, 106 cells were resuspended in 200 μl of phosphate-buffered saline (PBS) and the DNA was extracted by the Qiamp Blood kit (Qiagen, San Diego, Calif.). Fifteen microliters of PCR products was tested by Southern blot hybridization using the 32P-labeled oligonucleotide probe HV9 according to the method described elsewhere (2). When the hybridization signal was positive at the cell DNA dilution of 1:104 or lower, the culture was harvested, washed with sterile PBS, and used for immunoblotting assays. Wild-type Autographa californica nuclear polyhedrosis virus and derived recombinant viruses were used at a multiplicity of infection of approximately 10 PFU per cell to infect confluent monolayers of Sf21 cells. These cells were propagated in culture at 28°C in Grace’s medium (Gibco-BRL) supplemented with 5% heat-inactivated fetal calf serum.
Sera.
Six HHV-7-seronegative sera, designated N1 to N6, had been obtained from Japanese infants, and their seronegative status had been determined by means of both HHV-7-specific immunofluorescence and Western blot assays. Eight HHV-7-seropositive sera, designated P1 to P8, had been obtained from healthy French adults, and their seropositive status had been determined using HHV-7-specific Western blot assay. These samples were used in both HHV-7 immunoblotting and ELISA experiments as reference sera (see below). Twenty additional adult serum samples submitted for routine virological studies in the laboratory of Virology of Pitié-Salpêtrière Hospital were collected for the second step of the HHV-7 ELISA study. One hundred and seven samples from 61 infants and young children (from birth up to 5 years old) that had been routinely submitted for virological diagnostic procedures in the laboratory of virology of Trousseau Hospital were also tested by ELISA: 41 samples from distinct children were analyzed within a cross-sectional study; 66 sequential samples from 17 other distinct children, born from human immunodeficiency virus (HIV)-positive mothers and found uninfected by HIV at the end of their follow-up, were tested within a retrospective longitudinal study.
Production of recombinant proteins in bacteria and insect cells.
For the expression of gB in bacteria, the construct was generated in the pTrcHisC vector, carrying an N-terminal polyhistidine epitope tag (Invitrogen Corporation, Carlsbad, Calif.). The amplification of the HHV-7 sequence coding for the gB gene was performed using two primers derived from the nucleotide sequence of the HHV-7 strain JI (27): gB1Kpn (5′-acacggtaccATGAAAATTCTATTCCTTGAGTG-3′, nucleotides [nt] 56849 to 56871) and gB2Eco (5′-acacgaattcTCACAGTTCTTCTGTTGA-3′, nt 54401 to 54418); the lowercase letters denote nonviral sequences, while uppercase letters represent gB-specific sequences, and underlined residues are restriction sites. The final construct was designated pTr-gB-rec and contained the entire U39 open reading frame (ORF), 2,488 bp long, encoding a protein of 822 amino acids. Escherichia coli cells were grown in Luria-Bertani medium, and protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) overnight. For the expression of gB in Sf21 cells, two constructs were generated in the pBlueBacHis2A vector (Invitrogen Corporation), also carrying an N-terminal polyhistidine epitope tag. One of the constructs contained the entire U39 ORF and was designated Bac-gB-rec. The amplification of the insert was done using the primers gB1Bam (5′-acacggatccATGAAAATTCTATTCCTTGAGTG-3′; nt 56849 to 56871) and gB2Sal (5′-acacgtcgacTCACAGTTCTTCTGTTGA-3′; nt 54401 to 54418). The second construct contained the portion of U39 ORF coding for the N-terminal fragment of the gB (amino acids 1 to 396) and was designated Bac-gB.N-rec. The amplification of this portion of gB gene was carried out with the primers gB1Bam and gB2StopSal (5′-acacgtcgactcaCCTCCTTTTTCTAGAAGC-3′ [including an artificial translation stop codon denoted in boldface type], nt 55683 to 55700). The corresponding recombinant baculoviruses were generated and plaque purified using the Bac-N-Blue system (Invitrogen Corporation). The presence of specific HHV-7 insert sequences in purified virus clones was checked by PCR and nucleotide sequencing.
Immunofluorescence assay.
Sf21 cells were infected with A. california nuclear polyhedrosis virus, Bac-gB-rec, or Bac-gB.N-rec viruses for 72 h at 28°C. The cells were fixed and permeabilized in acetone for 10 min. Cells were then incubated for 30 min at 37°C with a mouse polyhistidine-specific antibody (Qiagen). Anti-mouse immunoglobulin (IgG) conjugated with fluorescein isothiocyanate was used to detect the binding of primary antibody.
Preparation of cell lysates.
HHV-7-infected SupT1 cells were harvested by centrifugation, washed three times in cold PBS, and lysed on ice in RIPA buffer (1 M Tris-HCl, pH 7.5; 150 mM NaCl; 10% sodium dodecyl sulfate [SDS]; 10% NP-40; 1% sodium deoxycholate supplemented with phenylmethylsulfonyl fluoride [20 μg/ml]). The bacteria were harvested by centrifugation and lysed on ice in lysis buffer (10 mM Tris-HCl, pH 7.5; 100 mM NaCl; 5 mM EDTA; 10 mM β-mercaptoethanol; 0.2 % Triton X-100; lysozyme [1 mg/ml]; DNase I [10 mg/ml]; RNase A [10 μg/ml]). Baculovirus-infected Sf21 cells expressing recombinant gB were washed twice with cold PBS and lysed on ice in IPB buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 10 mM EDTA, pH 8.0; 0.1% SDS; 1% Triton X-100; 1% sodium deoxycholate; aprotinin [2 μg/ml]) for at least 15 min (13).
Immunoblotting assays with cells lysates.
Immunoblotting was performed after proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose using the semidry technique. Membranes were then blocked for 30 min with blocking buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5% bovine serum albumin; 0.05% Tween 20) and incubated for 2 h at room temperature with either a mouse polyhistidine-specific monoclonal antibody or a 1:200 dilution of human sera in blotting buffer. The membrane was washed three times for 5 min in washing buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl) and incubated for another 2 h at room temperature with either an anti-mouse IgG conjugated with biotin or a 1:1,000 dilution of biotinylated anti-human antibody in blotting buffer. Avidin-biotinylated alkaline phosphatase complex (Vectastain ABC-AP kit; Vector Laboratories, Burlingame, Calif.) was added for 30 min. Positive bands were visualized after addition of 5-bromo-4-chloro-3-indoyl phosphate and nitroblue tetrazolium as substrates.
Reactivity of human sera with a synthetic peptide.
A synthetic peptide (Leu-Ser-Ser-Ile-Ser-Val-Lys-Arg-Ser-Glu-Glu-Glu-Glu-Tyr-Val-Ala-Tyr-His-Lys-Asp-Glu-Tyr-Val-Asn) corresponding to amino acid residues 129 to 152 of gB was obtained (Neosystem, Strasbourg, France) and designated H7GB129-152. ELISA microplates were coated with the peptide (100 μl per well of 0.5-μg/ml peptide solution in 0.05 M bicarbonate buffer, pH 9.6) by incubation for 20 h at 37°C. The wells were washed three times with PBS containing 0.5% Tween 20, and the nonspecific binding sites of the wells were blocked with PBS containing 2% newborn calf serum by incubation for 45 min at 37°C. Serum samples were diluted 1:100 in 0.01 M sodium phosphate buffer, pH 7.4, containing 0.75 M NaCl, 1% of newborn calf serum, and 0.05% Tween 20. Aliquots (100 μl) of diluted sera were added to each well and incubated for 30 min at room temperature, after which the wells were washed five times with PBS containing 0.5% Tween 20. One hundred microliters of goat F(ab′)2 anti-human IgG peroxidase-conjugated antibodies (TAGO, Burlingame, Calif.) diluted 1:5,000 was added, and plates were incubated for 30 min at room temperature. After three washings with PBS-0.5% Tween 20, the reaction was revealed by incubation with hydrogen peroxide-o-phenylenediamine (H2O2) for 15 min at room temperature. The color development was stopped with 2 N H2SO4, and the optic density (OD) was read at 492 nm.
Standardization of ELISA.
The variability of ELISA was studied by comparing the OD results from sample replicate testing either within the same run (intra-assay variability) or from distinct runs performed on different days (interassay variability). This study was done by testing distilled water instead of human serum (blank reaction), HHV-7-seronegative reference sera (negative controls), and HHV-7-seropositive reference sera (positive controls). In each run, the cutoff was established as the mean OD of at least three independent wells using the same negative control (one of the HHV-7-seronegative reference sera), plus 3 standard deviations (SD). The coefficient of variability (CV) was defined as the ratio of SD to mean OD. The specificity of the reactivity obtained with the HHV-7-seropositive sera was addressed by three different approaches: (i) in the absence of any peptide coated in the wells or in the presence of an irrelevant peptide (derived from the HHV-8 glycoprotein K8.1); (ii) after the sera had been incubated with lysates of cells infected with HHV-7 (SupT1 cells), HHV-6 (MT4 cells), HCMV (human embryonic fibroblasts [HF]) or lysates of Sf21 cells infected with Bac-gB-rec; and (iii) reactivity after prior incubation of sera with soluble H7GB129-152 at the final concentrations of 5 and 50 μg/ml. The ODs obtained with human serum samples in cross-sectional as well as longitudinal studies were expressed as their ratio to the cutoff determined in each run; samples were considered seropositive when this ratio was equal to or greater than 1.
Statistical analysis.
The comparison of ODs for paired sera was performed using the nonparametric Wilcoxon’s rank sum test. A P of less than 0.05 was considered statistically significant.
RESULTS
Reactivity of human sera to lysates of HHV-7-infected cells.
The eight HHV-7-seropositive human samples (P1 to P8) and one HHV-7-seronegative sample (N1) were repeatedly tested with lysates of HHV-7-infected SupT1 cells. All eight seropositive sera reacted with a protein of an apparent molecular mass of 89 kDa while the seronegative serum did not (data not shown). In addition, the seropositive sera specifically reacted with some other proteins which were present in HHV-7-infected SupT1 cells and apparently absent in uninfected ones. These results confirmed the serological HHV-7 status of the reference sera used in the study. However, none of the seropositive sera reacted specifically with a putative protein exhibiting an apparent molecular mass close to that expected for gB or gB cleavage product (approximately 100 to 110 kDa and 50 kDa, respectively). Moreover, numerous proteins, most of them corresponding to proteins also present in uninfected Sup T1 cells, were found to react unspecifically with both seropositive and seronegative sera (not shown). These unspecific bands tended to obscure the readouts of immunoblots.
Reactivity of human sera to recombinant forms of gB.
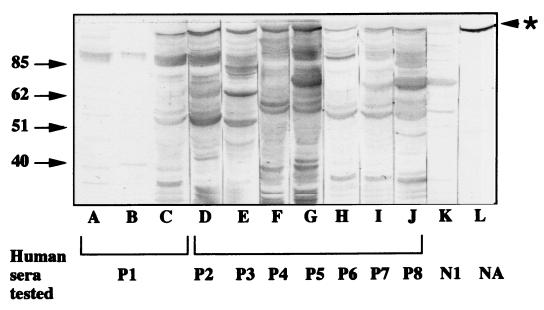
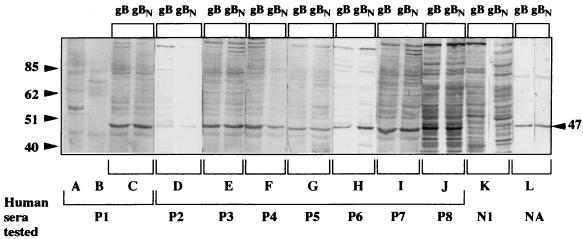
The limitations of immunoblot readout prompted us to test the reactivity of human sera against recombinant forms of gB. In a first step, the gB was expressed in bacteria after induction with IPTG overnight, thus providing the highest amount of protein in preliminary time course analyses. In immunoblot assays, all eight seropositive sera reacted with a protein of approximately 100 kDa (Fig. 1, lanes C to J), which was also recognized by the polyhistidine-specific mouse antibody and corresponded to the fusion protein polyhistidine-gB (Fig. 1, lane L). In contrast, the HHV-7-seronegative sera did not react with it, as exemplified in the case of N1 serum (Fig. 1, lane K). This result indicated that immune human sera contained gB-specific antibodies. However, the recombinant gB expressed in bacteria was not cleaved, as reflected by the absence of any protein fragment shorter than 100 kDa and carrying a polyhistidine-specific epitope (Fig. 1, lane L). In a second step, the gB was expressed in recombinant baculovirus-infected Sf21 cells. Two constructs carrying either the whole gB gene or a truncated form encoding amino acids 1 to 396 of the gB N-terminal part were used. Both constructs led to the prevailing expression of a fusion polyhistidine-gB protein of approximately 47 kDa which reacted with the polyhistidine-specific antibody (Fig. 2, lanes L) and appeared identical in both cases. Since the truncation of the gB gene had been designed to mimic the effects of gB cleavage at a canonic RKRR site, it was concluded that the expression of the whole gB gene in insect cells resulted in its cleavage at the expected site. Another protein with a higher molecular mass (about 80 kDa) consistently reacted with the polyhistidine-specific antibody, albeit to a much lower extent than the 47-kDa protein (Fig. 2, lanes L). The 80-kDa protein was not detected in either uninfected or wild-type baculovirus- infected cells (data not shown). It did not react with either HHV-7-seronegative or -seropositive sera (Fig. 2, lanes C to K), and its relationship with the predominant 47-kDa protein remained unclear. The eight HHV-7-seropositive reference sera did react with this N-terminal part of gB, obtained either by cellular cleavage of gB or by translation of the truncated gene (Fig. 2, lanes C to J), while seronegative sera did not (Fig. 2, lane K). This reactivity was absent when either uninfected Sf21 cells or cells infected with wild-type baculovirus were used as antigens (Fig. 2, lanes A and B, respectively). The N-terminal part of gB expressed in eucaryotic cells thus appeared to contain linear epitopes which remained accessible after denaturing SDS treatment and could react specifically with HHV-7-seropositive human sera. This reactivity was considered sufficiently potent and specific to permit the development of a serological routine assay. However, the great number of nonspecific bands observed with some sera (for instance, sera P7, P8, and N1 [Fig. 2]) as well as the difficulties of applying the immunoblot technique to large series of samples were obvious limitations for that project.
FIG. 1.
Immune reactivity of human sera to the recombinant gB expressed in bacteria. The recombinant protein gB was expressed in bacteria as a fusion protein with a polyhistidine tract at its N terminus. The proteins were separated by denaturing SDS-PAGE, transferred to a nitrocellulose sheet, and then allowed to react with distinct human sera (lanes C to K) or to the mouse antipolyhistidine antibody (lane L). In lanes A and B, P1 serum was tested against lysates of bacteria transformed with the plasmid pTrc-HisC containing no insert and the plasmid pTr-gB-rec in the absence of IPTG induction respectively, as negative controls. Numbers on the left of gel are in kilodaltons. The star to the right of gel indicates the position of recombinant gB. NA, not applicable.
FIG. 2.
Immune reactivity of human sera to the recombinant gB expressed in Sf21 insect cells. The recombinant proteins were expressed in Sf21 insect cells following infection with the recombinant baculoviruses Bac-gB-rec and Bac-gB.N-rec. The proteins, either the entire gB (gB) or its N-terminal part (gBN), were separated by denaturing SDS-PAGE, transferred to nitrocellulose, and then allowed to react with distinct human sera (lane pairs C to K) or the mouse antipolyhistidine antibody (lane pair L). In the lanes A and B, serum P1 was tested with either lysates of uninfected Sf21 or Sf21 cells infected with wild-type baculovirus, respectively, as negative controls. Numbers to the left and right of gel are in kilodaltons. NA, not applicable.
Reactivity of human sera to a peptide of HHV-7 gB.
In an attempt to obtain a purified antigen for the development of a routine assay, a peptide belonging to the N-terminal part of gB was selected. The criteria for the choice of this peptide were (i) a location within a region of high hydrophilicity, as predicted by the ProtScale program, and (ii) the absence of major homology with HHV-6 and HCMV gB. According to these criteria, the peptide H7GB129-152 was ultimately designed. Its sequence (24 residues) corresponding to amino acid residues 129 to 152 of HHV-7 gB included a predicted peak of hydrophilicity located on the central residues RSEEEE (amino acids 136 to 141) and absent from the gB sequence of other betaherpesviruses. The ELISA reactivity of this peptide in the absence of serum (blank reaction) provided weak background OD signals, with a mean value of 0.086 obtained when gathering the results of numerous independent assays (Table 1). The mean ODs obtained with the six HHV-7-seronegative reference sera were consistently higher than those in blank reactions but close to one another, ranging from 0.16 to 0.207. This low-OD signal was observed in spite of the fact that these sera had a high titer of HHV-6-specific antibodies, suggesting the absence of cross-reactivity of the H7GB129-152 peptide with these antibodies. Moreover, the variability of ELISA results was considered moderate (except in the case of serum N6) both in intra-assay and in interassay comparisons, with the CV ranging from 3.6 to 13.8%. This variability appeared to fit the development of a routine assay. When ELISA was applied to the eight seropositive reference sera (Table 2), ODs were significantly higher than those of seronegative sera and the derived cut-off within the same assay. The mean OD, computed from numerous independent assays, ranged from 0.489 (serum P2) to 1.18 (serum P3), with a mean cutoff of approximately 0.240. Again, the variability was low, with the interassay CV ranging from 6 to 14.6%.
TABLE 1.
Results of HHV-7-specific peptide-based ELISA with six reference HHV-7-seronegative sera
| Tested serum | Antibody titer
|
Intra-assay variabilitya
|
Interassay variabilitya
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HHV-6 | HHV-7 | No.b | OD
|
No. | OD
|
|||||
| Mean | SD | CV (%) | Mean | SD | CV (%) | |||||
| None (blank reaction) | NAc | NA | 8 | 0.089 | 0.063 | 7.1 | 14 | 0.086 | 0.015 | 17.1 |
| N1 | >1,280 | <10 | 4 | 0.199 | 0.019 | 9.5 | 5 | 0.207 | 0.018 | 8.8 |
| N2 | >1,280 | <10 | 4 | 0.174 | 0.014 | 8.3 | 3 | 0.160 | 0.020 | 13.8 |
| N3 | >1,280 | <10 | 3 | 0.182 | 0.006 | 3.3 | 2 | 0.177 | 0.006 | 3.6 |
| N4 | >1,280 | <10 | 3 | 0.173 | 0.019 | 11.0 | 2 | 0.173 | 0.019 | 11.0 |
| N5 | >1,280 | <10 | 4 | 0.183 | 0.024 | 13.2 | 2 | 0.177 | 0.008 | 4.8 |
| N6 | >1,280 | <10 | 3 | 0.217 | 0.097 | 44.7 | 2 | 0.162 | 0.077 | 47.0 |
The variability of OD signal was studied by comparing the values obtained from different wells of the same microplate within the same assay (intra-assay variability) and the values obtained in independent experiments (interassay variability).
Number of independent wells (intra-assay variability) or independent assays (interassay variability).
NA, not applicable.
TABLE 2.
Results of peptide ELISA with eight reference HHV-7-positive human sera
| ELISA parameter | Tested serum
|
|||||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
| Intra-assay variabilitya | ||||||||
| No.b | 2 | 2 | 4 | 2 | 2 | 4 | 4 | 2 |
| Mean OD | 1.107 | 0.563 | 0.960 | 0.598 | 0.692 | 1.009 | 0.642 | 0.885 |
| SD | 0.084 | 0.039 | 0.069 | 0.042 | 0.053 | 0.060 | 0.021 | 0.074 |
| CV (%) | 7.6 | 7.0 | 7.1 | 6.9 | 7.6 | 5.9 | 3.2 | 8.3 |
| Interassay variabilitya | ||||||||
| No.b | 8 | 13 | 11 | 7 | 7 | 7 | 7 | 8 |
| Mean OD | 1.142 | 0.489 | 1.188 | 0.669 | 0.698 | 1.091 | 0.717 | 0.866 |
| SD | 0.069 | 0.072 | 0.122 | 0.066 | 0.098 | 0.093 | 0.045 | 0.061 |
| CV (%) | 6.0 | 14.6 | 10.3 | 9.8 | 14.1 | 8.5 | 6.3 | 7.0 |
| OD in absence of H7GB129-152 | ||||||||
| No peptide | 0.052 | 0.085 | 0.075 | 0.062 | 0.063 | 0.061 | 0.053 | 0.044 |
| Irrelevant peptidec | 0.132 | 0.164 | 0.167 | 0.152 | 0.151 | 0.141 | 0.158 | 0.144 |
| % Reduction of OD following preincubation with cell lysates | ||||||||
| Uninfected SupT1 | 5 | 29 | 0 | 11 | 20 | 0 | 11 | 17 |
| HHV-7-infected SupT1 | 40 | 58 | 56 | 52 | 50 | 43 | 40 | 56 |
| Uninfected Sf21 | 11 | 22 | 0 | 0 | 17 | 0 | 0 | 0 |
| gB-expressing Sf21 | 46 | 66 | 30 | 26 | 57 | 26 | 60 | 42 |
| Uninfected MT4 | 20 | 2 | 4 | 10 | 13 | 7 | 11 | 0 |
| HHV-6-infected MT4 | 11 | 0 | 11 | 3 | 19 | 6 | 0 | 0 |
| Uninfected HF | 18 | 0 | 13 | 0 | 9 | 13 | 0 | 6 |
| HCMV-infected HF | 20 | 5 | 8 | 0 | 11 | 2 | 3 | 0 |
| % Reduction of OD following pre-incubation with H7GB129-152 at final concn (μg/ml) of: | ||||||||
| 5 | 27 | 30 | 32 | 48 | 14 | 23 | 37 | 24 |
| 50 | 75 | 45 | 66 | 61 | 70 | 67 | 63 | 61 |
The specificity of reactivity to H7GB129-152 peptide displayed by the eight seropositive sera was assessed by three additional distinct approaches (Table 2). First, the OD in the absence of the peptide was similar to that obtained in blank reactions while the reactivity to an irrelevant peptide (related to an HHV-8 glycoprotein and sharing no significant homology with H7GB129-152) was close to that observed with HHV-7 seronegative sera (see Table 1 for comparison). Second, the preliminary incubation of sera with cell lysates led to a significant reduction of reactivity to H7GB129-152 when these cells were infected with either HHV-7 or HHV-7 gB-expressing recombinant baculovirus. This reduction was absent or significantly lower when cells were either uninfected or infected with another betaherpesvirus (HHV-6 or HCMV). As an illustration, the median values of OD reduction for the panel of HHV-7-seropositive sera were 51 and 43% for HHV-7-infected SupT1 and HHV-7 gB-expressing Sf21 cells, respectively; 11, 0, 8.5, and 7.5% for uninfected SupT1, Sf21, MT4, and HF, respectively; 8.5 and 4% for HHV-6-infected MT4 cells and HCMV-infected HF, respectively. Third, the preincubation of sera with soluble H7GB129-152 at the final concentrations of 5 and 50 μg/ml led to a significant reduction of the OD signal (median reduction, 28.5 and 64.5%, respectively).
Application of ELISA to series of human serum samples.
Peptide ELISA was thus evaluated in a cross-sectional study of sera from adults and children whose serological HHV-7 status was unknown (Table 3). Among the 20 adult samples tested, 14 (70%) displayed an OD higher than cutoff and were therefore considered positive. For the six subjects considered negative, the range of ratios of OD to cutoff was 0.54 to 0.9. The rate of seropositivity did not seem to be influenced by either HCMV or HIV positivity, but the number of samples tested was too small to permit definite conclusions. These results confirmed the high rate of HHV-7 seropositivity observed among adults in other studies. However, among seropositive subjects, ODs were low (range of ratios of OD to cutoff, 1.12 to 1.97), suggesting either a low titer of antibodies directed to H7GB129–152 epitopes or a limited sensitivity of the assay. Taken together, these limitations might lead to an underestimation of seropositivity rate in this population group.
TABLE 3.
Results of peptide ELISA in cross-sectional studies
| Subject group | No. | Ratio of OD to cutoff
|
No. of sera with result above cutoff | |
|---|---|---|---|---|
| Median | Range | |||
| Adultsa | ||||
| HIV− HCMV− | 5 | 0.90 | 0.69–1.97 | 2 |
| HIV− HCMV+ | 5 | 1.56 | 0.54–1.70 | 4 |
| HIV+ HCMV− | 4 | 1.37 | 1.12–1.74 | 4 |
| HIV+ HCMV+ | 6 | 1.25 | 0.65–1.81 | 4 |
| Total | 20 | 1.25 | 0.54–1.97 | 14 |
| Children with indicated age (mo): | ||||
| 0–4 | 11 | 1.28 | 0.82–1.65 | 10 |
| 6–10 | 9 | 1.68 | 0.79–1.90 | 8 |
| 12–17 | 9 | 1.52 | 0.69–2.06 | 6 |
| 48–60 | 12 | 1.60 | 0.73–5.56 | 11 |
| Total | 41 | 1.42 | 0.69–5.56 | 35 |
Grouped according to HIV and HCMV status (+, positive; −, negative).
The overall rate of positivity among the 41 child samples tested was 85%, and the range of OD ratios was 0.69 to 5.56, which suggested a paradoxically higher frequency of HHV-7 infection and/or a greater intensity of immune response among young people. Surprisingly, the rate of positivity was not dramatically reduced among children of more than 6 months and less than 2 years of age, who were expected to have lost maternal antibodies but not yet acquired primary HHV-7 infection (Table 3). In order to clarify this apparent discrepancy with respect to the well-accepted schedule of HHV-7 infection, a longitudinal retrospective study was undertaken on the samples from 17 children for whom serial consecutive sera from birth to the 4th year of age were available. The overall results showed a significant decrease of OD signal after birth, followed by a significant reincrease starting from 1 month of age, as demonstrated by paired comparisons of sera obtained at different ages (Table 4). Accordingly, the rate of seropositivity, which was 100% at birth, dropped to 25% at 1 month of age and was followed by a reincrease to 100%. All together, these results suggested that, at least among the 17 children studied, the loss of maternal antibodies had been followed very early in the 1st year of life by the occurrence of primary infection.
TABLE 4.
Longitudinal analysis of ELISA reactivity in sequential samples from 17 children
| Agea (mo) | Ratio of OD to cutoff
|
% of samples with ratio of ≥1 | Paired comparisonb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | |||||||||
| 0–0.5 | 1.49 | 1.15–2.93 | 100 | X | X | X | ||||
| 0.5–1 | 0.88 | 0.6–1.96 | 25 | X | X | X | X | X | ||
| 1–3 | 1.04 | 0.74–2.5 | 54 | X | X | |||||
| 3–6 | 1.14 | 0.75–2.39 | 60 | X | X | |||||
| 6–12 | 1.51 | 1.09–2.89 | 100 | X | ||||||
| 12–48 | 1.6 | 1.0–2.85 | 100 | X | ||||||
| P for paired comparisons | NAc | NA | NA | 0.04 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | 0.04 |
Age expressed as intervals of time after birth, with the upper limit being excluded and birth noted as 0.
Xs show which age groups were compared. Indicated paired comparisons were performed using Wilcoxon’s rank sum test. Only significant differences are indicated (P < 0.05).
NA, not applicable.
DISCUSSION
The development of HHV-7-specific serological assays faces several obstacles. The marked genetic homology of HHV-7 with HHV-6, and, to a lesser extent, HCMV leads to the existence of common epitopes generating immune cross-reactivity. Moreover, the infections with the three distinct betaherpesviruses do coexist very frequently within the same individual, due to the high prevalence of each infection. To date, the most specific assay for detection of HHV-7 antibodies is immunoblot combined with preabsorption of sera with heterologous HHV-6 antigens, but it is, in many aspects, a cumbersome procedure. An HHV-7-specific epitope was recently defined in the tegument phosphoprotein pp85 and might constitute an elegant alternative to the use of crude infected cell lysates as antigens, but the peptide carrying this epitope could not be successfully used to develop an HHV-7-specific ELISA. The lack of a readily accessible specific assay is indeed detrimental for the diagnosis and understanding of HHV-7 infection, especially in situations of either co- or superinfection with HHV-6 and/or HCMV.
The results reported in this paper demonstrate that HHV-7 gB is a convenient target for the development of a specific ELISA. Although gB was poorly recognized by human sera in immunoblots prepared from crude infected cell lysates, this protein offers many theoretical advantages: (i) gB is highly conserved among HHV-7 strains; (ii) herpesvirus glycoproteins are generally good immune targets; (iii) the antibodies directed to glycoproteins are species specific and even, for some of their epitopes, type or subtype specific. Of note, this narrow immune specificity, which might be a limiting factor in terms of overall assay sensitivity, is not of concern in the case of HHV-7 due to the high genetic conservation of the virus, which does not include any characterized type or subtype. Experimentally, HHV-7-seropositive human sera reacted with HHV-7 gB expressed in bacteria although the protein was not cleaved and subjected to eucaryotic posttranslation modifications. This suggested the existence of simple linear epitopes as targets of HHV-7-specific serum antibodies. These results were confirmed by the reactivity of human sera to gB expressed in baculovirus-infected Sf21 insect cells and subjected to SDS-PAGE. The reactivity of the entire gB from Sf21 cells seemed to be focused on the N-terminal part of the protein obtained by spontaneous cleavage. A truncated gB gene coding for this part of gB permitted the expression of a protein which was indistinguishable from the spontaneously cleaved gB in SDS-PAGE and reacted identically with the eight HHV-7-seropositive reference sera. Again, this supported the idea of using some putative linear gB epitopes for the development of HHV-7-specific serological assays.
The choice of H7GB129-152 peptide first emerged from theoretical considerations rather than a precise epitope mapping. This peptide displays a limited homology with the gB sequences of HHV-6A, HHV-6B, and HCMV. The maximum number of strictly homologous consecutive amino acid residues is four, corresponding to the motif VAYH present both in HHV-7 and HCMV sequences. The peptide H7GB129-152 contains in its central part the original motif RSEEEE, which is specific to HHV-7 and corresponds to a major peak of hydrophilicity. When tested in ELISA with different human sera, this peptide provided a low background signal in blank reactions, a low nonspecific signal with HHV-7-seronegative samples, and a clear specific signal with HHV-7-seropositive samples. The OD signal did not seem adversely affected by the presence of either HHV-6 or HCMV antibodies. In contrast, this signal was absent when the coating of wells was done with an irrelevant peptide and significantly reduced when the reactivity of HHV-7 antibodies was partially blocked by preincubation with either extracts of cells expressing gB or the soluble form of the peptide. All the data converge to demonstrate that peptide ELISA is specific to HHV-7 antibodies and circumvents, to a great extent, many problems linked to cross-reactivity. The application of the peptide ELISA to human sera on a larger scale confirmed its validity. Although the specific signal was apparently lower than for children, the data confirmed the high prevalence of HHV-7 infection among the general adult population. The decrease of gB-specific antibodies with age, if confirmed by larger studies, will require further investigation but fits the data reported for other herpesviruses (30). More importantly in our opinion, the peptide ELISA was able to detect the disappearance of maternal antibodies after birth and their further reacquisition. Among the 17 children studied retrospectively, the loss of maternal antibodies was rapid, and the rate of seropositivity was 100% at the end of the first year of life, which is surprising since primary HHV-7 infection is classically assumed to occur later (7, 10, 23, 41, 47, 48). This group was the result of a particular selection consisting of children born from HIV-positive mothers but ultimately found to be uninfected by HIV. These children, who were carefully followed up in a hospital setting because of the risk of HIV infection, might have been exposed to HHV-7 earlier than healthy children in the general population. Whatever the explanation of this apparently earlier seroconversion may be, these results again illustrate the capacity of peptide assay to detect HHV-7 antibodies in a monospecific manner. This original property, which can be strengthened by technical improvements of ELISA procedures, might offer the opportunity to revisit the story of HHV-7 infection and its consequences in human pathology.
Acknowledgments
We very much thank Philip E. Pellett for the gift of the HHV-7 SB strain and SupT1 cells, Vincent Calvez for stimulating discussions about herpesvirus serology, Francis Barin for helpful advice in the design of the ELISA, Vincent Deubel for help in the handling of the baculovirus expression system, and Susan Orsoni for revising the English manuscript.
This work was supported in part by the Association Claude Bernard, the Action Concertée Coordonnée des Sciences du Vivant of the French Ministry of Research, the Association pour la Recherche sur le Cancer, and MRTC research grant 97-5-12172 to Michaël Franti. Haruhiko Kosuge was supported by a grant from the Japan Herpesvirus Infections Forum.
REFERENCES
- 1.Asano, T., S. Suga, T. Yoshikawa, T. Yasaki, and T. Uchikawa. 1995. Clinical features and viral excretion in an infant with primary human herpesvirus 7 infection. Pediatrics 95:187–190. [PubMed] [Google Scholar]
- 2.Berneman, Z. N., D. V. Ablashi, G. Lee, M. Eger-Fletcher, M. S. Reitz, C. L. Hung, I. Brus, A. L. Komaroff, and R. C. Gallo. 1992. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc. Natl. Acad. Sci. USA 89:10552–10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, J. B., T. F. Schwarz, J. L. Patton, K. Kite-Powell, P. E. Pellett, S. Wiersbitzky, R. Bruns, C. Muller, G. Jager, and J. A. Stewart. 1996. Evaluation of immunoassays for detection of antibodies to human herpesvirus 7. Clin. Diagn. Lab. Immunol. 3:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, J. B., D. A. Burns, C. S. Goldsmith, P. M. Feorino, K. Kite-Powell, R. F. Schinazi, P. W. Krug, and P. E. Pellett. 1997. Biologic properties of human herpesvirus 7. Virus Res. 52:25–41. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. J., and L. G. Vugler. 1989. Processing of the gp55–116 envelope glycoprotein complex (gB) of human cytomegalovirus. J. Virol. 63:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J., L. G. Vugler, E. J. Butfiloski, and E. B. Stephens. 1990. Cell surface expression of human cytomegalovirus gp55–116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis on the human neutralizing antibody response. J. Virol. 64:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cermelli, C., G. Fabio, M. Montorsi, A. M. Sabbatini, and M. Portolani. 1996. Prevalence of antibodies to human herpesviruses 6 and 7 in early infancy and age at primary infection. New Microbiol. 19:1–8. [PubMed] [Google Scholar]
- 8.Cermelli, C., P. Pietrosemoli, M. Meacci, M. Pecorari, A. M. Sabbatini, B. Colombari, and M. Portolani. 1997. SupT-1: a cell system suitable for an efficient propagation of both HHV-7 and HHV-6 variants A and B. New Microbiol. 20:187–196. [PubMed] [Google Scholar]
- 9.Chandran B., S. Tirawatnapong, B. Pfeiffer, and D. V. Ablashi. 1992. Antigenic relationships among human herpesvirus 6 isolates. J. Med. Virol. 37:247–253. [DOI] [PubMed] [Google Scholar]
- 10.Clark, D. A., M. L. Freeland, L. K. Mackie, R. F. Jarrett, and D. E. Onions. 1993. Prevalence of antibody to human herpesvirus 7 by age. J. Infect. Dis. 168:251–252. [DOI] [PubMed] [Google Scholar]
- 11.Drago, F., E. Ranieri, and A. Rebora. 1998. Pityriasis rosea and herpesvirus 7: action or interaction? Dermatology 197:275. [PubMed] [Google Scholar]
- 12.Ellinger K., F. Neipel, and L. Foa-Tomasi. 1993. The glycoprotein B homologue of human herpesvirus 6. J. Gen. Virol. 74:495–490. [DOI] [PubMed] [Google Scholar]
- 13.Flamand M, V. Deubel, and M. Girard. 1992. Expression and secretion of Japanese encephalitis virus nonstructural protein NS1 by insect cells using a recombinant baculovirus. Virology 191:826–836. [DOI] [PubMed] [Google Scholar]
- 14.Foa-Tomasi, L., E. Avitabile, L. Ke, and G. Campadelli-Fiume. 1994. Polyvalent and monoclonal antibodies identify major immunogenic proteins specific for human herpesvirus 7-infected cells and have weak cross-reactivity with human herpesvirus 6. J. Gen. Virol. 75:2719–2727. [DOI] [PubMed] [Google Scholar]
- 15.Foa-Tomasi, L., M. P. Fiorilli, E. Avitabile, and G. Campadelli-Fiume. 1996. Identification of an 85 kDa phosphoprotein as an immunodominant protein specific for human herpesvirus 7-infected cells. J. Gen. Virol. 77:511–518. [DOI] [PubMed] [Google Scholar]
- 16.Franti, M., J. T. Aubin, L. Poirel, A. Gautheret-Dejean, D. Candotti, J. M. Huraux, and H. Agut. 1998. Definition and distribution analysis of glycoprotein B gene alleles of human herpesvirus 7. J. Virol. 72:8725–8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franti, M., J. T. Aubin, L. Poirel, A. Gautheret-Dejean, I. Malet, A Cahour, J. M. Huraux, and H. Agut. 1999. Preferential associations of alleles of three distinct genes argue for the existence of two prototype variants of human herpesvirus 7. J. Virol. 73:9655–9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenkel, N., E. C. Schirmer, L. S. Wyatt, G. Katsafanas, E. Roffman, R. M. Danovich, and C. H. June. 1990. Isolation of a new herpesvirus from human CD4+ T cells. Proc. Natl. Acad. Sci. USA 87:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frenkel, N., and L. S. Wyatt. 1992. HHV-6 and HHV-7 as exogenous agents in human lymphocytes. Dev. Biol. Stand. 76:259–265. [PubMed] [Google Scholar]
- 20.Hata, A., T. Mukai, Y. Isegawa, and K. Yamanishi. 1996. Identification and analysis of glycoprotein B of human herpesvirus 7. Virus Res. 46:125–137. [DOI] [PubMed] [Google Scholar]
- 21.Hidaka, Y., Y. Liu, M. Yamamoto, R. Mori, C. Miyazaki, K. Kusuhara, K. Okada, and K. Ueda. 1993. Frequent isolation of human herpesvirus 7 from saliva samples. J. Med. Virol. 40:343–346. [DOI] [PubMed] [Google Scholar]
- 22.Hidaka, Y., K. Okada, K. Kusuhara, C. Miyazaki, K. Tokugawa, and K. Ueda. 1994. Exanthem subitum and human herpesvirus 7. Pediatr. Infect. Dis. 13:1010–1011. [PubMed] [Google Scholar]
- 23.Huang, LM, C. Y. Lee, M. Y. Liu, and P. L. Lee. 1997. Primary infections of human herpesvirus-7 and herpesvirus-6: a comparative, longitudinal study up to 6 years of age. Acta Paediatr. 86:604–608. [DOI] [PubMed] [Google Scholar]
- 24.Kasolo, F. C., E. Mpabalwani, and U. A. Gompels. 1997. Infection with AIDS-related herpesviruses in human immunodeficiency virus-negative infants and endemic childhood Kaposi’s sarcoma in Africa. J. Gen. Virol. 78:847–855. [DOI] [PubMed] [Google Scholar]
- 25.Lusso, P., P. Secchiero, R. W. Crowley, A. Garzino-Demo, Z. N. Berneman, and R. C. Gallo. 1994. CD4 is a critical component of the receptor for human herpesvirus-7: interference with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 91:3872–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megaw, A. G., D. Rapaport, B. Avidor, N. Frenkel, and A. J. Davison. 1998. The DNA sequence of the RK strain of human herpesvirus 7. Virology 244:119–132. [DOI] [PubMed] [Google Scholar]
- 27.Nicholas, J. 1996. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J. Virol. 70:5975–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portolani, M., C. Cermelli, P. Mirandola, and D. DiLuca. 1995. Isolation of human herpesvirus 7 from an infant with febrile syndrome. J. Med. Virol. 45:282–283. [DOI] [PubMed] [Google Scholar]
- 29.Quadri I, D. Navarro, P. Paz, and L. Pereira. 1992. Assembly of conformation-dependent neutralizing domains on glycoprotein B of human cytomegalovirus. J. Gen. Virol. 72:1985–1992. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen L. C. Matkin, R. Spaete, C. Pachl, and T. C. Merigan. 1991. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infections in humans. J. Infect. Dis. 164:835–842. [DOI] [PubMed] [Google Scholar]
- 31.Sada, E., M. Yasukawa, C. Ito, A. Takeda, T. Shiosaka, H. Tanioka, and S. Fujita. 1996. Detection of human herpesvirus 6 and human herpesvirus 7 in the submandibular gland, parotid gland, and lip salivary gland by PCR. J. Clin. Microbiol. 34:2320–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato, A., M. Nakagawa, K. Nishizawa, T. Narita, R. Nishikawa, A. Yamada, and T. Ishizaki. 1999. Thrombocytopenia after human herpesvirus-7 infection in a patient with DiGeorge syndrome. Pediatr. Hematol. Oncol. 21:171–172. [DOI] [PubMed] [Google Scholar]
- 33.Secchiero, P., L. D. Bonino, P. Lusso, M. C. Abele, G. Reato, S. Kerim, G. Palestro, G. Zauli, and G. Valente. 1998. Human herpesvirus type 7 in Hodgkin’s disease. Br. J. Haematol. 101:492–499. [DOI] [PubMed] [Google Scholar]
- 34.Secchiero, P., Z. N. Berneman, R. C. Gallo, and P. Lusso. 1994. Biological and molecular characteristics of human herpesvirus 7: in vitro growth optimization and development of a syncytia inhibition test. Virology 202:506–512. [DOI] [PubMed] [Google Scholar]
- 35.Secchiero, P., D. Sun, A. L. de Vico, R. W. Crowley, M. S. Reitz, G. Zauli, P. Lusso, and R. C. Gallo. 1997. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 71:4571–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skrincosky, D., P. Hocknell, L. Whetter, P. Secchiero, B. Chandran, and S. Dewhurst. 2000. Identification and analysis of a novel heparin-binding glycoprotein encoded by human herpesvirus 7. J. Virol. 74:4530–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefan, A., P. Secchiero, T. Baechi, W. Kempf, and G. Campadelli-Fiume. 1997. The 85-kilodalton phosphoprotein (pp85) of human herpesvirus 7 is encoded by open reading frame U14 and localizes to a tegument substructure in virion particles. J. Virol. 71:5758–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefan, A., M. De Lillo, G. Frascaroli, P. Secchiero, F. Neipel, and G. Campadelli-Fiume. 1999. Development of recombinant diagnostic reagents based on pp85(U14) and p86(U11) proteins to detect the human immune response to human herpesvirus 7 infection. J. Clin. Microbiol. 37:3980–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda, K., T. Okuno, Y. Isegawa, and K. Yamanishi. 1996. Identification of a variant A-specific neutralizing epitope on glycoprotein B (gB) of human herpesvirus 6. Virology 222:176–183. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, K., T. Kondo, S. Torigoe, S. Okada, T. Mukai, and K. Yamanishi. 1994. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J. Pediatr. 125:1–5. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka-Taya, K., T. Kondo, T. Mukai, H. Miyoshi, Y. Yamamoto, S. Okada, and K. Yamanishi. 1996. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J. Med. Virol. 48:88–94. [DOI] [PubMed] [Google Scholar]
- 42.Tugizov, S., D. Navarro, and P. Paz. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263–280. [DOI] [PubMed] [Google Scholar]
- 43.Van den Berg, J. S., J. H. van Zeijl, J. J. Rotteveel, W. J. Melchers, F. J. Gabreels, and J. M. Galama. 1999. Neuroinvasion by human herpesvirus type 7 in a case of exanthem subitum with severe neurologic manifestations. Neurology 52:1077–1079. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, B., B. Kropff, and H. Kalbacher. 1992. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J. Virol. 66:5290–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace, H. L., B. Natelson, W. Gause, and J. Hay. 1999. Human herpesviruses in chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 6:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt, L. S., and N. Frenkel. 1992. Human herpesvirus 7 is a constitutive inhabitant of adult human saliva. J. Virol. 66:3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyatt, L. S., W. J. Rodriguez, N. Balachandran, and N. Frenkel. 1991. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J. Virol. 65:6260–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikawa, T., Y. Asano, I. Kobayashi, T. Nakashima, T. Yazaki, S. Suga, T. Ozaki, L. S. Wyatt, and N. Frenkel. 1993. Seroepidemiology of human herpesvirus 7 in healthy children and adults in Japan. J. Med. Virol. 41:319–323. [DOI] [PubMed] [Google Scholar]