Abstract
Background
Acute respiratory infections (ARIs) are a leading cause of hospitalisation, severe morbidity, and mortality in children, representing a significant public health concern. This study aimed to evaluate the clinical features, laboratory findings, and outcomes of pediatric patients hospitalised due to ARIs caused by common respiratory viruses, including influenza virüs (IFV), human bocavirus (hBoV), human metapneumovirus(hMPV), human rhinovirus (hRV), and human respiratory syncytial virüs (hRSV).
Methods
We conducted a retrospective analysis of 1465 hospitalized pediatric patients at Ankara Bilkent City Hospital Children’s Hospital between August 2019 and March 2024. Nasopharyngeal swabs were analyzed using multiplex real-time PCR to identify viral pathogens. Clinical data, including demographics, intensive care needs, respiratory support, and chronic health conditions, were reviewed.
Results
A total of 1465 hospitalized children were included in the study, with a median age of 3 years and 61.8% being male. Of these patients, 32.9% (n = 482) had chronic health conditions. IFV was detected in 30.1% of patients, hBoV in 28.3%, hRSV in 18.4%, hRV in 14.4%, and hMPV in 8.8%. Over half of the infections occurred during the winter months, with IFV being the most commonly observed virus. Fever was most frequently observed in IFV cases, while cough and hypoxia were more prevalent in hBoV and hRSV infections. Admission to the Pediatric Intensive Care Unit was necessary in 19.5% of cases, with 33.1% of these requiring invasive mechanical ventilation. Invasive mechanical ventilation was most frequently required in hBoV cases. The mortality rate was 8.7%, predominantly observed in patients with chronic health conditions; hBoV was associated with the highest mortality.
Conclusion
This study provides a comprehensive analysis of the clinical, laboratory, and radiological characteristics of children hospitalized due to viral lower respiratory tract infections, offering valuable insights into common respiratory pathogens. The findings underscore a higher incidence of these infections during the winter months. It is recommended that hBoV and IFV infections be closely monitored in children with underlying chronic conditions. Moreover, the study highlights the importance of meticulous management of hBoV and hRSV infections, given their association with an increased need for intensive care support.
Keywords: Influenza virus, Human bocavirus, Human metapneumovirus, Human rhinovirus, Human respiratory syncytial virus, Lower respiratory tract infections
Introduction
Respiratory viruses are the most common pathogens encountered by clinicians. These pathogens can present in various clinical manifestations, ranging from uncomplicated upper respiratory tract infections to severe cases of respiratory failure [1, 2]. Acute respiratory infections (ARIs) are the leading cause of hospitalisation, morbidity, and mortality among children, thereby posing a significant global public health challenge [3]. A multitude of viruses has been identified as etiological agents of ARIs, including human respiratory syncytial virus (hRSV), human rhinovirus (hRV), human metapneumovirus (hMPV), human parainfluenza virus (hPIV), human enterovirus (EV), influenza virus (IFV), human coronavirus (hCoV), adenovirus (hAdV), and human bocavirus (hBoV). Collectively, these viruses are responsible for approximately 70% of all ARI cases [4].
Lower respiratory tract infections (LRTIs) affect the respiratory pathways below the level of the larynx and may be of bacterial or viral origin, with viral infections being more common [5]. Differential diagnosis between these causes is critical due to the distinct treatment approaches required, and diagnosis is primarily made by interpreting clinical features. Additionally, the viral pathogens can be identified using the multiplex PCR methods. Respiratory syncytial virus and IFV are the most frequent causes of LRTIs in children, typically observed during winter [6, 7]. Severe LRTIs can lead to millions of deaths annually in developing countries [8].
The objective of this study is to comprehensively evaluate the clinical and laboratory characteristics of pediatric patients hospitalised due to infections caused by common viral pathogens, including hBoV, IFV, hMPV, hRV, and hRSV. Furthermore, the study aims to elucidate the prognostic impact of these infections on morbidity and mortality rates. Emphasis is placed on the necessity of early diagnosis and timely intervention to manage these viral infections effectively.
Materials and methods
Study group and study design
This study was conducted at Ankara Bilkent City Hospital Children’s Hospital. A retrospective screening was performed on patients who presented to our center and underwent multiplex real-time PCR assay with nasopharyngeal swab samples between August 2019 and March 2024. From the collected data, patients who tested positive for IFV, hBoV, hMPV, hRV, and hRSV via PCR were identified. The study cohort comprised those patients who required hospitalisation. Exclusion criteria encompassed improperly collected samples, patients with positive test results for multiple viral agents, and cases where multiple nasopharyngeal swabs were obtained during the same hospital admission—only the initial admission samples were considered. Furthermore, patients hospitalized for conditions unrelated to respiratory distress and patients who underwent nasopharyngeal swabbing were excluded from the study.
Data collection
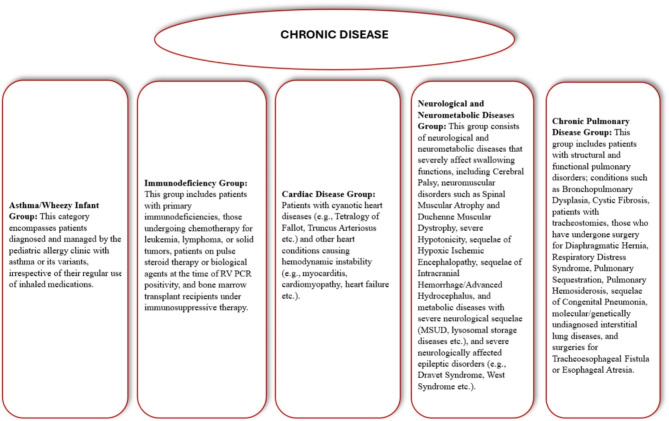
Data for this study were obtained from the hospital database. Patient demographics, including age and gender, along with the date and season of presentation, presenting complaints, and history of chronic illnesses (categorised into immunodeficiency, chronic pulmonary disease, cardiac disease, asthma/wheezy infant, and neurological and neurometabolic diseases) were retrospectively analyzed. Additional data points included the requirement for intensive care, length of hospital stay, need for respiratory support devices (invasive/non-invasive mechanical ventilation, high-flow systems), pulmonary imaging findings, and laboratory results. Chronic illnesses were classified as illustrated in Fig. 1. Nine patients with chronic lung disease had pre-existing tracheostomies and were using home mechanical ventilators prior to admission. As they continued to use their personal ventilators during hospitalization and did not require any additional or newly initiated respiratory support, they were excluded from the Respiratory Support group. Instead, these patients were classified under the chronic lung disease group for analysis purposes. Most of the patients with chronic conditions included in the study were regularly followed up at our center, and their previous chest X-rays were available in the hospital’s electronic medical records. The chest X-rays obtained during infection were evaluated by comparing them with the patients’ prior images. Only newly developed radiological abnormalities were considered. In patients with chronic conditions, pre-existing findings were not included if they were already present in earlier radiographs. Patients were evaluated for sputum cultures, and the results of those with available sputum cultures were analyzed.
Fig. 1.
Grouping of chronic diseases
Multiplex RT-PCR analysis
Respiratory viruses were identified using the multiplex real-time PCR assay (Rotor-Gene Q, QIAGEN, Germantown, Maryland, United States). This technique facilitates the detection of various pathogens, including IFV, hRSV, hCoV (Corana 229E, OC43, NL63, HKU1, SARS-COV2), hPIV, hMPV, hRV, EV, hBoV, hAdV, and human parechovirus. Additionally, bacterial pathogens including Mycoplasma pneumoniae, Bordetella pertussis, Chlamydophila pneumoniae, Haemophilus influenzae, and Streptococcus pneumoniae were also detected. Patients with multiple detected pathogens were excluded from the study to ensure the accuracy of the analysis.
Statistical analysis and ethics
The Statistical Package for the Social Sciences (SPSS) 23.0 (Chicago, Illinois, USA) was implemented for the statistical analysis. The Kolmogorov-Smirnov test and the examination of histograms were used to evaluate the compliance of numerical and continuous variables with normal distribution. Numerical data with a normal distribution were expressed as the mean Standard Deviation (SD), while data with a non-normal distribution were expressed as the median and interquartile range (IQR). Percentages (%) and numbers (n) were used to express categorical variables. In contrast, the Mann-Whitney U test was used to compare continuous variables that did not meet the normal distribution. The Kruskal-Wallis test evaluated continuous variables from many groups that did not fit into the normal distribution. Categorical variables were analyzed with the Pearson chi-square or Fisher’s Exact Test. When comparing more than one group, p values were calculated using the Bonferroni correction. The significance level was established at p < 0.05.
This study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the Ethics Committee of Ankara Bilkent City Hospital.
Results
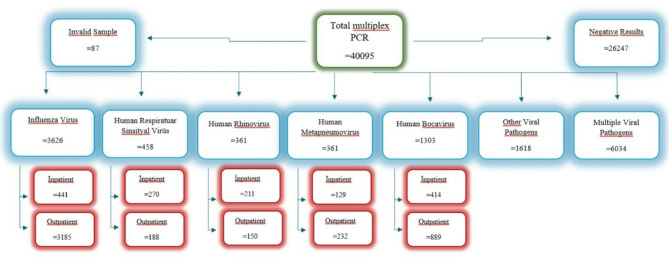
The study included a total of 1465 hospitalised patients. Of these, IFV was detected in 30.1% (n = 441) of patients, hRSV in 18.4% (n = 270), hRV in 14.4% (n = 211), hMPV in 8.8% (n = 129), and hBoV in 28.3% (n = 414) (Fig. 2). The cohort comprised 61.8% (n = 906) males, with a median age of 3 years (IQR 1–5 years). Fever was most frequently observed in patients with IFV, while cough was more prevalent in those with hRSV and hBoV infections. Sore throat was predominantly seen in patients with IFV. Presentation with hypoxia was most notably associated with hBoV and hMPV (Table 1).
Fig. 2.
Distribution of multiplex PCR test results for common respiratory viruses among hospitalized patients
Table 1.
Demographic and clinical characteristics of patients
| IFV 441(30,1%) |
hRSV 270(18,4%) |
hRV 211(14,4%) |
hMPV 129(8,8%) |
hBoV 414(28,3%) |
Overall 1465(100%) |
P value | |
|---|---|---|---|---|---|---|---|
| Gender (n, %) | |||||||
| Male |
260 (28,7%) |
170 (18,8%) |
123 (13,6%) |
85 (9,4%) |
268 (29,5%) |
906 (100%) |
|
| Female |
181 (32,4%) |
100 (17,9%) |
88 (15,7%) |
44 (7,9%) |
146 (26,1%) |
559 (100%) |
|
| Age (years) (median, IQR) | 5 (3–9) | 2 (1–3) | 1 (1–5) | 3 (2–5) | 2,5 (1–4) | 3 (1–5) | |
| Clinical presentation (n, %) | |||||||
| Fever | |||||||
| yes |
366a (39,6%) |
136b (14,7%) |
103b (11,1%) |
86c (9,3%) |
234b (25,3%) |
925 (100%) |
p < 0,001 |
| no |
75a (13,9%) |
134b (24,8%) |
108b (20%) |
43c (8%) |
180b (33,3%) |
540 (100%) |
|
| Cough | |||||||
| yes |
219a (20%) |
244b (22,2%) |
155c (14,1%) |
107d (9,8%) |
372b (33,9%) |
1097 (100%) |
p < 0,001 |
| no |
222a (60,3%) |
26b (7,1%) |
56c (15,2%) |
22d (6%) |
42b (11,4%) |
368 (100%) |
|
| Sore throat | |||||||
| yes |
85 (68,5%) |
- |
11 (8,9%) |
5 (4,1%) |
23 (18,5%) |
124 (100%) |
- |
| no |
199 (50,1%) |
1 (0,3%) |
60 (15,1%) |
27 (6,8%) |
110 (27,7%) |
397 (100%) |
|
| Season (n,%) | |||||||
| Winter |
319 (38,5%) |
193 (23,3%) |
55 (6,6%) |
76 (9,2%) |
186 (22,4%) |
829 (100%) |
|
| Spring |
88 (29,5%) |
61 (20,5%) |
68 (22,8%) |
35 (11,7%) |
46 (15,4%) |
298 (100%) |
|
| Summer |
0 (0%) |
3 (3,1%) |
39 (40,2%) |
9 (9,3%) |
46 (15,4%) |
97 (100%) |
|
| Autumn |
34 (14,1%) |
13 (5,4%) |
49 (20,3%) |
9 (9,3%) |
136 (56,4%) |
241 (100%) |
|
| PICU Requirement (n, %) |
45a (15,7%) |
72b (25,1%) |
43b (15%) |
28b (9,8%) |
99b (34,4%) |
287 (100%) |
p < 0,001 |
| Non-PICU |
396a (33,6%) |
198b (16,8%) |
168b (14,3%) |
101b (8,6%) |
315b (26,7%) |
1178 (100%) |
|
| Length of Stay (median, IQR) | |||||||
| PICU | 8 (5–13)a | 8 (5–10)a | 7 (4–14)a | 7 (4–14)a, c | 5 (3–7)c | 6 (4–10) | p < 0,001 |
| Total | 5 (2–10)a | 8 (6–11)b | 7 (5–12)b, c | 7 (4–11)c, d | 6 (4–10)d | 6 (4–11) | |
| Respiratory Support (n, %) | |||||||
| IMV |
20a (21,1%) |
9a (9,5%) |
21b (22,1%) |
12b (12,6%) |
33b (34,7%) |
95 (100%) |
p < 0,001 |
| NIMV |
35a (15,3%) |
59b (25,8%) |
40b (17,4%) |
17b (7,4%) |
78b (34,1%) |
229 (100%) |
p < 0,001 |
| HFNC |
16a (9,7%) |
41b (24,7%) |
36b (21,7%) |
19b (11,4%) |
54b (32,5%) |
166 (100%) |
p < 0,001 |
|
Home mechanical ventilatör (n,%) |
3 (33,4%) |
- |
2 (22,2%) |
- |
4 (44,4%) |
9 (100%) |
|
| Chronic Disease | |||||||
| Asthma/Wheezy Infant Group |
8 (6,7%) |
9 (7,5%) |
34 (28,3%) |
7 (5,8%) |
62 (51,7%) |
120 (100%) |
|
| Immunodeficiency Group |
42 (37,2%) |
12 (10,6%) |
18 (15,9%) |
4 (3,6%) |
37 (32,7%) |
113 (100%) |
|
| Cardiac Disease Group |
25 (44,6%) |
10 (17,9%) |
8 (14,3%) |
5 (8,9%) |
8 (14,3%) |
56 (100%) |
|
| Neurological and Neurometabolic Diseases Group |
46 (35,6%) |
14 (10,9%) |
25 (19,4%) |
10 (7,8%) |
34 (26,3%) |
129 (100%) |
|
| Chronic Pulmonary Disease Group |
8 (12,5%) |
4 (6,3%) |
13 (20,3%) |
5 (7,8%) |
34 (53,1%) |
64 (100%) |
PICU:Pediatric Intensive Care Unit, IVM:Invasive Mechanical Ventilation NIVM:Non-Invasive Mechanical Ventilation, HFNC:High Flow Nasal Cannula
IFV: Influenza Virus; hRSV: Human Respiratory Syncytial Virus; hRV: Human Rhinovirus; hMPV:human Metapneumovirus; hBoV:Human Bocavirus
Similar symbols indicating the same subscript letter denote no statistical difference, whereas different symbols indicate statistical significance
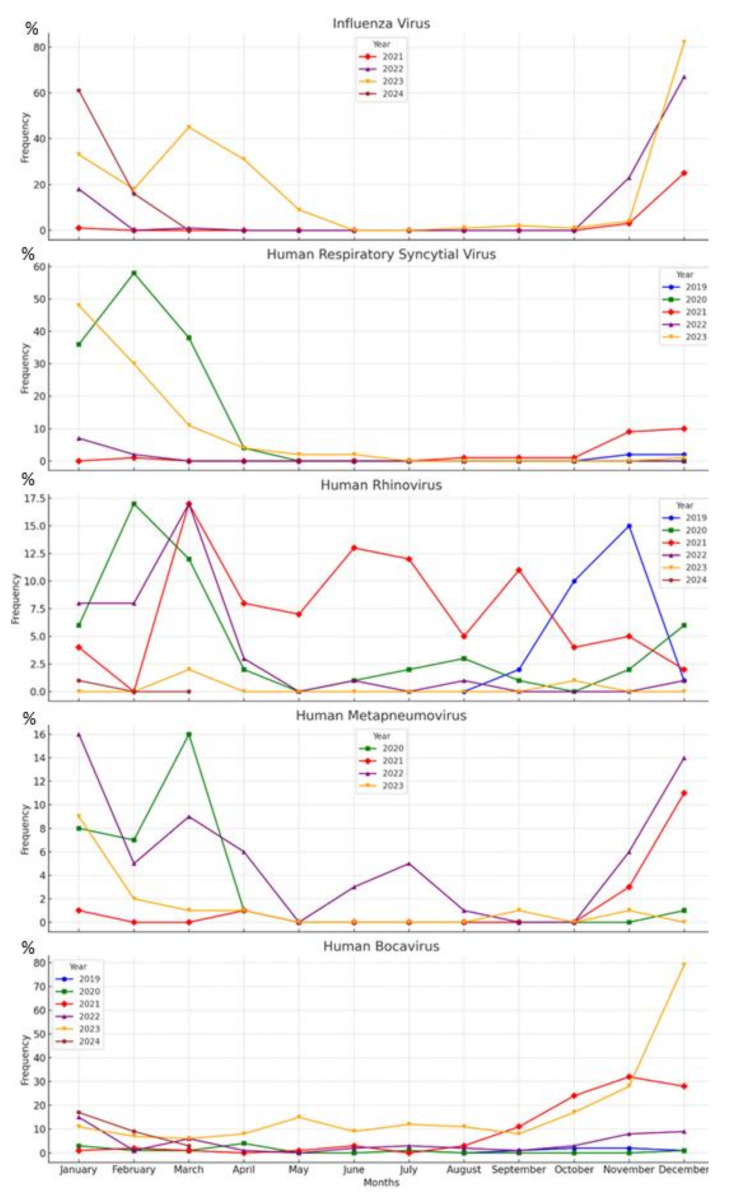
The research data indicate that over half of the viral pathogens were predominantly detected during the winter. IFV was identified as the most frequently occurring viral pathogen in the winter season, followed by hRSV and hBoV. In the spring, a similar distribution was observed across all viral pathogens. The distribution of hRV remained consistent throughout all seasons. The summer season exhibited the lowest detection rates for viral pathogens (Table 1). The monthly distribution of the pathogens is shown in Fig. 3.
Fig. 3.
Monthly Distribution of Various Respiratory Viruses Detected by Multiplex PCR from 2019 to 2024
It was found that 19.5% (n = 287) of patients required admission to the Pediatric Intensive Care Unit (PICU). Among those admitted to intensive care, 33.1% (n = 95) required invasive mechanical ventilation (IMV). When compared with other pathogens, the need for PICU admission was significantly lower in cases with IFV, while no significant differences were observed in PICU admission needs or length of stay among the other pathogens. The duration of PICU stay was notably shorter for patients with hBoV and hMPV, whereas it was similar across the other three pathogens. The need for IMV was lower in IFV and hRSV cases, while the requirement for non-invasive mechanical ventilation (NIMV) and high-flow nasal cannula (HFNC) was less frequent in IFV cases. Additionally, nine patients were already using home mechanical ventilators prior to hospital admission (Table 1). It was determined that all patients received oxygen support.
In the Asthma/Wheezy Infant and Chronic Pulmonary Disease groups, the highest prevalence of PCR positivity was observed for hBoV, whereas in the Immunodeficiency, Cardiac Disease, and Neurological and Neurometabolic Diseases groups, IFV PCR positivity was most frequently detected (Table 1).
Patients with chronic illnesses had a significantly higher rate of PICU admission for IFV, hRV, and hBoV compared to those without chronic conditions (p < 0.001). Similarly, the rate of intubation was also higher in patients with chronic illnesses for the same pathogens (p < 0.001) (Table 2).
Table 2.
Intensive care admissions and respiratory support based on chronic disease status
| IFV 441 (30,1%) |
hRSV 270 (18,4%) |
hRV 211 (14,4%) |
hMPV 129 (8,8%) |
hBoV 414 (28,3%) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chronic Disease |
No Chronic Disease |
P value |
Chronic Disease |
No Chronic Disease |
P value |
Chronic Disease |
No Chronic Disease |
P value |
Chronic Disease |
No Chronic Disease |
P value |
Chronic Disease |
No Chronic Disease |
P value | ||||
| n = 129 | n = 312 | n = 49 | n = 221 | n = 98 | n = 113 | n = 31 | n = 98 | n = 175 | n = 239 | |||||||||
|
PICU Requirement (n, %) |
23 (17,8%) |
22 (7,1%) |
p < 0.001 |
13 (26,5%) |
59 (26,7%) |
p > 0,05 |
29 (29,6%) |
14 (12,4%) |
p < 0.001 |
11 (35,5%) |
17 (17,3%) |
p > 0.05 |
59 (33,7%) |
40 (16,7%) |
p < 0.001 | |||
| Non-PICU |
106 (82,2%) |
290 (92,9%) |
36 (73,5%) |
162 (73,3%) |
69 (70,4%) |
99 (87,6%) |
20 (64,5%) |
81 (82,7%) |
116 (66,3%) |
199 (83,3%) |
||||||||
| Length of Stay (median, IQR) | ||||||||||||||||||
| PICU |
8 (5–33) |
6 (2–8) |
P = 0,017 |
8 (3–19,5) |
9 (6–10) |
P < 0.001 |
7 (4–15) |
7 (3,5–12) |
P > 0.05 |
7 (4–10) |
7 (3,5–8,5) |
p > 0.05 |
5 (3–9) |
5 (3–6,5) |
P > 0.05 | |||
| Total |
9 (4–20,5) |
4 (2–7) |
p < 0.001 |
10 (6–13,5) |
8 (6–10) |
p < 0.001 |
9 (6–15,25) |
7 (5–10) |
p < 0.001 |
9 (5–15) |
6 (4–10,25) |
p = 0.016 |
9 (5–18) |
5 (3–7) |
p < 0.001 | |||
|
Respiratory Support (n, %) |
||||||||||||||||||
| IMV |
14 (10,9%) |
6 (1,9%) |
p < 0.001 |
4 (8,2%) |
5 (2,3%) |
p > 0.05 |
18 (18,4%) |
3 (2,7%) |
p < 0.001 |
6 (19,4%) |
6 (6,1%) |
p > 0.05 |
27 (15,4%) |
6 (2,5%) |
p < 0.001 | |||
| NIMV |
16 (12,4%) |
19 (6,1%) |
P = 0,02 |
11 (22,4%) |
48 (21,7%) |
p > 0.05 |
21 (21,4%) |
19 (16,8) |
p > 0.05 |
5 (16,1%) |
12 (12,2%) |
p > 0.05 |
36 (20,6%) |
42 (17,6%) |
p > 0.05 | |||
| HFNC |
7 (5,4%) |
9 (2,9%) |
p > 0.05 |
5 (10,2%) |
36 (16,3%) |
p > 0.05 |
23 (23,5%) |
13 (11,5%) |
P = 0,021 |
7 (22,6%) |
12 (12,2%) |
p > 0.05 |
27 (15,4%) |
27 (11,3%) |
p > 0.05 | |||
PICU:Pediatric Intensive Care Unit, IVM:Invasive Mechanical Ventilation NIVM:Non-Invasive Mechanical Ventilation, HFNC:High Flow Nasal Cannula
IFV: Influenza Virus; hRSV: Human Respiratory Syncytial Virus; hRV: Human Rhinovirus; hMPV:human Metapneumovirus; hBoV:Human Bocavirus
It was determined that 8.7% (n = 25) of the patients in intensive care succumbed to their conditions. Among these fatalities, IFV was identified in 6 cases, hRSV in 1 case, hRV in 4 cases, hMPV in 2 cases, hBoV in 12 cases. Notably, 2 of the deceased patients had no chronic health conditions; both of these patients tested positive for hMPV. Within the deceased cohort, 10 patients were part of the Immunodeficiency group, 2 were in the Cardiac Disease group, 7 belonged to the Neurological and Neurometabolic Diseases group, and 4 were in the Chronic Pulmonary Disease group.
Sputum cultures were obtained from 31.5% (n = 461) of the patients, and no bacterial growth was detected in any of these samples. Additionally, all patients in the Immunodeficiency Group and the Chronic Pulmonary Disease Group had sputum cultures, none of which yielded any pathogenic organisms.
It was observed that the white blood cell count (WBC) was significantly elevated in cases with hRV and hBoV. The absolute neutrophil count (ANC) showed marked increases with hRV and hBoV, while the absolute lymphocyte count (ALC) was most significantly elevated in cases of hRSV. Platelet counts (PLT) were found to be within the normal range across all viral pathogens. Median values of C-reactive protein (CRP) were recorded as follows: 9.8 (range 3.6–26) for IFV, 3.4 (range 1-17.7) for hRSV, 5 (range 2-9.2) for hRV, 14.5 (range 1-38.5) for hMPV, and 11.3 (range 3.3–31.3) for hBoV, with significantly higher CRP levels observed in IFV, hMPV, and hBoV (p < 0.001). Reticular branching was the most commonly observed radiographic finding, occurring in 24.3% (n = 357) of cases, particularly with hBoV, followed by IFV and hRSV. Lobar infiltration was detected in 3.7% (n = 55) of cases. Lobar infiltration, pleural effusion, and atelectasis were predominantly seen in patients with hBoV. Pneumothorax was observed in 9 patients, with hBoV detected in 6 cases, IFV in 2, and hRSV in 1. Pneumomediastinum was noted in 5 patients: hBoV in 2, IFV in 2, and hRV in 1 (Table 3).
Table 3.
Laboratory and radiological findings of common respiratory viruses
| IFV (median, IQR) |
hRSV (median, IQR) |
hRV (median, IQR) |
hMPV (median, IQR) |
hBoV (median, IQR) |
P value | ||
|---|---|---|---|---|---|---|---|
| Laboratory results | |||||||
|
WBC (× 1000/mm3) |
6,8a (4,5–9,7) |
8,8b (6,6–11,7) |
10,7c (7,9–15) |
9,2b (6,4–12,8) |
10,5c (8–13,3) |
P < 0,001 | |
|
ANC (× 1000/mm3) |
3,8a (2–6,3) |
3,2b (2–5) |
5,5c (3,2–9) |
4,2a (2,6–6,6) |
6,4c (3,8–9,3) |
P < 0,001 | |
|
ALC (× 1000/mm3) |
1,6a (0,9 − 2,7) |
4b (2,5–5,3) |
2,8c (1,6 − 5) |
3,6c (1,9 − 5,3) |
2,4d (1,8 − 3,9) |
P < 0,001 | |
|
PLT (× 1000/mm3) |
257a (185–343) |
408b (314–503) |
402b (292–514) |
360b (281–505) |
360b (274–474) |
P < 0,001 | |
|
CRP mg/L |
9,8a (3,6–26) |
3,4b (1–17,7) |
5b (2–9,2) |
14,5a (1–38,5) |
11,3a (3,3–31,3) |
P < 0,001 | |
| Radiographic features | |||||||
| Reticular Branching |
253 (23,3%) |
238 (21,9%) |
151 (13,9%) |
87 (8%) |
357 (32,9%) |
< 0,001 | |
| Lobar Infiltration |
10 (8,7%) |
10 (8,7%) |
32 (27,8%) |
8 (7%) |
55 (47,8%) |
< 0,001 | |
| Pleural Effusion |
13 (20,3%) |
5 (7,8%) |
11 (17,2%) |
5 (7,8%) |
30 (46,9%) |
< 0,001 | |
| Atelectasis |
17 (10,6%) |
38 (23,8%) |
12 (7,5%) |
26 (16,3%) |
67 (41,9%) |
< 0,001 | |
| Pneumothorax |
2 (22,2%) |
1 (11,1%) |
- | - |
6 (66,7%) |
- | |
| Pneumomediastinum |
2 (40%) |
- |
1 (20%) |
- |
2 (40%) |
- | |
WBC, White Blood Cell; ANC absolute neutrophil count; ALC, absolute lymphocyte count; PLT, Platelet; CRP, C-reactive protein
IFV: Influenza Virus; hRSV: Human Respiratory Syncytial Virus; hRV: Human Rhinovirus; hMPV:human Metapneumovirus; hBoV:Human Bocavirus
Similar symbols indicating the same subscript letter denote no statistical difference, whereas different symbols indicate statistical significance
Discussion
In this study, the clinical and laboratory characteristics of the most prevalent pathogens causing LRTIs in children—specifically, IFV, hRSV, hRV, hMPV, and hBoV—were evaluated. Recognising the clinical features of these pathogens, which can manifest with severe clinical symptoms, is crucial for enabling early intervention that can significantly reduce morbidity and mortality. LRTIs are among the leading causes of hospital admissions in children and are associated with serious complications. Given the periodic variability in the prevalence of dominant pathogens, it is imperative to conduct surveillance studies at regular intervals. This study is one of the most comprehensive investigations conducted in recent years on common viral pathogens, providing detailed and generalizable findings thanks to its large patient population, thereby making significant contributions to the existing body of knowledge.
In the study group, the median age of the patients was 3 years. Among the viral pathogens, the IFV exhibited the highest median age value. Previous studies evaluating children hospitalised due to LRTIs have demonstrated that viral pathogens most frequently occur in children under the age of 4 years [9]. Similarly, our study indicates that the prevalent viral pathogens predominantly affect children under the age of 5. It has been established that LRTIs are a significant cause of mortality in children below 5 years of age [10]. Consequently, vigilant attention is necessary for children under this age, considering that detected viral pathogens can lead to hospitalisations and contribute to mortality rates.
The seasonal occurrence of viral pathogens can vary depending on the climatic and seasonal characteristics of countries as well as public awareness. Although the seasonal distribution identified in our study does not exactly match the literature, it exhibits similar characteristics [11, 12]. Over half of the common viral pathogens were observed predominantly in the winter season. It has been shown that the responses of interferon (IFN) genes and IFN-stimulated genes in the nasal mucosa decrease in cold weather, thereby facilitating the adhesion of viral pathogens to the mucosa [13]. Additionally, viruses have been demonstrated to be more resilient in colder weather, which may explain their frequent occurrence during winter months [14]. Nevertheless, hRV is observed throughout the year, as indicated in other studies [15]. While the peak period for each virus may vary, it should not be overlooked that the winter season is particularly risky for viral LRTIs. The seasonal distribution of viral pathogens is critical for clinicians to take necessary precautions and for the planning of public health interventions.
In a study conducted on intensive care patients, hRSV was identified as the most prevalent pathogen, followed by hRV and hBoV [11]. Li et al. concluded that intensive care admissions were similar between hMPV and hRSV [12]. In our study, it was similarly found that the need for intensive care among patients with hRV, hMPV, hRSV, and hBoV was high and showed no significant differences, consistent with previous studies. Furthermore, our study observed that IFV was associated with the lowest need for intensive care. This may be due to the fact that IFV infections tend to occur in older children and are more readily treatable. However, one of the critical findings of our study is that viral pathogens other than IFV similarly lead to intensive care admissions. Additionally, as demonstrated in our study, the presence of chronic conditions in patients with IFV, hRV, and hBoV infections is associated with an increased need for intensive care admission. Therefore, the clinical course in these patients should be carefully monitored, as it may be more severe. Given these findings, this underscores the importance of prompt and vigilant management by clinicians in all cases of viral pathogen-induced infections. Rapid intervention and close monitoring are essential in managing these infections to prevent severe outcomes.
In a study involving 115 hospitalised LRTI patients, it was shown that 63 patients required intensive care, with 25.4% of these patients needing IMV [11]. In our study, the requirement for IMV among intensive care patients was found to be 33.1%. Previous research has identified hBoV as the most frequent cause necessitating IMV in intensive care patients [11]. hBoV is known to be one of the most severe pathogens in children, leading to serious complications and mortality [16]. The high incidence of IMV in our study may be related to the substantial number of hBoV infections. Our study also found that the need for respiratory support was high for patients with hRV, hMPV, hRSV, and hBoV, with no significant differences among these pathogens. Conversely, the need for IMV, NIMV, and HFNC support was lowest in IFV cases. The highest intensive care admission rates and the need for mechanical ventilation and HFNC were most frequently associated with hBoV, highlighting the significance of hBoV infections. Additionally, it is noteworthy that 12 patients succumbed to hBoV infections.
Respiratory viruses may present with severe clinical manifestations, particularly in individuals with underlying chronic diseases. In a study conducted by Christensen et al., HBoV infections were identified as a significant risk factor in children with cardiovascular and pulmonary diseases, malignancies, and immunodeficiency diagnoses. Similarly, another study demonstrated an elevated risk of HBoV infections in children with cardiac conditions, neuromuscular diseases, and immunodeficiencies [17, 18]. Additionally, patients diagnosed with IFV have been reported to exhibit higher incidences of chronic pulmonary and neurological diseases [19]. While the data from our study shows similarities with findings in the literature, variations in study periods, as well as differences in the characteristics and sizes of study groups, may lead to divergent outcomes. In our findings, HBoV and IFV were the two most frequently observed viruses among patients with chronic conditions. It is imperative to ensure close monitoring and early intervention for patients with chronic diseases who are diagnosed with HBoV. To prevent IFV infections, the development and implementation of effective vaccination programs targeting high-risk groups are essential. Furthermore, in cases diagnosed with IFV, early initiation of antiviral therapy is recommended.
Previous studies have reported that various viral pathogens tend to cause more severe disease in patients with underlying conditions compared to those without [20–23]. In our study, when patients with and without chronic conditions were compared, it was observed that IFV, hRV, and hBoV infections were associated with an increased need for intensive care admission and a more severe clinical course in patients with underlying conditions. Additionally, the need for IMV was found to be higher in this group. Although the limited number of patients in each group may reduce the statistical power, a comparison with the existing literature suggests that particular caution should be exercised in children with chronic conditions. These children should be protected against viral infections and educated about preventive measures.
Mortality rates associated with LRTIs range from 2.3 to 12.6% [24, 25]. Another study reported a mortality rate of 12.7% [11]. In our study, the mortality rate was found to be 8.7%. It is noteworthy that nearly all mortalities occurred in children with chronic illnesses. Due to the small sample size and the study not being specifically designed to account for chronic conditions, drawing definitive conclusions in this regard is challenging. However, the presence of LRTIs in patients with chronic diseases necessitates heightened vigilance, early preventive measures, and close monitoring. Further research involving larger populations is essential to better understand and address this issue.
The clinical effects of commonly observed hRV and hBoV remain controversial. Some studies have reported their presence in asymptomatic individuals, suggesting that these viruses may act as mere bystanders rather than primary pathogens. Consequently, their role as a primary causative agent in patients with LTRIs remains debatable [26, 27]. However, several studies have also suggested that hBoV and hRV can serve as primary sources of infection [28–31]. In our study, only patients with a single detected pathogen were included, ensuring the absence of co-infections. Therefore, we considered hBoV and hRV as primary etiological agents. Nevertheless, clinicians should remain vigilant for other potential pathogens in patients testing positive for hRV or hBoV. In suspected cases, further investigation for additional pathogens is warranted.
The primary limitations of this study are its single-center design and retrospective nature. Climate changes, shifts in public awareness, public health programs, and infection prevention measures implemented during and after the SARS-CoV-2 pandemic may have influenced the seasonal distribution of viral pathogens. Additionally, the implementation of IFV vaccination programs and, although less common, hRSV vaccination efforts could have impacted the prevalence of these pathogens. In our study, we could not access vaccination information for the patients, limiting our ability to comment on this aspect. The relationship between chronic illness and mortality was not demonstrated for all viral pathogens. The low incidence of certain viral pathogens in the studied cases may have contributed to the inability to establish this relationship. Another issue is that, although we excluded co-infections, we could not rule out secondary bacterial infections solely based on negative sputum cultures, which hindered a clear distinction between viral and bacterial LRTIs. Despite these limitations, this large-population study provides valuable insights and can guide future research. Studies like this enhance the understanding and management of viral respiratory infections, offering significant contributions to public health strategies.
This study comprehensively evaluated the clinical and laboratory characteristics of common viral pathogens causing hospitalisation due to LRTIs in children. These data highlight the critical importance of early diagnosis and intervention in hBoV infections. Seasonal variations significantly influence the spread of viral pathogens, with infection rates markedly increasing during the winter months. Therefore, it is crucial to enhance seasonal awareness and develop early intervention strategies for managing these pathogens in clinical practice. The study provides valuable insights that can guide future research and inform public health policies, emphasizing the necessity of timely and targeted approaches to reduce the morbidity and mortality associated with viral LRTIs in children.
Abbreviations
- ALC
Absolute Lymphocyte Count
- ANC
Absolute Neutrophil Count
- ARIs
Acute Respiratory İnfections
- CRP
C-reactive protein
- EV
Human Enterovirus
- IFV
Influenza virüs
- hAdV
Adenovirus
- hBoV
Human Bocavirus
- hCoV
Human Coronavirus
- HFNC
High-Flow Nasal Cannula
- hMPV
Human Metapneumovirus
- hPIV
Human Parainfluenza Virüs
- hRSV
Human Respiratory Syncytial Virüs
- hRV
Human Rhinovirus
- IFN
Interferon
- IMV
Invasive Mechanical Ventilation
- IQR
Interquartile Range
- LRTIs
Lower respiratory tract infections
- NIMV
Non-invasive Mechanical Ventilation
- PICU
Pediatric Intensive Care Unit
- PLT
Platelet counts
- SD
Standard Deviation
- WBC
White Blood Cell Count
Author contributions
The concept and design of the study were developed by MY and AOP. GNG, NY, ANC, MDY, and EK took leading roles in data collection. Data analysis and statistical evaluation were conducted by FK and MY. KC and BDK contributed to the literature review and the writing of the manuscript. FK carried out the interpretation of the study findings and the drafting of the initial manuscript. Final revisions and critical content review were performed by OT and AOP. These contributions were significant at every stage of the study and were instrumental in shaping the final manuscript. All authors have reviewed and approved the final version of the manuscript, and each contributed significantly to its content.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The work was not supported or funded by any drug company.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the institutional and national research committees, and with the 1975 Helsinki Declaration and its later amendments. Ethical approval was granted by the Ethics Committee of Ankara Bilkent City Hospital. As the study was retrospective in nature, the requirement for obtaining informed consent from participants was waived by the Ethics Committee of Ankara Bilkent City Hospital, in accordance with. General consent for the use of medical records in research was obtained as part of the routine admission process where applicable.
Consent for publication
No interventions were performed on any patients for the purposes of this study; only existing medical records were utilized. Data was anonymized and confidentiality was maintained in compliance with ethical standards. The information used was in accordance with the general consent provided by patients at the time of their admission to the hospital.
Competing interests
The authors declare no competing interests.
Clinical trial registration
This study does not involve any clinical trials. Therefore, no clinical trial registration is required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicole LC, Regina P, Cecilia V, Constanza MV, Tania L, Marcela M, et al. Relevance of codetection of respiratory viruses in the severity of acute respiratory infection in hospitalized children. Andes Pediatrica: Revista Chil De Pediatria. 2021;92:349–58. [DOI] [PubMed] [Google Scholar]
- 2.Loubet P, Voiriot G, Houhou-Fidouh N, Neuville M, Bouadma L, Lescure FX, et al. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: A single-center retrospective study. J Clin Virol. 2017;91:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurskaya O, Ryabichenko T, Leonova N, Shi W, Bi H, Sharshov K et al. Viral etiology of acute respiratory infections in hospitalized children in Novosibirsk City, Russia (2013–2017). PLoS ONE. 2018;13. [DOI] [PMC free article] [PubMed]
- 4.Kusel MMH, De Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–6. [DOI] [PubMed] [Google Scholar]
- 5.Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the emergency department. Cochrane Database Syst Rev. 2014;2014. [DOI] [PMC free article] [PubMed]
- 6.Tamerius JD, Shaman J, Alonso WJ, Bloom-Feshbach K, Uejio CK, Comrie A, et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9:1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panozzo CA, Fowlkes AL, Anderson LJ. Variation in timing of respiratory syncytial virus outbreaks: lessons from National surveillance. Pediatr Infect Dis J. 2007;26 11 Suppl. [DOI] [PubMed]
- 8.Nair H, Simões EAF, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei L, Liu W, Zhang XA, Liu EM, Wo Y, Cowling BJ et al. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009–2013. Medicine. 2015;94. [DOI] [PMC free article] [PubMed]
- 10.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duyu M, Karakaya Z. Viral etiology and outcome of severe lower respiratory tract infections among critically ill children admitted to the PICU. Med Intensiva. 2021;45:447–58. [DOI] [PubMed] [Google Scholar]
- 12.Li YT, Liang Y, Ling YS, Duan MQ, Pan L, Chen ZG. The spectrum of viral pathogens in children with severe acute lower respiratory tract infection: A 3-year prospective study in the pediatric intensive care unit. J Med Virol. 2019;91:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxman EF, Storer JA, Fitzgerald ME, Wasik BR, Hou L, Zhao H, et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci U S A. 2015;112:827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ausar SF, Rexroad J, Frolov VG, Look JL, Konar N, Middaugh CR. Analysis of the thermal and pH stability of human respiratory syncytial virus. Mol Pharm. 2005;2:491–9. [DOI] [PubMed] [Google Scholar]
- 15.Eifan SA, Hanif A, Aljohani SM, Atif M. Respiratory Tract Viral Infections and Coinfections Identified by Anyplex™ II RV16 Detection Kit in Pediatric Patients at a Riyadh Tertiary Care Hospital. Biomed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed]
- 16.Chen YW, Huang YC, Ho TH, Huang CG, Tsao KC, Lin TY. Viral etiology of bronchiolitis among pediatric inpatients in Northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Infect. 2014;47:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen A, Kesti O, Elenius V, Eskola AL, Døllner H, Altunbulakli C, et al. Human bocaviruses and paediatric infections. Lancet Child Adolesc Health. 2019;3:418–26. [DOI] [PubMed] [Google Scholar]
- 18.Ademhan Tural D, Yalcin E, Emiralioglu N, Ozsezen B, Alp A, Sunman B, et al. Human bocavirus and human metapneumovirus in children with lower respiratory tract infections: effects on clinical, Microbiological features and disease severity. Pediatr Int. 2022;64:e15102. [DOI] [PubMed] [Google Scholar]
- 19.Biondo GF, Santana JC, Lago PM, Piva J, Souza PRA, Gaulke JG, et al. Impact of A/H1N1 influenza in children at a Brazilian university hospital. Brazilian J Infect Dis. 2018;22:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Herz W, Essa S. Spectrum of viral infections among primary immunodeficient children: report from a National registry. Front Immunol. 2019;10:MAY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi T, Vennard S, Mahdy S, Nair H. Risk factors for poor outcome or death in young children with respiratory syncytial Virus-Associated acute lower respiratory tract infection: A systematic review and Meta-Analysis. J Infect Dis. 2022;226(Suppl 1):S10–6. [DOI] [PubMed] [Google Scholar]
- 22.Helfrich AM, Nylund CM, Eberly MD, Eide MB, Stagliano DR. Healthy Late-preterm infants born 33–36 + 6 weeks gestational age have higher risk for respiratory syncytial virus hospitalization. Early Hum Dev. 2015;91:541–6. [DOI] [PubMed] [Google Scholar]
- 23.Papoff P, Moretti C, Cangiano G, Bonci E, Roggini M, Pierangeli A, et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farias JA, Fernández A, Monteverde E, Flores JC, Baltodano A, Menchaca A, et al. Mechanical ventilation in pediatric intensive care units during the season for acute lower respiratory infection: a multicenter study. Pediatr Crit Care Med. 2012;13:158–64. [DOI] [PubMed] [Google Scholar]
- 25.Hutton HK, Zar HJ, Argent AC. Clinical features and outcome of children with severe lower respiratory tract infection admitted to a pediatric intensive care unit in South Africa. J Trop Pediatr. 2019;65:46–54. [DOI] [PubMed] [Google Scholar]
- 26.Fry AM, Lu X, Olsen SJ, Chittaganpitch M, Sawatwong P, Chantra S, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE. 2011;6:e17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty M, Mishra B, Satapathy AK, Gulla KM, Das RR, Dwibedi B et al. Human bocavirus infection in childhood acute respiratory infection: is it an innocent bystander? Indian J Med Microbiol. 2023;46. [DOI] [PubMed]
- 28.Vandini S, Biagi C, Fischer M, Lanari M. Impact of rhinovirus infections in children. Viruses. 2019;11. [DOI] [PMC free article] [PubMed]
- 29.Wen X, Huang Q, Tao H, Zou W, Gao M, Guo H et al. Clinical characteristics and viral etiologies of outpatients with acute respiratory infections in Huzhou of China: a retrospective study. BMC Infect Dis. 2019;19. [DOI] [PMC free article] [PubMed]
- 30.Caporizzi A, Ravidà F, Barneschi S, Moriondo M, Nieddu F, Boscia S et al. Analysis of a cohort of 165 pediatric patients with human bocavirus infection and comparison between Mono-Infection and respiratory Co-Infections: A retrospective study. Pathogens. 2024;13. [DOI] [PMC free article] [PubMed]
- 31.Tarihi G, Alkan S, Yılmaz Ö, Yüksel H et al. Bayar Üniversitesi Tıp Fakültesi C, Sağlığı ve Hastalıkları ÇA,. New Etıologıcal Agents Of Chıldhood Lower Respıratory Tract Infectıons. Türkiye Çocuk Hastalıkları Dergisi. 2010;4:187–92.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.