Abstract
The mechanism of action of traditional Chinese medicine (TCM) remains unclear. Historically, research on TCM has mainly focused on exploring the mechanisms of active components acting on single targets. However, it is insufficient to explain the complex mechanisms by which these active components in TCM treat diseases. In recent years, the emergence of molecular glues (MGs) theory has provided new strategies to address this issue. MGs are small molecules that can promote interactions between proteins at their interface. The characteristic of MGs is to establish connections between diverse protein structures, thereby enabling a chemically-mediated proximity effect that triggers a wide spectrum of biological functions. Natural products are the result of billions of years of evolutionary processes in the natural environment. Thus, the extensive structural diversity of natural products renders them a rich source of MGs, including polyketides, terpenoids, steroids, lignans, organic acids, alkaloids and other classes. Currently, several well-known natural MGs, including the immunosuppressants cyclosporin A (CsA) and tacrolimus (FK506), as well as the anticancer agent taxol, have been incorporated into clinical practice. Meanwhile, the advancement of new technologies is propelling the discovery of novel MGs from natural products. Thus, we primarily summarize a growing variety of MGs from natural origins reported in recent years and categorize them based on the chemical structural types. Moreover, the main sources of TCM are natural products. The discovery of natural MGs promises to provide a new perspective for the elucidation of the molecular mechanism behind the efficiency of TCM. In summary, this review aims to provide insights from the perspective of natural products that could potentially influence TCM and modern drug development.
Keywords: chemically-induced proximity effect, drug development, molecular glues, nature products, protein degradation, protein-protein interactions
1. Concept of molecular glues
Molecular glues (MGs), are classified as small molecules that have the ability to bind to the interfaces of two proteins, thus facilitating protein–protein interactions (Domostegui et al., 2022, Geiger et al., 2022). In general, the proteins targeted by MGs consist of the protein-of-interest (POI) and the accessory protein (AP) (Geiger et al., 2022). The POI plays a central role in specific biological pathways (Geiger et al., 2022, Song et al., 2023). Meanwhile, AP is a protein that modulates the function of POI. Despite the fact that AP does not directly participate in biological processes, AP exerts regulatory control over the activity of POI, thereby affecting cellular function (Cooray et al., 2009). In general, MGs engage with POI and AP to form a ternary complex, which subsequently modulates protein–protein interactions to influence the functionality or stability of POI for cell phenotypes. Currently, from a molecular mechanism perspective, MGs can be divided into two categories, “non-degradative MGs” and “degradative MGs” (Domostegui et al., 2022).
Non-degradative MGs refer to a class of MGs that do not induce the degradation of POI. Non-degradative MGs establish a ternary complex with POI and AP, thereby modulating downstream signaling pathways without inducing degradation of POI (Domostegui et al., 2022, Guo, 2024). Non-degradative MGs such as cyclosporin A (CsA), tacrolimus (FK506), taxol and forskolin are of great significance for drug development (Jennewein and Croteau, 2001, Schreiber, 2021, Tesmer et al., 1997). In addition, MGs that cause the degradation of POI are degradative MGs. Degradative MGs, also known as MG degraders, promote the formation of a ternary complex involving the E3 ubiquitin ligase, which promotes ubiquitination and subsequent proteasomal degradation of POI to exert biological effects (Domostegui et al., 2022). For example, immunomodulatory drugs (IMiDs), such as thalidomide and its derivatives lenalidomide and pomalidomide, along with sulfonamides like indisulam and tasisulam, serve as MG degraders (Faust et al., 2020, Fischer et al., 2014). These agents selectively target pathogenic POI for degradation, thereby showing therapeutic potential. Thus, MG degraders present a novel avenue in drug discovery, highlighting advantages over traditional small molecule inhibitors. In particular, MG degraders can target a broader range of POI and facilitate ubiquitination-dependent degradation in a substoichiometric fashion (Dong et al., 2021). Both proteolysis-targeting chimeras (PROTACs) and MGs possess distinct advantages and limitations that enable them to target protein degradation effectively. PROTACs are particularly amenable to rational design, facilitating the development of these compounds (Yang et al., 2021). Besides, the use of PROTACs has facilitated the effective degradation of numerous pathogenic targets (Yang et al., 2021). However, PROTACs frequently exhibit high molecular weights and limited druggability (Dong et al., 2021, Yang et al., 2021). In contrast to PROTACs, MG degraders are monovalent small molecules that demonstrate superior cell permeability, reduced molecular weight, and enhanced pharmacokinetic properties. Consequently, these attributes position MG degraders as promising lead compounds in drug development (Dong et al., 2021). However, the design of MG degraders poses significant challenges due to a limited understanding of the controlling factors and a restricted number of available MG degraders (Dong et al., 2021, Yang et al., 2021).
The concept of MGs originated in the early 1990s, with the term first introduced in 1991 to explain the mechanisms of the immunosuppressive agents cyclosporine A (CsA) and FK506 (Schreiber, 2021). Previous studies have revealed that CsA and FK506 form the binary complexes CsA-cyclophilin and FK506-binding protein 12 (FKBP12), respectively. Here, calcineurin is the POI of binary complexes cyclophilin-CsA and FKBP12-FK506. The binding of cyclophilin-CsA or FKBP12-FK506 to calcineurin inhibits the enzyme activity of calcineurin, thereby causing T cell receptor inactivation in T lymphocytes and IgE receptor-mediated signaling pathways in mast cells (Liu et al., 1991, Schreiber, 1992, Schreiber, 2021). In 1994, rapamycin (RAPA) was reported to function as a MG by forming a ternary complex with FKBP12 and the mammalian target of rapamycin (mTOR), ultimately resulting in the inhibition of mTOR (Schreiber, 2021). Moreover, the plant hormone auxin has been found to serve as a MG to facilitate the interaction between the E3 ligase complex SCFTIR1 and the transcriptional regulatory factor Aux/IAA, subsequently resulting in the ubiquitination-dependent degradation of Aux/IAA (Tan et al., 2007). Furthermore, research has uncovered the MG properties of thalidomide, lenalidomide and pomalidomide, which promote the interaction between the E3 ubiquitin ligase CRL4CRBN and IKAROS family transcription factors IKZF1 and IKZF3. The interaction between CRL4CRBN and IKAROS family transcription factors triggers the ubiquitination and proteasomal degradation of IKZF1 and IKZF3, then resulting in the anti-proliferative effects in multiple myeloma cells (Fischer et al., 2014, Krönke et al., 2014). A retrospective review of the evolution of MG reveals a promising role of natural products as the source of MG discovery. Moreover, natural products possessing diverse pharmacological activities significantly contribute to the design, development, and application of pharmaceuticals. In addition, the active compounds in TCM potentially act as MGs to exert pharmacological effects. The exploration of natural MGs not only is expected to expand the target range of TCM but also is advantageous to elucidate the mechanism of action of TCM. Consequently, in recent years, researchers have intensified their efforts to identify MGs from natural sources and promote the development of TCM.
2. Working principle of molecular glues
There is a general hypothesis regarding the working principle of MGs. Upon binding to a protein, MGs undergo conformational changes by combination with specific amino acid residues of the protein to form a binding pocket. Specially, the binding pocket facilitates the interaction between POI and AP, enabling the formation of a stable complex. Thus, the unique structural adaptations of MGs facilitate the specific interaction between proteins, resulting in a chemically-induced proximity effect (Guo, 2024, Wu et al., 2022). For instance, the oxygen atoms in the sulfonyl groups of sulfonamide-based MGs like indisulam and tasisulam engage through hydrogen bonds with the amino acid residues Ala234 and Phe235 in the hydrophobic cavity of the DCAF15. The interaction between sulfonamide-based MGs and DCAF15 establishes a binding pocket that recruits the gene-splicing factors RBM39 and RBM23, thus promoting the ubiquitination-dependent protein degradation of RBM39 and RBM23 (Bussiere et al., 2020, Faust et al., 2020, Wei et al., 2022).
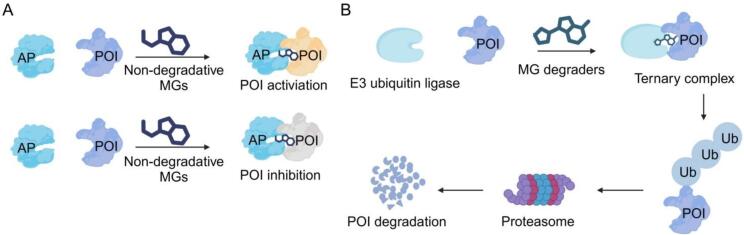
Of note, ternary complexes can regulate various biological functions of POI (Holdgate et al., 2024). For example, velcrin stabilizes the interaction between phosphodiesterase 3A and Schlafen family member 12 (SLFN12) to induce SLFN12 dephosphorylation, increasing RNase activity of SLFN12 and subsequently triggering cell death (Robinson et al., 2024, Yan et al., 2022). Moreover, antascomicin B acts as a MG to enhance the interaction between FKBP51 and Akt, thereby promoting Akt activation to regulate the downstream proteins involved in cellular survival, proliferation, and angiogenesis (Revathidevi and Munirajan, 2019, Schäfer et al., 2024, Tufano et al., 2023). Additionally, CR8 as a MG induces the interaction between complex cyclin-dependent kinase 12 (CDK12)-cyclin K and the E3 ubiquitin ligase CUL4 adaptor protein DDB1 by directly binding to CDK12 and DDB1, which results in the ubiquitination-dependent degradation of cyclin K for anti-tumor effect (Słabicki et al., 2020). In summary, MGs play a pivotal role in modulating disease progression by inhibiting, activating, or degrading POI (Fig. 1).
Fig. 1.
Working principle of MGs. Non-degradative MGs enhance the interaction between POI and AP by forming a ternary complex, thereby activating or inhibiting POI to affect downstream signaling pathway. In particular, the mechanism of non-degradative MGs does not involve the degradation of POI. POI: protein-of-interest, AP: accessory protein (A). MG degraders promote the interaction between the E3 ubiquitin ligase and POI to induce ubiquitination-dependent degradation (B).
3. Molecular glues from natural products
Natural products represent a valuable source of fundamental components for investigating MGs. MG-like natural products, characterized by different chemical structures, include polyketides, terpenoids, steroids, lignans, organic acids, alkaloids and others. In general, MG-like natural products display specific molecular structures that are responsible for selective interacting with cellular proteins to enable efficient molecular interactions (Andrei et al., 2017). Particularly, natural products-derived MGs display a series of pharmacological actions, such as anti-bacterial, anti-inflammatory, and anti-cancer properties for clinical application. Therefore, natural MGs play a crucial role in medicinal chemistry and offer opportunities for the advancement of novel pharmaceutical agents. This section will discuss the various structures and origins of MGs from natural sources to emphasize the unique biological functions.
3.1. Polyketide molecular glues
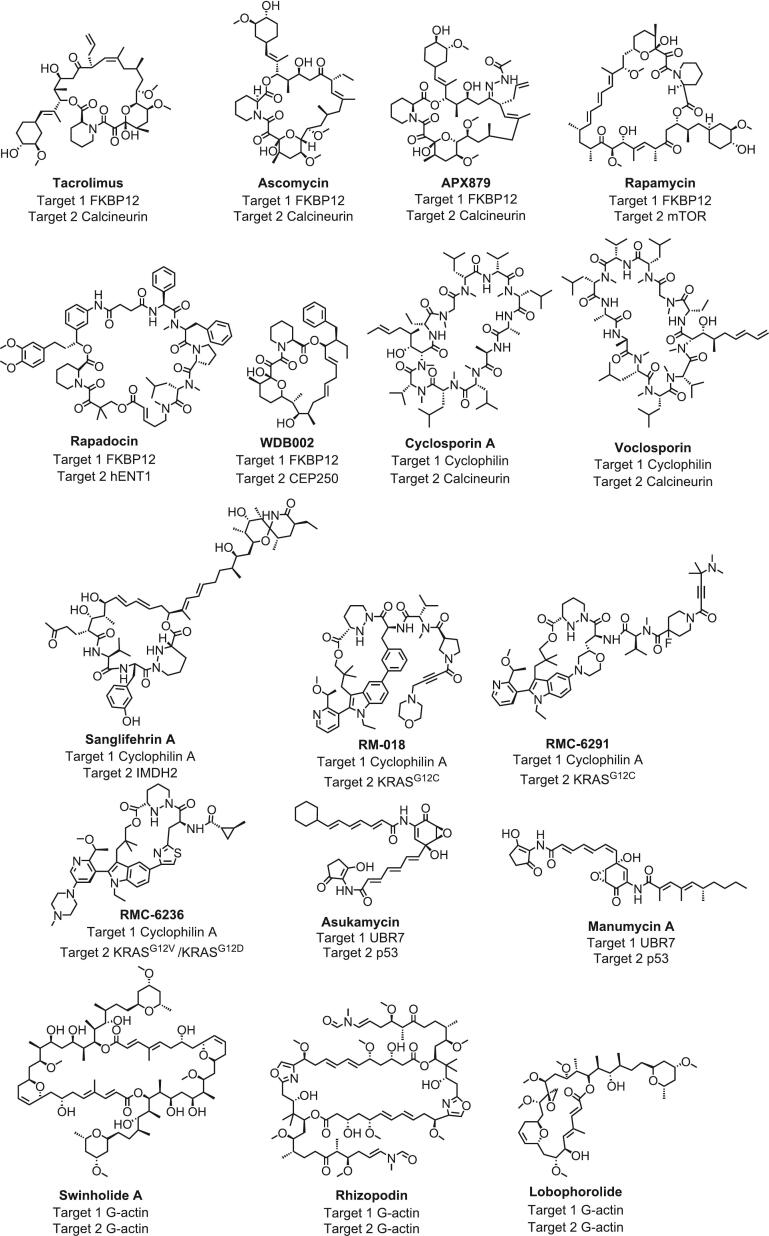
Polyketides represent a significant class of natural products, predominantly synthesized by bacteria, fungi, and plants. Polyketides are highly valued for significant clinical potential, including anti-cancer, anti-bacterial, anti-oxidant, and anti-inflammatory activities (Xu et al., 2021). From a biological synthesis perspective, polyketides are produced through a sequence of Claisen condensation reactions of short-chain acyl-CoA molecules like acetyl-CoA or malonyl-CoA, which results in the formation of structures characterized by a carbon skeleton decorated with β-keto groups (Yang et al., 2023). Thus, the structural variety of polyketides includes macrolide polyketides, polyether polyketides, xanthone polyketides, linear polyketides, hybrid polyketide nonribosomal peptides, and pyridine derivatives (Wang et al., 2023). Meanwhile, the diversity of polyketides not only enhances the functional flexibility, but also has potential to act as non-degradative MGs. Thus, the exploration and development of natural polyketides-derived MGs listed in Fig. 2 as therapeutic agents continue to be a promising field in pharmaceutical research.
Fig. 2.
Polyketide-derived natural molecular glues.
3.1.1. Polyketide molecular glues targeting FK506-binding protein 12
FKBP12 with peptidyl-prolyl isomerase activity is widely conserved among many eukaryotes (Kasahara, 2021). Polyketide MGs such as FK506 and RAPA can promote the interaction of FKBP12 with other proteins for regulating cellular function. One of the earliest discovered MGs targeting FKBP12 is FK506, isolated from the fungus Trichoderma polysporum. FK506 specifically targets FKBP12 to form a binary complex that subsequently recruits calcineurin to modulate the activation of T cells. The FK506-mediated interaction between FKBP12 and calcineurin significantly inhibits the biological function of calcineurin, which induces T cell inactivation (Domostegui et al., 2022, Guo, 2024, Liu et al., 1991). Furthermore, both calcineurin and FKBP12 are conserved in pathogenic fungi. Consequently, FK506 has anti-fungal activity by inducing the inhibitory ternary complex of FKBP12 with calcineurin (Geiger et al., 2022, Lee et al., 2018). Meanwhile, the structural analogs based on FK506 have also been reported to exhibit MG-like effects. For example, ascomycin, structurally analogous to FK506, similarly mediates interactions between FKBP12 and calcineurin, exhibiting obvious immunosuppressive and antifungal effects (Geiger et al., 2022, Sierra-Paredes and Sierra-Marcuño, 2008, Wang et al., 2019). Additionally, APX879, a derivative of FK506, exhibits decreased immunosuppressive activity, yet demonstrates enhanced antifungal effects (Geiger et al., 2022, Juvvadi et al., 2019).
Meanwhile, RAPA binds to FKBP12 to exert the immunosuppressive effects by targeting the mTOR instead of calcineurin. Further research demonstrates that the RAPA-FKBP12 complex directly interacts with the FRB domain in mTOR, thus inhibiting T cell proliferation for immunosuppressive effects (Benjamin et al., 2011, Gaali et al., 2011). Furthermore, RAPA-inspired macrocycles rapadocin can form the ternary complex with FKBP12 and human equilibrative nucleoside transporter 1 (hENT1), thereby inhibiting hENT1 to treat kidney ischemia–reperfusion injury (Guo et al., 2019). Moreover, WDB002, another FKBP12-binding polyketide, targets the centrosomal protein 250 (CEP250), which interacts with the Nsp13 protein of SARS-CoV-2, implicating potential antiviral activities by disrupting CEP250 function (Schreiber, 2021, Shigdel et al., 2020).
3.1.2. Polyketide molecular glues targeting cyclophilin
Cyclophilin is an 18 kD protein that exhibits peptidyl-prolyl isomerase activity (Schreiber, 2021). CsA, one of the earliest identified polyketide MG targeting cyclophilin, was isolated from the fungus Trichoderma polysporum. Initially, the exclusive inhibition of peptidyl-prolyl isomerase activity by CsA alone was inadequate in elucidating the biological effects of CsA, prompting the exploration of intricate mechanism of action. Then, the discovery of the interaction between the cyclophilin-CsA complex and calcineurin, leading to the formation of a ternary complex that structurally inhibits calcineurin activity, marks a significant advancement in understanding the molecular mechanisms involved. Thus, the inhibition of calcineurin blocks the dephosphorylation of NFAT, thereby diminishing effector T cell function and manifesting potent immunosuppressive effects. Similarly, the CsA analog voclosporin is able to act as a MG for the inhibition of calcineurin (Gaali et al., 2011, Liu et al., 1991, Milroy et al., 2014).
Moreover, sanglifehrin A (SfA) also binds to cyclophilin A to form the SfA-cyclophilin A binary complex. However, the SfA-cyclophilin A complex does not suppress calcineurin activity, but instead interacts with inosine monophosphate dehydrogenase 2 (IMPDH2). The interaction targets the cystathionine-β-synthase domain of IMPDH2 to induce a NF-κB mediated upregulation of the tumor suppression genes p53 and p21, thereby inhibiting T cell proliferation (Clarke et al., 2002, Geiger et al., 2022). In addition, RM-018 functions as a novel KRASG12C inhibitor. RM-018 induces a binary complex with cyclophilin A, which subsequently engages with the active KRASG12C to establish a covalent bond in a mutation-selective fashion. Of note, the interaction effectively inhibits KRASG12C by forming steric hindrance that prevents downstream effector protein binding for anti-KRASG12C mutant cancers (Geiger et al., 2022, Tanaka et al., 2021). Therefore, RM-018 contributes to the development of new targeted strategies for KRAS mutant cancers. Similarly, the RM-018 analog RMC-6291 exerts pharmacological effects by mediating the interaction between cyclophilin A and KRASG12C (Geiger et al., 2022, Mullard, 2023). In particular, RMC-6236 can promote the interaction between cyclophilin A and other mutant KRAS proteins such as KRASG12V and KRASG12D, thus inhibiting multiple RAS-driven cancers (Koltun et al., 2021, Mullard, 2023).
3.1.3. Other polyketide molecular glues
There are also some other types of polyketide MGs. Notable examples of polyketides, derived from Streptomyces, encompass asukamycin (ASU) and manumycin A (MANU A), both of which exhibit anti-tumor properties. Researchers have identified that ASU acts as a MG by covalently modifying the cysteine374 within the E3 ligase UBR7 domain, thereby forming the binary UBR7-ASU complex. The UBR7-ASU complex interacts with p53, leading to an enhancement of p53 transcriptional activity for tumor cell suppression. Similarly, the ASU analog, MANU A, also serves as a MG to mediate the interactions between UBR7 and p53, thereby revealing the anti-tumor mechanism for potential clinical utilization of polyketide MGs (Domostegui et al., 2022, Isobe et al., 2020). Additionally, macrolides like swinholide A, isolated from the sponge Theonella swinhoe, along with swinholide A analogs rhizopodin and lobophorolide, inhibit cancer progression by stabilizing G-actin homodimers to prevent actin polymerization. Thus, swinholide A represents a promising lead compound for cancer therapy, highlighting the vast potential of polyketide MGs with diverse biological activities (Andrei et al., 2017). Overall, polyketide MGs have already transitioned into clinical applications, highlighting the importance of developing MG-based therapies. The well-established research methods and techniques not only facilitate the subsequent study and development of polyketide MGs but also promote progress and innovation in related fields.
3.2. Terpenoid molecular glues
Terpenoids represent the largest class of natural products from plants. Terpenoids, composed of isoprene units with five carbon atoms, are classified into different subclasses, including hemiterpenoids, monoterpenoids, sesquiterpenoids, diterpenoids, and polyterpenoids (El-Baba et al., 2021, Tholl, 2015). Numerous terpenoids function as non-degradative MGs by stabilizing protein interactions to exert biological effects. Thus, the clarification of potential mechanisms of terpenoids-based MGs listed in Fig. 3 provides valuable insights for innovative drug development.
Fig. 3.
Terpenoid-derived natural molecular glues.
The MGs from terpenoids comprise a wide range of natural products. Previous study has indicated that the primary target of the fungal toxin fusicoccin is the plasma membrane H+-ATPase, which is responsible for the electrochemical proton concentration gradient. Meanwhile, the 14–3-3 protein, a ubiquitous AP in eukaryotic organisms, regulates various cellular processes, including cell cycle, signal transduction, and apoptosis. In particular, the 14–3-3 protein activates the H+-ATPase by binding to the autoinhibitory C-terminus of H+-ATPase. Moreover, fusicoccin stabilizes the interaction between the C-terminus of H+-ATPase and the 14-3-3 protein, leading to sustained activation of the proton pump for stomatal opening in plants (Anders et al., 2013, Tholl, 2015). Meanwhile, fusicoccin has the ability to impede the transcriptional activity of estrogen receptor α (ERα) by enhancing the interaction between 14-3-3 protein and ERα. This discovery presents a promising potential for the development of novel therapies for recurrent breast cancer (Anders et al., 2013, Milroy et al., 2014). Moreover, fusicoccin forms a ternary complex with a 14-3-3σ dimer and an murine double minute 2 (MDM2) di-phosphorylated peptide for promoting degradation of MDM2 to protect the activity of p53 tumor suppressor, thus exhibiting anti-tumor effect (Ward et al., 2024). Further, fusicoccin derivatives such as fusicoccin-THF (FC-THF) has been reported to enhance plasma membrane insertion of potassium channels TASK3 and TASK1 through interaction with the 14–3-3 protein, thereby influencing crucial physiological functions including heart electrical activity, oxygen sensing, and aldosterone secretion. Therefore, FC-THF may act as a lead compound for the regulation of potassium channel (Konstantinidou et al., 2023, Milroy et al., 2014). Similarly, cotylenin A, a metabolite from Cladosporium sp., continuously activates H+-ATPase by mediating interactions between the 14-3-3 protein and H+-ATPase, thus promoting potassium channel-mediated transpiration toxic to plants (Ottmann et al., 2009). In addition, cotylenin A provides treatment strategy for RAS mutant cancers by promoting physical interaction of C-RAF with the 14-3-3 protein (Molzan et al., 2013).
The antibiotic kirromycin exhibits the capability to stabilize elongation factor Tu (Ef-Tu) in the GTPase conformation, thereby impeding the entry of the receptor domain of aminoacyl tRNA into the peptide transferase center for bacteriostatic effects (Stark et al., 1997, Würtele et al., 2003). In addition, brefeldin A, a fungal metabolite derived from Eupenicillium brefeldianum blocks GDP/GTP exchange on ADP-ribosylation factor (ARF) by binding to the interface between the inactive small G protein ARF-GDP and the SEC7 domain of the ARF guanine nucleotide exchange factor for trapping the ARF-GDP/ SEC7 complex at the membrane. The action of brefeldin A ultimately hinders the generation of active ARF-GTP, leading to the inhibition of Golgi membrane transport function. Since ARF is primarily responsible for regulating cell membrane transport and the formation of the cell membrane skeleton, brefeldin A inhibits ARF-GTP formation to disrupt Golgi membrane transport function and protein secretion, which renders it a valuable tool for investigating these biological processes (N. Anders and Jürgens, 2008, Milroy et al., 2014, Peyroche et al., 1999).
Apart from fungal metabolites, the terpenoids in traditional medicine also have the potential to function as non-degradative MGs. Forskolin, derived from Coleus forskohlii, enhances cardiac function and reduces blood pressure by stabilizing the interaction between the C1a domain from adenylyl cyclase V and the C2 domain from adenylyl cyclase II (Maghsoudi et al., 2024, Milroy et al., 2014, Tesmer et al., 1997, Würtele et al., 2003). Moreover, taxol, the primary anti-tumor compound in Taxus, impedes the proliferation of tumor cells by stabilizing dynamic protein–protein interactions between α-tubulin and β-tubulin, thus resulting in microtubule stabilization (Dou et al., 2023, Jennewein and Croteau, 2001).
In summary, terpenoids possess significant research value due to the wide range of pharmacological activities, such as anti-inflammatory, antibacterial, anti-tumor properties, and potential for cardiovascular diseases. Thus, the identification of terpenoids as MGs underscores promising relevance in clinical applications.
3.3. Steroid molecular glues
Steroids are characterized by the basic skeleton of cyclopentano-perhydrophenanthrene, which comprises three cyclohexane rings (rings A, B, and C) and one cyclopentane ring (ring D) (Gomes et al., 2023, Zhou and Liu, 2021). Research indicates that certain steroids listed in Fig. 4 function as MGs for exerting significant pharmacological effects. For example, bufalin, an anti-tumor component from Bufonis venenum, specifically targets the transcription factor E2F2. Here, bufalin serves as a MG to promote the formation of a ternary complex of E2F2 and atypical E3 ligase ZFP91. The ternary complex induces the ubiquitination and subsequent degradation of E2F2, resulting in the suppression of c-Myc transcription for anti-liver cancer effect. Thus, the evidence highlights the potential of bufalin as a therapeutic MG in cancer treatment (Liu et al., 2022). Meanwhile, natural product physachenolide C, derived from the 17β-hydroxywithanolide class, demonstrates the ability as a steroid MG by linking the E3 ligases TRIM25, UBR4, UBR5, ZNF598, and HUWE1 to the bromodomain and extra-terminal (BET) domain family proteins BRD3 and BRD4, thereby promoting their degradation for overcoming resistance mechanisms of current prostate cancer therapies (Zerio et al., 2023). Furthermore, there are other steroid MG degraders for the treatment of prostate cancer. Galeterone and its analog VNPP433-3β enhance the interaction between the androgen receptor (AR), a key driver of prostate cancer, and the E3 ligases MDM2/CHIP in prostate cancer cells. This interaction leads to the ubiquitination-dependent degradation of AR and its splice variant AR-V7, thereby inhibiting activation of oncogenic eukaryotic translation initiation factor 4E phosphorylation, finally blocking the growth of prostate cancer cells (Thankan et al., 2023, Thomas et al., 2022, Thomas et al., 2023). In addition, brassinolide, a plant steroid hormone, significantly enhances the interaction between the cell surface receptor BRI1 and BRI1-associated kinase 1 (BAK1). As a MG, brassinolide promotes the heterodimerization of the leucine-rich repeat domains of BRI1 and BAK1, thereby initiating a BRI1 phosphorylation-mediated intracellular signaling pathway for plant growth (Santiago et al., 2013, Sun et al., 2013). In conclusion, steroids serve as an important source for natural MGs. Thus, the discovery of steroids-derived MGs underscores the underexplored potential of steroids as lead drugs.
Fig. 4.
Steroid-derived natural molecular glues.
3.4. Lignan molecular glues
Lignans belong to a class of natural compounds that are polymerized from two phenylpropanoid derivatives (Brito & Zang, 2018). Lignan compounds listed in Fig. 5 have the capacity to act as MGs by forming a ternary complex with two proteins. For example, schisandrol B (SolB) is a natural compound derived from the fruit of Schisandra chinensis (Turcz.) Baill. (Wuweizi in Chinese). SolB functions as a MG that boosts the interaction between MDM and p53, thereby enhancing p53 ubiquitination and degradation. Consequently, SolB exhibits significant anti-inflammatory and anti-senescence properties. Thus, SolB exhibits promising potential as a therapeutic candidate for calcific aortic valve disease (Liu et al., 2024). In addition, lignan derivatives MMH2 and GNE-0011 act as MGs by facilitating the interaction between the BET protein BRD4 and the E3 ubiquitin ligase DCAF16. This interaction ultimately leads to the targeted degradation of BRD4. The strategic degradation of BRD4 presents a novel therapeutic approach in the field of cancer treatment, potentially offering new avenues for intervention in oncogenic signaling pathways (Donati et al., 2018, Konstantinidou and Arkin, 2024). In particular, the relatively simple structure of lignans with fewer elements, which facilitates the synthesis of lignan-derivative MGs for the advancement of clinical translation.
Fig. 5.
Lignan-derived natural molecular glues.
3.5. Organic acid molecular glues
Some organic compounds with acidity listed in Fig. 6 from natural source demonstrate characteristics of MGs. Organic acid plant hormones play essential roles in exerting physiological effects through the MG-dependent protein degradation process. For example, indole-3-acetic acid (IAA), a growth-promoting hormone in plants, functions as a MG degrader by directly interacting with the E3 ligase complex SCFTIR1, thereby initiating the ubiquitin-dependent proteolysis of the transcriptional regulatory factor Aux/IAA for plant development (Dharmasiri et al., 2005, Gray et al., 2001, Tan et al., 2007). Similarly, the bioactive compound (3R,7S)-jasmonoyl-L-isoleucine (JA-Ile) is produced when the plant hormone jasmonic acid (JA) specifically binds with Ile. JA-Ile acts as a MG that enhances the interaction between the F-box protein subunit COI1 of SCFCOI1 and the JASATE ZIM DOMAIN (JAZ) family of transcriptional regulators, thereby leading to the ubiquitination-mediated degradation of JAZ. Then, the degradation of JAZ promotes MYC2 activity to upregulate the jasmonic acid-responsive genes for various plant regulatory processes (Sheard et al., 2010). Meanwhile, abscisic acid, as a MG, induces proximity of the monomeric receptor Pyl to protein phosphatase ABI1 for inducing leaves to abscise (Stanton et al., 2018, Yin et al., 2009). In addition to plant hormones, the small molecule inositol tetraphosphate (IP4) has also the capability to enhance the enzymatic activity of histone deacetylase 3 (HDAC3) by stabilizing the interaction of HDAC3 with the deacetylase activation domain of the SMRT co-repressor. HDAC has become a crucial focus in cancer treatment, as HDAC inhibitor has the potential to block cell growth. Hence, understanding the mechanism of IP4 presents an avenue for the development of innovative HDAC inhibitors (Khan and La Thangue, 2012, Milroy et al., 2014, Rui et al., 2023). Moreover, adenosine monophosphate acts as MG enhancing the interaction between the 14-3-3 protein and the carbohydrate-response element-binding protein (ChREBP) to regulate subcellular localization in response to changing glucose levels (Stevers et al., 2018). In the future, it is feasible to design new compounds for innovative drug development by modifying the structure of organic acid MGs.
Fig. 6.
Organic acid-derived molecular glues.
3.6. Alkaloid molecular glues
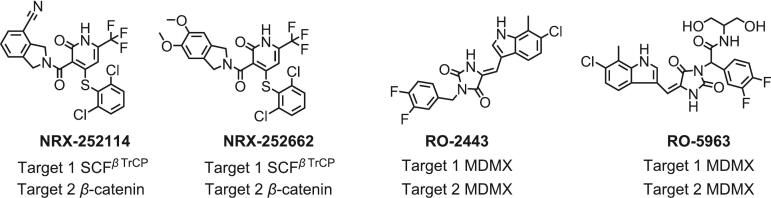
Alkaloids are nitrogenous organic compounds found in nature, encompassing diverse types such as pyrrole alkaloids, pyridine alkaloids, indole alkaloids, and benzylisoquinoline alkaloids (Yamada & Sato, 2021). Alkaloid-derived compounds listed in Fig. 7 undoubtedly possess the potential to function as MGs, demonstrating various physiological effects. Isoindoline alkaloids are widely found in a variety of natural products. The first naturally occurring isoindole is isolated from the sponge Reniera sp (Speck & Magauer, 2013). Compounds such as NRX-252114, and NRX-252262 that have dihydroindole nucleus structure facilitate the interaction between mutant β-catenin and its corresponding E3 ligase, SCFβTrCP, to enhance the ubiquitylation and subsequent degradation of mutant β-catenin. This process effectively hinders the progression of tumors characterized by elevated levels of β-catenin (Jaffry & Wells, 2023). Moreover, RO-2443 and RO-5963 with indole skeleton serves as a MG that facilitates MDMX dimerization, consequently impeding the interaction with downstream p53. This mechanism offers a potential therapeutic strategy for cancers characterized by MDMX overexpression (Graves et al., 2012, Holdgate et al., 2024). Therefore, alkaloids with various chemical structures and functions have the potential to serve as a crucial source of medically significant compounds.
Fig. 7.
Alkaloids-derived molecular glues.
3.7. Other natural molecular glues
Apart from the diverse structural categories of MGs mentioned above, ions also have a significant physiological impact similar to MGs. For example, Zn2+ acts as a specialized metal ion type cofactor to promote the interaction of E3 ubiquitin ligase CUL2FEM1B and the reduced form of FNIP1 protein. The interaction triggers the ubiquitination-dependent degradation of FNIP1 via the proteasome pathway, thus facilitating the restoration of mitochondrial oxidative phosphorylation to replenish cellular ATP levels. Therefore, these studies indicate that manipulating mitochondrial function through the MGs with Zn2+-like mechanism may represent a promising approach for cancer therapeutics (Domostegui et al., 2022, Manford et al., 2021). Moreover, proteins can also exert physiological functions similar to MGs. For example, the 14-3-3 protein functions as a molecular glue to bind to phosphorylated Cdc25C and Cdc25B phosphatases, regulating the progression of mitosis and facilitating repair of DNA damage (Forrest and Gabrielli, 2001, Robinson et al., 2024). Therefore, the discovery of novel MGs expands the conventional notion that MGs are restricted to organic small-molecular compounds. Further research should focus on unraveling the unique mechanisms of these specific types of ion and protein-based MGs, thus potentially enabling the exploration of therapeutic strategies.
4. Future trend of natural molecular glues
Natural products, with abundant and structurally diverse characteristics, have long been a vital source of lead compounds. Thus, the discovery of MGs from such natural sources is a crucial strategy. Natural MGs have the potential to enhance weak or establish new protein–protein interactions, leading to significant physiological effects on downstream signaling pathways (Holdgate et al., 2024, Wu et al., 2022). Therefore, natural MGs are promising lead compounds for drug design and do not necessarily rely on classical ligand-binding pockets to exert biological function. Consequently, the function characteristic of MGs expands the range of potential targets for drug development in pharmaceutical research (Wu et al., 2022).
The unique mechanism of MG degraders involves the enhancement and stabilization of interactions between E3 ligases and disease-related proteins, thereby facilitating the ubiquitination-mediated proteasomal degradation. In particular, the MG degraders operate through transient binding rather than competitive occupancy of target proteins, and subsequently dissociate after inducing the polyubiquitination of pathogenic proteins (Sasso et al., 2023). Consequently, a MG degrader is capable of eliminating multiple pathogenic proteins, leading to increased efficacy at significantly low dosages. Moreover, MG degraders can completely abolish the functions of pathogenic proteins, offering enhanced sensitivity towards drug-resistant targets and the potential to impact non-enzymatic protein functions (Sasso et al., 2023). However, the availability of natural MG degraders is presently constrained. Therefore, a comprehensive comprehension of current characteristics of MGs is crucial for the rational identification of MG degraders from natural sources.
Considering that natural products are an important source of MGs, it is necessary to develop a series of novel methods for the discovery of MGs. Firstly, the discovery of MGs often happens inadvertently, while intentional design presents significant challenges, which emphasizes the need for a deeper understanding of the working mechanisms (Wu et al., 2022). Secondly, the advancement of novel computational methodologies for forecasting the binding configuration of protein–protein interaction complexes triggered by MGs will enhance virtual screening and structure-based discovery of new MGs. Meanwhile, the integration of artificial intelligence technology may help to improve the efficiency of data mining and molecular design. For example, machine learning algorithms offer the potential to construct robust models from extensive high throughput screening, multi-omics, and protein–protein interactions network data, thereby enabling accurate prediction of potential protein-MGs-protein complexes (Dewey et al., 2023, Dong et al., 2021). In addition, MGs usually regulate protein–protein interactions through solvent exposure regions, thus novel MGs can be obtained by structural modification. Meanwhile, given the generally restricted dimensions of the protein–protein interaction interface, an effective strategy entails designing a MG that can promote the rearrangement of residues on the protein–protein binding surface, thus forming a more accommodating binding pocket for MGs (Dong et al., 2021, Wu et al., 2022).
In addition to the proteasomal pathway, lysosomes offer an alternative route for degrading disease-related proteins including membrane proteins, extracellular proteins, and protein aggregates. Therefore, investigating lysosomal pathway-mediated targeted protein degradation strategies will broaden the range of substrates available for targeted degradation (Sasso et al., 2023). Nowadays, there is a significant deficiency in both the variety and quantity of natural MGs from medicinal plants; therefore, exploring natural products that target lysosomal pathways is advantageous for expanding the sources of natural MGs.
In conclusion, the discovery of natural MGs is expected to increase due to commitment of scientists to developing advanced technologies and methodologies for studying these materials. A major challenge that remains is the systematic acquisition of targeted MGs through rational design, guided by a thorough understanding of the physical and chemical properties of existing natural MGs. Overcoming this challenge could facilitate the development of MGs that exhibit improved therapeutic efficacy, thereby establishing MGs as valuable pharmacological agents for treating a range of diseases. Consequently, the advancement of research focused on natural MGs is anticipated to contribute significantly to the evolution of modern medicine. Notably, numerous natural MGs constitute the primary active constituents identified within TCM. Thus, the comprehensive screening of effective natural medicinal agents from TCM presents an innovative strategy to enhance both the diversity and availability of medicinal agents. Furthermore, the identification of natural MGs holds the potential to elucidate the complex pharmacological mechanisms underlying the therapeutic effects of TCM, thereby facilitating the modernization and scientific validation of traditional healing practices.
CRediT authorship contribution statement
Lina Yin: Conceptualization, Software, Visualization, Writing – original draft, Writing – review & editing. Tingting Niu: Writing – review & editing. Ling Li: Writing – review & editing. Wei Yu: Writing – review & editing, Visualization. Bo Han: Writing – review & editing. Asma Rehman: Writing – review & editing, Visualization. Kewu Zeng: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was financially supported by Jinan New 20 Policies for Higher Education Funding (No. 202228048), Natural Science Foundation of Shandong Province (Joint Fundation for Innovation and Development) (No. ZR2022LZY021), the Special Fund for “Tian-Chi Talent Introduction Program”, and the Special Fund for Taishan Scholars Project in Shandong Province (No. tstp20230633).
References
- Anders C., Higuchi Y., Koschinsky K., Bartel M., Schumacher B., Thiel P.…Ottmann C. A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chemistry & Biology. 2013;20(4):583–593. doi: 10.1016/j.chembiol.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Anders N., Jürgens G. Large ARF guanine nucleotide exchange factors in membrane trafficking. Cellular and Molecular Life Sciences. 2008;65(21):3433–3445. doi: 10.1007/s00018-008-8227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei S.A., Sijbesma E., Hann M., Davis J., O'Mahony G., Perry M.W.D.…Ottmann C. Stabilization of protein-protein interactions in drug discovery. Expert Opinion on Drug Discovery. 2017;12(9):925–940. doi: 10.1080/17460441.2017.1346608. [DOI] [PubMed] [Google Scholar]
- Benjamin D., Colombi M., Moroni C., Hall M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nature Reviews Drug Discovery. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- Brito A.F., Zang Y. A review of lignan metabolism, milk enterolactone concentration, and antioxidant status of dairy cows fed flaxseed. Molecules. 2018;24(1):41. doi: 10.3390/molecules24010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere D.E., Xie L.L., Srinivas H., Shu W., Burke A., Be C.…Paulk J. Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nature Chemical Biology. 2020;16(1):15–23. doi: 10.1038/s41589-019-0411-6. [DOI] [PubMed] [Google Scholar]
- Clarke S.J., McStay G.P., Halestrap A.P. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. Journal of Biological Chemistry. 2002;277(38):34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- Cooray S.N., Chan L., Webb T.R., Metherell L., Clark A.J.L. Accessory proteins are vital for the functional expression of certain G protein-coupled receptors. Molecular and Cellular Endocrinology. 2009;300(1–2):17–24. doi: 10.1016/j.mce.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Dewey J.A., Delalande C., Azizi S.A., Lu V., Antonopoulos D., Babnigg G. Molecular glue discovery: Current and future approaches. Journal of Medicinal Chemistry. 2023;66(14):9278–9296. doi: 10.1021/acs.jmedchem.3c00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Domostegui A., Nieto-Barrado L., Perez-Lopez C., Mayor-Ruiz C. Chasing molecular glue degraders: Screening approaches. Chemical Society Reviews. 2022;51(13):5498–5517. doi: 10.1039/d2cs00197g. [DOI] [PubMed] [Google Scholar]
- Donati B., Lorenzini E., Ciarrocchi A. BRD4 and cancer: Going beyond transcriptional regulation. Molecular Cancer. 2018;17(1):164. doi: 10.1186/s12943-018-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G.Q., Ding Y., He S.P., Sheng C.Q. Molecular glues for targeted protein degradation: From serendipity to rational discovery. Journal of Medicinal Chemistry. 2021;64(15):10606–10620. doi: 10.1021/acs.jmedchem.1c00895. [DOI] [PubMed] [Google Scholar]
- Dou Z.H., Ding X., Feng Y., Zhou L.H., Gu Y.Q., Liu L. Systematic profiling of taxol and its analogues (taxalogues) binding to β-tubulin and molecular analysis of their effects on microtubule stabilization. Journal of the Chinese Chemical Society. 2023;70(4):825–836. [Google Scholar]
- El-Baba C., Baassiri A., Kiriako G., Dia B., Fadlallah S., Moodad S., Darwiche N. Terpenoids' anti-cancer effects: Focus on autophagy. Apoptosis. 2021;26(9–10):491–511. doi: 10.1007/s10495-021-01684-y. [DOI] [PubMed] [Google Scholar]
- Faust T.B., Yoon H., Nowak R.P., Donovan K.A., Li Z.N., Cai Q.…Fischer E.S. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nature Chemical Biology. 2020;16(1):7–14. doi: 10.1038/s41589-019-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E.S., Böhm K., Lydeard J.R., Yang H.D., Stadler M.B., Cavadini S.…Thomä N.H. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest A., Gabrielli B. Cdc25B activity is regulated by 14-3-3. Oncogene. 2001;20(32):4393–4401. doi: 10.1038/sj.onc.1204574. [DOI] [PubMed] [Google Scholar]
- Gaali S., Gopalakrishnan R., Wang Y., Kozany C., Hausch F. The chemical biology of immunophilin ligands. Current Medicinal Chemistry. 2011;18(35):5355–5379. doi: 10.2174/092986711798194342. [DOI] [PubMed] [Google Scholar]
- Geiger T.M., Schäfer S.C., Dreizler J.K., Walz M., Hausch F. Clues to molecular glues. Current Research in Chemical Biology. 2022;2 [Google Scholar]
- Gomes A.R., Pires A.S., Roleira F.M.F., Tavares-da-Silva E.J. The structural diversity and biological activity of steroid oximes. Molecules. 2023;28(4):1690. doi: 10.3390/molecules28041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B., Thompson T., Xia M.X., Janson C., Lukacs C., Deo D.…Vassilev L.T. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(29):11788–11793. doi: 10.1073/pnas.1203789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W.M., Kepinski S., Rouse D., Leyser O., Estelle M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;414(6861):271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- Guo Z.F., Hong S.Y., Wang J.X., Rehan S., Liu W.K., Peng H.J.…Liu J.O. Rapamycin-inspired macrocycles with new target specificity. Nature Chemistry. 2019;11(3):254–263. doi: 10.1038/s41557-018-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.R. Chemically induced proximity and molecular glue. Acta Pharmaceutica Sinica. 2024:1–17. [Google Scholar]
- Holdgate G.A., Bardelle C., Berry S.K., Lanne A., Cuomo M.E. Screening for molecular glues - Challenges and opportunities. SLAS Discovery. 2024;29(2) doi: 10.1016/j.slasd.2023.12.008. [DOI] [PubMed] [Google Scholar]
- Isobe Y., Okumura M., McGregor L.M., Brittain S.M., Jones M.D., Liang X.…Nomura D.K. Manumycin polyketides act as molecular glues between UBR7 and P53. Nature Chemical Biology. 2020;16(11):1189–1198. doi: 10.1038/s41589-020-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffry U., Wells G. Small molecule and peptide inhibitors of βTrCP and the βTrCP-NRF2 protein-protein interaction. Biochemical Society Transactions. 2023;51(3):925–936. doi: 10.1042/BST20220352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S., Croteau R. Taxol: Biosynthesis, molecular genetics, and biotechnological applications. Applied Microbiology and Biotechnology. 2001;57(1–2):13–19. doi: 10.1007/s002530100757. [DOI] [PubMed] [Google Scholar]
- Juvvadi P.R., Fox D., 3rd, Bobay B.G., Hoy M.J., Gobeil S.M.C., Venters R.A.…Steinbach W.J. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nature Communications. 2019;10(1):4275. doi: 10.1038/s41467-019-12199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K. Physiological function of FKBP12, a primary target of rapamycin/FK506: A newly identified role in transcription of ribosomal protein genes in yeast. Current Genetics. 2021;67(3):383–388. doi: 10.1007/s00294-020-01142-3. [DOI] [PubMed] [Google Scholar]
- Khan O., La Thangue N.B. HDAC inhibitors in cancer biology: Emerging mechanisms and clinical applications. Immunology and Cell Biology. 2012;90(1):85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- Koltun E., Cregg J., Rice M.A., Whalen D.M., Freilich R., Jiang J.…Smith J.A. First-in-class, orally bioavailable KRASG12V(ON) tri-complex inhibitors, as single agents and in combinations, drive profound anti-tumor activity in preclinical models of KRASG12V mutant cancers. Cancer Research. 2021;81(13_Supplement):1260. [Google Scholar]
- Konstantinidou M., Arkin M.R. Molecular glues for protein-protein interactions: Progressing toward a new dream. Cell Chemical Biology. 2024;31(6):1064–1088. doi: 10.1016/j.chembiol.2024.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou M., Visser E.J., Vandenboorn E., Chen S., Jaishankar P., Overmans M.…Arkin M.R. Structure-based optimization of covalent, small-molecule stabilizers of the 14-3-3σ/ERα protein-protein interaction from nonselective fragments. Journal of the American Chemical Society. 2023;145(37):20328–20343. doi: 10.1021/jacs.3c05161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke J., Udeshi N.D., Narla A., Grauman P., Hurst S.N., McConkey M.…Ebert B.L. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee K.T., Lee S.J., Beom J.Y., Hwangbo A., Jung J.A.…Bahn Y.S. In Vitro and in vivo assessment of FK506 analogs as novel antifungal drug candidates. Antimicrobial Agents and Chemotherapy. 2018;62(11):e01627–e10718. doi: 10.1128/AAC.01627-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J.D., Jr., Lane W.S., Friedman J., Weissman I., Schreiber S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Yang H., Zhuo F.F., Yang Z., Zhao M.M., Guo Q.…Tu P.F. Atypical E3 ligase ZFP91 promotes small-molecule-induced E2F2 transcription factor degradation for cancer therapy. EBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang K., Zheng Q., Liu X., Yang Y., Xie C.…Dong N. Schisandrol B inhibits calcification of aortic valve by targeting p53 related inflammatory and senescence. Biomedicine & Pharmacotherapy. 2024;178 doi: 10.1016/j.biopha.2024.117241. [DOI] [PubMed] [Google Scholar]
- Maghsoudi S., Shuaib R., Van Bastelaere B., Dakshinamurti S. Adenylyl cyclase isoforms 5 and 6 in the cardiovascular system: Complex regulation and divergent roles. Frontiers in Pharmacology. 2024;15 doi: 10.3389/fphar.2024.1370506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford A.G., Mena E.L., Shih K.Y., Gee C.L., McMinimy R., Martínez-González B.…Rape M. Structural basis and regulation of the reductive stress response. Cell. 2021;184(21):5375–5390.e16. doi: 10.1016/j.cell.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milroy L.G., Grossmann T.N., Hennig S., Brunsveld L., Ottmann C. Modulators of protein-protein interactions. Chemical Reviews. 2014;114(9):4695–4748. doi: 10.1021/cr400698c. [DOI] [PubMed] [Google Scholar]
- Molzan M., Kasper S., Röglin L., Skwarczynska M., Sassa T., Inoue T.…Ottmann C. Stabilization of physical RAF/14-3-3 interaction by cotylenin A as treatment strategy for RAS mutant cancers. ACS Chemical Biology. 2013;8(9):1869–1875. doi: 10.1021/cb4003464. [DOI] [PubMed] [Google Scholar]
- Mullard A. Glue-based KRAS inhibitors make their debut cancer trial mark. Nature Reviews Drug Discovery. 2023;22(12):942. doi: 10.1038/d41573-023-00169-8. [DOI] [PubMed] [Google Scholar]
- Ottmann C., Weyand M., Sassa T., Inoue T., Kato N., Wittinghofer A., Oecking C. A structural rationale for selective stabilization of anti-tumor interactions of 14-3-3 proteins by cotylenin A. Journal of Molecular Biology. 2009;386(4):913–919. doi: 10.1016/j.jmb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Peyroche A., Antonny B., Robineau S., Acker J., Cherfils J., Jackson C.L. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: Involvement of specific residues of the Sec7 domain. Molecular Cell. 1999;3(3):275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- Revathidevi S., Munirajan A.K. Akt in cancer: Mediator and more. Seminars in Cancer Biology. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Robinson S.A., Co J.A., Banik S.M. Molecular glues and induced proximity: An evolution of tools and discovery. Cell Chemical Biology. 2024;31(6):1089–1100. doi: 10.1016/j.chembiol.2024.04.001. [DOI] [PubMed] [Google Scholar]
- Rui H., Ashton K.S., Min J., Wang C., Potts P.R. Protein-protein interfaces in molecular glue-induced ternary complexes: Classification, characterization, and prediction. RSC Chemical Biology. 2023;4(3):192–215. doi: 10.1039/d2cb00207h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J., Henzler C., Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341(6148):889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- Sasso J.M., Tenchov R., Wang D.S., Johnson L.S., Wang X.M., Zhou Q.A. Molecular glues: The adhesive connecting targeted protein degradation to the clinic. Biochemistry. 2023;62(3):601–623. doi: 10.1021/acs.biochem.2c00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer S.C., Voll A.M., Bracher A., Ley S.V., Hausch F. Antascomicin B stabilizes FKBP51-Akt1 complexes as a molecular glue. Bioorganic & Medicinal Chemistry Letters. 2024;104 doi: 10.1016/j.bmcl.2024.129728. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L. The rise of molecular glues. Cell. 2021;184(1):3–9. doi: 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- Sheard L.B., Tan X., Mao H.B., Withers J., Ben-Nissan G., Hinds T.R.…Zheng N. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468(7322):400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigdel U.K., Lee S.J., Sowa M.E., Bowman B.R., Robison K., Zhou M.Y.…Verdine G.L. Genomic discovery of an evolutionarily programmed modality for small-molecule targeting of an intractable protein surface. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(29):17195–17203. doi: 10.1073/pnas.2006560117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Paredes G., Sierra-Marcuño G. Ascomycin and FK506: Pharmacology and therapeutic potential as anticonvulsants and neuroprotectants. CNS Neuroscience Therapeutics. 2008;14(1):36–46. doi: 10.1111/j.1527-3458.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słabicki M., Kozicka Z., Petzold G., Li Y.D., Manojkumar M., Bunker R.D.…Ebert B.L. The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature. 2020;585(7824):293–297. doi: 10.1038/s41586-020-2374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Jiao Z., Hou Z., Wang R., Lian C., Xing Y.…Yin F. Selective protein of interest degradation through the split-and-mix liposome proteolysis targeting Chimera approach. Journal of the American Chemical Society. 2023;145(40):21860–21870. doi: 10.1021/jacs.3c05948. [DOI] [PubMed] [Google Scholar]
- Speck K., Magauer T. The chemistry of isoindole natural products. Beilstein Journal of Organic Chemistry. 2013;9:2048–2078. doi: 10.3762/bjoc.9.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B.Z., Chory E.J., Crabtree G.R. Chemically induced proximity in biology and medicine. Science. 2018;359(6380) doi: 10.1126/science.aao5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H., Rodnina M.V., Rinke-Appel J., Brimacombe R., Wintermeyer W., van Heel M. Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature. 1997;389(6649):403–406. doi: 10.1038/38770. [DOI] [PubMed] [Google Scholar]
- Stevers L.M., Sijbesma E., Botta M., MacKintosh C., Obsil T., Landrieu I.…Ottmann C. Modulators of 14-3-3 protein-protein interactions. Journal of Medicinal Chemistry. 2018;61(9):3755–3778. doi: 10.1021/acs.jmedchem.7b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.D., Han Z.F., Tang J., Hu Z.H., Chai C.L., Zhou B., Chai J.J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Research. 2013;23(11):1326–1329. doi: 10.1038/cr.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Calderon-Villalobos L.I.A., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Lin J.J., Li C., Ryan M.B., Zhang J., Kiedrowski L.A.…Corcoran R.B. Clinical acquired resistance to KRASG12C inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discovery. 2021;11(8):1913–1922. doi: 10.1158/2159-8290.CD-21-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer J.J., Sunahara R.K., Gilman A.G., Sprang S.R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278(5345):1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- Thankan R.S., Thomas E., Purushottamachar P., Weber D.J., Njar V.C.O. Salinization dramatically enhance the anti-prostate cancer efficacies of AR/AR-V7 and Mnk1/2 molecular glue degraders, galeterone and VNPP433-3β which outperform docetaxel and enzalutamide in CRPC CWR22Rv1 xenograft mouse model. Bioorganic Chemistry. 2023;139 doi: 10.1016/j.bioorg.2023.106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D. Biosynthesis and biological functions of terpenoids in plants. Advances in Biochemical Engineering/Biotechnology. 2015;148:63–106. doi: 10.1007/10_2014_295. [DOI] [PubMed] [Google Scholar]
- Thomas E., Thankan R.S., Purushottamachar P., Huang W.L., Kane M.A., Zhang Y.J.…Njar V.C.O. Novel AR/AR-V7 and Mnk1/2 degrader, VNPP433-3β: Molecular mechanisms of action and efficacy in AR-overexpressing castration resistant prostate cancer in vitro and in vivo models. Cells. 2022;11(17):2699. doi: 10.3390/cells11172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E., Thankan R.S., Purushottamachar P., Weber D.J., Njar V.C.O. Targeted degradation of androgen receptor by VNPP433-3β in castration-resistant prostate cancer cells implicates interaction with E3 ligase MDM2 resulting in ubiquitin-proteasomal degradation. Cancers. 2023;15(4):1198. doi: 10.3390/cancers15041198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M., Marrone L., D’Ambrosio C., Di Giacomo V., Urzini S., Xiao Y.…Romano S. FKBP51 plays an essential role in Akt ubiquitination that requires Hsp90 and PHLPP. Cell Death & Disease. 2023;14(2):116. doi: 10.1038/s41419-023-05629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang J.H., Yuan J., Jiang L.Y., Jiang X.L., Yang B.…Huang D. Generation of Streptomyces hygroscopicus cell factories with enhanced ascomycin production by combined elicitation and pathway-engineering strategies. Biotechnology and Bioengineering. 2019;116(12):3382–3395. doi: 10.1002/bit.27158. [DOI] [PubMed] [Google Scholar]
- Wang L., Lu H., Jiang Y.Y. Natural polyketides act as promising antifungal agents. Biomolecules. 2023;13(11):1572. doi: 10.3390/biom13111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.A., Romartinez-Alonso B., Kay D.F., Bellamy-Carter J., Thurairajah B., Basran J.…Doveston R.G. Characterizing the protein-protein interaction between MDM2 and 14-3-3σ; proof of concept for small molecule stabilization. Journal of Biological Chemistry. 2024;300(2) doi: 10.1016/j.jbc.2024.105651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.F., Ouyang Y.X., Cheng Q.L., Guo X.H. Research progress of molecular glue degraders based on PROTAC technology. Journal of Gannan Medical University. 2022;42(11):1202–1208. [Google Scholar]
- Wu H.Y., Yao H., He C., Jia Y.L., Zhu Z.Y., Xu S.T.…Xu J.Y. Molecular glues modulate protein functions by inducing protein aggregation: A promising therapeutic strategy of small molecules for disease treatment. Acta Pharmaceutica Sinica B. 2022;12(9):3548–3566. doi: 10.1016/j.apsb.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtele M., Jelich-Ottmann C., Wittinghofer A., Oecking C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO Journal. 2003;22(5):987–994. doi: 10.1093/emboj/cdg104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Qu R., Wu W., Jiang C., Shao D., Shi J. Applications of microbial co-cultures in polyketides production. Journal of Applied Microbiology. 2021;130(4):1023–1034. doi: 10.1111/jam.14845. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Sato F. Transcription factors in alkaloid engineering. Biomolecules. 2021;11(11):1719. doi: 10.3390/biom11111719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Ding Z., Zhang W., Cai G., Han H., Ma Y.…Ai Y. Multiple PDE3A modulators act as molecular glues promoting PDE3A-SLFN12 interaction and induce SLFN12 dephosphorylation and cell death. Cell Chemical Biology. 2022;29(6):958–969.e5. doi: 10.1016/j.chembiol.2022.01.006. [DOI] [PubMed] [Google Scholar]
- Yang D., Eun H., Prabowo C.P.S. Metabolic engineering and synthetic biology approaches for the heterologous production of aromatic polyketides. International Journal of Molecular Sciences. 2023;24(10):8923. doi: 10.3390/ijms24108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.M., Sun Y.H., Ni Z.H., Yang C.L., Tong Y., Liu Y.J.…Rao Y. Merging PROTAC and molecular glue for degrading BTK and GSPT1 proteins concurrently. Cell Research. 2021;31(12):1315–1318. doi: 10.1038/s41422-021-00533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P., Fan H., Hao Q., Yuan X.Q., Wu D., Pang Y.X.…Yan N.E. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nature Structural Molecular Biology. 2009;16(12):1230–1236. doi: 10.1038/nsmb.1730. [DOI] [PubMed] [Google Scholar]
- Zerio C.J., Sivinski J., Wijeratne E.M.K., Xu Y.M., Ngo D.T., Ambrose A.J.…Chapman E. Physachenolide C is a potent, selective BET inhibitor. Journal of Medicinal Chemistry. 2023;66(1):913–933. doi: 10.1021/acs.jmedchem.2c01770. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., Liu X.Y. Synthesis of rearranged steroids pinnigorgiols B and E. Chinese Journal of Organic Chemistry. 2021;41(7):2925–2926. [Google Scholar]