Abstract
Ferroptosis is a unique regulated form of cell death that is distinct from apoptosis, necrosis, and other well-characterized regulated cell death types, and plays an important role in the occurrence and development of chronic metabolic diseases, including diabetes, hypertension, hyperlipidemia, and non-alcoholic steatohepatitis. Recently, increasing evidence has supported traditional Chinese medicine (TCM) as a new hot spot for the treatment of chronic metabolic diseases by mediating ferroptosis. Unfortunately, few systematic reviews have described the importance of TCM in treating chronic metabolic diseases through the ferroptosis pathway. In the current review, the mechanism of ferroptosis and the roles of ferroptosis in chronic metabolic diseases are summarized. Additionally, this review illustrates that the regulation of ferroptosis by TCM could be an effective approach for treating chronic metabolic diseases based on experimental evidence and clinical application. In summary, this work will improve the understanding of ferroptosis and the ability of TCM to regulate ferroptosis in chronic metabolic diseases, thereby promoting the development and application of natural TCM.
Keywords: chronic metabolic diseases, diabetes mellitus, ferroptosis, hyperlipidemia, hypertension, traditional Chinese medicine
1. Introduction
Cell death is essential for the normal development of multicellular organisms and is abnormally activated or inhibited in many diseases (Fuchs and Steller, 2011, Zhao et al., 2024), meaning the end of cellular life. Traditionally, cell death has been divided into programmed death and nonprogrammed death, and it has generally been considered unregulated (Jiang, Stockwell, & Conrad, 2021). The concept of programmed cell death (PCD), which was executed through a programmed developmental pathway, emerged in the 1950s. However, regulatory cell death (RCD) is broader and refers to a molecular-regulated death program. According to previous research, ferroptosis is a new form of RCD driven by highly iron-dependent lipid peroxidation (LPO) at the cell membrane (Lei, Zhuang, & Gan, 2022).
Ferroptosis was first discovered in 2003, and then in 2012, Dixon et al. (2012) reported that erastin-induced death involves a unique set of morphological, biochemical, and genetic features that distinguish it from apoptosis, necrosis, and other well-characterized types of RCD (Table 1). Thus, the word ferroptosis was formally used as a description of the phenotype. Moreover, these authors also discovered that a specific small molecule inhibitor of ferroptosis (ferrostatin-1) prevents ferroptosis in cancer cells.
Table 1.
Differences between different types of cell death.
| Cell death | Ferroptosis | Apoptosis | Autophagy | Necroptosis |
|---|---|---|---|---|
| MorphologicalFeatures | Small mitochondria with increased mitochondrial membrane densities, reduction or vanishing of mitochondria crista, normal cell nucleus | Cellular and nuclear volume reduction, chromatin agglutination, nuclear fragmentation, formation of apoptotic bodies, and cytoskeletal disintegration, no significant changes in mitochondrial structure | Formation of double-membranedautolysosomes | Plasma membrane breakdown, generalized swelling of the cytoplasm and organelles, moderate chromatin condensation, release of cell contents |
| BiochemicalFeatures | GSH depletion, iron-dependent lipid peroxidation | DNA fragmentation | Enhancing autophagic flux and lysosomal activity | Decrease in ATP levels |
| Core genes | P53, HSPB1, ACSL4, FSP1, GPX4, TFR1, SLC7A11, NRF2, NCOA4 | Caspase, Bcl-2, Bax, P53 | ATG5, ATG7, AMPK | RIP1, RIP3 |
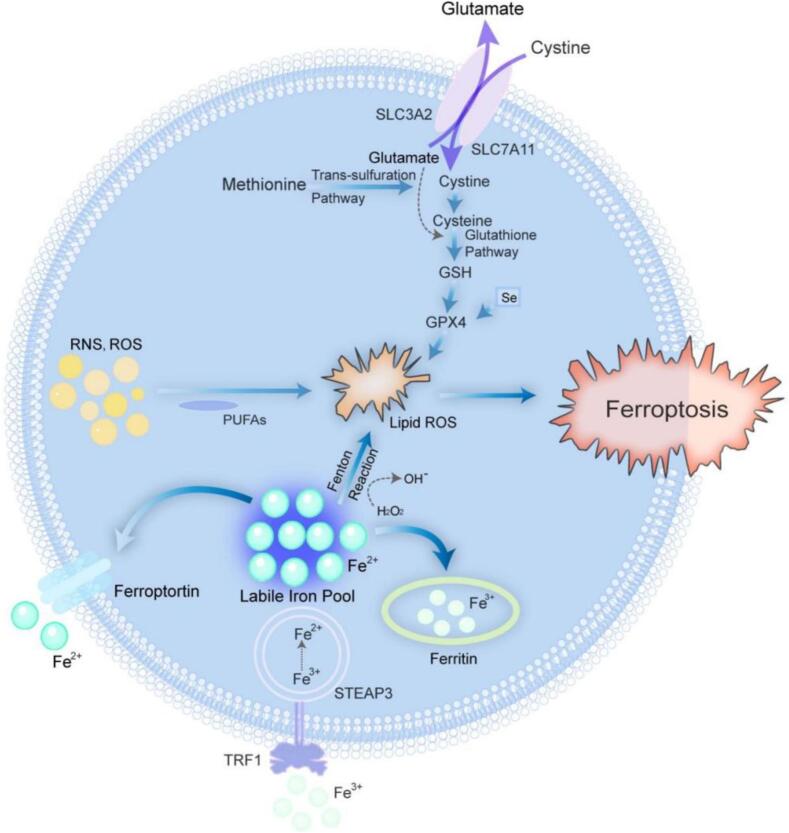
Ferroptosis is driven by lethal lipid peroxidation and is the result of imbalanced cellular metabolism and redox homeostasis. It can be inhibited not only by directly blocking lipid peroxidation or by consuming iron but also by pharmacological or genetic means. Ferroptosis is caused by the inhibition of the uptake of cystine, which is the raw material of glutathione (GSH). When cystine uptake is reduced, cellular antioxidant capacity decreases, leading to increased lipid peroxidation, and ROS production cannot be sufficiently blocked. This leads to the accumulation of deadly lipid ROS, and cell rupture and death. Ferroptosis has unique morphological and biological characteristics, including mitochondrial atrophy, decreased or even the absence of mitochondrial ridges, increased mitochondrial membrane density, GSH depletion, decreased cysteine uptake, and intracellular nicotinamide adenine dinucleotide (NADH) depletion, but ATP levels remain unchanged. In recent years, ferroptosis has been shown to play an important regulatory role in the development of many diseases and has become a research hotspot for improving the treatment and prognosis of related diseases. It is involved in Parkinson's disease, acute liver injury, neurodegenerative disease, myocardial ischemia, diabetes, and other diseases. Ferroptosis involves many parts and organs of the human body and is closely related to human health in Fig. 1.
Fig. 1.
Connections between ferroptosis and various diseases.
Chronic metabolic diseases (CMDs) are a general term for a group of diseases caused by metabolic problems. These diseases have a common cause and interact with each other. Often, patients can have two or more diseases at the same time. In recent years, with the continuous improvement in living standards, people’s lifestyles have changed greatly, and the incidence of CMDs, disability rates, and mortality rates are increasing. According to traditional Chinese medicine (TCM) theory, the metabolism and distribution of water and grain essence are closely related to the spleen, kidney, liver, and other organs, and it is emphasized that the imbalance of the internal environment is the root cause of CMDs. About the dialectical typing of CMDs, our scholars have proposed corresponding treatments such as “regulating the liver and spleen, eliminating phlegm and removing blood stasis” following the therapeutic principles of “balancing yin and yang, relieve stress, reconcile blood, and dredging the meridians and channels”. For chronic disease, long-term medication is needed to maintain therapeutic efficacy. Although Western drugs are fast-acting, the side effects are evident. Moreover, the disease easily rebounds and relapses.
TCM, as a natural treasure, has multiple active ingredients. Both the herb and the herbal extracts of TCM are known to have multitarget and multichannel effects. The pathogenesis of chronic metabolic diseases is complex, and pure chemical drug therapy only targets metabolic regulation at a certain node, which may not only be ineffective for other links but may even mask other pathological development processes, which at this moment highlights the advantages and potentials of TCM. As reported in clinical application, various natural compounds and herbal extracts have been confirmed to exhibit good preventive and ameliorative effects on CMDs, for example, Huanglian diarrhea heart soup (Aloe, Anemarrhenae Rhizoma, Monascus purpureus Went, Momordica charantia L., Salviae Miltiorrhizae Radix et Rhizoma, Schisandrae Chinensis Fructus, and Zingiberis Rhizoma) (Yu et al., 2018), hypoglycemic thirst-quenching granules (Zuo, 2017) as well as small-molecules compounds containing rhubarbic acid (Sheng et al., 2011), rhubarb phenol (Hu et al., 2018), rhubarbol (Shen et al., 2021), resveratrol (Ji & Xu, 2021) and honokiol (Liu et al., 2021a).
A growing body of research suggests that ferroptosis plays an important role in the development of CMDs (Feng et al., 2022), and an increasing number of herbal medicines have been found to modulate ferroptosis to inhibit or induce disease (Table 2). For example, Cyclocarya paliurus (Batal.) Iljinsk has strong activity in lowering blood lipid levels, blood pressure, and uric acid levels, and exerts hepatoprotective effects. Flavonol glycosides, the active ingredients of Cpaliurus, have anti-uric acid and anti-inflammatory effects, and their mechanism may involve enhancing cellular antioxidant capacity through regulation of the Nrf2 pathway (Xu, Zhang, & Xiang, 2023). Many effective TCM ingredients and formulas, such as artemisinin, baicalin, and Salvia miltiorrhiza Bge., have been found to have value in treating tumors and neurological diseases by intervening in ferroptosis (Wu et al., 2021a). Therefore, pharmacological modulation through the induction or inhibition of ferroptosis by herbal medicine has great potential in the treatment of chronic metabolic diseases. This paper presents a systematic review of the mechanisms of ferroptosis in CMDs and the treatment of CMDs by modulating ferroptosis via TCM, aiming to provide a reference for the development of drugs and new ideas for the treatment of CMDs.
Table 2.
Advances in treatment of diseases by modulation of ferroptosis by active ingredients in TCMs.
| Year | Discoveries | References |
|---|---|---|
| 2017 | Artemisia annua L. and artemisinin modulate ferroptosis to treat cancer | Efferth, 2017 |
| 2018 | Artemisinin analogs, baicalein, puerarin, modulation of ferroptosis to treat a variety of diseases | Xu, Li, & Jiang, 2018 |
| 2019 | Epimedium brevicomu Maxim. can inhibit iron aggregation | Liao, Ge, & Yu, 2019 |
| Astragalus flavonoids can fight oxidative stress | Liao, Ge, & Yu, 2019 | |
| 2020 | Tanshinone IIA induces ferroptosis in gastric cancer cells through P53-mediated SLCA11 downregulation | Guan, Chen, Li, & Dong, 2020 |
| The active active ingredient in Curcuma longa L. induces ferroptosis | Chen, & Liu, 2020 | |
| 2021 | Isorhinophylline in Lycium barbarum L. can inhibit nerve damage induced by ferroptosis | Cai et al., 2021 |
| Triptolide can induce ferroptosis | Zhao et al., 2021 | |
| 2022 | Astragaloside IV regulates ferroptosis through the Nrf2/SLC7A11/GPX4 axis to inhibit lung injury | Wang et al., 2022b |
| Salvianolic acid B protects against ferroptosisin rats with myocardial infarction by upregulating Nrf2 | Shen et al., 2022 | |
| Neuroprotective effects of berberine, gastrodin, baicalin, and tanshinone IIA on diabetic erectile dysfunction through inhibition of ferroptosis | Xu, Ma, & Zhang, 2022 | |
| Gastrodin, salidroside, corylifolia, osthole may combat Alzheimer's disease by inhibiting ferroptosis | Zang, Dong, & Hu, 2022 | |
| Resveratrol, naringenin, may combat myocardial ischemia–reperfusion injury by inhibiting ferroptosis | Li, Liu, Li, Xue, & Xu, 2022 | |
| Induction of ferroptosis by piperlongumine exerts anti-tumor effects | Zeng, Gao, & Lv, 2022 | |
| Saffron yellow pigment, digitonin, and galangal intervene in ferroptosis for ischemic stroke | Liu, Guo, Zhang, & Chang, 2022 | |
| 2023 | Tripterygium wilfordii ameliorates lung injury by inhibiting ferroptosis via the Nrf2/HO-1 pathway | Song et al., 2023 |
| Astragaloside IV attenuates cerebral ischemiareperfusion injury by inhibiting ferroptosis | Wang et al., 2023 | |
| Berberine improves iron levels and inhibits ferroptosis | Li et al., 2023 | |
| Chaihu saponin B2 inhibits ferroptosis and ameliorates depression-induced microglia activation | Wang et al., 2023 | |
| Icariin inhibits ferroptosis and attenuates nonalcoholic steatohepatitis in mice | Yuan, Zhang, & Zhang, 2023 | |
| Salvia miltiorrhiza Bge., Capsicumannuum L., Gastrodia elata Bl., Scutellaria baicalensis Georgi, Ginkgo biloba L., and Alpinia officinarum Hance reduce ferroptosis and treat ischemic stroke | Liao, Lin, & Wang, 2023 | |
| Artemisia annua L., S. baicalensis, Coptis chinensis Franch, Tripterygium wilfordii Hook., and Glycyrrhiza uralensis Fisch. modulate ferroptosis to inhibit hepatic fibrosis | Li, Wu, & Ma, 2023 | |
| Benzoyl aconitine, quercetin modulates ferroptosis and treats osteoporosis | Zuang, Guo, & Chen, 2023 | |
| Magnesium isoglycyrrhizinate, curcumol, berberine, all induce ferroptosis and thus inhibit liver fibrosis | Wang, Zhao, & Miao, 2023 | |
| Anwulignan, dihydroquercetin inhibits ferroptosis and improves pulmonary fibrosis | Wang, Zhao, & Miao, 2023 | |
| Iris sapogenins, calycosin, and rhodiola roseaglycosides inhibit ferroptosis and exert antifibrotic effects | Wang, Zhao, & Miao, 2023 | |
| Puerarin and chaetosaponin A modulate ferroptosis and treat cardiovascular disease | Fu et al., 2023 | |
| Porinic acid modulates ferroptosis to treat Alzheimer's disease | Fan, Dou, & Hu, 2024 | |
| Ginkgetin inhibits ferroptosis in the treatment of glioma | Zhao, Bao, & Sun, 2023 | |
| Leonurine inhibits ferroptosis and attenuates acute kidney injury | Hu, Gu, Ma, Fan, & Ci, 2022 |
2. Mechanisms of ferroptosis
Simply put, ferroptosis is the result of a dysfunctional balance of intracellular lipid ROS generation and degradation. This process is controlled by several metabolic pathways (Stockwell, 2022), and the major pathways involved include lipid peroxidation, iron metabolism, the Fenton reaction (an inorganic chemical reaction in which a mixed solution of H2O2 and Fe2+ oxidizes many organic compounds to the state of the art), the cystathionine-glutathione reverse transporter protein system (Xc-system)/GSH/glutathione peroxidase 4 (GPX4) axis, and the transsulfuration pathway. The most important of these are induced by a triad of iron metabolism, lipid peroxidation, and GSH and GPX4 in Fig. 2. However, sensitivity to ferroptosis is also regulated by other pathways and processes. In recent years, a series of key regulators of ferroptosis have been identified. Common inducers include erastin, glutamate, and cisplatin; common inhibitors include deferoxamine, Fer-1, and vitamin E (Table 3).
Fig. 2.
Mechanisms of ferroptosis. Ferroptosis occurs through iron metabolism, lipid metabolism, glutathione depletion, and GPX4 inactivation. GSH, glutathione; GPX4, glutathione peroxidase 4; RNS, reactive nitrogen species; ROS, reactive oxygen species; PUFAs, polyunsaturated fatty acids; TRF1, transferrin receptor 1.
Table 3.
Common inducers and inhibitors of ferroptosis.
| Drugs or compounds | Mechanisms | References | |
|---|---|---|---|
| Inducer | Erastin | GSH depletion | Dixon et al., 2012 |
| Sulfasalazine | GSH depletion | Dixon et al., 2012 | |
| Sorafenib | GSH depletion | Dixon et al., 2014 | |
| Glutamate | GSH depletion | Zhang et al., 2019 | |
| Imidazole ketone erastin | GSH depletion | Leu, Murphy, & George, 2019 | |
| Diaryl-isoxazoles | GSH depletion | Newell et al., 2014 | |
| INF-γ | Downregulating expression of system xc- | Zitvogel & Kroemer, 2019 | |
| RSL3 | GPX4 inactivation and GSH deletion | Yang et al., 2014 | |
| ML162 | GPX4 inactivation and GSH deletion | Yang et al., 2014 | |
| Diphenyleneiodonium chloride | Inhibiting GPX4 | Dächert, Ehrenfeld, Habermann, Dolgikh, & Fulda, 2020 | |
| Artemisinin derivatives | Inhibiting GPX4 | Zou & Schreiber, 2020 | |
| FINO2 | GPX4 inactivation and iron oxidation | Gaschler et al., 2018 | |
| FIN56 | Depleting CoQ10 and degrading GPX4 | Gaschler et al., 2018 | |
| Buthionine sulfoximine | GSH depletion | Zhang et al., 2018 | |
| Statins | CoQ10 deletion | Viswanathan et al., 2017 | |
| Siramesine, lapatinib | Increasing intracellular iron concentration | Ma et al., 2017, Ma et al., 2016 | |
| Withaferin | Degrading GPX4 and increasing HO-1 | Hassannia et al., 2018 | |
| BSO, DPI2 | GSH depletion | Yang et al., 2014 | |
| BAY 87‐2243 | Inhibition of mitochondrialrespiratory chain (CI) | Basit et al., 2017 | |
| Trigonelline, brusatol | Nrf2 inhibition | Xie et al., 2017 | |
| Cisplatin | Decreasing GSH levels | Guo et al., 2018 | |
| Artemisinins | Increasing cellular iron levels | Eling, Reuter, Hazin, Hamacher-Brady, & Brady, 2015 | |
| Inhibitor | Ferrostatin-1 | Inhibiting lipid peroxidation | Dixon et al., 2012 |
| Trolox | Blocking propagation of lipid peroxidation | Dächert, Ehrenfeld, Habermann, Dolgikh, & Fulda, 2020 | |
| Liproxstatins | Blocking lipid peroxidation | Eling, Reuter, Hazin, Hamacher-Brady, & Brady, 2015 | |
| CoQ10 | Blocking lipid peroxidation | Schreiber et al., 2019 | |
| Idebenone | Blocking lipid peroxidation | Schreiber et al., 2019 | |
| Glutathione | GPXs inactivation | Schreiber et al., 2019 | |
| Vitamin E | Inhibiting lipid peroxidation | Seiler et al., 2008 | |
| Liproxststatin-1 | Inhibiting lipid peroxidation | Seiler et al., 2008 | |
| α-Tocopherol | Blocking propagation of lipid peroxidation | Shin, Kim, Lee, & Roh, 2018 | |
| Amino-oxyacetic acid | Inhibiting glutaminase | Gao, Monian, Quadri, Ramasamy, & Jiang, 2015 | |
| GLS inhibitor Compound 968 | Inhibiting glutaminase | Gao, Monian, Quadri, Ramasamy, & Jiang, 2015 | |
| Troglitazone | Inhibiting ACSL4 | Doll et al., 2017 | |
| Rosiglitazone | Inhibiting ACSL4 | Doll et al., 2017 | |
| Pioglitazone | Inhibiting ACSL4 | Doll et al., 2017 | |
| Tetrahydrobiopterin | Lipid remodeling | Kraft et al., 2020 | |
| Dihydrobiopterin | Lipid remodeling | Kraft et al., 2020 | |
| Iron chelator (deferiprone, ciclopirox, deferoxamine and dexrazoxane) | Depleting iron | Li et al., 2020 |
2.1. Iron metabolism disorders
According to previous studies, iron is a major cofactor for many of the oxidoreductase enzymes associated with metabolism in the oldest living organisms on earth (Mayneris-Perxachs, Moreno-Navarrete, & Fernández-Real, 2022) and is an important component of our bodies. Iron is often found in the form of trivalent iron (Fe), which is converted to divalent iron upon entry into the cell and is often metabolized or exocytosed in three ways. Only a small portion of divalent iron ions in cells act as free iron. The majority are oxidized into trivalent iron ions and stored in ferritin (Fer). Additionally, a small portion of iron is excreted through the cell membrane ferroportin (FPN) (Wang et al., 2022a). However, excessive accumulation of divalent iron ions results in iron overload; Then, the Fenton reaction and hydrogen peroxide binding occur, leading to the production of hydroxyl radicals. At this time, hydroxyl radicals can interact directly with the cell membrane, where it reacts with a variety of unsaturated fatty acids to ultimately produce ROS. With the accumulation of ROS, the accumulation of lipid peroxides and ultimately, the production of malondialdehyde (MDA) cause cytotoxicity, cell rupture, and death. A large amount of free iron is a critical initiator of ferroptosis. MDA is a product of membrane lipid peroxidation reactions and an indicator of lipid peroxidation, as it reflects the degree of peroxidation of cell membrane lipids. Therefore, increased iron absorption, reduced iron storage, and iron efflux can all affect the sensitivity of cells to ferroptosis, and strict regulation of iron transport, metabolism, and storage is necessary.
2.2. Accumulation of lipid peroxides
Lipid peroxidation refers to the interaction of reactive nitrogen species (RNS), large amounts of ROS, and polyunsaturated fatty acids (PUFAs) on lipid membranes to form lipid reactive oxygen species (L-ROS). PUFAs are sites of oxidized lipid damage and are required for ferroptosis (Rochette et al., 2022). The reactive lipid species (RLS) produced by lipid peroxidation can initiate lipid peroxidation by itself, which determines cell signaling events through the modification of key proteins or by causing toxicity and triggering cell death cascades (Higdon, Diers, Oh, Landar, & Darley-Usmar, 2012). RLS, including 4-hydroxy-2-nonenal (4-HNE) and MDA, are associated with the progression of numerous diseases, including diabetes, cardiovascular disease, and liver disease (Ayala, Muñoz, & Argüelles, 2014). Ferroptosis occurs as a result of disruption of the structure and function of the phospholipid bilayer due to the accumulation of large amounts of lipid peroxides and their reactive degradation products in the cell, leading to cellular damage. However, the mechanism by which ferroptosis occurs remains unclear (Feng & Stockwell, 2018). High concentrations of L-ROS also trigger oxidative stress in cells, causing oxidative damage and ultimately triggering ferroptosis. Research has shown that there is a dihydrolactate dehydrogenase (DHODH) present on the inner membrane of mitochondria that can reduce ubiquinone to panthenol, scavenge free radicals, and ultimately inhibit membrane lipid peroxidation (Gan, 2021). Therefore, the regulation of lipid metabolism and maintenance of lipid metabolism homeostasis are important for the prevention of ferroptosis.
2.3. GSH depletion and GPX4 inactivation
Glutathione serves as a major buffer of intracellular antioxidants, mainly in the form of reduced GSH and oxidized glutathione (GSSG). GPX4 is primarily responsible for converting free hydrogen peroxide to water, reducing lipid hydroperoxides (L-OOH) to lipid hydroxyl derivatives (L-OH), and converting reduced GSH into GSSG, which are crucial for maintaining cellular redox homeostasis (Yang et al., 2014).
GSH is a general term for glutamate, cysteine, and glycine. It has antioxidant properties and helps remove various toxins and metallic elements from the body. Cystine is reduced intracellularly to cysteine (Cys) for GSH biosynthesis, which in turn affects GPX4 activity (Koppula, Zhuang, & Gan, 2021). When Cys levels are insufficient, certain cells can also biosynthesize Cys through methionine. When GSH levels are insufficient, the antioxidant defense ability decreases. This, leads to an increase in ROS and iron accumulation, resulting in ferroptosis.
GPX4, a selenium-containing protein (Stockwell, Jiang, & Gu, 2020), is the major neutralizing enzyme that catalyzes the reduction of phospholipid hydroperoxides (PLOOH) in mammalian cells, whereas the synthesis and conversion of PUFAs, precursors of PLOOH, regulate ferroptosis. It enables GSH to convert toxic L-OOH into nontoxic L-OH, which protect the cell membrane by scavenging iron-dependent lipid peroxides and inhibiting cellular oxidative stress while repairing oxidative damage to the cell membrane. Therefore, GPX4 is considered a major enzyme and key regulator in the prevention of ferroptosis. When GPX4 activity is reduced, lipid peroxides (e.g, MDA and ROS) increase. This in turn leads to GSH depletion, and ultimately ferroptosis is induced. However, studies have shown that the sensitivity of different cancer cells to GPX4 inhibitors varies widely (Ding et al., 2021), suggesting that other factors control resistance to ferroptosis.
3. Association of ferroptosis with CMDs
Generally CMDs include metabolic disorders such as diabetes (Brooks-Worrell & Palmer, 2012), hyperlipidemia (Yao, Li, & Zeng, 2020), hypertension (Zhu, Wang, & Ma, 2013), and liver disease (Zhu, Wang, & Ma, 2013). Ferroptosis is extensively involved in the physiopathologic processes of diabetes, hypertension, hyperlipidemia, NAFLD and other chronic metabolic diseases, and plays an important role in the development of these diseases, which are specifically described below.
3.1. Ferroptosis and diabetes mellitus
Diabetes mellitus (DM) is an endocrine metabolic disease characterized by markedly higher blood glucose levels than normal, and its complications can be life-threatening. The prevalence of DM is increasing annually. Existing hypoglycemic drugs cannot reverse its course and are not effective at treating it. Therefore, finding new ideas to prevent and treat diabetes is essential.
There is a strong association between hyperferritinemia and endocrine metabolic disorders (Wang & Chen, 2022). Increasing evidence indicates that ferroptosis is directly or indirectly involved in the development of diabetes and its various complications (Fong, Yang, & Zhi, 2022). Ferroptosis increases the risk of diabetes, which in turn causes an imbalance in iron metabolic homeostasis, ultimately creating a vicious cycle (Xiong, Ning, & Shi, 2019). The role and progression of ferroptosis in DM are now understood. The important links in the occurrence of ferroptosis have been explored to expand the understanding of DM and provide new ideas for clinical treatment involving ferroptosis-related regulation.
Ferroptosis is characterized by iron homeostasis dysregulation and lipid peroxidation. Iron overload reduces insulin secretion and increases the incidence of type 2 diabetes. The morphology of type 2 diabetic mice is consistent with the typical alterations of ferroptosis. MDA and ROS levels are elevated in both diabetic rats and patients with diabetes, suggesting the occurrence of lipid peroxidation (Zhou, 2020, Dham et al., 2021). During this process, lipid peroxides can be broken down into 4-HNE and MDA, which are inversely related to DNA, proteins, and other affinity factors that cannot undergo ferroptosis if they are not completely blocked by the antioxidant system, resulting in ferroptosis (Yan et al., 2021). ROS accumulation is a key step in the process of ferroptosis. Studies have shown that ROS can lead to pancreatic β-cell failure and insulin resistance, that ROS and oxidative stress contribute to the development of type 2 diabetes, and that lipid peroxidation is a causative factor for type 2 diabetes and its vascular complications (Rehman & Akash, 2017). In summary, ferroptosis is closely linked to diabetes.
Multiple studies have shown that the onset of ferroptosis can destroy islet cells and impair islet function, leading to abnormalities in islet signaling pathways. Erastin-treated islets showed impaired insulin secretion and reduced islet survival, suggesting that ferroptosis may lead to insufficient insulin secretion. Fer-1 can reduce serum TC and TG levels, ameliorate lipid metabolism disorders, reduce endothelial cell damage, and effectively reduce diabetic atherosclerotic lesions (Meng et al., 2021). When glucose deficiency occurs during glucose metabolism disorders, AMPK is activated. The protein kinase AMPK is a key factor in the regulation of energy metabolism, and it inhibits the onset of ferroptosis. However, erastin can also promote ferroptosis by activating AMPK; thus, AMPK has dual effects on ferroptosis.
3.2. Ferroptosis and hypertension
Hypertension (HTN) is a public health problem that threatens human health and is also an important risk factor for cardiovascular diseases such as atherosclerosis, heart failure, and myocardial infarction. HTN is a chronic disease with a high incidence rate in China, and national and international epidemiological studies have shown that there is a positive correlation between blood pressure in the population and the occurrence of cardiovascular and cerebrovascular diseases. As blood pressure increases, the incidence of cardiovascular and cerebrovascular diseases also increases. HTN is an independent risk factor for stroke that, can lead to cerebral hemorrhage, atherosclerotic cerebral infarction, and cerebral thrombosis, and the occurrence of cerebral damage can seriously affect the quality of life of patients. Therefore, to prevent and treat HTN, further research on new molecular targets involved in the pathogenesis of hypertension as well as the exploration of new therapeutic strategies are warranted.
In recent years, a growing body of research has suggested that ferroptosis is strongly associated with the development and progression of HTN (Yang et al., 2020, Zhang et al., 2022). Ferroptosis leads to endothelial cell dysfunction and ultimately to hypertension (Bai, Li, Liu, Qiao, & Wang, 2020). Serum ferritin levels are positively correlated with the incidence of HTN, and the brain tissue of hypertensive rats showed has elevated iron levels, elevated lipid peroxides, and altered other relevant indices of ferroptosis compared with those in the brain tissue of normotensive rats (Yang, Wang, Wang, Li, & Gao, 2020). Increased iron levels in the cardiac tissues of hypertensive mice increase the levels of lipid peroxides and inflammatory factors.
Reduced GPX4 activity decreased GSH content and increased MDA and ROS lead to the accumulation of intracellular lipid peroxides, which induces the onset of ferroptosis. GPX4 is a selenoprotein, and selenium is present in the form of selenocysteine in the active center of GPX4. Thus, selenium deficiency may impair the function of GPX4, cause lipid peroxidation, and ultimately lead to the development of ferroptosis (Ingold et al., 2018). Studies have shown that the serum levels of selenium are reduced in hypertensive patients (Gać et al., 2021). Moreover, in a rat model of adolescent alcoholism-induced hypertension where selenium levels in the kidney and serum were significantly reduced, selenium upplementation increased GPX4 activity and decreased serum aldosterone levels, subsequently lowering systolic blood pressure (Sobrino, Ojeda, Nogales, Murillo, & Carreras, 2019). GPX4 is considered a major enzyme involved in the prevention of ferroptosis. However, GPX4 expression in the aorta of hypertensive patients is significantly lower than that in the normal population. Hypertensive rats had significantly lower GPX4 levels and GSH and significantly greater total iron and MDA levels than normotensive rats. In HTN, the lipid peroxide MDA can significantly increase and cause endothelial damage. The plasma levels of lipid peroxides were significantly greater in hypertensive patients than in healthy control individuals. Increased levels of ROS can promote endothelial dysfunction, and accelerate VSMC value-addition and vascular remodeling, leading to increased peripheral resistance and elevated blood pressure.
In addition, mitochondrial dysfunction-induced impediments in energy metabolism increase mitochondrial ROS, and mitochondrial DNA mutations are involved in the pathophysiological process of HTN. Mitochondrial dysfunction increases oxidative stress, exacerbates endothelial cell dysfunction and vascular remodeling, and can mediate the onset of ferroptosis, which contributes to the development of HTN.
We also found that some inducers and inhibitors of ferroptosis influence the development of HTN. For example, Nrf2 plays a key role in redox regulation in HTN (Dovinova et al., 2020), and activation of Nrf2 inhibits the progression of HTN (Farooqui, Mohammad, Lokhandwala, & Banday, 2021). Coenzyme Q10 (CoQ10) is a mitochondrial coenzyme that inhibits ferroptosis and acts directly on endothelial cells to stimulate vasodilation and alleviate HTN (Doll et al., 2019). Fer-1 may alleviate HTN by inhibiting ferroptosis in VSMCs and endothelial cells. SLC7A11 may alleviate hypertensive cardiac hypertrophy by inhibiting ferroptosis and may serve as a potential therapeutic target for hypertensive cardiac hypertrophy.
3.3. Ferroptosis and hyperlipidemia
Hyperlipidemia (HLD), also known as a lipid metabolism disorder or abnormality, is induced by genetics, a high-fat diet, obesity, diabetes, and other factors; It is a common clinical disorder of lipid metabolism that manifests mainly as elevated levels of serum lipoproteins and lipids. HLD is one of the high-risk factors for many cardiovascular and cerebrovascular diseases. It can lead to a variety of complications and seriously jeopardize human health (Zang & Chen, 2022).
In recent years, the incidence of HLD has been increasing, and abnormal lipid metabolism and oxidative stress are important triggers of HLD. When the intracellular lipid metabolism is abnormal, the accumulation of peroxides may lead to ferroptosis. Ferroptosis inhibitors and iron chelators have been found to ameliorate fatty liver caused by HLD. Moreover, the occurrence of ferroptosis causes an increase in unsaturated fatty acids, and lipid metabolism disorders caused by large amounts of unsaturated fatty acids accelerate the development of HLD. Recent studies have confirmed that lipid metabolic disorders are closely related to ferroptosis and that disorders of lipid metabolism leading to increased lipid peroxidation may be one of the conditions necessary for the development of ferroptosis (Ma, Yu, Qing, Li & Hong, 2022). Thus, HLD is closely linked to ferroptosis, and the control of lipid metabolic homeostasis through the ferroptosis pathway provides a new perspective for the prevention and treatment of HLD.
3.4. Ferroptosis and nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is a chronic metabolic disease of the liver associated with lipid metabolism disorders. As the disease progresses, NAFLD can progress to liver fibrosis and even cirrhosis and liver cancer, making it the rarest chronic liver disease in the world today. Dyslipidemia is an important risk factor for the disease. Recent mechanistic studies of the disease have focused on insulin resistance (Watt, Miotto, De Nardo, & Montgomery, 2019), oxidative stress injury (Paradies, Paradies, Ruggiero, & Petrosillo, 2014), the inflammatory response (Forlano et al., 2020), gut flora disruption (Albillos, de Gottardi, & Rescigno, 2020), and ferroptosis (Wu et al., 2021b). Ferroptosis plays an important role in the pathogenesis of NAFLD, and the underlying mechanism is related to abnormal iron metabolism in fatty liver tissue and an increase in iron ions leading to ferroptosis. Ferroptosis is closely associated with NAFLD. The abnormal accumulation of intracellular lipids leads to dysregulation of redox homeostasis and triggers cellular ferroptosis. Recent studies have revealed that ferroptosis may be involved in the progression of NAFLD, and preliminary evidence suggests that ferroptosis in hepatocytes and intrahepatic macrophages may trigger nonalcoholic steatohepatitis (NASH) and that complex regulatory mechanisms exist (Chen & Liu, 2020). Studies have shown that hepatocyte ferroptosis precedes apoptosis at the onset of progression to NASH in NAFLD mice, leading to liver damage and inflammatory responses. Moreover, iron chelators and antioxidants can almost completely reverse hepatocyte death while inhibiting inflammatory responses and lipid peroxidation (Tsurusaki et al., 2019). In summary, targeting the ferroptosis pathway may be an effective strategy for the prevention and treatment of NAFLD (Wu et al., 2021b).
The levels of the metabolites of phospholipid peroxidation, MDA, and 4-hydroxynonenal, are elevated to varying degrees in patients with NAFLD, and lipid peroxidation and excess iron deposition can exacerbate the inflammatory response in patients by increasing the level of ferroptosis (Imai, Matsuoka, Kumagai, Sakamoto, & Koumura, 2017). The upregulation of transferrin receptor 1 was also detected in a mouse model of fatty liver, and this upregulation exacerbated the occurrence of ferroptosis by increasing iron intake (Datz, Müller, & Aigner, 2017). Based on the above studies, it is not surprising that iron deposition in NAFLD patients works in conjunction with the synthesis of large amounts of phospholipid peroxides to exacerbate ferroptosis and promote disease development. This finding also suggests that targeted inhibition of ferroptosis may be a potential treatment for patients with NAFLD.
4. TCM for treatment of CMDs by modulating ferroptosis
Evidence-based treatment is a feature of TCM, and in the face of the complex pathogenesis of CMDs and the problems of drug resistance and side effects of single-target drugs, the potential of TCM becomes apparent. Current research has found that a large number of herbal compounds and extracts can alleviate CMDs by modulating ferroptosis (Table 4). For example, the TCM compound Yitangkang and compound Danshen Drip Pill regulate ferroptosis and thus treat DM by modulating the Nrf2 pathway and inhibiting inflammatory responses; Pueraria mirifica and Angelica sinensis volatile oils, among others, can treat HTN by reducing iron overload and lipid peroxidation inhibiting the onset of ferroptosis; Didang Decoction and Jiawei Erzhi Pill can regulate ferroptosis by improving mitochondrial function and lipid metabolism levels thereby treating HLD; Liver Kangning and Ling Gui Zhu Gan Decoction can regulate ferroptosis by decreasing MDA activity and ameliorating oxidative stress thereby treating NAFLD.
Table 4.
Different herbal compounds and extracts that alleviate chronic metabolic diseases by regulating ferroptosis.
| Drugs | Dose | Subjects | Mechanisms | Effect | References |
|---|---|---|---|---|---|
| Compound Yitangkang | 10 g/(kg·d) | SPF grade Wistar rats | Regulating AMPK; Inhibiting ferroptosis. | Improvement of lipid metabolism disorders. | Ning et al, 2023 |
| Compound Danshen drip Pill | 810 mg/d | Humans | Inhibiting inflammatory responses; Inhibiting ferroptosis. | Lowering blood sugar levels in type 2 diabetes patients. | Liang, 2022 |
| Saffron | 30 mg/kg | Male SD rats | Induction of Nrf2 expression; Inhibition of ferroptosis. | Reducing blood glucose levels in rats. | Xing & Chan, 2020 |
| Cryptochlorogenic acid | N/A | N/A | Inhibition of ferroptosis. | Lowering blood glucose levels. | Huang, Han, & Yang, 2023 |
| Berberine | 7.5, 15, 30, 60, 90, 120, and 150 μmol/L | Glomerular podocyte MPC5 | Upregulating Nrf2; Inhibiting ferroptosis. | Stoping or delaying the onset of diabetic nephropathy. | Guan, Xie, & Ni, 2021 |
| Sennoside A | 30 mg/kg | Male C57BL/6J mice | Improving oxidative stress; Inhibiting ferroptosis. | Treatment of diabetic nephropathy. | Ding & Wang, 2021 |
| Curcumin | 12.5, 25.0, 30.0, 50.0 mmol/L | Neuro-2a cells | Activation of the Nrf2/HO-1 pathway, inhibiting ferroptosis. | Inhibition of diabetic nephropathy. | Wang, Sun, & Reng, 2023 |
| Quercetin | 5.4 g/kg | Male specific-pathogen-free C57BL/6J mice | Improving oxidative stress; Inhibition of ferroptosis. | Reducing iron levels in type 2 diabetes. | Li et al., 2020 |
| Pueraria Mirifica | N/A | Mice | Reducing iron overload and lipid peroxidation; Alleviating ferroptosis. | Lowering blood pressure. | Huang et al., 2022, Zhou et al., 2020, Shi et al., 2019 |
| Jiawei Erzhi Pills | 0.29 g/(kg·d) | Male apolipoprotein E deficient mice | Reducing the occurrence of hepatocyte lipid peroxidation; Inhibiting ferroptosis. | Protecting the liver in hyperlipidemia model mice. | Ma, Yu, & Qing, 2022 |
| Achyranthes aspera L. | 50, 75, 100, 125, and 150 mg/kg | Albino rats of both sexes | Significantly reducing serum cholesterol levels; Inhibiting ferroptosis. | Lipid-lowering drugs and treatment of obesity. | Khan et al., 2015 |
| Anthraquinones in rhubarb | N/A | Healthy male white rabbits | Promoting intestinal peristalsis and impeding intestinal absorption of cholesterol; Inhibiting ferroptosis. | Anti-hyperlipidemic activity. | Xu et al., 2007 |
| Cassia seeds | 10.69 mg/kg/day | Male SpragueDawley rats | Inhibiting cholesterol absorption and synthesis; Inhibiting ferroptosis. | Hypolipidemic effects. | Zhang et al., 2018a |
| Olea europaea | N/A | Human liver tumor cell | Repressing fatty acid synthesis and accelerating fatty acid oxidation; Inhibiting ferroptosis. | Anti-hyperlipidemic activity. | Di et al., 2018 |
| Tanshinone IIA | N/A | N/A | Modulating lipid peroxidation damage; Inhibiting ferroptosis. | Hypolipidemic effects. | Niu et al., 2000 |
| Artemisia iwayomogi Kitamura and Curcuma longa Linne | 50 mg/kg | Male apolipoprotein E deficient mice | Reducing oxidative stress and restoring the antioxidant capacities; Inhibiting ferroptosis. | Improvement in hyperlipidemia. | Shin et al., 2014 |
| Liver Kangning | 10 and 20 g/kg | Male Kunming breeder mice | Reducing MDA and increasing SOD activity; Inhibiting ferroptosis. | Treatment of NAFLD. | Chen, Lin, She, Kuang & Wang, 2019 |
| Zedoary Decoction | 1.3, 2.6, and 5.2 g/kg | Male C57BL/6J mice | Activating the Nrf2/HO-1 pathway and reducing lipid peroxide accumulation. | Activating the Nrf2/HO-1 pathway and reducing lipid peroxide accumulation. | Li et al, 2022 |
| Lipids and liver granules | 10 and 20 g/kg | Male C57 mice | Inducing ferroptosis via the TLR4/NF-κB pathway; Inhibiting ferroptosis. | Treatment of NAFLD and liver fibrosis. | Ye, Xue, & Li, 2021 |
| Astragaloside | 30, 60, and 120 mg/kg | Male SD rats | Antioxidative stress, regulating blood lipid levels, and reducing lipid accumulation; Inhibiting ferroptosis. | Delaying the progression of nonalcoholic fatty liver disease. | Han, 2016 |
| Dihydroartemisinin | N/A | Hepatocellular carcinoma cell lines SMMC-7721 and Huh-7 | Inhibiting intracellular GSH and GPX4 protein levels; Inducing ferroptosis. | Inhibiting hepatocellular carcinoma cell proliferation. | Li, Zhou, & Wang, 2019 |
| Ginkgolide B | 29 and 30 mg/kg | Male C57/BL6 ApoE−/−mice | Activating Nrf2; Inhibiting ferroptosis. | Reducing lipid droplet deposition, hepatic steatosis, and hepatocyte death. | Yang, Wang, Wang, Li, & Gao, 2020 |
| Protocatechuic acid and curcumin | N/A | N/A | Protecting against oxidative stress; Inhibiting ferroptosis. | Significantly ameliorating NAFLD. | Gao et al., 2022, Rahmani et al., 2016 |
| Total saponins of Panax ginseng | 4.5, 9, and 18 mg/kg | Male SD rats | Modulating inhibitory protein-binding expression of NO, NF-κB, and other signaling factor pathways; Inhibiting ferroptosis. | Prevention and treatment of NAFLD. | Feng, He, & Zhao, 2021 |
| Cassia anthraquinone glycosides | 5, 10, and 20 mg/kg/d | Male SD rats | Inhibits lipid peroxidation; Inhibiting ferroptosis. | Combating NAFLD. | Zhao, Wu, & Yao, 2019 |
| Gentianoside | 30, 60, and 120 mg/kg | Male SD rats | Activating the Nrf2 signaling pathway to inhibit oxidative stress; inhibiting ferroptosis. | Hepatoprotective effects. | Xu, Ma, & Zhang, 2022 |
4.1. TCM alleviates DM by regulating ferroptosis
In recent years, we have found that an increasing number of herbal medicines influence the course of diabetes through the modulation of ferroptosis. The TCM compound Yitangkang is composed of 12 herbs, including Astragalus membranaceus (Fisch.) Bge., Atractylodis Macrocephalae Rhizoma, etc, which strengthen the spleen, benefit qi, nourish yin, activate blood and prevent blood stasis. Moreover, mitochondrial structure and function are improved through the upregulation of Nrf2, which increases the expression of SLC7A11 and GPX4. Thus, ferroptosis is inhibited, and cognitive deficits are ultimately improved in db mice (Ning et al., 2023). Compound Danshen Dripping Pills inhibit the inflammatory response and attenuate cognitive impairment in type 2 diabetic mice (Liang, 2022). Saffron upregulated the expression of the Nrf2 protein in the nucleus of high glucose-induced HGMCs, promoted the expression of GPX4, promoted the expression of heme oxygenase-1 (HO-1) proteins reduced intracellular ROS production, and inhibited cellular ferroptosis (Xing & Shan, 2020). Cryptochlorogenic acid lowers blood glucose levels in type 1 diabetic rats by targeting ferroptosis. When the Nrf2 gene was knocked out in mice to inhibit ferroptosis, fasting blood glucose levels decreased (Huang, Han, & Yang, 2023). Berberine significantly reduced cellular ROS and GSH levels, upregulated NRF2 expression, and alleviated cellular ferroptosis (Guan, Xie, & Ni, 2021). Sennoside A significantly ameliorated oxidative stress and suppressed ferroptosis (Ding & Wang, 2021). Curcumin inhibits high glucose-induced cellular ferroptosis via the Nrf2/HO-1 pathway (Wang, Sun, & Reng, 2023). Quercetin, a naturally occurring inhibitor or modulator of iron metabolism, reduces iron levels in patients with type 2 diabetes. In a type 2 diabetes model, GSH is depleted, GPX4 is downregulated, and oxidative stress is induced in pancreatic tissue, but these effects are partially eliminated by quercetin (Li et al., 2020). In addition, recent studies have shown that polyphenols, including quercetin, curcumin, and gallocatechin-3-gallate, can act as potent inhibitors of ferroptosis (Xie et al., 2016, Kose et al., 2019). Moreover, berberine, baicalin, tanshinone di A, and isobutylamides all have ameliorative and therapeutic effects on diabetic complications. Therefore, modulating ferroptosis through the active ingredients of TCM to prevent or treat diabetes is undoubtedly a highly promising approach.
4.2. TCM alleviates HTN by modulating ferroptosis
An increasing number of studies have shown that the active ingredients of herbs are also involved in the development of ferroptosis through multiple pathways. For example, Pueraria lobata (Willd.) Ohwi can alleviate ferroptosis by reducing iron overload and lipid peroxidation (Huang et al., 2022) and can also lower blood pressure in mice with high salt-induced hypertension (SHR) (Zhou et al., 2020, Shi et al., 2019). We also found that many active ingredients of TCMs, such as astragaloside, Paeonia lactiflora Pall. total glycosides, angelica volatile oil, chuanxiongxizine, baicalin, gerberellin, and glochidonine have significant effects on the treatment of HTN. As research continues, lowering blood pressure by inhibiting cellular ferroptosis through herbal medicine is likely to be an effective strategy for the treatment of HTN. Relatively few studies have investigated the ability of modulation ferroptosis by Chinese herbs to attenuate HTN, which may be related to the difficulty of replicating models with the same mechanism. Therefore, we believe that this is certainly a highly promising direction for research.
4.3. TCM alleviates HLD by modulating ferroptosis
The current treatment for HLD mainly involves statins, fibrates, etc. However, the use of these drugs has been controversial due to their numerous adverse effects, so the use of herbal extracts as well as active ingredients for regulating blood lipid levels has broad application prospects. In recent years, research on the hypolipidemic effects of medicinal plants has progressed to some extent, but there are some limitations. TCMs alleviate HLD, although most of the recent studies have focused on the role of TCM monomers or potent extracts in the treatment of HLD. These monomers and extracts are more cost-effective because of their fewer adverse effects (Rauf et al., 2022). In recent years, many TCM compound formulas, such as Qi Dan Decoction, Hua Turbid Lipid Reducing Soup, and Compound Astragalus Ginseng Lipid Drink, have been applied to treat HLD. All of these compounds have achieved satisfactory therapeutic efficacy. Therefore, the application of TCM for the treatment of HLD is extremely promising. Jiawei Erzhi Pill (Aconiti Lateradix Praeparata, Cervi Cornu, Psoraleae Fructus, Eucommiae Cortex, Cervi Cornu Pantotrichum, Epimedii Folium) can reduce the expression of p53 in mouse liver tissues, decreasing the inhibitory effect of p53 on SLC7A11 and further increasing the expression of GSH in the serum and liver tissues of mice. This can lead to an increase in GPx4 expression and the inhibition of ferroptosis. Jiawei Erzhi Pills can also protect the liver of HLD model mice by antagonizing hepatocyte ferroptosis-related factors, which can reduce the occurrence of hepatocyte lipid peroxidation and attenuate hepatic lipid deposition (Ma, Yu, Qing, Li & Hong, 2022). According to the literature, herbs are more effective than modern lipid-lowering drugs at controlling lipid levels. More experiments are needed to confirm this idea in the future to promote the treatment of hyperlipidemia and related diseases.
Studies have shown that Achyranthes bidentata Bl. significantly reduces serum cholesterol levels and is a lipid-lowering agent that is also used to treat obesity (Khan et al., 2015). The anthraquinone component present in Rheum palmatum L. has antihyperlipidemic activity by promoting intestinal peristalsis and impeding intestinal absorption of cholesterol (Xu et al., 2007). The active components of Cassia obtusifolia L., protein, and anthraquinone glycosides, can inhibit cholesterol absorption and synthesis, thus exerting a hypolipidemic effect (Zhang et al., 2018a). Oral administration of total flavonoids from the stem and leaves of S. baicalensis glycosides to large diabetic rats suffering from hyperlipidemia lowered serum TG, LDL-C, and TC levels and elevated HDL-C levels (Megalli, Davies, & Roufogalis, 2006), suggesting that S. baicalensis has a hypolipidemic effect. Crataegus pinnatifida Bge. var. major N. E. Br. extract decreased blood TC, TG, and LDL-C levels in 45 hyperlipidemic volunteers (Guo, Liu, Gao, & Shi, 2014). Curcumin significantly decreased TC levels by decreasing lipid metabolism (Wo, Cui, & Tang, 2003). Olea europaea L. has potential antihyperlipidemic activity and could be a future drug candidate for the treatment of hyperlipidemia (Di et al., 2018). Gynostemma total saponins, tanshinone IIA, ginsenosides, curcumin, tea polyphenols, and other monomers or single-flavored herbs modulate lipid peroxidation damage, resulting in hypolipidemic effects (Niu et al., 2000). Plantago asiatica L. contained in Plantago asiatica L. inhibit TC synthesis and promote TC clearance. Anthraquinone in Polygonum multiflorum Thunb. promotes intestinal motility and increases TC excretion. Phytosterols in P. asiatica competitively inhibit the intestinal absorption of exogenous cholesterol, thereby increasing cholesterol excretion from the intestines. Ginsenosides promote TC conversion, degradation, and excretion. Studies have shown that the ethanol extract of Rhodiola rosea (Hook. f. et Thoms.) H. Ohba significantly reduces TC, TG, and LDL-C levels in hyperlipidemic mice (Shin et al., 2014). With rich resources, few side effects, and precise hypolipidemic effects, the research, pharmacology, and clinical application of the active ingredients of TCM are promising. In summary, the modulation of ferroptosis by TCM for the treatment of hyperlipidemia is a promising new approach idea, and we expect to provide more reliable theoretical and experimental support in the future.
4.4. TCM alleviates NAFLD by modulating ferroptosis
Currently, there is no recognized standard or definitive effective drug for the treatment of NAFLD. Therefore, the search for new and more effective drugs has become a priority. TCM is a natural treasure trove of compounds with a wide range of sources, active ingredients, and stable structures (Yao et al., 2021). Many studies have confirmed that TCM compound formulas can modulate ferroptosis and thus intervene in the development of NAFLD. For example, Liver Kangning (Scleromitrion diffusum Rhizoma, Polygoni Cuspidati Rhizoma et Radix, Schisandrae Chinensis Fructus, Bupleuri Radix, Ginseng Radix et Rhizoma) can reduce MDA and increase SOD activity, which can play a role in the treatment of NAFLD (Chen, Lin, She, Kuang & Wang, 2019). Zedoary Decoction, which consists of zedoary and atractylodes, can alleviate diabetes mellitus accompanied with nonalcoholic fatty liver disease by activating the Nrf2/HO-1 pathway and reducing lipid peroxide accumulation (Li, Liu, & Li, 2022). Chemolipids and liver recovery granules can induce fatty ferroptosis via the TLR4/NF-κB pathway, which provides a new concept for the treatment of nonalcoholic fatty liver and liver fibrosis (Ye, Xue, & Li, 2021).
In addition, many active ingredients of Chinese herbs affect the development of NAFLD. Astragaloside is an active ingredient purified from A. membranaceus that has been shown to exert antioxidative effects, regulate blood lipid levels, and reduce lipid accumulation (Han, 2016, Tong and Wang, 2014). These effects ultimately lead to the inhibition of ferroptosis and delay the progression of NAFLD in mice. Astragaloside can also activate AMPK and reduce fat synthesis in hepatocytes. AMPK plays a role in regulating energy metabolism and can improve the ability to resist oxidative stress. Therefore, astragaloside can effectively prevent and control NAFLD (Zang, 2022, Herzig and Shaw, 2018). Dihydroartemisinin inhibits intracellular GSH and GPX4 protein levels, thereby inducing hepatocarcinogenesis and ferroptosis and inhibiting hepatocellular carcinoma cell proliferation (Li et al, 2019). After ginkgolide B administration in NAFLD model mice, the liver showed a tendency toward reducetions in lipid droplet deposition, hepatic steatosis, and hepatocyte death, and the mechanism of action was related to the activation of the Nrf2 signaling pathway by ginkgolide B to block ferroptosis (Yang, Wang, Wang, Li, & Gao, 2020). Protocatechuic acid has pharmacological effects on lipid metabolism and antioxidative stress (Gao et al., 2022), and curcumin has pharmacological activity against oxidative stress, which significantly ameliorates NAFLD (Rahmani et al., 2016). Thus, we speculate that this effect may also be associated with ferroptosis. Ginsenoside Re has pharmacological effects such as regulating lipid metabolism, improving mitochondrial function, and alleviating oxidative stress (Li et al, 2022). Total saponins from Panax ginseng C. A. Mey. not only effectively reduce cholesterol, triglyceride, and low-density lipoprotein levels in the serum of NAFLD rats in the early stage but also significantly modulate the expression of inhibitory proteins that bind NO, NF-κB, and other signaling factors in fatty liver tissues. Inhibiting inflammatory cell-related response factors can also reduce the fat content of liver tissue and play a role in the prevention and treatment of NAFLD (Feng, He, Zhao, Yao, & Chen, 2021). Cassia anthraquinone glycoside is a naturally occurring immunogen associated with NAFLD. It is an important factor in the regulation of fatty liver epithelial cells, and can effectively activate a variety of naturally occurring immunogens associated with fatty liver (Chen, Wang, Huang, & Liu, 2016). The researchers found that cassia anthraquinone glycoside could play a role in significantly reducing the high expression of TLR4 and NF-κB induced by a high-fat diet and that adverse effects, such as skin inflammation, and lowering of blood lipids in NAFLD rats, could be significantly alleviated by cassia anthraquinone glycoside. Cassia anthraquinone glycosides also inhibit lipid peroxidation, preventing reinjury to liver cells to combat NAFLD (Zhao, Wu, & Yao, 2019). Gentianoside enhances the activities of superoxide dismutase, and glutathione peroxidase reduces MDA and activates the Nrf2 signaling pathway to inhibit oxidative stress, thus achieving hepatoprotective effects (Xu, Ma, & Zhang, 2022; Wu, Yang, Song, Yao & Chen, 2018).
At present, the relationship between ferroptosis and NAFLD is still in the preliminary stage of exploration, and there are still many problems to be solved. The molecular regulatory mechanism of ferroptosis is complex. Although the molecular mechanism of ferroptosis was initially revealed, the role of TCM in preventing NAFLD through the regulation of ferroptosis still needs to be further explored to provide the theoretical basis for a in-depth study of NAFLD.
5. Potential of ferroptosis in clinical management of CMDs
Currently, ferroptosis is involved in a wide range of diseases and cancers, so identifying more valid and reliable disease markers is of great clinical value. However, recognizing ferroptosis under physiological conditions has been a challenge due to the lack of specific markers;thus, there is an urgent need for more sensitive and specific markers to detect ferroptosis. Several studies have explored ferroptosis-related markers of disease.
Cui et al identified markers of ferroptosis-associated ulcerative colitis (UC) and ultimately revealed that two related genes, CD44 and DUC1, which may be ferroptosis-associated markers in UC. Besides, several ferroptosis-associated subtypes could be considered for potential treatment targets in patients with colorectal carcinoma (CRC) (Yue et al., 2022, Liu et al., 2021b). There are multiple ferroptosis-associated targets verified in cancer diagnosis, for example, four lncRNAs could be used in gastric cancer diagnosis (Cai, Wu, Jia, Pan, & Li, 2022); CD44 and FTL could be utilized in glioma diagnosis (Liu et al, 2020); PANX2 gene is reported to treat prostate cancer through Nrf2 signaling pathway (Liao et al, 2020); GCLC may be a new prognostic biomarker for lung adenocarcinoma (Luo, Zhang, Weng, & Zeng, 2022). Sae-Fung et al. (2023) constructed a new FRG signature model to predict the prognosis of patients with cholangiocarcinoma (CAA), which may serve as a prognostic biomarker and potential therapeutic target for these patients. Furthermore, ferroptosis-associated targets have been demonstrated to serve as biomarkers for diagnosis and treatment in other types of diseases. Li et al. (2021) found that CHAC1 was a valid indicator of poor prognosis in kidney renal clear cell carcinoma (KIRC). SLC40A1 is confirmed a diagnostic biomarker for osteoporosis and a possible hub gene for ferroptosis in osteoporosis patients (Cao, Xue, & Wang, 2023). Shi et al. (2022) demonstrated that P53 and c-Jun were able to distinguish PE patients from healthy pregnant women. These genes may be potential ferroptosis-related biomarkers for disease diagnosis and therapeutic monitoring. Liang et al. (2022) identified the ferroptosis-related gene SOCS1 as a marker for tuberculosis (TB) diagnosis and treatment. Three characteristic FRGs including ALOX15B, RPLPO and HP were illustrated to perform good accuracy in diagnosing major depression disorder (Chen et al, 2023).
This review is dedicated to exploring the use of Chinese herbal medicine for the prevention or treatment of CMDs by regulating ferroptosis and thus provides novel directions. During the course of our research, we found that there have been studies combining herbal medicines with ferroptosis and then looking for specific targets for CMDs. Ma et al. (2022) developed a scoring system for ferroptosis that serves a valid predictor of immune infiltration in diabetic kidney disease (DKD) patients. This approach can effectively validate the relationship between ferroptosis and immune cell infiltration in patients with DKD. Moreover, the active ingredients kaempferol and quercetin contained in TCMs ginseng and Astragalus may be potential drugs that modulate the immune and inflammatory mechanisms of DKD by affecting ferroptosis.
Hyperoxidized peroxiredoxin 3 (PRDX3) is a peroxidase located in mitochondria where lipid peroxides accumulate during the onset of ferroptosis. Some researchers have experimentally shown that oxidized PRDX3 is undetectable during apoptosis, necrosis, and mitochondrial oxidative stress and have identified PRDX3 as a marker of ferroptosis both in vitro and in vivo (Cui et al., 2023). Using this marker, it was also possible to determine that ferroptosis caused liver injury in the NAFLD mouse model. This is the first specific marker for ferroptosis, a major advance that predicts even more rapid growth in the field in the future. We believe that continuing to explore the relationship between ferroptosis and CMDs in depth, searching for ferroptosis-specific genes, and exploring the potential of TCM to regulate ferroptosis may lead to promising therapeutic targets for the precision treatment of patients with CMDs.
6. Discussion and future perspectives
CMDs are characterized by a long and recurrent course of illness and often require long-term clinical drug treatment. Therefore, the search for drugs with good efficacy and few side effects has always been a hot topic in research. TCMs are characterized by multiple targets, multiple pathways, and integrated regulation of body functions, and they have unique advantages in the treatment of CMDs. In recent decades, medicinal plants have been widely used in developing and developed countries due to their effectiveness and limited side effects.
Ferroptosis is a novel mode of programmed death that is iron-dependent and distinct from apoptosis, cell necrosis, and cell autophagy. Ferroptosis is closely related to intracellular GSH, lipid ROS, and iron levels, and a variety of signaling molecules such as GPX4, ACSLA, FTH1, SLC7A11, Nrf2, and HO-1 are involved in this process. Ferroptosis, a newly defined form of regulated cell death, is associated with several chronic metabolic diseases. Moreover, ferroptosis is expected to be a crucial target for the treatment of chronic metabolic diseases. In this study, the research progress on ferroptosis and its role in diabetes, hypertension, and other diseases was reviewed, hoping to provide clues for follow-up research on ferroptosis.
The process of ferroptosis is sophisticatedly regulated by multiple mechanisms and signaling pathways. Therefore, further in-depth studies on the mechanisms of ferroptosis in different disease types are important for the development of targeted drugs. Research on targeting ferroptosis in TCM is promising and, despite some undeniable limitations and difficulties, is important for the prevention and treatment of CMDs. For example, ferroptosis is involved in the onset and development of HLD and can serve as an independent predictor of early changes in HLD as well as a potentially important target for prevention and treatment in clinical practice. However, this approach still has many drawbacks. First, most of the recent studies have focused on animal models, and there is still little evidence demonstrating the mechanism of ferroptosis at the molecular level or in clinical patients. Whether ferroptosis or other forms of cell death during pathogenesis can be clearly distinguished for targeted prevention and treatment also deserves further exploration. Second, research on regulating ferroptosis through TCM or extracts to intervene in different stages of disease development is insufficient, and the related mechanisms also need to be further explored. Finally, there are few experimental studies on the therapeutic purpose of TCM through the regulation of ferroptosis, and little is known about their relationship, such as whether it is clinically feasible to treat chronic metabolic diseases through the inhibition of ferroptosis, how to choose specific drugs and their side-effects and adverse outcomes, and whether it is possible to seek relevant therapeutic measures by exerting multitargeted bidirectional regulation via natural medicines. Accordingly, we believe that we can conduct systematic studies on the treatment of chronic metabolic diseases by the regulation of ferroptosis by TCMs from the following aspects, in order to provide references for the development of TCMs, formulas and active compounds targeting the regulation of ferroptosis. To begin with, we need to make full use of modern scientific analysis techniques and data means to identify the lead compounds of TCM, in order to further discover the active ingredients that target the regulation of ferroptosis. Second, determining the relationship between disease and ferroptosis may require dialectically integrating ferroptosis with other relevant pathological processes to identify better targets for the treatment of CMDs. Finally, a series clinical trials and molecular biological varifications are necessary to conduct for improving the practicability of TCM to regulate the ferroptosis in the treatment of chronic metabolic diseases.
A variety of diseases can be alleviated or treated by inducing ferroptosis leading to cell death and inhibiting ferroptosis to block or slow disease progression. This review aimed to summarize and evaluate the mechanisms of ferroptosis and its role and progress in chronic metabolic diseases, to identify new antimetabolic drugs and targets and to provide a new strategy for improving metabolic disease diagnosis and treatment in the future. With further study, the value of the active ingredients of TCM in the treatment of metabolic diseases will be gradually revealed, and it is expected that TCM will become a commonly used anti-chronic metabolic disease drug in the clinic.
CRediT authorship contribution statement
Yuanyuan Zhang: Writing – review & editing, Writing – original draft, Data curation. Fazhi Su: Writing – review & editing, Project administration, Investigation, Supervision. En-lin Zhu: . Yanping Sun: Writing – review & editing. Haixue Kuang: Writing – review & editing, Conceptualization. Qiuhong Wang: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2018YFC1707100), the Chief Scientist of Qi-Huang Project of National Traditional Chinese Medicine Inheritance and Innovation “One Hundred Million” Talent Project (2021); the Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program (2018); Heilongjiang Touyan Innovation Team Program (2019); the National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of National Administration of TCM (Grant Number: [2022] No. 75); and the Seventh Batch of National Famous Old Traditional Chinese Medicine Experts Experience Heritage Construction Program of National Administration of TCM (Grant Number: [2022] No. 76).
Contributor Information
Haixue Kuang, Email: hxkuang@hljucm.net.
Qiuhong Wang, Email: qhwang668@sina.com.
References
- Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. Journal of Hepatology. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Li M., Liu Y., Qiao Z., Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radical Biology and Medicine. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- Basit F., van Oppen L.M., Schöckel L., Bossenbroek H.M., van Emst-de Vries S.E., Hermeling J.C.…Koopman W.J. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death & Disease. 2017;8(3):e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Worrell B., Palmer J.P. Immunology in the Clinic Review Series; focus on metabolic diseases: Development of islet autoimmune disease in type 2 diabetes patients: Potential sequelae of chronic inflammation. Clinical and Experimental Immunology. 2012;167(1):40–46. doi: 10.1111/j.1365-2249.2011.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Yi M., Tan Y., Li X., Li G., Zeng Z., Xiong W., Xiang B. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. Journal of Experimental & Clinical Cancer Research. 2021;40(1):190. doi: 10.1186/s13046-021-01995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Wu S., Jia Y., Pan X., Li C. Potential key markers for predicting the prognosis of gastric adenocarcinoma based on the expression of ferroptosis-related lncRNA. Journal of Immunology Research. 2022;2022 doi: 10.1155/2022/1249290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Xue Y., Wang J. Screening diagnostic markers of osteoporosis based on ferroptosis of osteoblast and osteoclast. Aging. 2023;15(18):9391–9407. doi: 10.18632/aging.204945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jiang X., Gao X., Wu W., Gu Z., Yin G.…Bi X. Ferroptosis-related genes as diagnostic markers for major depressive disorder and their correlations with immune infiltration. Frontiers in Medicine. 2023;10 doi: 10.3389/fmed.2023.1215180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.H., Lin H., She G.S., Kuang R.Z., Wang F. Therapeutical effect of GanKangNing on non-alcoholic fatty liver in mice. Strait Pharmaceutical Journal. 2019;31(9):43–45. [Google Scholar]
- Chen R., Liu Y. Mechanisms of iron death and their relation to nonalcoholic fatty liver disease. Journal of Hubei Minzu University (Medical Edition) 2020;37(3):80–83. [Google Scholar]
- Chen W., Wang X., Huang L.I., Liu B.O. Hepcidin in non-alcoholic fatty liver disease regulated by the TLR4/NF-κB signaling pathway. Experimental and Therapeutic Medicine. 2016;11(1):73–76. doi: 10.3892/etm.2015.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Ghai A., Deng Y., Li S., Zhang R., Egbulefu C.…Ye J. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Molecular Cell. 2023;83(21):3931–3939.e5. doi: 10.1016/j.molcel.2023.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dächert J., Ehrenfeld V., Habermann K., Dolgikh N., Fulda S. Targeting ferroptosis in rhabdomyosarcoma cells. International Journal of Cancer. 2020;146(2):510–520. doi: 10.1002/ijc.32496. [DOI] [PubMed] [Google Scholar]
- Datz C., Müller E., Aigner E. Iron overload and non-alcoholic fatty liver disease. Minerva Endocrinologica. 2017;42(2):173–183. doi: 10.23736/S0391-1977.16.02565-7. [DOI] [PubMed] [Google Scholar]
- Dham D., Roy B., Gowda A., Pan G., Sridhar A., Zeng X.…Palaniyandi S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radical Research. 2021;55(5):547–561. doi: 10.1080/10715762.2020.1866756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di T.M., Yang S.L., Du F.Y., Zhao L., Li X.H., Xia T., Zhang X.F. Oleiferasaponin A2, a novel saponin from Camellia oleifera Abel. seeds, inhibits lipid accumulation of HepG2 cells through regulating fatty acid metabolism. Molecules. 2018;23(12):3296. doi: 10.3390/molecules23123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang L. Mechanism exploration of sennoside A in treating DN based on Nrf2/HMOX-1 ferroptosis signaling pathway. Information on Traditional Chinese Medicine. 2021;38(7):36–39. [Google Scholar]
- Ding Y., Chen X., Liu C., Ge W., Hao X.…Zhang Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. Journal of Hematology & Oncology. 2021;14(1):19. doi: 10.1186/s13045-020-01016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E.…Stockwell B.R. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M.…Stockwell B.R. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3 doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I.…Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I.…Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovinova I., Kvandová M., Balis P., Gresova L., Majzunova M., Horakova L., Chan J.Y., Barancik M. The role of Nrf2 and PPARgamma in the improvement of oxidative stress in hypertension and cardiovascular diseases. Physiological Research. 2020;69(Suppl 4):S541–S553. doi: 10.33549/physiolres.934612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in Cancer Biology. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Eling N., Reuter L., Hazin J., Hamacher-Brady A., Brady N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Dou R., Hu J. Modulation of iron death by poric acid through Nrf2/SLC7A11/GPX4 signal pathway in the improvement of cognitive impairment of Alzheimer's disease rat. Chinese General Practice. 2024;27(2):177–183. [Google Scholar]
- Farooqui Z., Mohammad R.S., Lokhandwala M.F., Banday A.A. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clinical and Experimental Hypertension. 2021;43(2):175–180. doi: 10.1080/10641963.2020.1836191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H., Stockwell B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? Plos Biology. 2018;16(5) doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X.Y., He P.L., Zhao W., Yao Z., Chen W.H. Effects of Panax notoginseng total saponins on improving nonalcoholic fatty liver disease rats and NO/iNOS/NF-κB signaling pathway. Chinese Traditional Patent Medicine. 2021;43(1):50–55. [Google Scholar]
- Feng Y., Yang D., Zhi X., Deng H., Zhang W., Wang R., Wu W. Role of interaction between reactive oxygen species and ferroptosis pathway in methylglyoxal-induced injury in mouse embryonic osteoblasts. Journal of Southern Medical University. 2022;42(1):108–115. doi: 10.12122/j.issn.1673-4254.2022.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Yang D., Zhi X. Role of interaction between reactive oxygen species and ferroptosis pathway in methylglyoxal induced injury in mouse embryonic osteoblasts. Journal of Southern Medical University. 2022;42(1):108–115. doi: 10.12122/j.issn.1673-4254.2022.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano R., Mullish B.H., Mukherjee S.K., Nathwani R., Harlow C., Crook P.…Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.Q., Yu D.H., Chen P.P., Lu F., Wang Y., Liu S.M. Role of ferroptosis in cardiovascular diseases and research progress of traditional Chinese medicine intervention. Pharmacology and Clinics of Chinese Materia Medica. 2023;39(2):93–101. [Google Scholar]
- Fuchs Y., Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(7):1640. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gać P., Czerwińska K., Poręba M., Prokopowicz A., Martynowicz H., Mazur G., Poreba R. Serum zinc and selenium concentrations in patients with hypertrophy and remodelling of the left ventricle secondary to arterial hypertension. Antioxidants (Basel) 2021;10(11):1803. doi: 10.3390/antiox10111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B. Mitochondrial regulation of ferroptosis. Journal of Cell Biology. 2021;220(9) doi: 10.1083/jcb.202105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Molecular Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Tian R., Liu H., Xue H., Zhang R., Han S.…You Y. Research progress on intervention effect and mechanism of protocatechuic acid on nonalcoholic fatty liver disease. Critical Reviews in Food Science and Nutrition. 2022;62(32):9053–9075. doi: 10.1080/10408398.2021.1939265. [DOI] [PubMed] [Google Scholar]
- Gaschler M.M., Andia A.A., Liu H., Csuka J.M., Hurlocker B., Vaiana C.A.…Stockwell B.R. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nature Chemical Biology. 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X.M., Xie Y.S., Ni W.J., Tang L.Q. Influence of Nrf2/HO-1/GPX4 signaling pathway on high glucose-induced podocyte ferroptosis and intervention of berberine. Chinese Pharmacological Bulletin. 2021;37(3):396–403. [Google Scholar]
- Guan Z., Chen J., Li X., Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Bioscience Reports. 2020;40(8) doi: 10.1042/BSR20201807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C.…Wu G. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Research and Treatment. 2018;50(2):445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Liu Y., Gao Z.Y., Shi D.Z. Chinese herbal medicine on dyslipidemia: Progress and perspective. Evidence-Based Complementary and Alternative Medicine. 2014;2014 doi: 10.1155/2014/163036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D. Study of astragalosides on antihyperglycemia, regulating plasma lipid and improving antioxidant ability in experimental diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2016;25(4):360–364. [Google Scholar]
- Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y.…Vanden Berghe T. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. Journal of Clinical Investigation. 2018;128(8):3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Shaw R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon A., Diers A.R., Oh J.Y., Landar A., Darley-Usmar V.M. Cell signalling by reactive lipid species: New concepts and molecular mechanisms. Biochemical Journal. 2012;442(3):453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Gu W., Ma N., Fan X., Ci X. Leonurine alleviates ferroptosis in cisplatin-induced acute kidney injury by activating the Nrf2 signalling pathway. British Journal of Pharmacology. 2022;179(15):3991–4009. doi: 10.1111/bph.15834. [DOI] [PubMed] [Google Scholar]
- Hu Y.Z., Li J.M., Lu Z.Z., Li W., Jiang F.G., Gui J. The effect of chrysophanol on high fat diet-induced non-alcoholic fatty liver disease in neonatal rats. Immunological Journal. 2018;34(10):869–874. [Google Scholar]
- Huang M., Han X., Yang Z. Progress in the mechanism of ferroptosis in diabetes mellitus and its complications. Chinese Journal of Diabetes. 2023;31(2):155–157. [Google Scholar]
- Huang Y., Wu H., Hu Y., Zhou C., Wu J., Wu Y.…Sun J. Puerarin attenuates oxidative stress and ferroptosis via AMPK/PGC1α/Nrf2 pathway after subarachnoid hemorrhage in rats. Antioxidants (Basel) 2022;11(7):1259. doi: 10.3390/antiox11071259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Matsuoka M., Kumagai T., Sakamoto T., Koumura T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Current Topics in Microbiology and Immunology. 2017;403:143–170. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K.…Conrad M. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172(3):409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Ji Q.X., Xu X.L. Effect of polydatin on age-and diet-associated non-alcoholic steatohepatitis in LDL receptor knockout mice and its possible mechanisms. Chinese Traditional and Herbal Drugs. 2021;52(12):3602–3610. [Google Scholar]
- Jiang X., Stockwell B.R., Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Akhtar M.S., Khan B.A., Braga V.D., Reich A. Antiobesity, hypolipidemic, antioxidant and hepatoprotective effects of Achyranthes aspera seed saponins in high cholesterol fed albino rats. Archives of Medical Science. 2015;11(6):1261–1271. doi: 10.5114/aoms.2015.56353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P., Zhuang L., Gan B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein & Cell. 2021;12(8):599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose T., Vera-Aviles M., Sharp P.A., Latunde-Dada G.O. Curcumin and (-)- epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals (Basel) 2019;12(1):26. doi: 10.3390/ph12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F.…Schick J.A. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Central Science. 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nature Reviews Cancer. 2022;22(7):381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu J.I., Murphy M.E., George D.L. Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(17):8390–8396. doi: 10.1073/pnas.1821277116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu S., Xu J., Chen L., Xu C., Chen F.…Wang Y. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. Journal of Cellular and Molecular Medicine. 2021;25(7):3610–3621. doi: 10.1111/jcmm.16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E.W., Gao G., Wang M.Y., Wu L.M., Xie Z.S., Zhang Z.Q.…Xu J.Y. Mechanism of Zexie Decoction in inhibiting ferroptosis of hepatocytes to relieve non-alcoholic fatty liver disease. Acta Chinese Medicine. 2022;37(6):1243–1253. [Google Scholar]
- Li H., Wu M., Ma Y. Research progress of traditional Chinese medicine regulating ferroptosis to inhibit hepatic fibrosis. Journal of Nanjing University of Traditional Chinese Medicine. 2023;2023(6):587–593. [Google Scholar]
- Li N., Wang W., Zhou H., Wu Q., Duan M., Liu C.…Tang Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radical Biology and Medicine. 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Li X., Chen J., Feng W., Wang C., Chen M., Li Y.…Tian J. Berberine ameliorates iron levels and ferroptosis in the brain of 3 × Tg-AD mice. Phytomedicine. 2023;118 doi: 10.1016/j.phymed.2023.154962. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Zhou Y., Wang X., Wang X., Chen S.F., Wang Y., Du J. DHA inhibits proliferation of human hepatocellular carcinoma cells by inducing ferroptosis. Chinese Journal of Biochemistry and Molecular Biology. 2019;35(12):1361–1366. [Google Scholar]
- Li Z.J., Liu B.Y., Li H.R., Xue J.Q., Xu B. Research progress of traditional Chinese medicine glycosides in prevention and treatment of nonalcoholic fatty liver. Journal of Jilin Medical University. 2022;43(5):364–367. [Google Scholar]
- Liang K. Efficacy of compound Danshen Drip Pill in the treatment of patients with type 2 diabetes mellitus with hypertensive disease and its effect on related inflammatory cytokines. China's Naturopathy. 2022;30(14):70–73. [Google Scholar]
- Liang T., Chen J., Xu G., Zhang Z., Xue J., Zeng H.…Zhan X. Ferroptosis-related gene SOCS1 a marker for tuberculosis diagnosis and treatment, involves in macrophage polarization and facilitates bone destruction in tuberculosis. Tuberculosis. 2022;132 doi: 10.1016/j.tube.2021.102140. [DOI] [PubMed] [Google Scholar]
- Liao D., Yang G., Yang Y., Tang X., Huang H., Shao J., Pan Q. Identification of pannexin 2 as a novel marker correlating with ferroptosis and malignant phenotypes of prostate cancer cells. OncoTargets and Therapy. 2020;13:4411–4421. doi: 10.2147/OTT.S249752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Ge J., Yu Q. Current research on mechanism of ferroptosis. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2019;21(10):2151–2158. [Google Scholar]
- Liao Z., Lin X., Wang J. Progress of the mechanism of iron death in ischemic stroke treated by traditional Chinese medicine. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease. 2023;21(12):2230–2233. [Google Scholar]
- Liu C.X., Guo X.Y., Zhang L.L., Chang X. Research progress of ferroptosis and ischemic stroke and its Chinese medicine intervention. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2022;30(7):135–140. [Google Scholar]
- Liu J., Zhang T., Zhu J., Ruan S., Li R., Guo B., Lin L. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy. Chinese Medicine. 2021;16(1):115. doi: 10.1186/s13020-021-00528-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Guo F., Guo W., Wang Y., Song W., Fu T. Ferroptosis-related genes are potential prognostic molecular markers for patients with colorectal cancer. Clinical and Experimental Medicine. 2021;21(3):467–477. doi: 10.1007/s10238-021-00697-w. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu Z., Jin T., Xu K., Liu M., Xu H. Ferroptosis in low-grade glioma: A new marker for diagnosis and prognosis. Medical Science Monitor: International Medical Journal of Experimental And Clinical Research. 2020;26 doi: 10.12659/MSM.921947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Zhang Z., Weng Y., Zeng J. Ferroptosis-related gene GCLC is a novel prognostic molecular and correlates with immune infiltrates in lung adenocarcinoma. Cells. 2022;11(21):3371. doi: 10.3390/cells11213371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G.P., Yu Z.Y., Qing L.J., Li J.L., Hong C.X. Jiawei-Erzhi Pills attenuate liver lipid deposition in mice with hyperlipidemia by inhibiting ferroptosis. Chinese Journal of Pathophysiology. 2022;38(2):259–266. [Google Scholar]
- Ma J., Li C., Liu T., Zhang L., Wen X., Liu X., Fan W. Identification of markers for diagnosis and treatment of diabetic kidney disease based on the ferroptosis and immune. Oxidative Medicine and Cellular Longevity. 2022;2022 doi: 10.1155/2022/9957172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Dielschneider R.F., Henson E.S., Xiao W., Choquette T.R., Blankstein A.R.…Gibson S.B. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Henson E.S., Chen Y., Gibson S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death & Disease. 2016;7(7):e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayneris-Perxachs J., Moreno-Navarrete J.M., Fernández-Real J.M. The role of iron in host-microbiota crosstalk and its effects on systemic glucose metabolism. Nature Reviews Endocrinology. 2022;18(11):683–698. doi: 10.1038/s41574-022-00721-3. [DOI] [PubMed] [Google Scholar]
- Megalli S., Davies N.M., Roufogalis B.D. Roufogalis, anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. Journal of Pharmacy and Pharmaceutical Sciences. 2006;9(3):281–291. [PubMed] [Google Scholar]
- Meng Z., Liang H., Zhao J., Gao J., Liu C., Ma X.…Wang Y. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sciences. 2021;284 doi: 10.1016/j.lfs.2021.119935. [DOI] [PubMed] [Google Scholar]
- Newell J.L., Keyari C.M., McDaniel S.W., Diaz P.J., Natale N.R., Patel S.A., Bridges R.J. Novel di-aryl-substituted isoxazoles act as noncompetitive inhibitors of the system Xc(-) cystine/glutamate exchanger. Neurochemistry International. 2014;73:132–138. doi: 10.1016/j.neuint.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Ning S.Y., Ma Y.X., Liu J.T., Zhou Y., Yang Y.F., Shi Y. Effect of Yitangkang on hepatic AMPK/SREBP-1C/FAS in type 2 diabetic rats. Chinese Archives of Traditional Chinese Medicine. 2023;41(10):164–168. 285. [Google Scholar]
- Niu X.L., Ichimori K., Yang X., Hirota Y., Hoshiai K., Li M., Nakazawa H. Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radical Research. 2000;33(3):305–312. doi: 10.1080/10715760000301471. [DOI] [PubMed] [Google Scholar]
- Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2014;20(39):14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani S., Asgary S., Askari G., Keshvari M., Hatamipour M., Feizi A., Sahebkar A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytotherapy Research. 2016;30(9):1540–1548. doi: 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- Rauf A., Akram M., Anwar H., Daniyal M., Munir N., Bawazeer S.…Khan H. Therapeutic potential of herbal medicine for the management of hyperlipidemia: Latest updates. Environmental Science and Pollution Research International. 2022;29(27):40281–40301. doi: 10.1007/s11356-022-19733-7. [DOI] [PubMed] [Google Scholar]
- Rehman K., Akash M.S.H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? Journal of Cellular Biochemistry. 2017;118(11):3577–3585. doi: 10.1002/jcb.26097. [DOI] [PubMed] [Google Scholar]
- Rochette L., Dogon G., Rigal E., Zeller M., Cottin Y., Vergely C. Lipid peroxidation and iron metabolism: Two corner stones in the homeostasis control of ferroptosis. International Journal of Molecular Sciences. 2022;24(1):449. doi: 10.3390/ijms24010449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-Fung A., Mutirangura A., Jitkaew S. Identification and validation of a novel ferroptosis-related gene signature for prognosis and potential therapeutic target prediction in cholangiocarcinoma. Frontiers in Immunology. 2023;13 doi: 10.3389/fimmu.2022.1051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R., Buchholz B., Kraus A., Schley G., Scholz J., Ousingsawat J., Kunzelmann K. Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. Journal of the American Society of Nephrology. 2019;30(2):228–242. doi: 10.1681/ASN.2018010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A., Schneider M., Förster H., Roth S., Wirth E.K., Culmsee C.…Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metabolism. 2008;8(3):237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Shen C., Pan Z., Wu S., Zheng M., Zhong C., Xin X.…Xian S. Emodin palliates high-fat diet-induced nonalcoholic fatty liver disease in mice via activating the farnesoid X receptor pathway. Journal of Ethnopharmacology. 2021;279 doi: 10.1016/j.jep.2021.114340. [DOI] [PubMed] [Google Scholar]
- Shen Y., Shen X., Wang S., Zhang Y., Wang Y., Ding Y.…Shen J. Protective effects of Salvianolic acid B on rat ferroptosis in myocardial infarction through upregulating the Nrf2 signaling pathway. International Immunopharmacology. 2022;112 doi: 10.1016/j.intimp.2022.109257. [DOI] [PubMed] [Google Scholar]
- Sheng X., Wang M., Lu M., Xi B., Sheng H., Zang Y.Q. Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. American Journal of Physiology Endocrinology and Metabolism. 2011;300(5):E886–E893. doi: 10.1152/ajpendo.00332.2010. [DOI] [PubMed] [Google Scholar]
- Shi M., Yang X., Ding Y., Sun L., Zhang P., Liu M.…Li R. Ferroptosis-related proteins are potential diagnostic molecular markers for patients with preeclampsia. Biology-basel. 2022;11(7):950. doi: 10.3390/biology11070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Yuan R., Chen X., Xin Q., Wang Y., Shang X.…Chen K. Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting ENOS. The American Journal of Chinese Medicine. 2019;47(1):19–38. doi: 10.1142/S0192415X19500022. [DOI] [PubMed] [Google Scholar]
- Shin D., Kim E.H., Lee J., Roh J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radical Biology & Medicine. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- Shin H.S., Han J.M., Kim H.G., Choi M.K., Son C.G., Yoo H.R., Seol I.C. Anti-atherosclerosis and hyperlipidemia effects of herbal mixture, Artemisia iwayomogi Kitamura and Curcuma longa Linne, in apolipoprotein E-deficient mice. Journal of Ethnopharmacology. 2014;153(1):142–150. doi: 10.1016/j.jep.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Sobrino P., Ojeda M.L., Nogales F., Murillo M.L., Carreras O. Binge drinking affects kidney function, osmotic balance, aldosterone levels, and arterial pressure in adolescent rats: The potential hypotensive effect of selenium mediated by improvements in oxidative balance. Hypertension Research. 2019;42(10):1495–1506. doi: 10.1038/s41440-019-0265-z. [DOI] [PubMed] [Google Scholar]
- Song C.Y., Feng M.X., Li L., Wang P., Lu X., Lu Y.Q. Tripterygium wilfordii Hook. f. ameliorates paraquat-induced lung injury by reducing oxidative stress and ferroptosis via Nrf2/HO-1 pathway. Ecotoxicology and Environmental Safety. 2023;252 doi: 10.1016/j.ecoenv.2023.114575. [DOI] [PubMed] [Google Scholar]
- Stockwell B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B.R., Jiang X., Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends in Cell Biology. 2020;30(6):478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Wang J. Effects of astragaloside IV on lipopolysaccharide-induced hepatocytes injury in vitro. Chinese Journal of Public Health. 2014;30(6):753–755. [Google Scholar]
- Tsurusaki S., Tsuchiya Y., Koumura T., Nakasone M., Sakamoto T., Matsuoka M.…Tanaka M. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death & Disease. 2019;10(6):449. doi: 10.1038/s41419-019-1678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B.…Schreiber S.L. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen X.Z., Wang Y.H., Cheng X.L., Zhao Y., Zhou L.Y., Wang K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discovery. 2022;8(1):394. doi: 10.1038/s41420-022-01183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.D., Chen X. Research progress of ferroptosis in cardiovascular diseases. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery. 2022;29(4):497–501. [Google Scholar]
- Wang X.R., Zhao W.X., Miao M.S. Effect of ferroptosis on organ fibrosis and intervention progress of traditional Chinese medicine: A review. Chinese Journal of Experimental Traditional Medical Formulae. 2023;29(8):246–256. [Google Scholar]
- Wang X., Li S., Yu J., Wang W., Du Z., Gao S.…Deng X. Saikosaponin B2 ameliorates depression-induced microglia activation by inhibiting ferroptosis-mediated neuroinflammation and ER stress. Journal of Ethnopharmacology. 2023;316 doi: 10.1016/j.jep.2023.116729. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Y., Huang D., Shi S., Pei C., Wu Y.…Wang Z. Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice. International Immunopharmacology. 2022;112 doi: 10.1016/j.intimp.2022.109186. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun Z.Y., Ren X.H., Zhao Z.G. Inhibitory effect of curcumin on ferroptosis induced by high glucose in neuro-2a cells via Nrf2/HO-1 pathway. Journal of Fujian Medical University. 2023;57(2):79–88. [Google Scholar]
- Watt M.J., Miotto P.M., De Nardo W., Montgomery M.K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocrine Reviews. 2019;40(5):1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- Wo X.D., Cui X.Q., Tang L.H. Effects of curcumin on the activities of plasma lipase and the concentrations of lipids in hyperlipidemic rats induced by high lipid diet. Chinese Journal of Arterial Lerosis. 2003;11(3):223–226. [Google Scholar]
- Wu J.Z., Yang B., Song B., Yao Z., Chen W.H. Effect of gentiopicroside on AMPKα signaling pathway of rats with non-alcoholic fatty liver disease. Chinese Traditional and Herbal Drugs. 2018;49(21):5142–5148. [Google Scholar]
- Wu J., Wang Y., Jiang R., Xue R., Yin X., Wu M., Meng Q. Ferroptosis in liver disease: New insights into disease mechanisms. Cell Death Discovery. 2021;7(1):276. doi: 10.1038/s41420-021-00660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liu M., Liang J., Li N., Yang D., Cai J.…Ma T. Ferroptosis as a new mechanism in Parkinson’s disease therapy using traditional Chinese medicine. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.659584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M.T.…Tang D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochemical and Biophysical Research Communications. 2016;473(4):775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J.…Tang D. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Reports. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- Xing T.T., Shan W. Protective effect of crocin on renal injury in diabetic rats by inhibiting oxidative stress and inflammatory response. Shaanxi Medical Journal. 2020;49(9):1067–1070. [Google Scholar]
- Xiong L., Ning F., Shi Y.B. Research progress of iron overload in the pathogenesis of diabetes mellitus. Journal of Medical Research. 2019;48(7):164–167. [Google Scholar]
- Xu R.C., Zhang P., Xiang M. Research progress on chemical constituents and anti-metabolic disease pharmacological effects of Cyclocarya paliurus. Chinese Traditional and Herbal Drugs. 2023;54(9):2962–2977. [Google Scholar]
- Xu W., Li C., Jiang Y. Ferroptosis pathway and its intervention regulated by Chinese materia medica. China Journal of Chinese Materia Medica. 2018;43(20):4019–4026. doi: 10.19540/j.cnki.cjcmm.20180517.001. [DOI] [PubMed] [Google Scholar]
- Xu Z.L., Ma Z.Y., Zhang P.H. Neuroprotective mechanism of traditional Chinese medicine extracts in the treatment of diabetic ED by inhibiting the ferroptosis pathway. National Journal of Andrology. 2022;28(11):1049–1053. [PubMed] [Google Scholar]
- Xu Z.P., Lu Z.J., Chen J.H., Deng X.Y., Mao Y.Z., Huo X. The effect of rhubarb ethanol-extract on hyperlipidemia and liver fatty in rabbits. Chinese Journal of Applied Physiology. 2007;23(3):375–380. [PubMed] [Google Scholar]
- Yan H.F., Zou T., Tuo Q.Z., Xu S., Li H., Belaidi A.A., Lei P. Ferroptosis: Mechanisms and links with diseases. Signal Transduction and Targeted Therapy. 2021;6(1):49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang M., Wang S., Li G., Gao Y. Study on ferroptosis pathway that operates in hypertensive brain damage. Clinical and Experimental Hypertension. 2020;42(8):748–752. doi: 10.1080/10641963.2020.1783545. [DOI] [PubMed] [Google Scholar]
- Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S.…Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Chen J., Gao Q., Shan X., Wang J., Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445 doi: 10.1016/j.tox.2020.152599. [DOI] [PubMed] [Google Scholar]
- Yao C.L., Zhang J.Q., Li J.Y., Wei W.L., Wu S.F., Guo D.A. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Natural Product Reports. 2021;38(9):1618–1633. doi: 10.1039/d0np00057d. [DOI] [PubMed] [Google Scholar]
- Yao Y.S., Li T.D., Zeng Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids in Health and Disease. 2020;19(1):23. doi: 10.1186/s12944-019-1171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M.Q., Xue J.D., Li F.P., Yang Y.Q., He J.Y., Tang Y.H. Study on regulation and mechanism of Huazhi Fugan granules on TLR4/NF-κB pathway and fatty iron death in non-alcoholic fatty liver. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2021;30(30):3307–3312. [Google Scholar]
- Yu X., Xu L., Zhou Q., Wu S., Tian J., Piao C.…Tong X. The efficacy and safety of the Chinese herbal formula, JTTZ, for the treatment of type 2 diabetes with obesity and hyperlipidemia: A multicenter randomized, positive-controlled, open-label clinical trial. International Journal of Endocrinology. 2018;2018 doi: 10.1155/2018/9519231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.Y., Zhang Y., Zhang W. Role of ferroptosis in cardiovascular diseases and Chinese medicine treatment based on iron homeostasis: A review. Chinese Journal of Experimental Traditional Medical Formulae. 2023;29(18):198–208. [Google Scholar]
- Yue Q., Zhang Y., Wang F., Cao F., Duan X., Bai J. Classification of colorectal carcinoma subtypes based on ferroptosis-associated molecular markers. World Journal of Surgical Oncology. 2022;20(1):117. doi: 10.1186/s12957-022-02575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang C., Dong C., Hu X. Research progress on molecular mechanism of traditional Chinese medicine in treatment of Alzheimer's disease. Chinese Traditional and Herbal Drugs. 2022;53(13):4132–4145. [Google Scholar]
- Zang, X. (2022). Molecular mechanism of iron death-induced nonalcoholic fatty liver disease in mice and study of the regulatory role of astragaloside. Liaoning University of Traditional Chinese Medicine. Thesis of Master degree.
- Zang X., Chen W. Preliminary study on the potential molecular net work interaction of ferroptosis in livertranscriptome of ApoE-/-hyperlipidemic mice. Journal of Chongqing Medical University. 2022;47(5):548–553. [Google Scholar]
- Zeng T., Gao L., Lv Z. Molecular mechanism of ferroptosis and its role in tumor treatment. Journal of Oncology in Chinese Medicine. 2022;4(1):6–12. [Google Scholar]
- Zhang M., Li X., Liang H., Cai H., Hu X., Bian Y., Zhang Y. Semen Cassiae extract improves glucose metabolism by promoting GlUT4 translocation in the skeletal muscle of diabetic rats. Frontiers in Pharmacology. 2018;9:235. doi: 10.3389/fphar.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tan H., Daniels J.D., Zandkarimi F., Liu H., Brown L.M., Stockwell B.R. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chemical Biology. 2019;26(5):623–633.e9. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Tang J., Song J., Xie M., Liu Y., Dong Z.…Zhong J. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radical Biology and Medicine. 2022;181:130–142. doi: 10.1016/j.freeradbiomed.2022.01.020. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yao Z., Wang L., Ding H., Shao J., Chen A.…Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Li X., Yang L., Zhang L., Jiang X., Gao W.…Liu J. Isorhynchophylline relieves ferroptosis-induced nerve damage after intracerebral hemorrhage via miR-122-5p/TP53/SLC7A11 pathway. Neurochemical Research. 2021;46(8):1981–1994. doi: 10.1007/s11064-021-03320-2. [DOI] [PubMed] [Google Scholar]
- Zhao W.J., Fu H.X., Ying C.M., Liu X.Z. Research progress on regulating ferroptosis and inhibiting cerebral ischemia reperfusion injury by traditional Chinese medicine. Chinese Traditional and Herb Drugs. 2024;55(8):2812–2819. [Google Scholar]
- Zhao Y., Bao L., Sun T. Ginkgetin induces ferroptosis of glioma cells by inhibiting Nrf2/HO-1/GPX4 signaling pathway. Journal of Modern Oncology. 2023;31(18):3366–3370. [Google Scholar]
- Zhao Z., Wu J., Yao Z. Effects of Cassia glycosides on the rats with non-alcoholic fatty liver disease through Toll-likereceptor 4 and nuclear factor-Kb. The Chinese Journal of Clinical Pharmacology. 2019;35(22):2863–2867. [Google Scholar]
- Zhou T., Wang Z., Guo M., Zhang K., Geng L., Mao A.…Yu F. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food & Function. 2020;11(11):10137–10148. doi: 10.1039/d0fo02356f. [DOI] [PubMed] [Google Scholar]
- Zhou Y. The protective effects of cryptochlorogenic acid on β-cells function in diabetes in vivo and vitro via inhibition of ferroptosis. Diabetes Metabolic Syndrome and Obesity-targets and Therapy. 2020;13:1921–1931. doi: 10.2147/DMSO.S249382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Wang P., Ma S. Metabolic hypertension: Concept and practice. Frontiers of Medicine. 2013;7(2):201–206. doi: 10.1007/s11684-013-0264-4. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Kroemer G. Interferon-γ induces cancer cell ferroptosis. Cell Research. 2019;29(9):692–693. doi: 10.1038/s41422-019-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Schreiber S.L. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chemical Biology. 2020;27(4):463–471. doi: 10.1016/j.chembiol.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuang W., Guo H., Chen Z. The mechanism of ferroptosis regulation in osteoporosis and research progress on traditional Chinese medicine intervention. Chinese Journal of Osteoporosis. 2023;29(6):890–896. [Google Scholar]
- Zuo, J. (2017). Effects of hypoglycemic thirst-quenching granules and yiqi and warm-yang fractions on the browning of white fat in obese mice with C57BL/6J. Beijing University of Chinese Medicine. Thesis of Doctor Degree.