Abstract
A LightCycler-based PCR protocol was developed which targets the ospA gene for the identification and quantification of the different Borrelia burgdorferi sensu lato species in culture and in ticks, based on the use of a fluorescently labeled probe (HybProbe) and an internally labeled primer. The detection limit of the PCR was 1 to 10 spirochetes. A melting temperature determined from the melting curve of the amplified product immediately after thermal cycling allowed the differentiation of the three different B. burgdorferi sensu lato genospecies (B. burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii) that are clinically relevant in Europe in a single PCR run. This method represents a simplified approach to study the association of different Borrelia species in ticks, the risk of Lyme borreliosis, and the putatively species-specific clinical sequelae. To determine the reliability of the real-time PCR protocol, we studied the prevalence of B. burgdorferi sensu lato infection in Ixodes ricinus ticks. A total of 1,055 ticks were collected by flagging vegetation in five different sites in the region of Konstanz (south Germany) and were examined for the distribution of B. burgdorferi species by real-time PCR. The mean infection rate was 35%. Of 548 adult ticks, 40% were positive, and of 507 nymphs, 30% were positive. The predominant genospecies (with 18% mixed infections) in the examined areas was B. afzelii (53%), followed by B. garinii (18%) and B. burgdorferi sensu stricto (11%); 0.8% of the infecting Borrelia could not be identified.
Lyme borreliosis (LB)—the most common arthropod-borne infection in Europe (24) and the United States (25)—is a complex multisystem disorder caused by Borrelia burgdorferi sensu lato, a group of genetically diverse spirochetes. The principal vectors of these spirochetes are ticks belonging to the genus Ixodes (2).
The development of an erythema migrans rash at the site of the tick bite often characterizes the onset of LB. If left untreated, the infection can persist for years and may result in a range of clinical symptoms, which vary depending on the duration of the infection and the organs affected.
Isolates of B. burgdorferi sensu lato can be classified into different genomic species (1, 11). Only one of them, B. burgdorferi sensu stricto, has been implicated as the cause of disease in North America, but in Europe three genospecies, Borrelia afzelii, Borrelia garinii, and B. burgdorferi sensu stricto, are known to be pathogenic, and still others, such as Borrelia valaisiana and Borrelia lusitaniae, have been identified but are of unknown pathogenicity (7). Coinfections by two or more genomic groups of B. burgdorferi sensu lato have been found in ticks (13, 14) and patients with LB (5).
There is strong evidence that different species are involved in distinct clinical manifestations of the disease (28). Different studies have presented indirect evidence for the association of B. garinii with predominantly neurological symptoms (5), while infections by B. burgdorferi sensu stricto and B. afzelii tend to lead to arthritic symptoms (29) and cutaneous manifestations (3), respectively. New rapid and sensitive methods are therefore required for differentiating the three pathogenic Borrelia species to test the strengths of these associations.
PCR is increasingly employed for the detection of Borrelia (5, 17, 21, 23). Recently, the LightCycler PCR, which assesses the amount of amplified DNA after each PCR cycle, was introduced. Besides nonspecific DNA measurement by intercalating dyes (such as SYBR Green), specific gene probes labeled with fluorescent dyes allow the quantification of formed amplicon. Furthermore, calculation of the melting point of the DNA-probe adduct enables identification of the PCR product. This method can be exploited to distinguish sequence deviations, e.g., polymorphisms of different bacterial strains.
Recently, two LightCycler PCR-based assays for the differentiation of Borrelia species were described (15, 19). The second method, an amendment of the first, could distinguish among all three Borrelia species known to be pathogenic for humans. However, the method requires two LightCycler PCRs targeting the recA gene and the p66 gene, respectively. Melting curve analysis of the recA gene amplicon allows the separation of B. garinii from B. burgdorferi sensu stricto and B. afzelii, and melting curve analysis of the p66 gene amplicon is employed for the separation of B. burgdorferi sensu stricto from B. afzelii and B. garinii.
The LightCycler PCR described here allows rapid genotyping of the three B. burgdorferi species in a single PCR run. Therefore, a sequence of the ospA gene which results in zero, two, or three mismatches in the three species was chosen as a probe, allowing differentiation by melting point analysis. In contrast to the above-mentioned method of Mommert et al., a fluorescently labeled hybridization probe and an internally labeled primer were used rather than the fluorescent dye SYBR Green. This combination allows a rapid genotyping of the three B. burgdorferi species in a single PCR run.
In Europe, where all three genospecies of Borrelia known to be pathogenic for humans are found, the identification of the genospecies in patient specimens and ticks from patients is necessary to make a distinction among their respective roles in the pathophysiology of LB manifestations. Furthermore, field studies in areas of endemicity to assess the prevalence of B. burgdorferi sensu lato species in ticks will aid risk assessment and allow a determination of the infectivities of different species.
In the present study, we used real-time PCR to characterize the distribution of species of B. burgdorferi sensu lato in ticks collected in the field. We show that the three genomic groups were present in 1,055 Ixodes ricinus ticks collected in the southern part of Germany during 1999 and 2000 and that coinfections with two or three genomic groups of B. burgdorferi sensu lato occur among these ticks.
MATERIALS AND METHODS
Study area and tick collection.
A total of 1,478 I. ricinus ticks were collected by the flagging method in five locations (A, B, H, L, and M) in the region of Konstanz (south Germany) in 1999 and 2000. The distances between the different locations were less than 10 km. At sites A and L, ticks were collected from the edges of roads and trails with mixed woodland on one side and meadows on the other side. Site B was a barbecue site in a forest situated near a marsh. M was a kindergarten in a forest, and at site H, ticks were collected from within the woodland near a path. The ticks were separated into nymphs and mature females and males and stored at −80°C until use. At least 200 ticks (100 nymphs, 50 males, and 50 females) from each location were examined by PCR.
Bacterial isolates and culture conditions.
The Borrelia strains used in this study (B. burgdorferi sensu stricto N40, B. garinii PSth and B29, and B. afzelii VS461) were cultured in modified BSK-H medium (Sigma, Deisenhofen, Germany) at 33°C as described previously (6). All Borrelia strains were kindly provided by T. Kamradt (Berlin, Germany), with the exception of B. garinii A218, which was a gift from H. Martilla. Culture density was determined by microscopy using a modified Thoma counting chamber (Merck Eurolab, Ismaning, Germany).
DNA extraction.
DNA from each Borrelia culture was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions or by a Chelex-based method (31), which is a faster DNA extraction procedure. The bacterial culture was diluted 1:10 with 20% Chelex 100 (Bio-Rad Laboratories, Munich, Germany). After being thoroughly mixed, the sample was incubated at 56°C for 30 min. Then, the suspension was boiled for 10 min, and the debris was removed by centrifugation (13,800 × g; 3 min). The supernatant was either used directly for amplification or stored at −20°C until use. To extract the DNA from the ticks, they were mechanically crushed with sterile pestles, and nucleic acid extractions were performed by the Chelex-based method using 40 and 45 μl of Chelex 100 for nymphs and adults, respectively.
Real-time PCR.
Real-time PCR was performed using a LightCycler rapid thermal cycler system (Roche Diagnostics GmbH, Mannheim, Germany). A 296-bp fragment from the gene encoding OspA was amplified. The reverse primer used was published previously by Demaerschalck et al. (5). The forward primer, serving as the acceptor probe of the HybProbe detection system, was labeled internally with the fluorescent dye LC Red 640. Thus, first-strand DNA already contained fluorescent dye. For detection and differentiation of the species, we used a fluorescein-labeled probe. The forward primer was located further downstream than the originally published forward primer so that the fluorescein-labeled probe could bind the first-strand DNA close enough to the LC Red 640 for electron transfer to occur. The probe and primers were designed as shown in Fig. 1 and Table 1. The forward primer and probe were both designed by TIB Molbiol (DNA Synthesis Service, Roche Diagnostics, Berlin, Germany), who also synthesized all primers and the probe.
FIG. 1.
Design of forward primer and probe for the identification of Borrelia species in the LightCycler. The forward primer (OspA iLC) labeled with LC Red 640 is incorporated into the first strand of DNA amplicon (A), and the fluorescein-labeled probe (Probe Ba2) binds to this DNA strand (B). The proximity of the two fluorochromes allows induction of LC Red 640 fluorescence by FRET. Due to the surplus of probes, the FRET signal depends directly on the amount of amplicon formed.
TABLE 1.
Oligonucleotide primer and probe sequences used in PCR amplification and detection protocol of B. burgdorferi sensu lato strains
| Primer or probe | Sequencea |
|---|---|
| Reverse primer | 5′-CTA GTG TTT TGC CAT CTT CTT TGA AAA-3′ |
| Forward primer | 5′-AGC CTT AAT AGC ATG C/TAA GCA AAA X′TG-3′ |
| Hybridization probe | 5′-GCg CTG TTT TTT TCA TCA AGG CTG CTA ACX-3′ |
X, fluorescein-labeled base; X′, LC Red 640-labeled base.
The 10-μl (final volume) PCR mixture included 1 μl of a commercial ready-to-use reaction mixture for PCR (LightCycler-DNA Fast Start master hybridization probes; Roche Diagnostics) that contains Hot Start Taq DNA polymerase, deoxynucleoside triphosphate mix, reaction buffer, and 1 mM Mgcl2. Mgcl2 was added to a final concentration of 5 mM. The final concentrations of the probe and the primers were 0.1 and 0.5 μM, respectively. Finally, 1 μl of template DNA was added.
The reaction mixture was loaded into glass capillary tubes (Roche), which were snap sealed with plastic caps. The conditions for thermal cycling were as follows: initial denaturation for 10 min (to activate the fast-start Taq polymerase), followed by the amplification program, which included a denaturation step at 95°C for 10 s, an annealing step at 57°C for 10 s, and extension at 72°C for 13 s. Fluorescence was measured at the end of each annealing phase. The amplification was followed by a melting program, which started at 54°C for 45 s and then increased to 95°C at 0.1°C/s, with the fluorescence signal continuously monitored.
Agarose gel electrophoresis.
PCR amplification products were resolved on 1.5% agarose gels by electrophoresis and visualized under UV light with ethidium bromide. As a marker, a 100-bp ladder was used (Gibco BRL, Karlsruhe, Germany). The expected amplification product was 296 bp.
Nested PCR and sequencing.
Nested PCR targeting 5S-23S rRNA was performed by following the protocol of Rijpkema et al. (21).
The nucleotide sequences of PCR-amplified fragments were determined by the dideoxy chain termination technique (22) with the Prism Big Dye Terminator Cycle-Sequencing Ready-Reaction kit (Applied Biosystems) using the ABI Prism system 310 DNA sequencer.
Statistics.
The Fisher test, an option of GraphPad (San Diego, Calif.) Instat, was used to determine statistical significance. A P value of ≤0.05 was considered significant.
RESULTS
Real-time PCR of Borrelia in culture samples.
A total of 55 amplification cycles were performed with genomic DNA of each Borrelia genotype and a template-free control. The forward primer (internally labeled with LC Red 640) was incorporated into the first-strand DNA. After being annealed, the 3′ fluorescein-labeled probe bound to the first-strand DNA so that the fluorophores were separated by one base. This close proximity of the two dyes during hybridization allowed fluorescence resonance energy transfer (FRET) between the fluorophores. The resulting light emission of LC Red 640 was detected, providing real-time monitoring of the amplification process.
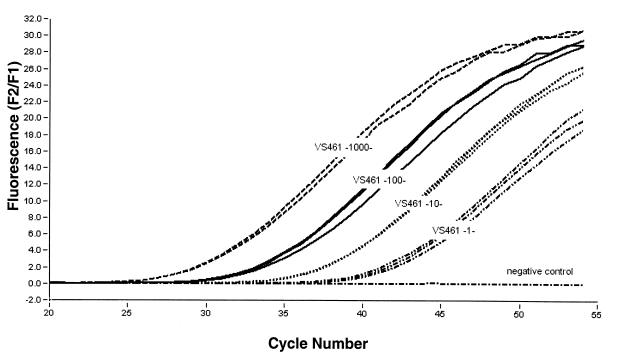
The fluorescence signal was measured at the end of each annealing phase and increased as the product accumulated in an exponential manner. No increase in fluorescence signal was observed in the absence of template. Figure 2 shows the progress of a PCR with Qiagen-extracted Borrelia DNA as the template. The DNA templates prepared by the Chelex method gave similar results (data not shown). The detection limit of the PCR was tested with serially diluted DNA templates of the three Borrelia strains from 108 Borrelia organisms/μl down to 100 Borrelia organisms/μl. It was found that a template DNA amount corresponding to 1 to 10 spirochetes (from strain N40 [data not shown], PSth [data not shown], or VS461 [Fig. 2]) was sufficient for detection. When uninfected ticks were spiked with known amounts of Borrelia, the recovery limit was also 1 to 10 spirochetes, despite attenuated PCR efficacy (slope of amplification), indicating the presence of inhibitors.
FIG. 2.
Detection limit of PCR assay for detection of OspA determined with serial dilutions of VS461 genomic DNA. The dilutions were utilized in doublets or triplets. All samples were amplified simultaneously. The DNA in the sample corresponded to 1,000, 100, 10, and 1 spirochete of B. afzelii (VS461). As a negative control, water was added to the reaction mixture instead of template.
A cutoff criterion was defined based on the fluorescence variations of 60 uninfected ticks in 10 different LightCycler runs: the distribution of absolute fluorescence maxima of these 60 uninfected ticks was assessed, and the cutoff was defined as the mean ± 3 standard deviations, resulting in 0.5 F2/F1, i.e., the quotient of LC Red 640 to fluorescein signal. Ticks which did not reach 0.5 F2/F1 until cycle 55 were considered negative.
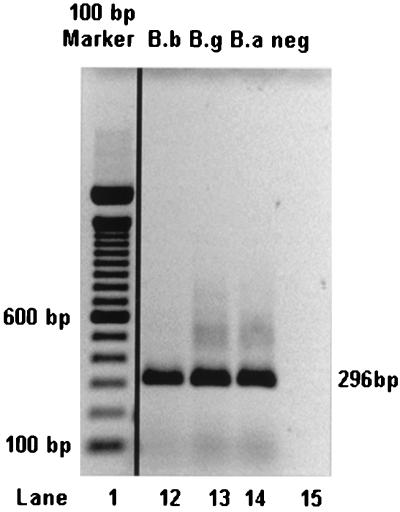
Real-time PCR allows the relative quantification of the amount of DNA template by using the cycle number at which the fluorescence signal starts to rise above a defined threshold (termed the ct value). In order to test the reproducibility of this measure, a Borrelia DNA standard was included in 55 independent LightCycler runs. The standard deviation was 1.32 cycles, showing excellent reproducibility of the method. The specificity of the PCR was confirmed by agarose (1.5%) gel electrophoresis (Fig. 3).
FIG. 3.
Gel electrophoresis of PCR products from three B. burgdorferi sensu lato strains. The predicted lengths of the products were confirmed on a 1.5% agarose gel. A 100-bp ladder was used as a size marker (first lane). The other lanes show PCR products obtained from B. burgdorferi sensu stricto (B.b; lane 12), B. garinii (B.g; lane 13), and B. afzelii (B.a; lane 14) and a water control (neg; lane 15).
Since no reference method (“gold standard”) is available and extraction of DNA from whole ticks excludes testing by methods other than PCR, the sensitivity and specificity of the assay cannot be assessed. In order to challenge the test and estimate these parameters, borderline positive-negative ticks were tested several times. Ticks (159) which were borderline in the first assay were analyzed repeatedly (a total of 410 measurements) until a definite classification was possible, i.e., the first result was either reproduced or falsified by repeated measurements. These data showed 3 false-negative (137 true-negative) and 8 false-positive (262 true-positive) measurements. From these data, a sensitivity of 98% and a specificity of 97% were estimated for this method.
Melting point analysis.
After amplification, a melting curve was generated for genotyping, i.e., fluorescence was monitored continuously while the temperature was raised from 54 to 95°C. This resulted in a sharp decrease in LC Red 640 fluorescence at the temperature at which the probe dissociates from the template. When the strand and the probe are perfectly matched, strong hybridization results and the melting temperature (Tm) is high. A mismatch, even of a single base, results in a lower Tm, due to the decreased hybridization stability that is reflected in the peak of the first derivative of the melting curve (−dF2/dT). Here, the probe was designed to match the sequence of the Borrelia species B. afzelii perfectly. The sequences of B. garinii and B. burgdorferi sensu stricto contained two and three mismatches with the probe, respectively. Therefore, a characteristic melting profile for each genotype was obtained.
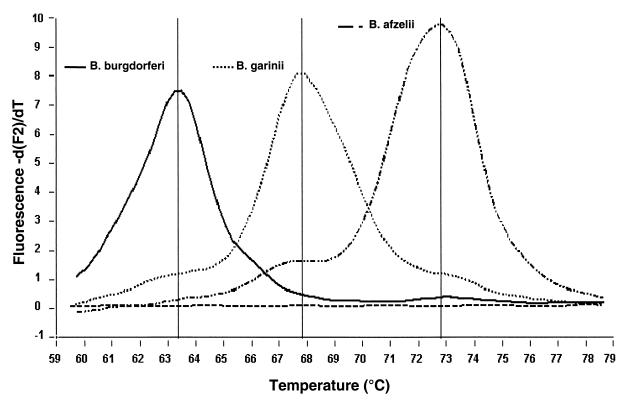
The temperatures at which the probes detached from PCR products during the melting program were calculated using the LightCycler software. The Tms of the three Borrelia species differed, as shown in Fig. 4. The average Tms of the reference strains of B. burgdorferi sensu stricto (N40), B. garinii (PSth), and B. afzelii (VS461) were 63, 68, and 72.5°C, respectively. Thus, the difference between the Tms of N40 and PSth was 5°C and the difference between those of PSth and VS461 was more than 4°C. As with this method each Tm corresponded specifically to one Borrelia species, we were also able to detect the different species in mixtures of two or three Borrelia genospecies (Fig. 5). Two criteria were used to define a positive signal in Tm analysis: (i) a relative maximum (i.e., a peak, to be distinguished from a plateau) of fluorescence at the characteristic temperature and (ii) a height of this peak of at least 10% of the peak of the control (representing about 100 Borrelia organisms). This procedure resulted in a cutoff around 0.3 −dF2/dT.
FIG. 4.
Identification of Tms for the three B. burgdorferi sensu lato species. The melting peak analysis which followed each PCR run showed that the Tms were 63°C for B. burgdorferi sensu stricto (N40), 68°C for B. garinii (PSth), and 72.5°C for B. afzelii (VS461).
FIG. 5.
Melting point analysis of mixtures of Borrelia species. Differentiation of species in coinfections with two (B. afzelii [B.a] VS461 plus B. garinii [B.g] PSth, VS461 plus B. burgdorferi sensu stricto [B.b] N40, or N40 plus PSth) or three (N40 plus PSth plus VS461) Borrelia species. In the negative control, water was used as a template.
Detection of B. burgdorferi sensu lato in ticks by real-time PCR.
The reliability of the real-time PCR protocol for the amplification of B. burgdorferi OspA was tested by assessing the infection rate in ticks collected in the field (Table 2). A total of 1,055 I. ricinus ticks collected at five different sampling sites in the region of Konstanz were investigated by real-time PCR. As summarized in Table 2, the infection rates for the ticks examined at the different sites varied significantly from 20 to 57% (P < 0.001). The overall prevalence of B. burgdorferi sensu lato in ticks was 35%. The highest infection rate (57%) was found in location B. Further, the overall infection rates in nymphs were significantly (P < 0.001) lower (30%, i.e., 152 out of 507) than those in the adult ticks (40%, i.e., 219 out of 548).
TABLE 2.
Summary of Borrelia infection rates in different maturation stages of I. ricinus ticks from different collection sites
| Tick | Infection rate
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa
|
B
|
H
|
L
|
M
|
Σ
|
|||||||
| No. + | % | No. + | % | No. + | % | No. + | % | No. + | % | No. + | % | |
| Female | 15/50 | 30 | 50/83 | 60 | 10/40 | 25 | 17/50 | 34 | 11/52 | 21 | 103/275 | 37 |
| Male | 18/50 | 36 | 43/67 | 64 | 16/56 | 29 | 18/60 | 36 | 21/50 | 42 | 116/273 | 42 |
| Adult | 33/100 | 33 | 93/150 | 62 | 26/96 | 27 | 35/100 | 35 | 32/102 | 31 | 219/548 | 40 |
| Nymph | 27/100 | 27 | 53/106 | 50 | 14/100 | 14 | 36/100 | 36 | 22/101 | 22 | 152/507 | 30 |
| Σ | 60/200 | 30 | 146/256 | 57 | 40/196 | 20 | 71/200 | 36 | 54/203 | 27 | 371/1,055 | 35 |
Five different collection sites in Konstanz. +, positive.
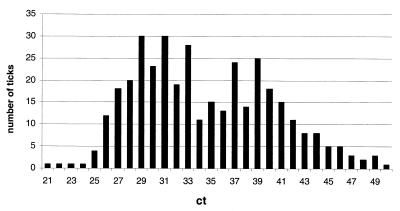
An estimation of Borrelia numbers in ticks was carried out employing the ct values of the individual runs. In spike experiments, 1,000 Borrelia genome equivalents corresponded to a ct value of 30, 100 genome equivalents corresponded to 34, 10 genome equivalents corresponded to 38, and 1 genome equivalent corresponded to more than 40. The distribution of ct values of all 371 positive ticks is shown in Fig. 6, with a median of 34, i.e., about 4,000 Borrelia organisms per tick.
FIG. 6.
Distribution of Borrelia counts in all 371 positive ticks. The cycle number at which the PCR fluorescence commences (ct value) allows the relative quantification of the number of genome equivalents present in the probe. Dilution series of Borrelia DNA showed that 1,000 Borrelia genome equivalents corresponded to a ct value of 30, 100 genome equivalents corresponded to 34, 10 genome equivalents corresponded to 38, and 1 genome equivalent corresponded to more than 40 cycles.
Genotyping (Table 3) indicated that B. afzelii was the predominant species in all of the areas studied. B. afzelii was detected in 70% of the infected ticks, followed by B. garinii (34%) and B. burgdorferi sensu stricto (12%). The Borrelia species infecting three ticks (0.8%) could not be identified, since they showed a Tm of 58.6°C, which did not correspond to any Tm of the other species. Mixed infections by two or three species were detected in 18% of the ticks characterized as positive by PCR. We detected double infections of B. afzelii with B. garinii (88%) or B. burgdorferi sensu stricto (9%). In one case, we could detect a mixed infection of all three species. A combination of B. garinii and B. burgdorferi sensu stricto alone was not found.
TABLE 3.
Identification of different B. burgdorferi sensu lato species in I. ricinus ticks collected at five different sites (A, B, H, L, and M) in Konstanz
| Speciesa | Infection rate
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A
|
B
|
H
|
L
|
M
|
Σ
|
|||||||
| No. +b | % | No. + | % | No. + | % | No. + | % | No. + | % | No. + | % | |
| B.b. | 7 | 12 | 16 | 11 | 7 | 18 | 0 | 0 | 9 | 17 | 39 | 11 |
| B.g. | 20 | 33 | 20 | 14 | 7 | 18 | 18 | 18 | 6 | 11 | 66 | 18 |
| B.a. | 23 | 38 | 80 | 55 | 18 | 45 | 46 | 65 | 28 | 52 | 195 | 53 |
| Unknown | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 4 | 1 |
| B.b. + B.g. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B.b. + B.a. | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 3 | 6 | 6 | 2 |
| B.g. + B.a. | 9 | 15 | 26 | 18 | 8 | 20 | 9 | 18 | 7 | 13 | 59 | 16 |
| B.a + unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0.3 |
| B.b + B.g + B.a | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 |
| Total positive | 60 | 30 | 146 | 57 | 40 | 20 | 71 | 36 | 54 | 27 | 371 | 35 |
| Total measured | 200 | 256 | 196 | 200 | 203 | 1,055 | ||||||
B.b., B. burgdorferi sensu stricto; B.g., B. garinii; B.a., B. afzelii.
+, positive.
The PCR products of 24 Borrelia isolates from ticks were sequenced to determine the genospecies. All 10 B. afzelii, 10 B. garinii, and 4 B. burgdorferi sensu stricto isolates showed the expected number of mismatches with the probe, stressing the validity of genotyping by melting point analysis. However, two well-characterized strains of B. garinii (B29 and A218) resulted in two peaks at 68 and 72.5°C in the melting point analysis, which would falsely indicate a double infection with B. garinii and B. afzelii. Sequencing of these strains did not clarify the occurrence of the two peaks, since both strains displayed the two mismatches only with the probe characteristic for B. garinii. Nevertheless, this finding might indicate that the frequency of double infection (5.6% of all ticks) with B. garinii and B. afzelii is overestimated. However, assuming independence of both infections, a very similar frequency of double infections of 3% can be calculated from the frequencies of either infection alone.
DISCUSSION
The aim of our study was the development of a new PCR method for the detection and differentiation of the B. burgdorferi sensu lato species B. afzelii, B. garinii, and B. burgdorferi sensu stricto. The use of the LightCycler system allowed the simultaneous differentiation of these species in one PCR run in a single tube, representing a much faster, less laborious, and less expensive method for genotype identification than the commonly used methods, including species-specific PCR, randomly amplified polymorphic DNA analysis, PCR-based sequencing, and restriction fragment length polymorphism.
The ospA gene was used as the target of real-time PCR. This gene is located on a linear 49-kb plasmid. The sequences of the ospA genes of the three major Borrelia species are different (33): ospA genotypes 1 and 2 correspond to B. burgdorferi sensu stricto and B. afzelii, respectively, and ospA genotypes 3 to 7 correspond to B. garinii. These genotypes correspond to the OspA serotypes 1 to 7 (34). Due to the hypothesized multiplicity of plasmid genes in clinical probes (18), portions of the ospA gene were frequently chosen as templates for PCR (5, 8, 16, 30).
A study by Will et al. (33) showed highly conserved ospA genes within the B. burgdorferi sensu stricto group as well as within the B. afzelii group but heterogeneity within the ospA genes of B. garinii-type strains. In line with this notion, a BLAST search in the National Center for Biotechnology Information (GenBank) databases (22 B. garinii sequences) for the B. garinii ospA sequence binding to the probe showed two distinct groups of B. garinii, each characterized by two mismatches with our probe (at positions 1 and 6 and positions 1 and 12, respectively). All 10 B. burgdorferi sensu stricto and 16 B. afzelii sequences showed zero or three mismatches, respectively. Since the method is extremely sensitive to changes in the sequence binding to the probe, misclassifications cannot be completely excluded.
The PCR assay described here is sensitive enough to detect fewer than 10 spirochetes of each of the three clinically relevant genospecies of B. burgdorferi sensu lato in a sample. Therefore, the detection limit is comparable to that of conventional nested PCRs. However, the real-time PCR is less laborious and considerably faster. The entire assay can be completed in approximately 1 h. Amplification, hybridization, and analysis are performed in one closed capillary tube, decreasing the risk of cross contamination. The melting curve analysis allows the differentiation of Borrelia species even in a mixture of all three species. Thus, the method presented here appears to be the first LightCycler-based PCR which allows the differentiation of the three Borrelia species B. burgdorferi sensu stricto, B. garinii, and B. afzelii in one PCR run.
Since any naturally occurring tick can be analyzed by only a single type of procedure and no gold standard for Borrelia detection is available, it was only possible to compare the new method to an established nested PCR in a block cycler targeting 5S-23S rRNA (21). Of 100 positive ticks, 86 were also positive in nested PCR, while all 50 negative ticks were negative in both assays. Taking nested PCR as the reference method, this would indicate a sensitivity of 100%, a specificity of 86%, and accuracy of 91%.
The observation that 14 ticks were positive only by real-time PCR might indicate either a higher sensitivity of the LightCycler or false-positive results. Employing serial dilutions of Borrelia DNA, no major difference in the limit of detection was observed. However, the nested PCR might contain higher concentrations of tick-borne inhibitors due to the larger amounts of tick extract used per tube (10 versus 20% of total volume).
There is an ongoing discussion about the clinical relevance of B. valaisiana and B. lusitaniae, which are occasionally found in ticks and birds (7). Due to sequence similarity, B. valaisiana yields the same melting point as B. afzelii in our system and thus cannot be distinguished. A total of 39 tick samples classified as B. afzelii infected were therefore subjected to Southern blot analysis according to the method described previously (21). Only a single case of B. valaisiana infection was found (data not shown), indicating that the prevalence of this genotype is very low in the investigated area.
The sensitivity and specificity of our method were estimated to be 98 and 97%, respectively, based on 159 ticks, which were analyzed two to six times. It is worth noting that ticks with low and borderline bacterial burdens were selected to challenge the method, thus actually underestimating the reproducibility in practice.
To test the feasibility and reliability of the real-time PCR protocol for the amplification of B. burgdorferi ospA, we investigated the distribution of B. burgdorferi sensu lato in ticks collected in Konstanz and determined the genomic groups present. Information about the prevalence of Borrelia infection in ticks in areas of endemicity is necessary for risk assessment. It has been shown that most habitats where ticks carrying Borrelia have been found are recreational sites (10). Therefore, we selected four sites with recreational functions within the five collection sites. Significant variability was observed in the prevalence of B. burgdorferi sensu lato among the sites examined, with infection rates ranging from 20 to 57%. These considerable differences in the prevalence of infected ticks even in habitats in close proximity indicates that the occurrence of Borrelia species in nature is affected by many ecological factors.
A very high infection rate (57%) was found at one of the five sites. Similar results showing high infection rates in particular sites have also been described by others (4, 12, 20). One possible explanation for this phenomenon could be that the transmission of the spirochetes to ticks is amplified by cofeeding on a vertebrate host, as has been proposed (9).
By using melting point analysis, we were able to differentiate the three genospecies of Borrelia known to be pathogenic for humans in the ticks collected. All three genomic groups (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) were found in tick isolates from the investigated sites. Our findings indicate that B. afzelii (70%) was present most abundantly, followed by B. garinii (34%). B. burgdorferi sensu stricto (12%) appears to be less common here. Similar findings were made in Slovenia, where out of 60 Borrelia-positive ticks, 53% were identified as infected by B. afzelii, 33% were identified as infected by B. garinii, and only 13% were identified as infected by B. burgdorferi sensu stricto (26).
Interestingly, three of the 1,055 ticks examined were positive for B. burgdorferi sensu lato but the isolates could not be classified into one of the three species, as their Tm (58.6°C) differed from those of the others: B. burgdorferi sensu stricto (63°C), B. garinii (68°C), and B. afzelii (72.5°C). A DNA sequence analysis (performed by MWG Biotech, Ebersberg, Germany) indicated that these spirochetes belong to the recently described Borrelia species strain A14S (32). A14S is phenotypically and genetically different from all other B. burgdorferi sensu lato species described and therefore most likely represents a new Borrelia genotype. Since it was cultured from a skin biopsy specimen of a patient with erythema migrans, it seems to be pathogenic for humans.
Mixed infections were found in 18% of the Borrelia-positive ticks—mainly double infections by B. afzelii and B. garinii. Double infections by B. garinii and B. burgdorferi sensu stricto were not found.
The quantification of Borrelia species in ticks showed a very heterogeneous distribution ranging from 1 to more than 1,000 Borrelia equivalents. Similar numbers were found by Stünzner et al. by microscopically counting Borrelia organisms in tick guts (27). This finding illustrates that a method with a low detection limit is required to assess all infected ticks. To our knowledge, the impact of different Borrelia burdens on infectivity for mammals has not yet been studied.
In conclusion, we have demonstrated that the use of the new real-time PCR method provides a rapid and sensitive tool for differentiating B. burgdorferi sensu lato species known to be pathogenic for humans. We have shown that the method can be applied for the detection and differentiation of Borrelia genospecies in ticks collected in the field and ticks removed from humans. The novel method appears to represent a versatile tool to assess the roles of different genospecies in the pathophysiology of Lyme disease in Europe.
Acknowledgments
We thank Lars Hareng for assistance and support with the LightCycler, Markus Müller for helpful discussion, and Sonja von Aulock for revision of the manuscript.
REFERENCES
- 1.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378–383. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319. [DOI] [PubMed] [Google Scholar]
- 3.Busch, U., C. Hizo-Teufel, R. Bohmer, V. Fingerle, D. Rossler, B. Wilske, and V. Preac-Mursic. 1996. Borrelia burgdorferi sensu lato strains isolated from cutaneous Lyme borreliosis biopsies differentiated by pulsed-field gel electrophoresis. Scand. J. Infect. Dis. 28:583–589. [DOI] [PubMed] [Google Scholar]
- 4.Cinco, M., D. Padovan, R. Murgia, L. Poldini, L. Frusteri, I. van de Pol, N. Verbeek-De Kruif, S. Rijpkema, and M. Maroli. 1998. Rate of infection of Ixodes ricinus ticks with Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii and group VS116 in an endemic focus of Lyme disease in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 17:90–94. [DOI] [PubMed] [Google Scholar]
- 5.Demaerschalck, I., A. BenMessaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diterich, I., L. Harter, D. Hassler, A. Wendel, and T. Hartung. 2001. Modulation of cytokine release in ex vivo-stimulated blood from borreliosis patients. Infect. Immun. 69:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escudero, R., M. Barral, A. Perez, M. M. Vitutia, A. L. Garcia-Perez, S. Jimenez, R. E. Sellek, and P. Anda. 2000. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 38:4026–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frossard, E., B. Rutti, J. Burgherr, E. Godfroid, M. Brossard, and N. J. Gerber. 1999. Detection of Borrelia DNA in synovial fluid for diagnosis of Lyme arthritis. Schweiz. Med. Wochenschr. 129:979–984. (In German.) [PubMed] [Google Scholar]
- 9.Gern, L., and O. Rais. 1996. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae). J. Med. Entomol. 33:189–192. [DOI] [PubMed] [Google Scholar]
- 10.Gray, J. S., O. Kahl, J. N. Robertson, M. Daniel, A. Estrada-Pena, G. Gettinby, T. G. Jaenson, P. Jensen, F. Jongejan, E. Korenberg, K. Kurtenbach, and P. Zeman. 1998. Lyme borreliosis habitat assessment. Zentbl. Bakteriol. 287:211–228. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson, M., L. Noppa, A. G. Barbour, and S. Bergstrom. 1992. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect. Immun. 60:1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junttila, J., M. Peltomaa, H. Soini, M. Marjamaki, and M. K. Viljanen. 1999. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J. Clin. Microbiol. 37:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebisch, G., B. Sohns, and W. Bautsch. 1998. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J. Clin. Microbiol. 36:3355–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misonne, M. C., G. Van Impe, and P. P. Hoet. 1998. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J. Clin. Microbiol. 36:3352–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mommert, S., R. Gutzmer, A. Kapp, and T. Werfel. 2001. Sensitive detection of Borrelia burgdorferi sensu lato DNA and differentiation of Borrelia species by LightCycler PCR. J. Clin. Microbiol. 39:2663–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moter, S. E., H. Hofmann, R. Wallich, M. M. Simon, and M. D. Kramer. 1994. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J. Clin. Microbiol. 32:2980–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 37:1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persing, D. H., B. J. Rutledge, P. N. Rys, D. S. Podzorski, P. D. Mitchell, K. D. Reed, B. Liu, E. Fikrig, and S. E. Malawista. 1994. Target imbalance: disparity of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J. Infect. Dis. 169:668–672. [DOI] [PubMed] [Google Scholar]
- 19.Pietila, J., Q. He, J. Oksi, and M. K. Viljanen. 2000. Rapid differentiation of Borrelia garinii from Borrelia afzelii and Borrelia burgdorferi sensu stricto by LightCycler fluorescence melting curve analysis of a PCR product of the recA gene. J. Clin. Microbiol. 38:2756–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijpkema, S., D. Golubic, M. Molkenboer, N. Verbeek-De Kruif, and J. Schellekens. 1996. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 20:23–30. [DOI] [PubMed] [Google Scholar]
- 21.Rijpkema, S. G., M. J. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanger, F., S. Nicklen, and A. R. Coulson. 1992. DNA sequencing with chain-terminating inhibitors. 1977. Bio/Technology 24:104–108. [PubMed] [Google Scholar]
- 23.Schwartz, J. J., A. Gazumyan, and I. Schwartz. 1992. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 174:3757–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanek, G., N. Satz, F. Strle, and B. Wilske. 1993. Epidemiology of Lyme borreliosis, p.358–370. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer, New York, N.Y.
- 25.Steere, A. C. 1994. Lyme disease: a growing threat to urban populations. Proc. Natl. Acad. Sci. USA 91:2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strle, F., Y. Cheng, J. A. Nelson, M. M. Picken, J. K. Bouseman, and R. N. Picken. 1995. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 14:994–1001. [DOI] [PubMed] [Google Scholar]
- 27.Stünzner, D., Z. Hubalek, J. Halouzka, D. Postic, K. Pierer, and E. Marth. 1998. Prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus ticks from Styria (Austria) and species identification by PCR-RFLP analysis. Zentbl. Bakteriol. 288:471–478. [DOI] [PubMed] [Google Scholar]
- 28.van Dam, H. K., K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. P. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708–717. [DOI] [PubMed] [Google Scholar]
- 29.van der Heijden, I. M., B. Wilbrink, S. G. Rijpkema, L. M. Schouls, P. H. Heymans, J. D. van Embden, F. C. Breedveld, and P. P. Tak. 1999. Detection of Borrelia burgdorferi sensu stricto by reverse line blot in the joints of Dutch patients with Lyme arthritis. Arthritis Rheum. 42:1473–1480. [DOI] [PubMed] [Google Scholar]
- 30.Vasiliu, V., P. Herzer, D. Rossler, G. Lehnert, and B. Wilske. 1998. Heterogeneity of Borrelia burgdorferi sensu lato demonstrated by an ospA-type-specific PCR in synovial fluid from patients with Lyme arthritis. Med. Microbiol. Immunol. 187:97–102. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, P. S., D. A. Metzger, and R. Higuchi. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506–513. [PubMed] [Google Scholar]
- 32.Wang, G., A. P. van Dam, and J. Dankert. 1999. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with lyme borreliosis. J. Clin. Microbiol. 37:3025–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Will, G., S. Jauris-Heipke, E. Schwab, U. Busch, D. Rossler, E. Soutschek, B. Wilske, and V. Preac-Mursic. 1995. Sequence analysis of ospA genes shows homogeneity within Borrelia burgdorferi sensu stricto and Borrelia afzelii strains but reveals major subgroups within the Borrelia garinii species. Med. Microbiol. Immunol. 184:73–80. [DOI] [PubMed] [Google Scholar]
- 34.Wilske, B., V. Preac-Mursic, U. B. Gobel, B. Graf, S. Jauris, E. Soutschek, E. Schwab, and G. Zumstein. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 31:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]