Abstract
Acute lung injury (ALI) of different etiologies has shared pathophysiologic process, from which we speculated that ALI of different etiologies may share common molecular features. While the shared genetic characteristics of ALI remain unclear. In this paper, we aimed to identify shared ferroptosis-associated and bottleneck genes from acute lung injury of different etiologies. Firstly, we extracted five groups of gene sets related to three distinct models of ALI from the Gene Expression Omnibus (GEO) database. Then, through the utilization of weighted gene co-expression network analysis (WGCNA), we identified 3 significant gene modules and ascertained 7 shared co-expressed genes affected by these models. Subsequently, through the utilization of differential gene expression analysis and protein-protein interaction network analysis for the 3 gene modules, the shared bottleneck gene Slc7a11 was identified. Moreover, the 7 shared co-expressed genes subjected to these three ALI models were used to identify shared ferroptosis-associated genes via the FerrDb database. Finally, the key gene Slc7a11 was confirmed and validated. In addition, we observed that Slc7a11 is both a driver and a suppressor gene in the FerrDb database. Interestingly, we found the expression level of Slc7a11 was significantly upregulated in the three ALI models. Experimentally, we confirmed the expression of Slc7a11 in rat ALI tissues by using immunofluorescence staining and real-time polymerase chain reaction (qRT-PCR) assays. Collectively, our findings complement the exploration of the shared pathogenesis of ALI. There are genetic features shared by ALI of different etiology and the increased expression of Slc7a11 was identified in the three different etiologies of ALI, which can improve our understanding of the shared molecular mechanisms underlying ALI.
Keywords: WGCNA, Shared gene, Ferroptosis, Acute lung injury, Bioinformatics
Subject terms: Biomedical engineering, Computational biology and bioinformatics
Introduction
Acute lung injury (ALI) and its more extreme manifestation, acute respiratory distress syndrome (ARDS), are a common cause of respiratory failure triggered by a group of diverse stimuli including, but not limited to, microbes, toxic gasses, burn, pollutants, gastric acids and fatty acids1. Although therapeutic strategies such as fluid management and supportive ventilation have led to improvements in clinical effects, ALI remains a significant morbidity and mortality2. Early recognition of patients and timely control of the progress of ALI are still vital to improve clinical outcomes.
Clinical responses in patients with ALI manifest as heterogeneous syndrome2–4and it is often difficult to predict for newly admitted patients in emergency room. However, ALI/ARDS shares similar pathophysiologic process including inflammation, oxidative stress and epithelial injury, which indicates the possibility of the existence of shared genetic features among these ALI/ARDS5,6. Ferroptosis is a type of oxidative stress-induced cell death characterized by lipid peroxidation and glutathione depletion7. Moreover, a series of molecular events involved in the process of ferroptosis overlap with oxidative stress, and which indicates that there is a crosstalk between ferroptosis and oxidative stress8. In recent years, researchers have conducted studies investigating the correlation between ferroptosis and ALI9, and the progression of ALI can be modulated by ferroptosis-associated genes, such as (nuclear factor-like 2) Nrf2, (glutathione peroxidase 4) GPX4, solute carrier family 7 member 11 (Slc7a11) and hemeoxygenase-1 (HO-1)10. Furthermore, Scl7a11 and Nrf2 along with PPARγ and glutathione, may interact with each other in a coordinated manner to combat oxidative stress induced lung injury11–13. Though the relationship between designated ALI and ferroptosis has been investigated9, the shared genetic features related to ALI from different etiologies remain less explored. Thus, the primary purpose of our paper is to identify shared ferroptosis-associated genes from ALI that have different etiology. Moreover, we are also concerned with the bottleneck gene, which refers to the gene with high betweenness. It is known that the bottleneck gene is essential in the biological network as the destruction of bottleneck genes could lead to the partition of the biological network, which is fatal to the cells14.
To this end, we first employed weighted gene co-expression network analysis (WGCNA) to identify biologically related gene expression modules closely relating to three different etiologies of ALI15,16. Subsequently, shared genes subjected to the three different clinically significant modules were screened out and from which the shared ferroptosis-associated gene Slc7a11 was identified. Through differential expression analysis, we further confirmed Slc7a11 as a key bottleneck gene among co-expressed differentially expressed genes (DEGs). In addition, we found that Slc7a11 is both a driver and a suppressor gene in the FerrDb database. It is remarkable that Slc7a11 was significantly upregulated in these models of ALI using GEO database and this phenomenon was validated with animal experiment. Combining our results with the experimental results of the recent researches17–19, where they have showed that the expression of Slc7a11 was significantly decreased, we reasoned that Slc7a11 may exhibit a temporal expression pattern over the developmental stages of ALI.
Materials and methods
Dataset information
Using the keyword ‘lung injury’ as the search condition for the GEO profile. Inclusion criteria were as follows: (1) dataset type is expression profiling by array, (2) the organism is limited to non-gene knockout mice, (3) the sample resource is lung and the sample collection window is 72 h, (4) the route of administration was via inhalation, (5) Sample size should be at least 6, (6) dataset must include normal control group and ALI group. After the screening, expression profiles of ALI-associated mRNAs in GSE1871, GSE18341, GSE2565, GSE75157, GSE109076, and GSE2411 were included and downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). The above datasets are classified into three distinct categories based on their etiological factors: lipopolysaccharide-induced ALI (LPS-ALI), styrene-induced ALI, and phosgene-induced ALI. These ALI models contribute to advancing our understanding of human disease pathogenesis. Specifically, the LPS-ALI model mimics clinical sepsis-associated ALI20. The styrene (STY)/phosgene (PHO) exposure model, which mimics industrial or occupational injury scenarios, facilitates the investigation of molecular mechanisms in chemical-induced ALI21,22.
GSE1871 and GSE18341 series were produced on the GPL570 platform (Affymetrix Mouse Genome 430 2.0 Array), the GSE75157 and GSE109076 series on the GPL11180 platform (Affymetrix HT MG-430 PM Array Plate), the GSE2565 series on the GPL339 platform (Affymetrix Mouse Expression 430 A Array), and the GSE2411 series on the GPL339 platform (Affymetrix Mouse Expression 430 A Array). The basic information of these datasets is shown in Supplementary Table S1.
Data preprocessing
Six datasets of gene expression matrixes and platform annotation tables were downloaded from the GEO. Firstly, the matrix files of GSE1871 and GSE18341 of LPS-ALI were combined into an integrated dataset, which was subsequently named the ‘merged LPS-ALI dataset’. Similarly, the integrated matrix file of GSE75157 and GSE109076 of styrene-induced ALI (STY-ALI) was named the ‘merged STY-ALI dataset’. Then, the two merged datasets as well as the individual dataset GSE2565 of phosgene-induce ALI (PHO-ALI), named as the ‘PHO-ALI dataset’, and the GSE2411 were processed by ‘Affy’ R package23for further analysis. The GSE2411 dataset was used for external validation24.
Identification of clinically significant module
WGCNA (version 1.72–5) works well for high-dimensional gene expression data. It has been widely used for identifying modules of highly correlated genes and relating modules to external sample traits25. The robust multi-array average (RMA) method was used for background correction, normalization, and expression calculation26. Moreover, the ‘ComBat’ function in the sva R package27was utilized to adjust the batch effect between two datasets. After data preprocessing, we used hierarchical clustering28–30 to visualize and quantify the batch effect, check sample distributions and detect potential outlier samples. Subsequently, we used the function ‘pickSoftThreshold’ (package WGCNA) to choose proper soft-thresholding power for constructing a weighted network. The function ‘blockwiseModules’ was carried out to identify significant modules. To reduce the gene information loss, the minimum module size was set as 40 and the threshold to merge the similar modules was set as 0.3. Module eigengene (first principal component of the module) was used as representative of the gene module. The correlation coefficient (Cor) between module eigengenes and sample trait was analyzed to identify the significant clinical module. Module with Cor ≥ 0.7 and p < 0.05 was designated as vital module. Modules with most positively relevant to ALI may contain key genes involved in the ALI’s pathology, which were prioritized for subsequent analysis due to their strong association with ALI. The WGCNA package was performed in R-Studio 3.6.0 software.
Identification of shared co-expressed genes and functional enrichment analysis
The merged LPS-ALI dataset, the merged STY-ALI dataset, and the PHO-ALI dataset were screened to obtain three clinically significant modules. The shared co-expressed genes in modules positively associated with LPS-ALI, STY-ALI and PHO-ALI were overlapped by Venn diagram (https://jvenn.toulouse.inra.fr/app/index.html). To explore potential roles of the genes in modules of ALI models (LPS-ALI, STY-ALI, and PHO-ALI) and the shared genes associated with the ALI of three models from the molecular level, Gene ontology (GO) enrichment analysis was performed by metascape31 (http://metascape.org/gp/index.htm), an online tool for annotation and visualization of genes and proteins. The default parameters, Min overlap of 3, Min Enrichment of 1.5 and p-value < 0.01 were selected as the thresholds of GO term enrichment analysis31.
Identification of co-expressed differentially expressed genes (DEGs)
The ‘limma’ R package (version 3.60.2) was adopted to screen co-expressed DEGs by comparing the expression levels between the ALI and Normal samples, with an adjusted P value less than 0.05 and |log2FC| greater than 0.5 for further evaluations, where log2FC > 0.5, p < 0.05 was upregulation, log2FC < −0.5, p < 0.05 was downregulation. Next, the ‘heatmap’ R package was used to visualize the results by generating a volcano plot.
PPI network construction and shared bottleneck genes identification
The online database STRING (https://cn.string-db.org/) was used to build PPI network of DEGs. Full STRING network as network type, Evidence as an indicator for the significance of network edges,
and setting 0.4 as the threshold of the active interaction score. The Cytoscape software was used for visualization of PPI network32. The cytoHubba plugin in Cytoscape was used to detect hub genes, from which top 15 bottleneck genes were identified by cytohubba-Betweenness plugin with default parameters. Genes with high betweenness centrality score act as bridges between different modules of a gene regulatory network33. The shared bottleneck genes were identified by intersection of top 15 bottleneck genes from 3 modules.
Shared ferroptosis-related genes identification, key genes identification and validation
We downloaded a list of ferroptosis-related genes from the Ferroptosis Database (http://www.zhounan.org/ferrdb/current/)34. The ferroptosis-related gene list, including gene drivers, suppressors and unclassified regulator, was used for further analysis. Then, we intersected these ferroptosis-related genes with the shared genes to identify shared ferroptosis-related genes using Venn diagram. The overlapping shared bottleneck genes and shared ferroptosis-related genes were defined as key genes. After all, key genes were identified, and a further step was taken to investigate the expression level of key genes on ALI. In addition, the GSE2411 dataset as an external validation set was utilized to evaluate the efficacy of the key genes.
The construction of ALI rat model
Twelve male Sprague-Dawley rats (male, 6–8 weeks, 220–280 g) were provided by the Experimental Animal Center of Zhengzhou University and kept in standard laboratory conditions under a 12-h light/dark cycle, with controlled temperature and humidity. The animal study was performed in accordance with the National Institutes of Health Guide for the Care and Use of laboratory animals and was approved by the Animal Ethics Committee of Zhengzhou University. License for Use of Laboratory Animals: SYXK (YU) 2020-0008. Sprague-Dawley rats were assigned randomly to the following two groups: ALI group (smoke exposure) and normal group (ambient air exposure), with 6 rats in each group.
The animal model of smoke inhalation-induced acute lung injury was established based on the previous method with few changes35. Briefly, the smoke was produced from the heated mixture of 50 g of dry wood shavings and 50 g of honeycomb paperboard chips and 30 ml of kerosene in a pot at the bottom of smoke-generating box. The smoke was wafted from the pot to a 20 × 20 × 30 cm container using an air blower. When the container was filled with smoke, rats were arranged in the container for 3 min. Next, the rats were moved from the container to normal environment for 3 min. Two rats were exposed each time to the smoke and this procedure was repeated three times. ALI rats were anesthetized and sacrificed at 8 h with intraperitoneal pentobarbital sodium overdose (100 mg/kg) and the lung tissues were collected for further analysis. The euthanasia method was an overdose of sodium pentobarbital by intraperitoneal injection according to the AVMA Guidelines for the Euthanasia of Animals (2020 Edition). Our current study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). All animal experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85 − 23, revised 1996).
Real-time polymerase chain reaction
Total RNA was isolated from lung tissues using Trizol Substitute (R1100; Solarbio, China) and converted to cDNA using UEIris RT mix with DNase (R2020; US EVERBRIGHT, China), and Real-time polymerase chain reaction (RT-PCR) was carried out by using Universal SYBR Green qPCR Supermix (S2024; US EVERBRIGHT, China) on an QuantStudio5 System (Applied Biosystems, Carlsbad, USA) following the manufacturer’s instructions. The rat PCR primers used were: Slc7a11, forward: 5-CCATCATCATCGGCACCGTCATC-3; Slc7a11, reverse: 5-TACTCCACAGGCAGACCAGAACAC-3; GAPDH, forward: 5-GAAGGTCGGTGTGAACGGATTTG-3; GAPDH, reverse: 5-CATGTAGACCATGTAGTTGAGGTCA-3. Relative mRNA expression of Slc7a11 were calculated by using the 2−ΔΔCt method. GAPDH was used as an internal control.
Immunofluorescence staining of lung tissues
After deparaffinized in dimethylbenzene and rehydrated in alcohol gradient, lung tissues sections were boiled in sodium citrate buffer (pH 6.0) and cooled at room temperature and washed three times with phosphate-buffered saline (PBS). Subsequently, the sections were permeabilized for 20 min with 0.3% Triton X-100 and blocked for one hour at room temperature with 5% goat serum. Then, the sections were incubated with anti-SLC7 A11 (1:250) (NB300-318, Novus, Centennial, CO, USA) at 4℃ overnight. After washing three times with PBS, the sections were treated with the anti-rabbit conjugated Alex 555 secondary antibody (1:200, ab150078, Abcam) for two hours at room temperature. The nuclei were stained with DAPI. Samples were imaged with a confocal microscope. Red and blue colors were represented as SLC7 A11 signals and nuclei respectively.
Statistical analysis
Parameters were presented as the mean standard deviation (SD). Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, USA). Statistical comparisons between the ALI and normal groups were carried out with Student’s t-test. P < 0.05 was considered as a statistically significant difference.
Results
Data Preparation and evaluation
After data preprocessing, a total of 21,814 genes were identified in the merged LPS-ALI dataset of GSE1871 and GSE18341, a total of 21,760 genes in the merged STY-ALI dataset of GSE1871 and GSE18341, and a total of 13,664 genes in the individual PHO-ALI dataset of GSE2565. After normalizing gene expression matrix and adjusting batch effect in these datasets, hierarchical clustering analysis was carried out. As for the merged STY-ALI dataset, 2 outliers were identified in the normal group and hence these 2 normal samples and the corresponding 2 matched ALI samples were excluded. While there are no outliers in the other two datasets. Finally, the merged LPS-ALI dataset included 11 ALI and 11 Normal samples, the merged STY-ALI dataset contained 9 ALI and 9 Normal samples, and the individual PHO-ALI dataset comprised 24 ALI and 24 Normal samples. As shown in Figs. 1A, 2A and 3A, the samples from each group were clustered, indicating that there were significant distinctions between the ALI and normal groups.
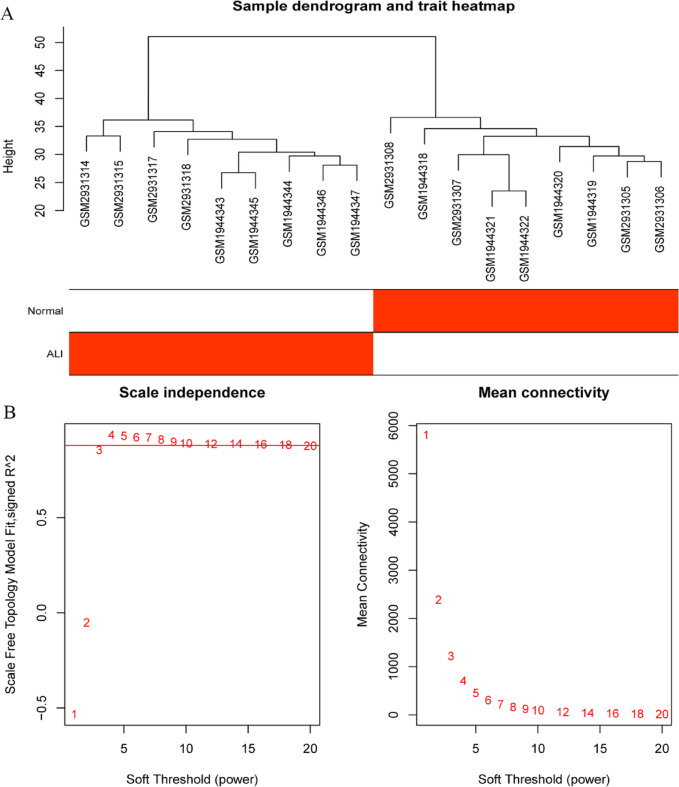
Fig. 1.
Hierarchical clustering analysis in the merged LPS-ALI dataset. (A) Clustering dendrogram of 22 samples. The branches of the dendrogram correspond to clustered samples. (B) Network topology of different soft-thresholding powers. The red line represents the value of the scale-free fit index (0.90).
Fig. 2.
Hierarchical clustering analysis in the merged STY-ALI dataset. (A) Clustering dendrogram of 18 samples. The branches of the dendrogram correspond to clustered samples. (B) Network topology of different soft-thresholding powers. The red line represents the value of the scale-free fit index (0.88).
Fig. 3.
Hierarchical clustering analysis in the PHO-ALI dataset. (A) Clustering dendrogram of 48 samples. The branches of the dendrogram correspond to clustered samples. (B) Network topology of different soft-thresholding powers. The red line represents the value of the scale-free fit index (0.85).
Construction of WGCNA and identification of clinically significant modules
The values of β = 8 (scale free R2 = 0.90), β = 9 (scale free R2 = 0.88), and β = 10 (scale free R2 = 0.85) were selected as the soft-threshold powers to construct scale-free networks using the ‘pickSoftThreshold’ function of WGCNA (Figs. 1B, 2B and 3B). The module-trait relationship presented the strength of association between modules and traits (Fig. 4A-C).
Fig. 4.
(A) Heatmap of the correlation coefficient (Cor) between modules and sample traits of the merged LPS-ALI dataset. Every color represents a co-expression module, and the values in the cells contain the corresponding Cor and P value. Module with Cor ≥ 0.7 and p < 0.05 was designated as vital module. (B) Heatmap of the correlation coefficient (Cor) between modules and sample traits of the merged STY-ALI dataset. Every color represents a co-expression module, and the values in the cells contain the corresponding Cor and P value. Module with Cor ≥ 0.7 and p < 0.05 was designated as vital module. (C) Heatmap of the correlation coefficient (Cor) between modules and sample traits of the PHO-ALI dataset. Every color represents a co-expression module, and the values in the cells contain the corresponding Cor and P value. Module with Cor ≥ 0.7 and p < 0.05 was designated as vital module. (D) Venn diagram showing the overlap of seven shared genes among the magenta module (LPS-ALI), turquoise module (STY-ALI), and brown module (PHO-ALI).
In our present study, we focused on the ALI-associated modules with the high correlation coefficient. For these three datasets, we found that the magenta module of the merged LPS-ALI dataset (Cor = 0.87, p = 1e-01, Fig. 4A), the turquoise module of the merged STY-ALI dataset (Cor = 0.95, p = 2e-09, Fig. 4B) and the brown module of the PHO-ALI dataset (Cor = 0.79, p = 3e-11, Fig. 4C) were most positively related to ALI. Therefore, the magenta module (366 genes) in LPS-ALI, the turquoise module (2948 genes) in STY-ALI and the brown module (146 genes) in PHO-ALI were identified as the most valuable modules and the genes in these modules were selected for subsequent research (Supplementary Table S2).
Identification of shared genes and functional analysis
We performed a Venn diagram to identify the shared genes from the three ALI-associated modules (the magenta module of LPS-ALI, the turquoise module of STY-ALI and the brown module of PHO-ALI). 7 shared genes associated with ALI were identified by Venn diagram (Fig. 4D).
To investigate the function mechanism of the three clinically significant modules and the 7 common genes, the top 20 of GO enrichment of genes in the three modules of LPS, STY, and PHO-ALI and the shared genes were analyzed, as shown in Fig. 5 (The detailed information of enrichment is shown in Supplementary Table S3 to S6). GO analysis showed that the genes in the magenta module of LPS-ALI were mainly involved in membrane organization, response to oxidative stress, regulation of smooth muscle cell migration and regulation of vascular endothelial growth factor production. The biological functions of the turquoise module of STY-ALI mainly focused on intracellular protein transport, regulation of cellular response to stress, positive regulation of programmed cell death and blood vessel development. Genes within the brown module of PHO-ALI were mainly enriched in positive regulation of cell death, regulation of protein kinase activity, response to oxidative stress, response to growth factor, and vasculature development. Through above enrichment analysis, biological pathways such as response to oxidative stress, positive regulation of cell death, and vascular development were enriched in the three gene datasets. Given the overlap in GO pathways, we investigated the genetic overlap among LPS-ALI, STY-ALI and PHO-ALI, and identified 7 common genes that were shared by them (Fig. 4D). To investigate which distinct biological pathways these genes are associated with, we performed GO enrichment analysis. These 7 shared genes were mainly enriched for skeletal muscle cell differentiation, cellular response to oxidative stress, and response to organic cyclic compound pathways (Fig. 5D).
Fig. 5.
The gene functional enrichment analysis of three clinically significant modules and shared genes. The top 20 GO enrichment terms in the magenta module (A), in the turquoise module (B), the in the brown module, and in the shared genes (D).
Differential gene analysis of module genes
In the magenta module, a total of 91 DEGs across the ALI and normal groups were acquired (Supplementary Table S7), with 66 up-regulated and 25 down-regulated genes (Fig. 6A). In the turquoise module, a total of 134 DEGs were acquired (Supplementary Table S7), with 104 up-regulated and 30 down-regulated genes (Fig. 6B). DEGs within the brown module were 51 (Supplementary Table S7), with 47 up-regulated and 4 down-regulated genes (Fig. 6C).
Fig. 6.
Differences analysis of DEGs. Volcano map of DEGs between ALI samples and normal samples in the magenta module of LPS-ALI (A), the turquoise module of STY-ALI (B), and the brown module of PHO-ALI (C). Green dots represent down-regulated genes with significant differences and red dots represent up-regulated genes with significant differences.
PPI network analysis and shared bottleneck genes identification
The 91 DEGs, 134 DEGs, and 51 DEGs obtained from the differential gene analysis were imported into the STRING database to construct a PPI network for hub gene connection, respectively (Fig. 7). These interaction networks were visualized by Cytoscape software. Next, based on the intersections of the top 15 bottleneck genes obtained using the cytoHubba-betweenness plugin, we identified the shared co-expressed bottleneck gene: Slc7a11 (Fig. 7D).
Fig. 7.
Identification of shared differentially expressed bottleneck gene Slc7a11. The 15 top bottleneck genes in the magenta module of LPS-ALI (A), the turquoise module of STY-ALI (B), and the brown module of PHO-ALI (C), where yellow-red means the deeper colors representing the higher-ranking score. The shared bottleneck gene Slc7a11 in the intersection of the 3 modules identified by Venn diagram (D).
Screening for shared ferroptosis-related genes
We also extracted three ferroptosis-associated gene datasets (Supplementary Table S8) including ferroptosis driver (264 genes), ferroptosis suppressor (238 genes), and ferroptosis unclassified regulator (110 genes) from the Ferroptosis Database (FerrDb) and intersected them with the 7 shared genes to identify shared ALI-associated ferroptosis genes. We observed that the gene Slc7a11 was the only one shared simultaneously by these 4 datasets, as was shown in the Venn diagram (Fig. 8A). Furthermore, we observed that Slc7a11 was both a driver and a suppressor gene in Fig. 8A.
Fig. 8.
Analysis of the shared ferroptosis-related gene Slc7a11. (A) Venn diagram to identify the shared ferroptosis gene Slc7a11 in the intersection of the 7 shared genes and the ferroptosis-associated gene lists. The expression level of Slc7a11 was significantly elevated in the ALI group compared to the control group in the merged LPS-ALI dataset (B), the merged STY-ALI dataset (C), and the PHO-ALI dataset (D). P < 0.05 is considered statistically significant.
Verification of Slc7a11 with microarray analysis
To evaluate the expression level of Slc7a11 in the three processed microarray datasets (the merged LPS-ALI dataset, the merged STY-ALI dataset and the PHO-ALI dataset) based on GEO database, the microarray analysis corresponding to the three datasets was used to get that the expression level of Slc7a11 was significantly higher in ALI group compared with the normal group (P < 0.05; Figure. 8B-D).
Slc7a11 is up-regulated in ALI tissue
To further confirm the expression of Slc7a11 between the ALI group and normal group, we conducted immunofluorescence staining and qRT-PCR for verification. In addition, the external dataset GSE2411 was also used to evaluate the expression of Slc7a11 which was significantly upregulated in ALI. The immunofluorescence staining revealed visibly increased Slc7a11 expression in the ALI group and the qRT-PCR results also indicated that the expression levels of Slc7a11 was significantly higher in the ALI group than in the normal group (Fig. 9). These findings were consistent with the results of the microarray analysis.
Fig. 9.
The upregulation of Slc7a11 expression in ALI. (A) The expression level of Slc7a11 was up-regulated in ALI groups compared with normal groups in GSE2411. (B) The expression level of Slc7a11 was up-regulated in ALI groups compared with Normal groups by qPCR. (C) The immunofluorescence of lung tissues showed that the expression of Slc7a11 (red) was increased in ALI groups compared with normal groups, magnification at 100×, scale bar 50 μm, Nuclei were stained with DAPI (blue), *p < 0.05, **p < 0.01.
Discussion
It is clear that ALI is a complex disorder of the lung, and the pathogenesis is multifactorial such as sepsis, trauma, burn and smoke36,37. Clinically, it is often difficult to predict which underlying pathogenesis or etiology plays a contributing cause at early stage of community acquired ALI. However, the lung reacts similarly to various types of insults, and it is known that different causes of ALI have shared features such as inflammation, lung endothelial injury, edema formation, and oxidative stress36,38. Researches have demonstrated that biomarkers increased in several forms of ALI39–41, but few have reported shared molecular features in different etiologies of ALI.
Our study is the first one to integrate the transcriptomes of three different etiologies of ALI for exploring the shared mechanism, revealing potential shared pathways and genes. Comprehensive bioinformatics analysis using WGCNA, Cytoscape and PPI network as well as experimental verification with a rat model of ALI revealed Slc7a11 as the shared ferroptosis-related bottleneck gene of ALI of different etiologies and revealed that pathways such as cellular response to oxidative stress, vascular growth and regulation of cell death may be the shared mechanism of ALI of different etiologies. Lipid peroxidation, particularly the formation of oxidized phospholipids, represents the initial event of oxidative stress. The crosstalk between lipid peroxidation and ferroptosis exerts profound effects on the pathogenesis and progression of ALI11. Oxidized phospholipids exhibits the potential to mitigate lung injury induced by diverse stimuli11,42. Furthermore, Nonas S. et al. verified the protective effects of oxidized phospholipids on endothelial cell injury via LPS-induced ALI rat model43. Therefore, these pathways shared by three distinct models of ALI may be critical mechanisms by which ferroptosis regulates ALI pathophysiology.
In our study, the overlap among the vital modules gave rise to seven shared co-expressed genes (Egr1, Slc7a11, Fos, Slc3a2, Srxn1, Atf3 and Zfp36). These genes have a strong correlation with ALI. WGCNA provides co-expression network information but cannot directly display differential gene expression information. So, we explored DEGs in modules. Among the seven shared co-expressed genes, six (Egr1, Slc7a11, Slc3a2, Srxn1, Atf3, and Zfp36) were identified as ferroptosis-associated genes ALI. Notably, three genes (Egr1, Atf3, and Slc7a11) were differentially expressed in ALI models (Supplementary Table S9 and Table S10). Importantly, Slc7a11 was uniquely identified as a shared ferroptosis-related and bottleneck gene across three distinct etiologies of ALI. Furthermore, Egr1, Atf3, and Slc7a11 were confirmed as differentially expressed ferroptosis-related genes. Egr1, which is expressed in the initial stages of various diseases, is involved in cell proliferation, angiogenesis and apoptosis44,45. Researchers have found that Egr1 can promote ferroptosis by regulating GLS2 or GPX4/SLC7 A11 pathway and affect the progression of the disease46. The expression of Atf3 can be induced by various cellular stresses, such as oxidative stress and cell damage, and it is involved in the regulation of ferroptosis. Studies have shown that Atf3 can promote ferroptosis by regulating the expression of Slc7a1147. These findings suggested that these differentially expressed ferroptosis-related genes may be involved in the shared molecular biological regulation and interacted with ALI.
Our study identifies Slc7a11 as a shared ferroptosis-associated bottleneck gene. Slc7a11 functions as a key regulator of ferroptosis by sustaining intracellular redox homeostasis. Jin Li et al. reported that the expression of Slc7a11 was down-regulated in LPS-ALI after 48 h48. Additionally, Pengfei Liu et al. also showed that a similar expression pattern of Slc7a11 in ALI group after 16 h LPS challenge49. Interestingly, Hui Dong et al. reported that Slc7a11 was up-regulated after 3 h of ALI induced by intestinal ischemia/reperfusion and they thought the expression of Slc7a11 was increased in a compensatory manner in ALI group50. Given that the lung reacts similarly to various types of insults, this study employed three distinct models of ALI to investigate shared ferroptosis-related genes. Notably, the shared ferroptosis-associated bottleneck gene Slc7a11 was identified and regardless of the etiology, Slc7a11 may be a key shared gene in ALI. In our study, the level of Slc7a11 was increased in ALI group after smoke-induced ALI for 8 h. In general, there are two main discrepancies between previous studies that showed downregulation of Slc7a11 in ALI and the current findings of upregulation, one is that the model is different, the other is the time point of harvesting. In the research of the roles of cholesterol-25-hydroxylase in LPS-induced ALI51, Madenspacher JH et al. found that cholesterol-25-hydroxylase exerts a dual function in maintaining lung tissue homeostasis, and its activity is strongly correlated with the severity of ALI. Moreover, the dual role of oxidized phospholipids is thought to present structure, concentration, and milieu dependent11. These perspectives provide us with an insight that Slc7a11 may exhibit dynamic expression pattern in the process of ALI. The severity of ALI represents another crucial factor that requires consideration. We also acknowledge that other factors may also have important effects on SLC7 A11, such as structural variations, concentration, and cellular environment. Our follow-up studies will primarily focus on the dynamic expression of Slc7a11.
During lung injury, the activation of profibrogenic TGF-β and AP-1 signaling, along with the induction of proinflammatory mediators such as ROS, leads to the downregulation of peroxisomes. This downregulation, in turn, generates additional ROS and further enhances the activation of TGF-β1 and AP-1 signaling. This creates a self-perpetuating vicious cycle, ultimately exacerbating the progression of pulmonary fibrosis52. So, research on antioxidant defense mechanisms in ALI provides critical insights for prognostic evaluation.
Under oxidative stress, Slc7a11 expression exhibits regulatory dynamics. The transcription factor Nrf2 is an essential modulator of anti-oxidative stress. Nrf2 mediates suppression of ferroptosis by orchestrating the expression of telomerase reverse transcriptase and modulating Slc7a11 functional activity53. Nrf2 knockdown decreased Slc7a11 expression, while Slc7a11 suppression reciprocally reduced Nrf2 level. Conversely, Slc7a11 overexpression upregulated Nrf2 expression relative to controls54. Under conditions where Nrf2 protein degradation is compromised, Nrf2 can bind to antioxidant response elements located in gene promoter regions, thereby regulating Slc7a11 transcriptional activation53Evidence indicated that aryl hydrocarbon receptor (AhR) can mediate transcriptional activation of Slc7a11 through binding to its promoter region, resulting in suppression of ferroptosis. Furthermore, the oxidative stress milieu within cells promotes endogenous AhR ligand production, leading to AhR activation and subsequent initiation of AhR-mediated transcriptional control of Slc7a11 expression, ultimately forming a cytoprotective feedback mechanism against oxidative damage55. Collectively, these findings suggest that a complex regulatory interplay may exist between SLC7 A11 expression dynamics and oxidative stress responses.
Slc7a11 as both a driver and suppressor of ferroptosis prompted us to consider the potential dual roles of Slc7a11, which potentially serves as a shared regulatory target in the ferroptosis signaling pathway. Accumulating evidence indicates that various transcription factors, such as NRF2, ATF3, and p53, can modulate cellular susceptibility to ferroptosis through regulation of Slc7a11. For example, elevated Slc7a11 expression is well-established as a critical anti-ferroptosis factor, while p53-mediated downregulation of Slc7a11 through promoter binding promotes ferroptosis53. Furthermore, studies have demonstrated that the overexpression of SLC7 A11 facilitates ferroptosis through a mechanism of post-transcriptional regulation. This supports the role of SLC7 A11 as a regulatory target for miR-27a-3p, where the suppression of miR-27a-3p results in the upregulation of SLC7 A11, subsequently augmenting the incidence of ferroptosis56. These findings suggest that Slc7a11 plays a dual role in ferroptosis, with its expression level regulated by various transcription factors and post-transcriptional regulators. These regulatory mechanisms collectively affect the cellular susceptibility to ferroptosis.
Elevated Slc7a11 expression functions as an initial adaptive strategy against oxidative stress, strengthening cellular defense against damage57. Slc7a11 is overexpressed in multiple cancer cell types and linked to metastasis and drug resistance, but it shows low expression in adjacent normal tissues58,59. Notably, Slc7a11 deletion suppresses tumor growth while sparing normal tissues58. In addition, recent research reported that Slc7a11 overexpression increased the proliferation of pulmonary artery smooth muscle cell, and this effect can be reversed by inhibiting Slc7a1160. These findings suggest that elevated Slc7a11 expression may serve a dual role, functioning not only as a compensatory response to oxidative stress but also potentially contributing to vascular smooth muscle cell proliferation in the process of ALI. Hence, the crosstalk between oxidative stress and Slc7a11 in the intrinsic mechanisms of ALI will also be the subject of intense exploration. Considering the underlying compensatory mechanism and cell proliferation function in Slc7a11, investigating the molecular mechanism of Slc7a11 in ALI provides important insights into drug selection, optimal timing for therapeutic intervention, and the development of novel drugs.
Our current study had several limitations. Firstly, only three different models of ALI were included, and other models of ALI had not been included partly because of a lack of microarray data and partly because of small sample size. Secondly, animal models in this study might not fully mimic gene expression in human ALI. Thirdly, we have only verified the shared gene with the smoke-induced model, and other models warrant investigation in the future. Finally, we did not build the transcriptional regulatory network, and the upstream transcriptional regulators also needed to be investigated.
Conclusion
By means of bioinformatics analysis, our paper was the first one to use three different models of ALI to screen significant gene modules, and we identified the shared genes subjected to these three ALI models. Importantly, the shared ferroptosis-associated bottleneck gene Slc7a11 was screed out and may exert dual function through oxidative stress. Furthermore, upregulated Slc7a11 was witnessed in all these three etiologies of ALI based on microarray analysis and was validated by external datasets. Moreover, the animal experiment was constructed to verify the result that Slc7a11 were upregulated in ALI rats. We hope that our results can shed light on the understanding of the shared pathogenesis of ALI.
Acknowledgements
Not applicable.
Author contributions
J. L. and Z. C. conceived and designed the work. J. L. performed the experiments, prepared figures and/or tables, and wrote the manuscript. Y. Y. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Cooperation Foundation of China, grant number U1604188.
Data availability
The data from the current study can be provided based on the request from the corresponding author, and the data supporting the findings of this study are openly available in the GEO database at http://www.ncbi.nlm.nih.gov/geo/ and in the FerrDb Database at http://www.zhounan.org/ferrdb/current/. The corresponding information of microarray datasets was obtained from the reference supplementary materials.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gonçalves-de-Albuquerque, C. F., Silva, A. R., Burth, P., Castro-Faria, M. V. & Castro-Faria-Neto, H. C. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediators Inflamm. 260465 (2015). (2015). [DOI] [PMC free article] [PubMed]
- 2.Meyer, N. J., Gattinoni, L. & Calfee, C. S. Acute respiratory distress syndrome. Lancet398, 622–637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long, M. E., Mallampalli, R. K. & Horowitz, J. C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci.136, 747–769 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu, F. et al. Identifying novel clinical phenotypes of acute respiratory distress syndrome using trajectories of daily fluid balance: a secondary analysis of randomized controlled trials. Eur. J. Med. Res.29, 299 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo, Y. et al. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun.12, 7094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezerra, F. S. et al. Oxidative stress and inflammation in acute and chronic lung injuries. Antioxid12, 548 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, F. et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell. Metab.35, 84–100e8 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Hu, Y. et al. Crosstalk of ferroptosis and oxidative stress in infectious diseases. Front. Mol. Biosci.10, 1315935 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu, T. & Sun, S. Role and mechanism of ferroptosis in acute lung injury. Cell. Cycle. 22, 2119–2129 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell185, 2401–2421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karki, P. & Birukov, K. G. Oxidized phospholipids in healthy and diseased lung endothelium. Cells9, 981 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jyotsana, N., Ta, K. T. & DelGiorno, K. E. The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of Cancer. Front. Oncol.12, 858462 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai, W., Yu, L. & Deng, Y. PPARγ alleviates preeclampsia development by regulating lipid metabolism and ferroptosis. Commun. Biol.7, 429 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, C. Y. et al. Spatial transcriptomics reveals gene interactions and signaling pathway dynamics in rat embryos with anorectal malformation. Cell. Biol. Toxicol.40, 34 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu, M. et al. Identification and validation of immune and oxidative stress-related diagnostic markers for diabetic nephropathy by WGCNA and machine learning. Front. Immunol.14, 1084531 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, J. et al. TLE4 downregulation identified by WGCNA and machine learning algorithm promotes papillary thyroid carcinoma progression via activating JAK/STAT pathway. J. Cancer. 15, 4759–4776 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, B., Wang, H. & Chen, Z. Puerarin inhibits ferroptosis and inflammation of lung injury caused by Sepsis in LPS induced lung epithelial cells. Front. Pediatr.9, 706327 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, Y. et al. Electroacupuncture alleviates LPS-Induced ARDS through Α7 nicotinic acetylcholine Receptor-Mediated Inhibition of ferroptosis. Front. Immunol.13, 832432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, Y. et al. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell. Death Dis.13, 530 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen, Y. et al. Role and mechanisms of autophagy, ferroptosis, and pyroptosis in sepsis-induced acute lung injury. Front. Pharmacol.15, 1415145 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer, K. C., Sharma, B., Kaufmann, B., Kupper, A. & Hodgson, M. Lung disease associated with occupational styrene exposure. Am. J. Ind. Med.10.1002/ajim.22867 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Cao, C., Zhang, L. & Shen, J. Phosgene-Induced acute lung injury: approaches for mechanism-based treatment strategies. Front. Immunol.13, 917395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautier, L., Cope, L., Bolstad, B. M. & Irizarry, R. A. affy–analysis of affymetrix genechip data at the probe level. Bioinformatics20, 307–315 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Zhang, Y. et al. Identification of key biomarkers associated with immune cells infiltration for myocardial injury in dermatomyositis by integrated bioinformatics analysis. Arthritis Res. Ther.25, 69 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform.9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samadishadlou, M. et al. Unlocking the potential of MicroRNAs: machine learning identifies key biomarkers for myocardial infarction diagnosis. Cardiovasc. Diabetol.22, 247 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The Sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics28, 882–883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao, W. et al. Weighted gene coexpression network analysis: state of the Art. J. Biopharm. Stat.20, 281–300 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Lazar, C. et al. Batch effect removal methods for microarray gene expression data integration: a survey. Brief. Bioinform. 14, 469–490 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Liu, S. et al. Weighted gene co-expression network analysis identifies FCER1G as a key gene associated with diabetic kidney disease. Ann. Transl Med.8, 1427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun.10, 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franz, M. et al. Cytoscape.js 2023 update: a graph theory library for visualization and analysis. Bioinformatics39, btad031 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacono, G., Massoni-Badosa, R. & Heyn, H. Single-cell transcriptomics unveils gene regulatory network plasticity. Genome Biol.20, 110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, N. & Bao, J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford). 2020, baaa021. 10.1093/database/baaa021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilin, Z., Yandong, N. & Faguang, J. Role of angiotensin-converting enzyme (ACE) and ACE2 in a rat model of smoke inhalation induced acute respiratory distress syndrome. Burns41 (7), 1468–1477. 10.1016/j.burns.2015.04.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning, L., Shishi, Z., Bo, W. & Huiqing, L. Targeting immunometabolism against acute lung injury. Clin. Immunol.249, 109289 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bos, L. D. J. & Ware, L. B. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet400, 1145–1156 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Li, W. et al. Classic Signaling Pathways in Alveolar Injury and Repair Involved in Sepsis-Induced ALI/ARDS: New Research Progress and Prospect. Dis. Markers. 6362344 (2022). (2022). [DOI] [PMC free article] [PubMed]
- 39.Hong, H. et al. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway. Phytomedicine101, 154143 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Jia, L., Hao, H., Wang, C. & Wei, J. Etomidate attenuates hyperoxia-induced acute lung injury in mice by modulating the Nrf2/HO-1 signaling pathway. Exp. Ther. Med.22, 785 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, S. W. et al. GPR84 regulates pulmonary inflammation by modulating neutrophil functions. Acta Pharmacol. Sin. 44, 1665–1675 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karki, P. & Birukov, K. G. Oxidized phospholipids in control of endothelial barrier function: mechanisms and implication in lung injury. Front. Endocrinol.12, 794437 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nonas, S. et al. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am. J. Respir Crit. Care Med.173, 1130–1138 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, B. et al. The role of the transcription factor EGR1 in Cancer. Front. Oncol.11, 642547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou, K. & Zeng, Z. Role of early growth response 1 in inflammation-associated lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol.325, L143–L154 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, M. et al. Arginine methyltransferase PRMT1 promotes ferroptosis through EGR1/GLS2 axis in sepsis-related acute lung injury. Commun. Biol.8, 159 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, Q. et al. Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis. Cell. Death Dis.14, 668 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, J. et al. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell. Mol. Biol. Lett.27, 29 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu, P. et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett.25, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong, H. et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY. 12, 12943–12959. 10.18632/aging.103378 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madenspacher, J. H. et al. 25-Hydroxycholesterol exacerbates vascular leak during acute lung injury. JCI Insight8, e155448 . [DOI] [PMC free article] [PubMed]
- 52.Oruqaj, G. et al. Compromised peroxisomes in idiopathic pulmonary fibrosis, a vicious cycle inducing a higher fibrotic response via TGF-β signaling. Proc. Natl. Acad. Sci. U S A. 112, E2048–2057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, T., Yi, J., Wu, H., Wang, K. & Zhou, B. SLC7A11 in hepatocellular carcinoma: potential mechanisms, regulation, and clinical significance. Am. J. Cancer Res.14, 2326–2342 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, X. et al. SLC7A11 reduces Laser-Induced choroidal neovascularization by inhibiting RPE ferroptosis and VEGF production. Front. Cell. Dev. Biol.9, 639851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kou, Z. et al. AhR signaling modulates ferroptosis by regulating SLC7A11 expression. Toxicol. Appl. Pharmacol.486, 116936 (2024). [DOI] [PubMed] [Google Scholar]
- 56.Lu, X. et al. MiR-27a-3p promotes Non-Small cell lung Cancer through SLC7A11-Mediated-Ferroptosis. Front. Oncol.11, 759346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li, P. et al. SLC7A11-associated ferroptosis in acute injury diseases: mechanisms and strategies. Eur. Rev. Med. Pharmacol. Sci.27, 4386–4398 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science368, 85–89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, M. et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J. Hepatol.76, 1138–1150 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Hu, P. et al. The mechanism of the imbalance between proliferation and ferroptosis in pulmonary artery smooth muscle cells based on the activation of SLC7A11. Eur. J. Pharmacol.928, 175093 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from the current study can be provided based on the request from the corresponding author, and the data supporting the findings of this study are openly available in the GEO database at http://www.ncbi.nlm.nih.gov/geo/ and in the FerrDb Database at http://www.zhounan.org/ferrdb/current/. The corresponding information of microarray datasets was obtained from the reference supplementary materials.