Abstract
Objective:
Cyclophosphamide (CTX) is an effective anticancer drug, but toxic effects against normal human tissues are its dose-limiting drawback. The aim of this research was to investigate the in vivo immunomodulatory activities of Allium ampeloprasum Subsp Iranicum against CTX-induced toxicity in mice.
Materials and Methods:
Extract of the whole plant of A. ampeloprasum Subsp Iranicum was obtained using the maceration technique, and its total phenolic and flavonoid contents were quantified through spectrophotometric analysis. Mice received daily oral administration (PO) of the extract (150 mg/kg) for a duration of 14 days, either as a standalone treatment or in conjunction with an intraperitoneal (IP) injection of 20 mg/kg CTX. The effects of the extract on body weight, spleen weight, white blood cell (WBC), serum antibody titer hemagglutination (HA), delayed-type hypersensitivity response (DTH), lymphocyte proliferation, cytokine production, and histopathological examinations were evaluated.
Results:
A. ampeloprasum Subsp Iranicum restored several parameters including spleen weight (p<0.001), WBC (p<0.001), lymphocytes (p<0.05), and monocytes (p<0.01), HA titer (p<0.05), and DTH response (p<0.01). A. ampeloprasum Subsp Iranicum notably stimulated lymphocyte proliferation to PHA (p<0.01) and LPS (p<0.05) and recovered interferon (IFN)-γ (p<0.001) and interleukin (IL)-4 (p<0.001) levels in the immunosuppressed mice. Also, CTX-induced histopathological changes were reversed by A. ampeloprasum Subsp Iranicum.
Conclusion:
Analyses revealed A. ampeloprasum Subsp Iranicum could regulate immunity and increase host immune responses. The observed strengthening effect can be attributed to the high amount of flavonoids and dipropyl trisulfide compounds present in A. ampeloprasum Subsp Iranicum.
Key Words: Cyclophosphamide, Allium ampeloprasum, Subsp Iranicum, Immunomodulatory
Introduction
The immune system is a complex web of mechanisms that play an important role in protecting the internal environment of the body against disease agents (Nicholson, 2016). In the immune system, two different types of reactions to pathogens and invading agents are observed: innate responses and acquired responses (Delves and Roitt, 2000). Immunodeficiency disorders may result from a primary genetic defect that can affect the function of the innate or acquired immune system, through inhibition of immune cells or pathways, or may result from a secondary immunodeficiency such as environmental conditions, viral or bacterial infections, malnutrition, autoimmunity or treatment with drugs that suppress the immune system (Marshall et al., 2018). In immunodeficiency, the ability of the immune system to fight infectious diseases is compromised or completely absent and people whose immune function is impaired show a greater susceptibility to infection and morbidity and even mortality (Descotes, 2005; Gleichmann et al., 1989).
Cyclophosphamide (CTX) is mainly used to control and treat cancer and autoimmune diseases, and is prescribed before transplantation to prevent graft rejection and transplant complications in the host. This drug is a type of nitrogen mustard drug that exerts its effects through DNA alkylation, inhibiting protein synthesis through DNA and RNA cross-linking, which eventually leads to programmed cell death. Nevertheless, the primary limitation in CTX therapy stems from its cytotoxic effects on healthy human tissues, which serve as the main factor restricting dosage and consequently impacting treatment protocols and overall quality of life. The prominent drawback associated with the utilization of synthetic immunomodulatory agents resides in their adverse reactions, including neutropenia, anorexia, and proteinuria (Ahlmann and Hempel, 2016; Emadi et al., 2009).
Medicinal plants have been used in health care since ancient times (Bayan et al., 2014). Due to the fact that some plants have immunomodulatory properties and low toxicity, they can be a suitable alternative to conventional chemical drugs (Abtahi Froushani et al., 2015; Shirani et al., 2022). Among these medicinal plants, Allium genus could ameliorate chemotherapy symptoms by stimulating both innate and adaptive immunity. Allium genus, belonging to the family Amaryllidaceae, is recognized as one of the most extensive monocotyledonous genera. This botanical group comprises diverse culinary herbs such as garlic, onion, shallot, leek, chives, and scallions. Allium genus has been used in the treatment of a diverse array of pathological conditions due to its antibacterial, antifungal, blood sugar lowering, blood pressure lowering, blood cholesterol lowering, and anti-atherosclerotic properties (Lanzotti et al., 2014; Ruhee et al., 2020; Teshika et al., 2019).
Allium ampeloprasum Subsp Iranicum is a fascinating plant that delights our taste buds with its onion-like flavor, making it a popular choice for raw consumption and culinary experimentation. With its ten long flower stalks and broad leaves, this plant displays a unique tuberous structure. This remarkable plant is a rich source of starch, dietary fibers, carbohydrates, proteins, minerals, and an array of bioactive phytochemicals. It is like a treasure trove of goodness! Not to mention, it boasts an impressive lineup of antioxidants and important vitamins such as A, B, and C. Drawing inspiration from other members of the Allium genus, A. ampeloprasum Subsp Iranicum exhibits a wide range of pharmacological activities. It has been found to possess remarkable potential as an antidiabetic, anti-inflammatory, hypolipidaemic, anticarcinogenic, and antimicrobial agent. Additionally, its powerful free radical scavenging abilities make it an excellent choice for combatting oxidative stress. This plant also shows promising effects as an antihelmintic, diuretic, and antihypertensive effects, and possesses digestive properties (Mehdizadeh et al., 2021; Fatoorechi et al., 2016; Shiehzadeh et al., 2022).
The aim of this research was to investigate the in vivo immunomodulatory activities of a hydroalcoholic extract of A. ampeloprasum Subsp Iranicum against CTX-induced immunosuppression in NMRI mice by investigating immune organ index, white blood cell count, serum antibody titer hemagglutination (HA), delayed-type hypersensitivity (DTH), splenocyte proliferation, NK cell activity, cytokine production, and histopathological examination.
Materials
Plant material
A. ampeloprasum Subsp Iranicum was gathered during the spring of 2023 from the scenic Chaharmahal VA Bakhtiari Mountains in Iran. A team of skilled botanists from the Islamic and Complementary Medicine department, Iran University of Medical Sciences, Tehran, Iran, precisely identified the specimen.
Plant extraction
All parts of A. ampeloprasum Subsp Iranicum were dried in the shade and ground into powder by a blender. The whole plant powder (10 g) was extracted using the maceration method with 100 ml of 80% aqueous ethanol. The solvent was filtered every 24 hr and fresh solvent was added for a total duration of 3 days. All the extracts were combined and underwent drying using both a rotary evaporator and freeze dryer at a temperature kept below 40℃ (Yousefsani et al., 2022).
Measurement of total phenolic compounds
The spectrophotometric determination of the total phenolic content was conducted using the Folin-Ciocalteu method, with gallic acid as the standard described previously (Fattahi et al., 2014).
Measurement of total flavonoid compounds
The total flavonoid content was measured using a colorimetric assay described previously (Chang et al., 2002).
Animals
The mice utilized in the study were acquired from the Faculty of Medical Sciences, Tarbiat Modares University. These mice, obtained from the Naval Medical Research Institute (NMRI), were male, aged 6 to 8 weeks, with a weight ranging from 19 to 21 g. Before any experiments began, the mice were housed in polystyrene cages accommodating five mice per cage. They resided in the laboratory environment for a duration of 1 week, adhering to standard conditions. These standard conditions entailed maintaining a temperature of 20–22°C, a relative humidity of 35%, and a light-dark cycle of 12 hr each. Throughout this observational period, the mice had unrestricted access to both food and water, provided ad libitum. All animal experiments conducted during this study obtained approval from the ethical committee of Tarbiat Modares University, with the assigned approval number IR.MODARES.AEC.1401.014.
Doses and exposure schedules
A total of sixty mice were grouped randomly with each group comprising twenty mice (n=20). Each of these groups was further subdivided into four subsets, where each subset consisted of five mice (n=5).
(i) In the first subset, the mice received 150 mg/kg A. ampeloprasum Subsp Iranicum (PO) for a duration of 14 days. (ii) The second subset of mice received 150 mg/kg A. ampeloprasum Subsp Iranicum (14 days) (PO) combined with IP injection of 20 mg/kg CTX (5 days). (iii) The third subset underwent IP injection of 20 mg/kg CTX for a period of 5 days, and this served as the positive control group. (iv) The fourth subset of mice received oral administration of normal saline for 14 days, representing the negative control group (Riahi-Zanjani et al., 2015; Shiehzadeh et al., 2022).
Body and spleen weight
In order to evaluate the weight of the mice, their weight was measured on the first day before administering the first dose and on the 14th day, 2 hr after the last dose. After measuring their weight, the animals were anesthetized using ketamine at a dosage of 100 mg/kg and xylazine at 10 mg/kg, then they were sacrificed by decapitation, and the weight of their spleen was recorded.
White blood cell count
On the 14th day, 2 hr after administering the last dose, 0.2 ml of blood was collected from the Retro-orbital plexus into tubes stained with EDTA-K2. Total WBC counts, lymphocytes, neutrophil and monocytes were determined.
Preparation of single-cell suspension
On the 14th day, two hours after the final dose administration, the mice were euthanized by severing the spinal cord. Subsequently, the spleen was aseptically extracted from the mice. Using a needle and syringe, the spleen was processed into a cell suspension using RPMI1640 medium. To separate any remaining spleen tissue debris, the cell suspension was passed through a cell strainer. The cells were then centrifuged at 224 g and 4°C for 10 min. The supernatant was discarded, and the remaining sediment was resuspended in approximately 3 ml of red blood cell (RBC) lysis solution for 3 min at room temperature. Afterward, the cells were centrifuged again at 224 g and 4°C for 5 min. The resulting supernatant was discarded. The cells were washed three times using RPMI1640 culture medium. To determine the percentage of cell viability, a cell suspension was prepared in 5% phosphate buffer saline (PBS) and mixed with 0.4% trypan blue. By examining the cells under an optical microscope, the percentage of cell viability was determined (Riahi-Zanjani et al., 2015).
HA test
To perform the experiment, 1 mL of washed sheep red blood cells (SRBCs) diluted using Freund's complete adjuvant until reaching a dilution of 5×108. On the 11th day, 5×108 SRBCs was administered to the mice via IP injection. At the end of experiment, after providing sera from collected blood samples, aliquots (50 µl) of two-fold dilutions of the sera (in PBS) . Then, we set up multiple test tubes with serial dilutions of the serum. In each test tube, we mixed 25 µl of serum with 25 µl of sheep red blood cells (SRBC) suspension in phosphate buffer. To observe the agglutination reaction, the tubes were incubated at 37°C for 1 hr. The antibody titer was determined by recording the highest dilution at which agglutination was observed (Riahi-Zanjani et al., 2015).
DTH response
The DTH test was conducted following the method by Fararjeh et al. with some modifications. The preparation of RBC followed a similar procedure as described previously, but with a variation. We continued the dilution process using Freund's complete adjuvant until reaching a dilution of 1×109. On the 11th day, the animals were IP sensitized with 1×109 SRBCs. Then, on the 14th day, a booster dose equivalent to 1×109 SRBCs was injected into the left hind footpad. To control non-specific swelling, a comparable volume of Freund's incomplete adjuvant was injected into the right hind footpad. After 24 and 48 hr, we measured the increase in volume in both paws using a Vernier caliper (Mitutoyo, Kawasaki, Japan). By calculating the difference between the volumes of the left and right hind footpads, we obtained data on the specific response and the impact of non-specific swelling. (Zimecki and Kruzel, 2000).
Lymphocyte proliferation
The 100 µl aliquots of the splenocytes were placed into wells of a 96-well microtiter plate. To promote cell attachment and stimulation, we did not add any mitogen, PHA, or LPS to the wells, maintaining a final concentration of 5 and 1 μg/ml. The plate was then placed in a controlled incubator at 37°C with 5% CO2 for 48 hr. This incubation period allowed the cells to attach to the substrate and induce the desired response. Afterwards, the MTT assay was employed to determine the cells' proliferation. To calculate the proliferation index (PI), we divided the absorbance value of the stimulated cells by the absorbance value of the unstimulated cells. This index provides valuable insights into the relative proliferation rates between the two conditions (Shirani et al., 2021).
Production of cytokines
To measure the levels of two important cytokines, IFN-γ and IL-4 in the collected supernatants, we utilized commercially available ELISA kits and followed the instructions provided by the manufacturer (Shirani et al., 2021).
Histopathological examinations
Spleen was collected from each mouse and fixed in 10% formalin. Following mounting, 5-μm thick sections of these tissues were stained with hematoxylin and eosin (H&E) and analyzed via light microscopy.
Statistical analysis
The data are expressed as the mean±SD and were analyzed using a one-way ANOVA, followed by Tukey's multiple comparison test with the utilization of PRISM, version 6.00 (GraphPad Software Inc., San Diego, CA, USA). A statistical significance of p<0.05 was deemed as indicating a notable difference.
Results
Amount of total phenolic compounds in the extract
The total phenolic content of the extract was 197.85 mg/l g of dry extract.
Amount of total flavonoid compounds in the extract
The total flavonoid compound of the extract was 334/66 mg/1 g of dry extract.
Effect on mouse body weight
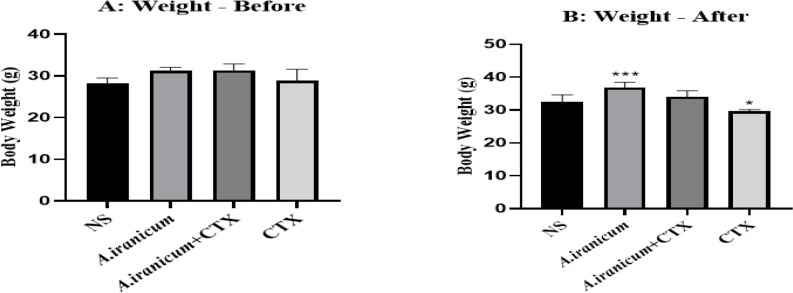
The average body weight showed a significant increase in mice that received A. ampeloprasum Subsp Iranicum plant extract compared to the normal saline group (p<0.001) (Figure 1A). In addition, in mice that received CTX, a significant decrease was observed compared to the normal saline group (p<0.05) (Figure 1B).
Figure 1.
The effects of A. ampeloprasum Subsp Iranicum extract on mice body weight before (A) and after (B) treatment. Data are expressed as the mean±SD (n=5). *p<0.05 and ***p<0.001 compared to control group (NS). Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline), CTX (Cyclophosphamide).
As shown in Figure 2, spleen weight in the group that received A. ampeloprasum Subsp Iranicum extract alone showed a significant enhance compared to the normal saline group (p<0.05). On the other hand, a meaningfully decrease was seen in the CTX group compared to the normal saline group (p<0.01). In addition, A. ampeloprasum Subsp Iranicum extract (150 mg/kg) markedly enhanced spleen weight compared to CTX group (p<0.001) (Figure 2).
Figure 2.
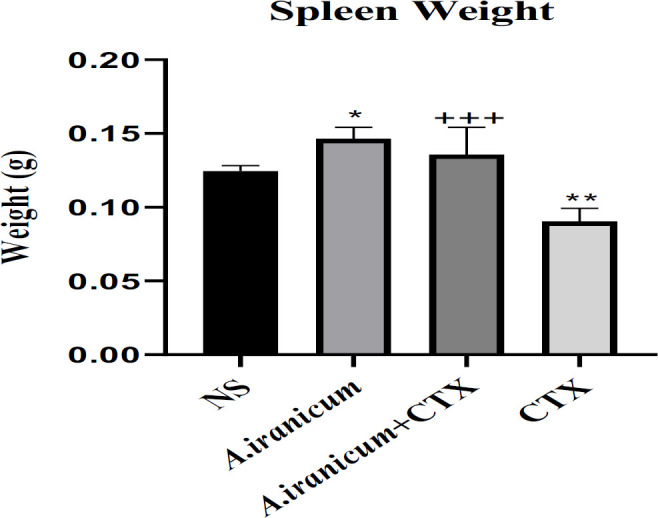
The effects of A. ampeloprasum Subsp Iranicum extract on spleen weight in CTX exposed mice. Data are expressed as the mean±SD (n=5). *p<0.05 and ***p<0.001 compared to control group (NS), +++p<0.001 compared to CTX group. Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline) and CTX (Cyclophosphamide).
White blood cell counts
The administration of CTX (50 mg/kg) resulted in a significant decrease in the number of WBC, lymphocytes, monocytes, and; when comparing to the control group. However, introducing A. ampeloprasum Subsp Iranicum at a dose of 150 mg/kg demonstrated a notable increase in white blood cell count. Moreover, the extract from this plant exhibited the ability to alleviate the reduction in white blood cells, lymphocytes, and monocytes induced by CTX, as summarized in Table 1.
Table 1.
Impacts of subacute orally exposure to A. ampeloprasum Subsp Iranicum for 14 days on white blood cell counts in cyclophosphamide-exposed mice
| CTX | A. iranicum +CTX | A. iranicum | NS | |
|---|---|---|---|---|
| 1.85±0.79** | 3.25±0.14**++ | 5.48±0.18***+++ | 2.44±1.08 | WBC (10 4 /µl) |
| 1.47±0.12* | 2.20±0.21*+ | 4.10±0.97***+++ | 1.86±0.90 | LYM (10 4 /µl) |
| 0.20±0.14* | 0.68±0.20**+++ | 0.97±0.21*+ | 0.38±0.13 | MONO (10 4 /µl) |
| 0.18±0.05 | 0.37±0.09*+ | 0.41±0.09*+ | 0.20±0.08 | NEU (10 4 /µl) |
Data are expressed as mean±SD. *p<0.05, **p<0.01 and ***p< 0.001: significant changes compared to the control group (NS). +p<0.05, ++p<0.01, and +++p<0.001 compared to CTX group. Monocytes (MONO; Neutrophils (NEU); Lymphocytes (LYM); White blood cell (WBC).
Spleen cell number
As observed in Figure 3, in mice that received A. ampeloprasum Subsp Iranicum extract, there was a significant increase in spleen cells compared to the normal saline group (p<0.05) whereas administration of CTX markedly decreased it. The decreased spleen cell numbers in the CTX group were reversed by A. ampeloprasum Subsp Iranicum (p<0.01) compared to the CTX only exposed group.
Figure 3.
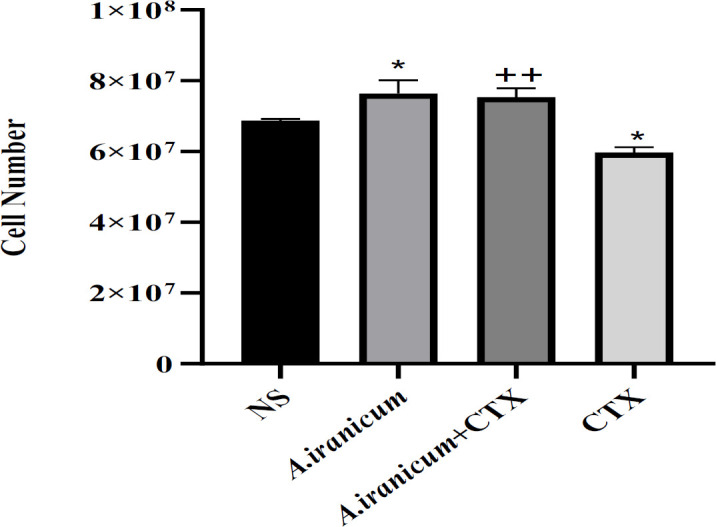
The effects of A. ampeloprasum Subsp Iranicum extract on spleen cell number in CTX-exposed mice. Data are expressed as the mean±SD (n=5 animals). *p<0.05 compared to control group (NS), ++p<0.01 compared to CTX group. Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline) and CTX (Cyclophosphamide).
Serum antibody titer
Results of the hemagglutination (HA) titer presented an obvious decline in antibody production in CTX - treated mice compared to the normal saline group (p<0.001). In addition, a significant increase in antibody production was observed in the group that received A. ampeloprasum Subsp Iranicum extract compared to the normal saline group (p<0.01). Also, the ability to produce antibodies in the mice that received the A. ampeloprasum Subsp Iranicum extract + CTX was significantly higher than the CTX group (p<0.05) (Figure 4).
Figure 4.
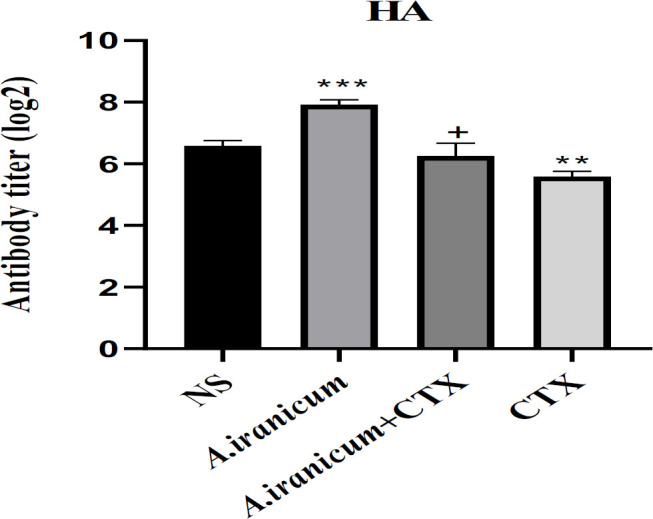
The effects of A. ampeloprasum Subsp Iranicum extract on hemagglutination (HA) titer in CTX-exposed mice. Data are expressed as the mean±SD (n=5). **p<0.01, ***p<0.001 compared to control group (NS), +p<0.05 compared to CTX group. Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline) and CTX (Cyclophosphamide).
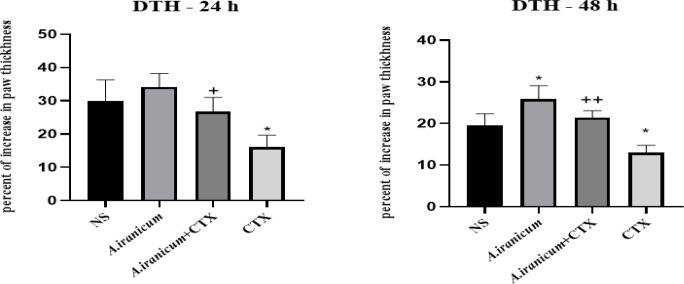
DTH response
As depicted in Figure 5A, a statistically significant decrease was seen in the CTX group compared to the normal saline group after 24 hr (p<0.05). Furthermore, there was a significant increase in the DTH response in the A ampeloprasum Subsp Iranicum extract + CTX group when compared to the CTX group. After 48 hr (Figure 5B), there was a significant elevation in the DTH response in the groups that received A. ampeloprasum Subsp Iranicum extract compared to the control group (p<0.05) (Figure 5B).
Figure 5.
The effects of A. ampeloprasum Subsp Iranicum extract on delayed-type hypersensitivity (DTH) response after 24 (A) and 48 hr (B) in CTX-exposed mice. Data are expressed as mean±SD (n=5). *p<0.05 compared to control group (NS), + p<0.05 and ++p<0.01 compared to CTX group. Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline) and CTX (Cyclophosphamide).
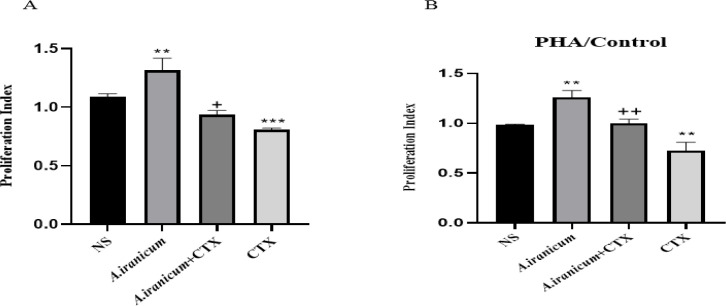
Lymphocyte proliferation
As presented in Figure (6), A. ampeloprasum Subsp Iranicum extract remarkably increased cell proliferation in response to PHA and LPS, compared to the normal saline group. On the contrary, a significant decline was observed in the CTX group compared to the normal saline group.
Figure 6.
The effects of exposure to A. ampeloprasum Subsp Iranicum on lymphocyte proliferation response to LPS (A) and PHA (B) in CTX-exposed mice. Data are expressed as mean±SD (n=5). *p<0.05 compared to control group (NS), +p<0.05 and ++p<0.01 compared to CTX group. Data were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test. NS (Normal Saline), CTX (Cyclophosphamide).
In addition, A. ampeloprasum Subsp Iranicum administration significantly attenuated CTX-induced cell proliferation suppression compared to the CTX group.
Effect on the secretion IFN-γ and IL-4
Mice exposed to CTX exhibited significantly reduced concentrations of IFN-𝛾 and IL-4. However, according to Table 2, the administration of A. ampeloprasum Subsp Iranicum restored the levels of IFN-𝛾 and IL-4 in the immunosuppressed mice.
Table 2.
Impacts of subacute orally exposure to A. ampeloprasum Subsp Iranicum for 14 days on cytokines concentrations in cyclophosphamide-exposed mice.
| CTX | A. ampeloprasum Subsp Iranicum + CTX | A. ampeloprasum Subsp Iranicum | NS | |
|---|---|---|---|---|
| 175±15*** | 255±21+++ | 238±18+++ | 264±18 | IFN-𝛾 (pg/ml) |
| 86±19*** | 150±21+++ | 141±27+++ | 154± 23 | IL-4 (pg/ml) |
Data are presented as mean±SD. ***p<0.001: significant changes compared to the control group (NS). +++p<0.001 compared to CTX group. NS (Normal Saline), CTX (Cyclophosphamide), interferon 𝛾 (IFN-𝛾), interleukin-4 (IL-4)
Histopathology
The spleen was evaluated for white pulp atrophy (or hyperplasia), red pulp: white pulp (Shirani et al., 2018). As observed in Figure 7A, there were no changes in the normal saline group. Analyses revealed that CTX induced splenic white pulp atrophy and an increase in the red: white pulp ratio, which was reversed by A. ampeloprasum Subsp Iranicum (Figure 7C and 7D).
Figure 7.
The effects of A. ampeloprasum Subsp Iranicum extract on spleen histopathology after 24 hr in mice. Data are expressed as the mean±SD (n=5). NS (Normal Saline) and CTX (Cyclophosphamide).
Discussion
Cyclophosphamide (CTX), a nitrogen mustard class anticancer drug, is utilized in the treatment of various cancer types. However, its usage is significantly constrained due to the induction of toxicity in non-cancerous cells. CTX exhibits the ability to impair DNA structure, eliminate immune cells, disrupt the proliferation and differentiation of B and T cells, and suppress both humoral and cellular immune responses. Administration of CTX results in myelosuppression and immunosuppression. Consequently, CTX has been employed in the establishment of immunosuppressed animal models across different research studies. Nevertheless, there exists considerable variation in the protocols and durations utilized in these studies aiming to induce CTX-induced immunosuppression (Haubitz, 2007; Shirani et al., 2015). In the current study, a murine model of CTX-induced immunosuppression was employed by intraperitoneally administration of CTX (50 mg/kg) from the beginning of the experiment for 5 days. The findings of the present study demonstrated that the mice treated with CTX showed significantly reduced body weight and immune organ indices, WBC count, spleen cell number, and lymphocyte proliferation ability, down-regulated cytokine (IFN-γ and IL-4,), and suppression of DTH and HA responses compared to the control group which indicated immunosuppression.
The Allium plants, which are widely distributed in the world, can increase host immune responses to various diseases by regulating immunity (Padiyappa et al., 2022). These findings have drawn considerable attention to investigating whether A. ampeloprasum Subsp Iranicum could be a promising natural immunomodulator. The present study showed that A. ampeloprasum Subsp Iranicum can restore several parameters including body and spleen weight, spleen cellularity, WBC counts, HA titer, DTH response, and lymphocyte proliferation ability, and cytokine production suggesting that A. ampeloprasum Subsp Iranicum can exert its protective effect in CTX-induced immunosuppressive mice.
The result of this study indicated that exposure to CTX resulted in a lower mass of the spleen, which might be associated with an overall decreased spleen cell number because of the direct cytolytic action of CTX on lymphocytes. Treatment with A. ampeloprasum Subsp Iranicum led to a higher spleen weight, possibly because of the increase in the population of different cells in these organs (Figures 2 and 3). Our findings support the conclusions of Radjabian et al., 2019; A. ampeloprasum Subsp Iranicum had the highest impact on the proliferation of splenocytes compared to other plants (A. sativum, A. elburzense, and A. asarense).
Lymphocyte proliferation is a critical aspect of lymphocyte activation, which is considered a significant milestone in the initiation of adaptive immunity (Heinzel et al., 2018). In this study, it was observed that A. ampeloprasum Subsp Iranicum notably enhanced the proliferation of T lymphocytes induced by PHA and B-lymphocytes induced by LPS in CTX-treated mice with immunosuppression. This observation suggests that A. ampeloprasum Subsp Iranicum exerts its protective effects on CTX-induced immunosuppressive mice by enhancing both cellular and humoral immunity.
Immunoglobulins are the basic member of humoral immunity, which can activate complement, specifically bind to antigens, and display an important function includes neutralizing infectious agents, and preventing bacterial bound and/or entry into host cells (Schroeder and Cavacini, 2010). Consist with the results of lymphocyte proliferation, the HA test in the current study showed a considerable decrease in the production of antibodies against SRBC in the group that received CTX compared to the normal saline group, while A. ampeloprasum Subsp Iranicum extract was able to improve the reduction of antibody production caused by CTX (Figure 4).
T lymphocytes play a vital function in host defense against microbial pathogens through the production of a wide range of cytokines. Specific immune responses play a crucial role in the immune system, and they can be classified into two main types: Type 1 T helper (Th1) and Type 2 T helper (Th2) cell responses. These two types of immune responses have distinct functions and serve different purposes within the body. Th1 lymphocytes are associated with cellular immune responses such as delayed hypersensitivity by producing cytokines predominantly IFN-γ, whereas Th2 stimulates the humoral response, promotes B cell proliferation, and induces antibody production by secreting cytokines including IL-4 (Rajaei, 2022).
According to Table 2, it was observed that A. ampeloprasum Subsp Iranicum effectively restored the levels of IFN-γ and IL-4 in immunosuppressed mice. This suggests that A. ampeloprasum Subsp Iranicum had a significant impact on activating Th1 and Th2 cells. DTH response assay is a valuable method for assessing the cellular immune response, which is triggered by direct stimulation of sensitive T cells upon contact with antigens. The outcomes of the DTH response demonstrated a strong immune response in mice treated with the hydroalcoholic extract of A. ampeloprasum Subsp Iranicum.
By measuring the DTH response, it is possible to indirectly assess the stimulation of Th2, Th1, and Th17 cells. It appears that A. ampeloprasum Subsp Iranicum, similar to other Allium species, has the potential to influence the balance of Th1/Th2 cells and even affect the activity of Th17 cells (Allen, 2013; Terhune et al., 2013).
Recent studies have delved into the fascinating realm of flavonoids and their impact on immune response. For instance, research by Farhadi et al. in 2014 highlighted how flavonoids can boost DTH and stimulate the secretion of IFNγ from Th1 cells (Farhadi et al., 2014).
In the hydroalcoholic extract of A. ampeloprasum Subsp Iranicum, the flavonoid content was found to be quite significant, with a measure of 334.66 mg/g of dry extract based on quercetin. This observation provides strong evidence linking the observed strengthening effect to this particular compound. Moving on to another fascinating component, phytol, it has been discovered in various Allium species, including A. ampeloprasum Subsp Iranicum. Presently, phytol is being utilized as an adjuvant to enhance immune responses triggered by vaccines. Exciting research conducted by Aachoui et al. in 2011 and Harke et al. in 2021 show cases the safety and high efficacy of this combined approach in stimulating the immune system when used in conjunction with different vaccines (Aachoui et al., 2011; Harke et al., 2021).
Additionally, the organosulfur compounds found in Allium species play a crucial role in their medicinal effects. In the case of A. ampeloprasum Subsp Iranicum, the most abundant bioactive compound is dipropyl trisulfide, constituting approximately 34.77% of the total chemical compounds in the plant. Intriguingly, a study by Arsenijevic et al. in 2021 indicated that a solution containing dipropyl polysulfide compounds (with 8.48% dipropyl trisulfide) could influence the balance of Th17/Treg cells in favor of regulatory T (Treg) cells. This finding, as demonstrated by Aryakia and Karimi in 2018, has significant implications for immune regulation. These studies shed light on the remarkable potential of compounds found in A. ampeloprasum Subsp Iranicum, paving the way for further investigation into their immunostimulatory properties and possible applications in optimizing immune responses (Aryakia and Karimi, 2018).
The present study revealed that the hydroalcoholic extract of A. ampeloprasum Subsp Iranicum has the potential to enhance the recovery from immunosuppression induced by CTX. This is achieved by regulating cytokine secretion levels and strengthening the immune system. The protective effect of the A. ampeloprasum Subsp Iranicum extract on the immune system may be attributed to the presence of polyphenols, flavonoids, and sulfur compounds. Conducting further comprehensive studies is necessary to gain a better understanding of the impact of A. ampeloprasum Subsp Iranicum on various immune system mechanisms.
Acknowledgment
This research was supported by Tarbiat Modares University and Iran University of Medical
Conflicts of interest
The authors have declared that there is no conflict of interest.
References
- 1.Aachoui Y, Chowdhury RR, Fitch RW, Ghosh SK. Molecular signatures of phytol-derived immunostimulants in the context of chemokine–cytokine microenvironment and enhanced immune response. Cell Immunol. 2011;271:227–238. doi: 10.1016/j.cellimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Abtahi Froushani SM, Gheibi S, Esmaeili Gouvarchin Ghaleh H, Mansori Motlagh B. Effects of Echinacea pupurea on the immunity system: from promise to fact. Razi J Med Sci. 2015;22:8–15. [Google Scholar]
- 3.Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78:661–671. doi: 10.1007/s00280-016-3152-1. [DOI] [PubMed] [Google Scholar]
- 4.Allen IC. Delayed-type hypersensitivity models in mice. Methods Mol Biol. 2013;1031:101–107. doi: 10.1007/978-1-62703-481-4_13. [DOI] [PubMed] [Google Scholar]
- 5.Arsenijevic D, Stojanovic B, Milovanovic J, Arsenijevic A, Simic M, Pergal M, Kodranov I, Cvetkovic O, Vojvodic D, Ristanovic E, Manojlovic D, Milovanovic M, Arsenijevic N. Hepatoprotective effect of mixture of dipropyl polysulfides in concanavalin A-induced hepatitis. Nutrients. 2021;13 doi: 10.3390/nu13031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aryakia E, Karimi HR. Evaluating essential oil constituent of four Allium species of the subgenus and section Allium. Iranian Field Crop Sci. 2018;49:115–123. [Google Scholar]
- 7.Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Delves PJ, Roitt IM. The immune system. N Engl J Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 9.Descotes J. Immunotoxicology: role in the safety assessment of drugs. Drug Saf. 2005;28:127–136. doi: 10.2165/00002018-200528020-00004. [DOI] [PubMed] [Google Scholar]
- 10.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 11.Farhadi L, Mohammadi-Motlagh HR, Seyfi P, Mostafaie A. Low concentrations of flavonoid - rich fraction of shallot extract induce delayed - type hypersensitivity and TH1 cytokine IFNγ expression in BALB/c mice. Int J Mol Cell Med. 2014;3:16–25. [PMC free article] [PubMed] [Google Scholar]
- 12.Fattahi S, Zabihi E, Abedian Z, Pourbagher R, Motevalizadeh Ardekani A, Mostafazadeh A, Akhavan-Niaki H. Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on Hela and BT-474 cell lines. Int J Mol Cell Med. 2014;3:102–107. [PMC free article] [PubMed] [Google Scholar]
- 13.Fatoorechi V, Rismanchi M, Nasrollahzadeh J. Effects of Persian leek (Allium ampeloprasum) on hepatic lipids and the expression of proinflammatory gene in hamsters fed a high-fat/ high-cholesterol diet. Avicenna J Phytomed. 2016;6:418–424. [PMC free article] [PubMed] [Google Scholar]
- 14.Gleichmann E, Kimber I, Purchase I. Immunotoxicology: suppressive and stimulatory effects of drugs and environmental chemicals on the immune system: a discussion. Archi Toxicol. 1989;63:257–273. doi: 10.1007/BF00278639. [DOI] [PubMed] [Google Scholar]
- 15.Harke M, Somkuwar A, Dubey S, Limsay R, Umap S. Qualitative and quantitative phytochemical evaluation of ethanolic extract of Mentha pipperita (Linn ) J Pharm Innov. 2021;10:996–1000. [Google Scholar]
- 16.Haubitz M. Acute and long-ter toxicity of cyclophosphamide. Tx Med. 2007;19:26–31. [Google Scholar]
- 17.Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–38. doi: 10.1016/j.coi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Lanzotti V, Scala F, Bonanomi G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem Rev. 2014;13:769–791. [Google Scholar]
- 19.Mehdizadeh T, Kaboudari A, Reale A. Stimulatory effect of Allium ampeloprasum L ssp iranicum Wendelbo on the probiotic Bifidobacterium bifidum in Iranian white cheese. J Dairy Sci. 2021: 10550–10557. doi: 10.3168/jds.2021-20371. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson LB. The immune system. Essays Biochem. 2016;60:275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padiyappa SD, Avalappa H, Somegowda M, Sridhara S, Venkatesh YP, Prabhakar BT, Pramod SN, Almujaydil MS, Shokralla Sh, Abdelbacki AMM, Elansary HO, El-Sabrout AM, Mahmoud EA. Immunoadjuvant and humoral immune responses of garlic (Allium sativum L ) lectins upon systemic and mucosal administration in BALB/c mice. Molecules. 2022;27:1375. doi: 10.3390/molecules27041375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radjabian T, Hosseinpur Yektaei Z, Ghazanfari T, Nasiri Z, Fotovvat M. The immunoregulatory effects of four Allium species on macrophages and lymphocytes viability. Immunoregulation. 2019;2:25–34. [Google Scholar]
- 24.Rajaei S. In: Cell-Mediated Immunity in Encyclopedia of Infection and Immunity. N Rezaei., editor. Oxford; 2022. pp. 56–63. [Google Scholar]
- 25.Riahi-Zanjani B, Balali-Mood M, Mohammadi E, Badie-Bostan H, Memar B, Karimi G. Safranal as a safe compound to mice immune system. Avicenna J Phytomed. 2015;5:441–449. [PMC free article] [PubMed] [Google Scholar]
- 26.Ruhee RT, Roberts LA, Ma S, Suzuki K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front Nutr. 2020;7:64. doi: 10.3389/fnut.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiehzadeh F, Vakili P, Shirani K. Evaluation of immune responses induced by hydroalcoholic extract of Allium ampeloprasum L subsp iranicum in mice: Allium iranicum as a powerful immune system booster. Iran J Pharmaceutical Sci. 2022: 176–182. [Google Scholar]
- 29.Shirani K, Hassani FV, Razavi-Azarkhiavi K, Heidari S, Zanjani BR, Karimi G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ Toxicol Pharmacol. 2015;39:1262–1275. doi: 10.1016/j.etap.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Shirani K, Riahi Zanjani B, Mehri S, Razavi-Azarkhiavi K, Badiee A, Hayes AW, Giesy JP, Karimi G. miR-155 influences cell-mediated immunity in Balb/c mice treated with aflatoxin M1. Drug Chem Toxicol. 2021;1:39–46. doi: 10.1080/01480545.2018.1556682. [DOI] [PubMed] [Google Scholar]
- 31.Shirani K, Riahi-Zanjani B, Omidkhoda SN, Barangi S, Karimi G. The hematopoietic potential of methanolic and aqueous extracts of Portulaca oleracea in a phenylhydrazine model of anemia. Avicenna J Phytomed. 2022;13:85–96. doi: 10.22038/AJP.2022.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirani K, Zanjani BR, Mahmoudi M, Jafarian AH, Hassani FV, Giesy JP, Karimi Gh. Immunotoxicity of aflatoxin M1: as a potent suppressor of innate and acquired immune systems in a subacute study. J Sci Food Agri. 2018;98:5884–5892. doi: 10.1002/jsfa.9240. [DOI] [PubMed] [Google Scholar]
- 33.Terhune J, Berk E, Czerniecki BJ. Dendritic cell-induced Th1 and Th17 cell differentiation for cancer therapy. Vaccines. 2013;1:527–549. doi: 10.3390/vaccines1040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, Pandian SK, Fawzi MM. Traditional and modern uses of onion bulb (Allium cepa L ): a systematic review. Crit Rev Food Sci Nutr. 2019;59:S39–S70. doi: 10.1080/10408398.2018.1499074. [DOI] [PubMed] [Google Scholar]
- 35.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]