Abstract
Two molecular fingerprinting techniques, pulsed-field gel electrophoresis (PFGE) and amplified fragment length polymorphism (AFLP), were used to investigate the epidemiological relatedness among Clostridium difficile isolates from suspected outbreaks in three general hospitals. Analysis by PFGE yielded inconclusive data as a result of extensive DNA degradation. Although this degradation could be prevented to a certain extent by the inclusion of thiourea in the electrophoresis buffer, the weak DNA banding patterns obtained in this way were still far from optimal. AFLP data were obtained by using fluorescently labeled PCR primers and analysis on an ABI PRISM automated DNA analysis platform. AFLP analysis yielded high resolution and highly reproducible DNA fingerprinting patterns from which the epidemiological relatedness among the isolates could easily be determined. AFLP results could be readily obtained within 24 h, whereas 3 to 4 days were routinely required to complete the lengthy PFGE protocol. AFLP clearly proved to be a much more fail-safe fingerprinting method for C. difficile isolates, especially for those isolates for which a standard PFGE procedure yielded inconclusive results due to DNA degradation.
Clostridium difficile is a well-recognized major cause of antibiotic-associated diarrhea and pseudomembranous colitis in hospitalized patients. However, it is often not clear what the epidemiological relationship is among isolates retrieved from different patients. Although pulsed-field gel electrophoresis (PFGE) has been widely used as a molecular fingerprinting technique for typing of clinical isolates, it is well documented that clostridial isolates have proven to be untypeable by PFGE due to extensive DNA degradation (9, 11). In fact, DNA degradation leading to untypeable isolates in PFGE procedures and inconclusive fingerprinting results has been reported in other genera as well, such as Escherichia (10), Pseudomonas (2), and Vibrio (13). Alternative techniques are therefore needed. In contrast to PFGE, several other mostly PCR-based typing techniques have been reported which have the advantage that they can also be performed on partially degraded DNA. One of these techniques is amplified fragment length polymorphism (AFLP) analysis. In AFLP analysis, a specific subfraction of multiple genomic restriction fragments is amplified by PCR, finally resulting in high-resolution subgenomic fingerprints (17). AFLP has reportedly been used for molecular fingerprinting of a variety of bacterial species (1, 6, 8, 15), but not yet for C. difficile. Because of the problems with the PFGE procedure described above, we decided to explore AFLP analysis as an alternative fingerprinting method for typing of C. difficile isolates.
MATERIALS AND METHODS
Strains.
A total of 30 C. difficile isolates were collected for this study from three different hospitals (A, B, and C). From hospital A, 15 isolates (A1 to A15) were randomly selected from a collection containing more than 100 isolates collected from 1998 onwards. From hospital B, 11 isolates (B1 to B11) were collected and stored in 1992, and from hospital C, 4 isolates (C1 to C4) were collected and stored in the spring of 2000. The strains were stored as pure cultures at −80°C according to standard procedures. All strains were isolated from patients with clinical symptoms of pseudomembranous colitis. Two unrelated C. difficile strains were included as internal controls in all experiments. All but four strains were toxin A and B positive as determined by PCR (12) prior to PFGE and AFLP analysis, the exceptions (B4, B6, B9, and B11) being negative for both toxins. It is unlikely that there has been a large exchange of patients among the three hospitals. If there had been an exchange of patients, it would most likely have been between hospitals A and B.
PFGE.
The isolates were routinely cultured anaerobically on Clostridium Difficile Moxalactam Norfloxacin agar (Oxoid, Haarlem, The Netherlands) for 48 h. Agarose plugs were made by mixing 250 μl of a freshly grown bacterial cell suspension (5 to 10% transmission at 450 nm) in lysis buffer (3 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 62.5 mM Na2EDTA, 0.25% Brij 58 [20 cetyl ether], 0.1% sodium deoxycholate, and 0.25% sodium lauryl sarcosine) with 10 μl of 1-mg/ml lysostaphine (Sigma, Zwijndrecht, The Netherlands) and 250 μl of 2% low-melting-point agarose (Bio-Rad, Veenendaal, The Netherlands) at 50°C. The mixture was immediately poured into the appropriate PFGE mold and allowed to solidify for 10 min at 4°C. Next, the plugs were incubated in 2 ml of EC buffer (6 mM Tris-HCl [pH 7.5], 100 mM Na2EDTA, 1 M NaCl, 0.5% Brij 58, 0.2% sodium deoxycholate, and 0.5% sodium lauryl sarcosine) for 5 h at 37°C. After the plugs were extensively washed with TE buffer (10 mM Tris-HCl [pH 7.5], 10 mM Na2EDTA), they were incubated with 2 ml of ES buffer (0.5 M Na2EDTA [pH 9.3], 1% sodium lauryl sarcosine, 1 mg of proteinase K/ml) overnight at 50°C. Next, the plugs were extensively washed with TE buffer and cut in 3- by 5-mm pieces. Individual pieces were washed with 100 mM Tris-HCl [pH 8.0]–5 mM MgCl2 and subsequently incubated in 100 μl of 1× restriction buffer with 30 U of SmaI (New England Biolabs, Beverly, Mass.) and 1 μg of RNase A for at least 1 h at 25°C. DNA fragments were separated in 1.2% PFGE-grade agarose in 0.5× Tris-borate-EDTA with or without an additional 50, 100, or 200 μM thiourea for 22 h at 14°C in a CHEF DR-III apparatus (Bio-Rad) at 6 V/cm with initial and final pulse times of 1 and 35 s, respectively. DNA fragments were stained using ethidium bromide and recorded using a Gel-Doc 1000 system (Bio-Rad).
AFLP.
Total bacterial DNA was isolated from the same cell suspensions used for the PFGE procedure using the method described by Boom et al. (4) with minor modifications. Adapters were made by mixing equimolar amounts of complementary oligonucleotides (Eurogentec, Seraing, Belgium) and heating the mixture to 95°C, with subsequent slow cooling to ambient temperature. Following isolation of the DNA, a combined restriction-ligation procedure was used in which 10 to 100 ng of genomic DNA was incubated with 5 pmol of EcoRI adapter, 50 pmol of MseI adapter, 2 U of EcoRI, 5 U of MseI (both from New England Biolabs), and 1 U of T4 DNA ligase (Promega, Leiden, The Netherlands) in a total volume of 20 μl of 1× reaction buffer for 1 h at 20°C, after which the mixture was diluted five times with ultrapure water. One microliter of the diluted restriction-ligation mixture was used for amplification in a volume of 25 μl under the following conditions: 1 μM EcoRI primer, 1 μM MseI primer, 0.2 mM each deoxynucleoside triphosphate, 1× reaction buffer, and 1 U of Taq DNA polymerase (Roche, Almere, The Netherlands). The cycling parameters were 94°C for 15 s, 56°C for 15 s, and 72°C for 15 s repeated 40 times, preceded by a 4-min incubation at 94°C and followed by an additional 10-min incubation at 72°C. The PCR products were analyzed on an ABI PRISM 3700 automated DNA analysis platform according to the instructions of the manufacturer. An overview of PCR primers and adapter sequences is shown in Table 1.
TABLE 1.
Overview of double-stranded adapter sequences and PCR primers used for the AFLP procedurea
| Adapter and primer | Sequence |
|---|---|
| EcoRI primer | 5′-Flu-GACTGCGTACCAATTCN-3′ |
| EcoRI adapter | 5′-CTCGTAGACTGCGTACC-3′ 3′-CTGACGCATGGTTAA-5′ |
| MseI primer | 5′-GATGAGTCCTGACTAAN-3′ |
| MseI adapter | 5′-GACGATGAGTCCTGAC-3′ 3′-TACTCAGGACTGAT-5′ |
The N residue in the PCR primers represents a selective residue which can be any of the four deoxynucleotides (A, G, C, or T). The specific nucleotide used for the experiments is mentioned in the text. Flu, fluorescein.
Determination of epidemiological relatedness.
Fingerprint patterns were analyzed for similarity using Gel Compar II software (Applied Maths, Kortrijk, Belgium). In order to compare AFLP patterns, The data obtained were analyzed by unweighted pair group method with arithmetic mean clustering using the Pearson correlation coefficient. DNA fragments smaller than 50 bp were ignored.
RESULTS
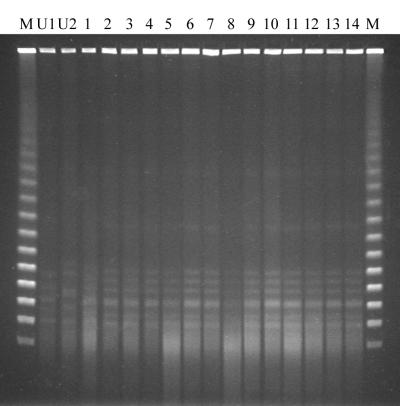
Initially, all strains were tested for epidemiological relationships using PFGE. When the standard PFGE protocol was used, 25 out of 30 isolates yielded no DNA banding pattern at all due to extensive DNA degradation, 3 isolates yielded a very faint pattern, and only 2 isolates yielded good DNA banding patterns (not shown). DNA degradation occurred independently of the origin of the isolates and independently of the storage time. As a result, the majority of the isolates could not be distinguished from each other using this technique. Addition of 50 μM thiourea to the electrophoresis buffer yielded no clear improvement (not shown). However, when the concentration of thiourea was raised to 100 μM, approximately 22 out of 23 tested strains yielded very faint but recognizable DNA fingerprinting patterns. In Fig. 1, a representative result obtained with 14 clinical isolates and 2 unrelated C. difficile strains is shown. As can be seen, the banding patterns obtained were still rather vague, showing primarily weak and diffuse DNA bands, very unlike the crisp, clear banding patterns that are usually obtained with other microbial isolates. A further increase of the thiourea concentration to 200 μM did not improve the results.
FIG. 1.
Representative PFGE results obtained for 14 clinical C. difficile isolates (lanes 1 to 14) and 2 unrelated C. difficile strains (lanes U1 and U2) after the inclusion of 100 μM thiourea to the electrophoresis buffer. The marker lanes (M) contain bacteriophage lambda concatemers (approximately 0.05 to 1.0 Mbp).
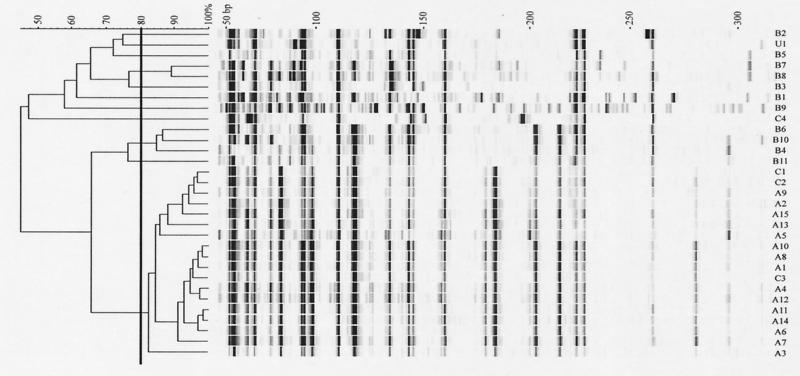
In order to circumvent the typing problems with degraded DNA in the PFGE procedure, we used AFLP as an alternative method to obtain molecular fingerprints from the C. difficile isolates. Preliminary experiments showed that high-quality fingerprints could be obtained with an EcoRI primer without selective extension and an MseI primer with extension G (not shown). We therefore used this combination of primers to test all C. difficile isolates collected. The results are presented in Fig. 2. From the results, it can be concluded that almost all C. difficile isolates from hospital A have a very high degree of similarity, suggesting they have a clonal origin. The fingerprints obtained with the isolates from hospital B do not have a high degree of similarity, suggesting that these isolates were mainly from incidental manifestations of C. difficile. Interestingly, three out of four isolates from hospital C also had a high degree of similarity with the majority of the isolates from hospital A.
FIG. 2.
AFLP fingerprints obtained from 30 clinical C. difficile isolates (A1 to A15, B1 to B11, and C1 to C4) from three general hospitals and an unrelated control strain (U1). The fingerprints were obtained using a fluorescently labeled EcoRI primer and an unlabeled MseI-plus-G primer. The dendrogram was created by unweighted pair group method with arithmetic mean clustering using the Pearson correlation coefficient.
DISCUSSION
In this study, we compared two typing techniques to obtain molecular fingerprints from clinical C. difficile isolates. PFGE yielded only poor data as a result of DNA degradation, whereas high-quality fingerprints could be obtained using AFLP as an alternative typing method. This makes AFLP the preferred method for fingerprinting of C. difficile isolates.
A total of 30 clinical C. difficile isolates from three hospitals were analyzed using both techniques in order to determine any epidemiological relationship between the isolates. From the AFLP results, it could be concluded that the majority of the isolates from hospital A had a very high degree of similarity, suggesting they have a clonal origin. The majority of the isolates from hospital B, however, did not show a high degree of similarity, suggesting that they were mainly the result of incidental manifestations of C. difficile. However, in a few cases, similar patterns were obtained with isolates collected from different patients, which could have resulted from transmission. Since the isolates from hospital A were collected over a 3-year period, the results indicate that this may be the fingerprint of an endogenous strain present in the hospital and that the manifestations of C. difficile are the result of nosocomial transmission. The same fingerprint was found in three out of four strains from hospital C, suggesting that this pattern may be from an epidemiological strain that has spread on a national level. Although there are no previous data supporting widespread geographic dissemination of C. difficile isolates, this assumption could be substantiated further by examining C. difficile isolates from more institutions nationwide and beyond. The faint patterns that were obtained upon inclusion of thiourea in the electrophoresis buffer in the PFGE procedure also revealed a large number of seemingly identical fingerprints, which also supports this theory.
Although PFGE is still considered to be the “gold standard” for molecular typing of bacterial isolates, one of its potential pitfalls is the necessity to isolate intact chromosomal DNA. Because of the sensitivity of very high molecular weight DNA to mechanical shearing, the DNA is protected from these effects by being liberated from bacterial cells while they are embedded in agarose plugs. This is done by lysis of the cells with detergent followed by subsequent degradation of proteins by incubation with a protease, usually proteinase K. Since these are all relatively slow processes, the liberated DNA is potentially much more exposed to nuclease activity. Therefore, it is understandable that genomic DNA degradation could occur to some extent in certain bacterial strains or isolates that produce high levels of nucleases. A number of adaptations to the standard PFGE procedure have been suggested which could minimize exposure of the DNA to nuclease activity. These adaptations have included (i) shortening of most of the incubation procedures, (ii) treatment of cells with formaldehyde, (iii) use of more concentrated cell suspensions, (iv) heat treatment of the cells, and (v) use of another proteinase. Although we have tried a number of these suggested adaptations, they did not provide improved fingerprinting results (not shown).
Recently, it was reported that degradation of genomic DNA during PFGE analysis did not occur exclusively during DNA isolation (7). Instead, the DNA was shown to be degraded upon the formation of free Tris radicals during the electrophoresis procedure. DNA degradation could be prevented by the addition of a free radical scavenger, particularly thiourea, to the electrophoresis buffer (5, 7, 14). However, free radicals themselves do not degrade DNA molecules. If this were the case, DNA degradation should occur independently of the particular bacterial strain under investigation. In fact, it should occur during all DNA electrophoresis procedures in which a Tris buffer is used. Since this is clearly not the case, this implies that another component, most likely a protein, must be involved and that this component is at least partially resistant to proteinase K digestion. This component should be present in bacterial strains in which degradation of DNA occurs during the standard PFGE procedure, and it should be absent in strains in which DNA degradation does not occur.
It should be clear that PFGE is certainly not a fail-safe method, particularly in cases where DNA degradation proves to be a problem. When other DNA purification techniques are used, like the method of Boom et al., in which there is a rapid inactivation of nucleases by chaotropic salts, enzymatic degradation of the DNA is hardly an issue (4). However, a disadvantage of that method could be the inability to isolate intact chromosomal DNA. Due to the influence of mechanical shearing forces, DNA isolated by the method of Boom et al. and many other isolation methods is usually degraded to fragments of around 50 kbp. Therefore, DNA isolated by the method of Boom et al. is unsuitable for analysis by PFGE, where the size of the genomic DNA fragments to be analyzed is usually on the order of 50 to 500 kbp. However, in AFLP analysis, the size of the genomic DNA fragments that are amplified and analyzed is on the order of 50 to 500 bp. One can calculate that if genomic DNA fragments are on average 50,000 bp in size, only 1% of the total population of a certain AFLP fragment of 500 bp will be affected by this degradation. For smaller fragments, these numbers are even lower. Even if the genomic DNA fragments were degraded to fragments with an average size of only 5,000 bp, approximately 90% of all large AFLP fragments would still remain unaffected. Therefore, unless the DNA is fragmented to a much greater extent, it is highly unlikely that degradation of genomic DNA will affect the outcome of an AFLP analysis. This makes the AFLP technique a much more robust method than PFGE analysis.
The AFLP technique offers a very high degree of flexibility. There is a free choice of restriction enzymes to be used, and also of the number and identity of selective residues added to the amplification primers to amplify a specific subset of genomic restriction fragments. Furthermore, the AFLP procedure is very easy to perform, can be performed on very small quantities of DNA, and is much faster than PFGE analysis. With AFLP, a fingerprinting result can theoretically be obtained within 8 h: 1 h for DNA isolation, 1 h for the combined digestion and adapter ligation, 2 h for amplification, and 1 to 4 h for electrophoresis, depending on the available electrophoresis equipment. However, the electrophoresis procedure is usually performed overnight, allowing results to be obtained immediately the next day. In contrast, most PFGE procedures take up to 2 to 4 days. Another advantage of the AFLP procedure is the potential to automate virtually the entire procedure, thereby reducing much of the workload of the laboratory technicians performing the fingerprinting experiments. Also, unlike other random amplification techniques, such as arbitrarily primed PCR (3), AFLP offers the highest level of reproducibility between experiments as an intrinsic feature of the technique (17).
Despite all the advantages that AFLP has to offer compared to PFGE, certain disadvantages also exist. One is the virtual inability to compare a large number of AFLP patterns by eye. In order to compare the complex AFLP patterns to each other, one has to depend on expensive computer software. In contrast, most people working with PFGE are familiar with the guidelines of Tenover et al. (16) for the manual interpretation of PFGE patterns in order to determine the epidemiological relatedness of bacterial isolates. This information is essential for the management of suspected microbial outbreaks in clinical settings. Furthermore, PFGE data are readily exchangeable between different laboratories because of the uniformity of equipment that is used. This is clearly a benefit of the use of PFGE data in interlaboratory comparisons. Primary AFLP data, however, can be generated on a large variety of platforms (fluorescent versus radioactive detection, gel-based automatic sequencers or capillary-based sequencers, plain agarose gels, etc.). Standardization of the technique will certainly be required in order to allow interlaboratory comparisons with AFLP data as well.
For distinquishing epidemiologically related strains from unrelated strains, PFGE is still considered to be the gold standard. However, it may very well be possible that in some instances the discriminatory power of PFGE exceeds that of AFLP and vice versa. For the typing of C. difficile isolates, this study indicates that the discriminatory power of AFLP is comparable to that of PFGE.
Acknowledgments
We thank J. W. Mouton for critical reading of the manuscript.
REFERENCES
- 1.Aarts, H. J., L. A. van Lith, and J. Keijer. 1998. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett. Appl. Microbiol. 26:131–135. [DOI] [PubMed] [Google Scholar]
- 2.Barth, A. L., and T. L. Pitt. 1995. Auxotrophic variants of Pseudomonas aeruginosa are selected from prototrophic wild-type strains in respiratory infections in patients with cystic fibrosis. J. Clin. Microbiol. 33:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidet, P., V. Lalande, B. Salauze, B. Burghoffer, V. Avesani, M. Delmée, A. Rossier, F. Barbut, and J.-C. Petit. 2000. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 38:2484–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corkill, J. E., R. Graham, C. A. Hart, and S. Stubbs. 2000. Pulsed-field gel electrophoresis of degradation-sensitive DNAs from Clostridium difficile PCR ribotype 1 strains. J. Clin. Microbiol. 38:2791–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijkshoorn, L., H. Aucken, P. Gerner-Smidt, P. Janssen, M. E. Kaufmann, J. Garaizar, J. Ursing, and T. L. Pitt. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, M., F. S. Kaczmarek, K. Stutzman-Engwall, and P. Dyson. 1994. Characterization of a Streptomyces-lividans-type site-specific DNA modification system in the avermectin-producer Streptomyces avermitilis permits investigation of two novel giant linear plasmids, pSA1 and pSA2. Microbiology 140:1367–1371. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, J. R., E. Slater, J. Xerry, D. S. Tompkins, and R. J. Owen. 1998. Use of an amplified-fragment length polymorphism technique to fingerprint and differentiate isolates of Helicobacter pylori. J. Clin. Microbiol. 36:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hielm, S., J. Björkroth, E. Hyytiä, and H. Korkeala. 1998. Genomic analysis of Clostridium botulinum group II by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 64:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumiya, H., J. Terajima, A. Wada, Y. Inagaki, K. I. Itoh, K. Tamura, and H. Watanabe. 1997. Molecular typing of enterohemorrhagic Escherichia coli O157:H7 isolates in Japan by using pulsed-field gel electrophoresis. J. Clin. Microbiol. 35:1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristjansson, M., M. H. Samore, D. N. Gerding, P. C. DeGirolami, K. M. Bettin, A. W. Karchmer, and R. D. Arbeit. 1994. Comparison of restriction endonuclease analysis, ribotyping, and pulsed-field gel electrophoresis for molecular differentiation of Clostridium difficile strains. J. Clin. Microbiol. 32:1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou, Q., S. K. F. Chong, J. F. Fitzgerald, J. A. Siders, S. D. Allen, and C.-H. Lee. 1997. Rapid and effective method for preparation of fecal specimens for PCR assays. J. Clin. Microbiol. 35:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall, S., C. G. Clark, G. Wang, M. Mulvey, M. T. Kelly, and W. M. Johnson. 1999. Comparison of molecular methods for typing Vibrio parahaemolyticus. J. Clin. Microbiol. 37:2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Römling, U., and B. Tümmler. 2000. Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J. Clin. Microbiol. 38:464–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloos, J. H., P. Janssen, C. P. van Boven, and L. Dijkshoorn. 1998. AFLP typing of Staphylococcus epidermidis in multiple sequential blood cultures. Res. Microbiol. 149:221–228. [DOI] [PubMed] [Google Scholar]
- 16.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, and M. Kuiper. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]