Abstract
Citrus is the world’s largest fruit category, yet it is frequently damaged by weeds during cultivation and management. As a green cultivation measure, covering crops in orchards effectively controls weeds and enhances soil quality. At present, the research on covering crops is mostly focused on soil, but there is still a lack of research on how crops affect citrus trees. This study aims to provide theoretical support for the widespread adoption of the green management practices. The previous research of us found that rattail fescue and vicia villosa had notably enhanced the organic matter and alkali-hydrolyzable nitrogen levels in orchard soils. Consequently, this study treated citrus orchards with sowing rattail fescue and vicia villosa between rows, with manual tillage serving as the control, to investigate the impact of two-year grass cultivation on N metabolism in citrus roots. Results indicated that both types of grass significantly enhanced amino acid metabolism in citrus roots at depths of 0–20 cm, significantly increasing activities of nitrate reductase, nitrite reductase, glutamine synthetase, NADH-glutamate synthetase, and NADPH-glutamate dehydrogenase, as well as expression levels of NR and NiR. Rattail fescue demonstrated superior effects. There was no discernible pattern in amino acid levels at depths of 20–40 cm, with both grass types significantly increasing NR, NADH-GOGAT enzyme activity, and also increasing gene expression levels for NiR, GDH1, and GDH2. Both types of grass significantly promoted N metabolism in citrus roots at depths of 0–20 cm, with rattail fescue outperforming vicia villosa.
Keywords: Citrus, Cover crops, Grass, N metabolism, Root
Introduction
Citrus is one of the world’s important cash crops and the world’s largest category of fruit. It is cultivated in more than 135 countries. The cultivation area and yield of Citrus in China ranks first in the world [1, 2]. Nitrogen (N) is one of the essential nutrient elements for plant growth and development, and is also a component of biological macromolecules such as amino acids, proteins and nucleotides in plants [3–6]. The main source of N uptake by plants is inorganic N absorbed by roots from soil, including nitrate nitrogen (NO3−-N) and ammonium nitrogen (NH4+-N) [7]. The inorganic N absorbed by plants needs to be converted into amino acids through the assimilation process of N metabolism before it can participate in the life metabolism of plants and be truly used by plants [8]. The roots of higher plants mainly absorb NO3, and a small amount of NH4+ [9, 10], but the N in NO3− is in a highly oxidized state, which can not be directly used by plants. Only when reduced to NH4+ under the action of nitrate reductase (NR) and nitrite reductase (NIR), can it participate in the subsequent assimilation process [11–13]. Under the action of glutamine synthase (GS), NH4+ absorbed by roots and reduced NH4+ together with Glutamate in plants generate glutamine. Glutamine forms Glutamate under the catalysis of glutamate synthase (GOGAT). One part of the generated Glutamate synthesizes amino acids needed by other plants through transamination [14], and the other part of glutamate continues to participate in NH4+ assimilation in the form of substrate. This process is called GS/GOGAT cycle [15], and plants can assimilate more than 95% of NH4+ through GS/GOGAT pathway [16]. Glutamate dehydrogenase (GDH) can also catalyze NH4+ and α- ketoglutarate to produce glutamate, but GDH has low affinity with NH4+.The GDH pathway mainly plays an auxiliary role in the synthesis of glutamate [17–22]. Enzyme related genes in the processes of NO3− reduction and NH4+ assimilation in N metabolism have been studied in several species [23–26].
Cover crops cultivation represents a modern green soil management practice, which can not only control weeds and reduce herbicide use but also enhance the input of organic matter in the orchard, thereby improving soil physical and chemical properties [27–31]. Cover crops primarily consist of gramineous and leguminous grass species, including ryegrass, white clover, and vetch [32–38]. Gramineous grass species, with their high C: N ratios and slow root degradation rates, provide a substantial amount of C to the soil, significantly enhancing soil organic matter levels. Leguminous grass species, with their low C: N ratios and rapid degradation rates, are capable of biological nitrogen fixation, adding more N to the soil, which is often used as the source of organic N input to the soil [39–42]. However, both gramineous and leguminous plants can effectively increase soil organic matter content enhance N input and mineralization, and reduce nutrient leaching losses. The specific effects vary according to the grass species [43].
Rattail fescue is a plant of the Poaceae family, belonging to the genus Vulpi, and vicia villosa belongs to the Vicia genus in the legume family. In prior research, we discovered that both grass species are highly effective in controlling weed growth and improving soil quality in citrus orchards, especially in elevating organic matter and nitrogen content [44, 45]. To achieve clarifying the effects of these two grass species on N metabolism in citrus roots and explore the mechanism of different grass species affecting N metabolism in citrus roots, in this work, we assessed the impact of two grasses covering and a control (tillage) on the citrus root nitrogen metabolites, nitrogen metabolism enzyme activity, and related gene expression levels in a citrus orchard after two years of cover crop establishment. As a result, we can offer a theoretical foundation for research on cover crops and the selection of suitable grass species for citrus orchards.
Materials and methods
Site description and plant materials
Field trials were conducted in an experimental citrus orchard located in Shehong city, Sichuan province, China (105.42°E, 30.69°N, 324.2 m above sea level). The study site experiences a subtropical humid climate with average annual temperature 17.2°C, average rainfall 908–993 mm, average annual sunshine hours 1306.9–1471.8 h, and usually 284 frost-free days per year. Moreover, it used three-year-old trees of Ehime 38 hybrid planted with spacing of 3 m × 4 m, and the tree height is 1.5–1.7 m, and the crown width is 1.1–1.4 M. The experiment was designed with rattail fescue and vicia villosa coverage as the treatment, and each treatment occupied 0.2 ha that had three replicates. Treatments were applied within a 2 m width centered in the tree row leaving 1 m from the trunk. Clean tillage used hoe and shovel at 0–5 cm soil depth in combination in March, May and August was employed as the control treatment (CK), to keep the control treatment soil in an exposed state. The seeds were sown manually in rotary tilled soil to a depth of 1–2.5 cm in October 2020. The experimental citrus orchard was fertilized four times in 2020, 375 kg ha− 1 nitrogenous fertilizer in February, 300 kg ha− 1 compound fertilizers in April, 450 kg ha− 1 compound fertilizers in June and 375 kg ha− 1 compound fertilizers in September. In the following year, no fertilizer was applied.
Root sampling
In October 2022, two years after grass coverage in citrus orchard, the lateral roots of the citrus trees were collected as samples for the four diagonal vertices of the citrus tree’s drip line at 0–20 cm and 20–40 cm depth using hoe and shovel. Three trees are a repetition, with three replicates for each treatment. The 0–20 cm and 20–40 cm root samples from the vicia villosa treatment (G) were labeled G1 and G2, while those from the rattail fescue treatment (S) were labeled S1 and S2. The two soil depth samples from the clean tillage treatment (CK) were labeled CK1 and CK2. These collected samples were then refrigerated and taken back to the laboratory. In the laboratory, the soil on the root surface was washed away, then cleaned using an ultrasonic cleaner, frozen with liquid nitrogen, and stored at -80°C for future use.
Root N metabolites analyses
An appropriate amount of sample was added to the pre-cooled MeOH: ACN: H2O solution containing internal standard (v: v: v = 2: 2: 1), and the tissue grinder was ground at 60 Hz for 120s and ultrasound for 10 min. Wait at -20 °C for 1 h, centrifuge at 13,000 rpm at 4 °C for 15 min. Take the supernatant and freeze-dry. During mass spectrometry analysis, an appropriate amount of ACN: H2O solution (v: v = 1: 1) was added for reconstitution, vortex oscillator oscillation for 30s, ultrasonic for 10 min. Centrifuge at 13,000 rpm at 4 °C for 15 min. Aspirate the supernatant into the injection bottle for LC-MS/MS analysis.
All samples were acquired by the LC-MS system followed machine orders. Firstly, all chromatographic separations were performed using an ultra performance liquid chromatography (UPLC) system (SCIEX, UK). ACQUITY UPLC T3 column (100 mm*2.1 mm, 1.8 μm, Waters, UK) was used for the reversed phase separation. The column oven was maintained at 50 °C. The flow rate was 0.3 ml/min and the mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in ACN). Gradient elution conditions were set as follows: 0–0.5 min, 5% B; 0.5–2.5 min, 5–70% B; 2.5–7.5 min, 70-100% B; 7.5–9.0 min, 100%, 9.0–9.5 min, 100%-5%; 9.5–12 min,5% B.
Enzyme activities related to root N metabolism analyses
Nitrate Reductase (NR), Nitrite Reductase (NiR), Glutamine synthetase (GS), NADH-glutamate synthase (NADH-GOGAT), NADPH-glutamate dehydrogenase (NADPH-GDH) and NADH-glutamate dehydrogenase (NADH-GDH) were measured using the Suzhou Geruisi Biotechnology Co., Ltd. test kit, according to the instructions.
Enzymes gene expression levels related to root N metabolic analyses
Total RNA of root samples was extracted using the RNAprep Pure Plant Kit of Tiangen biochemical technology company. The quality and concentration of extracted RNA were detected by 1% agarose gel electrophoresis and nucleic acid micro analyzer. The electrophoretic bands were clear without dragging, and the concentration was > 200 ng/uL, A260/A280 values between 1.8 ~ 2.1 are qualified RNA samples. The qualified root total RNA samples were synthesized with the reverse transcription kit from Mei5bio Biotechnology Co., Ltd. And according to the instructions. The cDNA samples with the end of reverse transcription were stored at -20 °C for future use.
Primer 5.0 software was used to design n metabolic enzyme related genes, and real-time quantitative PCR (qRT-PCR) was used to screen primers with strong specificity. Using the synthesized cDNA as template, qRT-PCR was performed on CFX 96 fluorescence quantitative PCR instrument using the screened primer sequence (Table 1). The total reaction system was 20 uL with the reaction procedure was pre denaturation at 95 ° C for 5 min, denaturation at 95 ° C for 10 s, annealing at 58 °C for 10 s, extension at 72 °C for 20 s, cycle was 45 times, and each template was repeated 3 times. The ΔΔCT method was used for data processing, and the relative expression of the target gene was expressed by the 2−∆∆Ct value.
Table 1.
Primers sequences for Real-time PCR
| Gene | Accession number | Sequence of primer(5´-3´) |
|---|---|---|
| NR | Cs3g19060 | F: TCATTACAGAAGCAATCCTCAG |
| R: CACCAGCAACGAATCCTT | ||
| NiR | Cs8g05970 | F: GAGTGGTATGGACAATGTTAGA |
| R: GCAAGTTAGTGACGGTAGG | ||
| GS | Cs6g17430 | F: GCCTAAGTGGAATTATGATGGT |
| R: ATTGTTGCCTCTCCTGAATG | ||
| GS2 | Cs6g17420 | F: CAACTACAGCACCAAGTCTAT |
| R: CCTCTCCATAAGCAGCAAT | ||
| FD-GOGAT | orange1.1t03572 | F: AGAAGCGGTCGTACTCCTGA |
| R: GGCGAAGACCATTCCTATCA | ||
| NADH-GOGAT2 | Cs4g16210 | F: TCAGGAGATTCTTCACAGGTT |
| R: TTCAAGGTCTTCCGATATGCTA | ||
| NADP-GDH | Cs5g15430 | F: GGAATGAAGGCAGTAGGAAT |
| R: ATAGGCTTGTCAGCAATGAT | ||
| GDH1 | Cs5g26650 | F: GACATCTCTGGAGCCATCAA |
| R: CGTCACAGTCCTCAATCAATATC | ||
| GDH2 | Cs7g19160 | F: ACAAGGAGTTGGCACATAG |
| R: CAGAAGGTTCAGGAAGAGG | ||
| actin | Cb250364 | F: ATCTGCTGGAAGGTGCTGAG |
| R: CCAAGCAGCATGAAGATCAA |
Statistical analysis
The data was sorted by Excel 2019 software. The significance of differences in root N metabolites, enzyme activities and gene expression levels were analyzed using one-way analysis of variance and the Student-Newman-Keuls test with IBM SPSS Statistics 22.0 software (IBM Corporation, Armonk, NY, USA). Differences were adjudged to be significant at the 5% significance level. Origin 2022 software and online platform were used for plotting.
Results
OPLS-DA analysis of root metabolites
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) is a statistical tool that integrates Orthogonal Signal Correction (OSC) with the PLS-DA approach. The OPLS-DA model was well-developed, effectively differentiating samples across various depth and treatments (Fig. 1), which suggested effective metabolite separation among treatments, with clear metabolic differences evident, thus enabling further analysis of the metabolite data.
Fig. 1.
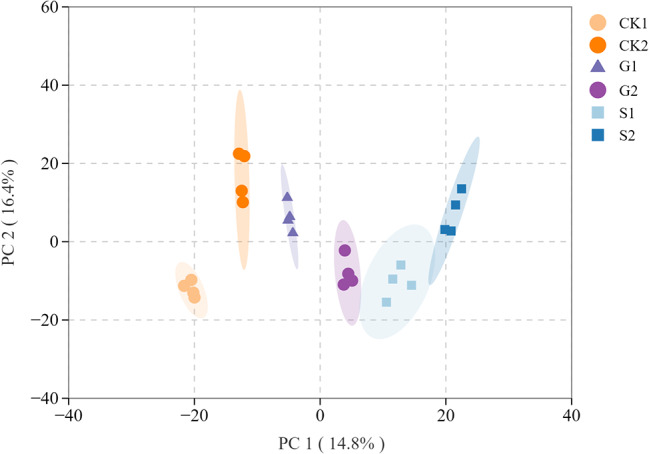
OPLS-DA analysis of root metabolites in citrus under different treatments. The horizontal axis represents the first principal component PC1, and the vertical axis represents the second principal component PC2. Each point in the graph represents a sample, and different colors represent different groups. CK1: Clean tillage at 0–20 cm, CK2: Clean tillage at 20–40 cm, G1: Vicia villosa at 0–20 cm, G2: Vicia villosa at 20–40 cm, S1: Rattail fescue at 0–20 cm, S2: Rattail fescue at 20–40 cm
Differential metabolites in root systems
The variable importance projection (VIP) values, P values, and fold-change values through the OPLS-DA analysis were used for data analysis along with the creation of a volcano map (Fig. 2) of differential metabolites. The map revealed that within the 0–20 cm root depth, there were 729 differential metabolites between the CK and vicia villosa treatments, with 417 being upregulated in the vicia villosa treatment and 312 in the CK treatment. Similarly, there were 536 differential metabolites between the CK and rattail fescue treatments, with 314 being upregulated in the rattail fescue treatment and 222 in the CK treatment. There were 588 differential metabolites between the vicia villosa and rattail fescue treatments, with 286 being upregulated in the rattail fescue treatment and 302 in the vicia villosa treatment. The up-regulated and down regulated metabolites in the vicia villosa and CK treatment were higher than those in the rattail fescue and CK treatments, indicating that at the 0–20 cm depth, the roots of the vicia villosa treatment had a higher number of differential metabolites compared to CK. However, there was little difference in the up-regulated and down regulated metabolites between the vicia villosa and rattail fescue treatments.
Fig. 2.
Volcano diagram of root metabolites in citrus fruits treated with different treatments. CK1 VS G1: CK1/G1 comparison group, CK1 VS S1: CK1/S1 comparison group, G1 VS S1: G1/S1 comparison group, CK2 VS G2: CK2/G2 comparison group, CK2 VS S2: CK2/S2 comparison group, G2 VS S2: G2/S2 comparison group. Each point in the volcano plot represents a metabolite, with the horizontal axis representing the fold change of each substance compared to the group (taking the logarithm based on 2), and the vertical axis representing the P-value of the Student T-test (taking the logarithm based on 10). Metabolites with significant upregulation are represented in red, metabolites with significant downregulation are represented in green, and metabolites with no significant differences are represented in gray
In the root system at a depth of 20–40 cm, there were 265 differential metabolites between the CK treatment and the vicia villosa treatment, with 158 upregulated in the vicia villosa treatment and 107 upregulated in the CK treatment. There were 334 differential metabolites between the CK treatment and the rat thatch treatment, with 94 upregulated in the rattail fescue treatment and 240 upregulated in the CK treatment. There were 255 differential metabolites between the vicia villosa and rattail fescue treatments, with 150 upregulated in the rattail fescue treatment and 105 upregulated in the vicia villosa treatment. The number of differential metabolites between the two treatments was the highest in the roots at a depth of 20–40 cm, but they were predominantly downregulated in the rat thatch treatment, while there were few differences in the differential metabolites between the two treatments.
KEGG metabolic pathways of differential metabolites in roots
The Differential Abundance Score (DA Score) is a pathway-based method for analyzing metabolic changes. The DA Score can capture the average and overall variations in all metabolites within a pathway. Analysis of differentially expressed metabolites in roots at varying depths between the cover crop and CK treatments was conducted using the KEGG database (Fig. 3). At depths of 0–20 cm, the plant hormone signal transduction pathway ranked first, followed by the D-amino acid metabolism pathway. Both pathways had identical DA values, with the D-amino acid metabolism pathway containing a greater number of metabolites. In the downregulation, the alpha − Linolenic acid metabolism pathway ranked first, followed by the arginine metabolism pathway. These results indicate significant differences in amino acid metabolism at depths of 0–20 cm across all treatments.
Fig. 3.
Difference abundance scores of different metabolic pathways in citrus root systems under different treatments. A, Cover crops/CK comparison group at 0–20 cm. B, Cover crops/CK comparison group at 20–40 cm. The vertical axis in the figure represents the name of the differential pathway, and the horizontal axis represents the differential abundance score (DA score). DA score represents the overall changes in metabolites in metabolic pathways. A score of 1 indicates an upregulation of the expression trend of metabolites identified in this pathway, while a score of -1 indicates a downregulation of the expression trend of metabolites identified in this pathway. The length of the line segment represents the absolute value of the DA score, the size of the dots at the endpoints of the line segment represents the number of metabolites in the pathway, and the larger the dots, the greater the number of metabolites. The color depth of line segments and dots is proportional to the DA score value. The darker the red color, the more likely the overall expression of the pathway is to be upregulated, while the darker the blue color, the more likely the overall expression of the pathway is to be downregulated
Within the root system at a depth of 20–40 cm, the primary pathway affected by an upregulation in DA values were the cyanoamino acid metabolism, followed by the tryptophan metabolism. This suggests that amino acid metabolism played a significant role in the upregulation pathways within this depth range.
Amino acid differences in root system
Based on the results, the amino acid metabolic pathways in root metabolites across treatments were analyzed, leading to the selection of 24 differentially expressed monomeric amino acids and their derivatives for further investigation. The Fig. 4 revealed that, within the 0–20 cm root system depth, 9 amino acids and their derivatives were upregulated in vicia villosa compared to CK, with L-Phenylalanine exhibiting the highest increase. There was a close proximity between L-Arginine and D-Norvaline, with 15 amino acids being upregulated in CK. Conversely, 15 amino acids were upregulated in rattail fescue compared to CK, with L-Arginine being the most elevated. In the comparison between vicia villosa and rattail fescue, all 24 monomeric amino acids and their derivatives were upregulated in rattail fescue, with L-Arginine being the most elevated and D-Tryptophan being the lowest. When compared to CK treatment, monomeric amino acids and their derivatives that were upregulated in rattail fescue were more pronounced than those in vicia villosa treatment. In all cases, they were predominantly upregulated in rattail fescue, indicating that rattail fescue enhances the metabolism of monomeric amino acids and their derivatives more significantly at 0–20 cm depth than vicia villosa and CK treatments.
Fig. 4.
Column chart of amino acids in citrus root systems under different treatments. CK1 VS G1: CK1/G1 comparison group, CK1 VS S1: CK1/S1 comparison group, G1 VS S1: G1/S1 comparison group, CK2 VS G2: CK2/G2 comparison group, CK2 VS S2: CK2/S2 comparison group, G2 VS S2: G2/S2 comparison group. The vertical axis represents the name of the differential metabolite, and the horizontal axis represents the logarithmic value of the differential metabolite fold (Fold Change). Different colored columns represent different expression patterns, with red representing upregulated expression and green representing downregulated expression
In the root system at a depth of 20–40 cm, 15 amino acids and their derivatives were upregulated in vicia villosa treatment, with DL-glutamic acid being the most elevated, and 9 were upregulated in CK treatment, with THF-L-Glutamate being the most elevated. In the comparison between CK and rattail fescue treatment, 8 amino acids and their derivatives were upregulated in rattail fescue, with L-Arginine being the most elevated, and 16 were upregulated in CK, with S-Adenosylmethionine being the most elevated. In the comparison between vicia villosa and rattail fescue treatments, 5 amino acids and their derivatives were upregulated in rattail fescue treatment, with THF-L-glutamate being the most elevated, while 19 were upregulated in vicia villosa treatment, with Vinylacetylglycine being the most elevated. Vicia villosa treatment had the highest number of upregulated amino acids and their derivatives, indicating that its promotional effect on root amino acids and their derivatives at a depth of 20–40 cm surpassed that of CK and rattail fescue treatments.
The VIP heat map (Fig. 5) was created by combining VIP values with the content of amino acids and their derivatives across each treatment. The Fig. 4 revealed that among the three treatments for roots at depths of 0–20 cm, DL-Proline exhibited the highest VIP value, followed closely by L-Arginine, and O-Phospho-L-Tyrosine had the lowest. Among the top 10 amino acids with VIP values greater than 1, DL-Proline, L-Arginine, THF-L-Glutamate, D-Histidine, N-Jasmonicisoleucine, L-Formylkynurenine, DL-Glutamic acid, L-Phenylalanine, D-Tryptophan, L-Tryptophan, and Proline were all most abundant in the rattail fescue treatment. The last seven amino acids with VIP values were the highest in CK treatment. The rattail fescue treatment upregulated the most amino acids and ranked highly in VIP, indicating that rattail fescue has a more significant role in promoting amino acid metabolism in citrus roots at depths of 0–20 cm.
Fig. 5.
VIP heat map of amino acids in citrus root systems with different treatments. (A) Citrus root amino acids at 0–20 cm. (B) Citrus root amino acids at 20–40 cm. CK1: Clean tillage at 0–20 cm, CK2: Clean tillage at 20–40 cm, G1: Vicia villosa at 0–20 cm, G2: Vicia villosa at 20–40 cm, S1: Rattail fescue at 0–20 cm, S2: Rattail fescue at 20–40 cm. The horizontal axis represents the VIP value calculated by the OPLS-DA model for differential metabolites, and the left vertical axis represents the names of differential metabolites (showing the top 20 differential metabolites with VIP values). The comparison group information is displayed on the right, with red indicating high expression of the metabolite in the corresponding group and green indicating low expression of the metabolite in the corresponding group. A, soil at a depth of 0–20 cm. B, soil at a depth of 20–40 cm
Among the three treatments with roots at a depth of 20–40 cm, L-Arginine exhibited the highest VIP value, particularly in the rattail fescue treatment, while DL-Norvaline had the lowest VIP value, nearly at zero. Of the top 12 amino acids with VIP values greater than 1, L-Arginine and D-Tryptophan were the highest in rattail fescue treatment, while L-Tryptophan was the highest in the control (CK). The other nine amino acids were the lowest in rattail fescue treatment. In the bottom nine amino acids, P-Fluorophenylalanine was the highest in the vicia villosa treatment. The findings suggested that at a depth of 20–40 cm, rattail fescue most significantly promotes L-Arginine and D-Tryptophan, while its contribution to other amino acid metabolism is not pronounced. Besides, vicia villosa treatment does not significantly contribute to amino acid metabolism in roots.
Citrus roots N-metabolizing enzyme activity
The Fig. 6 indicated that at a depth of 0–20 cm, the treatments with vicia villosa and rattail fescue significantly enhanced the enzyme activities of NR, NiR, GS, and NADPH-GDH compared to the control (CK). The rattail fescue treatment was notably more effective than the vicia villosa treatment. Both cover crop treatments significantly increased NADH-GOGAT enzyme activity, yet the difference between them was not significant. The NADH-GDH enzyme activity in the vicia villosa treatment was notably higher than that in CK, while it was notably lower in the rattail fescue treatment. These results suggest that at a depth of 0–20 cm, vicia villosa significantly boosts the activity of six N metabolism enzymes, while rattail fescue treatment notably enhances the activity of five N metabolism enzymes excluding NADH-GDH, thereby significantly bolstering the N uptake capacity of citrus roots and providing more N sources for the fruit.
Fig. 6.
A, Enzyme activity on the citrus root nitrogen metabolism pathway under different treatments. B, N-metabolizing enzyme activity in citrus roots under different treatments. Letters after numbers were generated from Student–Newman–Keuls test and different letters indicated significant differences (p < 0.05). Error bars represent the standard error of the mean (n = 3). CK1: Clean tillage at 0–20 cm, CK2: Clean tillage at 20–40 cm, G1: Vicia villosa at 0–20 cm, G2: Vicia villosa at 20–40 cm, S1: Rattail fescue at 0–20 cm, S2: Rattail fescue at 20–40 cm
At a depth of 20–40 cm, the rattail fescue treatment notably increased NR enzyme activity. There was no significant difference in NiR enzyme activity between CK and vicia villosa or rattail fescue treatments. The GS enzyme activity in both treatments was lower than that in CK. The rattail fescue treatment significantly enhanced the activity of NADH-DODAT enzyme compared to CK, and it was notably higher than that in the vicia villosa treatment. The NADPH-GDH enzyme activities among the three treatments were not significantly different. The NADH-GDH enzyme activity in the vicia villosa treatment was notably higher than that in CK and rattail fescue treatments, with no significant difference observed between CK and rattail fescue treatments. Only NADH-GOGAT enzyme activity exhibited a significant increase in the 20–40 cm root system under cover crop conditions, showing that the cover crops treatment did not notably enhance the N uptake capacity of 20–40 cm citrus roots, thereby significantly bolstering the N uptake capacity of citrus roots and providing more N sources for the fruit.
Citrus root N metabolism related enzymes gene expression
The Fig. 7 indicated that, within the 0–20 cm root system, there was no significant difference in NR expression between the two treatments, yet both were significantly higher than CK. NiR and NADH-GOGAT2 expression levels were similar, with the rattail fescue treatment significantly higher than the others, while there was no significant difference between CK and vicia villosa. GS and GDH2 expression levels were contrary to NR, but GS expression with significant differences observed in all treatments, and no notable difference between vicia villosa and rattail fescue. Vicia villosa’s GS2 expression was the lowest and significantly different from other treatments, while there was no significant difference between CK and rattail fescue. FD-GOGAT expression in CK was significantly higher than in rattail fescue and vicia villosa by more than 10 times, with no significant difference observed between the two grass treatments. In NADP-GDH, vicia villosa was higher than CK but not significantly, with both significantly higher than the rattail fescue treatment. Vicia villosa ‘s GDH1 expression was significantly higher than that of rattail fescue, with both treatments showing a significant increase over CK.
Fig. 7.
Expression of N-metabolizing enzyme genes in citrus roots treated with different treatments. Letters after numbers were generated from Student–Newman–Keuls test and different letters indicated significant differences (p < 0.05). Error bars represent the standard error of the mean (n = 3). CK1: Clean tillage at 0–20 cm, CK2: Clean tillage at 20–40 cm, G1: Vicia villosa at 0–20 cm, G2: Vicia villosa at 20–40 cm, S1: Rattail fescue at 0–20 cm, S2: Rattail fescue at 20–40 cm
In the root system at a depth of 20–40 cm, the expression levels of NR and GS2 followed similar trends. Vicia villosa exhibiting notably the lowest levels and the CK treatment the highest, though this difference was not significant in GS2. The expression of NiR followed an opposite trend to that of NR, with the vicia villosa treatment being the highest but not significantly different from the rattail fescue treatment, yet significantly higher than the CK treatment. In GS, the CK treatment was significantly higher than both the vicia villosa and rattail fescue treatments. In FD-GOGAT, the vicia villosa treatment led with the highest levels, followed by the rattail fescue treatment at the lowest, both with significant differences. In contrast to FD-GOGAT, in NADH-GOGAT2, the rattail fescue treatment stood out as significantly the highest, while the vicia villosa treatment was notably the lowest. The expression levels of GDH1 and GDH2 were similar to those of NiR, with no significant difference between the vicia villosa and rattail fescue treatments but significantly higher than the CK treatment.
Relationships among N-metabolite, enzyme activity, gene expression
A network (Fig. 8) diagram was depicted based on data with a correlation p-value less than 0.05, indicating significant correlation. As depicted, within the 0–20 cm root system, there were 97 lines, with 52 positively and 45 negatively correlated. Similarly, at the 20–40 cm depth, there were 108 lines, with 37 positively and 71 negatively correlated.
Fig. 8.
Network diagram of N metabolism correlation in citrus root systems at different depths under cover crop treatments. A, Citrus root amino acids at 0–20 cm. B, Citrus root amino acids at 20–40 cm. Orange circles represent metabolite, purple circles represent enzymatic activity, and blue circles represent gene expression level. The red line indicates a significant positive correlation, the green line indicates a significant negative correlation (P < 0.05), and the thicker the line, the stronger the correlation
Within the 0–20 cm root system, NIR enzyme activity was significantly positively correlated with NIR gene expression, while NADH-GDH enzyme activity was significantly positive correlated with NADP-GDH and GDH1 gene expression. The GS enzyme activity was significantly negatively correlated with GS gene expression. In both cover crop treatments, DL-Proline content was significantly positively correlated with NR, NIR, GS, NADPH-GDH enzyme activities, and gene expressions of NiR, GS2, and FD-GOGAT2. L-Arginine content was significantly positively correlated with NIR enzyme activity and negatively correlated with NADPH-GDH enzyme activity, as well as gene expressions of GS, FD-GOGAT, GDH1 and GDH2. DL-Glutamic acid content was similarly significantly positively correlated with DL-Proline content and exhibits significant positive correlations with NR, NiR, GS enzyme activities, and gene expressions of NiR, GS2, and FD-GOGAT2.
In the root system at a depth of 20–40 cm, significant positive correlations were observed between NiR enzyme activity and NiR gene expression, GS enzyme activity and GS2 gene expression, NADH-GOGAT enzyme activity and NADH-GOGAT2 gene expression. There was a significant negative correlation between NADH-GOGAT enzyme activity and FD-GOGAT2 gene expression. Concurrently, a significant negative correlation was observed between NADPH-GDH enzyme activity and NADP-GDH gene expression. Additionally, L-Arginine content was significantly positively correlated with NR, NADH-GOGAT, and NADPH-GDH enzyme activities, as well as with NR, FD-GOGAT2 gene expression. Conversely, there was a significant negative correlation between L-Arginine content and NiR, NADH-GDH enzyme activities, and NiR, NADP-GDH gene expression.
Discussion
Two types of covering crops have a promoting effect on amino acid metabolism in citrus roots
Plant roots primarily absorb nitrogen in the form of NO3− and NH4+ from the soil, and the absorbed NO3− and NH4+ are transformed into amino acids in the cytoplasm of roots through a series of enzymatic reactions, subsequently participating in other vital metabolic processes in the plant [46]. In this experiment, within the 0–20 cm depth of the citrus root system, the rattail fescue treatment demonstrated a stronger promotive effect on amino acid metabolism compared to the CK and vicia villosa. However, at depths of 20–40 cm, the promotive effects of the two cover crops treatments on amino acid metabolism were not clearly discernible, with the vicia villosa treatment contributing less significantly. One study found that that grass species planted in orchards would store a portion of the nitrogen in the soil, releasing organic matter and nitrogen for fruit trees to utilize following their own decomposition [47–49]. Rattail fescue, a grass belonging to the Poaceae family, has dense roots that can extend up to 60 cm, with most distributed within the 0–20 cm soil layer [50, 51]. Root exudates have a limited depth of action. Following rattail fescue’s decomposition, it releases nitrogen [52]. Rattail fescue degrades rapidly in highly fertile soil, resulting in a short retention time for nitrogen, which remains high [53]. This experiment did not involve deeply turning the residues of withered and fallen grass, rather, it allowed them to remain on the soil surface for natural degradation. It might take longer for these residues to reach deeper layers of soil following their natural breakdown. At the same time, citrus root systems primarily absorb N and other mineral elements through their fibrous root, with the main roots serving a more stabilizing function. The deeper the soil, the lower the porosity and the fewer the fibrous root. Citrus roots exhibit a higher number of fibrous root at depths of 0–20 cm [54] and exhibit stronger amino acid metabolism. This may account for the greater enhancement in amino acid metabolism observed in the 0–20 cm layer of citrus roots in this experiment, with no discernible pattern at depths of 20–40 cm.
DL-Proline contains equal amounts of D- Proline and L-Proline, and proline is an amino acid found in plants that plays a crucial role in responding to external environmental stresses [55–59]. In plants, proline synthesis primarily occurs through the glutamate and ornithine synthesis pathways, with glutamate and ornithine serving as the precursors [60–63]. Some studies suggest that glutamate is the primary pathway for proline synthesis, with increased activity in this pathway leading to higher proline levels [64, 65]. In our experimental results, both cover crop treatments at depths of 0–20 cm showed higher Dl-Proline levels than the control, along with increased activity in glutamate synthase. The levels and activity were highest in the rat-grass treatment. It was postulated that rattail fescue promoted the Glutamate synthesis pathway more effectively at 0–20 cm, leading to increased proline levels and enhanced nitrogen metabolism. L-Arginine is widely present in plants. Previous studies have linked Arginine to the nitrogen metabolism of fruit trees, and it is the primary form of soluble nitrogen within these plants [66–68]. Arginine is synthesized from glutamate via acetylation, followed by a series of enzymatic reactions to produce arginine [69–74]. In this experiment, both cover crop treatments enhanced Arginine levels in roots at depths of 0–20 cm and 20–40 cm, with significant contributions observed. It is speculated that at the depth of 0–40 cm both cover crop treatments can promote the N metabolism of citrus roots by promoting arginine synthesis, which is the most important way at the depth of 20–40 cm.
The activity of N metabolic enzymes and related gene expression in citrus roots are affected by covering crops
Nitrate reductase (NR) is a key rate-limiting enzyme in the assimilation of nitrogen in plant roots [75, 76], and its activity can be used to assess the strength of nitrogen metabolism [77]. In this study, both cover crop treatments enhanced the activity of NR enzyme and the expression of NR gene in roots at depths of 0–20 cm and 20–40 cm. The rattail fescue treatment showed a more pronounced effect than the vicia villosa treatment, suggesting that cover crop treatments could improve the N metabolism capacity of citrus roots at depths of 0–20 cm and 20–40 cm. This finding aligned with the results of this experiment, which showed that both cover crop treatments improved root amino acid metabolism. Nitrite reductase (NIR) is a key control enzyme in NO3− assimilation [78]. In our research, both cover crop treatments increased the activity of NIR in the 0–20 cm depth of the root system, with the rattail fescue treatment showing a significantly greater effect than the vicia villosa treatment. The trend in NIR gene expression was consistent, but reversed at the 20–40 cm depth, indicating that cover crop treatments did not effectively promote subsequent NO3− assimilation in citrus roots at this depth, which may had contributed to the insufficient N metabolism capacity observed in citrus roots at this depth in this experiment.
Over 95% of the NH4+ in higher plants is assimilated via the GS/GOGAT cycle [79, 80]. Both GS enzyme activity and NR are critical measures of a plant’s ability to absorb and utilize nitrogen [81]. GDH primarily contributes to NH4+ assimilation during the initial phase, serving a supplementary role [14]. In our research findings, at depths of 0–20 cm, the GS, NADH-GOGAT, and NADPH-GDH enzyme activities exhibited similar trends to those of NR and NiR. At depths of 20–40 cm, only NADH-GOGAT activity was comparable to NR. However, there was no discernible trend in gene expression. It is hypothesized that the cover crop treatment at depths of 0–20 cm intensifies the reduction of NO3−, thereby providing plants with an increased number of nitrogen sources for subsequent GS/GOGAT cycles to produce more amino acids. With the reduction in NiR activity during cover crop treatment at depths of 20–40 cm, the number of nitrogens sourced available to the roots decreases, leading to reduced activity in enzymes responsible for subsequent amino acid synthesis.
Two types of covering crops have significant effects on correlation among amino acids, N metabolic enzymes and related genes in citrus roots
In the root systems of higher plants, NO3− is the primary form of N absorbed, with a smaller amount of NH4+ absorbed. However, the absorbed NO3− cannot be directly utilized. It must be reduced to NH4+ by NR and NIR, which is then combined with the absorbed NH4+ to generate glutamate through the GS/GOGAT cycle, subsequently being converted into other amino acids to participate in plant life activities [82]. In the 0–20 cm layer of the root system from this experiment, the contents of DL-Proline and DL-Glutamic acid exhibited significant positive correlations with NR, NiR, and GS enzyme activities, as well as with the expression levels of NiR, GS2, and FD-GOGAT2 genes. This suggested that the cover crop treatment enhanced the activity of N-metabolizing enzymes and the expression of related genes in citrus roots at depths of 0–20 cm, thereby promoting the synthesis of glutamate. DL-Proline was the amino acid with the highest VIP contribution, and L-Arginine was not significantly different from it. Based on the results of this study, it was hypothesized that rattail fescue and vicia villosa increased the content of NO3− and NH4+ in the soil due to the intervention of the two crops coverage. This enhanced the activities of nitrogen metabolism related enzyme genes (whether positively or negatively regulated) in citrus roots, thereby increasing the activities of nitrogen metabolism related enzymes. That promoted the absorption of NO3− and NH4+ in citrus roots, and further converted them into L-Glutamic acid. L-Glutamic acid was primarily converted into DL-Proline and L-Arginine, the main form of nitrogen in fruit trees, through other pathways (not within the scope of this study), and participated in citrus life activities. The enhancement of enzyme activities such as NR, NiR, GS, and NDDH-GOGAT in the 0–20 cm depth of the two crops treatments improved the conversion of NO3− and NH4+ by citrus roots. However, the lack of significant improvement in NiR enzyme activity in the 20–40 cm depth might be one of the reasons for the overall weak nitrogen metabolism capacity and lower amino acid content in the 20–40 cm depth under cover crops treatment.
Conclusions
Rattail fescue and vicia villosa coverage in citrus orchards can improve the activity of enzymes and gene expression related to N metabolism in citrus roots, then promote nitrogen absorption, thus promote nitrogen and amino acid metabolism in ctrus roiots. The effect is more pronounced at the depth of 0–20 cm. In addition, rattail fescue showed superior performance compared to vicia villosa.
Acknowledgements
Not applicable.
Author contributions
First authorship: Hang Li and Zhenghua Jin share first authorship. Conceptualization: Hang Li, Bo Xiong, Xu Wang, Zhihui Wang. Formal analysis: Hang Li, Zhenghua Jin, Tie Wang, Lijun Deng. Funding acquisition: Zhihui Wang. Investigation: Hang Li, Zhenghua Jin, Lundong Tan, Guochao Sun. Methodology: Hang Li, Zhenghua Jin, Tie Wang. Supervision: Bo Xiong, Xu Wang, Ling Liao, Siya He, Zhihui Wang. Writing– original draft: Hang Li, Zhenghua Jin. Writing– review & editing: Hang Li, Bo Xiong, Zhihui Wang.
Funding
This study was financially supported by the National Key R&D Program of China (2021YFD1600802-02) and the Yaan Science and Technology Program, China (23CGZH0004).
Data availability
Data is provided within the manuscript files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hang Li and Zhenghua Jin are joint first authors.
References
- 1.Guo WW, Ye JL, Deng XX. Fruit scientific research in new China in the past 70 years, citrus. J Fruit Sci. 2019;36:1264–72. 10.13925j.cnki.gsxb.Z02. [Google Scholar]
- 2.Tang YN. Rhizosphere microecological mechanism of selenium enhancing phosphorus [Wuhan]ptake in citrus. [Wuhan]. Huazhong Agricultural University; 2023.
- 3.Gobert A, Plassard C. Kinetics of no3(-) net fluxes in Pinus Pinaster, rhizopogon Roseolus and their ectomycorrhizal association, as affected by the presence of no3(-) and nh4+. Plant Cell Environ. 2007;30:1309–19. 10.1111/j.1365-3040.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 4.Kraiser T, Gras DE, Gutierrez AG, Gonzalez B, Gutierrez RA. A holistic view of nitrogen acquisition in plants. J Exp Bot. 2011;62:1455–66. 10.1093/jxb/erq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bascuñán-Godoy L, Sanhueza C, Hernández CE, Cifuentes L, Pinto K, Álvarez R, et al. Nitrogen supply affects photosynthesis and photoprotective attributes during drought-induced senescence in quinoa(article). Front Plant Sci. 2018;9:994. 10.3389/fpls.2018.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Liu L, Sun H, Zhang Y, Zhu J, Zhang K, et al. Physiological and comparative transcriptomic analysis provide insight into cotton (gossypium hirsutum l.) root senescence in response. Front Plant Sci. 2021;12:748715. 10.3389/fpls.2021.748715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good AG, Shrawat AK, Muench DG. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004;9:597–605. 10.1016/j.tplants.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Liao L. Study on reasonable [Chengdu]itrogen [Chengdu]utrition requirement and regulation mechanism of carbon and [Chengdu]itrogen metabolism in Huangguogan. [Chengdu]. Sichuan Agricultural University; 2019.
- 9.Zhou X, Wang A, Hobbie EA, Zhu F, Qu Y, Dai L, et al. Mature conifers assimilate nitrate as efficiently as ammonium from soils in four forest plantations. New Phytol. 2021;229:3184–94. 10.1111/nph.17110. [DOI] [PubMed] [Google Scholar]
- 10.Xu GH, Fan XR, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63:153–82. [DOI] [PubMed] [Google Scholar]
- 11.Horchani F, Bouallegue A, Mabrouk L, Namsi A, Abbes Z. Nitrate reductase regulation in wheat seedlings by exogenous nitrate: A possible role in tolerance to salt stress. J Plant Nutr Soil Sci. 2023;186:1. 10.1002/jpln.202300101. [Google Scholar]
- 12.Lee S, Choi JH, Truong HA, Lee YJ, Lee H. Enhanced nitrate reductase activity offers Arabidopsis ecotype Landsberg erecta better salt stress resistance than col-0. Plant Biol. 2022;24:1. 10.1111/plb.13420. [DOI] [PubMed] [Google Scholar]
- 13.Chaukiyal SP, Khatri N, Kannojia P, Bhatia P. In-vivo nitrate reductase activity in the Myrica esculenta buch. Ham. D.Don seedlings under nursery conditions. Octa J Environ Res. 2015;3:185–95. [Google Scholar]
- 14.Miflin BJ, Habash DZ. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot. 2002;53:979–87. 10.1093/jexbot/53.370.979. [DOI] [PubMed] [Google Scholar]
- 15.Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nat Rev Microbiol. 2005;435:819–23. 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 16.Nunes-Nesi A, Fernie AR, Stitt M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant. 2010;3:973–96. [DOI] [PubMed] [Google Scholar]
- 17.Shao Q, Shu S, Du J, Xing W, Guo S, Sun J. Effects of Nacl stress on nitrogen metabolism of cucumber seedlings. Russ J Plant Physiol. 2015;62:595–603. 10.1134/s1021443715050155. [Google Scholar]
- 18.Fontaine JX, Tercé-Laforgue T, Armengaud P, Clément G, Renou JP, Pelletier S, et al. Characterization of a nadh-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism(article). Plant Cell. 2012;24:4044–65. 10.1105/tpc.112.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins M, Sousa B, Lopes J, Soares C, Machado J, Carvalho S, et al. Diclofenac shifts the role of root glutamine synthetase and glutamate dehydrogenase for maintaining nitrogen assimilation and proline production at the expense of shoot carbon reserves in solanum Lycopersicum L. Environ Sci Pollut Res. 2020;27:29130–42. 10.1007/s11356-020-09136-x. [DOI] [PubMed] [Google Scholar]
- 20.Li ZS, Zhang CF, Lin QH, Peng J, Li CJ, He GC, et al. Effect of exogenous ammonium on glutamine synthetase, glutamate synthase, and glutamate dehydrogenase in the root of rice seedling. Wuhan Univ J Nat Sci. 1999;4:358–62. 10.1007/bf02842373. [Google Scholar]
- 21.Krapp A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr Opin Plant Biol. 2015;25:115–22. 10.1016/j.pbi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Kwinta J, Bartoszewicz K, Bielawski W. Purification and characteristics of glutamate dehydrogenase (gdh) from triticale roots. Acta Physiol Plant. 2001;23:399–405. 10.1007/s11738-001-0049-2. [Google Scholar]
- 23.Han Z, Lu Y, Zhao Y, Wang Y, Han Z, Han Y, et al. Analysis of relative expression of key enzyme genes and enzyme activity in nitrogen metabolic pathway of two genotypes of potato (solanum tuberosum l.) under different nitrogen supply levels. Horticulturae. 2022;8:769. 10.3390/horticulturae8090769. [Google Scholar]
- 24.Yu XM, Li JF, Zhu LN, Wang B, Wang L, Bai Y, et al. Effects of root restriction on nitrogen and gene expression levels in nitrogen metabolism in Jumeigui grapevines (vitis viniferal.*vitis labruscal). Agricultural Sci China. 2015;14:67–79. [Google Scholar]
- 25.Zhao L, Cai B, Zhang X, Zhang B, Feng J, Zhou D, et al. Physiological and transcriptional characteristics of banana seedlings in response to nitrogen deficiency stress. Horticulturae. 2024;10:290. 10.3390/horticulturae10030290. [Google Scholar]
- 26.Xu XX, Liu GY, Liu JQ, Lyu MX, Wang F, Xing Y, et al. Potassium alleviated high nitrogen-induced Apple growth Inhibition by regulating photosynthetic nitrogen allocation and enhancing nitrogen utilization capacity. Hortic Plant J. 2024;10:1–14. 10.1016/j.hpj.2023.04.003. [Google Scholar]
- 27.Kou JC, Yang WQ, Han MY, Chen A, Li B, Zhang W. Research progress on interplanting grass in orchard in China. Pratacultural Sci. 2010;27:154–9. [Google Scholar]
- 28.Gómez JA, Sobrinho TA, Giráldez JV, Fereres E. Soil management effects on runoff, erosion and soil properties in an Olive grove of Southern Spain. Soil Tillage Res. 2009;102:5–13. 10.1016/j.still.2008.05.005. [Google Scholar]
- 29.Montanaro G, Celano G, Dichio B, Xiloyannis C. Effects of soil-protecting agricultural practices on soil organic carbon and productivity in fruit tree orchards. Land Degrad Dev. 2010;21:132–8. 10.1002/ldr.917. [Google Scholar]
- 30.Wooldridge J, Fourie J, Joubert ME. Effects of soil surface management practices on soil and tree parameters in a ‘cripps pink’/m7 Apple orchard 2. Tree performance and root distribution. South Afr J Plant Soil. 2013;30:171–7. 10.1080/02571862.2013.848948. [Google Scholar]
- 31.Wooldridge J, Fourie J, Joubert ME. Effects of soil surface management practices on soil and tree parameters in a ‘cripps pink’/m7 Apple orchard 1. Mineral Nutr South Afr J Plant Soil. 2013;30:163–70. 10.1080/02571862.2013.854416. [Google Scholar]
- 32.Rodrigues MA, Correia CM, Claro AM, Ferreira IQ, Barbosa JC, Moutinho-Pereira JM, et al. Soil nitrogen availability in Olive orchards after mulching legume cover crop residues. Sci Hort. 2013;158:45–51. 10.1016/j.scienta.2013.04.035. [Google Scholar]
- 33.Hogue EJ, Cline JA, Neilsen G, Neilsen D. Growth and yield responses to mulches and cover crops under low potassium conditions in drip-irrigated Apple orchards on coarse soils. HortScienc. 2010;45:1866–71. 10.21273/hortsci.45.12.1866. [Google Scholar]
- 34.Wells ML. Pecan tree productivity, fruit quality, and nutrient element status using clover and poultry litter as alternative nitrogen fertilizer sources. HortScience. 2012;47:927–31. 10.21273/hortsci.47.7.927. [Google Scholar]
- 35.Tworkoski TJ, Glenn DM. Weed suppression by grasses for orchard floor management. Weed Technol. 2012;26:559–65. 10.1614/wt-d-11-00044.1. [Google Scholar]
- 36.Rodrigues M, Dimande P, Pereira E, Ferreira I, Freitas S, Correia C, et al. Early-maturing annual legumes: an option for cover cropping in rainfed Olive orchards. Nutr Cycl Agrosyst. 2015;103:153–66. 10.1007/s10705-015-9730-5. [Google Scholar]
- 37.Ripoche A, Metay A, Celette F, Gary C. Changing the soil surface management in vineyards: immediate and delayed effects on the growth and yield of grapevine. Plant Soil. 2011;339:259–71. 10.1007/s11104-010-0573-1. [Google Scholar]
- 38.Gouthua S, Skinkisa PA, Morreb J, Maierb CS, Deluca LG. Berry nitrogen status altered by cover cropping: effects on berry hormone dynamics, growth and amino acid composition of Pinot noir(article). Food Chem. 2012;135:1–8. 10.1016/j.foodchem.2012.04.019. [Google Scholar]
- 39.Mcgourty GT, Reganold JP. Managing vineyard soil organic matter with cover crops. David R Smart. 2005;145–51.
- 40.Olmstead MA. Cover crops and a floor management strategy for Pacific Northwest vineyards. [Pullman]: Washington State University; 2006. [Google Scholar]
- 41.Faria CMB, Soares JM, LeÃO OPCS. Adubação Verde com Leguminosas Em Videira no submédio São Francisco. Revista Brasileira De Ciência Do Solo. 2004;28:641–8. [Google Scholar]
- 42.Fourie JC, Louw PJE, Agenbag GA. Cover crop management in a Chardonnay/99 Richter vineyard in the coastal wine grape region, South Africa. Part 1: effect of two management practices on selected grass and broadleaf species. S Afr J Enol Vitic. 2006;27:178–86. [Google Scholar]
- 43.Sanchez JE, Wilson TC, Kizilkaya K, Parker E, Harwood RR. Enhancing the mineralizable nitrogen pool through substrate diversity in long term cropping systems. Soil Sci Soc Am J. 2001;65:1442–1442. 10.2136/sssaj2001.6551442x. [Google Scholar]
- 44.Wang X, Li Y, Lai YH, Hou YH, Li H, Ma X, et al. The effect of grass mulching on soil properties and microbial community in citrus orchard. Prataculture Anim Husb. 2021;0:61–5. 10.3969/j.issn.2096-3971.2021.02.011. [Google Scholar]
- 45.Li H, Wang X, Li Y, Hou Y, Zhao Z, Meng L, et al. Cover crops control weed and improve soil qualities in citrus orchard. J Soil Sci Plant Nutr. 2023;23:6827–37. 10.1007/s42729-023-01545-4. [Google Scholar]
- 46.Li C, Tang Z, Wei J, Qu H, Xie Y, Xu G. The osamt1.1 gene functions in ammonium uptake and ammonium-potassium homeostasis over low and high ammonium concentration ranges. J Genet Genomics. 2016;43:639–49. [DOI] [PubMed] [Google Scholar]
- 47.Steenwerth K, Belina KM. Cover crops and cultivation: impacts on soil N dynamics and Microbiological function in a mediterranean vineyard agroecosystem. Appl Soil Ecol. 2008;40:370–80. 10.1016/j.apsoil.2008.06.004. [Google Scholar]
- 48.McCracken DV, Smith MS, Grove JH, MacKown CT, Blevins RL. Nitrate leaching as influenced by cover cropping and nitrogen source. Soil Sci Soc Am J. 1994;58:1476–83. 10.2136/sssaj1994.03615995005800050029x. [Google Scholar]
- 49.Drinkwater LE, Snapp SS. Nutrients in agroecosystems: rethinking the management paradigm(review). Adv Agron. 2007;92:163–86. 10.1016/s0065-2113(04)92003-2. [Google Scholar]
- 50.Zhang XY, Zou GY, Li ZR, Sun J. Ecological planting techniques for orchard mouse grass. Place: Published: China Agricultural Publishing House; 2020. [Google Scholar]
- 51.Ding YF. Effects of different inter-cropping modes on camellia Oleifera growth and woodland ecology. [Hefei]: Anhui Agricultural University; 2018. [Google Scholar]
- 52.Yang HX, Zhou MH, Li JL, Liang B, Sui FG. Decay and nutrient release in vulpia myuros grasses, a species suitable for soil conservation in temperate zone orchards. Acta Prataculturae Sinica. 2015;24:208–13. 10.11686/cyxb20150424. [Google Scholar]
- 53.Liang B, Yang X, He X, Murphy D, Zhou J. Long-term combined application of manure and Npk fertilizers influenced nitrogen retention and stabilization of organic C in loess soil. Plant Soil. 2012;353:249–60. 10.1007/s11104-011-1028-z. [Google Scholar]
- 54.Chen JZ. Various discussions on fruit tree cultivation: Southern edition. Beijing: China Agricultural Publishing House; 2011. [Google Scholar]
- 55.Ozturk M, Turkyilmaz B, Gucel S, Guvensen A. Proline accumulation in some coastal zone plants of the Aegean region of Turkey. Eur J Plant Sci Biotechnol. 2011;5:54–6. 10.1016/0370-2693(78)90111-9. [Google Scholar]
- 56.Unal B, Guvensen A, Dereboylu A, Ozturk M. Variations in the proline and total protein contents in Origanum sipyleum L. From different altitudes of spil mountain, Turkey. Pak J Bot. 2013;45:571–6. [Google Scholar]
- 57.Kavi Kishor PB, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37:300–11. 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- 58.Abdalla MM, El-Khoshiban NH. The influence of water stress on growth, relative water content, photosynthetic pigments, some metabolic and hormonal contents of two triticium aestivum cultivars. J Appl Sci Res. 2007;3:2062–74. [Google Scholar]
- 59.Kishor P, Kumari P, Sunita M, Sreenivasulu N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front Plant Sci. 2015;6:544. 10.3389/fpls.2015.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munir Ozturk BT, Unal P, García-Caparrós A, Khursheed A, Gul, Hasanuzzaman M. Osmoregulation and its actions during the drought stress in plants physiologia plantarum. 2021;172:1321–35. 10.1111/ppl.13297 [DOI] [PubMed]
- 61.Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants– a review plant. Soil Environ. 2008;54:89–99. 10.17221/2774-pse. [Google Scholar]
- 62.Koenigshofer H, Loeppert HG. The up-regulation of proline synthesis in the meristematic tissues of wheat seedlings upon short-term exposure to osmotic stress. J Plant Physiol. 2019;237:21–9. 10.1016/j.jplph.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad P, Hameed A, Abd-Allah EF, Sheikh SA, Wani MR, Rasool S et al. Biochemical and molecular approaches for drought tolerance in plants (book chapter). Physiological mechanisms and adaptation strategies in plants under changing environment. 2013;2:1–29. 10.1007/978-1-4614-8600-8_1
- 64.Parida AK, Dagaonkar VS, Phalak MS, Aurangabadkar LP. Differential responses of the enzymes involved in proline biosynthesis and degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery. Acta Physiol Plant. 2009;30:619–27. 10.1007/s11738-008-0157-3. [Google Scholar]
- 65.Rai AN, Penna S. Molecular evolution of plant p5cs gene involved in proline biosynthesis(article). Mol Biol Rep. 2013;40:6429–35. 10.1007/s11033-013-2757-2. [DOI] [PubMed] [Google Scholar]
- 66.Tromp J. Storage and mobilization of nitrogenous compounds in Apple trees with special reference to arginine. Plant Physiol. 1969;143–59.
- 67.Titus JS, Kang SM. Nitrogen metabolism, translocation, and recycling in Apple trees horticultural reviews, 4. 1982:204–46.
- 68.Canton FR, Suarez MF, Canovas FM. Molecular aspects of nitrogen mobilization and recycling in trees photosynthesis research. 2005;83:265–78. 10.1007/s11120-004-9366-9 [DOI] [PubMed]
- 69.Guak S, Neilsen D. Determining the role of N remobilization for growth of Apple (malus domestica borkh.) trees by measuring xylem-sap N flux. J Exp Bot. 2003;54:2121–32. 10.1093/jxb/erg228. [DOI] [PubMed] [Google Scholar]
- 70.Malaguti D, Millard P, Wendler R, Hepburn A, Tagliavini M. Regulation of growth, development and whole organism physiology. Translocation of amino acids in the xylem of Apple (malus domestica borkh.) trees in spring as a consequence of both N remobilization and root uptake. J Exp Bot. 2001;52:1665. 10.1093/jexbot/52.361.1665. [PubMed] [Google Scholar]
- 71.Slocum RD. Genes, enzymes and regulation of arginine biosynthesis in plants. Plant Physiol Biochem. 2005;43:729–45. 10.1016/j.plaphy.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Winter G, Todd C, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534. 10.3389/fpls.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbano-Gámez JA, Jorge El-Azaz CÁ, Torre FNDL, Cánovas FM. Enzymes involved in the biosynthesis of arginine from ornithine in maritime pine (pinus pinaster ait). Plants. 2020;9:E1271. 10.3390/plants9101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Locke M, Ghazaly E, Freitas MO, Mitsinga M, Lattanzio L, Nigro CL, et al. Inhibition of the polyamine synthesis pathway is synthetically lethal with loss of argininosuccinate synthase 1. Cell Rep. 2016;16:1604–13. 10.1016/j.celrep.2016.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aitlessov K, Zhumabekova B, Sagyndykov U, Tuyakbayeva A, Bitkeyeva A, Bazarbaeva KZ, et al. Foliar fertilization with molybdate and nitrate up-regulated activity of nitrate reductase in lemon balm leaves. Horticulturae. 2023;9:1325. 10.3390/horticulturae9121325. [Google Scholar]
- 76.Ye LT, Lu HJ, Song WJ, Tu ED, Shen QR, Zhang YL. Variation of activity of N metabolizing enzymes in rice plants with different N use efficiency at late growth stages. Turang Xuebao. 2011;48:132–40. [Google Scholar]
- 77.Sun MH, Lu XP, Cao XJ, Li J, Xiong J, Xie SX. Effect of different forms of nitrogen on the activity of nitrate reductase and expression of the relative genes in citrus sinensis ×poncirus trifoliate. J Fruit Trees. 2017;410–7. 10.13925/j.cnki.gsxb.20160314.
- 78.Takahashi M, Sasaki Y, Ida S, Morikawa H. Nitrite reductase gene enrichment improves assimilation of no(2) in Arabidopsis. Plant Physiol. 2001;126:731–41. 10.2307/4279934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lea P, Robinson S, Stewart G. The enzymology and metabolism of glutamine, glutamate, and asparagine. Biochem Plants. 1990;16. 10.1016/B978-0-08-092616-2.50010-3.
- 80.Hirel B, Bertin P, Quiller I, eacute, Bourdoncle W, Attagnant C, et al. Towards a better Understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001;125:1258–70. 10.1104/pp.125.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koranda M, Schnecker J, Kaiser C, Fuchslueger L, Kitzler B, Stange BF, et al. Microbial processes and community composition in the rhizosphere of European beech– the influence of plant C exudates. Soil Biol Biochem. 2011;43:551–8. 10.1016/j.soilbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tian J. The expression of [Yangling]itrogen uptake [Yangling]nd metabolism related [Yangling]enes [Yangling]n sophora Japonica L. And [Yangling]ts relationship with the [Yangling]ging of [Yangling]ncient trees. [Yangling]. North West Agriculture and Forestry University; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript files.









