Abstract
Increased levels of extracellular L-glutamate have been suggested to play a role in retinal damage in a number of blinding diseases such as glaucoma and diabetic retinopathy. Although glutamate can cause retinal damage, in part, by hyper-stimulating its receptors (“excitotoxicity”), the down-stream events that lead to retinal damage are poorly understood. In this study, we injected kainic acid (KA), a glutamate receptor agonist that specifically hyper-stimulates non-NMDA-type receptors, into the vitreous humor of CD-1 mice and have investigated the role of plasminogen activators (PAs) [tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA)] in excitotoxicity-induced retinal damage. Injection of KA into the vitreous humor led to an up-regulation in tPA and an induction in uPA activity in the retina and this was associated with activation of zymogen plasminogen to active plasmin. Immunocytochemical analysis indicated that retinal ganglion cells (RGCs), constitutively, express tPA and release it into the extracellular space upon KA injection. Immunocytochemical analysis also indicated an increase in uPA in the nerve fiber layer after KA injection which was absent in the control retinas. These events were associated with apoptotic death of cells initially in the ganglion cell layer and subsequently in the inner and outer nuclear layer, associated with loss of both RGCs and amacrine cells. These phenomena were inhibited when recombinant plasminogen activator inhibitor (rPAI-1) or tPA-STOP were injected into the vitreous humor with KA, whereas a plasmin inhibitor, alpha-2-antiplasmin, failed to attenuate KA-induced retinal damage. Taken together, these results suggest that inhibition of plasminogen activators might attenuate retinal damage in blinding retinal diseases in which hyper-stimulation of glutamate receptors is implicated as a causative factor to retinal damage.
Keywords: Retina, ganglion cells, excitotoxicity, kainic acid, tissue plasminogen activator, urokinase plasminogen activator, and non-NMDA receptors
Abbreviations: tPA: tissue plasminogen activator; uPA: urokinase plasminogen activator; PAI-1: plasminogen activator inhibitor-1; GCL: ganglion cell layer; RGC: retinal ganglion cells; CNS: central nervous system; KA, kainic acid, NMDA, N-methyl-D-aspartate
Introduction
Retinal damage is a major concern in a number of blinding retinal diseases including diabetic retinopathy and glaucoma (1–5). Although the mechanisms that underlie retinal damage in these diseases are poorly understood, previous studies have suggested that excitatory amino acid (EAA) such as L-glutamate might play a role (6–11). Accumulation of glutamate into the extracellular space activates two major classes of receptors, the ionotropic (N-methyl-D-aspartate [NMDA], alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA]/kainic acid [KA]-type) and the G-protein-coupled metabotropic receptors (12–14). A number of cells including ganglion cells and amacrine cells in the retina express these receptors (15, 16). Although activation of these receptors constitutes a physiological phenomenon, hyper-stimulation of NMDA and non-NMDA type ionotropic receptors can lead to cell death. Indeed, a number of previous studies have reported that glutamate and its receptor agonists such as NMDA and KA hyper-stimulate glutamate receptors (“excitotoxicity”) and contribute to retinal damage (10, 17–25). In addition, elevated levels of glutamate and glutamate-mediated excitotoxicity have been implicated in ganglion cell loss in animals models for retinal ischemia (7, 26, 27), axonal injury (28), and glaucoma (6, 29). Furthermore, previous studies have also reported the presence of elevated levels of glutamate in human glaucoma (6). In spite of these observations, the role of glutamate in glaucoma is controversial, the mechanisms that underlie glutamate-mediated excitotoxic-retinal damage are poorly understood and remain open to debate.
Due to the lack of a clear understanding of the mechanisms involved in excitotoxic-retinal damage, it is worth to investigate and possibly draw some parallels with other neurodegenerative diseases in which the role of excitotoxicity has been investigated and continue to be investigated. In this regard, recent investigations into the mechanisms of excitotoxicity in the central nervous system (CNS) have identified tissue plasminogen activator (tPA) as one of the contributing factors to neuronal damage (30–32). Plasminogen activators (PAs), uPA and tPA are serine proteases that convert plasminogen, a physiological zymogen, into active plasmin, a trypsin-like endopeptidase of broad substrate specificity (33). Endogenous plasminogen activator inhibitors (PAIs) control plasminogen activation by regulating the activity of plasminogen activators. In the central nervous system (CNS) tPA plays a role in neuronal plasticity (34, 35) and neuronal regeneration (36–38), whereas uPA plays a role in astrocyte proliferation and tissue remodeling (39, 40). A number of previous studies have demonstrated the presence of tPA and uPA in various eye tissues including, retinal neovascular membranes (41, 42), and in normal (43–46) and diabetic retinas (47). In a clinical setting, tPA has been used to treat stroke patients (48, 49), as well as patients with submacular hemorrhage (50–54). Although the functional roles of these proteases in the retina are unclear, mounting evidence indicates that tPA and uPA might play a degenerative role in the CNS (30–32, 55–60) and retina (42, 61–64).
Previous studies from our laboratory have suggested that hyper-stimulation of non-NMDA receptors (excitotoxicity) in the retina by kainic acid leads to an up-regulation of extracellular modulating proteases such as matrix metalloproteinase-9 (MMP-9) and this in turn plays a role in retinal damage (65). However, inhibition of MMP activity by a synthetic inhibitor failed to offer complete protection against KA-induced cell loss indicating the possible role of other proteases such as plasminogen activators uPA and tPA in retinal damage. Therefore, we injected kainic acid (KA) into the vitreous humor of normal CD-1 mice (to hyper-stimulate non-NMDA-type glutamate receptors in the retina) and investigated the role of tPA and uPA in retinal damage. We provide some novel findings that hyper-stimulation of non-NMDA-type receptors leads to an increase in both tPA and uPA activity and protein levels in the retina. In addition, we show that the degenerative events associated with hyper-stimulation of non-NMDA type glutamate receptors can be attenuated by injection of rPAI-1 or tPA-STOP into the vitreous humor along with KA. These results for the first time, to our knowledge, indicate that up-regulation of plasminogen activators (both tPA and uPA) play a causative role in retinal damage mediated by hyper-stimulation of glutamate receptors.
Methods
Intravitreal injections
All the experiments on animals were performed under general anesthesia according to institutional protocol guidelines and the Association for Research in Vision & Ophthalmology (ARVO) statement for the use of Animals in Ophthalmology and Vision Research. Normal adult CD-1 mice (6–8 weeks old; Charles River Breeding Labs, Wilmington, MA) were anesthetized by an intraperitoneal injection of 1.25% avertin (2,2,2-tribromoethanol in tert-amyl alcohol; 0.017 ml/g body weight). Kainic acid was injected in to the vitreous humor as previously described (65). Unless otherwise indicated, intravitreal injections were performed in a final volume of 2 ul. For control experiments, eyes (n=6 retinas each; 3 independent experiments) were injected with 2 ul of 0.1M phosphate buffered saline (PBS, pH 7.4) alone and for treatment groups, eyes (n=6 retinas each; 3 independent experiments) were injected with 2 ul of 5, 10, and 20 mM (corresponding to 10, 20, and 40 nmoles) kainic acid (Sigma, St. Louis, MO) prepared in PBS. For time course studies, 20 nmoles KA was injected into the vitreous humor and proteins were extracted from 6 retinas each at each time point (4 independent experiments). In separate experiments (n=6 retinas each; 3 independent experiments), 2 ul of 10 mM KA (corresponding to 20 nmoles) plus rPAI-1 (corresponding to 0.25 and 5 ug; American Diagnostica, Stamford, CT) were injected into the vitreous humor. In separate experiments (n=6 retinas each; 3 independent experiments), 1 ul of 20 mM KA (corresponding to 20 nmoles) plus 1 ul of a synthetic tPA inhibitor, tPA-STOP (2, 7-bis-(4-amidonobenzylidene)-cycloheptanone-1-one dihydrochloride salt (corresponding to 1 and 4 uM; American Diagnositica, Stamford, CT) or 1 ul of KA (20 nmoles) plus 1 ul of plasmin inhibitor, alpha-2-antiplasmin (corresponding to 4 ug; ICN Biochemicals, Aurora, OH) were injected into the vitreous humor.
Protein extraction
Following 6 h, 12 h, 1 day and 2 days after intravitreal injection, animals were anesthetized with an overdose of avertin, and their eyes were enucleated. Retinas were carefully removed and washed three times with phosphate buffered saline (pH 7.4) to remove any vitreous humor that may have adhered to the retina. Three to four retinas each were placed in eppendorff tubes containing 40 ul of extraction buffer (1% nonidet-P40, 20 mM Tris-HCl, 150 mM NaCl, 1 mM Na3VO4, pH 7.4) and the tissues were homogenized. Tissue homogenates were centrifuged at 10,000 rpm for 5 min at 4°C and the supernatants were collected. Protein concentration in supernatants was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA).
Zymography assays
Activities of plasminogen activators (both tPA and uPA) and plasmin were determined by substrate zymography according to methods described previously (66). Briefly, retinal extracts containing equal amounts of protein (25 ug) were mixed with SDS gel-loading buffer and loaded without reduction or heating onto 10% SDS polyacrylamide gels containing fibrinogen (5.5 mg/ml) and plasminogen (50 ug/ml) to determine the activity of plasminogen activators (both tPA and uPA), and gels containing 0.2% beta-casein to determine the activity of plasmin. After electrophoresis, the gels were washed three times with 2.5% Triton-X 100 (15 min each time), and placed in 0.1M glycine-buffer (pH 8.0; for detection of tPA and uPA) or 0.1M Tris-buffer (pH 8.0; for detection of plasmin) and incubated overnight at 37 C to allow proteolysis of the substrates in the gels. The gels were stained with 0.1% Coomassie Brilliant Blue-R250 and then de-stained with a solution containing 25% methanol and 10% acetic acid. Samples containing standard recombinant tPA or plasmin were co-electrophoresed for comparison. tPA activity in zymograms was confirmed by incubating the gels with rPAI-1 or tPA-STOP (data not shown) and by western blot analysis (as described below). A reduced molecular weight size standard was also included on all gels (data not shown; Life Technologies, Gaithersburg, MD). The area cleared by tPA and uPA in the zymograms was scanned by a flat-bed scanner, relative protease activity level were determined using image analysis software (Scion Corporation, Frederick, MD), and the results from four independent experiments were represented as mean arbitrary denistometric units +/− SEM. Statistical significance was analyzed by using a non-parametric Newman-Keuls analog procedure (GB-Stat Software, Dynamic Microsystems, Silver Spring, MD) and expressed as the mean +/− SEM.
Immunohistochemistry
Eyes enucleated after KA injection were fixed with 4% paraformaldehyde for 1 h at room temperature and embedded in OCT compound (Sakura Finetek USA, Torrance, CA). Traverse, 10 micron-thick cryostat sections were cut and placed onto super-frost plus slides (Fisher Scientific, Pittsburgh, PA). Sections were incubated with antibodies against uPA (1:100 dilution in PBS, Innovative Research, MI), calretinin (amacrine cell marker, 1:100 dilution in PBS, Chemicon, CA), and neurofilament-light (ganglion cell marker, 1:100 dilution in PBS, SantaCruz, CA) to determine the tissue localization of these proteins. Sections were washed three times (15 min each) with Tris-HCl buffer (pH 7.4) and incubated with appropriate Alexa FluorR-568-conjugated secondary antibodies (1:200 dilution in PBS for NF-L and calretinin; Molecular Probes, Eugene, OR) or Alexa FluorR-486-conjugated secondary antibodies (1:200 dilution in PBS for uPA) for 1 h at room temperature. Sections were washed again with Tris-HCl buffer (three times, 15 min each) and mounted with a cover slip. Sections were observed under a Nikon bright field microscope equipped with epifluorescence, and digitized images were obtained using a SPOT digital camera. Images were converted into gray scale images using Adobe Photoshop Software, versions 5.5 and 7.0 (Adobe system Inc., Mountain View, CA).
Immuno-gold labeling of tPA
For tPA immuno-gold labeling (Figure 2), 10-micron thick cryostat sections (prepared as described above) were incubated with a solution containing 10% bovine serum albumin (in phosphate buffered saline, pH 7.4) and 0.3% Triton X-100 for 1 h at room temperature. Sections were washed three times with PBS (15 min each) and incubated overnight with polyclonal antibodies against tPA (1:100 dilution; Innovative Research, Southfield, MI; antibody was diluted in a solution containing 1% BSA and 0.3% Triton X-100 in PBS). The next morning, sections were washed three times with PBS (30 min each) and incubated with goat anti-rabbit IgG conjugated to 5 nm gold particles (1:100 dilution; Accurate Chemical & Scientific Corporation, Westbury, NY; in 1% BSA containing 0.3% Tritin-X-100) for 1 h at room temperature. Sections were washed four times (20 min each) with PBS and four times (20 min each) with distilled water and Silver enhancement was performed on the sections according to manufacturer’s instructions (Silver Enhancement Kit-Light Microscopy, Accurate Chemical & Scientific Corporation, Westbury, NY). Finally, sections were mounted using standard aqueous mounting solution and observed under bright field microscope. Digitized images were obtained using a SpotR digital camera attached to the microscope and compiled them using Photoshop software (Adobe System Incorporated, CA).
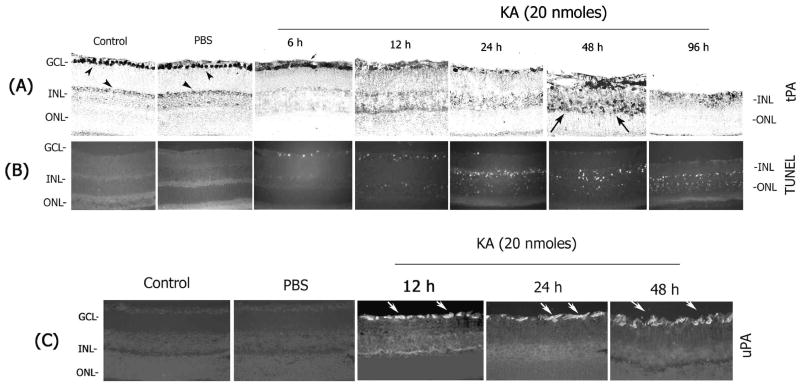
Figure 2. Increase in tPA and uPA associates with apoptotic cell death in the retina.
Retinal cross sections prepared from un-injected control or PBS- or KA-injected (20 nmoles) eyes were subjected to immunohistochemistry using antibodies against tPA (A) and uPA (C). Apoptotic cell death in the retinal cross sections was determined by using an In situ cell death detection kit (TUNEL) (B). tPA immunoreactivity was constitutively observed in the ganglion cells (arrowheads) and to some extent in the inner nuclear layer (arrowheads). At 6h after KA injection, tPA was found in the extracellular space (small arrow). tPA remaining in the retina was localized in extracellular space in the ganglion cell layer and in the inner plexiform layer in a diffusive pattern one day after KA injection. Two days after KA treatment tPA was detected in cells that are scattered throughout the inner retina (long arrows). (C) uPA was absent in un-injected control or PBS-injected retinas but it was up-regulated in the nerve fiber layer after KA injection (arrows). An up-regulation in uPA and tPA was associated with TUNEL-positive cells initially in the ganglion cell layer and subsequently in the inner and outer nuclear layers (B). All the images were taken at 40× magnification.
Apoptosis assay
Apoptotic cell death in retinal cross sections was determined using a commercially available kit. Briefly, 10 micron-thick cryostat sections (n=8–10 sections for each treatment from 4 independent experiments) prepared as described above and apoptotic cell death was detected by TdT-mediated dUTP nick-end labeling (TUNEL) assay, using an In situ cell death detection kit with fluorescein (Roche Biochemicals, Mannheim, Germany) and the protocol provided by the manufacturer. Tissue sections were examined using a Nikon microscope equipped with epifluorescence, and digital images were obtained with a SPOT digital camera and images were compiled using Adobe Photoshop Software, versions 5.5 and 7.0 (Adobe System Incorporated, CA). The remaining number of TUNEL-positive cells in retinal cross sections was quantitated using image analysis software (Scion Corporation, Frederick, MD) and the results were represented as total number of TUNEL-positive cells, mean+/− SEM. Statistical significance was analyzed by using a non-parametric Newman-Keuls analog procedure (GB-Stat Software, Dynamic Microsystems, Silver Spring, MD).
Westernblot analysis
Aliquots containing an equal amount of protein (25 ug) were mixed with gel loading buffer, and separated on 10% SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred onto nylon membranes and non-specific binding was blocked with 10% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T). Membranes were then probed with antibodies against tPA (1:1000 dilution; Innovative Research, Southfield, MI), uPA (1:2000 dilution; Research, Southfield, MI) and plasminogen (1:2000; Innovative Research, Southfield, MI). After incubation with the primary antibodies, membranes were washed with TBS-T, and incubated with appropriate horse radish peroxidase (HRP)-conjugated secondary antibodies (1:4000 dilution; Santacruz Biotechnology, Santacruz, CA) for 1 h at room temperature. Finally, the proteins on the membranes were detected using an ECL chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ) and exposing the membranes to X-ray film. Recombinant uPA, tPA, and plasminogen were co-electrophoresed as positive standards (data not shown).
Retrograde labeling of retinal ganglion cells
Ganglion cells were retrogradely labeled as previously described (65, 67). Briefly, 1.5 ul of a 5% solution of Aminostilbamidine (Molecular Probes, Eugene, OR) in PBS was injected into the superior colliculi of anesthetized mice using a stereotaxic apparatus. KA was injected into the vitreous humor one week after Aminostilbamidine application. Various times after KA injection, the animals were anesthetized and their eyes were enucleated and fixed in 4% paraformaldehyde for 1 h at room temperature. Retinas were detached from the eye cups and rinsed with PBS (two times, 15 min each). After rinsing, retinas were overlaid on a glass slide and four small incisions were made at the periphery to flatten the retina. The retinas were mounted with coverslips using an aqueous mounting medium (containing an anti-fading agent; GEL/MOUNT; Biomeda Corporation, Foster City, CA). Alternatively retinas were detached from the eyecups, embedded in OCT compound and processed for the preparation of retinal cross sections as described in immunohistochemistry section above. Ten micron-thick retinal cross sections were prepared and aminostilbamidine-positive ganglion cells were observed under a fluorescence microscope (Nikon, Tokyo, Japan). Retinal ganglion cells, located approximately at the same distance from the optic disk, were observed under a fluorescence microscope (~155 sq microns, 40× magnification) and photographed using a SpotR digital camera attached to the fluorescence microscope.
Results
Kainic acid up-regulates tPA and uPA in the retina and contributes to retinal damage
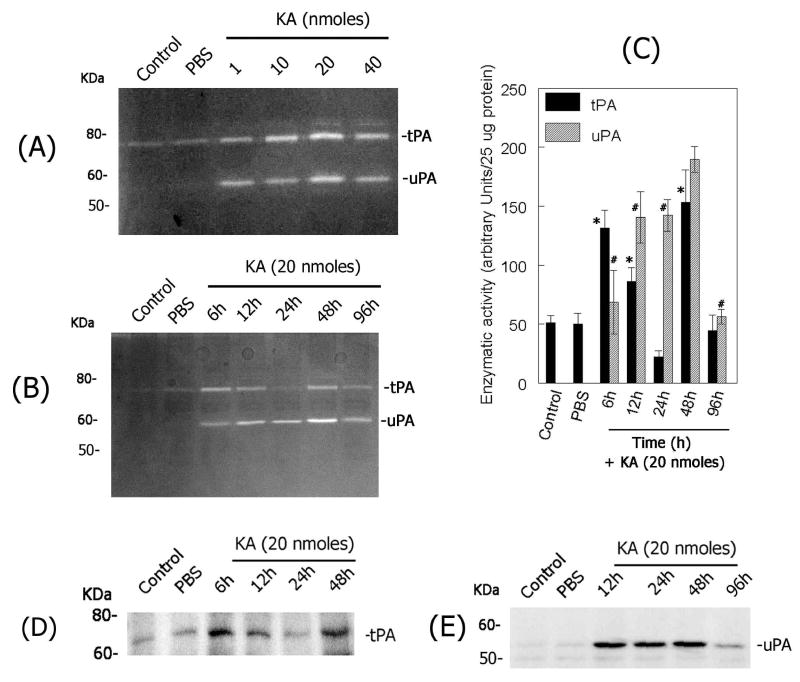
To determine whether hyper-stimulation of non-NMDA-type glutamate receptors modulates tPA and uPA levels in the retina, KA was injected into the vitreous humor in CD-1 mice and total retinal proteins were extracted. tPA and uPA activity in retinal protein extracts was determined by fibrinogen/plasminogen zymography assays. Zymography assays indicated a very low and constitutive level of tPA activity in control or PBS-injected eyes (Figure 1A). In contrast, KA injected eyes showed a dose-dependent (Figure 1A) and time-related (Figure 1B) increase in tPA activity in the retina. tPA activity was increased as early as 6 h after KA injection, returned to lower levels by 1 day and increased again at 2 days after KA injection (Figure 1B). This type of tPA activity profile was consistently observed in four independent experiments as shown by arbitrary densitometric units (Figure 1C). Western blot analysis of retinal protein extracts from a similar experiment confirmed the expression pattern of tPA in the retina (Figure 1D). Although low levels of PAI-1 and PAI-2 were observed, constitutively, in retinal proteins extracted from un-injected control or PBS-injected eyes, western blot analysis indicated no significant change in PAI-1 and PAI-2 proteins in KA injected eyes (at 6h, 12h, 24h, 48h and 96h) when compared to un-injected control or PBS-injected eyes (data not shown). Zymography assays indicated the absence of uPA activity in retinal proteins extracted from un-injected control retinas (Figure 1A & B). In contrast, intravitreal injection of KA led to a transient up-regulation in uPA activity in the retina. uPA levels were induced as early as 6h after KA injection, reached a peak between 1–2 days and returned to lower levels at day 4 (Figure 1B & C).
Figure 1. KA-induces plasminogen activators in the retina.
(A) The activity of plasminogen activators was determined by fibrinogen/plasminogen zymography in retinal proteins (aliquots containing 25 ug total proteins) extracted from un-injected control eyes or from eyes injected with PBS- or 1, 10, 20 and 40 nmoles of KA (at 48h). (B) The activity of plasminogen activators was also determined by fibrinogen/plasminogen zymography in retinal proteins (aliquots containing 25 ug total proteins) extracted from un-injected control or PBS-injected eyes and from KA-injected eyes (20 nmoles). (C) Proteolytic activity of uPA and tPA in zymograms was scanned by a flat-bed scanner and results from four independent experiments were represented as arbitrary densitometric units per 25 ug protein. tPA, *P<0.05 PBS vs KA at 6h, 12h, 24h; uPA, #P<0.05 PBS vs KA at 6h, 12h, 24h, 48h and 96 h. Aliquots containing an equal amount of retinal proteins (25 ug) from un-injected control or PBS- or KA-injected (20 nmoles) eyes were subjected to western blot analysis to determine tPA (D) and uPA (E) proteins in the retina.
To determine the cell types that synthesize tPA and uPA, immunohistochemical analysis was performed on retinal cross sections prepared at 6 h, 12h, 24h, 48h and 96 h after KA injection. An intense tPA immunostaining (by immuno-gold labeling) was, constitutively, observed in the ganglion cells in un-injected control or PBS-injected eyes (Figure 2A). A faint tPA immunostaining was also observed in the anterior portion of the inner plexiform layer. Interestingly, tPA immunoreactivity present in ganglion cells was found in the extracellular space as early as 6 h after KA injection (possibly due to depolarization) and one day after KA injection most of the cells that expressed tPA disappeared in the ganglion cell layer (Figure 2A) and this observation correlated with a reduction in tPA activity in zymograms (Figure 1B). In addition, at two days after KA injection, tPA immunolocalization was noticed in cells that are scattered in the inner plexiform layer and in the ganglion cell layer. However, the morphological appearance of these tPA-immunoreactive cells was different when compared to the morphology of either ganglion or amacrine cells. uPA was absent in retinal sections prepared from un-injected control or PBS-injected eyes (Figure 2C) but uPA protein was transiently up-regulated in the nerve fiber layer after intravitreal injection of KA (Figure 2C). An up-regulation in tPA (in ganglion cells) and an induction in uPA (in the nerve fiber layer) after KA injection was associated with the presence of TUNEL-positive apoptotic cells in the retina (Figure 2B). Twelve hours after KA injection, increased expression of plasminogen activators was associated with the appearance of increased number of TUNEL-positive cells in the ganglion cell layer and with few TUNEL-positive cells in the inner nuclear layer (Figure 2B). At one day after KA injection, tPA expression was reduced in the retina because majority of the ganglion cells that expressed tPA have undergone apoptosis at this time, while increased expression of uPA was still observed in the nerve fiber layer in cells that are resemble activated astrocytes. At this time, TUNEL-positive cells were also observed in the inner and outer nuclear layers due to secondary retinal damage (Figure 2B). At 48 h after KA injection, TUNEL-positive cells were also found in the outer nuclear layer, although the expression of tPA and uPA does not correlate with localization of TUNEL positive cells. At 48 h after KA injection, most of the tPA was expressed by cells that migrated into the inner nuclear and ganglion cell layers but these cells do not seem to be either ganglion cells or amacrine cells; they rather seem to be microglial cells.
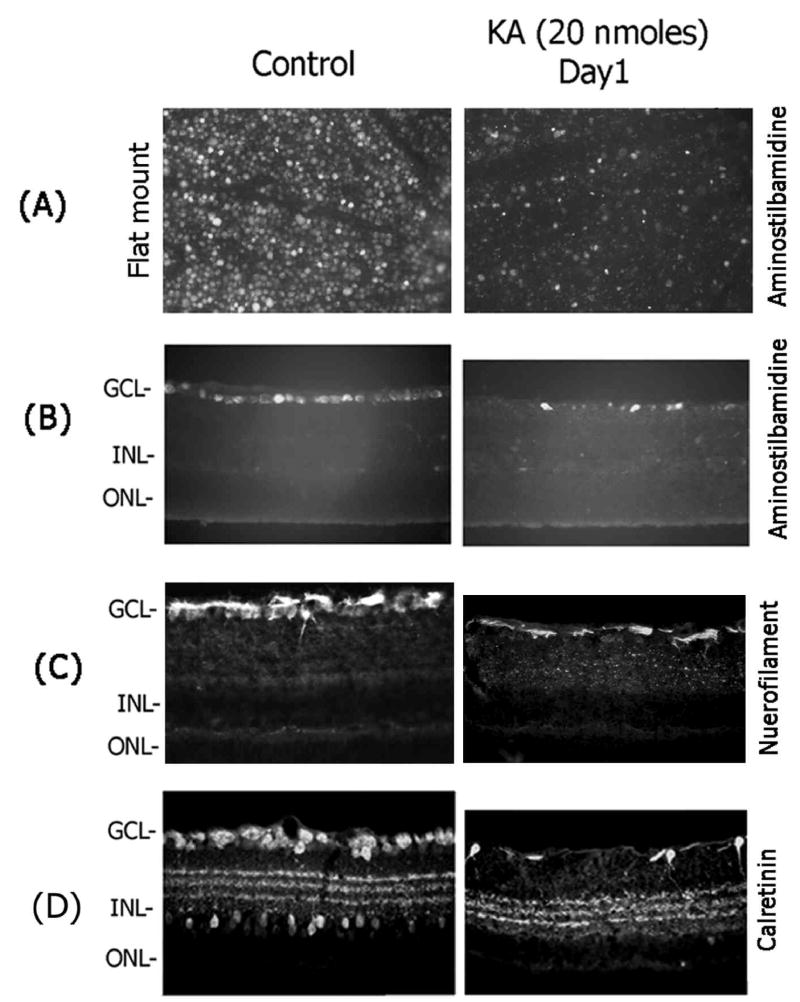
Since cells in the ganglion cell layer and inner nuclear layer showed TUNEL-positive staining as early as 6–12 h, two different experiments were performed to determine the cell types that underwent apoptotic death (TUNEL-positive) in these two layers. In the first experiment, ganglion cells were retrogradely-labeled with aminostilbamidine and KA was injected into the vitreous humor one week after labeling. Retinas were removed from the enucleated eyes one day after KA injection, prepared as flat mounted retinas and the loss of aminostilbamidine-positive ganglion cells was determined by fluorescence microscopy (Figure 3A). In addition, retinal cross sections were also prepared at one day after KA injection (from retrogradely-labeled retinas) and the loss of aminostilbamidine-positive ganglion cells was determined by fluorescence microscopy (Figure 3B). Retinal cross sections prepared from a similar experiment were also immunostained with antibodies against neurofilament-light (NF-L) to determine ganglion cell loss (Figure 3C). Examination of retinal cross sections indicated a significant decrease in the number of aminostilbamidine-positive and NF-L–positive ganglion cells one day after KA injection (Figure 3A, B, and C), compared to un-injected controls. Since amacrine cells in the retina can also respond to hyper-stimulation of glutamate receptors by KA, in a second experiment, immunolocalization experiments were performed on retinal cross sections prepared one day after KA injection to determine whether KA also causes loss of amacrine cells (Figure 3D). Immunolocalization of retinal cross sections with calretinin antibodies (labels AII amacrine cells) indicated a decrease in the number of calretinin-positive amacrine cells both in the ganglion cell layer (presumably, displaced amacrine cells) and in the inner nuclear layer (Figure 3D). These results indicate that intravitreal injection of KA leads to an up-regulation in tPA and uPA activity in the retina and causes loss of both ganglion cells and amacrine cells by an apoptotic mechanism.
Figure 3. Effect of KA on retinal damage.
Retinal ganglion cells were retrogradely labeled with aminostilbamidine one week before KA injection, and their loss in retinal flat mounts (A) or retinal cross sections (B) was determined at 1 day after KA injection. (B) Retinal cross sections prepared from un-injected control and KA injected eyes (at one day) from a separate experiments were also immunostained with antibodies against neurofilament-light (NF-L) (C) or calretinin (D). Note a decrease in the number of aminostilbamidine-positive ganglion cells, a decrease in nerve fiber layer-associated NF-L immunostaining, and a decrease in calretinin-positive immunostaining at 1 day after KA injection. Images in panel A are taken at 20× magnification. All other images were obtained at 40× magnification.
Kainic acid induces plasminogen activation
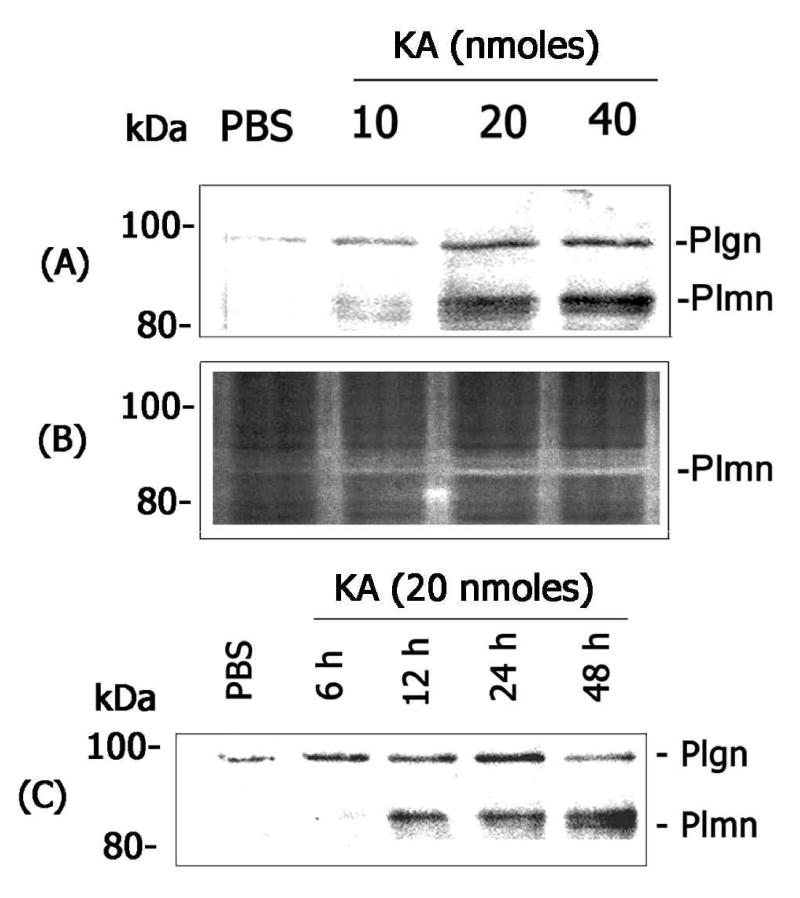
One of the roles of plasminogen activators (tPA and uPA) is to activate a physiological zymogen plasminogen into active plasmin. Active plasmin in turn can modulate extracellular matrix proteins such as laminin and contribute to neuronal cell loss by removing critical cell-matrix interactions. Although the data presented above show an increase in tPA and uPA activity (Figure 1), it was not completely clear whether increased levels of plasminogen activators play a role in plasminogen activation in the retina. Therefore, retinal proteins extracted from KA-or PBS-injected eyes were subjected to western blot analysis using an antibody that detects both plasminogen and its active product plasmin (Figure 4 A&C). In addition, casein-zymography assays were performed using retinal protein extracts to determine whether plasmin, which might be generated from plasminogen, is proteolytically active (Figure 4B). Western blot analysis indicated a low and constitutive level of plasminogen protein in retinal proteins extracted from control eyes (Figure 4A). Western blot analysis also indicated an increase in plasminogen levels and activation of plasminogen to plasmin in retinal proteins extracted from KA-injected eyes in a dose- (Figure 4A) and time-related fashion (Figure 4C). Casein-zymography assays indicated that the lower molecular weight plasmin band is, indeed, proteolytically active (Figure 4B). These results indicate that KA- mediated up-regulation in uPA and tPA associates with plasminogen activation in the retina.
Figure 4. Effect of KA on plasminogen activation in the retina.
(A) Aliquots containing an equal amount of retinal proteins (25 ug) extracted from PBS- or KA-injected eyes (at 48 h) were subjected to western blot analysis using antibodies that detect both plasminogen and plasmin. (B) Retinal proteins from a similar experiment were also subjected to casein-zymography to determine the activity of plasmin. (C) Aliquots containing an equal amount of retinal proteins (25 ug) extracted from PBS- or KA-injected eyes were subjected to western blot analysis to detect plasminogen activation. The migration position of the bands representing plasminogen (Plgn) and plasmin (Plmn) were indicated in the figures. Note that KA-induced an activation of plasminogen into active plasmin in a dose- and time-related fashion.
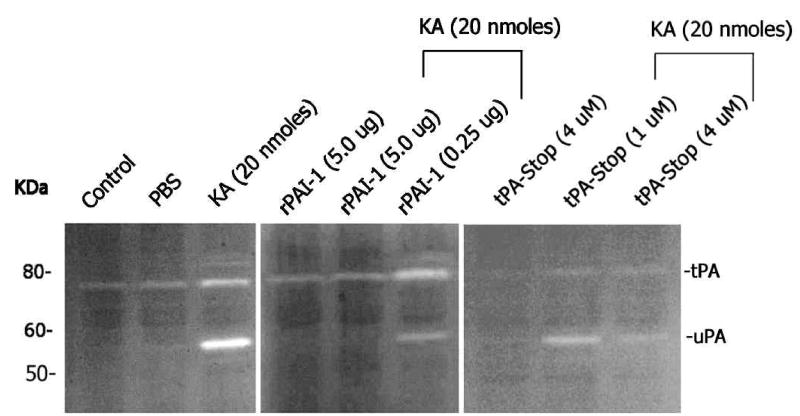
rPAI-1 and tPA-STOP attenuate KA-induced retinal damage
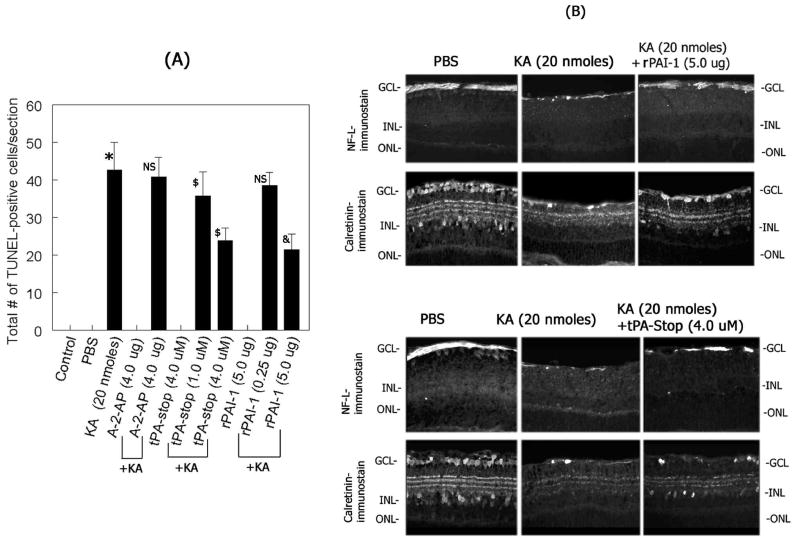
Since an up-regulation in uPA and tPA and activation of plasminogen to plasmin were associated with retinal damage (Figure 3), we reasoned that inhibition of either plasminogen activators or plasmin might protect retina against KA-induced damage. Three different experiments were performed to determine these possibilities. First, the effect of rPAI-1 and tPA-STOP (which can inhibit both uPA and tPA) on uPA as well as tPA activity was determined by zymography assays on retinal proteins extracted from KA or KA plus rPAI-1 or KA plus tPA-STOP-injected eyes. Zymography assays indicated that higher concentration of both rPAI-1 (5.0 ug) and tPA-STOP (4 uM) inhibited tPA and uPA activity in the retina (Figure 5). In the second experiment, the effect of rPAI-1 and tPA-STOP or alpha-2-antiplasmin (plasmin inhibitor) on KA-induced cell loss was determined by TUNEL assays at two days post-injection. Quantification of of total number of TUNEL-positive cells in retinal cross sections indicated a significant increase in TUNEL-positive cells in the retina after KA-injection, as expected (Figure 6A). Significantly less number of TUNEL-positive cells was observed in retinal cross sections after injection of KA with rPAI-1 or tPA-STOP. In contrast, intravitreal injection of KA with alpha-2-antiplasmin (4.0 ug) failed to offer significant protection against KA-induced cell loss (Figure 6A). In the third experiment, the effect of rPAI-1 and tPA-STOP on retinal degeneration was determined by immunostaining of retinal cross sections with antibodies against ganglion cells (NF-L) and amacrine cells (calretinin). Examination of retinal cross sections indicated a significant loss of both ganglion cells (Figure 6B) and amacrine cells (Figure 6B) after KA-injection, as expected. In contrast, injection of rPAI-1 and tPA-STOP with KA resulted in significant protection against KA-induced cell loss as observed by the remaining NF-L-positive immunostaining in the ganglion cell layer (Figure 6B) and calretinin-positive immunostaining in the ganglion cell layer (displaced amacrine cells) and in the inner nuclear layer (Figure 6B). These results indicate that rPAI-1 and tPA-STOP attenuate retinal degeneration by inhibiting the activities of uPA and tPA in the retina.
Figure 5. Effect of rPAI-1 and tPA-STOP on tPA and uPA activity in the retina.
Two days after intravitreal injection of KA (20 nmoles) with or without indicated concentrations of rPAI-1 or tPA-STOP, retinal proteins were extracted and aliquots containing an equal amount of proteins (25 ug) were subjected to fibrinogen/plasminogen zymography. Note that both rPAI-1 and tPA-STOP inhibited KA-induced tPA and uPA activity in the retina.
Figure 6. Effect of plasminogen activator and plasmin inhibitors on KA-induced retinal damage.
(A) Retinal cross sections were prepared two days after intravitreal injection of KA (20 nmoles) with or without indicated concentrations of rPAI-1, tPA-STOP, and alpha-2-antiplasmin (A-2-AP) and apoptotic cell death was determined by TUNEL assays. The remaining number of TUNEL-positive cells in retinal cross sections was quantitated using Scion-image analysis software and the results from four independent experiments were represented as mean TUNEL-positive cells +/− SEM. *P<0.05 PBS vs KA; NS, not significant, KA vs KA plus A-2-AP (4.0 ug); $P<0.05 KA vs KA plus tPA-STOP (1.0 uM); NS, not significant, KA vs KA plus rPAI-1 (0.25 ug); $P<0.05 KA vs KA plus tPA-STOP (4.0 uM), & P<0.05 KA vs KA plus rPAI-1 (5.0 ug). (B) Two days after intravitreal injection of KA (20 nmoles) with or without indicated concentrations of rPAI-1 or tPA-STOP, retinal cross sections were prepared and immunohistochemical analysis was performed using antibodies against neurofilament light (NF-L) and calretinin. Note that rPAI-1 and tPA-STOP (but not alpha-2-antiplasmin) inhibited uPA and tPA and attenuated the loss of both ganglion cells and amacrine cells in the retina.
Discussion
A number of previous studies have indicated that retinal damage in diseases such as glaucoma and diabetic retinopathy might be due to hyper-stimulation of ionotropic glutamate receptors (“excitotoxicity”) mediated by excessive levels of extracellular glutamate in the retina (1, 2, 13, 68, 69). The mechanisms involved in retinal damage, however, are poorly understood and many of them are still speculative. Therefore, in this study, we have determined the role of plasminogen activators in excitotoxic retinal damage.
We show that kainic acid that hyper-stimulates non-NMDA type glutamate receptors, induces retinal damage through an up-regulation not only in tPA but also in uPA activity in the retina. Activity and protein levels of both tPA and uPA were transiently increased in the retina after intravitreal injection of KA. uPA was absent in un-injected control or PBS-injected retinas, while tPA was constitutively expressed by ganglion cells in un-injected control or PBS-injected retinas. tPA present in the ganglion cells was up-regulated after intravitreal injection of KA and most of the tPA was released into the extracellular space in the inner retina after KA treatment. In contrast, uPA which was absent in un-injected control retinas was induced after intravitreal injection of KA and it was localized in the nerve fiber layer. Once up-regulated, both tPA and uPA can perform distinct functions in the retina. They can contribute to retinal damage via activation of plasminogen into active plasmin. Plamin in turn can contribute to retinal damage by modulating or degrading extracellular matrix proteins such as laminin (present in the nerve fiber layer) and this in turn might result in detachment-induced apoptosis of both ganglion cells and amacrine cells (66). In addition, plasminogen activators tPA and uPA can cause retinal damage independent of plasminogen activation. To determine these possibilities, we have injected KA with inhibitors of uPA and tPA (rPAI-1 and tPA-STOP) or an inhibitor of plasmin (alpha-2-antiplasmin) into the vitreous humor and determined the retinal damage by TUNEL assays.
We found that intravitreal injection of rPAI-1 and tPA-STOP not only inhibited the activities of tPA and uPA (zymography assays, figure 5) but also offered significant protection against KA-induced retinal damage (TUNEL assays, figure 6). In contrast, intravitreal injection of KA with alpha-2-antiplasmin (4.0 ug) failed to offer significant protection against KA-induced retinal damage. These results suggest that KA-induced retinal damage is mediated by an increase in plasminogen activators (both uPA and tPA) but independent of plasminogen activation. For example, tPA can activate NMDA-type glutamate receptors as previously shown in the central nervous system (49, 70) and uPA can control calcium influx (71) and both tPA and uPA might perform similar functions in the retina and contribute to excitotoxic retinal damage. Although at first glance, this might seem an unlikely possibility because retinal damage in this study was determined after hyper-stimulation of non-NMDA type receptors (by KA), it is worth to mention that ganglion cells and amacrine cells express both NMDA and non-NMDA type glutamate receptors and tPA that was up-regulated in the retina after KA injection can proteolytically process NMDA-type glutamate receptors and might contribute directly to retinal damage. Although we found an increase in uPA activity and protein levels and an association of uPA with plasminogen activation, little is known about the additional roles of uPA in the retina at this time. Studies aimed in this direction are necessary to delineate additional roles of plasminogen activators in the retina. Although inhibition of plasmin activity can attenuate detachment-induced apoptosis (presumably by maintaining cell-ECM contacts), in the presence of increased amounts of excitotoxin such as kainic acid and in the presence of increased levels of uPA and tPA, inhibition of plasmin alone does not seem to confer protection against retinal damage.
In a previous study we have observed that the optic nerve ligation-induced retinal damage is dependent on plasminogen activation whereas in this study, we have found that KA-induced retinal damage is independent of plasminogen activation. Although the exact mechanisms for the differential role of plasminogen activation in retinal damage are not clear, there is one major difference between the animal model employed in this study (intravitreal injection of kainic acid) and the animal model employed in our previous study (optic nerve ligation). In our previous study, relatively higher plasminogen levels were found in the retina after optic nerve ligation compared to the levels found in the retina after kainic acid injection into the vitreous humor. The increase in plasminogen levels found in the retina after optic nerve ligation was due to the damage to the central retinal artery, compromise in blood retinal barrier (BRB, determined indirectly by albumin western blot analysis) and leakage of plasminogen into the retina, whereas similar compromise in BRB was not observed after intravitreal injection of kainic acid (data not shown). Clearly, plasminogen activation seems to play a differential role in retinal damage depending on the model system used.
An intriguing observation made in this study is regarding the activity and protein profile of tPA during time course experiments. Constitutive levels of tPA present in the un-injected control or PBS-injected eyes were increased as early 6–12 h after KA injection, decreased by 24 h and increased again at 48 h (Figure 1). This could be because of two possibilities. First, a reduction in tPA activity could be simply due to the loss of ganglion cells that contributed to tPA production in the retina. This possibility was supported by immunohistochemical analysis of tPA in Figure 2. Data in figure 2 shows that tPA present in the ganglion cells was gradually released into the extracellular space starting at 6–12 h possibly due to membrane depolarization (72). At 24 h after KA injection, majority of the ganglion cells that expressed tPA were killed by KA and hence zymography and western blot assays indicated reduced levels of tPA in the retina. Also, tPA remaining in the extracellular space at 24 h KA injection could be recycled by glial cells (48) or degraded by additional proteolytic mechanisms or inhibited by protease inhibitors such as neuroserpin. Although it is speculative at this time, KA could activate metabotropic glutamate receptors (due to the release of endogenous L-glutamate by dying neurons) and these receptors in turn could counteract up-regulation of tPA mediated by non-NMDA receptors.
In this study we found that two different cell types synthesize tPA in the retina. This was supported by immunolocalization studies for tPA on retinal cross sections prepared at 48 and 96 h after KA injection (Figure 2). The results presented in Figure 2 indicate that at 48 h after KA injection, a different cell type showed tPA-positive staining in the retina. tPA-positive cells were found throughout the inner retina in a scattered fashion, they were morphologically different from ganglion cells, and morphology of tPA-positive cells resembles that of microglial cells. Although co-immunolocalization experiments could have been a better approach to determine the origin of tPA in the retina, these experiments were unsuccessful due to technical difficulties and non-availability of suitable antibodies. The results presented above, however, indicate that two different cell types, ganglion cells and microglial cells (73), contribute to the origin of tPA in the retina. Although the exact reason why microglial cells synthesize tPA after KA injection is not clear, they might synthesize tPA and use it to migrate into the inner retina to remove the debris from dying cells (74–76) but they can contribute also to secondary retinal damage. It is also possible that microglial cells can be migrated into the inner retina independent of tPA up-regulation (32, 73, 77). Although spatial expression of plasminogen activators in the retina (at 24 h to 96 h) does not seem to correlate with the localization of TUNEL-positive cells, the presence of TUNEL-positive cells in the inner and outer nuclear layers could be due to secondary events of damage subsequent to the primary events initiated in the ganglion cell layer which then continues to affect cells in remaining retinal layers. Secondary degeneration can be mediated by a number of factors that could be released by degenerating ganglion and amacrine cells including endogenous glutamate and cytokines such as tumor necrosis factor-alpha and interleukin-1beta.
In addition to the up-regulation of tPA and uPA, hyper-stimulation of non-NMDA receptors by KA can result in up-regulation of other proteases such as matrix metalloproteinases (MMPs), as previously reported (65). This seems to be quite plausible because both rPAI-1 and tPA-STOP failed to protect, completely, the KA-mediated retinal damage in this study. In addition, previous studies from this laboratory have reported that inhibition of MMP activity alone also do not offer complete protection against KA-mediated retinal damage (65). These results suggest that both MMPs and plasminogen activators might collectively play a role in excitotoxic retinal damage. At this time, we cannot rule out the possible role of other proteases that can contribute to retinal damage.
Although there is some evidence in the CNS regarding the role of tPA in neuronal damage (30, 32), the novelty of the observations made in this study is that up-regulation of not only tPA but also uPA plays a causative role in retinal damage in response to intravitreal injection of KA that hyper-stimulates glutamate receptors. As indicated above, in the absence of a clear understanding of the mechanisms involved in ischemia or/and excitotoxic retinal damage (due to hyper-stimulation of glutamate receptors), the results provided in this study suggest that strategies that are aimed at reducing the activity of plasminogen activators might offer protection against retinal damage in blinding retinal diseases in which glutamate receptor-mediated excitotoxicity has been implicated as a causative factor (6, 8, 28, 68, 69).
Acknowledgments
This work was supported by National Institute project grant EY13643 (to SKC) and Vision Research Infrastructure Development Grant EY014803.
References
- 1.Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001;85:1252–1259. doi: 10.1136/bjo.85.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 4.Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gomez JM, Vidal-Sanz M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:2002–2014. [PubMed] [Google Scholar]
- 5.Osborne NN, Larsen AK. Antigens associated with specific retinal cells are affected by ischaemia caused by raised intraocular pressure: effect of glutamate antagonists. Neurochem Int. 1996;29:263–270. doi: 10.1016/0197-0186(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 7.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48(Suppl 1):S38–46. doi: 10.1016/s0039-6257(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 8.Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115:1161–1166. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- 9.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 10.Schwarcz R, Coyle JT. Kainic acid: neurotoxic effects after intraocular injection. Invest Ophthalmol Vis Sci. 1977;16:141–148. [PubMed] [Google Scholar]
- 11.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 13.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 14.Osborne NN, Larsen A, Barnett NL. Influence of excitatory amino acids and ischemia on rat retinal choline acetyltransferase-containing cells. Invest Ophthalmol Vis Sci. 1995;36:1692–1700. [PubMed] [Google Scholar]
- 15.Brandstatter JH, Hartveit E, Sassoe-Pognetto M, Wassle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci. 1994;6:1100–1112. doi: 10.1111/j.1460-9568.1994.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EL, Kalloniatis M. Localisation of amino acid neurotransmitters during postnatal development of the rat retina. J Comp Neurol. 1997;380:449–471. doi: 10.1002/(sici)1096-9861(19970421)380:4<449::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Lucas DR, Newhouse JP. The toxic effects so sodium L-glutamate on the inner layers of the retina. Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 18.Olney JW. Glutamate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969;28:455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sisk DR, Kuwabara T, Kirsch AD. Behavioral recovery in albino rats with glutamate-damaged retinas. Invest Ophthalmol Vis Sci. 1984;25:1124–1128. [PubMed] [Google Scholar]
- 20.Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223:250–258. doi: 10.1007/BF02153655. [DOI] [PubMed] [Google Scholar]
- 21.Morgan IG, Ingham CA. Kainic acid affects both plexiform layers of chicken retina. Neurosci Lett. 1981;21:275–280. doi: 10.1016/0304-3940(81)90216-0. [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y, Benz AM, Kurby CO, Labruyere J, Zorumski CF, Price MT, Olney JW. An ex vivo rat retinal preparation for excitotoxicity studies. J Neurosci Methods. 1995;60:219–225. doi: 10.1016/0165-0270(95)00015-m. [DOI] [PubMed] [Google Scholar]
- 23.Izumi Y, Kirby-Sharkey CO, Benz AM, Labruyere J, Price MT, Wozniak DF, Zorumski CF, Olney JW. Age dependent sensitivity of the rat retina to the excitotoxic action of N-methyl-D-aspartate. Neurobiol Dis. 1995;2:139–144. doi: 10.1006/nbdi.1995.0015. [DOI] [PubMed] [Google Scholar]
- 24.Siliprandi R, Canella R, Carmignoto G, Schiavo N, Zanellato A, Zanoni R, Vantini G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8:567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- 25.Manabe S, Lipton SA. Divergent NMDA signals leading to proapoptotic and antiapoptotic pathways in the rat retina. Invest Ophthalmol Vis Sci. 2003;44:385–392. doi: 10.1167/iovs.02-0187. [DOI] [PubMed] [Google Scholar]
- 26.Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- 27.Kim TW, Kang KB, Choung HK, Park KH, Kim DM. Elevated glutamate levels in the vitreous body of an in vivo model of optic nerve ischemia. Arch Ophthalmol. 2000;118:533–536. doi: 10.1001/archopht.118.4.533. [DOI] [PubMed] [Google Scholar]
- 28.Vorwerk CK, Zurakowski D, McDermott LM, Mawrin C, Dreyer EB. Effects of axonal injury on ganglion cell survival and glutamate homeostasis. Brain Res Bull. 2004;62:485–490. doi: 10.1016/S0361-9230(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 29.Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE. Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res. 1997;58:864–867. [PubMed] [Google Scholar]
- 30.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 31.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- 33.Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 34.Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- 35.Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci U S A. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman GC, Seeds NW. Tissue plasminogen activator mRNA expression in granule neurons coincides with their migration in the developing cerebellum. J Comp Neurol. 1995;360:658–670. doi: 10.1002/cne.903600410. [DOI] [PubMed] [Google Scholar]
- 37.Krystosek A, Seeds NW. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981;78:7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 39.Moonen G, Grau-Wagemans MP, Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982;298:753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima K, Reddington M, Kohsaka S, Kreutzberg GW. Induction of urokinase-type plasminogen activator in rat facial nucleus by axotomy of the facial nerve. J Neurochem. 1996;66:2500–2505. doi: 10.1046/j.1471-4159.1996.66062500.x. [DOI] [PubMed] [Google Scholar]
- 41.Majka S, McGuire P, Colombo S, Das A. The balance between proteinases and inhibitors in a murine model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2001;42:210–215. [PubMed] [Google Scholar]
- 42.Das A, McGuire PG, Eriqat C, Ober RR, DeJuan E, Jr, Williams GA, McLamore A, Biswas J, Johnson DW. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999;40:809–813. [PubMed] [Google Scholar]
- 43.Tripathi RC, Tripathi BJ, Park JK. Localization of urokinase-type plasminogen activator in human eyes: an immunocytochemical study. Exp Eye Res. 1990;51:545–552. doi: 10.1016/0014-4835(90)90085-9. [DOI] [PubMed] [Google Scholar]
- 44.Tripathi RC, Park JK, Tripathi BJ, Millard CB. Tissue plasminogen activator in human aqueous humor and its possible therapeutic significance. Am J Ophthalmol. 1988;106:719–722. doi: 10.1016/0002-9394(88)90707-6. [DOI] [PubMed] [Google Scholar]
- 45.Tripathi BJ, Geanon JD, Tripathi RC. Distribution of tissue plasminogen activator in human and monkey eyes. An immunohistochemical study. Ophthalmology. 1987;94:1434–1438. doi: 10.1016/s0161-6420(87)33278-6. [DOI] [PubMed] [Google Scholar]
- 46.Schacke W, Beck KF, Pfeilschifter J, Koch F, Hattenbach LO. Modulation of tissue plasminogen activator and plasminogen activator inhibitor-1 by transforming growth factor-beta in human retinal glial cells. Invest Ophthalmol Vis Sci. 2002;43:2799–2805. [PubMed] [Google Scholar]
- 47.Lutty GA, keda K, Chandler C, McLeod DS. Immunolocalization of tissue plasminogen activator in the diabetic and nondiabetic retina and choroid. Invest Ophthalmol Vis Sci. 1991;32:237–245. [PubMed] [Google Scholar]
- 48.Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, Leveille F, Cacquevel M, Plawinski L, MacKenzie ET, Bu G, Buisson A, Vivien D. Is tissue-type plasminogen activator a neuromodulator? Mol Cell Neurosci. 2004;25:594–601. doi: 10.1016/j.mcn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Benchenane K, Lopez-Atalaya JP, Fernandez-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Johnson MW, Olsen KR, Hernandez E. Tissue plasminogen activator thrombolysis during surgical evacuation of experimental subretinal hemorrhage. Ophthalmology. 1992;99:515–521. doi: 10.1016/s0161-6420(92)31939-6. [DOI] [PubMed] [Google Scholar]
- 51.Lewis H, Resnick SC, Flannery JG, Straatsma BR. Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Am J Ophthalmol. 1991;111:197–204. doi: 10.1016/s0002-9394(14)72259-7. [DOI] [PubMed] [Google Scholar]
- 52.Lewis, H., and VanderBrug Medendorp, S. (1997) Tissue plasminogen activator-assisted surgical excision of subfoveal choroidal neovascularization in age-related macular degeneration: a randomized, double-masked trial. Ophthalmology 104, 1847–1851; discussion 1852 [DOI] [PubMed]
- 53.Hesse L, Schmidt J, Kroll P. Management of acute submacular hemorrhage using recombinant tissue plasminogen activator and gas. Graefes Arch Clin Exp Ophthalmol. 1999;237:273–277. doi: 10.1007/s004170050232. [DOI] [PubMed] [Google Scholar]
- 54.Hesse L, Schroeder B, Heller G, Kroll P. Quantitative effect of intravitreally injected tissue plasminogen activator and gas on subretinal hemorrhage. Retina. 2000;20:500–505. doi: 10.1097/00006982-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 56.Traynelis SF, Lipton SA. Is tissue plasminogen activator a threat to neurons? Nat Med. 2001;7:17–18. doi: 10.1038/83289. [DOI] [PubMed] [Google Scholar]
- 57.Masos T, Miskin R. mRNAs encoding urokinase-type plasminogen activator and plasminogen activator inhibitor-1 are elevated in the mouse brain following kainate-mediated excitation. Brain Res Mol Brain Res. 1997;47:157–169. doi: 10.1016/s0169-328x(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 58.Tsirka SE. Clinical implications of the involvement of tPA in neuronal cell death. J Mol Med. 1997;75:341–347. doi: 10.1007/s001090050119. [DOI] [PubMed] [Google Scholar]
- 59.Tsirka SE, Bugge TH, Degen JL, Strickland S. Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci U S A. 1997;94:9779–9781. doi: 10.1073/pnas.94.18.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 61.Irvine WD, Johnson MW, Hernandez E, Olsen KR. Retinal toxicity of human tissue plasminogen activator in vitrectomized rabbit eyes. Arch Ophthalmol. 1991;109:718–722. doi: 10.1001/archopht.1991.01080050134044. [DOI] [PubMed] [Google Scholar]
- 62.Hrach CJ, Johnson MW, Hassan AS, Lei B, Sieving PA, Elner VM. Retinal toxicity of commercial intravitreal tissue plasminogen activator solution in cat eyes. Arch Ophthalmol. 2000;118:659–663. doi: 10.1001/archopht.118.5.659. [DOI] [PubMed] [Google Scholar]
- 63.Chen SN, Ho CL, Kuo YH, Ho JD. Intravitreous tissue plasminogen activator injection and pneumatic displacement in the management of submacular hemorrhage complicating scleral buckling procedures. Retina. 2001;21:460–463. doi: 10.1097/00006982-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Esser P, Heimann K, Bartz-Schmidt KU, Walter P, Krott R, Weller M. Plasminogen in proliferative vitreoretinal disorders. Br J Ophthalmol. 1997;81:590–594. doi: 10.1136/bjo.81.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–2383. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Chaudhry A, Chintala SK. Inhibition of plasminogen activation protects against ganglion cell loss in a mouse model of retinal damage. Mol Vis. 2003;9:238–248. [PubMed] [Google Scholar]
- 67.Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem. 2002;277:47461–47468. doi: 10.1074/jbc.M204824200. [DOI] [PubMed] [Google Scholar]
- 68.Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43(Suppl 1):S142–150. doi: 10.1016/s0039-6257(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 69.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37:1618–1624. [PubMed] [Google Scholar]
- 70.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 71.Christow SP, Bychkov R, Schroeder C, Dietz R, Haller H, Dumler I, Gulba DC. Urokinase activates calcium-dependent potassium channels in U937 cells via calcium release from intracellular stores. Eur J Biochem. 1999;265:264–272. doi: 10.1046/j.1432-1327.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 72.Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci. 1999;112 (Pt 22):4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- 74.Thanos S. The Relationship of Microglial Cells to Dying Neurons During Natural Neuronal Cell Death and Axotomy-induced Degeneration of the Rat Retina. Eur J Neurosci. 1991;3:1189–1207. doi: 10.1111/j.1460-9568.1991.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 75.Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thanos S, Naskar R. Correlation between retinal ganglion cell death and chronically developing inherited glaucoma in a new rat mutant. Exp Eye Res. 2004;79:119–129. doi: 10.1016/j.exer.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]