Abstract
Group B streptococcus (GBS; Streptococcus agalactiae) is the most common cause of neonatal and obstetric sepsis and is an increasingly important cause of septicemia in elderly individuals and immunocompromised patients. Ongoing surveillance to monitor GBS serotype distribution will be needed to guide the development and use of GBS conjugate vaccines. We designed sequencing primers based on the previously published sequences of the capsular polysaccharide (cps) gene clusters to further define partial cps gene clusters for eight of the nine GBS serotypes (serotypes Ia to VII). Subsequently, we designed and evaluated primers to identify serotypes Ia, Ib, III, IV, V, and VI directly by PCR and all eight serotypes (serotypes Ia to VII) by sequence heterogeneity. A total of 206 clinical GBS isolates were used to compare our molecular serotype (MS) identification method with conventional serotyping (CS). All clinical isolates were assigned an MS, whereas 188 of 206 (91.3%) were assigned a serotype by use of antisera. A small number of isolates (serosubtypes III-3 and III-4) showed different serotype specificities between PCR and sequencing, but the PCR results correlated with those obtained by CS. The overall agreement between the MS identification method and CS for isolates for which results of both tests were available was 100% (188 of 188 isolates). The MS identification method is a specific and practical alternative to conventional GBS serotyping and will facilitate epidemiological studies.
Group B streptococcus (GBS; Streptococcus agalactiae) is the most common cause of neonatal and obstetric sepsis and is an increasingly important cause of septicemia in elderly individuals and immunocompromised patients (24, 28). The incidence of neonatal GBS sepsis has been reduced in recent years by the use of intrapartum antibiotic prophylaxis (23), but there are many problems with this approach (16, 26). In future, vaccination is likely to be preferred, and there has been considerable progress in the development of conjugate polysaccharide GBS vaccines (21).
Before the introduction of vaccines, extensive epidemiological studies will be required to assess not only the burden of disease but also the distributions of GBS serotypes to determine the optimal formulations of vaccine antigens (7). A serotype distribution based on the serotypes for one geographic location or small numbers of patients may not be generally applicable (9, 17). Continued monitoring will be necessary to assess the suitability of combinations of GBS vaccine antigens for different target populations in different geographic locations (7, 9).
Nine capsular polysaccharide GBS serotypes have been described (7, 9). Various serotyping methods have been used, including immunoprecipitation (29), enzyme immunoassay (10), coagglutination (6), counterimmunoelectrophoresis and capillary precipitation (27), latex agglutination (31), fluorescence microscopy (5), and inhibition enzyme-linked immunosorbent assay (2). These methods are labor-intensive and require high-titer serotype-specific antisera, which are expensive and difficult to make and which are commercially available for only six serotypes, serotypes Ia to V (2). Molecular biology-based methods of genotyping, such as pulsed-field gel electrophoresis (22) and restriction endonuclease analysis (19), are useful for epidemiological studies but do not generally identify serotypes. Molecular serotype (MS) identification methods are theoretically attractive because of their potentially high discriminatory powers and reproducibilities (25). PCR-based assays have been used to detect and genotype GBS isolates, but further development is needed to make them practicable for use for serotype identification (12, 25).
In the study described here, we used the published sequences of the capsular polysaccharide synthesis (cps) gene clusters of GBS serotypes Ia and III (3, 30) and our own sequencing results to analyze partial cps gene cluster sequences of eight serotypes (serotypes Ia to VII) and to develop an MS identification method. Recently published sequences of cps gene clusters of serotypes Ib (K. Miyake et al., submitted to GenBank, 2001 [GenBank accession number AB050723]) and serotypes IV, V, and VI (K. McKinnon et al., submitted to GenBank, 2001 [GenBank accession numbers AF355776, AF349539, and AF337958, respectively]) confirmed the results of our sequence analysis. Subsequently, we used these sequences to design PCR primers specific for serotypes Ib, IV, V, and VI.
MATERIALS AND METHODS
GBS reference strains and clinical isolates.
A panel of nine GBS serotypes (serotypes Ia to VIII; reference panel 1) was kindly provided by Lawrence Paoletti, Channing Laboratory, Boston, Mass. Diana Martin, Streptococcus Reference Laboratory, Institute of Environmental Science and Research (ESR), Porirua, Wellington, New Zealand, provided another panel of nine international reference GBS type strains including those of serotypes Ia to VI (reference panel 2) (Table 1). In addition, we tested isolates from 205 patients, including 146 which had been referred from various laboratories in New Zealand for serotyping and 59 isolated from normally sterile sites over a period of 10 years in one diagnostic laboratory in Sydney, Australia. One culture was subsequently shown to be mixed, so 206 different isolates were examined. Conventional serotyping (CS) was performed at the Streptococcus Reference Laboratory, ESR, and the MS identification method was performed at the Centre for Infectious Diseases and Microbiology Laboratory Services, Institute of Clinical Pathology and Medical Research, Sydney, Australia.
TABLE 1.
GBS reference panels used in the study
| Laboratory strain panel and strain numbera | Source | Serotype | MS andsubtype | GenBank accession no. |
|---|---|---|---|---|
| Reference panel 1 | ||||
| 090 | Channing | Ia | Ia | AF332893 |
| H36B | Channing | Ib | Ib | AF332903 |
| 18RS21 | Channing | II | II | AF332905 |
| M781 | Channing | III | III-2b | AF332896 |
| 3139 | Channing | IV | IV | AF332908 |
| CJB 111 | Channing | V | V | AF332910 |
| SS1214 | Channing | VI | VI | AF332901 |
| 7271 | Channing | VII | VII | AF332913 |
| JM9 130013 | Channing | VIII | VIII | |
| Reference panel 2 | ||||
| NZRM 908 (NCDC SS615) | ESR | Ia | Ia | AF332894 |
| NZRM 909 (NCDC SS618) | ESR | Ib | Ib | AF332904 |
| NZRM 910 (NCDC SS700) | ESR | Ia | Ia | AF332914 |
| NZRM 911 (NCDC SS619) | ESR | II | II | AF332906 |
| NZRM 912 (NCDC SS620) | ESR | III | III-3b | AF332897 |
| NZRM 2217 (Prague 25/60) | ESR | Nontypeable (R) | II | AF332907 |
| NZRM 2832 (Prague 1/82) | ESR | IV | IV | AF332909 |
| NZRM 2833 (Prague 10/84) | ESR | V | V | AF332911 |
| NZRM 2834 (Prague 118754) | ESR | VI | VI | AF332902 |
The strains in reference panel 1 were supplied by Lawrence Paoletti, Channing Laboratory. Reference panel 2 consisted of New Zealand Reference Medical Culture Collection strains, supplied by Diana Martin, ESR.
The numbers indicate MS III subtypes based on sequence heterogeneity; see the text for more detail.
The two panels of GBS reference strains and 63 selected clinical isolates were studied in more detail by sequencing >2,200 bp of each to identify appropriate sequences for use in the MS identification method. These and the remaining clinical isolates were then used to evaluate the MS identification method and to compare the results with those obtained by CS. Typing by both methods was initially done without knowledge of the results of the other method.
Bacterial isolates were retrieved from storage by subculture on blood agar plates (Columbia II agar base supplemented with 5% horse blood) and were incubated overnight at 37°C.
CS.
CS was performed by a standard methodology (29). Briefly, an acid-heated (56°C) extract was prepared for each isolate and the serotype was determined by immunoprecipitation of type-specific antiserum in agarose. An isolate was considered positive for a particular serotype when the precipitation that occurred formed a line of identity with that of the control strain. The antisera used were prepared against serotypes Ia, Ib, II, III, IV, and V in rabbits at ESR.
Fourteen selected isolates, including six isolates that were nontypeable with antisera against serotypes I to V, six isolates that initially gave discrepant results between CS and the MS identification method, and two separate isolates from a mixed culture, were kindly tested with antisera against all serotypes by Abbie Weisner and Androulla Efstratiou at the Central Public Health Laboratory, Colindale, London, United Kingdom.
Development of MS identification method. (i) Oligonucleotide primers.
Four previously published oligonucleotide primers and a series of new primers designed by us were used to sequence the genes of interest, namely, the 16S-23S rRNA intergenic spacer region and the partial cps gene cluster, or to amplify unique sequences of cps clusters of each GBS serotype. The sequences, target sites, and melting temperatures of the primers used in the present study are shown in Table 2. Some were designed with high melting temperatures to be used in a rapid-cycle PCR (12, 14).
TABLE 2.
Oligonucleotide primers used in the study
| Primer | Target | Melting temp (°C)a | GenBank accession no(s). | Sequenceb |
|---|---|---|---|---|
| CFBS | cfb gene | 56.7 | X72754 | 328-GAT GTA TCT ATC TGG AAC TCT AGT G-352 |
| Sag59c | cfb gene | 77.4 | X72754 | 350-GTGGCTGGTGCATTGTTAT TTT CAC CAG CTG TAT TAG AAG TA-391 |
| Sag190c | cfb gene | 76.8 | X72754 | 545-CATTAACCGGTTTTTCATAATCT GTT CCC TGA ACA TTA TCT TTG AT-500 |
| CFBA | cfb gene | 63.2 | X72754 | 568-TTT TTC CAC GCT AGT AAT AGC CTC-545 |
| 16SS | 16S rRNA gene | 69.3 | AB023574 | 1441-GCC GCC TAA GGT GGG ATA GAT G-1462 |
| 23SA | 23S rRNA gene | 65.7 | X68427 | 70-CGT CGT TTG TCA CGT CCT TC-51 |
| DSF2d | 16S rRNA gene | 75.9 | AB023574 | 975-CATCCTTCTGACC GGC CTA GAG ATA GGC TTT CT-1007 |
| DSR1d | 16S rRNA gene | 81.5 | AB023574 | 1250-CGTCACCGG CTT GCG ACT CGT TGT ACC AA-1222 |
| cpsES3 | cpsE gene | 71.5 | AB028896 (Ia), AF163833 (III) | 6410/6020-GTT AGA TGT TCA ATA TAT CAA TGA ATG GTC TAT TTG GTC AG-6450/6060 |
| cpsFS | cpsF gene | 75.0 | AB028896 (Ia), AF163833 (III) | 6777/6387-CAT CTG GTG CCG CTG TAG CAG TAC CAT T-6804/6414 |
| cpsFA | cpsF gene | 73.2 | AB028896 (Ia), AF163833 (III) | 6859/6469-GTC GAA AAC CTC TAT A/GTA AAC/T GGT CTT ACA A/GCC AAA TAA CTT ACC-6815/6425 |
| cpsGA | cpsG gene | 54.7 | AB028896 (Ia), AF163833 (III) | 7162/6772-AAG/C AGT TCA TAT CAT CAT ATG AGA G-7138/6748 |
| cpsGA1 | cpsG gene | 74.5 | AB028896 (Ia), AF163833 (III) | 7199/6809-CCG CCA/G TGT GTG ATA ACA ATC TCA GCT TC-7171/6781 |
| IacpsHS1 | cpsH gene | 77.9 | AB028896 (Ia) | 8463-GGC CTG CTG GGA TTA ATG AAT ATA GTT CCA GGT TTG C-8499 |
| cpsIA | cpsI gene | 70.3 | AB028896 (Ia), AF163833 (III) | 8816/8312-GTA TAA CTT CTA TCA ATG GAT GAG TCT GTT GTA GTA CGG-8778/8274 |
| IbcpsIS | cpsI gene | 71.1 | AB050723 (Ib) | 4116-GAT AAT AGT GGA GAA ATT TGT GAT AAT TTA TCT CAA AAA GAC G-4158 |
| IbcpsIA1 | cpsI gene | 78.6 | AB050723 (Ib) | 4638-CCT GAT TCA TTG CAG AAG TCT TTA CGA TGC GAT AGG TG-4601 |
| IIIcpsHS | cpsH gene | 72.1 | AF163833 (III) | 7672-GAA TAC TAT TGG TCT GTA TGT TGG TTT TAT TAG CAT CGC-7710 |
| IVcpsHS1 | cpsH gene | 71.2 | AF355776 (IV) | 7887-CCC AAG TAT AGT TAT GAA TAT TAG TTG GAT GGT TTT TGG-7925 |
| IVcpsMA | cpsH gene | 80.7 | AF355776 (IV) | 8265-GGG TCA ATT GTA TCG TCG CTG TCA ACA AAA CCA ATC AAA TC-8225 |
| VcpsHS2 | cpsH gene | 74.0 | AF349539 (V) | 7871-CCC AGT GTG GTA ATG AAT ATT AGT TGG CTA GTT TTT GG-7908 |
| VcpsMA | cpsM gene | 73.1 | AF349539 (V) | 8244-CCC CCC ATA AGT ATA AAT AAT ATC CAA TCT TGC ATA GTC AG-8204 |
| VIcpsHS1 | cpsH gene | 77.2 | AF337958 (VI) | 7767-CCT TAT TGG GCA AGG TAT AAG AGT TCC CTC CAG TGT G-7803 |
| VIcpsIA | cpsI gene | 74.5 | AF337958 (VI) | 8126-GAA GCA AAG ATT CTA CAC AGT TCT CAA TCA CTA ACT CCG-8088 |
The primer melting temperatures were provided by a primer synthesizer (Sigma-Aldrich).
Numbers represent the numbered base positions at which primer sequences start and finish (numbering start point 1 refers to start points 1 of the GenBank accession numbers for the corresponding genes). Underlined sequences indicate the bases added to modify previously published primers. Slashes between letters indicate the alternative nucleotides in different serotypes. Slashes between numbers indicate alternative positions according to two GenBank references.
From Ke et al. (12).
From Ahmet et al. (1).
(ii) DNA preparation and PCR.
Five individual GBS colonies or a sweep of culture was sampled with a disposable loop and resuspended in 1 ml of digestion buffer (10 mM Tris-HCl [pH 8.0], 0.45% Triton X-100, 0.45% Tween 20) in 2-ml Eppendorf tubes. The tubes containing GBS suspensions were heated at 100°C (in a dry block heater or water bath) for 10 min and then quenched on ice and centrifuged at 16,000 × g in an Eppendorf centrifuge 5415c for 2 min to pellet the cell debris. A total of 5 μl of each supernatant containing extracted DNA was used as a template for PCR (18).
PCR systems (25 μl for detection only, 50 μl for detection and sequencing) were used as described previously (13). The denaturation, annealing, and elongation temperatures and times used were 96°C for 1 s, 55 to 72°C (according to the primer melting temperatures) for 1 s, and 74°C for 1 to 30 s (according to the lengths of the amplicons), respectively, for 35 cycles.
A total of 10 μl of each of the PCR products was analyzed by electrophoresis on 1.5% agarose gels, which were stained with 0.5 μg of ethidium bromide ml−1. For detection and/or serotype identification, the presence of PCR amplicons of the expected length, shown by UV transillumination, was accepted as a positive result. For sequencing, 40 μl of each of the PCR products was further purified by the polyethylene glycol precipitation method (1).
(iii) Sequencing.
The PCR products were sequenced with Applied Biosystems Taq DyeDeoxy terminator cycle-sequencing kits by standard protocols. The corresponding amplification primers or inner primers were used as the sequencing primers.
(iv) Multiple-sequence alignments.
Multiple-sequence alignments were performed with the Pileup and Pretty programs in the Multiple Sequence Analysis program group. Both programs are provided in WebANGIS (3rd version; Australian National Genomic Information Service).
Nucleotide sequence accession numbers.
The new sequence data reported in this paper appear in the GenBank Nucleotide Sequence Database under accession numbers AF291411 to AF291419 for the 16S-23S rRNA intergenic spacer regions for serotype Ia to VIII reference strains from reference panel 1 and accession numbers AF332893 to AF332917, AF363032 to AF363060, AF367973, AF381030, and AF381031 for partial cps gene clusters for the two panels of reference strains [Table 1] and selected representative clinical isolates. The previously reported sequence data used in this paper have appeared in the GenBank Nucleotide Sequence Database with the following accession numbers: AB023574 for the 16S rRNA gene, U39765 and L31412 for the 16S-23S rRNA intergenic spacer regions, X68427 for the Streptococcus oralis 23S rRNA gene, X72754 for the cfb gene, AB028896 for the cps gene cluster for serotype Ia, AB050723 for the partial cps gene cluster for serotype Ib, AF163833 for the cps gene cluster for serotype III, AF355776 for the cps gene cluster for serotype IV, AF349539 for the cps gene cluster for serotype V, and AF337958 for the cps gene cluster for serotype VI.
RESULTS
PCR.
With two exceptions, all GBS-specific primer pairs produced amplicons of the expected sizes from all reference strains and clinical isolates tested (Table 2). The exceptions were Sag59-Sag190 and CFBS-CFBA. Both of these primer pairs target the cfb gene but failed to produce amplicons from one clinical isolate, despite repeated attempts. We assumed either that this isolate lacked the cfb gene or that the gene was present in a mutant form. It has been suggested previously that a PCR that targets the cfb gene will not identify all GBS isolates (8) and that another primer pair based on the 16S rRNA gene, DSF2-DSR1 (1), is not entirely specific. Therefore, in the present study we used both primer pairs (DSF2-DSR1 and Sag59-Sag190) to confirm that all the isolates were GBS isolates.
Sequence heterogeneity of 16S-23S rRNA intergenic spacer regions.
The 16S-23S rRNA intergenic spacer regions were sequenced for strains of serotypes Ia to VIII from reference panel 1. Multiple-sequence alignment showed differences between the sequences of the serotypes at only two positions: position 207 (serotype V has a T or a C residue, serotypes VII and VIII have a C residue, and the other serotypes have a T residue) and position 272 (serotype III has a T residue, and the other serotypes have a G residue). These regions are therefore unsuitable for use in the MS identification method. (Throughout this report, the position numbers in the sequences refer to the numbers in the corresponding GenBank sequences.)
Sequence heterogeneity at 3′ end of cpsD-cpsE-cpsF and 5′ end of cpsG.
Using a series of primers that target the 3′ end of cpsD-cpsE-cpsF and the 5′ end of cpsG, we amplified and sequenced 2,226 or 2,217 bp, depending on the presence or absence of a 9-base repetitive sequence, from both panels of reference strains (serotypes Ia to VII) and 63 selected clinical isolates. Representative sequences were deposited in GenBank. See Table 1 for the GenBank accession numbers of the strains from the reference panel.
Repetitive sequence.
At the 3′-end region of cpsD, we found a 9-base repetitive sequence (TTA CGG CGA) in most isolates of MSs Ia and II; some isolates of MS III; all isolates of MS IV, V, and VII; but none of the isolates of MS Ib or VI examined (Table 3). The presence or absence of this repetitive sequence can be used to further subtype MS Ia, II, and III (see below).
TABLE 3.
Heterogeneity of eight GBS MSs and subtypes in regions of 3′ end of cpsD and 5′ end of cpsE
| Gene and sitesa | Nucleotide
|
Specificity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ia | Ib | II and III-4e | III | IV | V | VI | VII | ||
| cpsD | |||||||||
| 62 | G | A | Gb | A | A | A | A | G | Ia, II, VII |
| 78–86 | − in Ia-2c and + in Ia-1c | − | − in II-2b and + in II-1d | − in III-2; + in III-1 and in III-3e | + | + | − | + | See text |
| cpsD-cpsE spacer | |||||||||
| 138 | G | G | G | G | G | Af | G | G | V |
| 139 | G | G | G | A in III-2; G in III-1 and III-3 | G | G | G | G | III-2 |
| 144 | T | T | T | G in III-2; T in III-1 and III-3 | T | T | T | T | III-2 |
| cpsE | |||||||||
| 198 | A | C | Ab | A | C | Cf | A | A | Ib, IV, V |
| 204 | G | G | G | A in III-2 and III-3; G in III-1 | G | G | G | G | III-2, III-3 |
| 211 | T | T | T | T | T | T | G | T | VI |
| 218 | C | C | C | C | C | C | T | C | VI |
| 240 | T | T | T | T | T | T | C | T | VI |
| 249 | T | C | Tb | T | C | Cf | T | T | Ib, IV, V |
| 300 | C | C | C | T in III-2; C in III-1 and III-3 | C | C | C | C | III-2 |
| 321 | C | C | C | T in III-1; C in III-2 and III-3 | C | C | C | C | III-1 |
| 419 | T | C | Tb | T | T | T | T | T | Ib |
| 429 | A | T | Ab | T | T | T | T | A | Ia, II, VII |
| 437 | C | C | C; T in III-4 | C | C | C | C | T | VII, III-4 |
| 457 | T | A | Cb | A | A | A | A | C | Ia, II, VII |
| 466 | G | G | G | G | A | G | G | A | IV |
| 486 | G | A | A | G in III-3; A in III-2 and III-1 | A | A | A | A | Ia, III-3 |
| 602 | G | G | Ab | G | G | G | G | A | II, VII |
| 606 | T | T | T | T | T | T | C | T | VI |
| 627 | T | C | C | C | C | C | C | C | Ia |
| 636 | C | T | T | C in III-1; T in III-2 and III-3 | T | T | T | T | Ia, III-1 |
| 645 | C | T | Cb | C | T | T | C | C | Ib, IV, V |
| 803 | A | A | A | A | A | A | T | A | VI |
| 971 | C | T | T | C | C | C | T | T | Ia, III, IV, V |
| 1026 | A | G | G | G in III-2 and III-1; A in III-3 | A | A | G | G | Ia, III-3, IV, V |
| 1044 | T | T | T | T | T | T | C | T | VI |
| 1173 | A | G | A | A | A | A | A | A | Ib |
| 1194 | C | C | C | A | A | C | A | C | III, IV, VI |
| 1251 | G | G | G | G | G | G | A | G | VI |
| 1278 | A | A | A | A | A | G | A | A | V |
Numbering start point 1 refers to start point 1 of GenBank accession number AF332908 (for serotype IV reference strain 3139). A repetitive sequence (TTACGGCGA) is found at in gene cpsD at positions 78 to 86.
One serotype II strain, as determined by CS, has mutations at the nine sites (see text).
Repetitive sequence is present (+) in Ia-1 and absent (−) in Ia-2 (see text).
Repetitive sequence is present (+) in II-1 and absent (−) in II-2 (see text).
Repetitive sequence is present (+) in III-1 and III-3 and absent (−) in III-2; it is variable in III-4 (see text).
The nucleotides at positions 138, 198, and 249 in one serotype V reference strain, as determined by CS (Prague 10/84), are identical to the corresponding nucleotides in GenBank (GenBank accession number AF349539); the nucleotides are G, A, and T, respectively. In another serotype V reference strain as determined by CS (CJB 111) and all the other sequenced serotype V strains, as determined by CS, the nucleotides are identical; the nucleotides are A, C, and C, respectively.
Intraserotype heterogeneity.
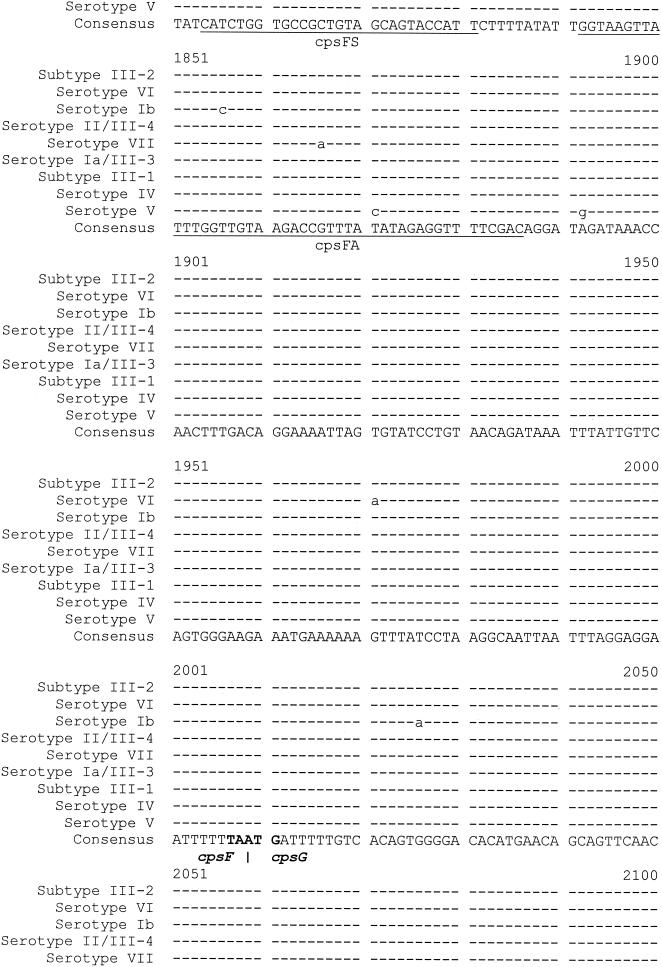
In general, the level of intraserotype heterogeneity was low; there were minor random variations in a few isolates of all serotypes except MS III, in which the intraserotype heterogeneity was more complex. MS III could be divided into four sequence subtypes on the basis of the heterogeneity of the sequence at 22 positions (positions −62, 139, 144, 204, 300, 321, 429, 437, 457, 486, 602, 636, 971, 1026, 1194, 1413, 1501, 1512, 1518, 1527, 1629, and 2134) and the presence or absence of the repetitive sequence (at positions 78 to 86) (Fig. 1; Table 3).
FIG. 1.
Molecular serotype identification based on the sequence heterogeneity of the 790-bp fragment (positions 1437 to 2226, corresponding to the regions of the amplicon obtained with primer pair cpsES3-cpsGA1) at the 3′ end of cpsE-cpsF and the 5′ end of cpsG (the relevant primers are indicated). Numbering start point 1 refers to start point 1 of the sequence with GenBank accession number AF332908 (for serotype reference strain 3139). Dashes indicate the same sequence as the consensus sequence; *, for serotype Ib, position 2221 refers to the sequence with GenBank accession number AB050723.
Among 60 MS III isolates (58 clinical isolates and 2 reference strains), subtypes III-1 (30 isolates) and III-2 (22 isolates) were predominant. The repetitive sequence was present in subtype III-1 but not subtype III-2; there were differences at seven other sites (positions 139, 144, 204, 300, 321, 636, and 1629) (Table 3).
Five isolates belonged to subtype III-3, which contained the repetitive sequence, and had nucleotides identical to those of subtype III-1 at three sites (positions 139, 144, and 300) and those of subtype III-2 at four sites (positions 204, 321, 626, and 1629). The sequence of subtype III-3 differed from those of both subtypes III-1 and III-2 at seven sites (positions 486, 1026, 1413, 1512, 1518, 1527, and 2134). The nucleotides at these seven sites in subtype III-3 were identical to those at the corresponding sites of MS Ia.
There were three subtype III-4 isolates, whose sequences were nearly identical to the corresponding sequence of MS II. The only exception was at position 437, where the nucleotide was T in subtype III-4 (as in MS VII) and C in MS II. This difference can be used (in addition to PCR; see below) to differentiate subtype III-4 from MS II. Two subtype III-4 isolates contained the repetitive sequence, but the other one did not. Because of the small number of subtype III-4 isolates, we did not use the repetitive sequence to subtype them further.
Interserotype heterogeneity.
There were 56 sites of heterogeneity among the eight MSs. The sites most suitable for use in PCR and sequencing for the MS identification method were a group of 23 sites nearest the 3′ end of the region (Fig. 1); the sites were contained in the regions corresponding to the amplicons of the cpsES3-cpsGA1 primer pair. First, they were consistent across two panels of reference strains and most clinical isolates (the only exceptions were the small number of subtype III-3 and III-4 isolates; see below). Second, they were relatively concentrated within a 790-bp region (positions 1437 to 2226), which is a convenient length for sequencing in a single reaction. Third, they contained enough heterogeneous sites to allow differentiation, with few exceptions, of MSs Ia to VII. Subtype III-3 cannot be distinguished from MS Ia only on the basis of the sequence of this 790-bp region, nor can subtype III-4 be distinguished from MS II. However, they can be identified by an MS III-specific PCR (see below).
Serotype VIII does not form amplicons with primer pairs that target the 790-bp region but can be identified by exclusion after PCR identification of GBS. The present study identified one MS VIII isolate for which none of the primer pairs that amplify the 2,226-bp region (in addition to those that amplify the 790 bp region) produced amplicons.
Mixed serotype specificities in single isolates.
Eleven isolates were identified as one MS on the basis of the MS-specific PCR and their overall sequences (within the 2,226- or 2,217-bp segment), but their sequences differed at some sites from those of isolates of the same MS and shared site-specific characteristics with those of isolates of another MS. They included five subtype III-3 isolates and three subtype III-4 isolates (see above). The sequence of one nonserotypeable reference strain (Prague 25/60), which Swas identified as MS II, differed from those of the other MS II isolates at five sites at the 5′ end of the region, but the nucleotides at three of these sites were identical to those of MS III. The MS III-specific PCR was negative for Prague 25/60. One clinical isolate identified as serotype II by CS and MS II on the basis of its overall sequence had bases at nine sites at the 5′ end of the region that were characteristic of serotype Ib isolates; the MS Ib-specific PCR was negative. Finally, one reference strain that was serotype V by CS (Prague 10/84) had the same sequence as the corresponding sequence in GenBank (accession number AF349539), but at the 5′ end of the region the sequences of both strains were different at three sites from the sequences of the other MS V strains that we studied.
All of these mixed-serotype specificities except for those associated with subtypes III-3 and III-4 occurred at the 5′-end region of the 2,226- or 2,217-bp fragment at position 2226 or 2217. This supported our selection of the 790-bp sequence at the 3′ end as the sequencing target for the MS identification method. By using this target, the MSs of all isolates except MS III isolates of subtypes III-3 and III-4 were correctly identified; the MSs of subtypes III-3 and III-4 can be identified by the MS III-specific PCR (see below).
MS identification method based on MS-specific PCR targeting the 3′ end of cpsG-cpsH-cpsI/cpsM.
Our sequence alignment results showed that there was significant sequence heterogeneity at the 3′ end of cpsG-cpsH-cpsI or cpsM (data not shown), which makes it appropriate for use of the 3′ end of cpsG-cpsH-cpsI or cpsM in the design of specific primer pairs for differentiation of serotypes Ia, Ib, III, IV, V, and VI directly by PCR (Table 2). By using two panels of reference strains and the specified conditions, all MS-specific primer pairs amplified DNA only from isolates of the corresponding serotypes. When clinical isolates were tested, an MS was assigned by PCR only to 179 of 206 (86.9%) clinical isolates, as follows: MS Ia n = 40; MS Ib, n = 35; MS III, n = 58; MS IV, n = 7; MS V, n = 36; MS VI, n = 3.
Comparison of serotype identification results between MS identification method and CS.
After CS and the MS identification method had been completed, the results were compared. The initial results were discrepant for 15 isolates; the results for all 15 isolates were resolved by retesting and/or correction of clerical errors.
The CS and the MS identification method and sequence subtyping results are shown in Table 4. An MS was assigned to all isolates by PCR and/or sequencing, whereas a serotype was assigned to 188 of 206 (91.3%) isolates by CS. A specific PCR has not yet been developed for MSs II and VIII, so the serotypes of all MS II isolates were determined by sequencing only and the serotype of one MS VIII isolate was determined by exclusion (see above). For all other isolates except those of subtypes III-3 and III-4 and those with other minor sequence differences described above, the results of PCR and sequencing were consistent. The CS results correlated well with the PCR results.
TABLE 4.
Comparison of results of CS and MS identification method and subtyping of 206 clinical GBS isolates
| Serotype by CS | No. of strains of MS and subtypea:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ia | Ib | II | III-1 | III-2 | III-3 | III-4 | IV | V | VI | VIII | |
| Ia | 38 | ||||||||||
| Ib | 30 | ||||||||||
| II | 25 | ||||||||||
| III | 27 | 20 | 4 | 3 | |||||||
| IV | 7 | ||||||||||
| V | 31 | ||||||||||
| VI | 2 | ||||||||||
| VIII | 1 | ||||||||||
| NTb | 2 | 5 | 1 | 3 | 1 | 5 | 1 | ||||
| Total (206)c | 40 | 35 | 26 | 30 | 21 | 4 | 3 | 7 | 36 | 3 | 1 |
For details about the MS III sequence subtypes (subtypes III-1, III-2, III-3, and III-4), see text.
NT, nontypeable.
One mixed culture was included as two separate isolates (one serotype II and one subtype III-2).
The final results of CS and the MS identification method were the same for all 188 isolates (100%) for which results by both methods were available. Eighteen clinical isolates that were nonserotypeable by CS were assigned the following MSs: Ia, two isolates; Ib, five isolates; II, one isolate; subtype III-1, three isolates; subtype III-2, one isolate; V, five isolates; and VI, one isolate.
Mixed culture.
Four clinical isolates gave positive results by the MS III-specific PCR but were provisionally identified as MS II by sequencing. Three were serotype III by CS and one was serotype II by CS, with a weak cross-reaction with serotype III antiserum. These isolates were studied further by subculturing 12 individual colonies of each isolate. All subcultures were tested by the MS III-specific PCR. All 12 subcultures of colonies of the three serotype III isolates by CS were positive by the MS III-specific PCR, and the isolates were therefore classified as subtype III-4 (see above). However, 11 of 12 subcultures of the colony of the fourth isolate were negative by the MS III-specific PCR and 1 subculture was positive by the MS III-specific PCR. It was therefore assumed that this was a mixed culture, predominantly of MS II and serotype II by CS. The serotypes of these two isolates obtained by CS were confirmed by retesting each isolate individually. The one colony that was MS III-specific PCR positive and serotype III by CS was subsequently identified as subtype III-2, and the isolate was included as an additional clinical isolate (for a total of 206).
Algorithm for GBS serotype assignment by PCR and sequencing.
In order to make GBS serotype identification by our PCR and sequencing method practicable, we designed an algorithm for clinical use (Table 5). All the primers (except the inner sequencing primers) used (see Table 2 for the primer sequences) had high melting temperatures (>70°C), so a rapid-cycle PCR could be used.
TABLE 5.
Algorithm for GBS MS identification by PCR and sequencing
| Amplification primer pairsa | PCR product size (bp) | Interpretation |
|---|---|---|
| GBS identification primer pairs | ||
| Sag59-Sag190 | 196 | GBS (S. agalactiae) |
| DSF2-DSR1 | 276 | GBS (S. agalactiae) |
| GBS MS identification by MS-specific PCR | ||
| IacpsHS1-cpsIA | 354 | Serotype Ia |
| IbcpsIS-IbcpsIA1 | 523 | Serotype Ib |
| IIIcpsHS-cpsIA | 641 | Serotype III |
| IVcpsHS1-IVcpsMA | 379 | Serotype IV |
| VcpsHS2-VcpsMA | 374 | Serotype V |
| VIcpsHS1-VIcpsIA | 360 | Serotype VI |
| GBS MS identification by sequencing | ||
| cpsES3-cpsGA1 | 790 | See Fig. 1b |
| cpsES3-cpsFA | 450 | See Fig. 1b |
| cpsFS-cpsGA1 | 423 | See Fig. 1b |
DISCUSSION
Capsule production in GBS isolates is controlled by the capsular polysaccharide synthesis (cps) gene cluster (3), which had been sequenced for serotype Ia (30) and serotype III (3) strains before we began our study. Differences between the two serotypes and serotype-specific definition regions of cpsH (capsular polysaccharide repeating unit polymerase gene, serotype definition gene) have been identified previously (3, 30). The corresponding sequences for serotype Ib (Miyake et al., submitted to GenBank, 2001 [GenBank accession number AB050723]) and serotypes IV, V, and VI (McKinnon et al., submitted to GenBank, 2001 [GenBank accession numbers AF355776, AF349539, and AF337958, respectively]) were released when the project was nearly finished; but the sequences of the cps gene clusters for the other three serotypes (serotypes II, VII, and VIII) have not previously been published.
The published sequences of the cps gene clusters for serotypes Ia and III showed considerable homology at the 3′ end of cpsD-cpsE-cpsF and the 5′ end of cpsG (3, 30), and we hypothesized that these regions would also be relatively conserved in other serotypes. Our study and the recently released sequences for the cps gene clusters of serotypes Ib, IV, V, and VI have supported this hypothesis. We designed a series of primers to amplify a 2,226- or 2,217-bp segment in this region and found that amplicons were obtained from all serotypes except serotype VIII (3, 30). This confirmed a previous suggestion that the sequence of serotype VIII in this region is significantly different from those of other serotypes (4).
Using eight serotype (serotype Ia to VII) reference strains, we showed more than 50 points of heterogeneity between serotypes (Fig. 1). Using 63 selected clinical isolates that had been serotyped by conventional methods, we found that these interserotype differences were generally consistent and specific, especially those at the 23 sites clustered at the 3′ ends of the regions (corresponding to the regions amplified by primer pair cpsES3-cpsGA1). We used these differences to assign serotypes to the remaining clinical isolates collected for the present study without knowledge of the serotype obtained by conventional methods.
Sequence analysis of the 3′ end of cpsG-cpsH-cpsI or cpsM for serotypes Ia, III, Ib, IV, V, and VI showed that this region is highly variable, and cpsH also contains serotype-specific definition sites (3), making this region a suitable target for direct serotype identification by PCR. We designed MS-specific primers for MSs Ia, Ib, III, IV, V, and VI and confirmed their specificities, initially using two serotype reference panels for CS. When the MS-specific PCR alone was used to test 206 clinical isolates, it correctly identified 86.9% of the isolates. The results of the rapid-cycle MS-specific PCR are available within 1 working day. In future, it will be possible to extend this method to all MSs when the sequences of this region of the cps gene cluster are available for serotypes II, VII, and VIII.
MSs II and VII can be identified by sequencing of the 790-bp PCR amplicons of the 3′ end of cpsE-cpsF and the 5′ end of cpsG. A positive GBS-specific PCR result and negative PCR results with all the primers that amplify the 790 bp identified MS VIII by exclusion. In future, and in some laboratories at present, sequencing of the 790-bp PCR amplicons of the 3′ end of cpsE-cpsF and the 5′ end of cpsG for all isolates may be more convenient, as only one method and fewer primers are needed. However, if sequencing is not available in-house, the turnaround time is longer and a small proportion of serotypes would be assigned to the incorrect serotype (subtypes III-3 and III-4 would be assigned MSs Ia and II, respectively). This could be avoided by first screening by the MS III-specific PCR. Sequencing of the 790-bp PCR amplicon allows MS III to be subtyped on the basis of the sequence heterogeneity.
Previous studies in the United States and several other countries have shown that serotypes Ia, Ib, II, III, and V are those most frequently isolated from normally sterile sites (9, 11). Serotypes VI and VIII are the predominant serotypes isolated from patients in Japan (15) but are uncommon elsewhere. Although our isolates were selected, they were probably representative of those that cause disease in Australasia; serotypes Ia, Ib, II, III, and V were the most common serotypes identified, although there were small numbers of isolates of serotypes IV, VI, and VIII.
Up to 13% of GBS isolates are nonserotypeable (28); in our study the proportion was 8.7% (18 of 206 isolates) when antisera against all nine serotypes were used. Failure to react with antisera may be due to decreased levels of synthesis of type-specific antigen (20), nonencapsulated phase variation, or insertion or mutation in genes of cps gene clusters (4, 25). One nonserotypeable GBS strain in our study had a deletion of a T base in the cpsG gene; this caused a change in the cpsG reading frame.
In summary, we have developed an alternative to CS for GBS isolates which is accurate and reproducible, can be performed by any laboratory with access to PCR and sequencing capabilities, and, importantly, does not require panels of serotype-specific antisera, which are increasingly difficult to maintain. All isolates are serotypeable, and sequencing of a relatively limited 790-bp region can provide additional subtyping information for MS III. In future we will combine this method with further PCR typing to identify members of the family of variable surface proteins that are important virulence factors for GBS isolates and known GBS mobile genetic elements (which will be reported separately). These extended typing methods will provide a comprehensive means of strain identification that will be useful for the epidemiological studies that will be needed to monitor GBS isolates before and after introduction of GBS conjugate vaccines.
Acknowledgments
We thank Ansuiya Sharma, Rebecca Hoile, Leanne Montgomery, David Smith, and Gordana Nedeljkovic for help in culturing GBS isolates; Moana Ngatai for some serotyping; and Mark Wheeler for precious help in sequencing.
REFERENCES
- 1.Ahmet, Z., P. Stanier, D. Harvey, and D. Holt. 1999. New PCR primers for the sensitive detection and specific identification of group B beta-hemolytic streptococci in cerebrospinal fluid. Mol. Cell. Probes 13:349–357. [DOI] [PubMed] [Google Scholar]
- 2.Arakere, G., A. E. Flores, P. Ferrieri, and C. E. Frasch. 1999. Inhibition enzyme-linked immunosorbent assay for serotyping of group B streptococcal isolates. J. Clin. Microbiol. 37:2564–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaffin, D. O., S. B. Beres, H. H. Yim, and C. E. Rubens. 2000. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J. Bacteriol. 182:4466–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139–146. [DOI] [PubMed] [Google Scholar]
- 5.Cropp, C. B., R. A. Zimmerman, J. Jelinkova, A. H. Auernheimer, R. A. Bolin, and B. C. Wyrick. 1974. Serotyping of group B streptococci by slide agglutination fluorescence microscopy and microimmunodiffusion. J. Lab. Clin. Med. 84:594–603. [PubMed] [Google Scholar]
- 6.Hakansson, S., L. G. Burman, J. Henrichsen, and S. E. Holm. 1992. Novel coagglutination method for serotyping group B streptococci. J. Clin. Microbiol. 30:3268–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison, L. H., J. A. Elliott, D. M. Dwyer, J. P. Libonati, P. Ferrieri, L. Billmann, and A. Schuchat. 1998. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program. J. Infect. Dis. 177:998–1002. [DOI] [PubMed] [Google Scholar]
- 8.Hassan, A. A., A. Abdulmawjood, A. O. Yildirim, K. Fink, C. Lammler, and R. Schlenstedt. 2000. Identification of streptococci isolated from various sources by determination of cfb gene and other CAMP-factor genes. Can. J. Microbiol. 46:946–951. [PubMed] [Google Scholar]
- 9.Hickman, M. E., M. A. Rench, P. Ferrieri, and C. J. Baker. 1999. Changing epidemiology of group B streptococcal colonization. Pediatrics 104:203–209. [DOI] [PubMed] [Google Scholar]
- 10.Holm, S. E., and S. Hakansson. 1988. A simple and sensitive enzyme immunoassay for determination of soluble type-specific polysaccharide from group B streptococci. J. Immunol. Methods 106:89–94. [DOI] [PubMed] [Google Scholar]
- 11.Kalliola, S., J. Vuopio-Varkila, A. K. Takala, and J. Eskola. 1999. Neonatal group B streptococcal disease in Finland: a ten-year nationwide study. Pediatr. Infect. Dis. J. 18:806–810. [DOI] [PubMed] [Google Scholar]
- 12.Ke, D., C. Menard, F. J. Picard, M. Boissinot, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2000. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin. Chem. 46:324–331. [PubMed] [Google Scholar]
- 13.Kong, F., X. Zhu, W. Wang, X. Zhou, S. Gordon, and G. L. Gilbert. 1999. Comparative analysis and serovar-specific identification of the multiple banded antigen genes of Ureaplasma urealyticum biovar 1. J. Clin. Microbiol. 37:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong, F., S. Gordon, and G. L. Gilbert. 2000. Rapid-cycle PCR for detection and typing of Mycoplasma pneumoniae in clinical specimens. J. Clin. Microbiol. 38:4256–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachenauer, C. S., D. L. Kasper, J. Shimada, Y. Ichiman, H. Ohtsuka, M. Kaku, L. C. Paoletti, P. Ferrieri, and L. C. Madoff. 1999. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J. Infect. Dis. 179:1030–1033. [DOI] [PubMed] [Google Scholar]
- 16.Levine, E. M., V. Ghai, J. J. Barton, and C. M. Strom. 1999. Intrapartum antibiotic prophylaxis increases the incidence of gram-negative neonatal sepsis. Infect. Dis. Obstet. Gynecol. 7:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, F. Y., J. D. Clemens, P. H. Azimi, J. A. Regan, L. E. Weisman III, J. B. Philips, G. G. Rhoads, P. Clark, R. A. Brenner, and P. Ferrieri. 1998. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J. Infect. Dis. 177:790–792. [DOI] [PubMed] [Google Scholar]
- 18.Mawn, J. A., A. J. Simpson, and S. R. Heard. 1993. Detection of the C protein gene among group B streptococci using PCR. J. Clin. Pathol. 46:633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagano, Y., N. Nagano, S. Takahashi, K. Murono, K. Fujita, F. Taguchi, and Y. Okuwaki. 1991. Restriction endonuclease digest patterns of chromosomal DNA from group B beta-haemolytic streptococci. J. Med. Microbiol. 35:297–303. [DOI] [PubMed] [Google Scholar]
- 20.Palacios, G. C., E. K. Eskew, F. Solorzano, and S. J. Mattingly. 1997. Decreased capacity for type-specific-antigen synthesis accounts for high prevalence of nontypeable strains of group B streptococci in Mexico. J. Clin. Microbiol. 35:2923–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti, L. C., J. Pinel, K. D. Johnson, B. Reinap, R. A. Ross, and D. L. Kasper. 1999. Synthesis and preclinical evaluation of glycoconjugate vaccines against group B Streptococcus types VI and VIII. J. Infect. Dis. 180:892–895. [DOI] [PubMed] [Google Scholar]
- 22.Rolland, K., C. Marois, V. Siquier, B. Cattier, and R. Quentin. 1999. Genetic features of Streptococcus agalactiae strains causing severe neonatal infections, as revealed by pulsed-field gel electrophoresis and hylB gene analysis. J. Clin. Microbiol. 37:1892–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrag, S. J., S. Zywicki, M. M. Farley, A. L. Reingold, L. H. Harrison, L. B. Lefkowitz, J. L. Hadler, R. Danila, P. R. Cieslak, and A. Schuchat. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N. Engl. J. Med. 342:15–20. [DOI] [PubMed] [Google Scholar]
- 24.Schuchat, A. 1999. Group B streptococcus. Lancet 353:51–56. [DOI] [PubMed] [Google Scholar]
- 25.Sellin, M., C. Olofsson, S. Hakansson, and M. Norgren. 2000. Genotyping of the capsule gene cluster (cps) in nontypeable group B streptococci reveals two major cps allelic variants of serotypes III and VII. J. Clin. Microbiol. 38:3420–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towers, C. V., M. H. Carr, G. Padilla, and T. Asrat. 1998. Potential consequences of widespread antepartal use of ampicillin. Am. J. Obstet. Gynecol. 179:879–883. [DOI] [PubMed] [Google Scholar]
- 27.Triscott, M. X., and G. H. Davis. 1979. A comparison of four methods for the serotyping of group B streptococci. Aust. J. Exp. Biol. Med. Sci. 57:521–527. [DOI] [PubMed] [Google Scholar]
- 28.Tyrrell, G. J., L. D. Senzilet, J. S. Spika, D. A. Kertesz, M. Alagaratnam, M. Lovgren, and J. A. Talbot. 2000. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study—1996. Sentinel Health Unit Surveillance System Site Coordinators. J. Infect. Dis. 182:168–173. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson, H. W., and M. D. Moody. 1969. Serological relationships of type I antigens of group B streptococci. J. Bacteriol. 97:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto, S., K. Miyake, Y. Koike, M. Watanabe, Y. Machida, M. Ohta, and S. Iijima. 1999. Molecular characterization of type-specific capsular polysaccharide biosynthesis genes of Streptococcus agalactiae type Ia. J. Bacteriol. 181:5176–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuerlein, T. J., B. Christensen, and R. T. Hall. 1991. Latex agglutination detection of group-B streptococcal inoculum in urine. Diagn. Microbiol. Infect. Dis. 14:191–194. [DOI] [PubMed] [Google Scholar]