Abstract
We used Tellegen’s Multidimensional Personality Questionnaire (MPQ) harm avoidance (fear) scale and the constraint superfactor as personality measures of inhibitory control and examined their association with glucose metabolism in the orbitofrontal gyrus at rest in 14 recently abstinent methamphetamine-dependent subjects and 22 comparison subjects. Higher MPQ scores were associated with higher relative orbitofrontal gyrus metabolism in the methamphetamine-dependent subjects. There was a tendency towards a negative association for the comparison subjects (test of coincidence of regression lines for the two subject groups: F = 3.3, df = 2,32; p = 0.051). These results suggest that the role of the orbitofrontal cortex in inhibitory control can be manifested in stable personality predispositions and further implicate this region in the core characteristics of drug addiction.
Keywords: Constraint superfactor, Drug addiction, Fear, Glucose metabolism, Harm avoidance scale, Inhibitory control, Methamphetamine dependence, Normalized/relative metabolism, Orbitofrontal cortex, PET FDG, Resting baseline, Tellegen’s Multidimensional Personality Questionnaire
INTRODUCTION
Compulsive drug self-administration in addicted individuals occurs even when the drug is no longer perceived as pleasurable and in the presence of adverse physical reactions to the drug [1]. We previously suggested that this process of loss of control and drug bingeing, a core characteristic of drug addiction, could be attributed to impairments in response inhibition [2] and a disrupted function of the striato-thalamo-orbitofrontal circuit [3,4]. Indeed, we recently documented that higher relative orbitofrontal gyrus glucose metabolism at rest, measured using 2-deoxy-2[18F]fluoro-d-glucose (FDG) PET, was associated with higher inhibitory control (higher Stroop interference score) in cocaine-addicted subjects and alcoholics. In contrast, for comparison subjects matched on age, education, IQ and performance on the Stroop task, higher metabolism was associated with lower control (lower score) [5].
The goal of the current report was to validate these results using self-report personality measures of inhibitory control. Tellegen’s Multidimensional Personality Questionnaire (MPQ) [6] includes a three-dimensional constraint superfactor, defined as a threshold variable that modulates stimulus elicitation of behavior, affect, and cognition, providing a measure of individual differences in behavioral inhibition [7]. Following the suggestion that a neurobiological basis for personality traits can be identified most clearly by analyzing more homogeneous as compared to heterogeneous traits [7], and to contain Type I (experimenter-wise) error probability, we chose to target the scale that has the highest loadings on the constraint superfactor, the harm avoidance scale. This scale measures the motivational trait that underlies specific approach and avoidance tendencies, linked to basic individual differences in fearfulness of physically dangerous situations; high-scoring persons report restricting themselves to situations that are relatively safe and avoiding physically dangerous activities and situations [6].
We performed correlational analyses between measures of global and relative orbitofrontal gyrus glucose metabolism obtained at baseline using PET FDG and MPQ harm avoidance scale in 14 recently abstinent methamphetamine-dependent subjects and 22 comparison subjects. We hypothesized that similar to our findings with the Stroop task, the direction of the association between orbitofrontal gyrus metabolism and inhibitory control will be reversed for the methamphetamine-dependent subjects compared with control subjects. Specifically, we predicted that increased control (higher scores on the harm avoidance scale) would be associated with greater relative glucose metabolism in the orbitofrontal gyrus for the methamphetamine-dependent subjects but lower metabolism for the controls. Correlations with the constraint superfactor were also conducted.
MATERIALS AND METHODS
Subjects: Fourteen subjects (three men and 11 women), who fulfilled DSM-IV criteria for methamphetamine dependence, and 22 healthy volunteers (17 men and five women), had completed the MPQ within two weeks of a PET FDG study. The characteristics of the complete methamphetamine-dependent sample were described previously [8]. In brief, the methamphetamine-dependent subjects were recruited from drug rehabilitation centers in the Los Angeles area; they used ≥0.5 g/day, at least 5 days/week, for ≥2 years and abstained from any drug use for ≥2 weeks prior to study entry.
Detailed medical and drug use histories, physical, neurological, and psychiatric evaluations were performed for all subjects, including blood test screenings and HIV testing (HIV serology was only performed for the methamphetamine-dependent subjects). Subjects were excluded for history of psychiatric or neurological disorder, history of head trauma, medical illness, and current or past history of dependence on drugs other than nicotine, caffeine, and methamphetamine (dependence on methamphetamine was exclusionary for the comparison subjects). No subject was taking medication at the time of the study, and a prescan urine test was done to ensure the absence of psychoactive drug use in all subjects. Written informed consent was obtained from the subjects after the procedures had been fully explained. The study was approved by the Institutional Review Boards at both Brookhaven National Laboratory and Harbor-UCLA Medical Center.
Personality assessment: The psychometric properties of the MPQ, a factor-analytically developed 240-item instrument [6], are within the well acceptable range. Alpha coefficients for the MPQ harm avoidance scale averaged 0.83 across four samples of college and community men and women. A 30-day test–retest stability coefficient was 0.88 for the harm avoidance scale and 0.89 for the constraint superfactor. External correlations were also high: 0.56 and 0.53 with trait ratings and 0.55 and 0.58 with heritability data, for the harm avoidance scale and constraint superfactor, respectively [6].
The harm avoidance scale and constraint superfactor were highly correlated in both study groups (r = 0.82, p < 0.0001 for the comparison subjects and r = 0.75, p < 0.01 for the methamphetamine-dependent subjects). The harm avoidance scale and constraint superfactor also had similar correlations with the negative emotion (NEM) superfactor (r = −0.53, p < 0.0001 for harm avoidance with NEM; r = −0.54, p < 0.0001 for constraint with NEM across all subjects; these correlations were similar within each study group). There were no significant correlations between the harm avoidance scale or constraint superfactor and the positive emotion superfactor for any of the groups or across all subjects.
PET scanning: PET scans were performed with a CTI 931 scanner (Siemens, Knoxville, TN; 15 slices, spatial resolution: 6 × 6 × 6.5 mm full width at half maximum) in all but two subjects, who were scanned with a Siemens Exact HR+ scanner. Details on procedures for positioning, arterial and venous catheterization, quantification of radiotracer, and transmission and emission scans have been published [9]. Briefly, one 20-min emission scan was taken 35 min after an i.v. injection of 4–6 mCi of FDG. During the study, subjects were kept lying in the PET camera with their eyes open; the room was dimly lit and noise was kept to a minimum. A nurse remained with the subjects throughout the procedure to ensure that the subjects did not fall asleep during the study.
Regions of interest were selected by using a previously published template that locates 115 non-overlapping regions of interest (ROIs) [9]. In brief, we used small ROIs to minimize the contribution of partial volume effects on the metabolic values. The orbitofrontal gyrus averaged 0.7 cm3. The size and orientation of the ROIs were the same in all subjects. Placement of the regions was determined by reference to an atlas of axial tomographic anatomy [10] by an experienced investigator (G.J.W). Three ROIs in the frontal lobe 12 mm below an oblique plane, parallel to the canthomeatal line, were averaged to obtain measures for the orbitofrontal gyrus (Fig. 1). Global metabolism (average metabolism in the 15 planes scanned) was also obtained. Due to the large variability of the absolute regional metabolic measures, we computed ratios of the absolute regional to the global metabolic measures, thus obtaining relative regional measures of metabolism.
Fig. 1.
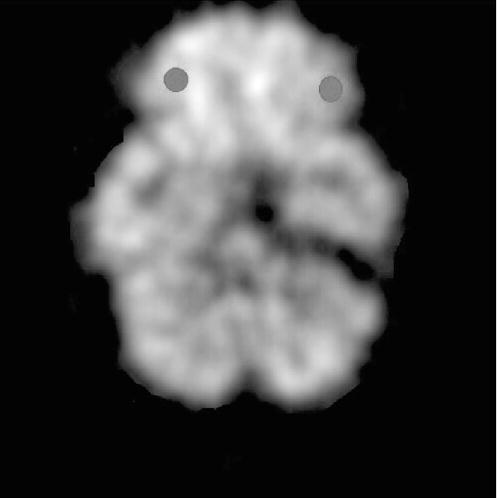
Location of the regions of interest used to assess metabolic activity in the orbitofrontal cortex.
Statistical analyses: The methamphetamine-dependent subjects were compared to the controls on select variables. Group differences in continuous variables were examined using unpaired Student’s t-tests (two-tailed). The corrected t statistic and degrees of freedom were used whenever Levene’s test for equality of variance was significant. For dichotomous variables, χ2 tests with Fisher’s exact statistic were used. Pearson product–moment correlation analyses were conducted between the MPQ variables and global and relative (normalized) brain metabolism in the orbitofrontal gyrus separately in each group. All correlations were one-tailed.
RESULTS
Demographics, MPQ variables, and orbitofrontal gyrus metabolism for the two groups are presented in Table 1. There were significant differences between the methamphetamine-dependent and comparison subjects in distributions of sex and handedness, and in age and education. There were no significant differences between the groups in distributions of race, in the MPQ variables, or in the metabolism measures.
Table 1.
Demographics, personality variables and PET FDG glucose metabolism in the orbitofrontal gyrus in study participants.
| Methamphetamine abusers (n = 14) | Comparison subjects (n = 22) | |
|---|---|---|
| Sex (% male)a | 21% | 84% |
| Race (% caucasian) | 93% | 65% |
| Handedness (% right)b | 64% | 97% |
| Agec | 32.9 (5.5) | 42.7 (15.3) |
| Educationd | 12.3 (1.4) | 15.7 (2.3) |
| MPQ harm avoidance scale | 18 (6.1) | 18.8 (5.5) |
| MPQ constraint superfactor | 49.8 (10) | 53.8 (12.2) |
| Orbitofrontal gyrus global metabolism | 53.2 (13.4) | 46.8 (7.3) |
| Orbitofrontal gyrus relative metabolism | 1.35 (0.1) | 1.38 (0.1) |
χ2 (df = 1, corrected for continuity) = 13.8, p < 0.0001;
χ2 (df = 1, corrected) = 6.2, p < 0.05;
t (df = 41.8, corrected for heterogeneity of variance) = 3.2, p < 0.01;
t (df = 38.7, corrected) = 6.0, p < 0.001.
The correlation between the MPQ harm avoidance scale and relative orbitofrontal gyrus metabolism was significant for the methamphetamine-dependent subjects (r = 0.57, p < 0.05) but not for the comparison subjects (r = 0.07, p > 0.1). To examine whether our definition of the orbitofrontal gyrus region influenced these correlations, we expanded it to include two more ROIs situated dorsally (2.8 and 3.4 mm above the canthomeatal line, respectively) to the original ROI. The correlation for the methamphetamine-dependent subjects was unchanged (r = 0.50), while for the controls it showed a trend towards a negative association (r = −0.18). When using a different normalization method (to decrease extreme values we divided the absolute metabolism in this expanded region by the metabolism only in gray matter, instead of by the global metabolism, which includes both gray and white matter), the correlation for the methamphetamine-dependent subjects was again unchanged (r = 0.56, p < 0.05). The negative correlation for the comparison subjects increased even further (r = −0.25) although it still did not reach the nominal significance threshold (Fig. 2). The magnitude of this correlation increased even further (r = −0.39, p = 0.087), when selecting the 14 comparison subjects that best matched the methamphetamine subjects on age and education.
Fig. 2.
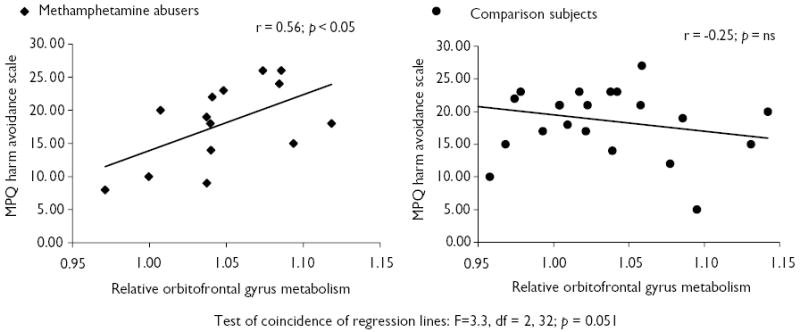
Association between Tellegen’s Multidimensional Personality Questionnaire (MPQ) harm avoidance and relative orbitofrontal gyrus metabolism in 14 methamphetamine abusers and 22 comparison subjects.
To examine whether the two regression lines that best fit the data for the methamphetamine-dependent subjects vs controls are significantly different, we performed a test for their coincidence. Results revealed that the null hypothesis specifying that the two regression lines are coincident, that is, have the same slope and intercept, was rejected with almost 95% confidence (F = 3.3, df = 2,32; p = 0.051).
Similar results were documented for the constraint superfactor; its positive correlation with relative orbitofrontal gyrus metabolism approached significance for the methamphetamine-dependent subjects (r = 0.40, p < 0.1) but not for the comparison subjects (r = −0.02, p > 0.1). These correlations increased for the methamphetamine-dependent subjects (r = 0.46, p = 0.05) and controls (r = −0.29, p = 0.09) when expanding the definition of the orbitofrontal gyrus as described above.
Finally, we examined whether the differences between the study groups on demographics can account for these results. Student’s t-tests revealed no significant differences between males (n = 20) and females (n = 16) and between right-handers (n = 30) and non-right-handers (n = 6) on all MPQ and metabolism measures. Pearson analyses revealed no significant correlations between education and any of the MPQ or metabolism variables for any of the groups (separately or combined). No further action was therefore taken to correct for differences in sex, handedness, and education between the groups. Age was negatively correlated with global orbitofrontal metabolism for the methamphetamine-dependent subjects only (r = −0.62, p < 0.05, two-tailed). We therefore conducted additional correlations between the MPQ and metabolism variables, controlling for age (partial correlations). As anticipated, these analyses revealed no change in the pattern of results for the comparison subjects. For the methamphetamine-dependent subjects, the strength of the correlations between relative orbitofrontal gyrus (as originally defined) metabolism and MPQ harm avoidance scale (r = 0.66, p < 0.01) and the constraint superfactor (r = 0.62, p < 0.05) was increased. No additional changes were observed.
DISCUSSION
For recently abstinent methamphetamine-dependent subjects, higher scores on the MPQ harm avoidance scale were associated with greater relative orbitofrontal gyrus glucose metabolism at rest. Controlling for age, metabolism in this region was also positively correlated with the MPQ constraint superfactor. These results implicate the orbitofrontal gyrus in behavioral inhibition where the higher the glucose metabolism at rest, the higher the self-reported avoidance of potentially harmful situations and inhibitory constraint on inappropriate approach behaviors [7]. These results are consistent with our previous study [5], in which higher relative orbitofrontal gyrus glucose metabolism at rest correlated with higher Stroop interference scores, indicating better inhibitory control of prepotent responses, in cocaine-addicted subjects and alcoholics.
Overall, these results are consistent with the role of the orbitofrontal cortex in evaluating response–reinforcement relationships; patients with orbitofrontal lesions show perseverative touching of a previously rewarded stimulus, unable to change their behavior in response to a change in reward contingencies [11]. Similarly, neuroimaging studies have documented orbitofrontal hypermetabolism in individuals with obsessive–compulsive disorder (for review see [12]) and Tourette syndrome [13], disorders that are characterized by perseverative behavior or the decreased ability to respond appropriately to changing contingencies in the environment. Intriguingly, a recent functional magnetic resonance imaging study demonstrated hypoactivity of the orbitofrontal cortex during aversive conditioning in criminal psychopathy and hyperactivity in social phobia, implicating under-responsiveness of the fear circuitry in the former and over-responsiveness in the latter [14]. A positive correlation between metabolism in the orbitofrontal gyrus at resting baseline and a self-reported trait of behavioral constraint and harm avoidance (fear) in the methamphetamine-dependent subjects in the present report, might indicate a greater disposition or vulnerability to experience periodic lack of control and compulsive drug intake (perseverative behavior) and increased behavioral impulsivity (including over-responsiveness to immediate rewards and decreased avoidance of longer-term punishment) [15].
For the comparison subjects, there was a tendency towards a negative correlation between relative orbitofrontal gyrus metabolism and the MPQ variables, such that higher harm avoidance and constraint scores were associated with lower orbitofrontal metabolism at rest. However, these results were not statistically significant. It is possible that glucose metabolism during activation would be more reliably associated with neuropsychological measures than metabolism at rest, as implicated in a recent PET study where metabolism in the visual and auditory regions was correlated with neurocognitive function (dementia severity) during stimulation but not at rest in 15 Alzheimer’s disease patients [16]. An alternative explanation pertains to the methodology we used to define this ROI, which was based on pre-selection of small regions [9] possibly contributing to a Type II error. Performing correlational analyses on a pixel-by-pixel basis, a significant positive correlation between regional cerebral blood flow in the ventromedial prefrontal cortex (a region that is located in close proximity to where we obtained the ROIs for the orbitofrontal gyrus) with negative affect was demonstrated in two large samples (n = 51 and n = 38) of healthy subjects in a recent PET study [17]. The positive direction of this association is consistent with the observed trends for the comparison subjects in the current report (NEM was negatively associated with harm avoidance and constraint, which in turn were negatively associated with the orbitofrontal gyrus in this group).
There were no significant correlations between the MPQ variables and the absolute orbitofrontal gyrus metabolism for any of the study groups, which may reflect the fact that regional absolute measures are strongly influenced by the overall metabolic activity in the brain of a given subject; in contrast, relative measures reflect much better regional differences between brain areas [18]. In addition, the increased variability in measures of absolute regional metabolism would necessitate a larger sample size to document significant correlations or group differences.
A limitation of this study is our inability to match the comparison group to the methamphetamine-dependent subjects on the demographic variables that are known to affect neurocognitive function. However, our results cannot be accounted for by the differences in sex, handedness and education because these variables were not significantly correlated with our dependent variables. When controlling for age, which was significantly correlated with orbitofrontal metabolism, the correlations between our dependent variables became stronger. Nevertheless, these results need to be validated using a larger sample size with matched control subjects.
CONCLUSION
Orbitofrontal gyrus metabolism is associated with the aspects of behavioral control that are identified on self-report measures of personality predispositions in recently abstinent methamphetamine dependent individuals. Taken together with our previous findings [5], we suggest that the role of the orbitofrontal cortex in the control of behavior is not limited to purely cognitive–behavioral functions, such as those measured by standard neuropsychological reaction-time or performance-based tools (e.g. Stroop), but also manifests in stable personality traits. Our results further implicate the orbitofrontal gyrus in the core characteristics of drug addiction, emphasizing its role in the failure to properly inhibit excessive drug consumption and develop aversive reactions to potentially dangerous situations.
Acknowledgments
This study was supported in part by grants from the National Institute on Drug Abuse (to N.D.V.: DA06891-06; to L.C.: 1R01 DA12734 and K20-DA00280; and to R.Z.G.: 1K23 DA15517-01), U.S. Department of Energy (OBER), and National Institute on Alcohol Abuse and Alcoholism (AA/ODO9481-04), Harbor-UCLA General Clinical Research Center (M01 RR00425), SUNY-Stony Brook General Clinical Research Center (M01 RR10710) and ONDCP).
References
- 1.Fischman MW, Schuster CR, Javaid J. J Pharmacol Exp Ther. 1985;235:677–682. [PubMed] [Google Scholar]
- 2.Volkow ND, Ding Y–S, Fowler JS. J Addict Dis. 1996;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Fowler JS, Wang G-J. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Fowler JS. Cerebr Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Volkow ND, Wang G-J. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tellegen A and Waller NG. Exploring personality through test construction: development of the multidimensional personality questionnaire. In: Briggs SR and Cheek JM (eds.), Personality Measures: Development and Evaluation, Vol. 1, Greenwich: JAI Press; 1997.
- 7.Depue RA, Collins PF. Behav Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Chang L, Wang G-J. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 9.Wang GJ, Volkow ND, Roque CT. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T and Hirano A. An Atlas of the Human Brain for Computerized Tomography. Stuttgart: Gustav Fisher Verlag; 1978.
- 11.Rolls ET, Hornack J, Wade D, McGrath J. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena S, Brody AL, Schwartz JM, Baxter LR. Br J Psychiatry. 1998;35:26–37. [PubMed] [Google Scholar]
- 13.Chase TN, Geoffrey V, Gillespie M. Rev Neurol. 1986;142:851–855. [PubMed] [Google Scholar]
- 14.Veit R, Flor H, Erb M. Neurosci Lett. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein RZ, Volkow ND. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrini P, Furey ML, Alexander GE. Am J Psychiatry. 1999;156:470–473. doi: 10.1176/ajp.156.3.470. [DOI] [PubMed] [Google Scholar]
- 17.Zald DH, Mattson DL, Pardo JV. Proc Natl Acad Sci. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Brodie JD, Wolf AP. J Cerebr Blood Flow Metab. 1986;6:441–446. doi: 10.1038/jcbfm.1986.77. [DOI] [PubMed] [Google Scholar]


